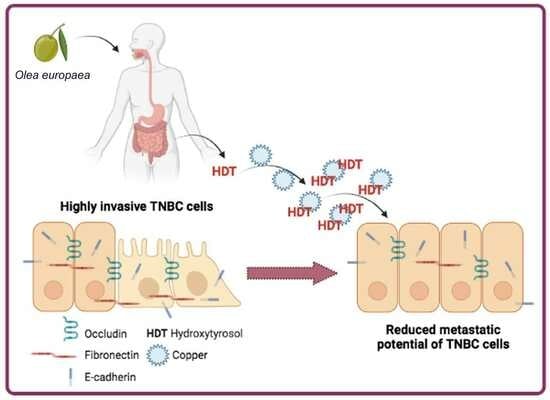
Hydroxytyrosol Counteracts Triple Negative Breast Cancer Cell Dissemination via Its Copper Complexing Properties
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Cultures and Treatments
2.3. Cell Viability Assessment by MTT Assay
2.4. Western Blot Analysis
2.5. Immunofluorescence Analysis
2.6. Wound Healing Assay
2.7. TNBC Aggressiveness Evaluation by Boyden Chamber Migration and Invasion Assay
2.8. Real-Time-Quantitative PCR (RT-qPCR)
2.9. UV-VIS and EPR Spectroscopy
2.10. Molecular Modeling of the HDT–Copper Coordination Complexes
2.11. Statistical Analysis of Data
3. Results
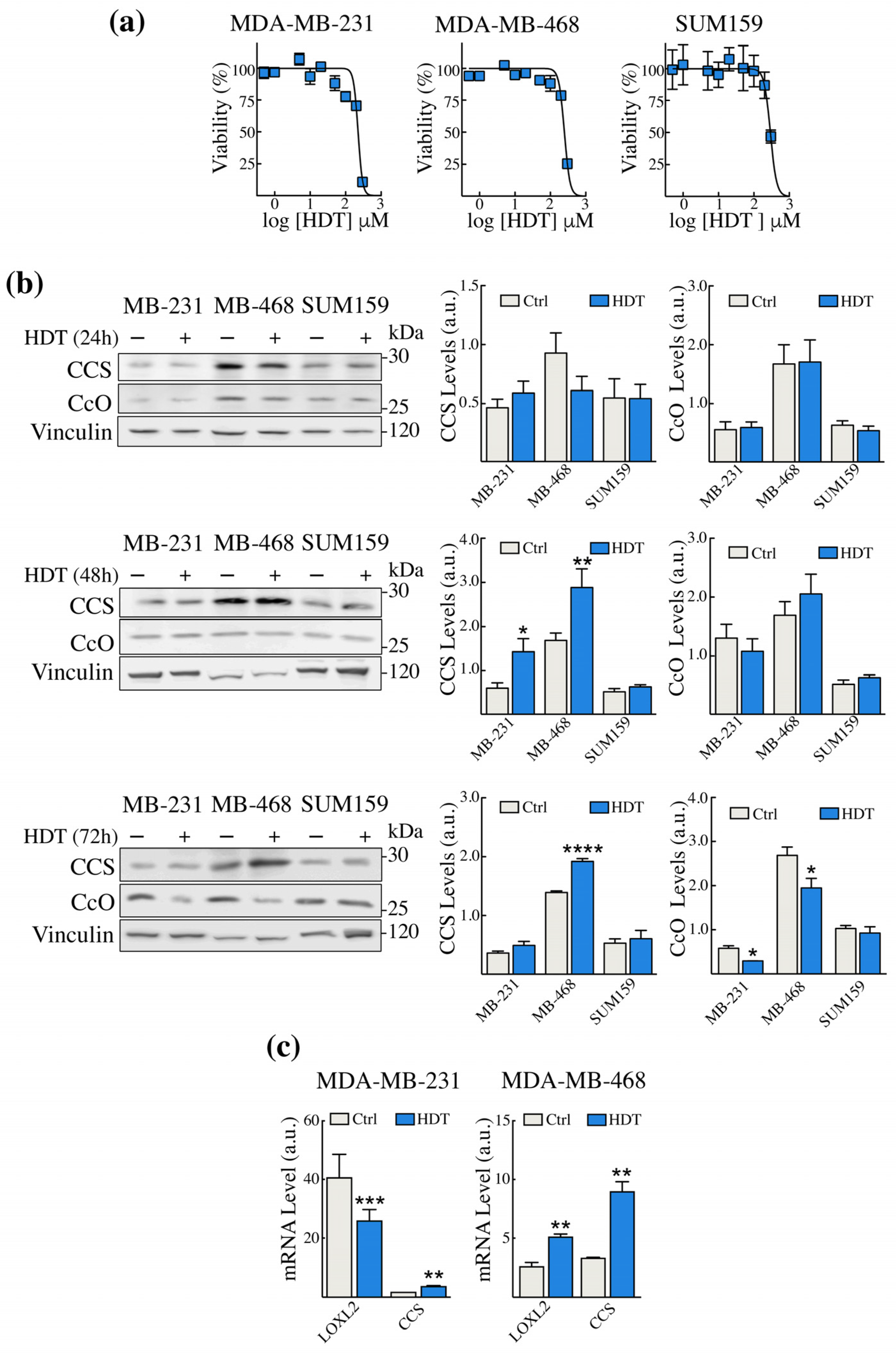
3.1. HDT Treatment of TNBC Cells Modulates the Levels of Intracellular Copper Sensors CCS and CcO
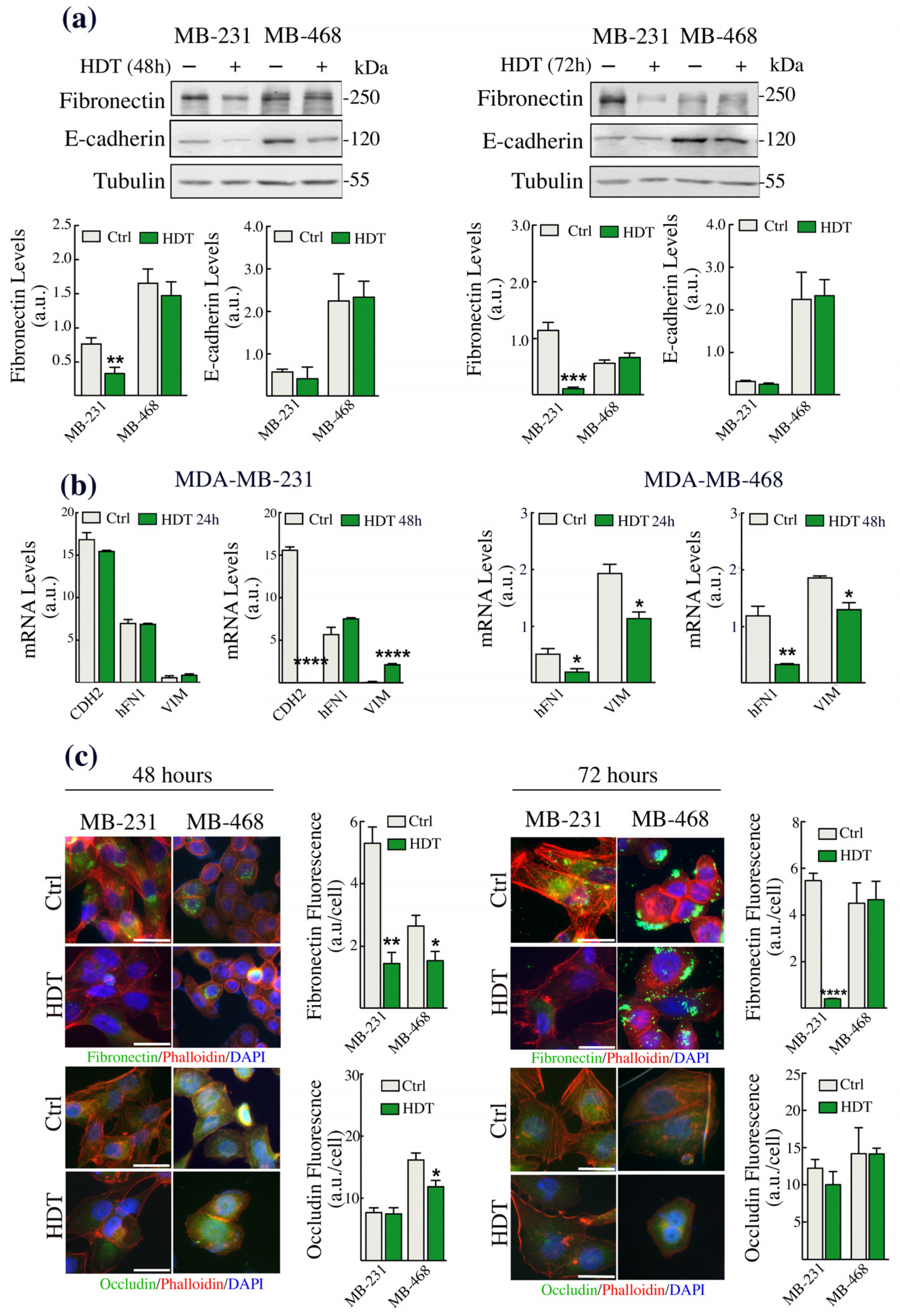
3.2. The Epithelial/Mesenchymal Phenotype of TNBC Cells Is Strongly Affected by HDT
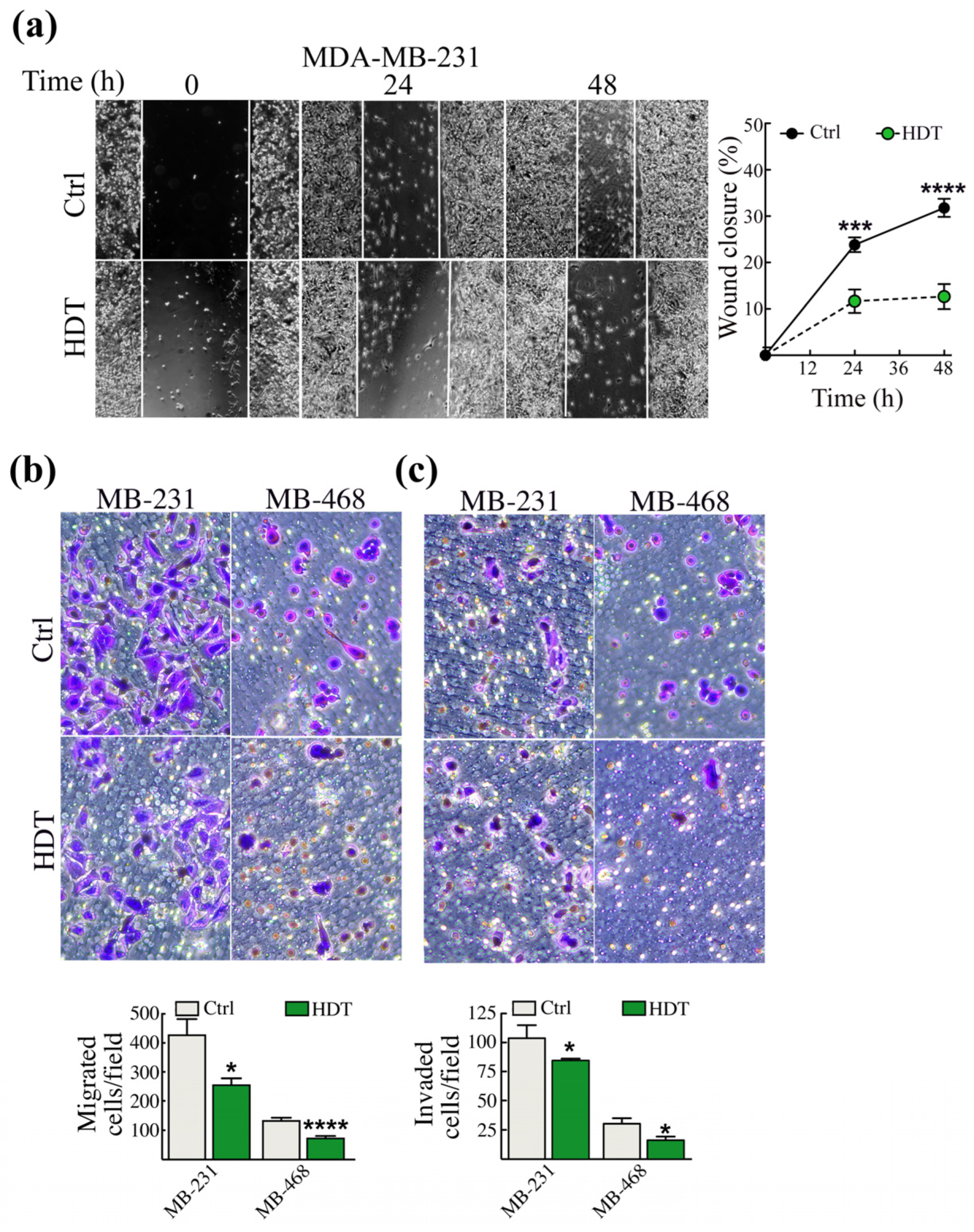
3.3. The Modulation of EMT Hallmarks Prompted by HDT Reflects a Reduced Aggressiveness of TNBC Cells
3.4. HDT Triggers EMT by Modulating the Phosphorylation of the Copper-Dependent Kinase AKT
3.5. Formation of HDT: Copper Complex
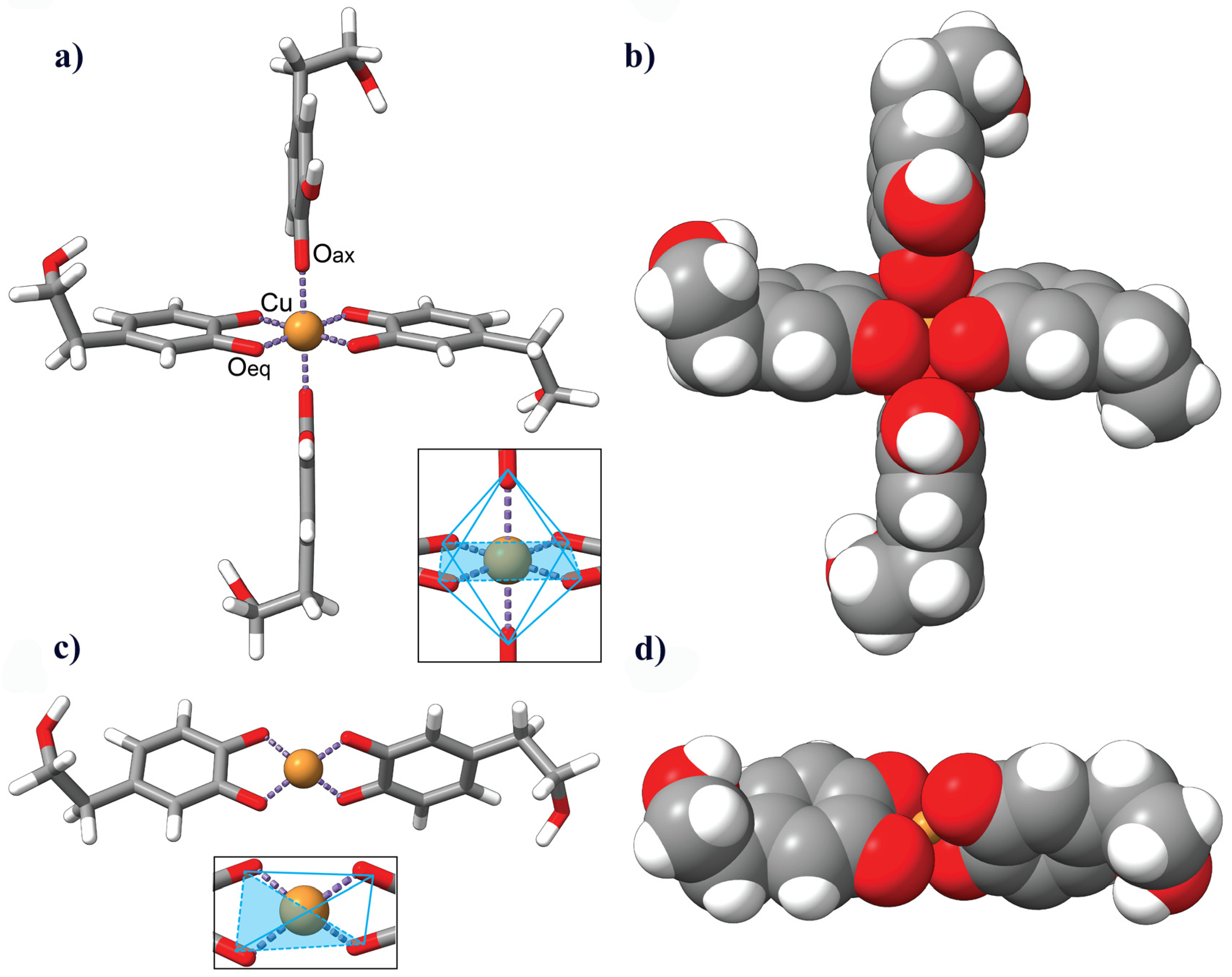
3.6. Description of the HDT–Copper Coordination Complexes by Molecular Modeling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Song, X.; Xu, W.; Lei, S.; Zhang, H.; Yang, L. Stand Up to Stand Out: Natural Dietary Polyphenols Curcumin, Resveratrol, and Gossypol as Potential Therapeutic Candidates against Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Nutrients 2023, 15, 3885. [Google Scholar] [CrossRef] [PubMed]
- Sharma, E.; Attri, D.C.; Sati, P.; Dhyani, P.; Szopa, A.; Sharifi-Rad, J.; Hano, C.; Calina, D.; Cho, W.C. Recent Updates on Anticancer Mechanisms of Polyphenols. Front. Cell Dev. Biol. 2022, 10, 1005910. [Google Scholar] [CrossRef]
- Mileo, A.M.; Miccadei, S. Polyphenols as Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies. Oxid. Med. Cell. Longev. 2016, 2016, 6475624. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Abolmaesoomi, M.; Junit, S.M.; Ali, J.M.; Chik, Z.B.; Aziz, A.A. Effects of Polyphenolic-Rich Extracts from Citrus Hystrix on Proliferation and Oxidative Stress in Breast and Colorectal Cancer. Turkish J. Biochem. 2023, 48, 110–118. [Google Scholar] [CrossRef]
- Benvenuto, M.; Mattera, R.; Taffera, G.; Giganti, M.G.; Lido, P.; Masuelli, L.; Modesti, A.; Bei, R. The Potential Protective Effects of Polyphenols in Asbestos-Mediated Inflammation and Carcinogenesis of Mesothelium. Nutrients 2016, 8, 275. [Google Scholar] [CrossRef]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Choi, H.Y.; Yang, G.-M.; Kim, K.; Saha, S.K.; Cho, S.-G. The Anti-Cancer Effect of Polyphenols against Breast Cancer and Cancer Stem Cells: Molecular Mechanisms. Nutrients 2016, 8, 581. [Google Scholar] [CrossRef]
