Genista tridentata Phytochemical Characterization and Biological Activities: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Methods for Identification of Studies
2.1.1. Electronic Searches
2.1.2. Searching Other Resources
2.2. Criteria for Considering Studies for This Review
2.2.1. Types of Studies
2.2.2. Inclusion and Exclusion Criteria
2.2.3. Outcomes
2.3. Data Collection and Analysis
2.3.1. Selection of Studies
2.3.2. Data Extraction and Management
2.4. Quality Assessment
3. Results
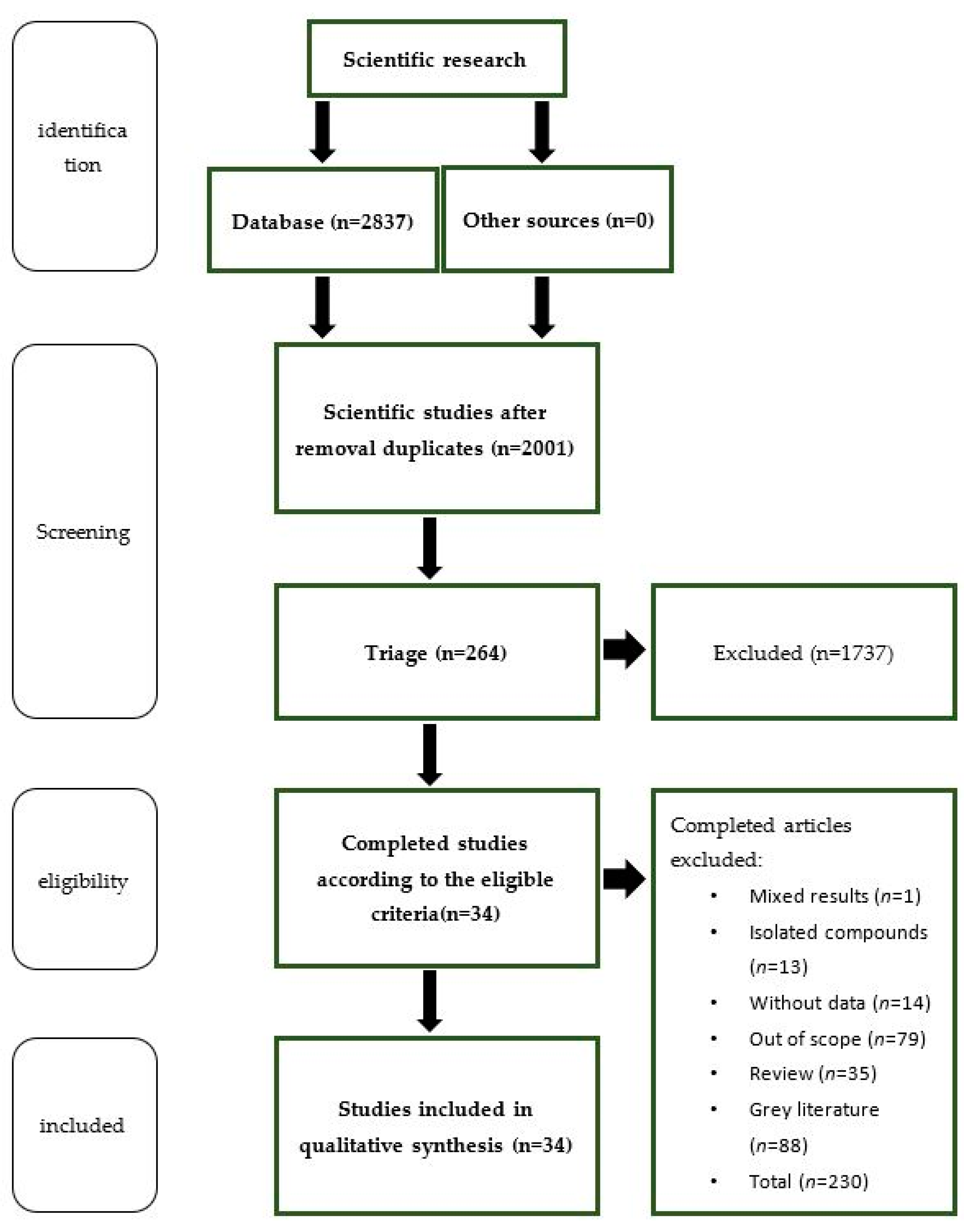
3.1. Literature Search
3.2. Extraction Procedures, Plant Parts Used, and Sampling
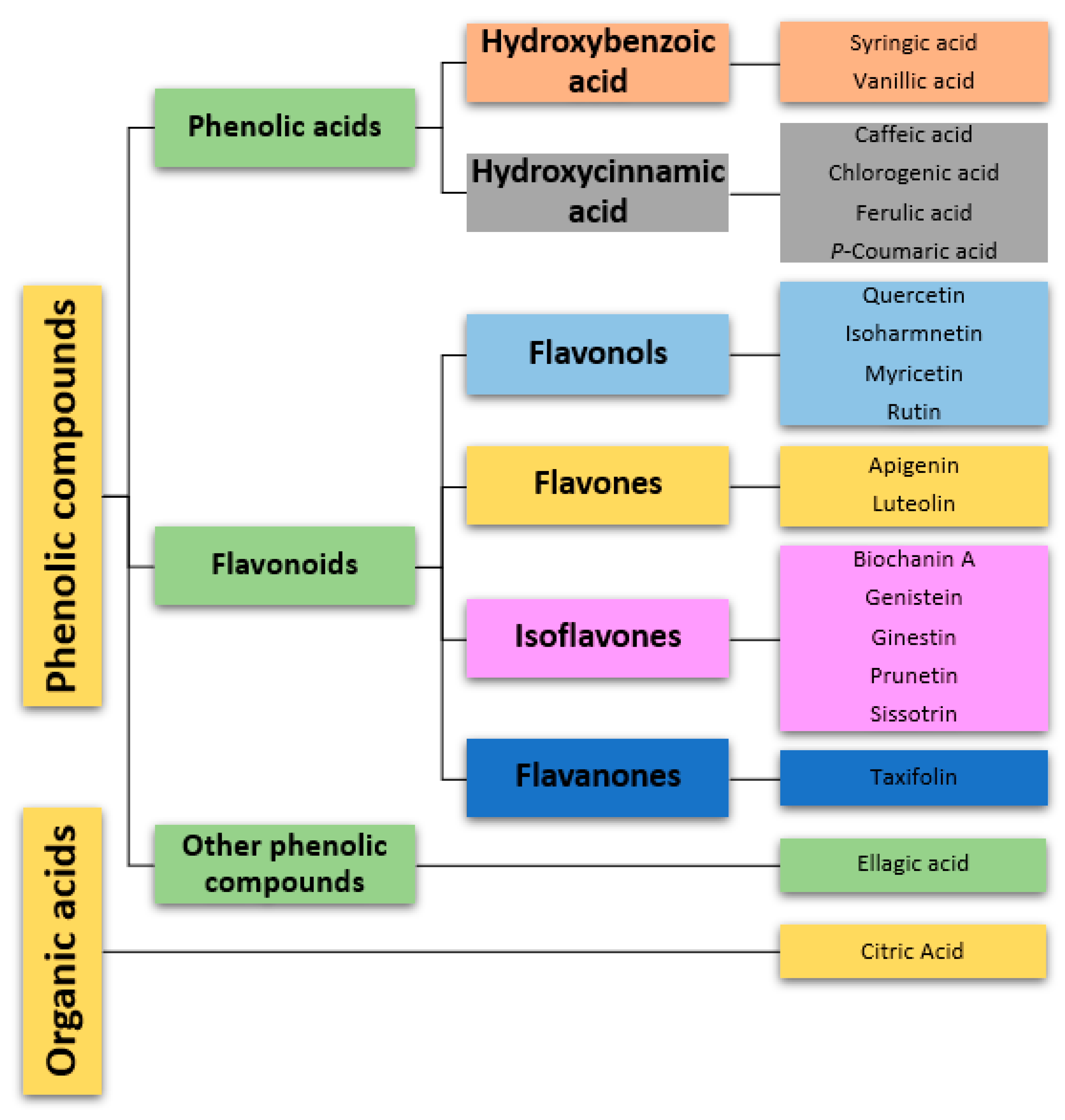
3.3. Phytochemical Characterization
3.4. Biological Activities
3.4.1. Antioxidant Activity
3.4.2. Anti-Inflammatory Activity
3.4.3. Antifungal and Antibacterial Activity
3.4.4. Cytotoxicity
3.4.5. Nematocidal/Nematotoxicity Activity
3.4.6. Other Activities
3.5. Quality Assessment
4. Discussion
4.1. Phytochemical Characterization
4.2. Biological Activities
4.2.1. Plant Bioactive Compounds as Antioxidants
4.2.2. Anti-Inflammatory Activity
4.2.3. Antifungal and Antibacterial Activity
4.2.4. Cytotoxicity
4.2.5. Nematocidal/Nematotoxicity Activity
4.2.6. Other Activities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinela, J.; Carvalho, A.M.; Ferreira, I.C. Wild edible plants: Nutritional and toxicological characteristics, retrieval strategies and importance for today’s society. Food Chem. Toxicol. 2017, 110, 165–188. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Donno, D.; Enri, S.R.; Lonati, M.; Scariot, V. Exploring wild edible flowers as a source of bioactive compounds: New perspectives in horticulture. Folia Hortic. 2021, 33, 27–48. [Google Scholar] [CrossRef]
- Pinto, D.C.; Simões, M.A.; Silva, A.M. Genista tridentata L.: A rich source of flavonoids with anti-inflammatory activity. Medicines 2020, 7, 31. [Google Scholar] [CrossRef]
- Simões, M.A.; Pinto, D.C.; Neves, B.M.; Silva, A.M. Flavonoid profile of the Genista tridentata L., a species used traditionally to treat inflammatory processes. Molecules 2020, 25, 812. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.T.; Gonçalves, J.C.; Alves, V.; Moldão-Martins, M. Antioxidant activity and phenolic content of extracts from different Pterospartum tridentatum populations growing in Portugal. Procedia Food Sci. 2011, 1, 1454–1458. [Google Scholar] [CrossRef]
- Ferreira, F.M.; Dinis, L.T.; Azedo, P.; Galhano, C.I.; Simões, A.; Cardoso, S.M.; Rosário, M.; Domingues, M.; Pereira, O.R.; Palmeira, C.M.; et al. Antioxidant capacity and toxicological evaluation of Pterospartum tridentatum flower extracts. CyTA-J. Food 2012, 10, 92–102. [Google Scholar] [CrossRef]
