Mitochondrial Functionality Is Regulated by Alkylphospholipids in Human Colon Cancer Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Treatments
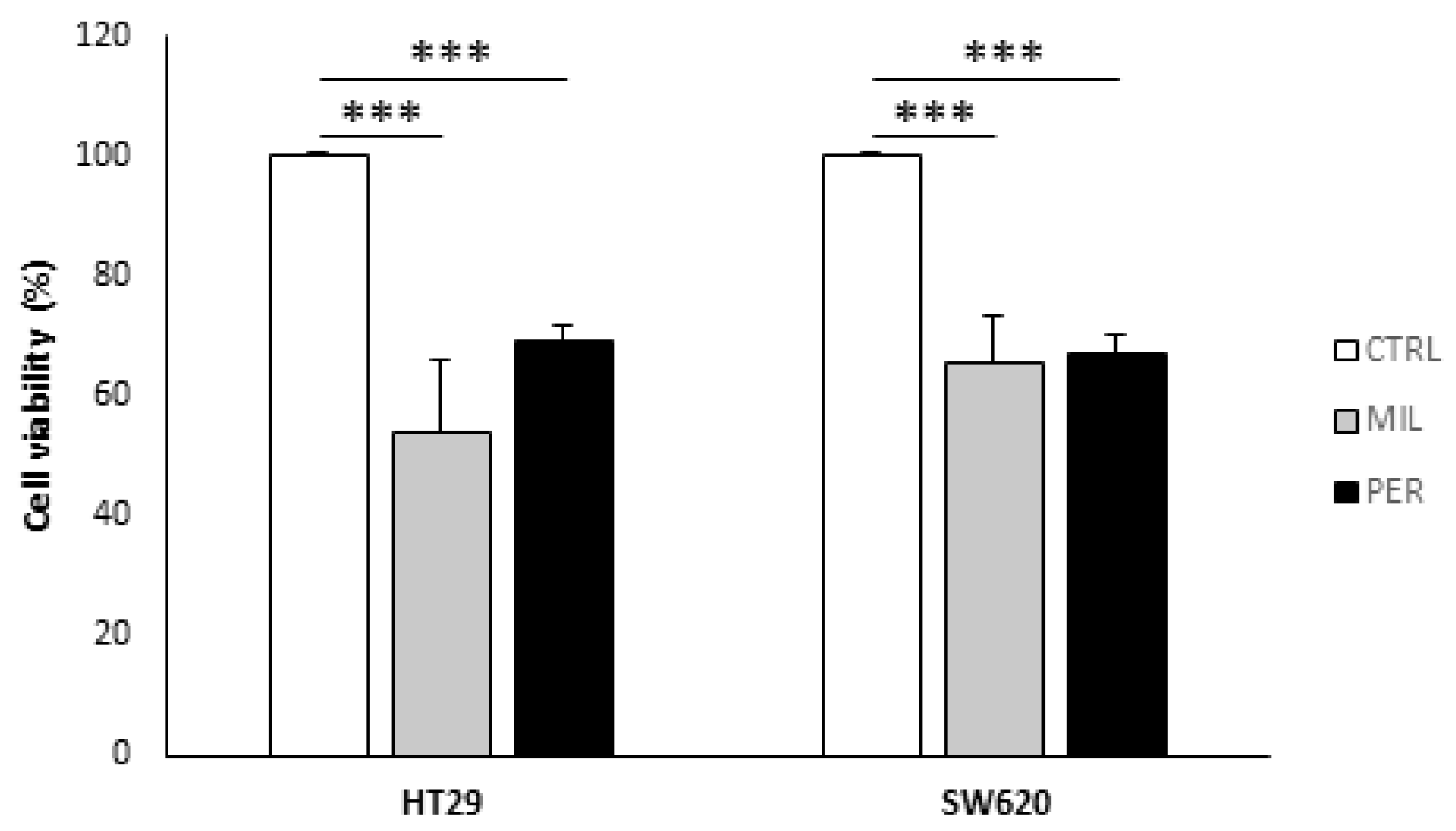
2.3. Determination of Cell Viability
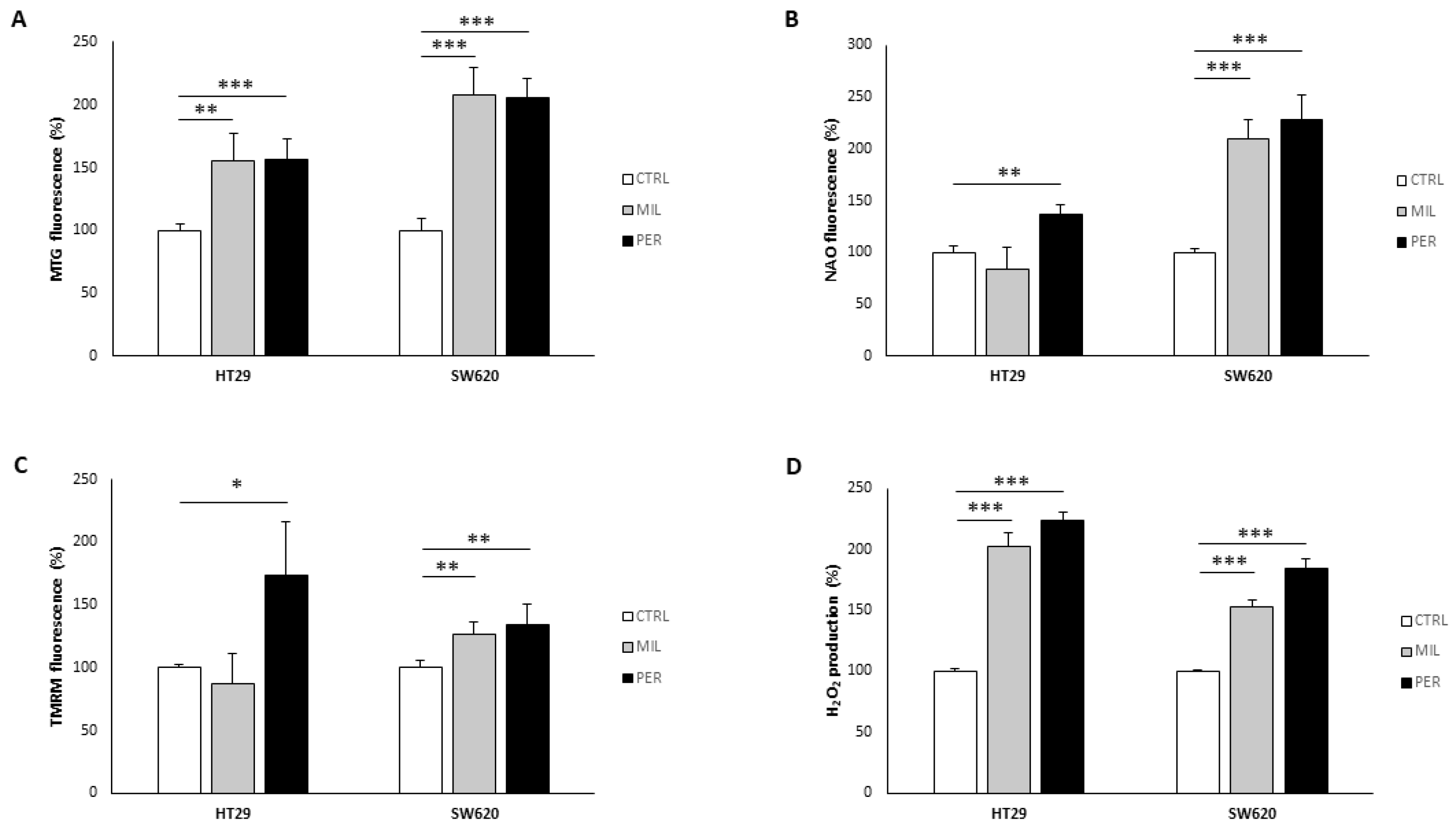
2.4. Analysis of Mitochondrial Parameters
2.5. Measurement of H2O2 Production
2.6. Western Blot
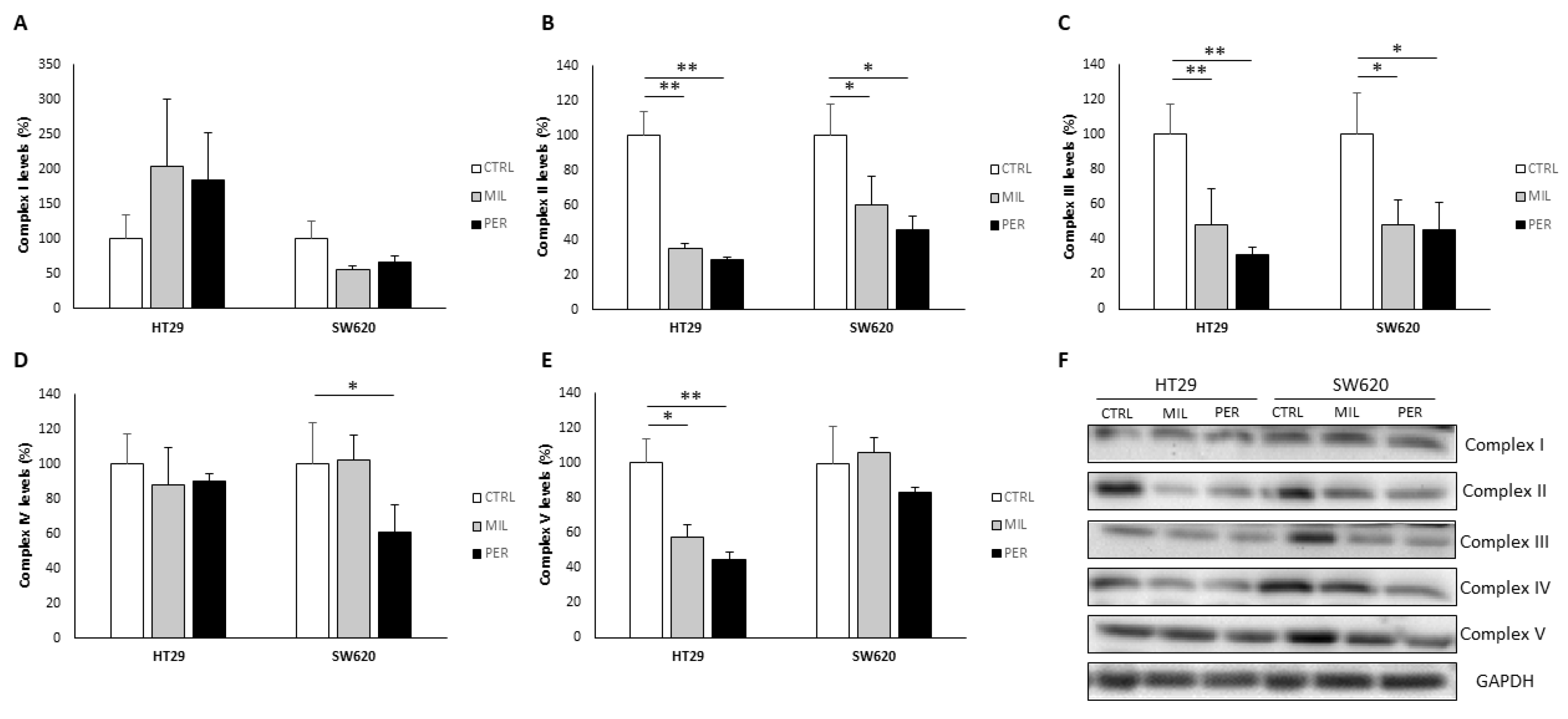
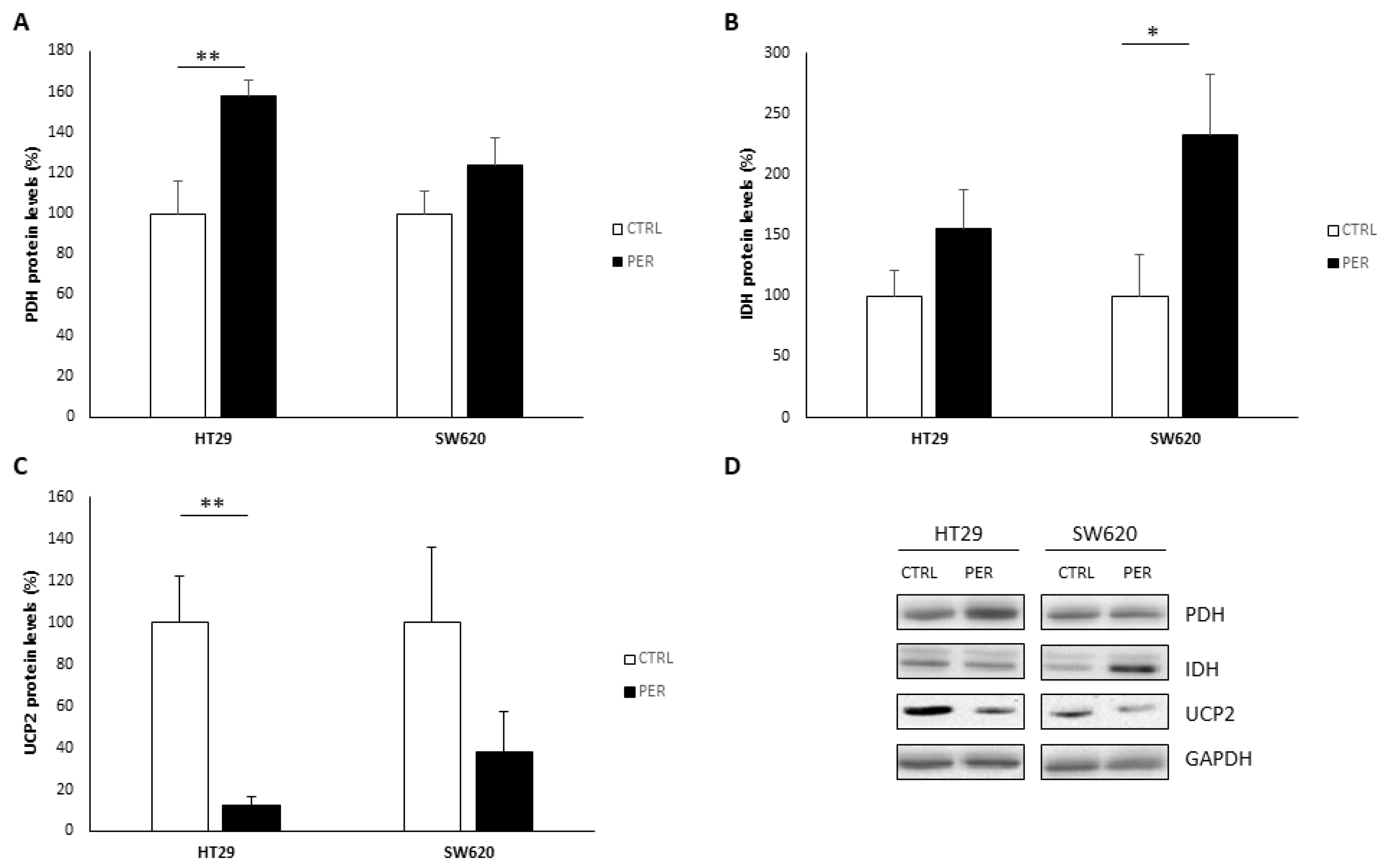
2.7. Enzymatic Activities
2.8. Oxygen Consumption Rate (OCR)
2.9. Statistical Analyses
3. Results
3.1. APLs Decrease Cell Viability in Colon Cancer Cells
3.2. APLs Increase Mitochondrial Mass and H2O2 Production
3.3. Oxidative Phosphorylation Complexes Are Affected by APL Treatment
3.4. Perifosine Alters Mitochondrial Function
3.5. Perifosine Increases PARP Cleavage and LC3-II Levels
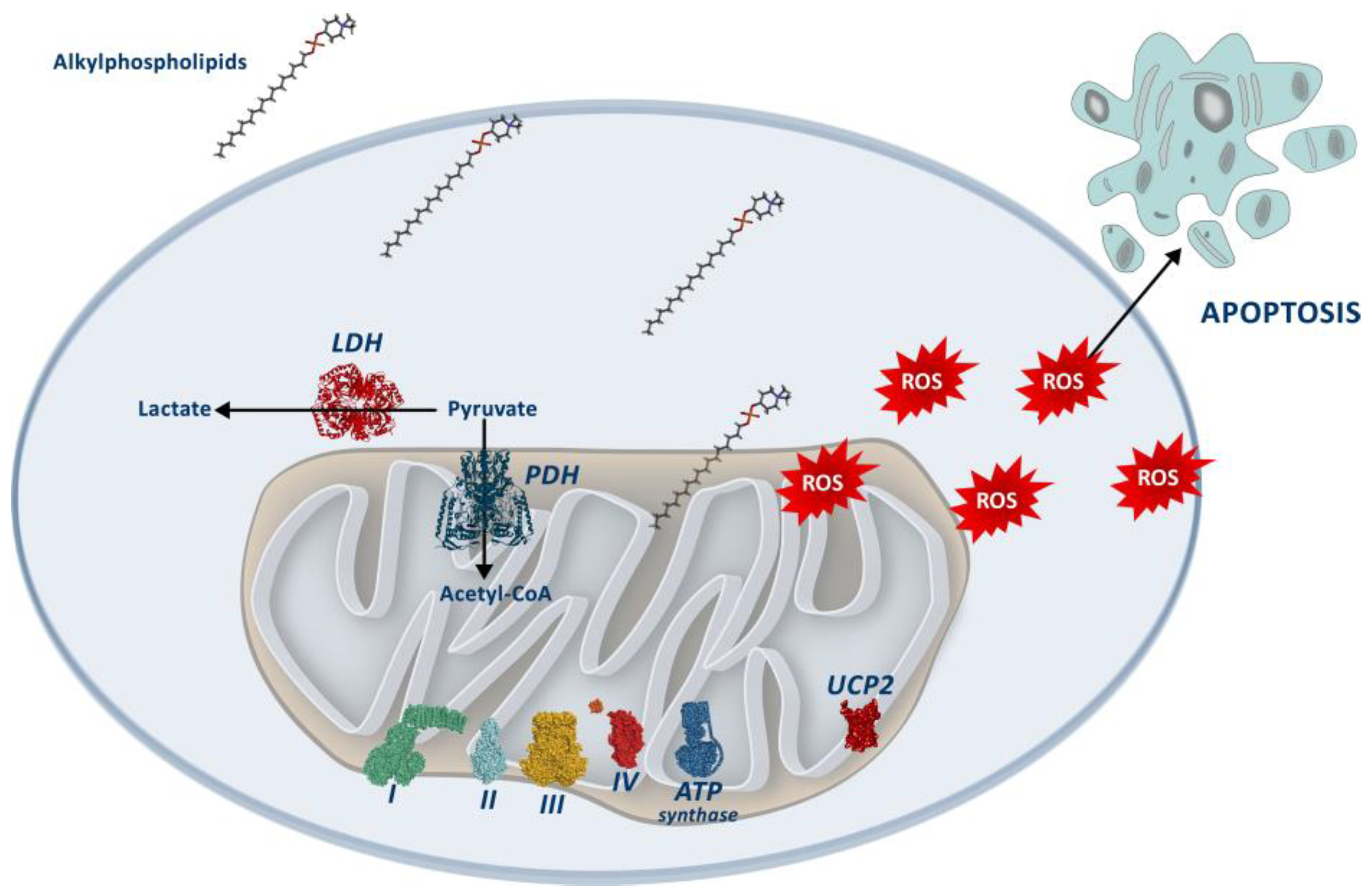
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ríos-Marco, P.; Marco, C.; Gálvez, X.; Jiménez-López, J.M.; Carrasco, M.P. Alkylphospholipids: An Update on Molecular Mechanisms and Clinical Relevance. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Burgeiro, A.; Pereira, C.V.; Carvalho, F.S.; Pereira, G.C.; Mollinedo, F.; Oliveira, P.J. Edelfosine and Perifosine Disrupt Hepatic Mitochondrial Oxidative Phosphorylation and Induce the Permeability Transition. Mitochondrion 2013, 13, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Kostadinova, A.; Topouzova-Hristova, T.; Momchilova, A.; Tzoneva, R.; Berger, M.R. Antitumor