- Avtanski, D.; Poretsky, L. Phyto-Polyphenols as Potential Inhibitors of Breast Cancer Metastasis. Mol. Med. 2018, 24, 29. [Google Scholar] [CrossRef]
- Kronski, E.; Fiori, M.E.; Barbieri, O.; Astigiano, S.; Mirisola, V.; Killian, P.H.; Bruno, A.; Pagani, A.; Rovera, F.; Pfeffer, U.; et al. MiR181b Is Induced by the Chemopreventive Polyphenol Curcumin and Inhibits Breast Cancer Metastasis via Down-Regulation of the Inflammatory Cytokines CXCL1 and -2. Mol. Oncol. 2014, 8, 581–595. [Google Scholar] [CrossRef]
- Farghadani, R.; Naidu, R. The Anticancer Mechanism of Action of Selected Polyphenols in Triple-Negative Breast Cancer (TNBC). Biomed. Pharmacother. 2023, 165, 115170. [Google Scholar] [CrossRef]
- Rowe, D.L.; Ozbay, T.; O’Regan, R.M.; Nahta, R. Modulation of the BRCA1 Protein and Induction of Apoptosis in Triple Negative Breast Cancer Cell Lines by the Polyphenolic Compound Curcumin. Breast Cancer Basic Clin. Res. 2009, 3, 61–75. [Google Scholar] [CrossRef]
- Mahbub, A.A.; Le Maitre, C.L.; Haywood-Small, S.L.; McDougall, G.J.; Cross, N.A.; Jordan-Mahy, N. Differential Effects of Polyphenols on Proliferation and Apoptosis in Human Myeloid and Lymphoid Leukemia Cell Lines. Anticancer. Agents Med. Chem. 2013, 13, 1601–1613. [Google Scholar] [CrossRef]
- Li, C.X.; Lin, Z.X.; Zhao, X.H.; Zuo, W.F.; Wang, N.; Zhang, Z.Y.; Chen, X. Sen Differential Effects of Phenolic Extracts from Red-Fleshed Apple Peels and Flesh induced G1 Cell Cycle Arrest and Apoptosis in Human Breast Cancer MDA-MB-231 Cells. J. Food Sci. 2021, 86, 4209–4222. [Google Scholar] [CrossRef]
- Chimento, A.; De Luca, A.; D’Amico, M.; De Amicis, F.; Pezzi, V. The Involvement of Natural Polyphenols in Molecular Mechanisms Inducing Apoptosis in Tumor Cells: A Promising Adjuvant in Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 1680. [Google Scholar] [CrossRef] [PubMed]
- Wen Zhang Peng Zhang, X.X.M.L.S.W.H.M.; Sun, K. Synergy Effects of Copper Ion in Doxorubicin-Based Chelate Prodrug for Cancer Chemo-Chemodynamic Combination Therapy. Drug Deliv. 2023, 30, 2219426. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, M.; Liu, Y.; Si, Z. Cope with Copper: From Copper Linked Mechanisms to Copper-Based Clinical Cancer Therapies. Cancer Lett. 2023, 561, 216157. [Google Scholar] [CrossRef] [PubMed]
- Vitaliti, A.; De Luca, A.; Rossi, L. Copper-Dependent Kinases and Their Role in Cancer Inception, Progression and Metastasis. Biomolecules 2022, 12, 1520. [Google Scholar] [CrossRef] [PubMed]
- Pękal, A.; Biesaga, M.; Pyrzynska, K. Interaction of Quercetin with Copper Ions: Complexation, Oxidation and Reactivity towards Radicals. BioMetals 2011, 24, 41–49. [Google Scholar] [CrossRef]
