The Role of the Insulin/Glucose Ratio in the Regulation of Pathogen Biofilm Formation
Abstract
Simple Summary
Abstract
1. Introduction
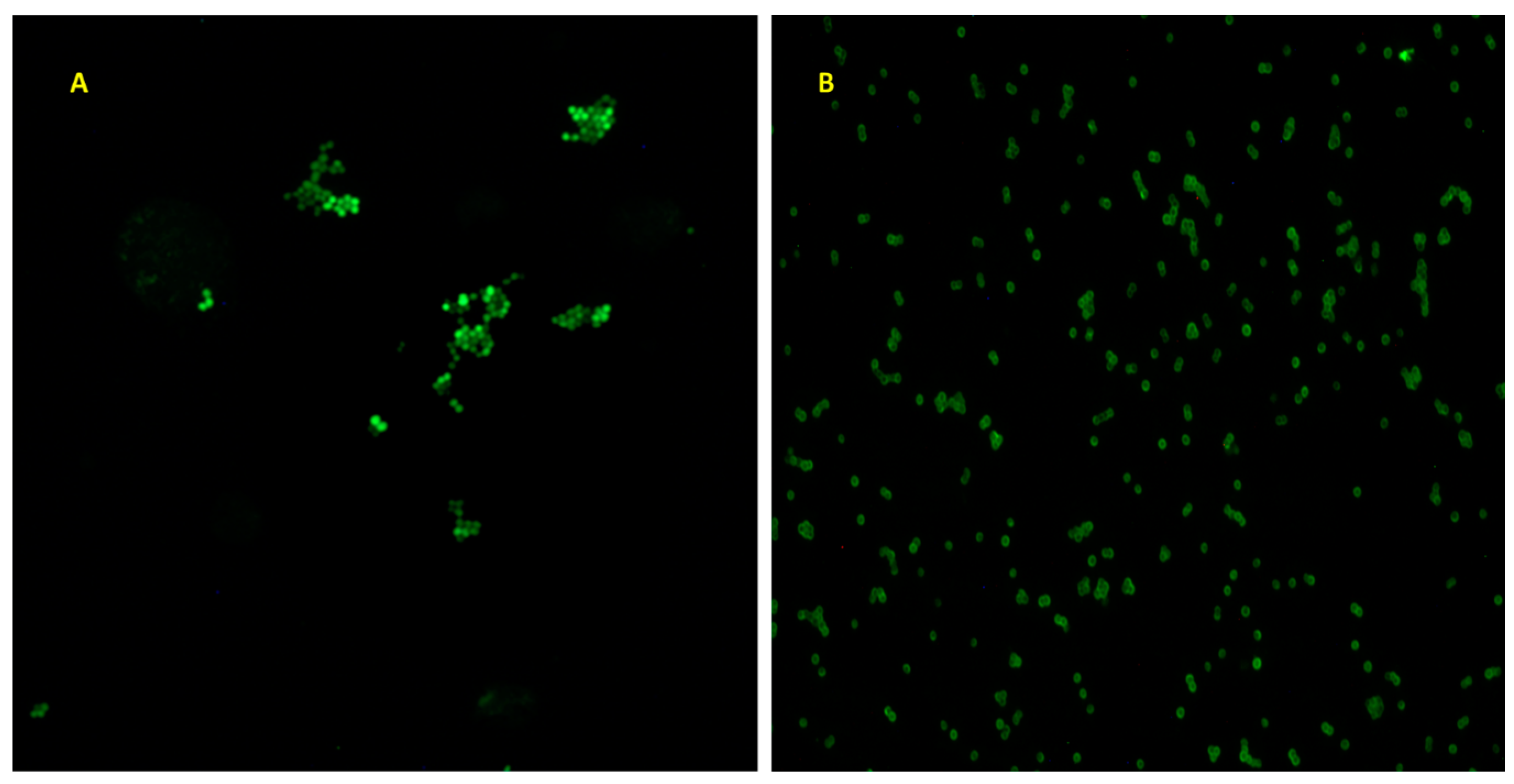
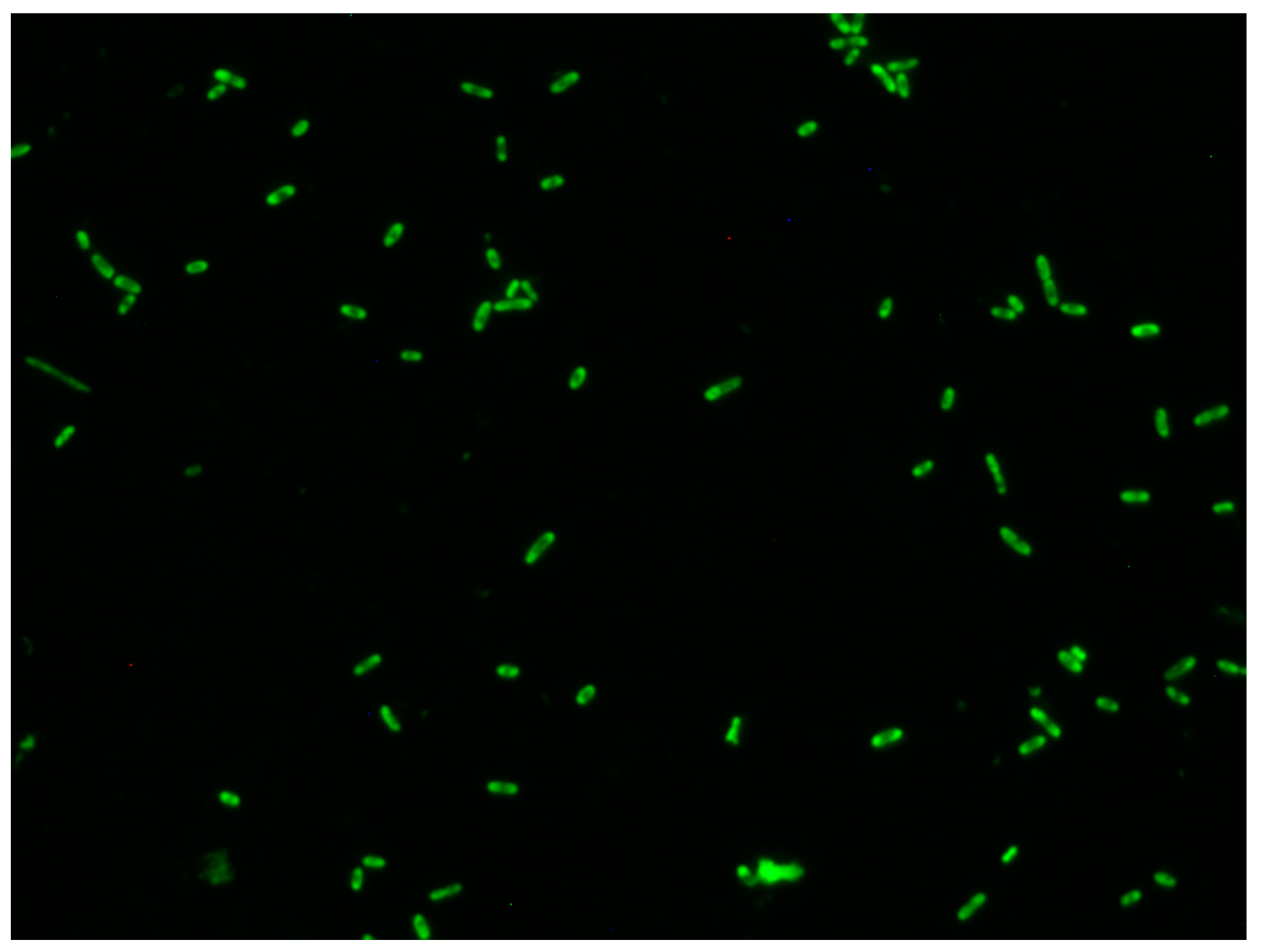
2. Materials and Methods
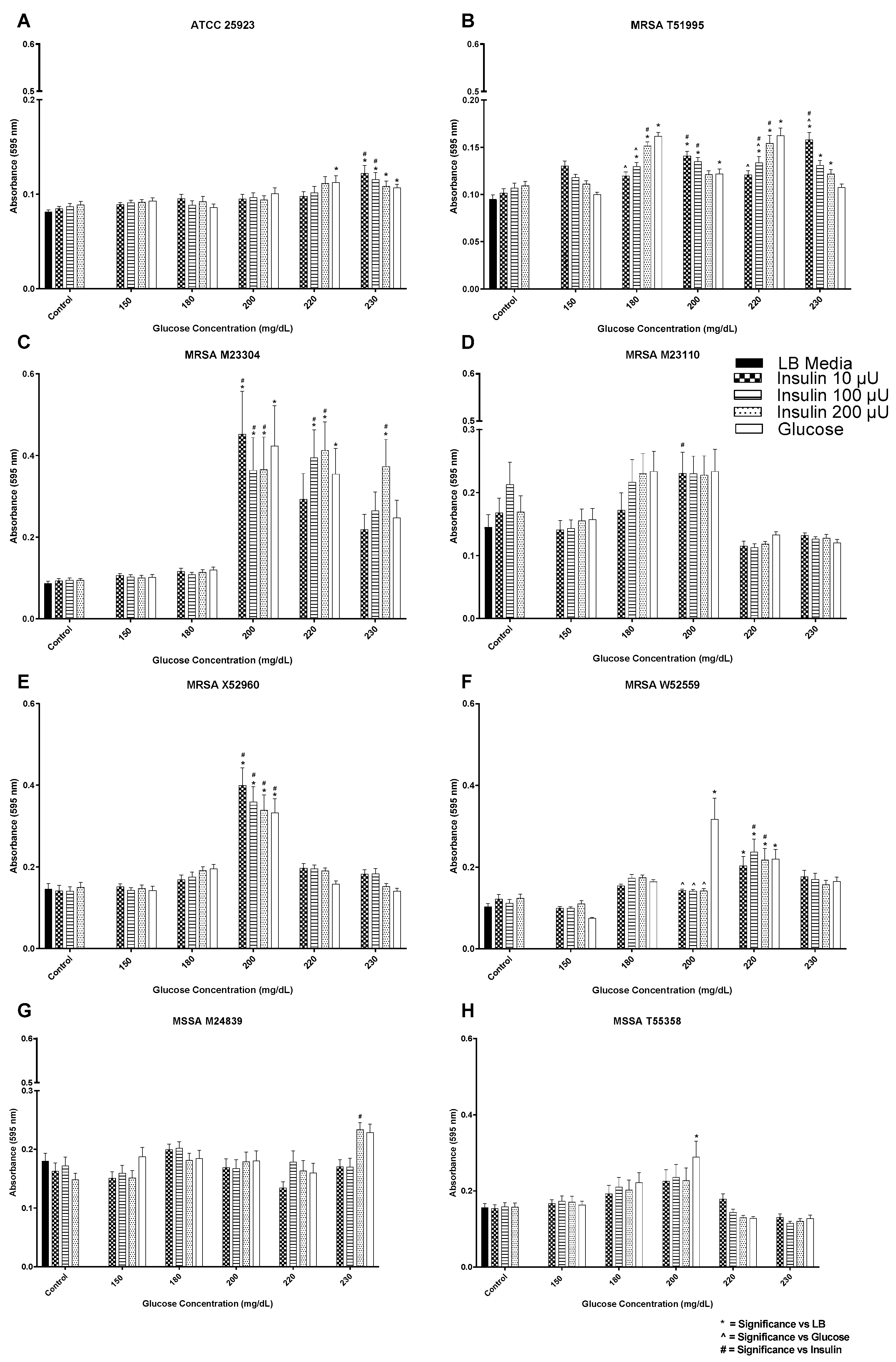
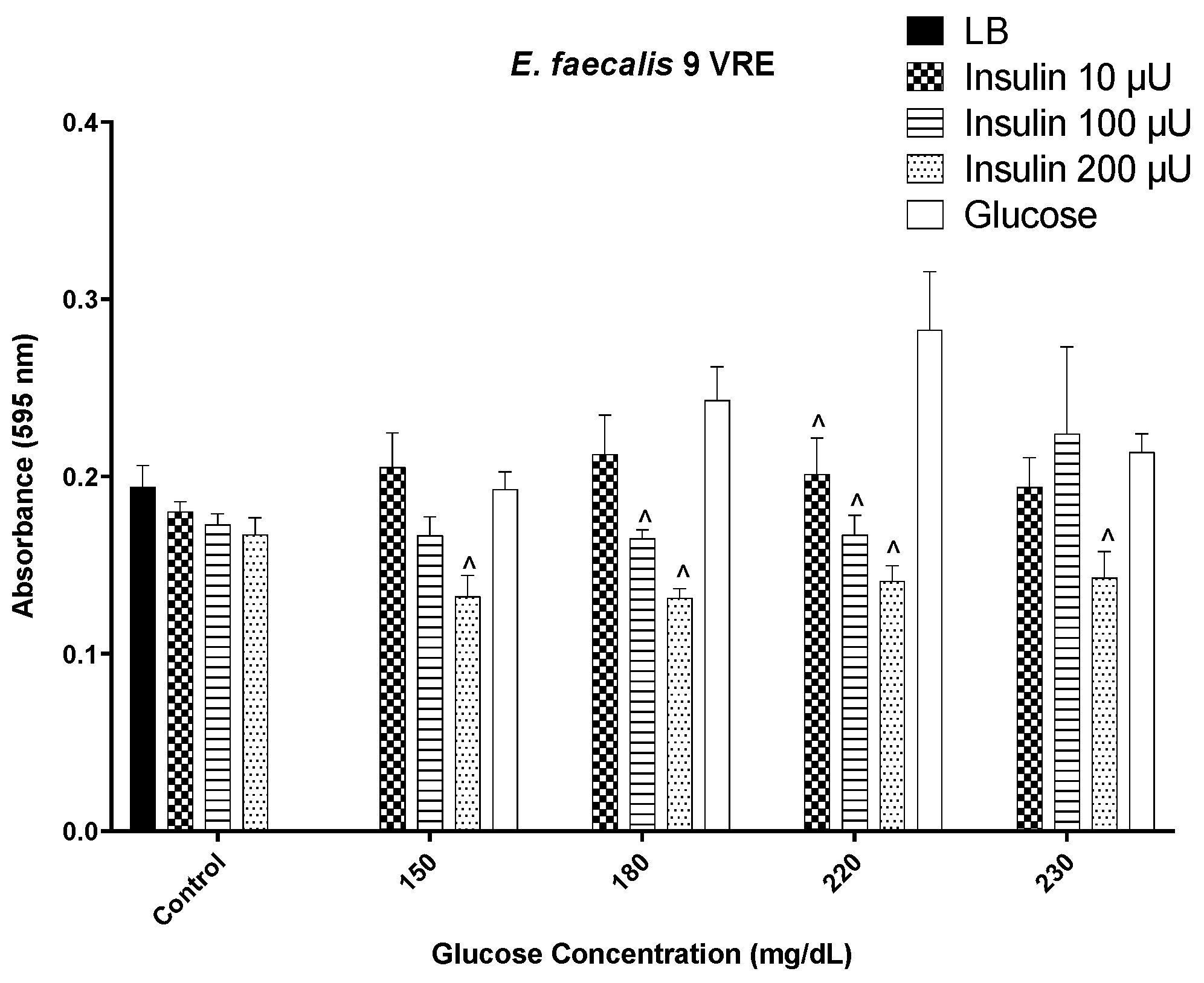
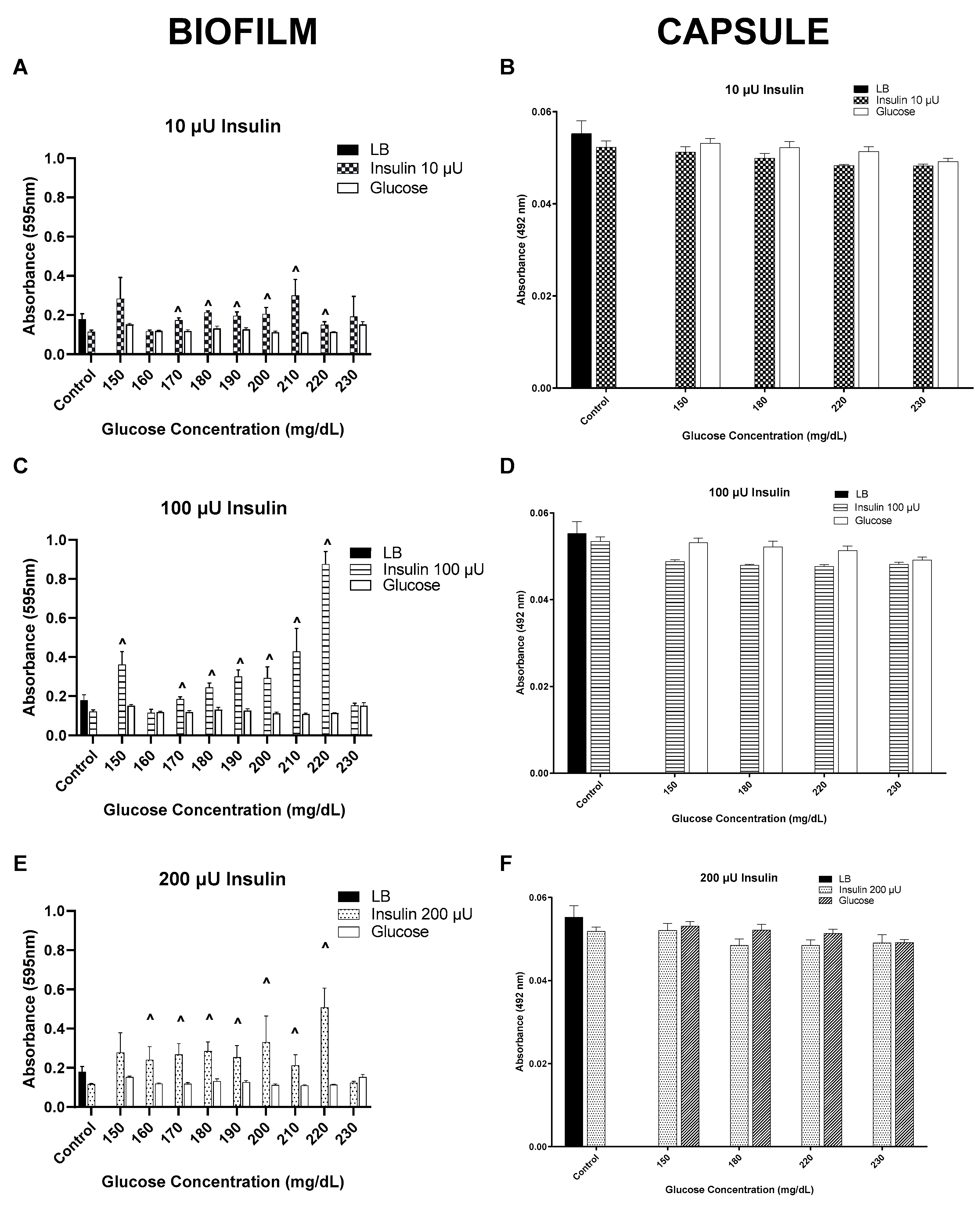
3. Results
3.1. Insulin/Glucose Concentration Effect on Biofilm Formation by MSSA, MRSA, and VRE
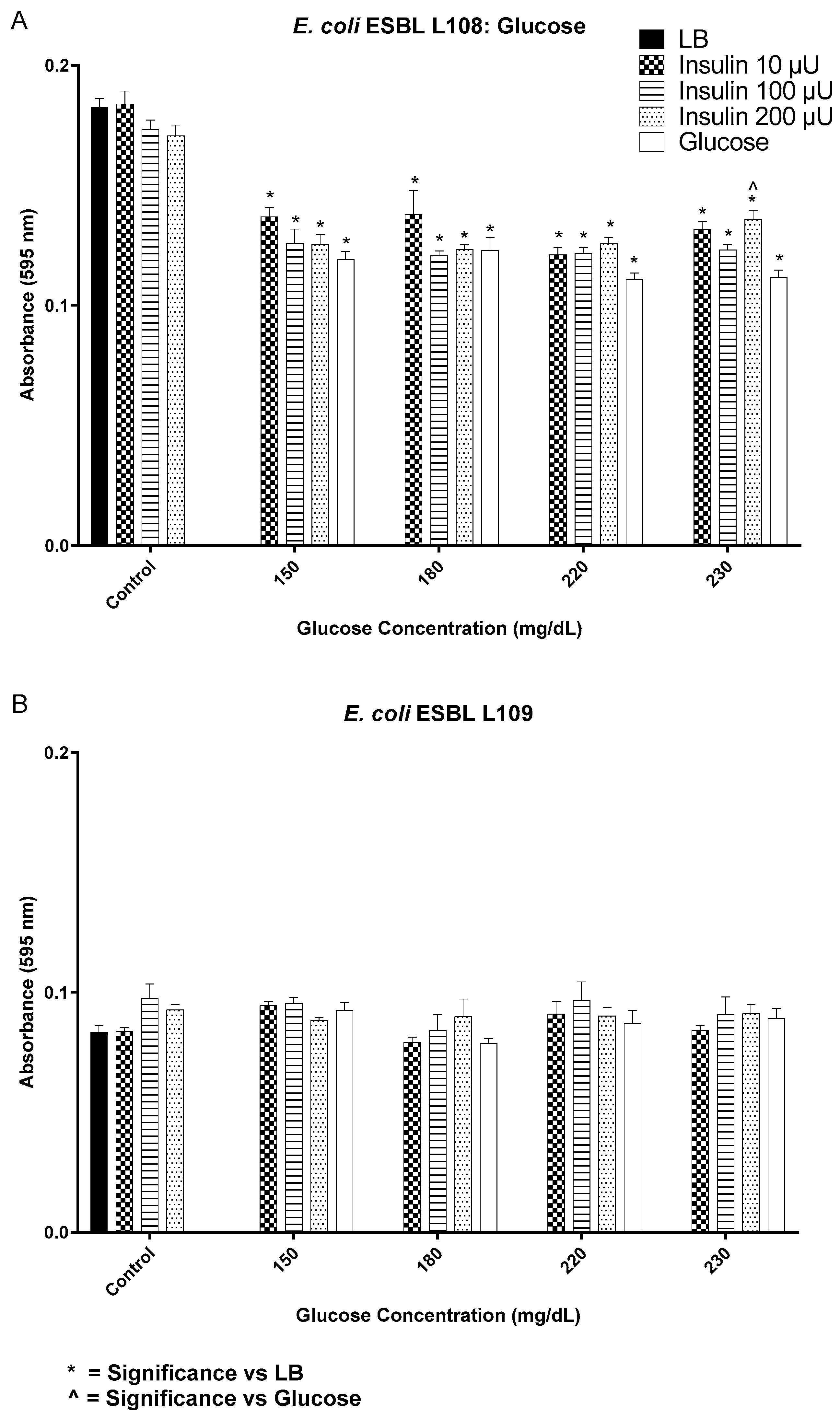
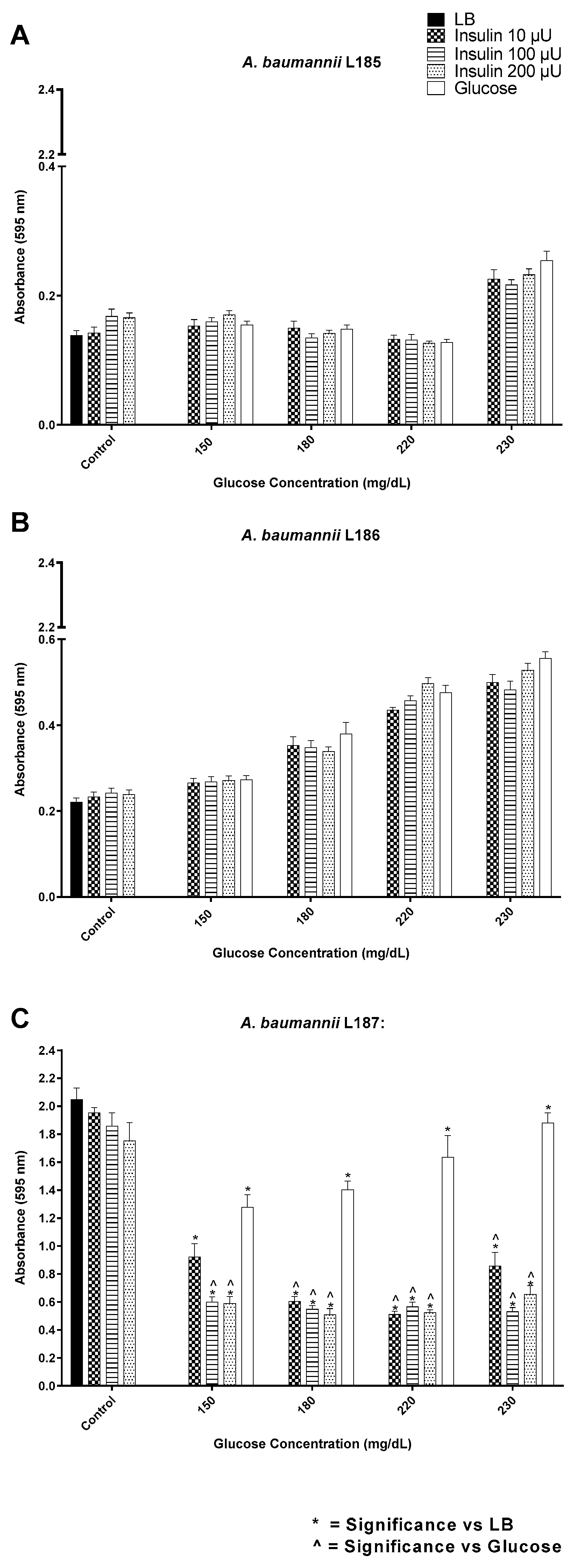
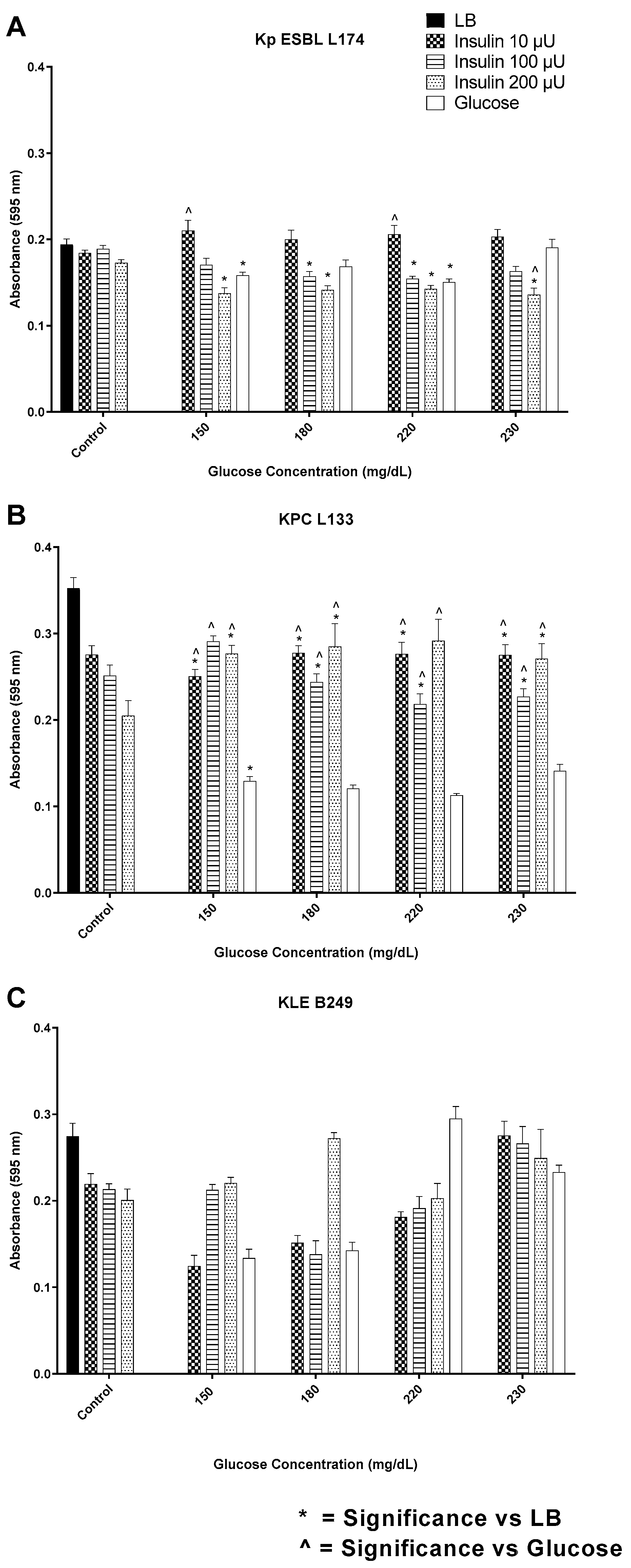
3.2. Insulin/Glucose Concentration Effect on Biofilm Formation by Gram-Negative Pathogens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qaseem, A.; Humphrey, L.L.; Chou, R.; Snow, V.; Shekelle, P. Use of Intensive Insulin Therapy for the Management of Glycemic Control in Hospitalized Patients: A Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2011, 154, 260–267. [Google Scholar] [CrossRef]
- Van den Berghe, G. What’s New in Glucose Control in the ICU? Intensive Care Med. 2013, 39, 823–825. [Google Scholar] [CrossRef][Green Version]
- Ellahham, S. Insulin Therapy in Critically Ill Patients. Vasc. Health Risk Manag. 2010, 1089–1101. [Google Scholar] [CrossRef]
- Ellahham, S. Molecular Mechanisms of Hyperglycemia and Cardiovascular-Related Events in Critically Ill Patients: Rationale For The Clinical Benefits of Insulin Therapy. Clin. Epidemiol. 2010, 2, 281–288. [Google Scholar] [CrossRef]