- GBIF—Global Biodiversity Information Facility. Available online: https://www.gbif.org/search?q=Pterospartum%20tridentatum (accessed on 10 October 2023).
- Plants of the World Online (POWO). Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:496422-1#synonyms (accessed on 10 October 2023).
- Vitor, R.F.; Mota-Filipe, H.; Teixeira, G.; Borges, C.; Rodrigues, A.I.; Teixeira, A.; Paulo, A. Flavonoids of an extract of Pterospartum tridentatum showing endothelial protection against oxidative injury. J. Ethnopharmacol. 2004, 93, 363–370. [Google Scholar] [CrossRef]
- Novais, M.H.; Santos, I.; Mendes, S.; Pinto-Gomes, C. Studies on pharmaceutical ethnobotany in Arrábida natural park (Portugal). J. Ethnopharmacol. 2004, 93, 183–195. [Google Scholar] [CrossRef]
- Grosso, A.C.; Costa, M.M.; Ganço, L.; Pereira, A.L.; Teixeira, G.; Lavado, J.M.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Essential oil composition of Pterospartum tridentatum grown in Portugal. Food Chem. 2007, 102, 1083–1088. [Google Scholar] [CrossRef]
- Pinela, J.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. Influence of the drying method in the antioxidant potential and chemical composition of four shrubby flowering plants from the tribe Genisteae (Fabaceae). Food Chem. Toxicol. 2011, 49, 2983–2989. [Google Scholar] [CrossRef]
- Gião, M.S.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Infusions of Portuguese medicinal plants: Dependence of final antioxidant capacity and phenol content on extraction features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Bremner, P.; Rivera, D.; Calzado, M.A.; Obón, C.; Inocencio, C.; Beckwith, C.; Fiebich, B.L.; Munoz, E.; Heinrich, M. Assessing medicinal plants from south-eastern Spain for potential anti-inflammatory effects targeting nuclear factor-Kappa B and other pro-inflammatory mediators. J. Ethnopharmacol. 2009, 124, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Oliveira, P.; Carreira-Casais, A.; Pereira, E.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Stojkovic, D.; Sokovic, M.; Simal-Gandara, J.; Prieto, M.A.; et al. From tradition to health: Chemical and bioactive characterization of five traditional plants. Molecules 2022, 27, 6495. [Google Scholar] [CrossRef]
- Gonçalves, S.; Gomes, D.; Costa, P.; Romano, A. The phenolic content and antioxidant activity of infusions from Mediterranean medicinal plants. Indust. Crops Prod. 2013, 43, 465–471. [Google Scholar] [CrossRef]