- Lomozová, Z.; Catapano, M.C.; Hrubša, M.; Karlíčková, J.; Macáková, K.; Kučera, R.; Mladěnka, P. Chelation of Iron and Copper by Quercetin B-Ring Methyl Metabolites, Isorhamnetin and Tamarixetin, and Their Effect on Metal-Based Fenton Chemistry. J. Agric. Food Chem. 2021, 69, 5926–5937. [Google Scholar] [CrossRef]
- Teng, Y.; Zhao, J.; Ding, L.; Ding, Y.; Zhou, P. Complex of EGCG with Cu(II) Suppresses Amyloid Aggregation and Cu(II)-Induced Cytotoxicity of α-Synuclein. Molecules 2019, 24, 2940. [Google Scholar] [CrossRef]
- Flieger, J.; Tatarczak-Michalewska, M.; Blicharska, E.; Świeboda, R.; Banach, T. HPLC Identification of Copper (II)-Trans-Resveratrol Complexes in Ethanolic Aqueous Solution. J. Chromatogr. Sci. 2016, 55, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Maghool, F.; Emami, M.H.; Alipour, R.; Mohammadzadeh, S.; Sereshki, N.; Dehkordi, S.A.E.; Fahim, A.; Tayarani-Najaran, Z.; Sheikh, A.; Kesharwani, P.; et al. Rescue Effect of Curcumin against Copper Toxicity. J. Trace Elem. Med. Biol. 2023, 78, 127153. [Google Scholar] [CrossRef] [PubMed]
- Capo, C.R.; Pedersen, J.Z.; Falconi, M.; Rossi, L. Oleuropein Shows Copper Complexing Properties and Noxious Effect on Cultured SH-SY5Y Neuroblastoma Cells Depending on Cell Copper Content. J. Trace Elem. Med. Biol. 2017, 44, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Messeha, S.S.; Zarmouh, N.O.; Asiri, A.; Soliman, K.F.A. Gene Expression Alterations Associated with Oleuropein-Induced Antiproliferative Effects and S-Phase Cell Cycle Arrest in Triple-Negative Breast Cancer Cells. Nutrients 2020, 12, 3755. [Google Scholar] [CrossRef]
- Moral, R.; Escrich, E. Influence of Olive Oil and Its Components on Breast Cancer: Molecular Mechanisms. Molecules 2022, 27, 477. [Google Scholar] [CrossRef]
- Asgharzade, S.; Sheikhshabani, S.H.; Ghasempour, E.; Heidari, R.; Rahmati, S.; Mohammadi, M.; Jazaeri, A.; Amini-Farsani, Z. The Effect of Oleuropein on Apoptotic Pathway Regulators in Breast Cancer Cells. Eur. J. Pharmacol. 2020, 886, 173509. [Google Scholar] [CrossRef]
- Calahorra, J.; Martínez-Lara, E.; De Dios, C.; Siles, E. Hypoxia Modulates the Antioxidant Effect of Hydroxytyrosol in MCF-7 Breast Cancer Cells. PLoS ONE 2018, 13, e0203892. [Google Scholar] [CrossRef]
- Razali, R.A.; Lokanathan, Y.; Yazid, M.D.; Ansari, A.S.; Saim, A.B.; Hj Idrus, R.B. Modulation of Epithelial to Mesenchymal Transition Signaling Pathways by Olea Europaea and Its Active Compounds. Int. J. Mol. Sci. 2019, 20, 3492. [Google Scholar] [CrossRef]
- Cruz-Lozano, M.; González-González, A.; Marchal, J.A.; Muñoz-Muela, E.; Molina, M.P.; Cara, F.E.; Brown, A.M.; García-Rivas, G.; Hernández-Brenes, C.; Lorente, J.A.; et al. Hydroxytyrosol Inhibits Cancer Stem Cells and the Metastatic Capacity of Triple-Negative Breast Cancer Cell Lines by the Simultaneous Targeting of Epithelial-to-Mesenchymal Transition, Wnt/β-Catenin and TGFβ Signaling Pathways. Eur. J. Nutr. 2019, 58, 3207–3219. [Google Scholar] [CrossRef]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.; Camacho-Corencia, P.; Sanchez-Rovira, P.; Vera-Ramirez, L.; Ramirez-Tortosa, M. Hydroxytyrosol Inhibits Growth and Cell Proliferation and Promotes High Expression of Sfrp4 in Rat Mammary Tumours. Mol. Nutr. Food Res. 2011, 55, S117–S126. [Google Scholar] [CrossRef] [PubMed]
