Age-Associated Changes of Sirtuin 2 Expression in CNS and the Periphery
Abstract
:Simple Summary
Abstract
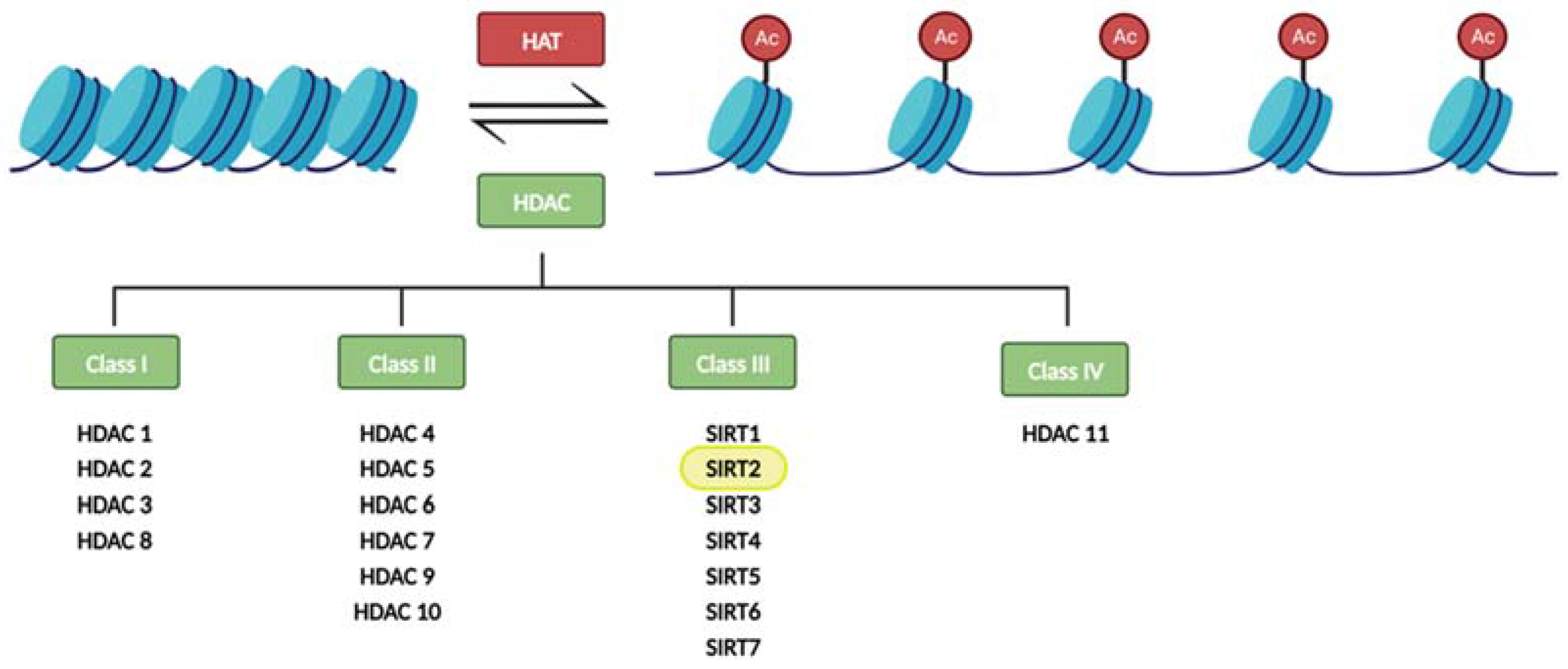
1. SIRT2 and Aging
2. CNS SIRT2 Expression in Aging
3. Peripheral SIRT2 Expression in Aging
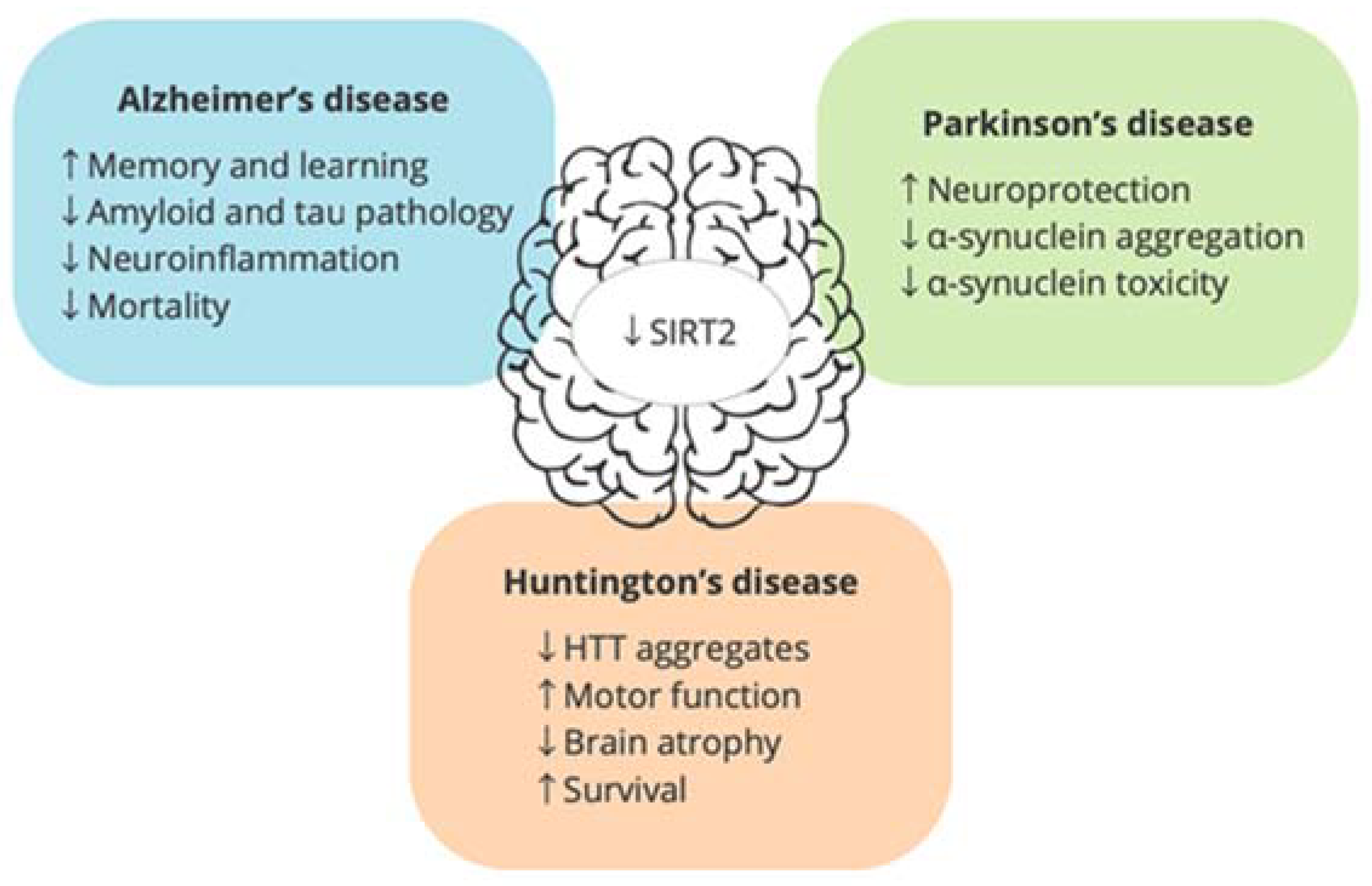
4. Is SIRT2 a Good Pharmacological Target for Age-Related Neurodegenerative Diseases?
4.1. Alzheimer’s Disease
4.2. Parkinson’s Disease
4.3. Huntington’s Disease
4.4. Amyotrophic Lateral Sclerosis
5. SIRT2 Pharmacological Inhibitors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 13 October 2023).
- Wu, C.T.; Morris, J.R. Genes, Genetics, and Epigenetics: A Correspondence. Science 2001, 293, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Pagiatakis, C.; Musolino, E.; Gornati, R.; Bernardini, G.; Papait, R. Epigenetics of Aging and Disease: A Brief Overview. Aging Clin. Exp. Res. 2021, 33, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.P. Epigenetics: Principles and Practice. Dig. Dis. 2011, 29, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Kim, J.-S. A Short Guide to Histone Deacetylases Including Recent Progress on Class II Enzymes. Exp. Mol. Med. 2020, 52, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Bahl, S.; Seto, E. Regulation of Histone Deacetylase Activities and Functions by Phosphorylation and Its Physiological Relevance. Cell. Mol. Life Sci. 2021, 78, 427–445. [Google Scholar] [CrossRef] [PubMed]
- Shoba, B.; Lwin, Z.M.; Ling, L.S.; Bay, B.-H.; Yip, G.W.; Kumar, S.D. Function of Sirtuins in Biological Tissues. Anat. Rec. 2009, 292, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mahur, P.; Muthukumaran, J.; Singh, A.K.; Jain, M. Shedding Light on Structure, Function and Regulation of Human Sirtuins: A Comprehensive Review. 3 Biotech. 2023, 13, 29. [Google Scholar] [CrossRef]
- Ziętara, P.; Dziewięcka, M.; Augustyniak, M. Why Is Longevity Still a Scientific Mystery? Sirtuins—Past, Present and Future. Int. J. Mol. Sci. 2023, 24, 728. [Google Scholar] [CrossRef]
- Chen, X.; Lu, W.; Wu, D. Sirtuin 2 (SIRT2): Confusing Roles in the Pathophysiology of Neurological Disorders. Front. Neurosci. 2021, 15, 614107. [Google Scholar] [CrossRef]
- Carafa, V.; Rotili, D.; Forgione, M.; Cuomo, F.; Serretiello, E.; Hailu, G.S.; Jarho, E.; Lahtela-Kakkonen, M.; Mai, A.; Altucci, L. Sirtuin Functions and Modulation: From Chemistry to the Clinic. Clin. Epigenetics 2016, 8, 61. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Hong, T.; Chen, X.; Cui, L. SIRT2: Controversy and Multiple Roles in Disease and Physiology. Ageing Res. Rev. 2019, 55, 100961. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-J.; Zhang, T.-N.; Chen, H.-H.; Yu, X.-F.; Lv, J.-L.; Liu, Y.-Y.; Liu, Y.-S.; Zheng, G.; Zhao, J.-Q.; Wei, Y.-F.; et al. The Sirtuin Family in Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Park, S.-H.; Imbesi, M.; Nathan, W.J.; Zou, X.; Zhu, Y.; Jiang, H.; Parisiadou, L.; Gius, D. Loss of NAD-Dependent Protein Deacetylase Sirtuin-2 Alters Mitochondrial Protein Acetylation and Dysregulates Mitophagy. Antioxid. Redox Signal. 2017, 26, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Deng, X.; Chen, Z.; Ba, X.; Qin, K.; Huang, Y.; Huang, Y.; Li, T.; Yan, J.; Tu, S. SIRT1: A Potential Therapeutic Target in Autoimmune Diseases. Front. Immunol. 2021, 12, 779177. [Google Scholar] [CrossRef] [PubMed]
- Manjula, R.; Anuja, K.; Alcain, F.J. SIRT1 and SIRT2 Activity Control in Neurodegenerative Diseases. Front. Pharmacol. 2021, 11. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, M.; Liang, J.; Wang, H.; Sun, D.; Li, H.; Chen, L. SIRT6 Mediates Multidimensional Modulation to Maintain Organism Homeostasis. J. Cell Physiol. 2022, 237, 3205–3221. [Google Scholar] [CrossRef]