- Aires, A.; Marrinhas, E.; Carvalho, R.; Dias, C.; Saavedra, M.J. Phytochemical composition and antibacterial activity of hydroalcoholic extracts of Pterospartum tridentatum and Mentha pulegium against Staphylococcus aureus isolates. BioMed Res. Int. 2016, 2016, 5201879. [Google Scholar] [CrossRef]
- Balanč, B.; Kalušević, A.; Drvenica, I.; Coelho, M.T.; Djordjević, V.; Alves, V.D.; Sousa, I.; Moldão-Martins, M.; Rakic, V.; Nedovic, V.; et al. Calcium–alginate–inulin microbeads as carriers for aqueous carqueja extract. J. Food Sci. 2016, 81, E65–E75. [Google Scholar] [CrossRef] [PubMed]
- Gião, M.S.; González-Sanjosé, M.L.; Muñiz, P.; Rivero-Pérez, M.D.; Kosinska, M.; Pintado, M.E.; Malcata, F.X. Protection of deoxyribose and DNA from degradation by using aqueous extracts of several wild plants. J. Sci. Food Agric. 2008, 88, 633–640. [Google Scholar] [CrossRef]
- Serralheiro, M.L.M.; Falé, P.L.; Ferreira, C.; Rodrigues, A.M.; Cleto, P.; Madeira, P.J.A.; Florêncio, M.H.; Frazão, F.N.; Serralheiro, M.L.M. Antioxidant and anti-acetylcholinesterase activity of commercially available medicinal infusions after in vitro gastrointestinal digestion. J. Med. Plants Res. 2013, 7, 1370–1378. [Google Scholar] [CrossRef]
- Coelho, C.M.M.; de Mattos Bellato, C.; Santos, J.C.P.; Ortega, E.M.M.; Tsai, S.M. Effect of phytate and storage conditions on the development of the ‘hard-to-cook’ phenomenon in common beans. J. Sci. Food Agric. 2007, 87, 1237–1243. [Google Scholar] [CrossRef]
- Falé, P.L.; Ferreira, C.; Rodrigues, A.; Frazão, F.; Serralheiro, M. Studies on the molecular mechanism of cholesterol reduction by Fraxinus angustifolia, Peumus boldus, Cynara cardunculus and Pterospartum tridentatum infusions. J. Med. Plants Res. 2014, 8, 9–17. [Google Scholar] [CrossRef]
- Luis, A.; Domingues, F.; Duarte, A.P. Bioactive compounds, RP-HPLC analysis of phenolics, and antioxidant activity of some portuguese shrub species extracts. Nat. Product Commun. 2011, 6, 1863–1872. [Google Scholar] [CrossRef]
- Gonçalves, J.C.; Coelho, M.T.; da Graça Diogo, M.; Alves, V.D.; Bronze, M.R.; Coimbra, M.A.; Martins, V.M.; Moldão-Martins, M. In vitro shoot cultures of Pterospartum tridentatum as an alternative to wild plants as a source of bioactive compounds. Nat. Product Commun. 2018, 13, 439–442. [Google Scholar] [CrossRef]
- Selçuk, A.A. A guide for systematic reviews: PRISMA. Turkish Arch. Otorhinolaryngol. 2019, 57, 57–58. [Google Scholar] [CrossRef]
- Sousa, N.; Almeida, O.F.X.; Wotjak, C.T. A hitchhiker’s guide to behavioral analysis in laboratory rodents. Genes Brain Behav. 2006, 5, 5–24. [Google Scholar] [CrossRef]
