Vascular Growth Factor Inhibition with Bevacizumab Improves Cardiac Electrical Alterations and Fibrosis in Experimental Acute Chagas Disease
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Infection of Animals with Trypomastigote Forms of T. cruzi
2.2. Anti-VEGF Antibody Treatment
2.3. Parasitological Parameters
2.4. Electrocardiographic Studies
2.5. Histopathology and Immunofluorescence
2.6. Immunoblotting
2.7. Ethics Statement
3. Results
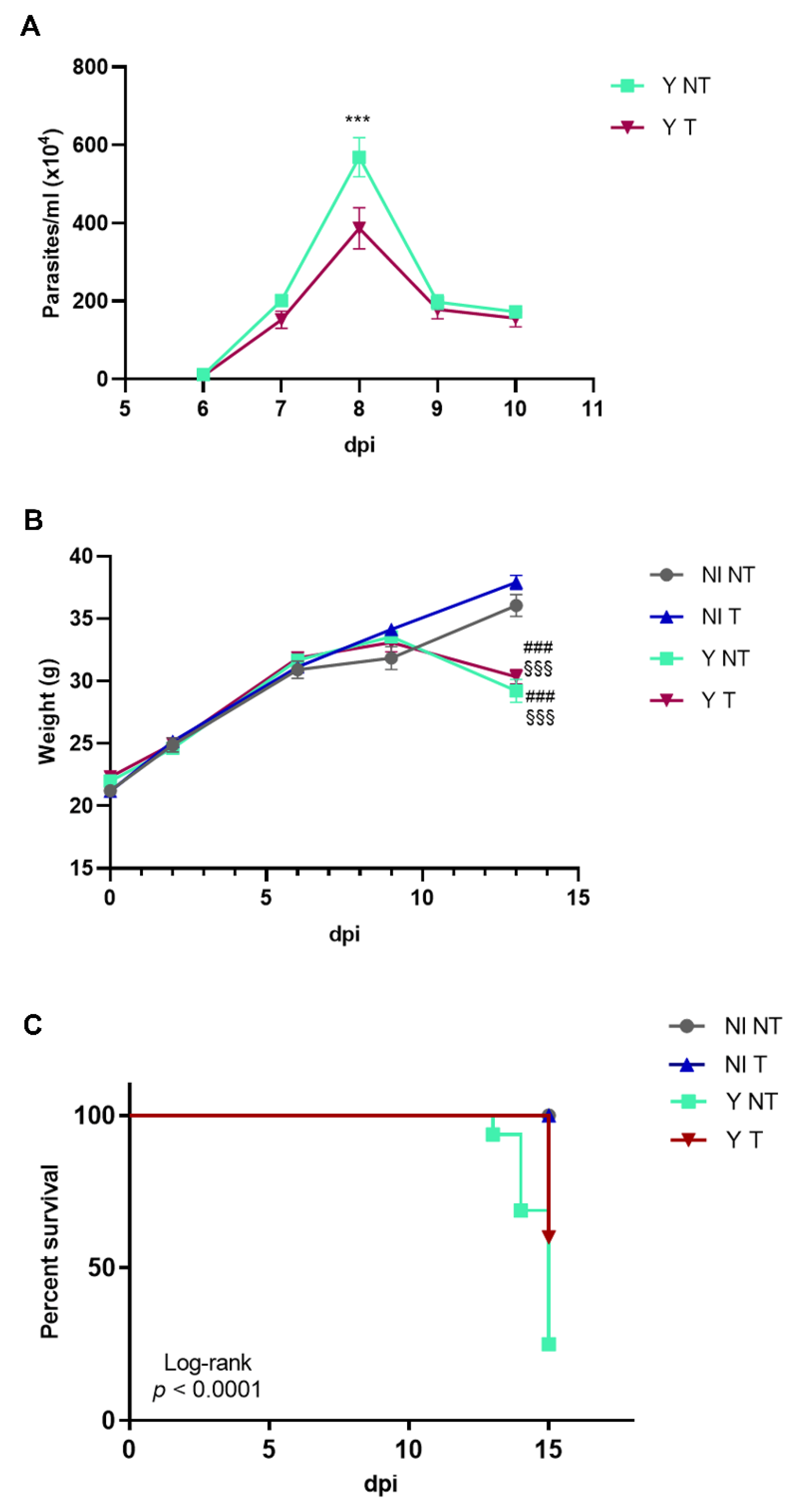
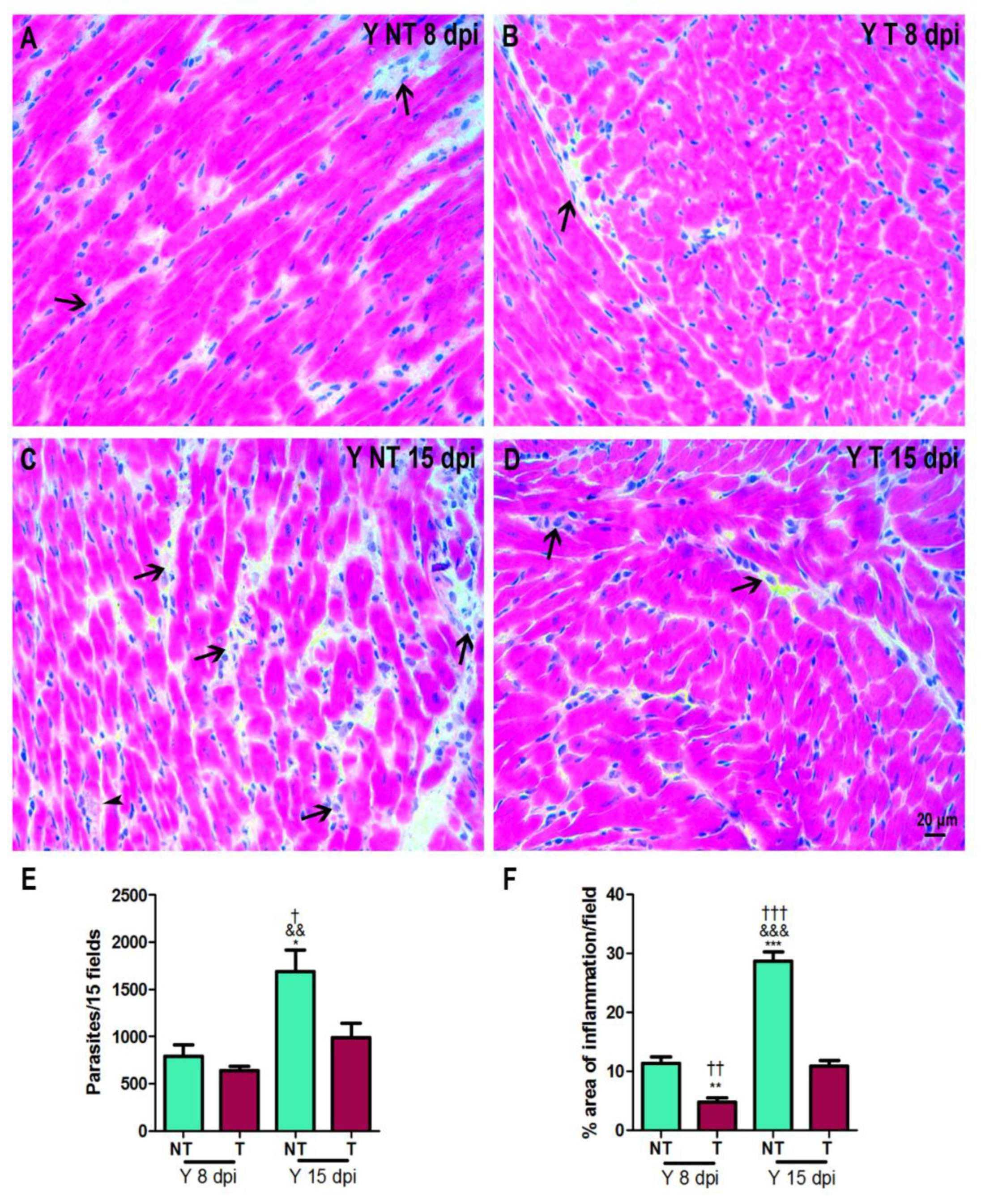
3.1. Anti-VEGF Treatment Improves Survival Rate and Inflammation
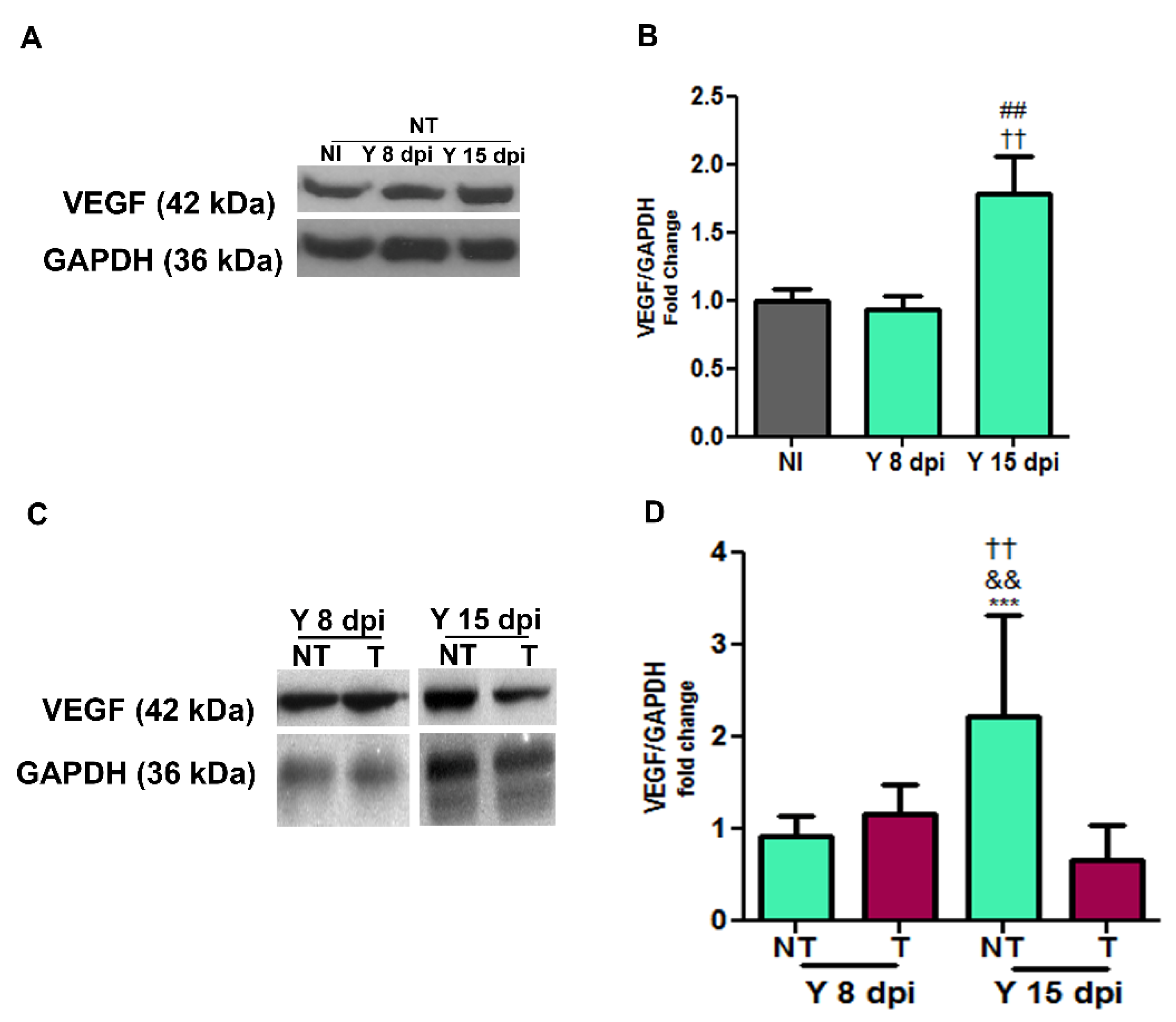
3.2. VEGF Protein Expression in Cardiac Tissue
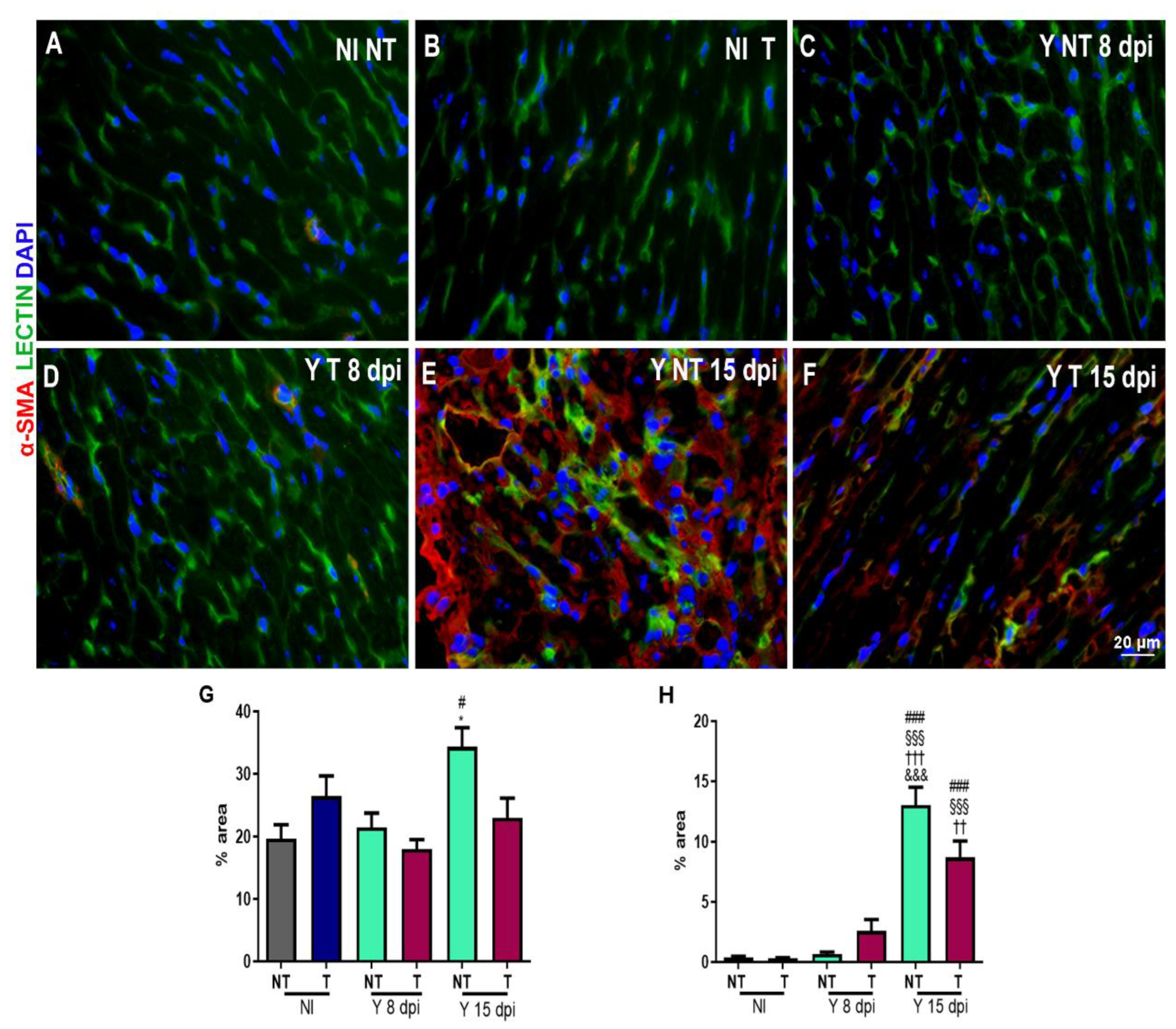
3.3. Anti-VEGF Treatment Reduces the Blood Vessel Abundance in Cardiac Tissue
3.4. Treatment Prevents Heart Fibrosis Development in T. cruzi-Infected Mice
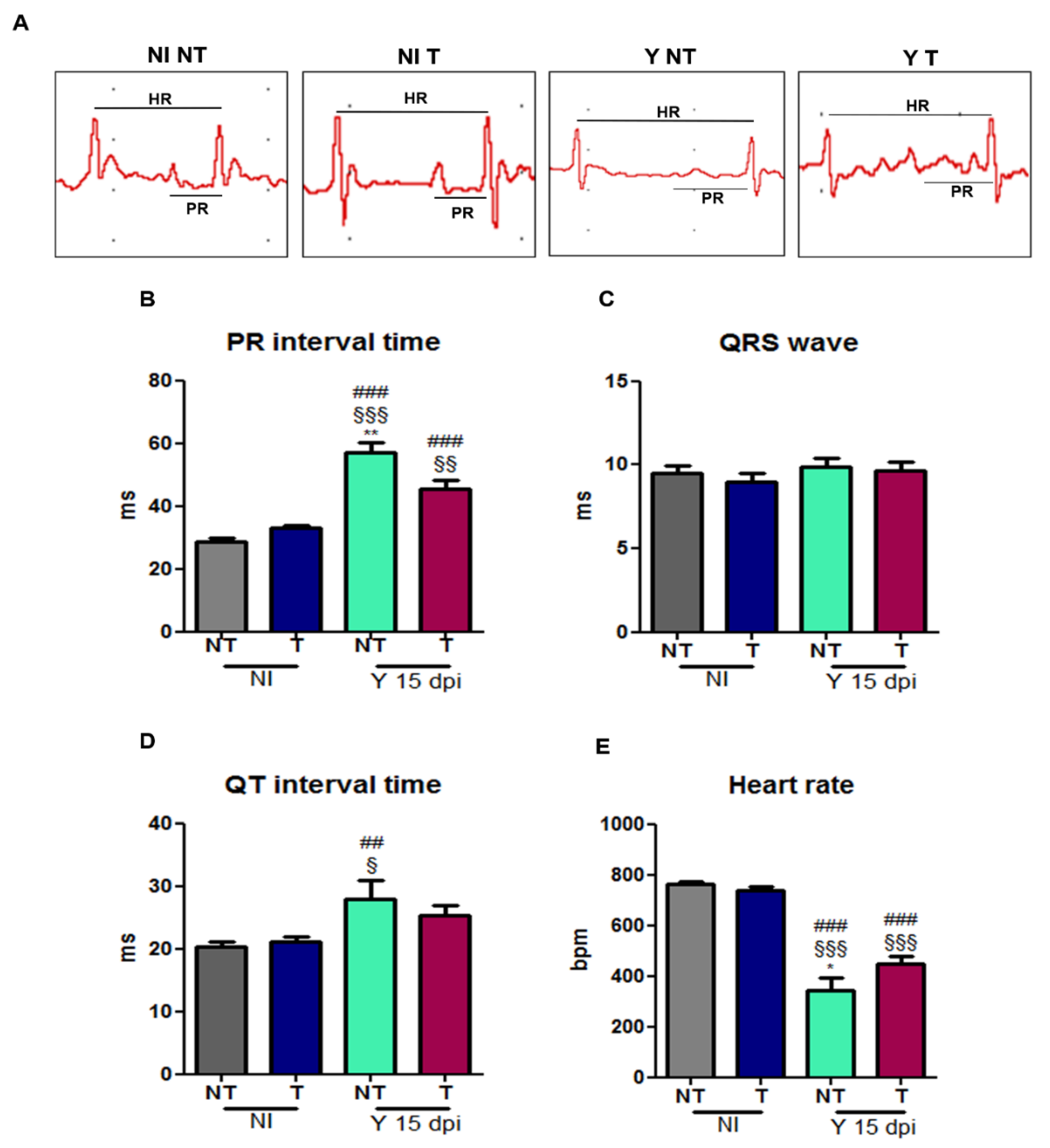
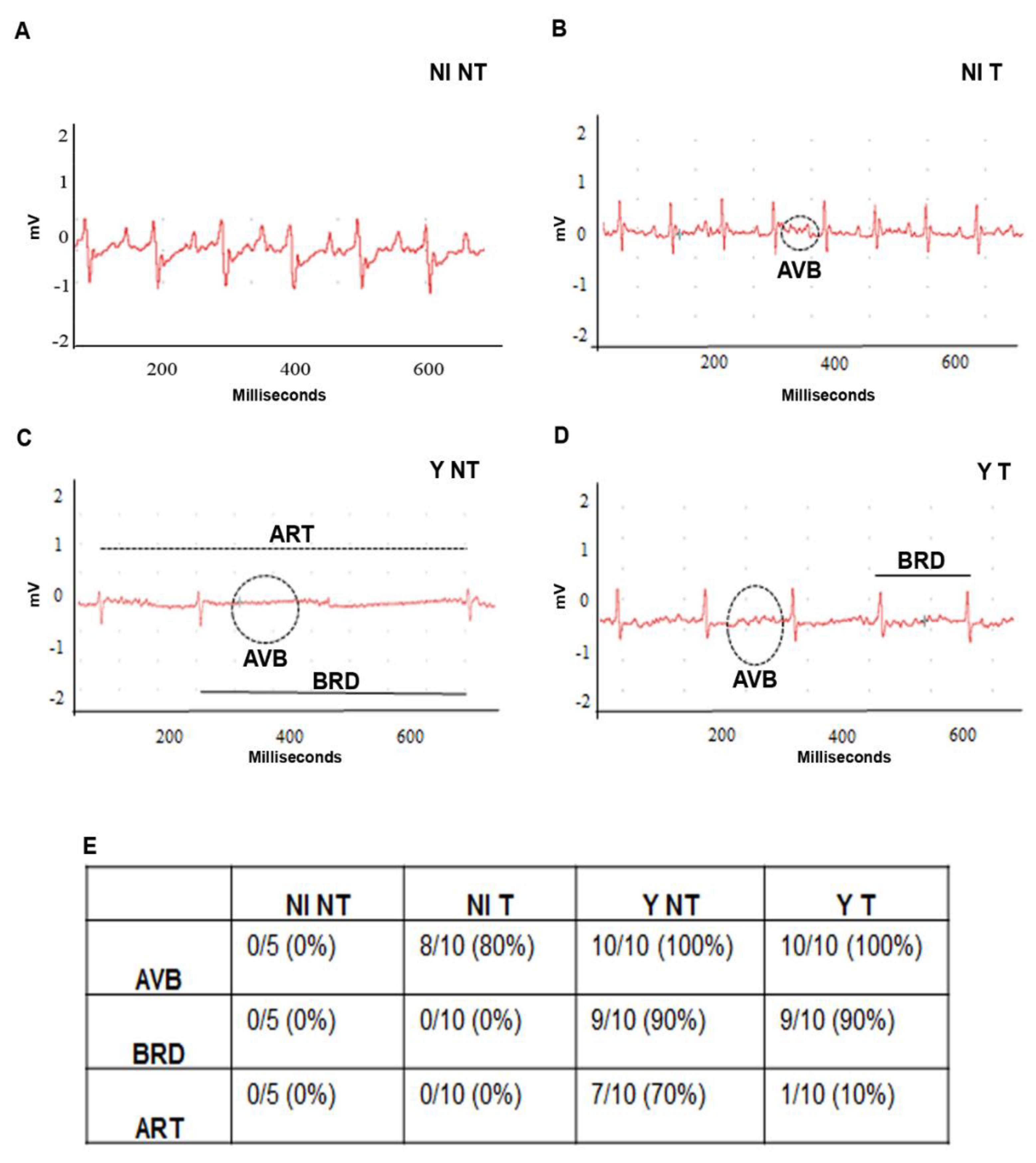
3.5. VEGF Blockage Ameliorates Electrocardiographic Alterations Caused by T. cruzi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030. Available online: https://apps.who.int/iris/rest/bitstreams/1326801/retrieve (accessed on 10 July 2023).
- Cançado, J.R. Long term evaluation of etiological treatment of chagas disease with benznidazole. Rev. Inst. Med. Trop. 2002, 44, 29–37. [Google Scholar] [CrossRef]
- Dias, J.C.; Ramos, A.N.; Gontijo, E.D., Jr.; Luquetti, A.; Shikanai-Yasuda, M.A.; Coura, J.R.; Torres, R.M.; Melo, J.R.; Almeida, E.A.; Oliveira, W.D., Jr.; et al. Brazilian Consensus on Chagas Disease, 2015. Epidemiol. Serv. Saude 2016, 25, 7–86. [Google Scholar] [CrossRef]
- Tanowitz, H.B.; Burns, E.R.; Sinha, A.K.; Kahn, N.N.; Morris, S.A.; Factor, S.M.; Hatcher, V.B.; Bilezikian, J.P.; Baum, S.G.; Wittner, M. Enhanced platelet adherence and aggregation in chagas’ disease: A potential pathogenic mechanism for cardiomyopathy. Am. J. Trop. Med. Hyg. 1990, 43, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Wittner, M.; Christ, G.J.; Huang, H.; Weiss, L.M.; Hatcher, V.B.; Morris, S.A.; Orr, G.A.; Berman, J.W.; Zeballos, G.A.; Douglas, S.A.; et al. Trypanosoma cruzi Induces Endothelin Release from Endothelial Cells. J. Infect. Dis. 1995, 171, 493–497. [Google Scholar] [CrossRef]
- Ashton, A.W.; Mukherjee, S.; Nagajyothi, F.; Huang, H.; Braunstein, V.L.; Desruisseaux, M.S.; Factor, S.M.; Lopez, L.; Berman, J.W.; Wittner, M.; et al. Thromboxane A2 is a key regulator of pathogenesis during Trypanosoma cruzi infection. J. Exp. Med. 2007, 204, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Factor, S.M.; Wittner, M.; Tanowitz, H.; Cho, S. Abnormalities of the Coronary Microcirculation in Acute Murine Chagas’ Disease. Am. J. Trop. Med. Hyg. 1985, 34, 246–253. [Google Scholar] [CrossRef]
- Shrestha, D.; Bajracharya, B.; Paula-Costa, G.; Salles, B.C.; Leite, A.L.J.; Menezes, A.P.J.; Souza, D.M.; Oliveira, L.A.; Talvani, A. Expression and production of cardiac angiogenic mediators depend on the Trypanosoma cruzi-genetic population in experimental C57BL/6 mice infection. Microvasc. Res. 2016, 110, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.D.L.; Fukasawa, S.; De Brito, T.; Parzianello, L.C.; Bellotti, G.; Ramires, J.A.F. Different microcirculatory and interstitial matrix patterns in idiopathic dilated cardiomyopathy and Chagas’ disease: A three dimensional confocal microscopy study. Heart 1999, 82, 279–285. [Google Scholar] [CrossRef]
- Heinke, J.; Patterson, C.; Moser, M. Life is a pattern: Vascular assembly within the embryo. Front. Biosci. (Elite Ed.) 2012, 4, 2269–2288. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The immune system and cardiac repair. Pharmacol. Res. 2008, 58, 88–111. [Google Scholar] [CrossRef]
- Marrelli, A.; Cipriani, P.; Liakouli, V.; Carubbi, F.; Perricone, C.; Perricone, R.; Giacomelli, R. Angiogenesis in rheumatoid arthritis: A disease specific process or a common response to chronic inflammation? Autoimmun. Rev. 2011, 10, 595–598. [Google Scholar] [CrossRef]