Lipids—Structure, Functions, and Medical Applications. In Advances in Protein Chemistry and Structural Biology; Academic Press Inc.: Cambridge, MA, USA, 2015; Volume 101, pp. 27–66. [Google Scholar]
- Kaleağasıoğlu, F.; Ali, D.M.; Berger, M.R. Multiple Facets of Autophagy and the Emerging Role of Alkylphosphocholines as Autophagy Modulators. Front. Pharmacol. 2020, 11, 547. [Google Scholar] [CrossRef]
- Leighl, N.B.; Dent, S.; Clemons, M.; Vandenberg, T.A.; Tozer, R.; Warr, D.G.; Crump, R.M.; Hedley, D.; Pond, G.R.; Dancey, J.E.; et al. A Phase 2 Study of Perifosine in Advanced or Metastatic Breast Cancer. Breast Cancer Res. Treat. 2008, 108, 87–92. [Google Scholar] [CrossRef] [PubMed]
- El-Sheridy, N.A.; El-Moslemany, R.M.; Ramadan, A.A.; Helmy, M.W.; El-Khordagui, L.K. Enhancing the in Vitro and in Vivo Activity of Itraconazole against Breast Cancer Using Miltefosine-Modified Lipid Nanocapsules. Drug Deliv. 2021, 28, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.; Choi, J.; Lee, C.; Lee, W.; Park, S.; Park, Z.; Baek, J.; Nam, J. Lipid Raft-disrupting Miltefosine Preferentially Induces the Death of Colorectal Cancer Stem-like Cells. Clin. Transl. Med. 2021, 11, e552. [Google Scholar] [CrossRef]
- Morii, Y.; Tsubaki, M.; Takeda, T.; Otubo, R.; Seki, S.; Yamatomo, Y.; Imano, M.; Satou, T.; Shimomura, K.; Nishida, S. Perifosine Enhances the Potential Antitumor Effect of 5-Fluorourasil and Oxaliplatin in Colon Cancer Cells Harboring the PIK3CA Mutation. Eur. J. Pharmacol. 2021, 898, 173957. [Google Scholar] [CrossRef]