- Barsoumian, A.E.; Mende, K.; Sanchez, C.J.; Beckius, M.L.; Wenke, J.C.; Murray, C.K.; Akers, K.S. Clinical Infectious Outcomes Associated with Biofilm-Related Bacterial Infections: A Retrospective Chart Review. BMC Infect. Dis. 2015, 15, 223. [Google Scholar] [CrossRef]
- Waldrop, R.; McLaren, A.; Calara, F.; McLemore, R. Biofilm Growth Has a Threshold Response to Glucose In Vitro. Clin. Orthop. Relat. Res. 2014, 472, 3305–3310. [Google Scholar] [CrossRef]
- Jacobi, J.; Bircher, N.; Krinsley, J.; Agus, M.; Braithwaite, S.S.; Deutschman, C.; Freire, A.X.; Geehan, D.; Kohl, B.; Nasraway, S.A.; et al. Guidelines for the Use of An Insulin Infusion for The Management of Hyperglycemia in Critically Ill Patients. Crit. Care Med. 2012, 40, 3251–3276. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Q.-H.; Tang, R.; Liu, H.-Y.; Ji, Z.-S.; Gao, Z.; Wang, Y.; You, H.-Y.; Hao, J.-W.; Zhou, M. Prognostic Significance of Plasma Insulin Level for Deep Venous Thrombosis in Patients with Severe Traumatic Brain Injury in Critical Care. Neurocrit. Care 2023, 38, 263–278. [Google Scholar] [CrossRef]
- Minasyan, H. Sepsis: Mechanisms of Bacterial Injury to the Patient. Scand. J. Trauma Resusc. Emerg. Med. 2019, 27, 19. [Google Scholar] [CrossRef]
- Klosowska, K.; Plotkin, B. Human Insulin Modulation of Escherichia coli Adherence and Chemotaxis. Am. J. Infect. Dis. 2006, 2, 197–200. [Google Scholar] [CrossRef]
- Plotkin, B.; Wu, Z.; Ward, K.; Nadella, S.; Green, J. Effect of Human Insulin on the Formation of Catheter-Associated E. coli Biofilms. Open J. Urol. 2014, 4, 49–56. [Google Scholar] [CrossRef]
