Challenges and Approaches of Culturing the Unculturable Archaea
Abstract
Simple Summary
Abstract
1. Introduction
2. Significance of Archaea and Archaeal Culturomics
3. Challenges in Archaeal Culturomics
3.1. Environmental Conditions/Abiotic Conditions
3.2. Suitable Nutritional Requirements (Concentration of Substrates)
3.3. Consortium and Co-Culturing
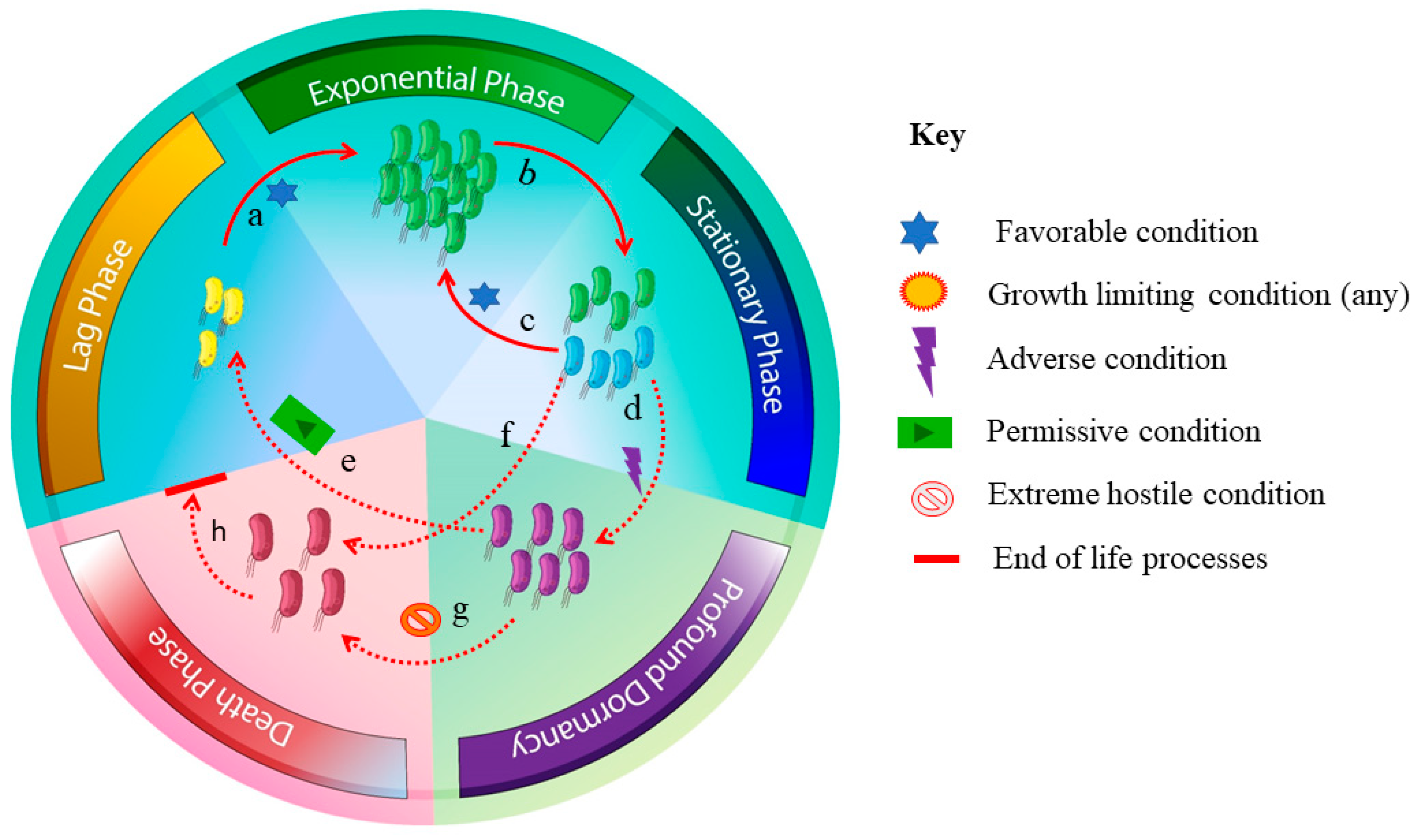
3.4. Inappropriate Incubation Time
3.5. Low Abundance, Presence of Persisters, and Dead Cells
3.6. Small Genome Size of Archaea (Symbiotic Association)
3.7. Incorrect Genome Annotation
4. Current Status of Culturomics for Archaea
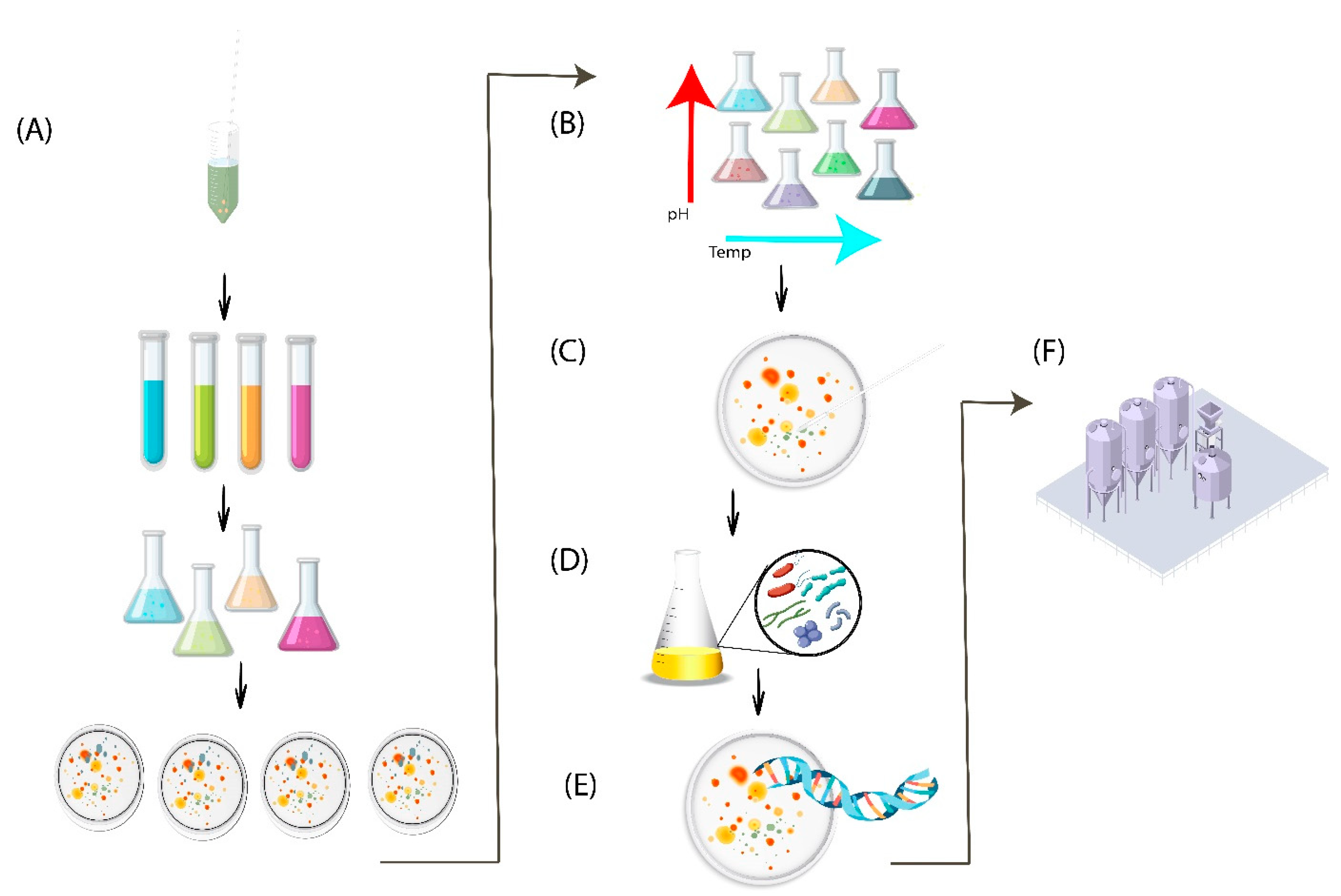
5. Culturomics Strategies for Archaea
5.1. Simulation Strategies of Natural Habitat (High-Throughput Culturing)
5.2. Co-Culturing
5.3. Single-Cell Sorting
5.4. Culture Media for Halophilic Archaea
5.5. Culture Media for Methanogenic Archaea
5.6. Culture Media for Thermophilic and Hyperthermophilic Archaea
5.7. Culture Media for Mesophilic Archaea
5.8. Culture Media for Acidophiles Archaea
5.9. Proposed Basal Media for Archaeal Cultivation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Woese, C.R.; Fox, G.E. Phylogenetic structure of the prokaryotic domain: The primary kingdoms. Proc. Natl. Acad. Sci. USA 1977, 74, 5088–5090. [Google Scholar] [CrossRef]
- Woese, C.R.; Magrum, L.J.; Fox, G.E. Archaebacteria. J. Mol. Evol. 1978, 11, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.S. The Archaea: A personal overview of the formative years. In The prokaryotes: A Handbook on the Biology of Bacteria; Springer: New York, NY, USA, 2006; Volume 3, pp. 3–9. [Google Scholar]
- Eme, L.; Spang, A.; Lombard, J.; Stairs, C.W.; Ettema, T.J.G. Archaea and the origin of eukaryotes. Nat. Rev. Microbiol. 2017, 15, 711–723. [Google Scholar] [CrossRef]
- Baker, B.J.; De Anda, V.; Seitz, K.W.; Dombrowski, N.; Santoro, A.E.; Lloyd, K.G. Diversity, ecology and evolution of Archaea. Nat. Microbiol. 2020, 5, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Bonfá, M.R.L.; Grossman, M.J.; Mellado, E.; Durrant, L.R. Biodegradation of aromatic hydrocarbons by Haloarchaea and their use for the reduction of the chemical oxygen demand of hypersaline petroleum produced water. Chemosphere 2011, 84, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Valentine, D.L. Adaptations to energy stress dictate the ecology and evolution of the Archaea. Nat. Rev. Microbiol. 2007, 5, 316–323. [Google Scholar] [CrossRef]
- Guy, L.; Ettema, T.J.G. The archaeal ‘TACK’superphylum and the origin of eukaryotes. Trends Microbiol. 2011, 19, 580–587. [Google Scholar] [CrossRef]
- Takai, K.; Nakamura, K.; Toki, T.; Tsunogai, U.; Miyazaki, M.; Miyazaki, J.; Hirayama, H.; Nakagawa, S.; Nunoura, T.; Horikoshi, K. Cell proliferation at 122 C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc. Natl. Acad. Sci. USA 2008, 105, 10949–10954. [Google Scholar] [CrossRef]
- Méndez-García, C.; Peláez, A.I.; Mesa, V.; Sánchez, J.; Golyshina, O.V.; Ferrer, M. Microbial diversity and metabolic networks in acid mine drainage habitats. Front. Microbiol. 2015, 6, 475. [Google Scholar]
- Baker, B.J.; Banfield, J.F. Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 2003, 44, 139–152. [Google Scholar] [CrossRef]
- Golyshina, O.V.; Lünsdorf, H.; Kublanov, I.V.; Goldenstein, N.I.; Hinrichs, K.-U.; Golyshin, P.N. The novel extremely acidophilic, cell-wall-deficient archaeon Cuniculiplasma divulgatum gen. nov., sp. nov. represents a new family, Cuniculiplasmataceae fam. nov., of the order Thermoplasmatales. Int. J. Syst. Evol. Microbiol. 2016, 66 Pt 1, 332–340. [Google Scholar] [CrossRef]
- Simankova, M.V.; Parshina, S.N.; Tourova, T.P.; Kolganova, T.V.; Zehnder, A.J.B.; Nozhevnikova, A.N. Methanosarcina lacustris sp. nov., a new psychrotolerant methanogenic archaeon from anoxic lake sediments. Syst. Appl. Microbiol. 2001, 24, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Kendall, M.M.; Wardlaw, G.D.; Tang, C.F.; Bonin, A.S.; Liu, Y.; Valentine, D.L. Diversity of Archaea in marine sediments from Skan Bay, Alaska, including cultivated methanogens, and description of Methanogenium boonei sp. nov. Appl. Environ. Microbiol. 2007, 73, 407–414. [Google Scholar] [CrossRef]