- Pavlatovská, B.; Machálková, M.; Brisudová, P.; Pruška, A.; Štěpka, K.; Michálek, J.; Nečasová, T.; Beneš, P.; Šmarda, J.; Preisler, J.; et al. Lactic Acidosis Interferes With Toxicity of Perifosine to Colorectal Cancer Spheroids: Multimodal Imaging Analysis. Front. Oncol. 2020, 10, 581365. [Google Scholar] [CrossRef]
- Ríos-Marco, P.; Ríos, A.; Jiménez-López, J.M.; Carrasco, M.P.; Marco, C. Cholesterol Homeostasis and Autophagic Flux in Perifosine-Treated Human Hepatoblastoma HepG2 and Glioblastoma U-87 MG Cell Lines. Biochem. Pharmacol. 2015, 96, 10–19. [Google Scholar] [CrossRef]
- Ríos-Marco, P.; Martín-Fernández, M.; Soria-Bretones, I.; Ríos, A.; Carrasco, M.P.; Marco, C. Alkylphospholipids Deregulate Cholesterol Metabolism and Induce Cell-Cycle Arrest and Autophagy in U-87 MG Glioblastoma Cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 1322–1334. [Google Scholar] [CrossRef]
- Richardson, P.G.; Nagler, A.; Ben-Yehuda, D.; Badros, A.; Hari, P.N.; Hajek, R.; Spicka, I.; Kaya, H.; LeBlanc, R.; Yoon, S.; et al. Randomized, Placebo-controlled, Phase 3 Study of Perifosine Combined with Bortezomib and Dexamethasone in Patients with Relapsed, Refractory Multiple Myeloma Previously Treated with Bortezomib. EJHaem 2020, 1, 94–102. [Google Scholar] [CrossRef]
