Reliability of Stereotactic Radiofrequency Ablation (SRFA) for Malignant Liver Tumors: Novice versus Experienced Operators
Abstract
Simple Summary
Abstract
1. Introduction
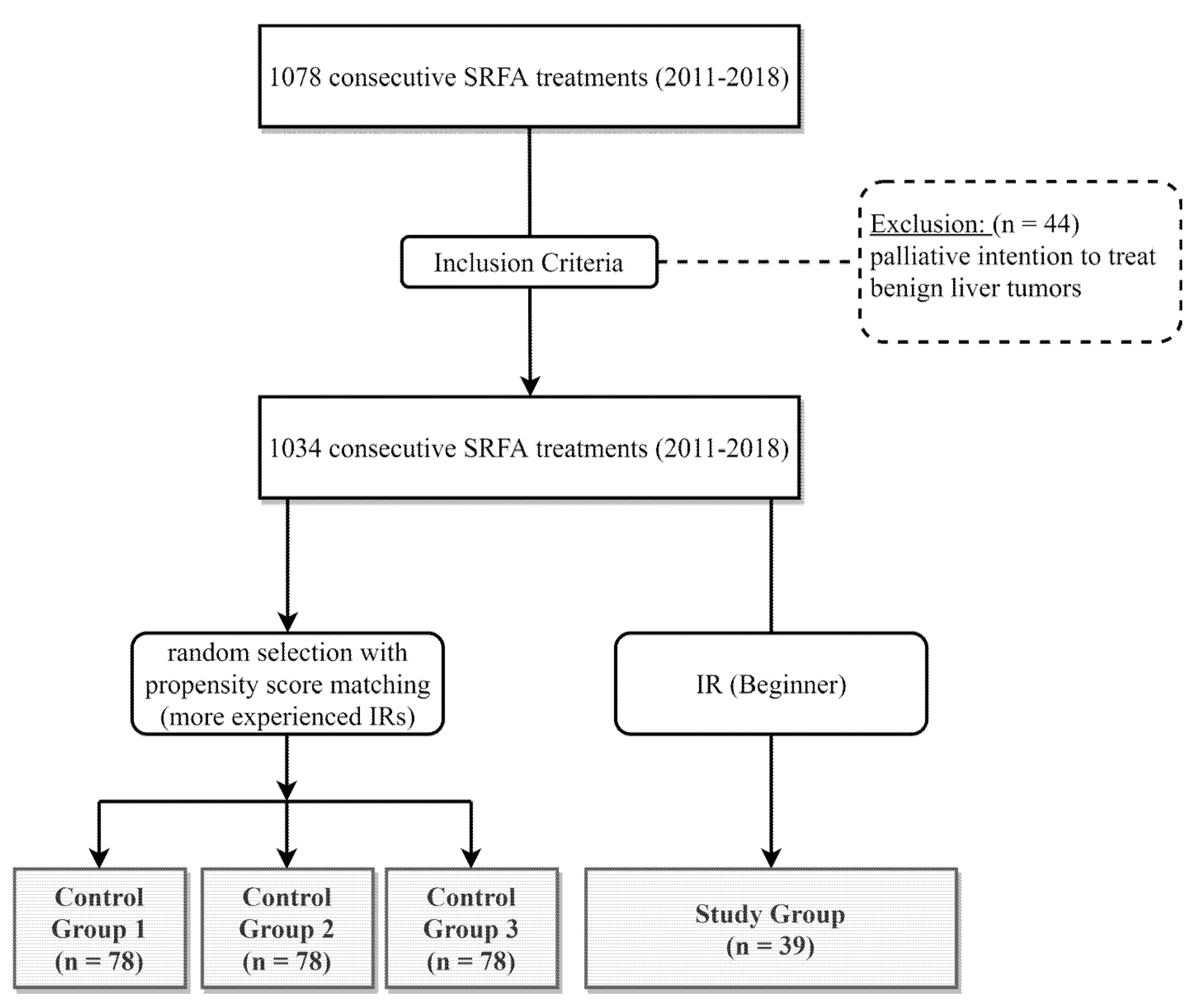
2. Materials and Methods
2.1. Patient Selection
2.2. Patient Characteristics
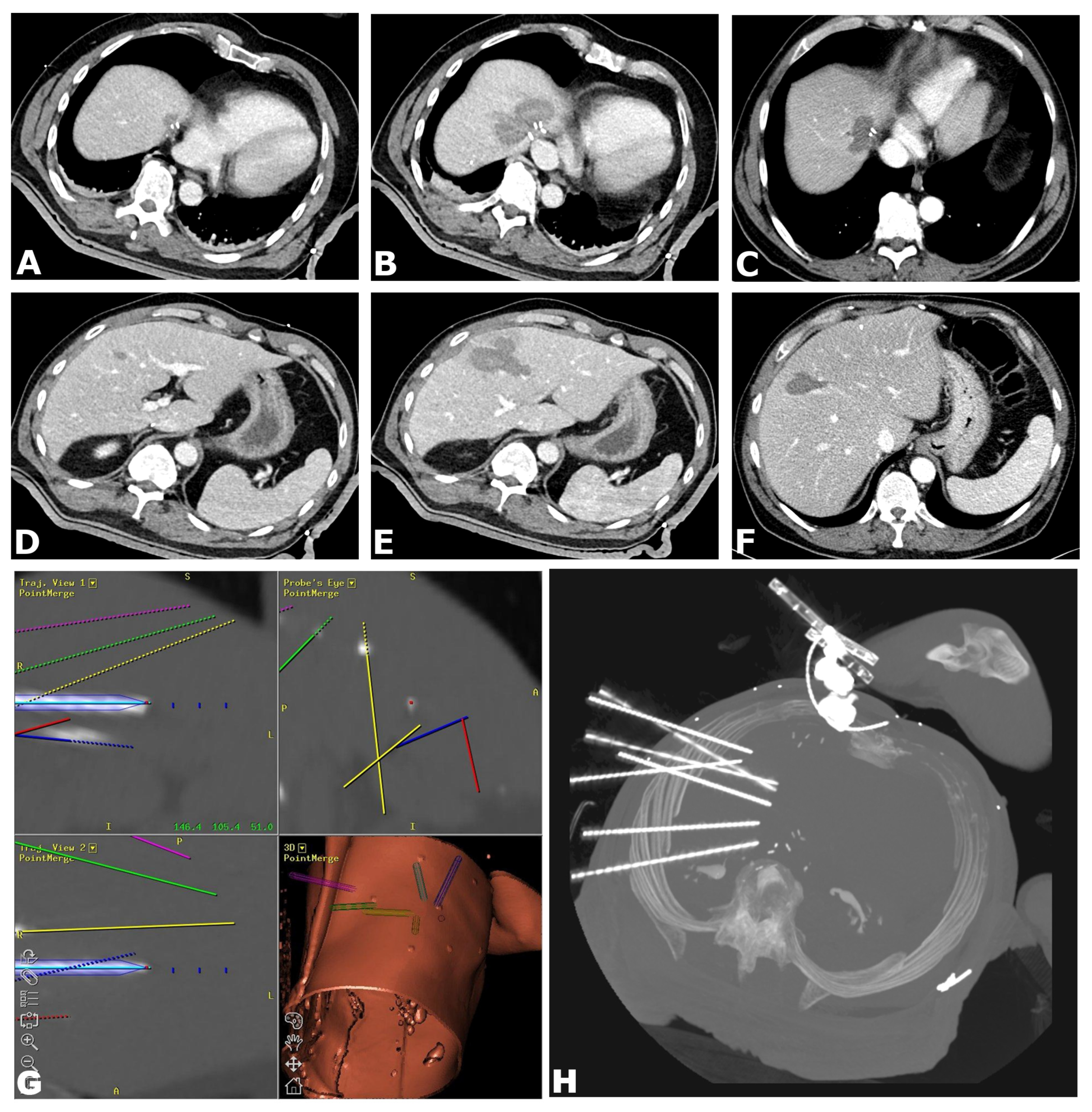
2.3. SRFA Procedure
- Preparation: The entire procedure is carried out under general anesthesia with full muscle relaxation. Immobilization is provided by a single (Bluebag, Interventional Systems, Kitzbühel, Austria) or double vacuum fixation technique (BodyFix, Medical Intelligence, Schwabmünchen, Germany). For image-to-patient registration, 10–15 registration markers (Beekley Spots, Beekley Corporation, Bristol, CT, USA), are broadly attached to the skin.
- Planning: A contrast-enhanced CT scan is acquired (Siemens SOMATOM Sensation Open, 82 cm bore size diameter, sliding gantry, Siemens AG, Erlangen, Germany) with 3 mm slice thickness in arterial and portal-venous phases. Datasets are transferred to an optical navigation system (Stealth Station Treon plus, Medtronic Inc., Boulder, CO, USA) and one or multiple antenna trajectories are planned with multiplanar and 3D reconstructed images using the navigation systems’ software.