- Plotkin, B.J.; Viselli, S.M. Effect of Insulin on Microbial Growth. Curr. Microbiol. 2000, 41, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Xavier-Filho, J.; Oliveira, A.E.A.; Silva, L.B.D.; Rocha, A.; Venancio, T.; Machado, O.; Oliva, M.L.; Fernandes, K.; Xavier-Neto, J. Plant Insulin or Glucokinin: A Conflicting Issue. Braz. J. Plant Physiol. 2003, 15, 67–78. [Google Scholar] [CrossRef]
- Fawell, S.E.; Lenard, J. A Specific Insulin Receptor and Tyrosine Kinase Activity in the Membranes of Neurospora crassa. Biochem. Biophys. Res. Commun. 1988, 155, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S. Insulin Rescues the Unicellular Eucaryote Tetrahymena from Dying in A Complete, Synthetic Nutrient Medium. Cell Biol. Intern. 1993, 17, 833–837. [Google Scholar] [CrossRef]
- LeRoith, D.; Shiloach, J.; Roth, J.; Lesniak, M.A. Insulin or a Closely Related Molecule Is Native to Escherichia coli. J. Biol. Chem. 1981, 256, 6533–6536. [Google Scholar] [CrossRef]
- Patel, N.; Curtis, J.C.; Plotkin, B.J. Insulin Regulation of Escherichia coli Abiotic Biofilm Formation: Effect of Nutrients and Growth Conditions. Antibiotics 2021, 10, 1349. [Google Scholar] [CrossRef]
- Konaklieva, M.; Plotkin, B. Chemical Communication—Do We Have a Quorum? Mini-Rev. Med. Chem. 2006, 6, 817–825. [Google Scholar] [CrossRef]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of Coagulase-Negative Staphylococci To Plastic Tissue Culture Plates: A Quantitative Model for the Adherence of Staphylococci to Medical Devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef]