- Jesus, L.d.O.P.; de Souza, A.A.; Torquato, H.F.V.; Gontijo, V.S.; Pereira de Freitas, R.; Gesteira, T.F.; Coulson-Thomas, V.J.; Torquato, R.J.S.; Tanaka, A.S.; Paredes-Gamero, E.J.; et al. In Vitro Study of Cytotoxic Mechanisms of Alkylphospholipids and Alkyltriazoles in Acute Lymphoblastic Leukemia Models. Molecules 2022, 27, 8633. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Tu, X.; Zhou, Q.; Huang, J.; Chen, Y.; Liu, J.; Lee, S.B.; Kim, W.; Nowsheen, S.; Luo, K.; et al. A Novel UCHL3 Inhibitor, Perifosine, Enhances PARP Inhibitor Cytotoxicity through Inhibition of Homologous Recombination-Mediated DNA Double Strand Break Repair. Cell Death Dis. 2019, 10, 398. [Google Scholar] [CrossRef]
- Adamová, B.; Říhová, K.; Pokludová, J.; Beneš, P.; Šmarda, J.; Navrátilová, J. Synergistic Cytotoxicity of Perifosine and ABT-737 to Colon Cancer Cells. J. Cell Mol. Med. 2023, 27, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Villa-Pulgarín, J.A.; Gajate, C.; Botet, J.; Jimenez, A.; Justies, N.; Varela-M, R.E.; Cuesta-Marbán, Á.; Müller, I.; Modolell, M.; Revuelta, J.L.; et al. Mitochondria and Lipid Raft-Located FOF1-ATP Synthase as Major Therapeutic Targets in the Antileishmanial and Anticancer Activities of Ether Lipid Edelfosine. PLoS Negl. Trop. Dis. 2017, 11, e0005805. [Google Scholar] [CrossRef] [PubMed]