- Paulo, A.; Martins, S.; Branco, P.; Dias, T.; Borges, C.; Rodrigues, A.I.; Costa, M.C.; Teixeira, A.; Mota-Filipe, H. The opposing effects of the flavonoids isoquercitrin and sissotrin, isolated from Pterospartum tridentatum, on oral glucose tolerance in rats. Phytother. Res. 2008, 22, 539–543. [Google Scholar] [CrossRef]
- Luis, A.; Domingues, F.; Gil, C.; Duarte, A.P. Antioxidant activity of extracts of portuguese shrubs: Pterospartum tridentatum, Cytisus scoparius and Erica spp. J. Med. Plants Res. 2009, 3, 886–893. [Google Scholar]
- Barbosa, P.; Lima, A.S.; Vieira, P.; Dias, L.S.; Tinoco, M.T.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C.; Mota, M. Nematicidal activity of essential oils and volatiles derived from portuguese aromatic flora against the pinewood nematode. J. Nematol. 2010, 42, 8–16. [Google Scholar]
- Gil, D.M.; Falé, P.L.; Serralheiro, M.L.; Rebelo, M.J. Herbal infusions bioelectrochemical polyphenolic index: Green tea–the gallic acid interference. Food Chem. 2011, 129, 1537–1543. [Google Scholar] [CrossRef]
- Faria, J.M.; Barbosa, P.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Bioactivity against Bursaphelenchus xylophilus: Nematotoxics from essential oils, essential oils fractions and decoction waters. Phytochemistry 2013, 94, 220–228. [Google Scholar] [CrossRef]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Scientific validation of synergistic antioxidant effects in commercialised mixtures of Cymbopogon citratus and Pterospartum tridentatum or Gomphrena globosa for infusions preparation. Food Chem. 2015, 185, 16–24. [Google Scholar] [CrossRef][Green Version]
- Faria, J.M.S.; Sena, I.; Ribeiro, B.; Rodrigues, A.M.; Maleita, C.M.N.; Abrantes, I.; Bennet, R.; Mota, M.; Figueiredo, A.C.D.S. First report on Meloidogyne chitwoodi hatching inhibition activity of essential oils and essential oils fractions. J. Pest Sci. 2016, 89, 207–217. [Google Scholar] [CrossRef]
- Caleja, C.; Finimundy, T.C.; Pereira, C.; Barros, L.; Calhelha, R.C.; Sokovic, M.; Ivanov, M.; Carvalho, A.M.; Rosa, E.; Ferreira, I.C. Challenges of traditional herbal teas: Plant infusions and their mixtures with bioactive properties. Food Funct. 2019, 10, 5939–5951. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Bento, C.; Nunes, A.R.; Simões, M.; Alves, G.; Silva, L.R. Multitarget protection of Pterospartum tridentatum phenolic-rich extracts against a wide range of free radical species, antidiabetic activity and effects on human colon carcinoma (Caco-2) cells. J. Food Sci. 2020, 85, 4377–4388. [Google Scholar] [CrossRef]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. HPLC-Profiles of tocopherols, sugars, and organic acids in three medicinal plants consumed as infusions. Int. J. Food Sci. 2014, 2014, 241481. [Google Scholar] [CrossRef]