- Needle Placement: To compensate for respiratory motion, temporary disconnections of the endotracheal tube (ETT) are carried out during each CT scan and for needle placement. After registration and sterile draping, an ATLAS aiming device (Interventional Systems, Kitzbühel, Austria) is used for navigated trajectory alignment and the placement of 15G/17.2 cm coaxial needles (Bard Inc., Murray Hill, NJ, USA) without real-time imaging control, serving as guides and placeholders for the RF electrodes. After co-axial needle placement, a non-enhanced CT-scan is acquired to verify needle placement by image fusion with the planning CT scan using the navigation system’s image 3D registration algorithm.
- RF Ablation: Up to three 17G RF probes (Cool-tip, Medtronic, Boulder, CO, USA, 3 cm exposure, 25 cm length) are inserted through the coaxial needles for serial tumor ablation, using the unipolar Cool-tip RF generator (Cool-tip, Medtronic, Boulder, CO, USA) and the Cool-tip RF switching controller for RF ablation. The standard ablation time for three RF probes is 16 min or until a significant increase in impedance (“roll-off effect”) is observed. Needle track ablation is performed prior to repositioning and final removal to reduce bleeding and potential tumor seeding.
- Finalization: After ablation, a final contrast-enhanced CT scan is carried out in both arterial and portal venous phases for the assessment of complications and evaluation of the ablative safety margins in 3D. If needed, the intervention may be continued in the same session by additional placement of coaxial needles and subsequent ablation (e.g., residual tumor, lack of sufficient safety margin).
2.4. Endpoints
2.5. IR Experience
2.6. Procedural Time Measurements
2.7. Statistical Analysis
3. Results
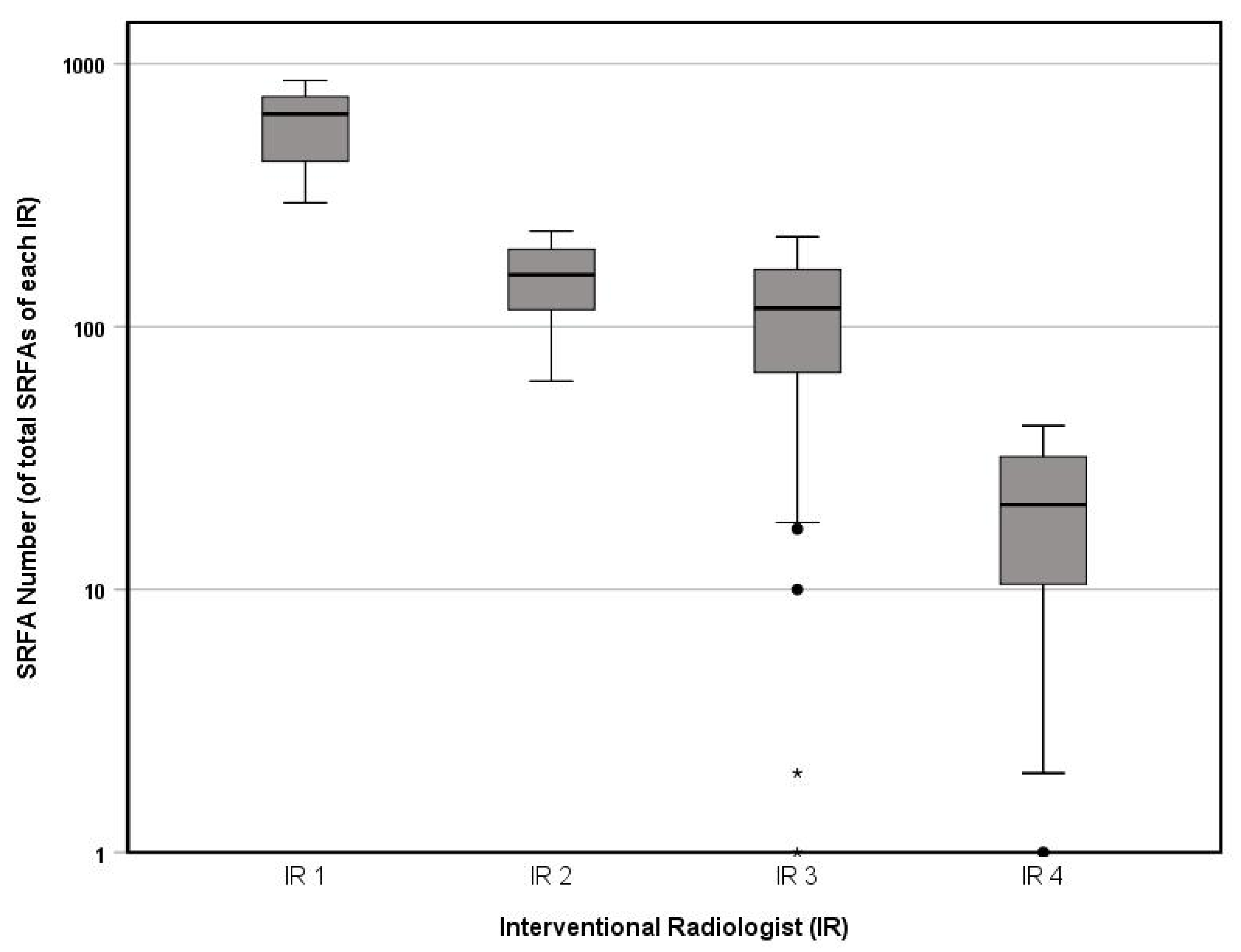
3.1. IR Experience
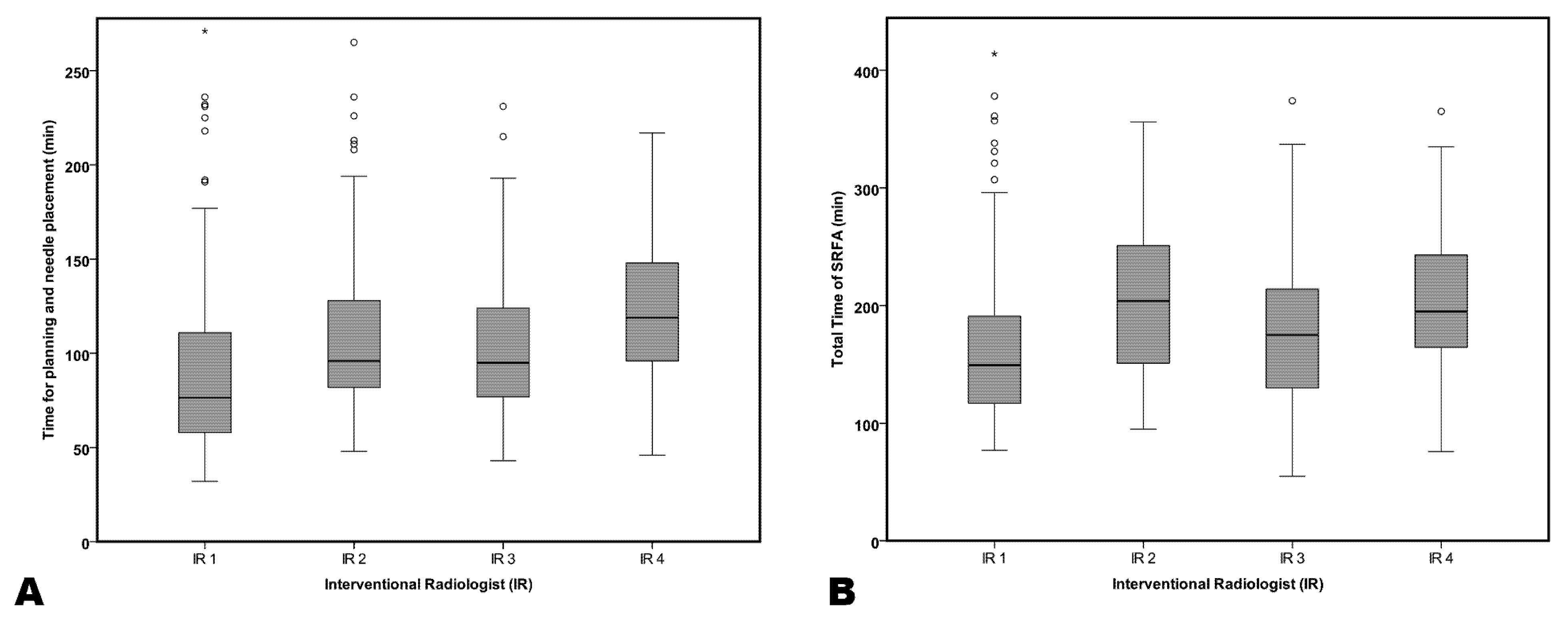
3.2. Procedural Time Efforts
3.3. Safety
3.4. Technical Success and Local Tumor Control
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Livraghi, T.; Meloni, F.; Di Stasi, M.; Rolle, E.; Solbiati, L.; Tinelli, C.; Rossi, S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008, 47, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Solbiati, L. Percutaneous treatment: Radiofrequency ablation of hepatic metastases in colorectal cancer. Tumori 2001, 87, S69. [Google Scholar] [PubMed]