- Marco, C.; Ríos-Marco, P.; Jiménez-López, J.; Segovia, J.; Carrasco, M. Antitumoral Alkylphospholipids Alter Cell Lipid Metabolism. Anti-Cancer Agents Med. Chem. 2014, 14, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Gajate, C.; Mollinedo, F. The Antitumor Ether Lipid ET-18-OCH3 Induces Apoptosis through Translocation and Capping of Fas/CD95 into Membrane Rafts in Human Leukemic Cells. Blood 2001, 98, 3860–3863. [Google Scholar] [CrossRef]
- Gomide, A.B.; Thomé, C.H.; dos Santos, G.A.; Ferreira, G.A.; Faça, V.M.; Rego, E.M.; Greene, L.J.; Stabeli, R.G.; Ciancaglini, P.; Itri, R. Disrupting Membrane Raft Domains by Alkylphospholipids. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Kuerschner, L.; Richter, D.; Hannibal-Bach, H.K.; Gaebler, A.; Shevchenko, A.; Ejsing, C.S.; Thiele, C. Exogenous Ether Lipids Predominantly Target Mitochondria. PLoS ONE 2012, 7, e31342. [Google Scholar] [CrossRef]
- García-Ruiz, C.; Ribas, V.; Baulies, A.; Fernández-Checa, J.C. Mitochondrial Cholesterol and the Paradox in Cell Death. In Handbook of Experimental Pharmacology; Springer: New York, NY, USA, 2017; Volume 240. [Google Scholar]
- Ribas, V.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondria, Cholesterol and Cancer Cell Metabolism. Clin. Transl. Med. 2016, 5, e22. [Google Scholar] [CrossRef]
- Martinez-Bernabe, T.; Sastre-Serra, J.; Ciobu, N.; Oliver, J.; Pons, D.G.; Roca, P. Estrogen Receptor Beta (ERβ) Maintains Mitochondrial Network Regulating Invasiveness in an Obesity-Related Inflammation Condition in Breast Cancer. Antioxidants 2021, 10, 1371. [Google Scholar] [CrossRef] [PubMed]