- Tong, Z.; Wang, Y.; Zhang, X.; Kim, D.D.; Sadhukhan, S.; Hao, Q.; Lin, H. SIRT7 Is Activated by DNA and Deacetylates Histone H3 in the Chromatin Context. ACS Chem. Biol. 2016, 11, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Liu, G.-H.; Qu, J. Mitochondrial Sirtuins, Metabolism, and Aging. J. Genet. Genom. 2022, 49, 287–298. [Google Scholar] [CrossRef]
- Zhu, C.; Dong, X.; Wang, X.; Zheng, Y.; Qiu, J.; Peng, Y.; Xu, J.; Chai, Z.; Liu, C. Multiple Roles of SIRT2 in Regulating Physiological and Pathological Signal Transduction. Genet. Res. 2022, 2022, 9282484. [Google Scholar] [CrossRef]
- Chambers, S.M.; Shaw, C.A.; Gatza, C.; Fisk, C.J.; Donehower, L.A.; Goodell, M.A. Aging Hematopoietic Stem Cells Decline in Function and Exhibit Epigenetic Dysregulation. PLoS Biol. 2007, 5, e201. [Google Scholar] [CrossRef]
- Anwar, T.; Khosla, S.; Ramakrishna, G. Increased Expression of SIRT2 Is a Novel Marker of Cellular Senescence and Is Dependent on Wild Type P53 Status. Cell Cycle 2016, 15, 1883–1897. [Google Scholar] [CrossRef] [PubMed]
- Lehallier, B.; Gate, D.; Schaum, N.; Nanasi, T.; Lee, S.E.; Yousef, H.; Moran Losada, P.; Berdnik, D.; Keller, A.; Verghese, J.; et al. Undulating Changes in Human Plasma Proteome Profiles across the Lifespan. Nat. Med. 2019, 25, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Poljak, A.; Grant, R.; Jayasena, T.; Mansour, H.; Chan-Ling, T.; Smythe, G.; Sachdev, P.; Guillemin, G.J. Differential Expression of Sirtuins in the Aging Rat Brain. Front. Cell Neurosci. 2015, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, M.M.; Tomkinson, E.M.; Nobles, J.; Wizeman, J.W.; Amore, A.M.; Quinti, L.; Chopra, V.; Hersch, S.M.; Kazantsev, A.G. The Sirtuin 2 Microtubule Deacetylase Is an Abundant Neuronal Protein That Accumulates in the Aging CNS. Hum. Mol. Genet. 2011, 20, 3986–3996. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Perdigon, T.; Belloch, F.B.; Ricobaraza, A.; Elboray, E.E.; Suzuki, T.; Tordera, R.M.; Puerta, E. Early Sirtuin 2 Inhibition Prevents Age-Related Cognitive Decline in a Senescence-Accelerated Mouse Model. Neuropsychopharmacology 2020, 45, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Keskin-Aktan, A.; Akbulut, K.G.; Abdi, S.; Akbulut, H. SIRT2 and FOXO3a Expressions in the Cerebral Cortex and Hippocampus of Young and Aged Male Rats: Antioxidant and Anti-Apoptotic Effects of Melatonin. Biol. Futur. 2022, 73, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Wongchitrat, P.; Pakpian, N.; Kitidee, K.; Phopin, K.; Dharmasaroja, P.A.; Govitrapong, P. Alterations in the Expression of Amyloid Precursor Protein Cleaving Enzymes MRNA in Alzheimer Peripheral Blood. Curr. Alzheimer Res. 2019, 16, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Mu, W.-C.; Karki, R.; Chiang, H.-H.; Mohrin, M.; Shin, J.J.; Ohkubo, R.; Ito, K.; Kanneganti, T.-D.; Chen, D. Mitochondrial Stress-Initiated Aberrant Activation of the NLRP3 Inflammasome Regulates the Functional Deterioration of Hematopoietic Stem Cell Aging. Cell Rep. 2019, 26, 945–954.e4. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, K.; Liu, H.; Yu, Y.; Song, M.; Huang, D.; Lei, J.; Zhang, Y.; Liu, Z.; Chu, Q.; et al. SIRT2 Counteracts Primate Cardiac Aging via Deacetylation of STAT3 That Silences CDKN2B. Nat. Aging 2023, 3, 1269–1287. [Google Scholar] [CrossRef]
- North, B.J.; Marshall, B.L.; Borra, M.T.; Denu, J.M.; Verdin, E. The Human Sir2 Ortholog, SIRT2, Is an NAD+-Dependent Tubulin Deacetylase. Mol. Cell 2003, 11, 437–444. [Google Scholar] [CrossRef]