- Taner, T.; Atwell, T.D.; Zhang, L.; Oberg, T.N.; Harmsen, W.S.; Slettedahl, S.W.; Kendrick, M.L.; Nagorney, D.M.; Que, F.G. Adjunctive radiofrequency ablation of metastatic neuroendocrine cancer to the liver complements surgical resection. HPB 2013, 15, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Wang, C.C.; Hung, C.H.; Chen, C.L.; Lu, S.N. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J. Hepatol. 2012, 56, 412–418. [Google Scholar] [CrossRef]
- Solbiati, L.; Ahmed, M.; Cova, L.; Ierace, T.; Brioschi, M.; Goldberg, S.N. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: Local response rate and long-term survival with up to 10-year follow-up. Radiology 2012, 265, 958–968. [Google Scholar] [CrossRef]
- Hildebrand, P.; Leibecke, T.; Kleemann, M.; Mirow, L.; Birth, M.; Bruch, H.P.; Burk, C. Influence of operator experience in radiofrequency ablation of malignant liver tumours on treatment outcome. Eur. J. Surg. Oncol. 2006, 32, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Lin, J.T.; Ho, H.J.; Wu, M.S.; Wu, C.Y. Evaluation of the Effect of Cumulative Operator Experience on Hepatocellular Carcinoma Recurrence after Primary Treatment with Radiofrequency Ablation. Radiology 2015, 276, 294–301. [Google Scholar] [CrossRef]
- Poon, R.T.; Ng, K.K.; Lam, C.M.; Ai, V.; Yuen, J.; Fan, S.T.; Wong, J. Learning curve for radiofrequency ablation of liver tumors: Prospective analysis of initial 100 patients in a tertiary institution. Ann. Surg. 2004, 239, 441–449. [Google Scholar] [CrossRef]
- Bale, R.; Widmann, G.; Stoffner, D.I. Stereotaxy: Breaking the limits of current radiofrequency ablation techniques. Eur. J. Radiol. 2010, 75, 32–36. [Google Scholar] [CrossRef]
- Widmann, G.; Schullian, P.; Haidu, M.; Bale, R. Stereotactic radiofrequency ablation (SRFA) of liver lesions: Technique effectiveness, safety, and interoperator performance. Cardiovasc. Intervent. Radiol. 2012, 35, 570–580. [Google Scholar] [CrossRef]
- Bale, R.; Widmann, G.; Haidu, M. Stereotactic radiofrequency ablation. Cardiovasc. Intervent. Radiol. 2011, 34, 852–856. [Google Scholar] [CrossRef]