- Bouallagui, Z.; Han, J.; Isoda, H.; Sayadi, S. Hydroxytyrosol Rich Extract from Olive Leaves Modulates Cell Cycle Progression in MCF-7 Human Breast Cancer Cells. Food Chem. Toxicol. 2011, 49, 179–184. [Google Scholar] [CrossRef]
- Lu, H.-Y.; Zhu, J.-S.; Zhang, Z.; Shen, W.-J.; Jiang, S.; Long, Y.-F.; Wu, B.; Ding, T.; Huan, F.; Wang, S.-L. Hydroxytyrosol and Oleuropein Inhibit Migration and Invasion of MDA-MB-231 Triple-Negative Breast Cancer Cell via Induction of Autophagy. Anticancer. Agents Med. Chem. 2019, 19, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Stoll, S.; Schweiger, A. EasySpin, a Comprehensive Software Package for Spectral Simulation and Analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Stroet, M.; Caron, B.; Visscher, K.M.; Geerke, D.P.; Malde, A.K.; Mark, A.E. Automated Topology Builder Version 3.0: Prediction of Solvation Free Enthalpies in Water and Hexane. J. Chem. Theory Comput. 2018, 14, 5834–5845. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Romagnoli, A.; D’Agostino, M.; Pavoni, E.; Ardiccioni, C.; Motta, S.; Crippa, P.; Biagetti, G.; Notarstefano, V.; Rexha, J.; Perta, N.; et al. SARS-CoV-2 Multi-Variant Rapid Detector Based on Graphene Transistor Functionalized with an Engineered Dimeric ACE2 Receptor. Nano Today 2023, 48, 101729. [Google Scholar] [CrossRef]
- Di Marino, D.; Chillemi, G.; De Rubeis, S.; Tramontano, A.; Achsel, T.; Bagni, C. MD and Docking Studies Reveal That the Functional Switch of CYFIP1 Is Mediated by a Butterfly-like Motion. J. Chem. Theory Comput. 2015, 11, 3401–3410. [Google Scholar] [CrossRef]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.I.I.I.; Skiff, W.M. UFF, a Full Periodic Table Force Field for Molecular Mechanics and Molecular Dynamics Simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Salha, D.; Andaç, M.; Denizli, A. Molecular Docking of Metal Ion Immobilized Ligands to Proteins in Affinity Chromatography. J. Mol. Recognit. 2021, 34, e2875. [Google Scholar] [CrossRef]
- Persson, I.; Lundberg, D.; Bajnóczi, É.G.; Klementiev, K.; Just, J.; Sigfridsson Clauss, K.G.V. EXAFS Study on the Coordination Chemistry of the Solvated Copper(II) Ion in a Series of Oxygen Donor Solvents. Inorg. Chem. 2020, 59, 9538–9550. [Google Scholar] [CrossRef]
- Daniele Di Marino Tilmann Achsel, C.L.M.F.; Bagni, C. Molecular Dynamics Simulations Show How the FMRP Ile304Asn Mutation Destabilizes the KH2 Domain Structure and Affects its Function. J. Biomol. Struct. Dyn. 2014, 32, 337–350. [Google Scholar] [CrossRef]