- Vaquero, A.; Scher, M.B.; Lee, D.H.; Sutton, A.; Cheng, H.-L.; Alt, F.W.; Serrano, L.; Sternglanz, R.; Reinberg, D. SirT2 Is a Histone Deacetylase with Preference for Histone H4 Lys 16 during Mitosis. Genes Dev. 2006, 20, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Michan, S.; Sinclair, D. Sirtuins in Mammals: Insights into Their Biological Function. Biochem. J. 2007, 404, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kim, S.; Ren, X. The Clinical Significance of SIRT2 in Malignancies: A Tumor Suppressor or an Oncogene? Front. Oncol. 2020, 10, 1721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Head, P.E.; Daddacha, W.; Park, S.-H.; Li, X.; Pan, Y.; Madden, M.Z.; Duong, D.M.; Xie, M.; Yu, B.; et al. ATRIP Deacetylation by SIRT2 Drives ATR Checkpoint Activation by Promoting Binding to RPA-SsDNA. Cell Rep. 2016, 14, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Luo, Y.; Warncke, K.; Sun, Y.; Yu, D.S.; Fu, H.; Behera, M.; Ramalingam, S.S.; Doetsch, P.W.; Duong, D.M.; et al. Acetylation Regulates Ribonucleotide Reductase Activity and Cancer Cell Growth. Nat. Commun. 2019, 10, 3213. [Google Scholar] [CrossRef]
- Muth, V.; Nadaud, S.; Grummt, I.; Voit, R. Acetylation of TAF(I)68, a Subunit of TIF-IB/SL1, Activates RNA Polymerase I Transcription. EMBO J. 2001, 20, 1353–1362. [Google Scholar] [CrossRef]
- Ishfaq, M.; Maeta, K.; Maeda, S.; Natsume, T.; Ito, A.; Yoshida, M. Acetylation Regulates Subcellular Localization of Eukaryotic Translation Initiation Factor 5A (EIF5A). FEBS Lett. 2012, 586, 3236–3241. [Google Scholar] [CrossRef]
- Cha, Y.; Han, M.-J.; Cha, H.-J.; Zoldan, J.; Burkart, A.; Jung, J.H.; Jang, Y.; Kim, C.-H.; Jeong, H.-C.; Kim, B.-G.; et al. Metabolic Control of Primed Human Pluripotent Stem Cell Fate and Function by the MiR-200c-SIRT2 Axis. Nat. Cell Biol. 2017, 19, 445–456. [Google Scholar] [CrossRef]
- Zhao, D.; Zou, S.-W.; Liu, Y.; Zhou, X.; Mo, Y.; Wang, P.; Xu, Y.-H.; Dong, B.; Xiong, Y.; Lei, Q.-Y.; et al. Lysine-5 Acetylation Negatively Regulates Lactate Dehydrogenase A and Is Decreased in Pancreatic Cancer. Cancer Cell 2013, 23, 464–476. [Google Scholar] [CrossRef]
- Gomes, P.; Fleming Outeiro, T.; Cavadas, C. Emerging Role of Sirtuin 2 in the Regulation of Mammalian Metabolism. Trends Pharmacol. Sci. 2015, 36, 756–768. [Google Scholar] [CrossRef]
- Zhang, H.; Dammer, E.B.; Duong, D.M.; Danelia, D.; Seyfried, N.T.; Yu, D.S. Quantitative Proteomic Analysis of the Lysine Acetylome Reveals Diverse SIRT2 Substrates. Sci. Rep. 2022, 12, 3822. [Google Scholar] [CrossRef] [PubMed]
- Crocco, P.; Montesanto, A.; Passarino, G.; Rose, G. Polymorphisms Falling Within Putative MiRNA Target Sites in the 3’UTR Region of SIRT2 and DRD2 Genes Are Correlated With Human Longevity. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 586–592. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Chiang, H.-H.; Luo, H.; Zheng, Z.; Qiao, Q.; Wang, L.; Tan, M.; Ohkubo, R.; Mu, W.-C.; Zhao, S.; et al. An Acetylation Switch of the NLRP3 Inflammasome Regulates Aging-Associated Chronic Inflammation and Insulin Resistance. Cell Metab. 2020, 31, 580–591.e5. [Google Scholar] [CrossRef] [PubMed]
- Sola-Sevilla, N.; Ricobaraza, A.; Hernandez-Alcoceba, R.; Aymerich, M.S.; Tordera, R.M.; Puerta, E. Understanding the Potential Role of Sirtuin 2 on Aging: Consequences of SIRT2.3 Overexpression in Senescence. Int. J. Mol. Sci. 2021, 22, 3107. [Google Scholar] [CrossRef]
- Wang, F.; Chan, C.-H.; Chen, K.; Guan, X.; Lin, H.-K.; Tong, Q. Deacetylation of FOXO3 by SIRT1 or SIRT2 Leads to Skp2-Mediated FOXO3 Ubiquitination and Degradation. Oncogene 2012, 31, 1546–1557. [Google Scholar] [CrossRef]
- Wang, F.; Nguyen, M.; Qin, F.X.-F.; Tong, Q. SIRT2 Deacetylates FOXO3a in Response to Oxidative Stress and Caloric Restriction. Aging Cell 2007, 6, 505–514. [Google Scholar] [CrossRef]