- Bale, R.; Widmann, G.; Schullian, P.; Haidu, M.; Pall, G.; Klaus, A.; Weiss, H.; Biebl, M.; Margreiter, R. Percutaneous stereotactic radiofrequency ablation of colorectal liver metastases. Eur. Radiol. 2012, 22, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Schullian, P.; Johnston, E.W.; Putzer, D.; Eberle, G.; Laimer, G.; Bale, R. Stereotactic radiofrequency ablation of subcardiac hepatocellular carcinoma: A case-control study. Int. J. Hyperth. 2019, 36, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Omary, R.A.; Bettmann, M.A.; Cardella, J.F.; Bakal, C.W.; Schwartzberg, M.S.; Sacks, D.; Rholl, K.S.; Meranze, S.G.; Lewis, C.A. Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J. Vasc. Interv. Radiol. 2003, 14, S293–S295. [Google Scholar] [CrossRef] [PubMed]
- Takai Takamatsu, R.; Okano, A.; Yamakawa, G.; Mizukoshi, K.; Obayashi, H.; Ohana, M. Impact of an ultrasound-guided radiofrequency ablation training program on the outcomes in patients with hepatocellular carcinoma. Diagn. Interv. Imaging 2019, 100, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Schullian, P.; Putzer, D.; Laimer, G.; Levy, E.; Bale, R. Feasibility, safety, and long-term efficacy of stereotactic radiofrequency ablation for tumors adjacent to the diaphragm in the hepatic dome: A case-control study. Eur. Radiol. 2019, 30, 950–960. [Google Scholar] [CrossRef]
- Schullian, P.; Laimer, G.; Putzer, D.; Effenberger, M.; Bale, R. Stereotactic radiofrequency ablation of primary liver tumors in the caudate lobe. HPB 2019, 22, 470–478. [Google Scholar] [CrossRef]
- Kong, W.T.; Zhang, W.W.; Qiu, Y.D.; Zhou, T.; Qiu, J.L.; Zhang, W.; Ding, Y.T. Major complications after radiofrequency ablation for liver tumors: Analysis of 255 patients. World J. Gastroenterol. 2009, 15, 2651–2656. [Google Scholar] [CrossRef]
- Livraghi, T.; Solbiati, L.; Meloni, M.F.; Gazelle, G.S.; Halpern, E.F.; Goldberg, S.N. Treatment of focal liver tumors with percutaneous radio-frequency ablation: Complications encountered in a multicenter study. Radiology 2003, 226, 441–451. [Google Scholar] [CrossRef]
- Mulier, S.; Mulier, P.; Ni, Y.; Miao, Y.; Dupas, B.; Marchal, G.; De Wever, I.; Michel, L. Complications of radiofrequency coagulation of liver tumours. Br. J. Surg. 2002, 89, 1206–1222. [Google Scholar] [CrossRef]
- Ding, H.; Su, M.; Zhu, C.; Wang, L.; Zheng, Q.; Wan, Y. CT-guided versus laparoscopic radiofrequency ablation in recurrent small hepatocellular carcinoma against the diaphragmatic dome. Sci. Rep. 2017, 7, 44583. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | All | IR 1 | IR 2 | IR 3 | IR 4 | p-Value |