- Fligny, C.; Duffield, J.S. Activation of pericytes: Recent insights into kidney fibrosis and microvascular rarefaction. Curr. Opin. Rheumatol. 2013, 25, 78–86. [Google Scholar] [CrossRef]
- Fabris, L.; Strazzabosco, M. Epithelial-Mesenchymal Interactions in Biliary Diseases. Semin. Liver Dis. 2011, 31, 11. [Google Scholar] [CrossRef]
- Lemos, Q.T.; A Andrade, Z. Angiogenesis and experimental hepatic fibrosis. Mem. Inst. Oswaldo Cruz 2010, 105, 611–614. [Google Scholar] [CrossRef]
- Lin, S.-L.; Kisseleva, T.; Brenner, D.A.; Duffield, J.S. Pericytes and Perivascular Fibroblasts Are the Primary Source of Collagen-Producing Cells in Obstructive Fibrosis of the Kidney. Am. J. Pathol. 2008, 173, 1617–1627. [Google Scholar] [CrossRef]
- Andrade, Z.A.; Santana, T.S. Angiogenesis and schistosomiasis. Mem. Inst. Oswaldo Cruz 2010, 105, 436–439. [Google Scholar] [CrossRef]
- Dakowicz, D.; Zajkowska, M.; Mroczko, B. Relationship between VEGF Family Members, Their Receptors and Cell Death in the Neoplastic Transformation of Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 3375. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, X.; Cui, H.; Shi, J.; Yuan, G.; Shi, S.; Hu, Y. The Role of the VEGF Family in Coronary Heart Disease. Front. Cardiovasc. Med. 2021, 8, 738325. [Google Scholar] [CrossRef]
- Okutani, D.; Lodyga, M.; Han, B.; Liu, M. Src protein tyrosine kinase family and acute inflammatory responses. Am. J. Physiol. Cell. Mol. Physiol. 2006, 291, L129–L141. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Novotny, W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005, 333, 328–335. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, X.; Liu, R.; Wu, S.; Liu, Q.; Li, J.; Ma, J. Bevacizumab is an Efficient Therapeutic Approach with Low Side Effects in Patient-Derived Xenografts of Adenoid Cystic Carcinoma of the Lacrimal Gland. Cancer Manag. Res. 2022, 14, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Uyeturk, U.; Gucuk, A.; Firat, T.; Kemahli, E.; Kukner, A.; Ozyalvacli, M.E. Effect of Mitomycin, Bevacizumab, and 5-Fluorouracil to Inhibit Urethral Fibrosis in a Rabbit Model. J. Endourol. 2014, 28, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Feng, H.; Kan, T.; Huang, B.; Zhang, M.; Li, Y.; Shi, C.; Wu, M.; Luo, Y.; Yang, J.; et al. Bevacizumab Attenuates Hepatic Fibrosis in Rats by Inhibiting Activation of Hepatic Stellate Cells. PLoS ONE 2013, 8, e73492. [Google Scholar] [CrossRef]
- Zhang, M.; Chu, S.; Zeng, F.; Xu, H. Bevacizumab modulates the process of fibrosis in vitro. Clin. Exp. Ophthalmol. 2015, 43, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Emami, M.; Jaberi, F.; Azarpira, N.; Vosoughi, A.; Tanideh, N. Prevention of arthrofibrosis by monoclonal antibody against vascular endothelial growth factor: A novel use of bevacizumab in rabbits. Orthop. Traumatol. Surg. Res. 2012, 98, 759–764. [Google Scholar] [CrossRef]
- Segerström, L.; Fuchs, D.; Bäckman, U.; Holmquist, K.; Christofferson, R.; Azarbayjani, F. The Anti-VEGF Antibody Bevacizumab Potently Reduces the Growth Rate of High-Risk Neuroblastoma Xenografts. Pediatr. Res. 2006, 60, 576–581. [Google Scholar] [CrossRef][Green Version]
- von Baumgarten, L.; Brucker, D.; Tirniceru, A.; Kienast, Y.; Grau, S.; Burgold, S.; Herms, J.; Winkler, F. Bevacizumab Has Differential and Dose-Dependent Effects on Glioma Blood Vessels and Tumor Cells. Clin. Cancer Res. 2011, 17, 6192–6205. [Google Scholar] [CrossRef]
- Walker, E.J.; Su, H.; Shen, F.; Degos, V.; Amend, G.; Jun, K.; Young, W.L. Bevacizumab Attenuates VEGF-Induced Angiogenesis and Vascular Malformations in the Adult Mouse Brain. Stroke 2012, 43, 1925–1930. [Google Scholar] [CrossRef]
- Brener, Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev. Inst. Med. Trop. Sao Paulo 1962, 4, 389–396. [Google Scholar]