- Morozova, D.; Wagner, D. Stress response of methanogenic archaea from Siberian permafrost compared with methanogens from nonpermafrost habitats. FEMS Microbiol. Ecol. 2007, 61, 16–25. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E. Archaeal means and extremes. Science 1998, 280, 542–543. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Y.; Pan, J.; Wang, F.; Li, M. Perspectives on cultivation strategies of archaea. Microb. Ecol. 2020, 79, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Brochier-Armanet, C.; Forterre, P.; Gribaldo, S. Phylogeny and evolution of the Archaea: One hundred genomes later. Curr. Opin. Microbiol. 2011, 14, 274–281. [Google Scholar] [CrossRef]
- Farkas, J.A.; Picking, J.W.; Santangelo, T.J. Genetic Techniques for the Archaea. Annu. Rev. Genet. 2013, 47, 539–561. [Google Scholar] [CrossRef]
- Rappé, M.S.; Giovannoni, S.J. The uncultured microbial majority. Annu. Rev. Microbiol. 2003, 57, 369–394. [Google Scholar] [CrossRef]
- Woese, C.R.; Kandler, O.; Wheelis, M.L. Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA 1990, 87, 4576–4579. [Google Scholar] [CrossRef]
- Barns, S.M.; Fundyga, R.E.; Jeffries, M.W.; Pace, N.R. Remarkable archaeal diversity detected in a Yellowstone National Park hot spring environment. Proc. Natl. Acad. Sci. USA 1994, 91, 1609–1613. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, J.A.; McCallum, K.; Davis, A.A. Novel major archaebacterial group from marine plankton. Nature 1992, 356, 148–149. [Google Scholar] [CrossRef]
- Tahon, G.; Geesink, P.; Ettema, T.J.G. Expanding archaeal diversity and phylogeny: Past, present, and future. Annu. Rev. Microbiol. 2021, 75, 359–381. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Xie, T.; Wu, Z.; Zheng, W.; Zhang, T.; Howe, S.; Chai, J.; Deng, F.; Li, Y.; Zhao, J. Archaea: An under-estimated kingdom in livestock animals. Front. Vet. Sci. 2022, 9, 973508. [Google Scholar] [CrossRef] [PubMed]
- Rinke, C.; Chuvochina, M.; Mussig, A.J.; Chaumeil, P.-A.; Davín, A.A.; Waite, D.W.; Whitman, B.W.; Parks, H.D.; Hugenholts, P. A standardized archaeal taxonomy for the Genome Taxonomy Database. Nat. Microbiol. 2021, 6, 946–959. [Google Scholar] [CrossRef]
- Parks, D.H.; Chuvochina, M.; Rinke, C.; Mussig, A.J.; Chaumeil, P.-A.; Hugenholtz, P. GTDB: An ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 2022, 50, D785–D794. [Google Scholar] [CrossRef]
- Rinke, C.; Schwientek, P.; Sczyrba, A.; Ivanova, N.N.; Anderson, I.J.; Cheng, J.-F.; Darling, A.; Malfatti, S.; Swan, K.B.; Gies, A.E.; et al. Insights into the phylogeny and coding potential of microbial dark matter. Nature 2013, 499, 431–437. [Google Scholar] [CrossRef]
- Spang, A.; Saw, J.H.; Jørgensen, S.L.; Zaremba-Niedzwiedzka, K.; Martijn, J.; Lind, A.E.; Eijk, V.R.; Schleper, C.; Guy, L.; Ettema, J.G.T. Complex archaea that bridge the gap between prokaryotes and eukaryotes. Nature 2015, 521, 173–179. [Google Scholar] [CrossRef]
- Seitz, K.W.; Lazar, C.S.; Hinrichs, K.-U.; Teske, A.P.; Baker, B.J. Genomic reconstruction of a novel, deeply branched sediment archaeal phylum with pathways for acetogenesis and sulfur reduction. ISME J. 2016, 10, 1696–1705. [Google Scholar] [CrossRef]
- Lazar, C.S.; Baker, B.J.; Seitz, K.W.; Teske, A.P. Genomic reconstruction of multiple lineages of uncultured benthic archaea suggests distinct biogeochemical roles and ecological niches. ISME J. 2017, 11, 1118–1129. [Google Scholar] [CrossRef]
- Zaremba-Niedzwiedzka, K.; Caceres, E.F.; Saw, J.H.; Bäckström, D.; Juzokaite, L.; Vancaester, E.; Seitz, W.K.; Anantharaman, K.; Starnawski, P.; Kjeldson, U.K.; et al. Asgard archaea illuminate the origin of eukaryotic cellular complexity. Nature 2017, 541, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, N.; Teske, A.P.; Baker, B.J. Expansive microbial metabolic versatility and biodiversity in dynamic Guaymas Basin hydrothermal sediments. Nat. Commun. 2018, 9, 4999. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Rinke, C.; Chuvochina, M.; Chaumeil, P.-A.; Woodcroft, B.J.; Evans, P.N.; Hugenholtz, P.; Tyson, W.G. Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nat. Microbiol. 2017, 2, 1533–1542. [Google Scholar] [CrossRef]
- Castelle, C.J.; Banfield, J.F. Major new microbial groups expand diversity and alter our understanding of the tree of life. Cell 2018, 172, 1181–1197. [Google Scholar] [CrossRef]
- Hug, L.A.; Baker, B.J.; Anantharaman, K.; Brown, C.T.; Probst, A.J.; Castelle, C.J.; Butterfield, N.C.; Hernsdorf, W.A.; Amano, Y.; Ise, K.; et al. A new view of the tree of life. Nat. Microbiol. 2016, 1, 16048. [Google Scholar] [CrossRef]
- Lloyd, K.G.; Steen, A.D.; Ladau, J.; Yin, J.; Crosby, L. Phylogenetically novel uncultured microbial cells dominate earth microbiomes. MSystems 2018, 3, e00055-18. [Google Scholar] [CrossRef]