- Gómez-Crisóstomo, N.P.; Rodríguez Martínez, E.; Rivas-Arancibia, S. Oxidative Stress Activates the Transcription Factors FoxO 1a and FoxO 3a in the Hippocampus of Rats Exposed to Low Doses of Ozone. Oxid. Med. Cell Longev. 2014, 2014, 805764. [Google Scholar] [CrossRef]
- Lynn, E.G.; McLeod, C.J.; Gordon, J.P.; Bao, J.; Sack, M.N. SIRT2 Is a Negative Regulator of Anoxia–Reoxygenation Tolerance via Regulation of 14-3-3 ζ and BAD in H9c2 Cells. FEBS Lett. 2008, 582, 2857–2862. [Google Scholar] [CrossRef]
- Nie, H.; Hong, Y.; Lu, X.; Zhang, J.; Chen, H.; Li, Y.; Ma, Y.; Ying, W. SIRT2 Mediates Oxidative Stress-Induced Apoptosis of Differentiated PC12 Cells. Neuroreport 2014, 25, 838–842. [Google Scholar] [CrossRef]
- Sarikhani, M.; Mishra, S.; Desingu, P.A.; Kotyada, C.; Wolfgeher, D.; Gupta, M.P.; Singh, M.; Sundaresan, N.R. SIRT2 Regulates Oxidative Stress-Induced Cell Death through Deacetylation of c-Jun NH2-Terminal Kinase. Cell Death Differ. 2018, 25, 1638–1656. [Google Scholar] [CrossRef]
- She, D.T.; Wong, L.J.; Baik, S.-H.; Arumugam, T.V. SIRT2 Inhibition Confers Neuroprotection by Downregulation of FOXO3a and MAPK Signaling Pathways in Ischemic Stroke. Mol. Neurobiol. 2018, 55, 9188–9203. [Google Scholar] [CrossRef] [PubMed]
- Pino, E.; Amamoto, R.; Zheng, L.; Cacquevel, M.; Sarria, J.-C.; Knott, G.W.; Schneider, B.L. FOXO3 Determines the Accumulation of α-Synuclein and Controls the Fate of Dopaminergic Neurons in the Substantia Nigra. Hum. Mol. Genet. 2014, 23, 1435–1452. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Zhao, W.; Ho, L.; Wang, J.; Walsh, K.; Gandy, S.; Pasinetti, G.M. Regulation of Forkhead Transcription Factor FoxO3a Contributes to Calorie Restriction-Induced Prevention of Alzheimer’s Disease-Type Amyloid Neuropathology and Spatial Memory Deterioration. Ann. N.Y. Acad. Sci. 2008, 1147, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Garg, G.; Singh, S.; Singh, A.K.; Rizvi, S.I. Antiaging Effect of Metformin on Brain in Naturally Aged and Accelerated Senescence Model of Rat. Rejuvenation Res. 2017, 20, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Gal, J.; Bang, Y.; Choi, H.J. SIRT2 Interferes with Autophagy-Mediated Degradation of Protein Aggregates in Neuronal Cells under Proteasome Inhibition. Neurochem. Int. 2012, 61, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Nakayama, Y.; Li, Y.; Matsumori, H.; Takahashi, H.; Kojima, H.; Wanibuchi, H.; Katoh, M.; Oshimura, M. SIRT2 Knockdown Increases Basal Autophagy and Prevents Postslippage Death by Abnormally Prolonging the Mitotic Arrest That Is Induced by Microtubule Inhibitors. FEBS J. 2014, 281, 2623–2637. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Bai, N.; Zhao, X.; Cheng, R.; Wu, X.; Jiang, B.; Li, X.; Xue, M.; Xu, H.; Guo, Q.; et al. Cooperative Effects of SIRT1 and SIRT2 on APP Acetylation. Aging Cell 2023, 22, e13967. [Google Scholar] [CrossRef] [PubMed]
- Kireev, R.A.; Vara, E.; Tresguerres, J.A.F. Growth Hormone and Melatonin Prevent Age-Related Alteration in Apoptosis Processes in the Dentate Gyrus of Male Rats. Biogerontology 2013, 14, 431–442. [Google Scholar] [CrossRef]
- Yudoh, K.; Karasawa, R.; Ishikawa, J. Age-Related Decrease of Sirtuin 2 Protein in Human Peripheral Blood Mononuclear Cells. Curr. Aging Sci. 2015, 8, 256–258. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, X.-K.; Lv, S.-J.; Wang, H.-P.; Liu, Y.; Zhou, J.; Gong, H.; Chen, X.-F.; Ren, S.-C.; et al. Sirtuin 2 Deficiency Aggravates Ageing-Induced Vascular Remodelling in Humans and Mice. Eur. Heart J. 2023, 44, 2746–2759. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L. Promoting Health and Longevity through Diet: From Model Organisms to Humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [PubMed]