- Martins, N.; Ferreira, I.C.; Barros, L.; Carvalho, A.M.; Henriques, M.; Silva, S. Plants used in folk medicine: The potential of their hydromethanolic extracts against Candida species. Ind. Crops Prod. 2015, 66, 62–67. [Google Scholar] [CrossRef]
- Abreu, A.C.; Paulet, D.; Coqueiro, A.; Malheiro, J.; Borges, A.; Saavedra, M.J.; Choi, Y.H.; Simões, M. Antibiotic adjuvants from Buxus sempervirens to promote effective treatment of drug-resistant Staphylococcus aureus biofilms. RSC Adv. 2016, 6, 95000–95009. [Google Scholar] [CrossRef]
- Gomes, F.; Martins, N.; Barros, L.; Rodrigues, M.E.; Oliveira, M.B.P.; Henriques, M.; Ferreira, I.C. Plant phenolic extracts as an effective strategy to control Staphylococcus aureus, the dairy industry pathogen. Ind. Crops Prod. 2018, 112, 515–520. [Google Scholar] [CrossRef]
- Mota, F.A.; Pereira, S.A.; Araújo, A.R.; Gullón, B.; Passos, M.L.; Saraiva, M.L.M. Automatic identification of myeloperoxidase natural inhibitors in plant extracts. Molecules 2022, 27, 1825. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Hernandez, M.P.; Karchesy, J.; Starkey, E.E. Research observation: Hydrolyzable and condensed tannins in plants of northwest Spain forests. Rangel. Ecol. Manag./J. Range Manag. Arch. 2003, 56, 461–465. [Google Scholar] [CrossRef][Green Version]
- Martinez, A.; Estévez, J.C.; Silva-Pando, F.J. Antioxidant activity, total phenolic content and skin care properties of 35 selected plants from Galicia (NW Spain). Front. Life Sci. 2012, 6, 77–86. [Google Scholar] [CrossRef]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Pterospartum tridentatum, Gomphrena globosa and Cymbopogon citratus: A phytochemical study focused on antioxidant compounds. Food Res. Int. 2014, 62, 684–693. [Google Scholar] [CrossRef]
- Takaishi, K.; Izumi, M.; Baba, N.; Kawazu, K.; Nakajima, S. Synthesis and biological evaluation of alkoxycoumarins as novel nematicidal constituents. Bioorganic Med. Chem. Lett. 2008, 18, 5614–5617. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef]
- Vicente, O.; Boscaiu, M. Flavonoids: Antioxidant compounds for plant defence and for a healthy human diet. Not. Bot. Hort. Agrobot. 2018, 46, 14–21. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- de Guzman, R.; Tang, H.; Salley, S.; Ng, K.Y. Synergistic effects of antioxidants on the oxidative stability of soybean oil-and poultry fat-based biodiesel. J. Am. Oil Chem. Soc. 2009, 86, 459–467. [Google Scholar] [CrossRef]