- Gill, A. The importance of bacterial culture to food microbiology in the age of genomics. Front. Microbiol. 2017, 8, 777. [Google Scholar] [CrossRef] [PubMed]
- Lagier, J.-C.; Edouard, S.; Pagnier, I.; Mediannikov, O.; Drancourt, M.; Raoult, D. Current and past strategies for bacterial culture in clinical microbiology. Clin. Microbiol. Rev. 2015, 28, 208–236. [Google Scholar] [CrossRef]
- Elkins, J.G.; Podar, M.; Graham, D.E.; Makarova, K.S.; Wolf, Y.; Randau, L.; Hedlund, B.P.; Brochier-Armanet, C.; Kunin, V.; Anderson, I.; et al. A korarchaeal genome reveals insights into the evolution of the Archaea. Proc. Natl. Acad. Sci. USA 2008, 105, 8102–8107. [Google Scholar] [CrossRef]
- Liu, C.-T.; Miyaki, T.; Aono, T.; Oyaizu, H. Evaluation of methanogenic strains and their ability to endure aeration and water stress. Curr. Microbiol. 2008, 56, 214–218. [Google Scholar] [CrossRef]
- Hassani, Y.; Saad, J.; Terrer, E.; Aboudharam, G.; Giancarlo, B.; Silvestri, F.; Raoult, D.; Drancourt, M.; Grine, G. Introducing clinical nanoarchaeaology: Isolation by co-culture of Nanopusillus massiliensis sp. nov. Curr. Res. Microb. Sci. 2022, 3, 100100. [Google Scholar] [CrossRef] [PubMed]
- Allers, T.; Mevarech, M. Archaeal genetics—The third way. Nat. Rev. Genet. 2005, 6, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.; Cassarini, C.; Lens, P.N.L. Physiology and distribution of archaeal methanotrophs that couple anaerobic oxidation of methane with sulfate reduction. Microbiol. Mol. Biol. Rev. 2019, 83, e00074-18. [Google Scholar] [CrossRef] [PubMed]
- Spang, A.; Stairs, C.W.; Dombrowski, N.; Eme, L.; Lombard, J.; Caceres, E.F.; Greening, C.; Baker, B.J.; Ettema, T.J. Proposal of the reverse flow model for the origin of the eukaryotic cell based on comparative analyses of Asgard archaeal metabolism. Nat. Microbiol. 2019, 4, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Reji, L.; Tolar, B.B.; Smith, J.M.; Chavez, F.P.; Francis, C.A. Differential co-occurrence relationships shaping ecotype diversification within Thaumarchaeota populations in the coastal ocean water column. ISME J. 2019, 13, 1144–1158. [Google Scholar] [CrossRef]
- Zhang, C.L.; Xie, W.; Martin-Cuadrado, A.-B.; Rodriguez-Valera, F. Marine Group II Archaea, potentially important players in the global ocean carbon cycle. Front. Microbiol. 2015, 6, 1108. [Google Scholar] [CrossRef]
- Haro-Moreno, J.M.; Rodriguez-Valera, F.; López-García, P.; Moreira, D.; Martin-Cuadrado, A.-B. New insights into marine group III Euryarchaeota, from dark to light. ISME J. 2017, 11, 1102–1117. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Pan, J.; Baker, B.J.; Gu, J.-D.; Li, M. Comparative genomic inference suggests mixotrophic lifestyle for Thorarchaeota. ISME J. 2018, 12, 1021–1031. [Google Scholar] [CrossRef]
- Vanwonterghem, I.; Evans, P.N.; Parks, D.H.; Jensen, P.D.; Woodcroft, B.J.; Hugenholtz, P.; Tyson, G.W. Methylotrophic methanogenesis discovered in the archaeal phylum Verstraetearchaeota. Nat. Microbiol. 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Dombrowski, N.; Lee, J.-H.; Williams, T.A.; Offre, P.; Spang, A. Genomic diversity, lifestyles and evolutionary origins of DPANN archaea. FEMS Microbiol. Lett. 2019, 366, fnz008. [Google Scholar] [CrossRef]
- Castelle, C.J.; Wrighton, K.C.; Thomas, B.C.; Hug, L.A.; Brown, C.T.; Wilkins, M.J.; Frischkorn, K.R.; Tringe, S.G.; Singh, A.; Markillie, L.M.; et al. Genomic expansion of domain archaea highlights roles for organisms from new phyla in anaerobic carbon cycling. Curr. Biol. 2015, 25, 690–701. [Google Scholar] [CrossRef]
- Thrash, J.C. Culturing the uncultured: Risk versus reward. Msystems 2019, 4, e00130-19. [Google Scholar] [CrossRef] [PubMed]
- Tyson, G.W.; Banfield, J.F. Cultivating the uncultivated: A community genomics perspective. Trends Microbiol. 2005, 13, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Carini, P. A “cultural” renaissance: Genomics breathes new life into an old craft. Msystems 2019, 4, e00092-19. [Google Scholar] [CrossRef] [PubMed]
- Alain, K.; Querellou, J. Cultivating the uncultured: Limits, advances and future challenges. Extremophiles 2009, 13, 583–594. [Google Scholar] [CrossRef]
- Achtman, M.; Wagner, M. Microbial diversity and the genetic nature of microbial species. Nat. Rev. Microbiol. 2008, 6, 431–440. [Google Scholar] [CrossRef]
- Adam, P.S.; Borrel, G.; Brochier-Armanet, C.; Gribaldo, S. The growing tree of Archaea: New perspectives on their diversity, evolution and ecology. ISME J. 2017, 11, 2407–2425. [Google Scholar] [CrossRef]