- Botelho, A.F.; Joviano-Santos, J.V.; Santos-Miranda, A.; Menezes-Filho, J.E.; Soto-Blanco, B.; Cruz, J.S.; Guatimosim, C.; Melo, M.M. Non-invasive ECG recording and QT interval correction assessment in anesthetized rats and mice. Pesqui. Vet. Bras. 2019, 39, 409–415. [Google Scholar] [CrossRef]
- Grishagin, I.V. Automatic cell counting with ImageJ. Anal. Biochem. 2015, 473, 63–65. [Google Scholar] [CrossRef]
- Sousa, M.A.; Alencar, A.A. On the tissular parasitism of Trypanosoma cruzi y strain in Swiss mice. Rev. Inst. Med. Trop. São Paulo 1984, 26, 6. [Google Scholar] [CrossRef] [PubMed]
- Guedes-Da-Silva, F.H.; Batista, D.G.J.; Meuser, M.B.; Demarque, K.C.; Fulco, T.O.; Araújo, J.S.; Da Silva, P.B.; Da Silva, C.F.; Patrick, D.A.; Bakunova, S.M.; et al. In Vitro and In Vivo Trypanosomicidal Action of Novel Arylimidamides against Trypanosoma cruzi. Antimicrob. Agents Chemother. 2016, 60, 2425–2434. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Berzal, C.; da Silva, C.F.; Batista, D.d.G.J.; de Oliveira, G.M.; Cumella, J.; Batista, M.M.; Peres, R.B.; Nefertiti, A.S.d.G.; Escario, J.A.; Gómez-Barrio, A.; et al. Activity profile of two 5-nitroindazole derivatives over the moderately drug-resistant Trypanosoma cruzi Y strain (DTU TcII): In vitro and in vivo studies. Parasitology 2020, 147, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Queiroga, T.B.D.; Pereira, N.d.S.; Silva, D.D.; Andrade, C.d.M.; Araújo Júnior, R.F.d.; Brito, C.R.D.N.; Galvão, L.M.d.C.; Câmara, A.C.J.; Nascimento, M.S.L.; Guedes, P.M.M. Virulence of Trypanosoma cruzi Strains Is Related to the Differential Expression of Innate Immune Receptors in the Heart. Front. Cell. Infect. Microbiol. 2021, 11, 696719. [Google Scholar] [CrossRef]
- Rossi, M.A.; Tanowitz, H.B.; Malvestio, L.M.; Celes, M.R.; Campos, E.C.; Blefari, V.; Prado, C.M. Coronary Microvascular Disease in Chronic Chagas Cardiomyopathy Including an Overview on History, Pathology, and Other Proposed Pathogenic Mechanisms. PLoS Negl. Trop. Dis. 2010, 4, e674. [Google Scholar] [CrossRef]