- North, B.J.; Rosenberg, M.A.; Jeganathan, K.B.; Hafner, A.V.; Michan, S.; Dai, J.; Baker, D.J.; Cen, Y.; Wu, L.E.; Sauve, A.A.; et al. SIRT2 Induces the Checkpoint Kinase BubR1 to Increase Lifespan. EMBO J. 2014, 33, 1438–1453. [Google Scholar] [CrossRef] [PubMed]
- 2022 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2022, 18, 700–789. [CrossRef] [PubMed]
- Sola-Sevilla, N.; Mesa-Lombardo, A.; Aleixo, M.; Expósito, S.; Diaz-Perdigón, T.; Azqueta, A.; Zamani, F.; Suzuki, T.; Maioli, S.; Eroli, F.; et al. SIRT2 Inhibition Rescues Neurodegenerative Pathology but Increases Systemic Inflammation in a Transgenic Mouse Model of Alzheimer’s Disease. J. Neuroimmune Pharmacol. 2023, 18, 529–550. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.F.; Esteves, A.R.; Oliveira, C.R.; Cardoso, S.M. Mitochondrial Metabolism Power SIRT2-Dependent Deficient Traffic Causing Alzheimer’s-Disease Related Pathology. Mol. Neurobiol. 2017, 54, 4021–4040. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Hanson, P.S.; Morris, C.M. Sirtuin-2 Protects Neural Cells from Oxidative Stress and Is Elevated in Neurodegeneration. Parkinsons Dis. 2017, 2017, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yun, T.; Ko, H.R.; Jo, D.-G.; Park, K.W.; Cho, S.-W.; Kim, J.; Ahn, J.-Y. Inhibitor of DNA Binding 2 (Id2) Mediates Microtubule Polymerization in the Brain by Regulating AK40 Acetylation of α-Tubulin. Cell Death Discov. 2021, 7, 257. [Google Scholar] [CrossRef]
- Gaetani, L.; Bellomo, G.; Parnetti, L.; Blennow, K.; Zetterberg, H.; Di Filippo, M. Neuroinflammation and Alzheimer’s Disease: A Machine Learning Approach to CSF Proteomics. Cells 2021, 10, 1930. [Google Scholar] [CrossRef]
- Sola-Sevilla, N.; Puerta, E. SIRT2 as a Potential New Therapeutic Target for Alzheimer’s Disease. Neural Regen. Res. 2024, 19, 125–131. [Google Scholar] [CrossRef]
- Biella, G.; Fusco, F.; Nardo, E.; Bernocchi, O.; Colombo, A.; Lichtenthaler, S.F.; Forloni, G.; Albani, D. Sirtuin 2 Inhibition Improves Cognitive Performance and Acts on Amyloid-β Protein Precursor Processing in Two Alzheimer’s Disease Mouse Models. J. Alzheimer’s Dis. 2016, 53, 1193–1207. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.-Q.; Hong, T.-T.; Sun, Y.-H.; Huang, H.-L.; Chen, F.; Chen, X.-J.; Chen, H.-Y.; Dong, S.-S.; Cui, L.-L.; et al. RTN4B-Mediated Suppression of Sirtuin 2 Activity Ameliorates β-Amyloid Pathology and Cognitive Impairment in Alzheimer’s Disease Mouse Model. Aging Cell 2020, 19, e13194. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Li, N.; Cheng, R.; Guan, Y.; Zhao, X.; Song, Z.; Xu, H.; Yi, F.; Jiang, B.; Li, X.; et al. Inhibition of SIRT2 Promotes APP Acetylation and Ameliorates Cognitive Impairment in APP/PS1 Transgenic Mice. Cell Rep. 2022, 40, 111062. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.J.; West, A.B.; Dawson, V.L.; Dawson, T.M. Molecular Pathophysiology of Parkinson’s Disease. Annu. Rev. Neurosci. 2005, 28, 57–87. [Google Scholar] [CrossRef] [PubMed]
- Shihabuddin, L.S.; Brundin, P.; Greenamyre, J.T.; Stephenson, D.; Sardi, S.P. New Frontiers in Parkinson’s Disease: From Genetics to the Clinic. J. Neurosci. 2018, 38, 9375–9382. [Google Scholar] [CrossRef]
- Wang, X.; Guan, Q.; Wang, M.; Yang, L.; Bai, J.; Yan, Z.; Zhang, Y.; Liu, Z. Aging-Related Rotenone-Induced Neurochemical and Behavioral Deficits: Role of SIRT2 and Redox Imbalance, and Neuroprotection by AK-7. Drug Des. Devel Ther. 2015, 9, 2553–2563. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, M.; Chen, H.; Yang, L.; Yan, Z.; Wang, X. Aging-Related 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Neurochemial and Behavioral Deficits and Redox Dysfunction: Improvement by AK-7. Exp. Gerontol. 2016, 82, 19–29. [Google Scholar] [CrossRef]