- Alorda-Clara, M.; Torrens-Mas, M.; Morla-Barcelo, P.M.; Roca, P.; Sastre-Serra, J.; Pons, D.G.; Oliver, J. High Concentrations of Genistein Decrease Cell Viability Depending on Oxidative Stress and Inflammation in Colon Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 7526. [Google Scholar] [CrossRef] [PubMed]
- Torrens-Mas, M.; González-Hedström, D.; Abrisqueta, M.; Roca, P.; Oliver, J.; Sastre-Serra, J. PGC-1α in Melanoma: A Key Factor for Antioxidant Response and Mitochondrial Function. J. Cell Biochem. 2017, 118, 4404–4413. [Google Scholar] [CrossRef]
- Böttinger, L.; Ellenrieder, L.; Becker, T. How Lipids Modulate Mitochondrial Protein Import. J. Bioenerg. Biomembr. 2016, 48, 125–135. [Google Scholar] [CrossRef]
- Mårtensson, C.U.; Doan, K.N.; Becker, T. Effects of Lipids on Mitochondrial Functions. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.A.; Kennedy, B.E.; Karten, B. Mitochondrial Cholesterol: Mechanisms of Import and Effects on Mitochondrial Function. J. Bioenerg. Biomembr. 2016, 48, 137–151. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial Membrane Potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Robbins, D.; Zhao, Y. New Aspects of Mitochondrial Uncoupling Proteins (UCPs) and Their Roles in Tumorigenesis. Int. J. Mol. Sci. 2011, 12, 5285–5293. [Google Scholar] [CrossRef]