|---|---|---|---|---|---|---|
| Ablations, n | 273 | 78 | 78 | 78 | 39 | 0.924 |
| HCC, n (%) | 170 (62.3) | 45 (57.7) | 48 (61.5) | 53 (67.9) | 24 (61.5) | |
| ICC, n (%) | 9 (3.3) | 3 (3.8) | 3 (3.8) | 2 (2.6) | 1 (2.6) | |
| Mets, n (%) | 94 (34.3) | 30 (38.5) | 27 (34.6) | 23 (29.5) | 14 (35.9) | |
| Age, median years (range) | 69 (18–88) | 70 (38–84) | 71 (38–88) | 69 (44–84) | 66 (18–80) | 0.117 |
| Sex (female/male), n (%) | 91/182 (33/67) | 32/46 (41/59) | 22/56 (28/72) | 24/54 (31/69) | 13/26 (33/67) | 0.357 |
| Cirrhosis, n (%) | 162/273 (59.3) | 43/78 (55.1) | 45/78 (57.7) | 50/78 (64.1) | 25/39 (64.1) | 0.701 |
| Child A, n (%) | 135/162 (83.3) | 38/43 (88.4) | 33/45 (73.3) | 41/50 (82.0) | 24/25 (61.5) | |
| Child B, n (%) | 24/162 (14.8) | 5/43 (11.6) | 11/45 (24.4) | 7/50 (14.0) | 1/25 (4.0) | |
| Child C, n (%) | 3/162 (1.9) | - | 1/45 (2.2) | 2/50 (4.0) | - | |
| Max. Tumor Size, median (range) | 2.8 cm (0.5–15) | 3.0 cm (0.5–15) | 3.0 cm (0.5–10) | 2.4 cm (0.5–13) | 2.5 cm (0.9–13.5) | 0.373 |
| HCC, n (%) | 2.6 cm (0.8–15) | 3.0 cm (0.5–15) | 2.9 cm (1–8.3) | 2.3 cm (1.1–9.0) | 2.4 cm (0.9–6.3) | |
| ICC, n (%) | 5.5 cm (0.5–8.0) | 2.0 cm (0.5–5.5) | 5.4 cm (2.8–8.0) | 7.0 cm (6.0–8.0) | 6.7 cm (6.7–6.7) | |
| Mets, n (%) | 2.7 cm (0.8–13.5) | 3.0 cm (0.8–13.5) | 3.5 cm (0.8–10.0) | 2.4 cm (1.0–13.0) | 3.0 cm (1.1–13.5) | |
| Tumor Number, n (range) | 2 (1–11) | 1 (1–11) | 2 (1–6) | 2 (1–11) | 1 (1–8) | 0.133 |
| HCC, n (%) | 2 (1–9) | 2 (1–9) | 1 (1–4) | 2 (1–6) | 2 (1–8) | |
| ICC, n (%) | 2 (1–3) | 2 (1–3) | 1.5 (1–2) | 1.5 (1–2) | 1 (1–1) | |
| Mets, n (%) | 1 (1–11) | 1 (1–11) | 2 (1–6) | 3 (1–11) | 1 (1–5) | |
| Needles, n (range) | 4 (1–28) | 4 (1–28) | 5 (1–12) | 4 (1–14) | 5 (1–20) | 0.159 |
| HCC, n (%) | 4 (1–20) | 4 (1–20) | 5 (1–12) | 4 (1–12) | 4 (1–20) | |
| ICC, n (%) | 6 (1–11) | 4 (4–7) | 8 (6–11) | 4 (1–7) | 10 (10–10) | |
| Mets, n (%) | 4 (1–28) | 5 (1–28) | 5 (1–12) | 4 (2–14) | 6 (3–19) |
| Overall | IR 1 | IR 2 | IR 3 | IR 4 | p-Value | |
|---|---|---|---|---|---|---|
| Mortality rate, n (%) | 3/273 (1.1) | 1/78 (1.3) | 1/78 (1.3) | 1/78 (1.3) | 0/39 (0) | 0.918 |
| Major Complication Rate, n (%) | 18/273 (6.6) | 8/78 (10.3) | 4/78 (5.1) | 4/78 (5.1) | 2/39 (5.1) | 0.498 |
| HCC, n (%) | 12/170 (7.1) | 6/45 (13.3) | 1/48 (2.1) | 3/53 (5.7) | 2/22 (8.3) | 0.193 |