- Hronowski, L.; Anastassiades, T.P. Quantitation and Interaction of Glycosaminoglycans with Alcian Blue in Dimethyl Sulfoxide Solutions. Anal. Biochem. 1979, 93, 60–72. [Google Scholar] [CrossRef]
- Farzam, F.; Plotkin, B.J. Effect of Sub-Mics of Antibiotics on The Hydrophobicity and Production of Acidic Polysaccharide by Vibrio vulnificus. Chemotherapy 2001, 47, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Mann-Salinas, E.A.; Baun, M.M.; Meininger, J.C.; Murray, C.K.; Aden, J.K.; Wolf, S.E.; Wade, C.E. Novel Predictors of Sepsis Outperform the American Burn Association Sepsis Criteria in the Burn Intensive Care Unit Patient. J. Burn. Care Res. 2013, 34, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Laird, A.M.; Miller, P.R.; Kilgo, P.D.; Meredith, J.W.; Chang, M.C. Relationship of Early Hyperglycemia to Mortality in Trauma Patients. J. Trauma-Inj. Infect. Crit. Care 2004, 56, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Mayberry, J.C. The Importance of Hyperglycemia Control in Orthopedic Trauma Patients. J. Orthop. Trauma 2013, 27, 21. [Google Scholar] [CrossRef]
- Sung, J.; Bochicchio, G.V.; Joshi, M.; Bochicchio, K.; Tracy, K.; Scalea, T.M. Admission Hyperglycemia Is Predictive of Outcome in Critically III Trauma Patients. J. Trauma-Inj. Infect. Crit. Care 2005, 59, 80–83. [Google Scholar] [CrossRef]
- Turina, M.; Fry, D.E.; Polk, H.C. Acute Hyperglycemia and the Innate Immune System: Clinical, Cellular, and Molecular Aspects. Crit. Care Med. 2005, 33, 1624–1633. [Google Scholar] [CrossRef]