- Roszak, D.B.; Colwell, R. Survival strategies of bacteria in the natural environment. Microbiol. Rev. 1987, 51, 365–379. [Google Scholar] [CrossRef]
- Staley, J.T.; Konopka, A. Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu. Rev. Microbiol. 1985, 39, 321–346. [Google Scholar] [CrossRef]
- van den Bosch, T.J.M.; Rijkers, R.; van Kessel, M.A.H.J.; Jetten, M.S.M.; Welte, C.U. Co-cultivation of the strictly anaerobic methanogen Methanosarcina barkeri with aerobic methanotrophs in an oxygen-limited membrane bioreactor. Appl. Microbiol. Biotechnol. 2018, 102, 5685–5694. [Google Scholar]
- Meulepas, R.J.W.; Jagersma, C.G.; Gieteling, J.; Buisman, C.J.N.; Stams, A.J.M.; Lens, P.N.L. Enrichment of anaerobic methanotrophs in sulfate-reducing membrane bioreactors. Biotechnol. Bioeng. 2009, 104, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.H.; Kelly, R.M. Cultivation Techniques for Hyperthermophilic Archaebacteria: Continuous Culture of Pyrococcus furiosus at Temperatures near 100 C. Appl. Environ. Microbiol. 1989, 55, 2086–2088. [Google Scholar] [CrossRef] [PubMed]
- Shirsalimian, M.S.; Amoozegar, M.A.; Sepahy, A.A.; Kalantar, S.M.; Dabbagh, R. Isolation of extremely halophilic Archaea from a saline river in the Lut Desert of Iran, moderately resistant to desiccation and gamma radiation. Microbiology 2017, 86, 403–411. [Google Scholar] [CrossRef]
- Overmann, J. Principles of enrichment, isolation, cultivation and preservation of prokaryotes. Prokaryotes 2006, 1, 80–136. [Google Scholar]
- Davis, K.E.R.; Joseph, S.J.; Janssen, P.H. Effects of growth medium, inoculum size, and incubation time on culturability and isolation of soil bacteria. Appl. Environ. Microbiol. 2005, 71, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Kappler, A.; Bryce, C. Cryptic biogeochemical cycles: Unravelling hidden redox reactions. Environ. Microbiol. 2017, 19, 842–846. [Google Scholar] [CrossRef]
- Schneegurt, M.A. Media and conditions for the growth of halophilic and halotolerant bacteria and archaea. In Advances in Understanding the Biology of Halophilic Microorganisms; Springer: Dordrecht, The Netherlands, 2012; pp. 35–58. [Google Scholar]
- Balch, W.E.; Fox, G.E.; Magrum, L.J.; Woese, C.R.; Wolfe, R. Methanogens: Reevaluation of a unique biological group. Microbiol. Rev. 1979, 43, 260–296. [Google Scholar] [CrossRef]
- Kashefi, K.; Tor, J.M.; Holmes, D.E.; Van Praagh, C.V.G.; Reysenbach, A.-L.; Lovley, D.R. Geoglobus ahangari gen. nov., sp. nov., a novel hyperthermophilic archaeon capable of oxidizing organic acids and growing autotrophically on hydrogen with Fe (III) serving as the sole electron acceptor. Int. J. Syst. Evol. Microbiol. 2002, 52, 719–728. [Google Scholar]
- Slobodkina, G.B.; Kolganova, T.V.; Querellou, J.; Bonch-Osmolovskaya, E.A.; Slobodkin, A.I. Geoglobus acetivorans sp. nov., an iron (III)-reducing archaeon from a deep-sea hydrothermal vent. Int. J. Syst. Evol. Microbiol. 2009, 59, 2880–2883. [Google Scholar] [CrossRef]
- Steen, A.D.; Crits-Christoph, A.; Carini, P.; DeAngelis, K.M.; Fierer, N.; Lloyd, K.G.; Thrash, C. High proportions of bacteria and archaea across most biomes remain uncultured. ISME J. 2019, 13, 3126–3130. [Google Scholar] [CrossRef]
- Murray, A.E.; Freudenstein, J.; Gribaldo, S.; Hatzenpichler, R.; Hugenholtz, P.; Kämpfer, P.; Konstantinidis, K.T.; Lane, C.E.; Papke, R.T.; Parks, D.H.; et al. Roadmap for naming uncultivated Archaea and Bacteria. Nat. Microbiol. 2020, 5, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, B.S.; Eichorst, S.A.; Wertz, J.T.; Schmidt, T.M.; Breznak, J.A. New strategies for cultivation and detection of previously uncultured microbes. Appl. Environ. Microbiol. 2004, 70, 4748–4755. [Google Scholar] [CrossRef] [PubMed]
- Welte, C.U. Revival of archaeal methane microbiology. Msystems 2018, 3, e00181-17. [Google Scholar] [CrossRef] [PubMed]
- Vaksmaa, A.; Guerrero-Cruz, S.; van Alen, T.A.; Cremers, G.; Ettwig, K.F.; Lüke, C.; Jetten, M.S. Enrichment of anaerobic nitrate-dependent methanotrophic ‘Candidatus Methanoperedens nitroreducens’ archaea from an Italian paddy field soil. Appl. Microbiol. Biotechnol. 2017, 101, 7075–7084. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Yoon, J.-C.; Shin, K.-S.; Kim, E.-H.; Yim, S.-B.; Cho, Y.-J.; Sung, G.M.; Lee, D.G.; Kim, S.B.; Lee, D.U. Dominance of endospore-forming bacteria on a rotating activated Bacillus contactor biofilm for advanced wastewater treatment. J. Microbiol. 2007, 45, 113–121. [Google Scholar]