- Sun, S.; Han, X.; Li, X.; Song, Q.; Lu, M.; Jia, M.; Ding, J.; Hu, G. MicroRNA-212-5p Prevents Dopaminergic Neuron Death by Inhibiting SIRT2 in MPTP-Induced Mouse Model of Parkinson’s Disease. Front. Mol. Neurosci. 2018, 11, 381. [Google Scholar] [CrossRef]
- Chen, X.; Mai, H.; Chen, X.; Cai, Y.; Cheng, Q.; Chen, X.; Li, X.; Fan, W.; Tang, P.; Ou, M.; et al. Rs2015 Polymorphism in MiRNA Target Site of Sirtuin2 Gene Is Associated with the Risk of Parkinson’s Disease in Chinese Han Population. Biomed. Res. Int. 2019, 2019, 1498034. [Google Scholar] [CrossRef]
- Chen, X.; Wales, P.; Quinti, L.; Zuo, F.; Moniot, S.; Herisson, F.; Rauf, N.A.; Wang, H.; Silverman, R.B.; Ayata, C.; et al. The Sirtuin-2 Inhibitor AK7 Is Neuroprotective in Models of Parkinson’s Disease but Not Amyotrophic Lateral Sclerosis and Cerebral Ischemia. PLoS ONE 2015, 10, e0116919. [Google Scholar] [CrossRef]
- Esteves, A.R.; Arduíno, D.M.; Silva, D.F.; Viana, S.D.; Pereira, F.C.; Cardoso, S.M. Mitochondrial Metabolism Regulates Microtubule Acetylome and Autophagy Trough Sirtuin-2: Impact for Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 1440–1462. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, P.; Tan, J.; Li, M.; Xu, X.; Shao, X.; Fang, F.; Zou, Z.; Zhou, Y.; Tian, B. Cdk5 Phosphorylation-Induced SIRT2 Nuclear Translocation Promotes the Death of Dopaminergic Neurons in Parkinson’s Disease. NPJ Parkinsons Dis. 2022, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Outeiro, T.F.; Kontopoulos, E.; Altmann, S.M.; Kufareva, I.; Strathearn, K.E.; Amore, A.M.; Volk, C.B.; Maxwell, M.M.; Rochet, J.-C.; McLean, P.J.; et al. Sirtuin 2 Inhibitors Rescue Alpha-Synuclein-Mediated Toxicity in Models of Parkinson’s Disease. Science 2007, 317, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Di Fruscia, P.; Zacharioudakis, E.; Liu, C.; Moniot, S.; Laohasinnarong, S.; Khongkow, M.; Harrison, I.F.; Koltsida, K.; Reynolds, C.R.; Schmidtkunz, K.; et al. The Discovery of a Highly Selective 5,6,7,8-Tetrahydrobenzo[4,5]Thieno[2,3-d]Pyrimidin-4(3H)-One SIRT2 Inhibitor That Is Neuroprotective in an in Vitro Parkinson’s Disease Model. ChemMedChem 2015, 10, 69–82. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.M.; Vicente Miranda, H.; Francelle, L.; Pinho, R.; Szegö, É.M.; Martinho, R.; Munari, F.; Lázaro, D.F.; Moniot, S.; Guerreiro, P.; et al. The Mechanism of Sirtuin 2-Mediated Exacerbation of Alpha-Synuclein Toxicity in Models of Parkinson Disease. PLoS Biol. 2017, 15, e2000374. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lv, X.; Zhai, K.; Xu, R.; Zhang, Y.; Zhao, S.; Qin, X.; Yin, L.; Lou, J. MicroRNA-7 Inhibits Neuronal Apoptosis in a Cellular Parkinson’s Disease Model by Targeting Bax and Sirt2. Am. J. Transl. Res. 2016, 8, 993–1004. [Google Scholar] [PubMed]
- Wang, Y.; Cai, Y.; Huang, H.; Chen, X.; Chen, X.; Chen, X.; Mai, H.; Li, X.; Zhao, J.; Yang, J.; et al. MiR-486-3p Influences the Neurotoxicity of a-Synuclein by Targeting the SIRT2 Gene and the Polymorphisms at Target Sites Contributing to Parkinson’s Disease. Cell Physiol. Biochem. 2018, 51, 2732–2745. [Google Scholar] [CrossRef] [PubMed]
- Young, A.B. Huntingtin in Health and Disease. J. Clin. Investig. 2003, 111, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Baldo, B.; Gabery, S.; Soylu-Kucharz, R.; Cheong, R.Y.; Henningsen, J.B.; Englund, E.; McLean, C.; Kirik, D.; Halliday, G.; Petersén, Å. SIRT1 Is Increased in Affected Brain Regions and Hypothalamic Metabolic Pathways Are Altered in Huntington Disease. Neuropathol. Appl. Neurobiol. 2019, 45, 361–379. [Google Scholar] [CrossRef]
- Luthi-Carter, R.; Taylor, D.M.; Pallos, J.; Lambert, E.; Amore, A.; Parker, A.; Moffitt, H.; Smith, D.L.; Runne, H.; Gokce, O.; et al. SIRT2 Inhibition Achieves Neuroprotection by Decreasing Sterol Biosynthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 7927–7932. [Google Scholar] [CrossRef]