- Kuai, X.-Y.; Ji, Z.-Y.; Zhang, H.-J. Mitochondrial Uncoupling Protein 2 Expression in Colon Cancer and Its Clinical Significance. World J. Gastroenterol. 2010, 16, 5773–5778. [Google Scholar] [CrossRef] [PubMed]
- Azadmanesh, J.; Borgstahl, G. A Review of the Catalytic Mechanism of Human Manganese Superoxide Dismutase. Antioxidants 2018, 7, 25. [Google Scholar] [CrossRef]
- Rigoulet, M.; Yoboue, E.D.; Devin, A. Mitochondrial ROS Generation and Its Regulation: Mechanisms Involved in H2O2 Signaling. Antioxid. Redox Signal 2011, 14, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Onukwufor, J.O.; Berry, B.J.; Wojtovich, A.P. Physiologic Implications of Reactive Oxygen Species Production by Mitochondrial Complex I Reverse Electron Transport. Antioxidants 2019, 8, 285. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The Hallmarks of Cancer Metabolism: Still Emerging. Cell Metab. 2022, 34, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, J.D.; Subramanian, T.; Liu, K.; King, M.R. Rafting Down the Metastatic Cascade: The Role of Lipid Rafts in Cancer Metastasis, Cell Death, and Clinical Outcomes. Cancer Res. 2021, 81, 5–17. [Google Scholar] [CrossRef] [PubMed]
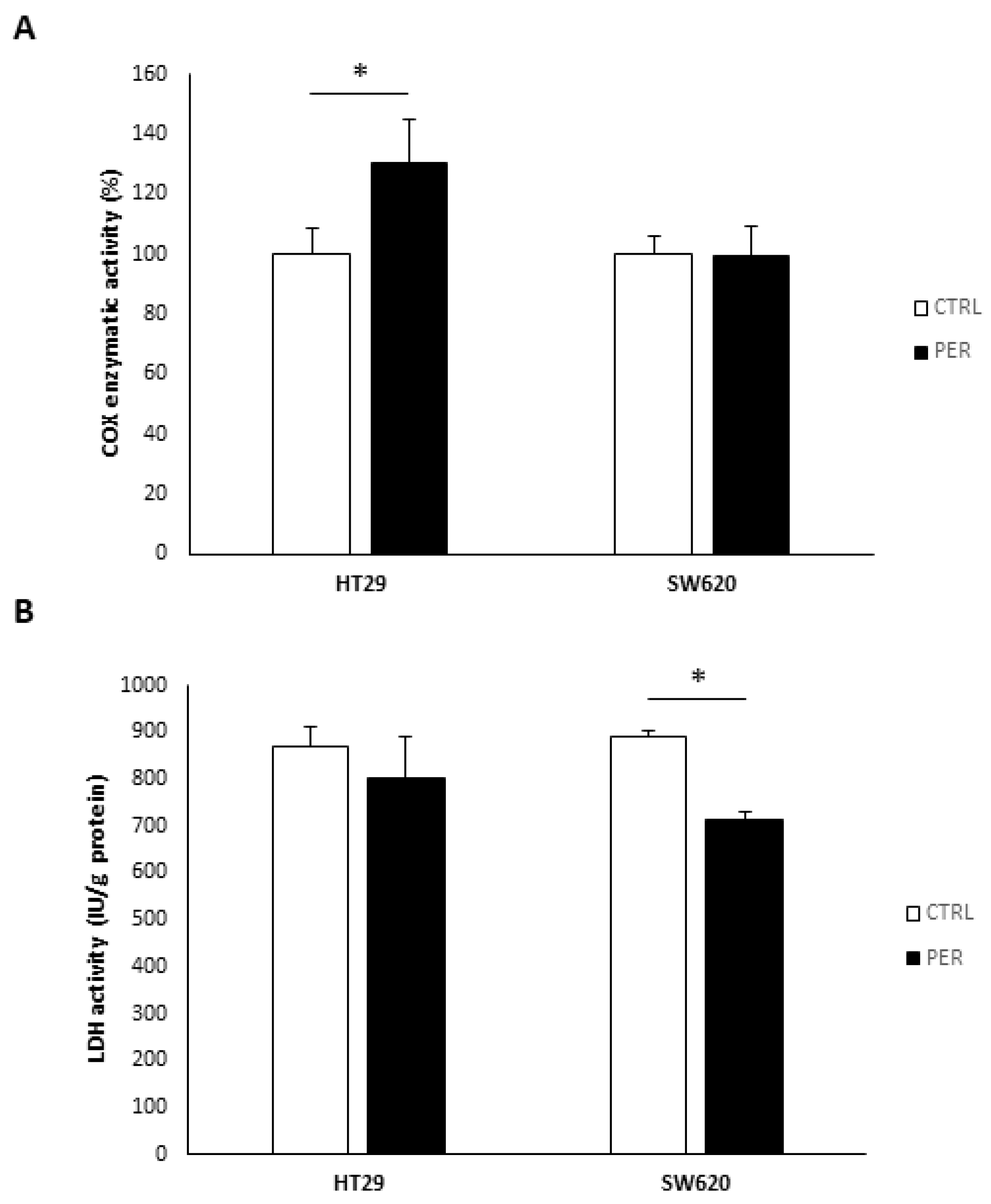

| HT29 | SW620 | |
|---|---|---|
| CTRL | 1.00 ± 0.08 | 1.00 ± 0.13 |
| MIL | 1.18 ± 0.34 | 0.81 ± 0.18 |
| PER | 1.72 ± 0.37 * | 0.66 ± 0.06 * |
| HT29 | SW620 | |||
|---|---|---|---|---|
| CTRL | PER | CTRL | PER | |
| Basal OCR | 46.2 ± 10.2 | 46.0 ± 16.1 | 24.6 ± 1.3 | 15.18 ± 7.9 |