- Yu, T.; Wu, W.; Liang, W.; Lever, M.A.; Hinrichs, K.-U.; Wang, F. Growth of sedimentary Bathyarchaeota on lignin as an energy source. Proc. Natl. Acad. Sci. USA 2018, 115, 6022–6027. [Google Scholar] [CrossRef]
- Huber, H.; Hohn, M.J.; Rachel, R.; Fuchs, T.; Wimmer, V.C.; Stetter, K.O. A new phylum of Archaea represented by a nanosized hyperthermophilic symbiont. Nature 2002, 417, 63–67. [Google Scholar] [CrossRef]
- Bruns, A.; Cypionka, H.; Overmann, J. Cyclic AMP and acyl homoserine lactones increase the cultivation efficiency of heterotrophic bacteria from the central Baltic Sea. Appl. Environ. Microbiol. 2002, 68, 3978–3987. [Google Scholar] [CrossRef]
- Button, D.K.; Robertson, B.R. Use of high-resolution flow cytometry to determine the activity and distribution of aquatic bacteria. In Handbook of Methods in Aquatic Microbial Ecology; CRC Press: Boca Raton, FL, USA, 2018; pp. 163–173. [Google Scholar]
- Colwell, R.R.; Grimes, D.J. Nonculturable Microorganisms in the Environment; ASM Press: Washington, DC, USA, 2000. [Google Scholar]
- Burns, D.G.; Camakaris, H.M.; Janssen, P.H.; Dyall-Smith, M.L. Combined use of cultivation-dependent and cultivation-independent methods indicates that members of most haloarchaeal groups in an Australian crystallizer pond are cultivable. Appl. Environ. Microbiol. 2004, 70, 5258–5265. [Google Scholar] [CrossRef]
- Lynch, M.D.J.; Neufeld, J.D. Ecology and exploration of the rare biosphere. Nat. Rev. Microbiol. 2015, 13, 217–229. [Google Scholar] [CrossRef]
- Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 2007, 5, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Hoehler, T.M.; Jørgensen, B.B. Microbial life under extreme energy limitation. Nat. Rev. Microbiol. 2013, 11, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Megaw, J.; Gilmore, B.F. Archaeal persisters: Persister cell formation as a stress response in Haloferax volcanii. Front. Microbiol. 2017, 8, 1589. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.S. The phenomenon of microbial uncultivability. Curr. Opin. Microbiol. 2013, 16, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.J.; Comolli, L.R.; Dick, G.J.; Hauser, L.J.; Hyatt, D.; Dill, B.D.; Land, M.L.; VerBerkmoes, N.C.; Hettich, R.L.; Banfield, J.F. Enigmatic, ultrasmall, uncultivated Archaea. Proc. Natl. Acad. Sci. USA 2010, 107, 8806–8811. [Google Scholar] [CrossRef]
- Narasingarao, P.; Podell, S.; Ugalde, J.A.; Brochier-Armanet, C.; Emerson, J.B.; Brocks, J.J.; Heidelberg, K.B.; Banfield, J.F.; Allen, E.E. De novo metagenomic assembly reveals abundant novel major lineage of Archaea in hypersaline microbial communities. ISME J. 2012, 6, 81–93. [Google Scholar] [CrossRef]
- Podar, M.; Makarova, K.S.; Graham, D.E.; Wolf, Y.I.; Koonin, E.V.; Reysenbach, A.-L. Insights into archaeal evolution and symbiosis from the genomes of a nanoarchaeon and its inferred crenarchaeal host from Obsidian Pool, Yellowstone National Park. Biol. Direct 2013, 8, 9. [Google Scholar] [CrossRef]
- Vavourakis, C.D.; Ghai, R.; Rodriguez-Valera, F.; Sorokin, D.Y.; Tringe, S.G.; Hugenholtz, P.; Muyzer, G. Metagenomic insights into the uncultured diversity and physiology of microbes in four hypersaline soda lake brines. Front. Microbiol. 2016, 7, 211. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Towards functional characterization of archaeal genomic dark matter. Biochem. Soc. Trans. 2019, 47, 389–398. [Google Scholar] [CrossRef]
- Zhou, Z.; Pan, J.; Wang, F.; Gu, J.-D.; Li, M. Bathyarchaeota: Globally distributed metabolic generalists in anoxic environments. FEMS Microbiol. Rev. 2018, 42, 639–655. [Google Scholar] [CrossRef]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glöckner, F.O.; Ludwig, W.; Schleifer, K.-H.; Whitman, W.B.; Euzéby, J.; Amann, R.; Rosselló-Móra, R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Curtis, T.P.; Sloan, W.T.; Scannell, J.W. Estimating prokaryotic diversity and its limits. Proc. Natl. Acad. Sci. USA 2002, 99, 10494–10499. [Google Scholar] [CrossRef] [PubMed]
- Lennon, J.T.; Locey, K.J. More support for Earth’s massive microbiome. Biol. Direct 2020, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Locey, K.J.; Lennon, J.T. Scaling laws predict global microbial diversity. Proc. Natl. Acad. Sci. USA 2016, 113, 5970–5975. [Google Scholar] [CrossRef] [PubMed]
- Starr, M.P.; Stolp, H.; Trüper, H.G.; Balows, A.; Schlegel, H.G. The Prokaryotes: A Handbook on Habitats, Isolation and Identification of Bacteria; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Sehgal, S.N.; Gibbons, N.E. Effect of some metal ions on the growth of Halobacterium cutirubrum. Can. J. Microbiol. 1960, 6, 165–169. [Google Scholar] [CrossRef]