- Chopra, V.; Quinti, L.; Kim, J.; Vollor, L.; Narayanan, K.L.; Edgerly, C.; Cipicchio, P.M.; Lauver, M.A.; Choi, S.H.; Silverman, R.B.; et al. The Sirtuin 2 Inhibitor AK-7 Is Neuroprotective in Huntington’s Disease Mouse Models. Cell Rep. 2012, 2, 1492–1497. [Google Scholar] [CrossRef]
- Pallos, J.; Bodai, L.; Lukacsovich, T.; Purcell, J.M.; Steffan, J.S.; Thompson, L.M.; Marsh, J.L. Inhibition of Specific HDACs and Sirtuins Suppresses Pathogenesis in a Drosophila Model of Huntington’s Disease. Hum. Mol. Genet. 2008, 17, 3767–3775. [Google Scholar] [CrossRef] [PubMed]
- Quinti, L.; Casale, M.; Moniot, S.; Pais, T.F.; Van Kanegan, M.J.; Kaltenbach, L.S.; Pallos, J.; Lim, R.G.; Naidu, S.D.; Runne, H.; et al. SIRT2- and NRF2-Targeting Thiazole-Containing Compound with Therapeutic Activity in Huntington’s Disease Models. Cell Chem. Biol. 2016, 23, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.M.; Balabadra, U.; Xiang, Z.; Woodman, B.; Meade, S.; Amore, A.; Maxwell, M.M.; Reeves, S.; Bates, G.P.; Luthi-Carter, R.; et al. A Brain-Permeable Small Molecule Reduces Neuronal Cholesterol by Inhibiting Activity of Sirtuin 2 Deacetylase. ACS Chem. Biol. 2011, 6, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Bobrowska, A.; Donmez, G.; Weiss, A.; Guarente, L.; Bates, G. SIRT2 Ablation Has No Effect on Tubulin Acetylation in Brain, Cholesterol Biosynthesis or the Progression of Huntington’s Disease Phenotypes in Vivo. PLoS ONE 2012, 7, e34805. [Google Scholar] [CrossRef] [PubMed]
- Liscic, R.M.; Alberici, A.; Cairns, N.J.; Romano, M.; Buratti, E. From Basic Research to the Clinic: Innovative Therapies for ALS and FTD in the Pipeline. Mol. Neurodegener. 2020, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Valle, C.; Salvatori, I.; Gerbino, V.; Rossi, S.; Palamiuc, L.; René, F.; Carrì, M.T. Tissue-Specific Deregulation of Selected HDACs Characterizes ALS Progression in Mouse Models: Pharmacological Characterization of SIRT1 and SIRT2 Pathways. Cell Death Dis. 2014, 5, e1296. [Google Scholar] [CrossRef] [PubMed]
- Taes, I.; Timmers, M.; Hersmus, N.; Bento-Abreu, A.; Van Den Bosch, L.; Van Damme, P.; Auwerx, J.; Robberecht, W. Hdac6 Deletion Delays Disease Progression in the SOD1G93A Mouse Model of ALS. Hum. Mol. Genet. 2013, 22, 1783–1790. [Google Scholar] [CrossRef]
- Banerjee, P.; Elliott, E.; Rifai, O.M.; O’Shaughnessy, J.; McDade, K.; Abrahams, S.; Chandran, S.; Smith, C.; Gregory, J.M. NLRP3 Inflammasome as a Key Molecular Target Underlying Cognitive Resilience in Amyotrophic Lateral Sclerosis. J. Pathol. 2022, 256, 262–268. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the Role of NLRP3 Inflammasome in Diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef]
- Lünemann, J.D.; Malhotra, S.; Shinohara, M.L.; Montalban, X.; Comabella, M. Targeting Inflammasomes to Treat Neurological Diseases. Ann. Neurol. 2021, 90, 177–188. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, T.; Zhu, Q.; Xu, Y.; Zha, X. Recent Advances in Inhibitors of Sirtuin1/2: An Update and Perspective. Future Med. Chem. 2018, 10, 907–934. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Khan, M.N.A.; Sawada, H.; Imai, E.; Itoh, Y.; Yamatsuta, K.; Tokuda, N.; Takeuchi, J.; Seko, T.; Nakagawa, H.; et al. Design, Synthesis, and Biological Activity of a Novel Series of Human Sirtuin-2-Selective Inhibitors. J. Med. Chem. 2012, 55, 5760–5773. [Google Scholar] [CrossRef] [PubMed]