- Di Marino, D.; D’Annessa, I.; Tancredi, H.; Bagni, C.; Gallicchio, E. A Unique Binding Mode of the Eukaryotic Translation Initiation Factor 4E for Guiding the Design of Novel Peptide Inhibitors. Protein Sci. 2015, 24, 1370–1382. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Romagnoli, A.; Moretti, P.; D’Agostino, M.; Rexha, J.; Perta, N.; Piccinini, A.; Di Marino, D.; Spinozzi, F.; La Teana, A. Structural–Functional Relationship of the Ribonucleolytic Activity of AIF5A from Sulfolobus Solfataricus. Biomolecules 2022, 12, 1432. [Google Scholar] [CrossRef]
- Bertinato, J.; L’Abbé, M.R. Copper Modulates the Degradation of Copper Chaperone for Cu, Zn Superoxide Dismutase by the 26 S Proteosome. J. Biol. Chem. 2003, 278, 35071–35078. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, V.C.; Gudekar, N.; Jasmer, K.; Papageorgiou, C.; Singh, K.; Petris, M.J. Copper Metabolism as a Unique Vulnerability in Cancer. Biochim. Biophys. Acta Mol. cell Res. 2021, 1868, 118893. [Google Scholar] [CrossRef] [PubMed]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global Quantification of Mammalian Gene Expression Control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Dangelmaier, C.; Manne, B.K.; Liverani, E.; Jin, J.; Bray, P.; Kunapuli, S.P. PDK1 Selectively Phosphorylates Thr(308) on Akt and Contributes to Human Platelet Functional Responses. Thromb. Haemost. 2014, 111, 508–517. [Google Scholar] [CrossRef]
- Vitaliti, A.; Roccatani, I.; Iorio, E.; Perta, N.; Gismondi, A.; Chirico, M.; Pisanu, M.E.; Di Marino, D.; Canini, A.; De Luca, A.; et al. AKT-Driven Epithelial-Mesenchymal Transition Is Affected by Copper Bioavailability in HER2 Negative Breast Cancer Cells via a LOXL2-Independent Mechanism. Cell. Oncol. 2023, 46, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Árkosi, Z.; Szabó-Plánka, T.; Rockenbauer, A.; Nagy, N.V.; Lázár, L.; Fülöp, F. An Electron Paramagnetic Resonance Study of Copper(II)−β-Substituted β-Amino Acid Systems by the Two-Dimensional Simulation Method: First Evidence of Primarily Steric Effects of Substituents on Equilibria of Metal Complexes. Inorg. Chem. 2003, 42, 4842–4848. [Google Scholar] [CrossRef]
- Ge, E.J.; Bush, A.I.; Casini, A.; Cobine, P.A.; Cross, J.R.; DeNicola, G.M.; Dou, Q.P.; Franz, K.J.; Gohil, V.M.; Gupta, S.; et al. Connecting Copper and Cancer: From Transition Metal Signalling to Metalloplasia. Nat. Rev. Cancer 2022, 22, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.N.; Chang, C.J. Metalloallostery and Transition Metal Signaling: Bioinorganic Copper Chemistry Beyond Active Sites. Angew. Chemie Int. Ed. 2023, 62, e202213644. [Google Scholar] [CrossRef]
- Baldari, S.; Di Rocco, G.; Toietta, G. Current Biomedical Use of Copper Chelation Therapy. Int. J. Mol. Sci. 2020, 21, 1069. [Google Scholar] [CrossRef] [PubMed]