| ICC, n (%) | 2/9 (22.2) | 0/3 (0) | 2/3 (66.7) | 0/2 (0) | 0/1 (0) | 0.162 |
| METS, n (%) | 4/94 (4.3) | 2/30 (6.7) | 1/27 (3.7) | 1/23 (4.3) | 0/14 (0) | 0.944 |
| Hospital Days, median (range) | 5 (1–31) | 4 (1–24) | 5 (2–21) | 4 (1–31) | 5 (2–24) | 0.257 |
| HCC, n (%) | 4 (1–20) | 4 (1–20) | 5 (3–14) | 5 (1–18) | 5 (2–10) | 0.195 |
| ICC, n (%) | 6 (2–19) | 3 (2–7) | 12 (5–19) | 4.5 (3–6) | 7 (7–7) | 0.206 |
| METS, n (%) | 5 (1–31) | 6 (2–24) | 5 (2–21) | 4 (1–31) | 6 (2–24) | 0.944 |
| Technical Success, n (%) | 628/628 (100) | 176/176 (100) | 149/149 (100) | 208/208 (100) | 95/95 (100) | - |
| Primary Technical Efficacy, n (%) | 619/628 (98.6) | 171/176 (97.2) | 149/149 (100) | 205/208 (98.6) | 94/95 (98.9) | 0.192 |
| HCC, n (%) | 355/363 (98.8) | 98/102 (96.1) | 81/81 (100) | 111/114 (97.4) | 65/66 (98.5) | 0.325 |
| ICC, n (%) | 14/14 (100) | 6/6 (100) | 4/4 (100) | 3/3 (100) | 1/1 (100) | - |
| METS, n (%) | 250/251 (99.6) | 67/68 (98.5) | 64/64 (100) | 91/91 (100) | 28/28 (100) | 0.440 |
| Secondary Technical Efficacy, n (%) | 625/628 (99.5) | 176/176 (100) | 149/149 (100) | 206/208 (99.0) | 94/95 (98.9) | 0.355 |
| HCC, n (%) | 360/363 (99.2) | 102/102 (100) | 81/81 (100) | 112/114 (98.2) | 65/66 (98.5) | 0.376 |
| ICC, n (%) | 14/14 (100) | 6/6 (100) | 4/4 (100) | 3/3 (100) | 1/1 (100) | - |
| METS, n (%) | 251/251 (100) | 68/68 (100) | 64/64 (100) | 91/91 (100) | 28/28 (100) | - |
| Local Recurrence, n (%) | 35/628 (5.6) | 7/176 (4.0) | 15/149 (10.1) | 5/95 (5.3) | 5/95 (5.3) | 0.051 |
| HCC, n (%) | 16/363 (4.4) | 2/102 (2.0) | 7/81 (8.6) | 5/114 (4.5) | 2/66 (3.0) | 0.158 |
| ICC, n (%) | 1/14 (7.1) | 0/6 (0) | 0/4 (0) | 1/3 (33.3) | 0/1 (0) | 0.267 |
| METS, n (%) | 18/251 (7.2) | 5/68 (7.4) | 8/64 (12.5) | 2/91 (2.2) | 3/28 (10.7) | 0.084 |
| Time Efforts | ||||||
| Planning and Placement, min (range) | 76.5 (32–271) | 96.0 (48–361) | 76.5 (32–271) | 76.5 (32–271) | 119 (46–292) | 0.000 |
| Total Time, min (range) | 149.5 (77–491) | 204 (95–454) | 95.0 (43–231) | 149.5 (77–491) | 195 (76–365) | 0.000 |
| Experienced IRs | IR 4 | p-Value | |
|---|---|---|---|
| Mortality rate, n (%) | 3/234 (1.3) | 0/39 (0) | 0.477 |
| Major Complication Rate, n (%) | 16/234 (6.8) | 2/39 (5.1) | 0.690 |
| HCC, n (%) | 10/146 (6.8) | 2/24 (8.3) | 0.793 |
| ICC, n (%) | 2/8 (25.0) | 0/1 (0) | 0.571 |
| METS, n (%) | 4/80 (5.0) | 0/14 (0) | 0.393 |