- Karunakar, M.A.; Staples, K.S. Does Stress-Induced Hyperglycemia Increase the Risk of Perioperative Infectious Complications in Orthopaedic Trauma Patients? J. Orthop. Trauma 2010, 24, 752–756. [Google Scholar] [CrossRef]
- Richards, J.E.; Kauffmann, R.M.; Obremskey, W.T.; May, A.K. Stress-Induced Hyperglycemia as a Risk Factor for Surgical-Site Infection in Nondiabetic Orthopedic Trauma Patients Admitted to the Intensive Care Unit. J. Orthop. Trauma 2013, 27, 16–21. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Klein, D.; Herndon, D.N. Insulin Treatment Improves the Systemic Inflammatory Reaction to Severe Trauma. Ann. Surg. 2004, 239, 553–560. [Google Scholar] [CrossRef]
- Klein, D.; Schubert, T.; Horch, R.E.; Jauch, K.W.; Jeschke, M.G. Insulin Treatment Improves Hepatic Morphology and Function through Modulation of Hepatic Signals after Severe Trauma. Ann. Surg. 2004, 240, 340–349. [Google Scholar] [CrossRef]
- Tannuri, U.; Coelho, M.C.M.; Maksoud, J.G. Serum-Insulin Glucose Ratio and Urinary Loss of Insulin After Trauma in Young Animals—Effect of Adrenergic Blockage. Pediatr. Surg. Int. 1993, 8, 210–214. [Google Scholar] [CrossRef]
- Hospenthal, D.R.; Green, A.D.; Crouch, H.K.; English, J.F.; Pool, J.; Yun, H.C.; Murray, C.K.; Prevention Combat-Related, I. Infection Prevention and Control in Deployed Military Medical Treatment Facilities. J. Trauma-Inj. Infect. Crit. Care 2011, 71, S290–S298. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Kinoshita, M.; Fujino, K.; Nakashima, M.; Yamamoto, Y.; Miyazaki, H.; Hamada, K.; Ono, S.; Iwaya, K.; Saitoh, D.; et al. Insulin Treatment Directly Restores Neutrophil Phagocytosis and Bactericidal Activity in Diabetic Mice and Thereby Improves Surgical Site Staphylococcus aureus Infection. Infect. Immun. 