- López de las Hazas, M.-C.; Piñol, C.; Macià, A.; Romero, M.-P.; Pedret, A.; Solà, R.; Rubió, L.; Motilva, M.-J. Differential Absorption and Metabolism of Hydroxytyrosol and Its Precursors Oleuropein and Secoiridoids. J. Funct. Foods 2016, 22, 52–63. [Google Scholar] [CrossRef]
- Kitsati, N.; Mantzaris, M.D.; Galaris, D. Hydroxytyrosol Inhibits Hydrogen Peroxide-Induced Apoptotic Signaling via Labile Iron Chelation. Redox Biol. 2016, 10, 233–242. [Google Scholar] [CrossRef]
- Tagliafierro, L.; Officioso, A.; Sorbo, S.; Basile, A.; Manna, C. The Protective Role of Olive Oil Hydroxytyrosol against Oxidative Alterations Induced by Mercury in Human Erythrocytes. Food Chem. Toxicol. 2015, 82, 59–63. [Google Scholar] [CrossRef]
- Rossi, L.; Lombardo, M.F.; Ciriolo, M.R.; Rotilio, G. Mitochondrial Dysfunction in Neurodegenerative Diseases Associated with Copper Imbalance. Neurochem. Res. 2004, 29, 493–504. [Google Scholar] [CrossRef]
- Liao, T.-T.; Yang, M.-H. Hybrid Epithelial/Mesenchymal State in Cancer Metastasis: Clinical Significance and Regulatory Mechanisms. Cells 2020, 9, 623. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, C.; Lee, J.-E.; Bartholdy, B.A.; Yang, D.; Liu, Y.; Erler, P.; Galbo, P.M.; Hodge, D.Q.; Huangfu, D.; et al. MLL3 Loss Drives Metastasis by Promoting a Hybrid Epithelial–Mesenchymal Transition State. Nat. Cell Biol. 2023, 25, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Schmidleitner, L.; Moreno, H.D.; Donato, E.; Falcone, M.; Bartsch, J.M.; Klein, C.; Vogel, V.; Würth, R.; Pfarr, N.; et al. Resistance to Mesenchymal Reprogramming Sustains Clonal Propagation in Metastatic Breast Cancer. Cell Rep. 2023, 42, 112533. [Google Scholar] [CrossRef]
- Guo, J.; Cheng, J.; Zheng, N.; Zhang, X.; Dai, X.; Zhang, L.; Hu, C.; Wu, X.; Jiang, Q.; Wu, D.; et al. Copper Promotes Tumorigenesis by Activating the PDK1-AKT Oncogenic Pathway in a Copper Transporter 1 Dependent Manner. Adv. Sci. 2021, 8, 202004303. [Google Scholar] [CrossRef] [PubMed]
- Bayascas, J.R.; Alessi, D.R. Regulation of Akt/PKB Ser473 Phosphorylation. Mol. Cell 2005, 18, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Tosato, M.; Pelosato, M.; Franchi, S.; Isse, A.A.; May, N.V.; Zanoni, G.; Mancin, F.; Pastore, P.; Badocco, D.; Asti, M.; et al. When Ring Makes the Difference: Coordination Properties of Cu2+/Cu+ Complexes with Sulfur-Pendant Polyazamacrocycles for Radiopharmaceutical Applications. New J. Chem. 2022, 46, 10012–10025. [Google Scholar] [CrossRef]
- Oess, A.; Cheshire, M.V.; McPhail, D.B.; Stoll, S.; El Alaili, M.; Vedy, J.C. Elucidation of Phenol-Cu Interaction Mechanisms by Potentiometry, ESR, UV Absorption Spectroscopy and Molecular Simulations. Sci. Total Environ. 1999, 228, 49–58. [Google Scholar] [CrossRef]
- Borges, F.; Guimarães, C.; Lima, J.L.F.C.; Pinto, I.; Reis, S. Potentiometric Studies on the Complexation of Copper(II) by Phenolic Acids as Discrete Ligand Models of Humic Substances. Talanta 2005, 66, 670–673. [Google Scholar] [CrossRef]