- Dundas, I.D.; Srinivasan, V.R.; Halvorson, H.O. A chemically defined medium for Halobacterium salinarium strain 1. Can. J. Microbiol. 1963, 9, 619–624. [Google Scholar] [CrossRef]
- Oren, A. Halobacterium sodomense sp. nov., a Dead Sea halobacterium with an extremely high magnesium requirement. Int. J. Syst. Evol. Microbiol. 1983, 33, 381–386. [Google Scholar] [CrossRef]
- Hamana, K.; Kamekura, M.; Onishi, H.; Akazawa, T.; Matsuzaki, S. Polyamines in photosynthetic eubacteria and extreme-halophilic archaebacteria. J. Biochem. 1985, 97, 1653–1658. [Google Scholar] [CrossRef]
- Mevarech, M.; Werczberger, R. Genetic transfer in Halobacterium volcanii. J. Bacteriol. 1985, 162, 461–462. [Google Scholar] [CrossRef]
- Oren, A. The ecology of the extremely halophilic archaea. FEMS Microbiol. Rev. 1994, 13, 415–439. [Google Scholar] [CrossRef]
- Zillig, W.; Holz, I.; Janekovic, D.; Klenk, H.P.; Imsel, E.; Trent, J.; Wunderl, S.; Forjaz, V.H.; Coutinho, R.; Ferreira, T. Hyperthermus butylicus, a hyperthermophilic sulfur-reducing archaebacterium that ferments peptides. J. Bacteriol. 1990, 172, 3959–3965. [Google Scholar] [CrossRef] [PubMed]
- Brock, T.D.; Brock, K.M.; Belly, R.T.; Weiss, R.L. Sulfolobus: A new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch. Für Mikrobiol. 1972, 84, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.B. Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Arch. Für Mikrobiol. 1959, 32, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Kester, D.R.; Duedall, I.W.; Connors, D.N.; Pytkowicz, R.M. Preparation of artificial seawater 1. Limnol. Oceanogr. 1967, 12, 176–179. [Google Scholar] [CrossRef]
- Huber, R.; Kristjansson, J.K.; Stetter, K.O. Pyrobaculum gen. nov., a new genus of neutrophilic, rod-shaped archaebacteria from continental solfataras growing optimally at 100 C. Arch. Microbiol. 1987, 149, 95–101. [Google Scholar] [CrossRef]
- Hamm, J.N.; Erdmann, S.; Eloe-Fadrosh, E.A.; Angeloni, A.; Zhong, L.; Brownlee, C.; Williams, T.J.; Barton, K.; Carswell, S.; Smith, M.A.; et al. Unexpected host dependency of Antarctic Nanohaloarchaeota. Proc. Natl. Acad. Sci. USA 2019, 116, 14661–14670. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Oliveira, T.; Wollweber, F.; Ponce-Toledo, R.I.; Xu, J.; Rittmann, S.K.-M.R.; Klingl, A.; Pilhofer, M.; Schleper, C. Actin cytoskeleton and complex cell architecture in an Asgard archaeon. Nature 2023, 613, 332–339. [Google Scholar] [CrossRef]
- Imachi, H.; Nobu, M.K.; Nakahara, N.; Morono, Y.; Ogawara, M.; Takaki, Y.; Takano, Y.; Uematsu, K.; Ikuta, T.; Ito, M.; et al. Isolation of an archaeon at the prokaryote–eukaryote interface. Nature 2020, 577, 519–525. [Google Scholar] [CrossRef]
- Hahn, C.J.; Laso-Pérez, R.; Vulcano, F.; Vaziourakis, K.-M.; Stokke, R.; Steen, I.H.; Teske, A.; Boetius, A.; Liebeke, M.; Amann, R.; et al. “Candidatus Ethanoperedens,” a thermophilic genus of archaea mediating the anaerobic oxidation of ethane. MBio 2020, 11, e00600-20. [Google Scholar] [CrossRef]
- Chen, S.-C.; Musat, N.; Lechtenfeld, O.J.; Paschke, H.; Schmidt, M.; Said, N.; Popp, D.; Calabrese, F.; Stryhanyuk, H.; Jaekel, U.; et al. Anaerobic oxidation of ethane by archaea from a marine hydrocarbon seep. Nature 2019, 568, 108–111. [Google Scholar] [CrossRef]
- Cross, K.L.; Campbell, J.H.; Balachandran, M.; Campbell, A.G.; Cooper, C.J.; Griffen, A.; Heaton, M.; Joshi, S.; Klingeman, D.; Leys, E.; et al. Targeted isolation and cultivation of uncultivated bacteria by reverse genomics. Nat. Biotechnol. 2019, 37, 1314–1321. [Google Scholar] [CrossRef]
- Liu, S.; Moon, C.D.; Zheng, N.; Huws, S.; Zhao, S.; Wang, J. Opportunities and challenges of using metagenomic data to bring uncultured microbes into cultivation. Microbiome 2022, 10, 76. [Google Scholar] [CrossRef]
- Bollmann, A.; Lewis, K.; Epstein, S.S. Incubation of environmental samples in a diffusion chamber increases the diversity of recovered isolates. Appl. Environ. Microbiol. 2007, 73, 6386–6390. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.; Cahoon, N.; Trakhtenberg, E.M.; Pham, L.; Mehta, A.; Belanger, A.; Kanigan, T.; Lewis, K.; Epstein, S. Use of ichip for high-throughput in situ cultivation of “uncultivable” microbial species. Appl. Environ. Microbiol. 2010, 76, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Seo, E.-Y.; Epstein, S.S.; Joung, Y.; Han, J.; Parfenova, V.V.; Belykh, O.I.; Gladkikh, A.S.; Ahn, T.S. Application of a new cultivation technology, I-tip, for studying microbial diversity in freshwater sponges of Lake Baikal, Russia. FEMS Microbiol. Ecol. 2014, 90, 417–423. [Google Scholar] [CrossRef]