| Hospital Days, median (range) | 4 (1–31) | 5 (2–24) | 0.852 |
| HCC, n (%) | 4 (1–20) | 5 (2–10) | 0.977 |
| ICC, n (%) | 5.5 (2–19) | 7 (7–7) | 0.667 |
| METS, n (%) | 5 (1–31) | 6 (2–24) | 0.873 |
| Technical Success, n (%) | 533/533 (100) | 95/95 (100) | - |
| Primary Technical Efficacy, n (%) | 525/533 (98.5) | 94/95 (98.9) | 0.735 |
| HCC, n (%) | 290/297 (97.6) | 65/66 (98.5) | 0.674 |
| ICC, n (%) | 13/13 (100) | 1/1 (100) | - |
| METS, n (%) | 222/223 (99.6) | 28/28 (100) | 0.674 |
| Secondary Technical Efficacy, n (%) | 531/533 (99.6) | 94/95 (98.9) | 0.378 |
| HCC, n (%) | 360/297 (99.2) | 65/66 (98.5) | 0.494 |
| ICC, n (%) | 13/13 (100) | 1/1 (100) | - |
| METS, n (%) | 223/223 (100) | 28/28 (100) | - |
| Local Recurrence, n (%) | 30/533 (5.6) | 5/95 (5.3) | 0.886 |
| HCC, n (%) | 14/297 (4.7) | 2/66 (3.0) | 0.547 |
| ICC, n (%) | 1/13 (7.7) | 0/1 (0) | 0.773 |
| METS, n (%) | 15/223 (6.7) | 3/28 (10.7) | 0.441 |
| Time Efforts | |||
| Planning and Placement, min (range) | 92 (32–361) | 119 (46–292) | 0.002 |
| Total Time, min (range) | 173 (55–491) | 195 (76–365) | 0.059 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schullian, P.; Laimer, G.; Johnston, E.; Putzer, D.; Eberle, G.; Widmann, G.; Scharll, Y.; Bale, R. Reliability of Stereotactic Radiofrequency Ablation (SRFA) for Malignant Liver Tumors: Novice versus Experienced Operators. Biology 2023, 12, 175. https://doi.org/10.3390/biology12020175
Schullian P, Laimer G, Johnston E, Putzer D, Eberle G, Widmann G, Scharll Y, Bale R. Reliability of Stereotactic Radiofrequency Ablation (SRFA) for Malignant Liver Tumors: Novice versus Experienced Operators. Biology. 2023; 12(2):175. https://doi.org/10.3390/biology12020175
Chicago/Turabian StyleSchullian, Peter, Gregor Laimer, Edward Johnston, Daniel Putzer, Gernot Eberle, Gerlig Widmann, Yannick Scharll, and Reto Bale. 2023. "Reliability of Stereotactic Radiofrequency Ablation (SRFA) for Malignant Liver Tumors: Novice versus Experienced Operators" Biology 12, no. 2: 175. https://doi.org/10.3390/biology12020175
APA StyleSchullian, P., Laimer, G., Johnston, E., Putzer, D., Eberle, G., Widmann, G., Scharll, Y., & Bale, R. (2023). Reliability of Stereotactic Radiofrequency Ablation (SRFA) for Malignant Liver Tumors: Novice versus Experienced Operators. Biology, 12(2), 175. https://doi.org/10.3390/biology12020175