- Pirker, K.F.; Baratto, M.C.; Basosi, R.; Goodman, B.A. Influence of PH on the Speciation of Copper(II) in Reactions with the Green Tea Polyphenols, Epigallocatechin Gallate and Gallic Acid. J. Inorg. Biochem. 2012, 112, 10–16. [Google Scholar] [CrossRef]
- Tosato, M.; Dalla Tiezza, M.; May, N.V.; Isse, A.A.; Nardella, S.; Orian, L.; Verona, M.; Vaccarin, C.; Alker, A.; Mäcke, H.; et al. Copper Coordination Chemistry of Sulfur Pendant Cyclen Derivatives: An Attempt to Hinder the Reductive-Induced Demetalation in 64/67Cu Radiopharmaceuticals. Inorg. Chem. 2021, 60, 11530–11547. [Google Scholar] [CrossRef]
- Barik, A.; Mishra, B.; Kunwar, A.; Kadam, R.M.; Shen, L.; Dutta, S.; Padhye, S.; Satpati, A.K.; Zhang, H.-Y.; Indira Priyadarsini, K. Comparative Study of Copper(II)–Curcumin Complexes as Superoxide Dismutase Mimics and Free Radical Scavengers. Eur. J. Med. Chem. 2007, 42, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, B.; Håkansson, M.; Jagner, S. Copper(Ii) Is Harder than Copper(i): A Novel Mixed-Valence Example from Alkoxide Chemistry. New J. Chem. 2003, 27, 459–461. [Google Scholar] [CrossRef]
- Pirsiavash, F.; Amani, V.; Abedi, A. Coordination Number in Copper(II) Complexes with Bipyridine-Dicarboxylate Anion and Diamine Derivatives. Res. Chem. Intermed. 2018, 44, 7411–7426. [Google Scholar] [CrossRef]
- Modec, B.; Podjed, N.; Lah, N. Beyond the Simple Copper(II) Coordination Chemistry with Quinaldinate and Secondary Amines. Molecules 2020, 25, 1573. [Google Scholar] [CrossRef] [PubMed]
- Shtepliuk, I.; Vagin, M.; Yakimova, R. Electrochemical Deposition of Copper on Epitaxial Graphene. Appl. Sci. 2020, 10, 1405. [Google Scholar] [CrossRef]
- Dávalos, J.Z.; Valderrama-Negrón, A.C.; Barrios, J.R.; Freitas, V.L.S.; Ribeiro da Silva, M.D.M.C. Energetic and Structural Properties of Two Phenolic Antioxidants: Tyrosol and Hydroxytyrosol. J. Phys. Chem. A 2018, 122, 4130–4137. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- de Vega, M.J.; Moreno-Fernández, S.; Pontes-Quero, G.M.; González-Amor, M.; Vázquez-Lasa, B.; Sabater-Muñoz, B.; Briones, A.M.; Aguilar, M.R.; Miguel, M.; González-Muñiz, R. Characterization of Novel Synthetic Polyphenols: Validation of Antioxidant and Vasculoprotective Activities. Antioxidants 2020, 9, 787. [Google Scholar] [CrossRef]
- Liu, R.; Mabury, S.A. Synthetic Phenolic Antioxidants: A Review of Environmental Occurrence, Fate, Human Exposure, and Toxicity. Environ. Sci. Technol. 2020, 54, 11706–11719. [Google Scholar] [CrossRef]
- Onychina, K.K.; Radosteva, E.R.; Gazizullina, E.R.; Gerasimova, E.L.; Sharafutdinova, E.N.; Ivanova, A.V. Research Antioxidant Properties of Natural and Synthetic Polyphenols. AIP Conf. Proc. 2022, 2390, 20056. [Google Scholar] [CrossRef]