- Carod-Artal, F.J. Trypanosomiasis, cardiomyopathy and the risk of ischemic stroke. Expert Rev. Cardiovasc. Ther. 2010, 8, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.R.; Waghabi, M.C.; Bailly, S.; Feige, J.-J.; Hasslocher-Moreno, A.M.; Saraiva, R.M.; Araujo-Jorge, T.C. The Search for Biomarkers and Treatments in Chagas Disease: Insights from TGF-Beta Studies and Immunogenetics. Front. Cell. Infect. Microbiol. 2022, 11, 767576. [Google Scholar] [CrossRef]
- Lannes-Vieira, J. Multi-therapeutic strategy targeting parasite and inflammation-related alterations to improve prognosis of chronic Chagas cardiomyopathy: A hypothesis-based approach. Mem. Inst. Oswaldo Cruz 2022, 117, e220019. [Google Scholar] [CrossRef]
- Nisimura, L.M.; Estato, V.; de Souza, E.M.; Reis, P.A.; Lessa, M.A.; Castro-Faria-Neto, H.C.; Pereira, M.C.d.S.; Tibiriçá, E.; Garzoni, L.R. Acute Chagas Disease Induces Cerebral Microvasculopathy in Mice. PLoS Negl. Trop. Dis. 2014, 8, e2998. [Google Scholar] [CrossRef]
- Tanowitz, H.B.; Wittner, M.; Morris, S.A.; Zhao, W.; Weiss, L.M.; Hatcher, V.B.; Braunstein, V.L.; Huang, H.; Douglas, S.A.; Valcic, M.; et al. The Putative Mechanistic Basis for the Modulatory Role of Endothelin-1 in the Altered Vascular Tone Induced by Trypanosoma cruzi. Endothelium 1999, 6, 217–230. [Google Scholar] [CrossRef]
- Pereira, I.R.; Vilar-Pereira, G.; Moreira, O.C.; Ramos, I.P.; Gibaldi, D.; Britto, C.; Moraes, M.O.; Lannes-Vieira, J. Pentoxifylline Reverses Chronic Experimental Chagasic Cardiomyopathy in Association with Repositioning of Abnormal CD8+ T-Cell Response. PLoS Negl. Trop. Dis. 2015, 9, e0003659. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.R.; Abreu, R.D.S.; Vilar-Pereira, G.; Degrave, W.; Meuser-Batista, M.; Ferreira, N.V.C.; Moreira, O.D.C.; Gomes, N.L.D.S.; de Souza, E.M.; Ramos, I.P.; et al. TGF-β inhibitor therapy decreases fibrosis and stimulates cardiac improvement in a pre-clinical study of chronic Chagas’ heart disease. PLoS Negl. Trop. Dis. 2019, 13, e0007602. [Google Scholar] [CrossRef]