- Jeong, D.; Ahn, T.S.; Lee, H.K.; Yim, J.H.; Joung, Y.; Epstein, S.S.; Seo, E.Y. A new method for microbial cultivation and its application to bacterial community analysis in Buus Nuur, Mongolia. Fundam. Appl. Limnol. 2013, 182, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Owen, J.S.; Seo, E.-Y.; Jung, D.; He, S. Microbial Interaction Is Among the Key Factors for Isolation of Previous Uncultured Microbes. J. Microbiol. 2023, 61, 655–662. [Google Scholar] [CrossRef]
- Fröhlich, J.; König, H. New techniques for isolation of single prokaryotic cells. FEMS Microbiol. Rev. 2000, 24, 567–572. [Google Scholar] [CrossRef]
- Zengler, K.; Walcher, M.; Clark, G.; Haller, I.; Toledo, G.; Holland, T.; Mathur, E.J.; Woodnutt, G.; Short, J.M.; Keller, M. High-throughput cultivation of microorganisms using microcapsules. Methods Enzymol. 2005, 397, 124–130. [Google Scholar]
- Huber, R.; Burggraf, S.; Mayer, T.; Barns, S.M.; Rossnagel, P.; Stetter, K.O. Isolation of a hyperthermophilic archaeum predicted by in situ RNA analysis. Nature 1995, 376, 57–58. [Google Scholar] [CrossRef]
- Stan-Lotter, H.; Fendrihan, S. Halophilic archaea: Life with desiccation, radiation and oligotrophy over geological times. Life 2015, 5, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Molecular ecology of extremely halophilic Archaea and Bacteria. FEMS Microbiol. Ecol. 2002, 39, 1–7. [Google Scholar] [CrossRef]
- Fendrihan, S.; Legat, A.; Pfaffenhuemer, M.; Gruber, C.; Weidler, G.; Gerbl, F.; Stan-Lotter, H. Extremely halophilic archaea and the issue of long-term microbial survival. In Life in Extreme Environments; Amils, R., Ellis-Evans, C., Hinghofer-Szalkay, H., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 125–140. [Google Scholar] [CrossRef]
- Ventosa, A.; Nieto, J.J.; Oren, A. Biology of moderately halophilic aerobic bacteria. Microbiol. Mol. Biol. Rev. 1998, 62, 504–544. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.M.; Mohammadian, J. Isolation and characterization of Halobacterium salinarum from saline lakes in Iran. Jundishapur J. Microbiol. 2011, 4 (Suppl. S1), S59–S65. [Google Scholar]
- Khelaifia, S.; Lagier, J.-C.; Nkamga, V.D.; Guilhot, E.; Drancourt, M.; Raoult, D. Aerobic culture of methanogenic archaea without an external source of hydrogen. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Khelaifia, S.; Raoult, D.; Drancourt, M. A versatile medium for cultivating methanogenic archaea. PLoS ONE 2013, 8, e61563. [Google Scholar] [CrossRef]
- Kelly, R.M.; Deming, J.W. Extremely thermophilic archaebacteria: Biological and engineering considerations. Biotechnol. Prog. 1988, 4, 47–62. [Google Scholar] [CrossRef]
- Rakhely, G.; Kovacs, K.L. Plating hyperthermophilic archaea on solid surface. Anal. Biochem. 1996, 243, 181–183. [Google Scholar] [CrossRef]
- Pikuta, E.V. Overview of Archaea. In Instruments, Methods, and Missions for Astrobiology XIV; International Society for Optics and Photonics: Bellingham, WA, USA, 2011; Volume 8152, p. 81520N. [Google Scholar]
- Simon, H.M.; Jahn, C.E.; Bergerud, L.T.; Sliwinski, M.K.; Weimer, P.J.; Willis, D.K.; Goodman, R.M. Cultivation of mesophilic soil crenarchaeotes in enrichment cultures from plant roots. Appl. Environ. Microbiol. 2005, 71, 4751–4760. [Google Scholar] [CrossRef]
- Brochier-Armanet, C.; Boussau, B.; Gribaldo, S.; Forterre, P. Mesophilic Crenarchaeota: Proposal for a third archaeal phylum, the Thaumarchaeota. Nat. Rev. Microbiol. 2008, 6, 245–252. [Google Scholar] [CrossRef]
- Giaveno, M.A.; Urbieta, M.S.; Ulloa, J.R.; Toril, E.G.; Donati, E.R. Physiologic versatility and growth flexibility as the main characteristics of a novel thermoacidophilic Acidianus strain isolated from Copahue geothermal area in Argentina. Microb. Ecol. 2013, 65, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-J.; Park, S.-J.; Yoon, D.-N.; Schouten, S.; Sinninghe Damsté, J.S.; Rhee, S.-K. Cultivation of autotrophic ammonia-oxidizing archaea from marine sediments in co-culture with sulfur-oxidizing bacteria. Appl. Environ. Microbiol. 2010, 76, 7575–7587. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tian, J.; Xiang, M.; Liu, X. Living strategy of cold-adapted fungi with the reference to several representative species. Mycology 2017, 8, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.D.; Ciulla, R.A.; Roberts, M.F. Osmoadaptation in archaea. Appl. Environ. Microbiol. 1999, 65, 1815–1825. [Google Scholar] [CrossRef]