| Primary Antibody | Origin | Company | Diluition |
|---|---|---|---|
| E-cadherin | Mouse | BD Transduction Laboratories(Milan, Italy) #610181 | 1:1000 |
| Fibronectin | Rabbit | Merck Life Science S.r.l #F3648 | 1:3000 |
| CCS | Rabbit | Santa Cruz Biotechnology (Dallas, TX, USA) #517412 | 1:1000 |
| Subunit II complex IV | Mouse | Molecular Probes (Eugene, OR, USA) #A-6404 | 1:1000 |
| ERK1/2 | Rabbit | CellSignaling #4695 | 1:1000 |
| Phospho ERK1/2 (Thr202/Tyr204) | Rabbit | CellSignaling #9101 | 1:1000 |
| AKT | Rabbit | CellSignaling #4691 | 1:1000 |
| Phospho-Akt (Ser473) | Rabbit | CellSignaling #4058 | 1:1000 |
| GAPDH | Rabbit | Merck Life Science S.r.l #G9545 | 1:2000 |
| Vinculin | Mouse | Santa Cruz Biotechnology # sc-5286 | 1:3000 |
| Tubulin | Mouse | Santa Cruz Biotechnology #25336 | 1:1000 |
| Gene | Primers |
|---|---|
| SNAI1 | F: 5′-CCAGTGCCTCGACCACTATG-3′ R: 5-CTGCTGGAAGGTAAACTCTGG-3′ |
| SNAI2 | F: 5′-CCAAGCTTTCAGACCCCCAT-3′ R: 5′-GAAAAAGGCTTCTCCCCCGT-3′ |
| TWIST | F: 5′-GCTTGAGGGTCTGAATCTTGCT-3′ R: 5′-GTCCGCAGTCTTACGAGGAG-3′ |
| ZEB1 | F: 5′-CAGCTTGATACCTGTGAATGGG-3′ R: 5′-TATCTGTGGTCGTGTGGGACT-3′ |
| MEMO1 | F: 5′-GCCGGAGTTTGTGGTGATTG-3′ R: 5′-CATTCAGCTGCGGTCCTGAG-3′ |
| LOXL2 | F: 5′-TACAAGCCAGAGCAACCCCT-3′ R: 5-CAGTGACTGCCTCTTTGGCA-3′ |
| ATOX1 | F: 5′-TGGTGGTATTGACGGTGTG-3′ R: 5′-CGTGATCAGAACCACGTCCA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perta, N.; Torrieri Di Tullio, L.; Cugini, E.; Fattibene, P.; Rapanotti, M.C.; Borromeo, I.; Forni, C.; Malaspina, P.; Cacciamani, T.; Di Marino, D.; et al. Hydroxytyrosol Counteracts Triple Negative Breast Cancer Cell Dissemination via Its Copper Complexing Properties. Biology 2023, 12, 1437. https://doi.org/10.3390/biology12111437
Perta N, Torrieri Di Tullio L, Cugini E, Fattibene P, Rapanotti MC, Borromeo I, Forni C, Malaspina P, Cacciamani T, Di Marino D, et al. Hydroxytyrosol Counteracts Triple Negative Breast Cancer Cell Dissemination via Its Copper Complexing Properties. Biology. 2023; 12(11):1437. https://doi.org/10.3390/biology12111437
Chicago/Turabian StylePerta, Nunzio, Laura Torrieri Di Tullio, Elisa Cugini, Paola Fattibene, Maria Cristina Rapanotti, Ilaria Borromeo, Cinzia Forni, Patrizia Malaspina, Tiziana Cacciamani, Daniele Di Marino, and et al. 2023. "Hydroxytyrosol Counteracts Triple Negative Breast Cancer Cell Dissemination via Its Copper Complexing Properties" Biology 12, no. 11: 1437. https://doi.org/10.3390/biology12111437
APA StylePerta, N., Torrieri Di Tullio, L., Cugini, E., Fattibene, P., Rapanotti, M. C., Borromeo, I., Forni, C., Malaspina, P., Cacciamani, T., Di Marino, D., Rossi, L., & De Luca, A. (2023). Hydroxytyrosol Counteracts Triple Negative Breast Cancer Cell Dissemination via Its Copper Complexing Properties. Biology, 12(11), 1437. https://doi.org/10.3390/biology12111437