- Wang, Y.; Da, G.; Li, H.; Zheng, Y. Avastin Exhibits Therapeutic Effects on Collagen-Induced Arthritis in Rat Model. Inflammation 2013, 36, 1460–1467. [Google Scholar] [CrossRef]
- Waghabi, M.C.; Ferreira, R.R.; Abreu, R.d.S.; Degrave, W.; de Souza, E.M.; Bailly, S.; Feige, J.-J.; de Araújo-Jorge, T.C. Transforming growth factor-ß as a therapeutic target for the cardiac damage of Chagas disease. Mem. Inst. Oswaldo Cruz 2022, 117, e210395. [Google Scholar] [CrossRef] [PubMed]
- Waghabi, M.C.; Keramidas, M.; Calvet, C.M.; Meuser, M.; Soeiro, M.N.C.; Mendonça-Lima, L.; Araújo-Jorge, T.C.; Feige, J.J.; Bailly, S. SB-431542, a transforming growth factor beta inhibitor, impairs Trypanosoma cruzi infection in cardiomyocytes and parasite cycle completion. Antimicrob. Agents Chemother. 2007, 8, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Sabino, B.M.; A Lessa, M.; Nascimento, A.R.M.; AB Rodrigues, C.; Henriques, M.d.G.; Garzoni, L.R.; I Levy, B.; Tibiriçá, E. Effects of Antihypertensive Drugs on Capillary Rarefaction in Spontaneously Hypertensive Rats: Intravital Microscopy and Histologic Analysis. J. Cardiovasc. Pharmacol. 2008, 51, 402–409. [Google Scholar] [CrossRef]
- López de Padilla, C.M.; Coenen, M.J.; Tovar, A.; De la Vega, R.E.; Evans, C.H.; Müller, S.A. Picrosirius Red Staining: Revisiting Its Application to the Qualitative and Quantitative Assessment of Collagen Type I and Type III in Tendon. J. Histochem. Cytochem. 2021, 69, 633–643. [Google Scholar] [CrossRef]
- Ada, S.; Ersan, S.; Sifil, A.; Unlu, M.; Kolatan, E.; Sert, M.; Sarioglu, S.; Yilmaz, O.; Camsari, T. Effect of bevacizumab, a vascular endothelial growth factor inhibitor, on a rat model of peritoneal sclerosis. Int. Urol. Nephrol. 2015, 47, 2047–2051. [Google Scholar] [CrossRef]