- Yadav, A.N.; Sharma, D.; Gulati, S.; Singh, S.; Dey, R.; Pal, K.K.; Kaushik, R.; Saxena, A.K. Haloarchaea endowed with phosphorus solubilization attribute implicated in phosphorus cycle. Sci. Rep. 2015, 5, 12293. [Google Scholar] [CrossRef]
- Atlas, R.M. Handbook of Microbiological Media; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Manikandan, M.; Pašić, L.; Kannan, V. Optimization of growth media for obtaining high-cell density cultures of halophilic archaea (family Halobacteriaceae) by response surface methodology. Bioresour. Technol. 2009, 100, 3107–3112. [Google Scholar] [CrossRef]
- Lizama, C.; Monteoliva-Sánchez, M.; Prado, B.; Ramos-Cormenzana, A.; Weckesser, J.; Campos, V. Taxonomic study of extreme halophilic archaea isolated from the “Salar de Atacama”, Chile. Syst. Appl. Microbiol. 2001, 4, 464–474. [Google Scholar] [CrossRef]
- Torreblanca, M.; Rodriguez-Valera, F.; Juez, G.; Ventosa, A.; Kamekura, M.; Kates, M. Classification of non-alkaliphilic halobacteria based on numerical taxonomy and polar lipid composition, and description of Haloarcula gen. nov. and Haloferax gen. nov. Syst. Appl. Microbiol. 1986, 8, 89–99. [Google Scholar] [CrossRef]
- Cuadros-Orellana, S.; Pohlschröder, M.; Durrant, L.R. Isolation and characterization of halophilic archaea able to grow in aromatic compounds. Int. Biodeterior. Biodegrad. 2006, 57, 151–154. [Google Scholar] [CrossRef]
- Asakawa, S.; Akagawa-Matsushita, M.; Morii, H.; Koga, Y.; Hayano, K. Characterization of Methanosarcina mazeii TMA isolated from a paddy field soil. Curr. Microbiol. 1995, 31, 34–38. [Google Scholar] [CrossRef]
- Whitman, W.B.; Bowen, T.L.; Boone, D.R. The methanogenic bacteria. Prokaryotes 2006, 9, 165–207. [Google Scholar]
- Rufener, W.H., Jr.; Nelson, W.O.; Wolin, M.J. Maintenance of the rumen microbial population in continuous culture. Appl. Microbiol. 1963, 11, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M.P.; Tzeng, S.F.; Robinson, I.M.; Joyner, A.E., Jr. Nutrient requirements of methanogenic bacteria. Adv. Chem. Ser. 1971, 105, 23–40. [Google Scholar]
- Harmsen, H.; Prieur, D.; Jeanthon, C. Distribution of microorganisms in deep-sea hydrothermal vent chimneys investigated by whole-cell hybridization and enrichment culture of thermophilic subpopulations. Appl. Environ. Microbiol. 1997, 63, 2876–2883. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Dombard, D.R.; Price, R.E.; Pichler, T.; Amend, J.P. Prokaryotic populations in arsenic-rich shallow-sea hydrothermal sediments of Ambitle Island, Papua New Guinea. Geomicrobiol. J. 2012, 29, 1–17. [Google Scholar] [CrossRef]
- Grant, C.L.; Pramer, D. Minor element composition of yeast extract. J. Bacteriol. 1962, 84, 869–870. [Google Scholar] [CrossRef]
- Repaske, R. Lysis of gram-negative bacteria by lysozyme. Biochim. Et Biophys. Acta 1956, 22, 189–191. [Google Scholar] [CrossRef]
- Miot, J.; Bernard, S.; Bourreau, M.; Guyot, F.; Kish, A. Experimental maturation of Archaea encrusted by Fe-phosphates. Sci. Rep. 2017, 7, 16984. [Google Scholar] [CrossRef]
- Quehenberger, J.; Albersmeier, A.; Glatzel, H.; Hackl, M.; Kalinowski, J.; Spadiut, O. A defined cultivation medium for Sulfolobus acidocaldarius. J. Biotechnol. 2019, 301, 56–67. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafiq, M.; Hassan, N.; Rehman, M.; Hayat, M.; Nadeem, G.; Hassan, F.; Iqbal, N.; Ali, H.; Zada, S.; Kang, Y.; et al. Challenges and Approaches of Culturing the Unculturable Archaea. Biology 2023, 12, 1499. https://doi.org/10.3390/biology12121499
Rafiq M, Hassan N, Rehman M, Hayat M, Nadeem G, Hassan F, Iqbal N, Ali H, Zada S, Kang Y, et al. Challenges and Approaches of Culturing the Unculturable Archaea. Biology. 2023; 12(12):1499. https://doi.org/10.3390/biology12121499
Chicago/Turabian StyleRafiq, Muhammad, Noor Hassan, Maliha Rehman, Muhammad Hayat, Gullasht Nadeem, Farwa Hassan, Naveed Iqbal, Hazrat Ali, Sahib Zada, Yingqian Kang, and et al. 2023. "Challenges and Approaches of Culturing the Unculturable Archaea" Biology 12, no. 12: 1499. https://doi.org/10.3390/biology12121499
APA StyleRafiq, M., Hassan, N., Rehman, M., Hayat, M., Nadeem, G., Hassan, F., Iqbal, N., Ali, H., Zada, S., Kang, Y., Sajjad, W., & Jamal, M. (2023). Challenges and Approaches of Culturing the Unculturable Archaea. Biology, 12(12), 1499. https://doi.org/10.3390/biology12121499