| Maximal respiration | 81.2 ± 19.2 | 53.9 ± 11.0 | 37.4 ± 0.9 | 25.7 ± 8.2 |
| Proton leak | 46.1 ± 10.8 | 42.7 ± 16.4 | 17.4 ± 1.6 | 17.89 ± 8.8 |
| ATP production | 2.05 ± 0.71 | 3.31 ± 1.39 | 7.17 ± 0.34 | 0.77 ± 0.11 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrens-Mas, M.; Collado-Solé, A.; Sola-Leyva, A.; Carrasco-Jiménez, M.P.; Oliver, J.; Pons, D.G.; Roca, P.; Sastre-Serra, J. Mitochondrial Functionality Is Regulated by Alkylphospholipids in Human Colon Cancer Cells. Biology 2023, 12, 1457. https://doi.org/10.3390/biology12121457
Torrens-Mas M, Collado-Solé A, Sola-Leyva A, Carrasco-Jiménez MP, Oliver J, Pons DG, Roca P, Sastre-Serra J. Mitochondrial Functionality Is Regulated by Alkylphospholipids in Human Colon Cancer Cells. Biology. 2023; 12(12):1457. https://doi.org/10.3390/biology12121457
Chicago/Turabian StyleTorrens-Mas, Margalida, Alejandro Collado-Solé, Alberto Sola-Leyva, María Paz Carrasco-Jiménez, Jordi Oliver, Daniel Gabriel Pons, Pilar Roca, and Jorge Sastre-Serra. 2023. "Mitochondrial Functionality Is Regulated by Alkylphospholipids in Human Colon Cancer Cells" Biology 12, no. 12: 1457. https://doi.org/10.3390/biology12121457
APA StyleTorrens-Mas, M., Collado-Solé, A., Sola-Leyva, A., Carrasco-Jiménez, M. P., Oliver, J., Pons, D. G., Roca, P., & Sastre-Serra, J. (2023). Mitochondrial Functionality Is Regulated by Alkylphospholipids in Human Colon Cancer Cells. Biology, 12(12), 1457. https://doi.org/10.3390/biology12121457