- Economopoulou, P.; Kotsakis, A.; Kapiris, I.; Kentepozidis, N. Cancer therapy and cardiovascular risk: Focus on bevacizumab. Cancer Manag. Res. 2015, 7, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Totzeck, M.; Mincu, R.I.; Rassaf, T. Cardiovascular Adverse Events in Patients with Cancer Treated with Bevacizumab: A Meta-Analysis of More Than 20,000 Patients. J. Am. Heart Assoc. 2017, 6, e006278. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisimura, L.M.; Ferreira, R.R.; Coelho, L.L.; de Oliveira, G.M.; Gonzaga, B.M.; Meuser-Batista, M.; Lannes-Vieira, J.; Araujo-Jorge, T.; Garzoni, L.R. Vascular Growth Factor Inhibition with Bevacizumab Improves Cardiac Electrical Alterations and Fibrosis in Experimental Acute Chagas Disease. Biology 2023, 12, 1414. https://doi.org/10.3390/biology12111414
Nisimura LM, Ferreira RR, Coelho LL, de Oliveira GM, Gonzaga BM, Meuser-Batista M, Lannes-Vieira J, Araujo-Jorge T, Garzoni LR. Vascular Growth Factor Inhibition with Bevacizumab Improves Cardiac Electrical Alterations and Fibrosis in Experimental Acute Chagas Disease. Biology. 2023; 12(11):1414. https://doi.org/10.3390/biology12111414
Chicago/Turabian StyleNisimura, Lindice Mitie, Roberto Rodrigues Ferreira, Laura Lacerda Coelho, Gabriel Melo de Oliveira, Beatriz Matheus Gonzaga, Marcelo Meuser-Batista, Joseli Lannes-Vieira, Tania Araujo-Jorge, and Luciana Ribeiro Garzoni. 2023. "Vascular Growth Factor Inhibition with Bevacizumab Improves Cardiac Electrical Alterations and Fibrosis in Experimental Acute Chagas Disease" Biology 12, no. 11: 1414. https://doi.org/10.3390/biology12111414
APA StyleNisimura, L. M., Ferreira, R. R., Coelho, L. L., de Oliveira, G. M., Gonzaga, B. M., Meuser-Batista, M., Lannes-Vieira, J., Araujo-Jorge, T., & Garzoni, L. R. (2023). Vascular Growth Factor Inhibition with Bevacizumab Improves Cardiac Electrical Alterations and Fibrosis in Experimental Acute Chagas Disease. Biology, 12(11), 1414. https://doi.org/10.3390/biology12111414