2012, 80, 4409–4416. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Lesniak, M.; Roth, J. Insulin in Insects and Annelids. Diabetes 1981, 30, 70–76. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Shiloach, J.; Heffron, R.; Rubinovitz, C.; Tanenbaum, R.; Roth, J. Insulin-Related Material in Microbes: Similarities and Differences from Mammalian Insulins. Can. J. Biochem. Cell Biol. 1985, 63, 839–849. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Shiloach, J.; Roth, J.; Lesniak, M. Evolutionary Origins of Vertebrate Hormones: Substances Similar to Mammalian Insulins are Native to Unicellular Eukaryotes. Proc. Natl. Acad. Sci. USA 1980, 77, 6184–6188. [Google Scholar] [CrossRef]
- Roth, J.; Leroith, D.; Collier, E.; Watkinson, A.; Lesniak, M. The Evolutionary Origins of Intercellular Communication and the Maginot Lines of the Mind. Ann. N. Y. Acad. Sci. 1986, 463, 1–11. [Google Scholar] [CrossRef]
- Dietz, E.; Uhlenbruck, G. An Insulin Receptor in Microorganisms: Fact or Fiction? Naturwissensenschaften 1989, 76, 269–270. [Google Scholar] [CrossRef]
- Feraco, D.; Blaha, M.; Khan, S.; Green, J.M.; Plotkin, B.J. Host Environmental Signals and Effects on Biofilm Formation. Microb. Pathog. 2016, 99, 253–263. [Google Scholar] [CrossRef]
- Miller, M.; Bassler, B. Quorum Sensing in Bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef]