- Erburu, M.; Muñoz-Cobo, I.; Diaz-Perdigon, T.; Mellini, P.; Suzuki, T.; Puerta, E.; Tordera, R.M. SIRT2 Inhibition Modulate Glutamate and Serotonin Systems in the Prefrontal Cortex and Induces Antidepressant-like Action. Neuropharmacology 2017, 117, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Matsumoto, Y.; Ishikawa, M.; Sugita, K.; Hashimoto, Y.; Wakai, N.; Kitao, A.; Morishita, E.; Toyoshima, C.; Hayashi, T.; et al. Design, Synthesis and Structure–Activity Relationship Studies of Novel Sirtuin 2 (SIRT2) Inhibitors with a Benzamide Skeleton. Bioorganic Med. Chem. 2015, 23, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Yeong, K.Y.; Khaw, K.Y.; Takahashi, Y.; Itoh, Y.; Murugaiyah, V.; Suzuki, T. Discovery of Gamma-Mangostin from Garcinia Mangostana as a Potent and Selective Natural SIRT2 Inhibitor. Bioorganic Chem. 2020, 94, 103403. [Google Scholar] [CrossRef]
- Singh, A.P.; Nigam, L.; Yadav, Y.; Shekhar, S.; Subbarao, N.; Dey, S. Design and in Vitro Analysis of SIRT2 Inhibitor Targeting Parkinson’s Disease. Mol. Divers. 2021, 25, 2261–2270. [Google Scholar] [CrossRef]
- Djokovic, N.; Rahnasto-Rilla, M.; Lougiakis, N.; Lahtela-Kakkonen, M.; Nikolic, K. SIRT2i_Predictor: A Machine Learning-Based Tool to Facilitate the Discovery of Novel SIRT2 Inhibitors. Pharmaceuticals 2023, 16, 127. [Google Scholar] [CrossRef]
| Authors and Year | Analyzed Model | Sample | Sirtuin 2 Expression with Aging | |
|---|---|---|---|---|
| Specie | Ages Compared in Months | |||
| Maxwell et al., 2011 [25] | C57BL/6 mouse | 4–5 vs. 19–22 | Spinal cord and cortex | Increase |
| Kireev et al., 2013 [59] | Male Wistar rat | 2 vs. 22 | Hippocampus (dentate gyrus) | Decrease |
| Braidy et al., 2015 [24] | Female Wistar rat | 3 vs. 12 vs. 24 | Occipital lobe | Increase |
| Garg et al., 2017 [55] | Male Wistar rat | 4 vs. 24 | Whole brain | Increase |
| Diaz-Perdigon et al., 2020 [26] | Male SAMR1 and SAMP8 mice | 2 vs. 9 | Hippocampus | Increase |
| Keskin-Atkan et al., 2022 [27] | Male Wistar rat | 3 vs. 22 | Hippocampus and cortex | Increase |
| Li et al., 2023 [58] | C57BL/6 mouse | 3 vs. 6 vs. 12 vs. 24 | Hippocampus and cortex | Increase |
| Authors and Year | Analyzed Model | Sample | Sirtuin 2 Expression with Aging | |
|---|---|---|---|---|
| Specie | Ages Compared | |||
| Chambers et al., 2007 [21] | C57BL/6 mouse | 2- vs. 21-month-old | HSCs isolated from BM | Decrease |
| Yudoh et al., 2015 [60] | Human | 22- to 66-year-old | PBMCs | Decrease |
| Luo et al., 2019 [29] | C57BL/6 mouse | 3- vs. 24-month-old | HSCs isolated from BM | Decrease |
| Wongchitrat et al., 2019 [28] | Human | 25- to 35-year-old vs. ≥65-year-old | Peripheral blood (plasma) | Increase |
| Lehallier et al., 2019 [23] | Human | 18- to 95-year-old | Peripheral blood (plasma) | Decrease |
| He et al., 2020 [44] | Male C57BL/6 mouse | 3- vs. 24-month-old | Macrophages isolated from BM | Decrease |
| Ye et al., 2023 [30] | Cynomolgus macaque | 4- to 6- vs. 18- to 21-year-old | Cardiomyocytes | Decrease |
| Zhang et al., 2023 [61] | C57BL/6 mouse | 4- vs. 24-month-old | Aorta and VSMCs | Decrease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garmendia-Berges, M.; Sola-Sevilla, N.; Mera-Delgado, M.; Puerta, E. Age-Associated Changes of Sirtuin 2 Expression in CNS and the Periphery. Biology 2023, 12, 1476. https://doi.org/10.3390/biology12121476
Garmendia-Berges M, Sola-Sevilla N, Mera-Delgado M, Puerta E. Age-Associated Changes of Sirtuin 2 Expression in CNS and the Periphery. Biology. 2023; 12(12):1476. https://doi.org/10.3390/biology12121476
Chicago/Turabian StyleGarmendia-Berges, Maider, Noemi Sola-Sevilla, MCarmen Mera-Delgado, and Elena Puerta. 2023. "Age-Associated Changes of Sirtuin 2 Expression in CNS and the Periphery" Biology 12, no. 12: 1476. https://doi.org/10.3390/biology12121476
APA StyleGarmendia-Berges, M., Sola-Sevilla, N., Mera-Delgado, M., & Puerta, E. (2023). Age-Associated Changes of Sirtuin 2 Expression in CNS and the Periphery. Biology, 12(12), 1476. https://doi.org/10.3390/biology12121476