- Taga, M.E.; Bassler, B.L. Chemical Communication Among Bacteria. Proc. Natl. Acad. Sci. USA 2003, 100, 14549–14554. [Google Scholar] [CrossRef] [PubMed]
- Waters, C.M.; Bassler, B.L. Quorum Sensing: Cell-To-Cell Communication in Bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, A.; Wood, T.K. Bacterial Quorum Sensing: Signals, Circuits, and Implications for Biofilms and Disease. Annu. Rev. Biomed. Eng. 2008, 10, 145–167. [Google Scholar] [CrossRef]
- Kirby, D.; Raino, C.; Rabor, S.F., Jr.; Wasson, C.; Plotkin, B. Semi-Automated Method for Multi-Tasking Measurement of Microbial Growth, Capsule, and Biofilm Formation. Adv. Microbiol. 2012, 2, 623–628. [Google Scholar] [CrossRef][Green Version]
- Shiomi, D.; Banno, S.; Homma, M.; Kawagishi, I. Stabilization of Polar Localization of a Chemoreceptor Via Its Covalent Modifications and Its Communication with a Different Chemoreceptor. J. Bacteriol. 2005, 187, 7647–7654. [Google Scholar] [CrossRef] [PubMed]
- Sourjik, V. Receptor Clustering and Signal Processing in E. coli Chemotaxis. Trends Microbiol. 2004, 12, 569–576. [Google Scholar] [CrossRef]
- Tatsuno, I.; Homma, M.; Oosawa, K.; Kawagishi, I. Signaling by the Escherichia coli Aspartate Chemoreceptor Tar with a Single Cytoplasmic Domain Per Dimer. Science 1996, 274, 423. [Google Scholar] [CrossRef]
- Zhang, P.; Khursigara, C.M.; Hartnell, L.M.; Subramaniam, S. Direct Visualization of Escherichia coli Chemotaxis Receptor Arrays Using Cryo-Electron Microscopy. Proc. Natl. Acad. Sci. USA 2007, 104, 3777–3781. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Ghosh, A.; Das, D.; Dey, A.; Mukherjee, D.; Garai, S.; Ray, R.R. Biofilm and Antimicrobial Resistance. Biofilm-Mediat. Dis. Causes Control. 2021, 437, 183–208. [Google Scholar]
- Clark, J.; Webb, R. The Site of Action of the Crystal Violet Nuclear Stain. Stain. Technol. 1955, 30, 89–92. [Google Scholar] [CrossRef]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T. Biofilms: Formation, Drug Resistance and Alternatives to Conventional Approaches. AIMS Microbiol. 2022, 8, 239–277. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plotkin, B.J.; Halkyard, S.; Spoolstra, E.; Micklo, A.; Kaminski, A.; Sigar, I.M.; Konaklieva, M.I. The Role of the Insulin/Glucose Ratio in the Regulation of Pathogen Biofilm Formation. Biology 2023, 12, 1432. https://doi.org/10.3390/biology12111432
Plotkin BJ, Halkyard S, Spoolstra E, Micklo A, Kaminski A, Sigar IM, Konaklieva MI. The Role of the Insulin/Glucose Ratio in the Regulation of Pathogen Biofilm Formation. Biology. 2023; 12(11):1432. https://doi.org/10.3390/biology12111432
Chicago/Turabian StylePlotkin, Balbina J., Scott Halkyard, Emily Spoolstra, Amanda Micklo, Amber Kaminski, Ira M. Sigar, and Monika I. Konaklieva. 2023. "The Role of the Insulin/Glucose Ratio in the Regulation of Pathogen Biofilm Formation" Biology 12, no. 11: 1432. https://doi.org/10.3390/biology12111432
APA StylePlotkin, B. J., Halkyard, S., Spoolstra, E., Micklo, A., Kaminski, A., Sigar, I. M., & Konaklieva, M. I. (2023). The Role of the Insulin/Glucose Ratio in the Regulation of Pathogen Biofilm Formation. Biology, 12(11), 1432. https://doi.org/10.3390/biology12111432