- Hajimehdipoor, H.; Shahrestani, R.; Shekarchi, M. Investigating the synergistic antioxidant effects of some flavonoid and phenolic compounds. Res. J. Pharmacogn. 2014, 1, 35–40. [Google Scholar]
- Pinto, D.C.G.A.; Silva, A.M.S. Valorisation of portuguese natural resources. Phytochem. Rev. 2021, 20, 249–258. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Ferrandiz, M.L.; Alcaraz, M. Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids. Agents Act 1991, 32, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Ding, M.; Zhai, B.; Xiao, L.; Piao, T.; Liu, M. Biochanin A inhibits lipopolysaccharide-induced inflammation in human umbilical vein endothelial cells. Life Sci. 2015, 136, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, W. Biochanin A inhibits lipopolysaccharide-induced inflammatory cytokines and mediators production in BV2 microglia. Neurochem. Res. 2015, 40, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tang, L.; Li, Y.; Wang, Y. Biochanin A protects against focal cerebral ischemia/reperfusion in rats via inhibition of p38-mediated inflammatory responses. J. Neurol. Sci. 2015, 348, 121–125. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Liu, X.; Cai, L.; Qi, J.; Zhang, P.; Li, Y. Biochanin A protects lipopolysaccharide/D-galactosamine-induced acute liver injury in mice by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Int. Immunopharmacol. 2016, 38, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Ham, I.; Choi, H.Y. Anti-inflammatory effect of prunetin via the suppression of NF-κB pathway. Food Chem. Toxicol. 2013, 58, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Piegholdt, S.; Pallauf, K.; Esatbeyoglu, T.; Speck, N.; Reiss, K.; Ruddigkeit, L.; Stocker, A.; Huebbe, P.; Rimbach, G. Biochanin A and prunetin improve epithelial barrier function in intestinal CaCo-2 cells via downregulation of ERK, NF-κB, and tyrosine phosphorylation. Free Radic. Biol. Med. 2014, 70, 255–264. [Google Scholar] [CrossRef]
- Du, Z.R.; Feng, X.Q.; Li, N.; Qu, J.X.; Feng, L.; Chen, L.; Chen, W.F. G protein-coupled estrogen receptor is involved in the anti-inflammatory effects of genistein in microglia. Phytomedicine 2018, 43, 11–20. [Google Scholar] [CrossRef]
- Wang, A.; Wei, J.; Lu, C.; Chen, H.; Zhong, X.; Lu, Y.; Li, L.; Huang, H.; Dai, Z.; Han, L. Genistein suppresses psoriasis-related inflammation through a STAT3–NF-κB-dependent mechanism in keratinocytes. Int. Immunopharmacol. 2019, 69, 270–278. [Google Scholar] [CrossRef]
- Khajevand-Khazaei, M.R.; Mohseni-Moghaddam, P.; Hosseini, M.; Gholami, L.; Baluchnejadmojarad, T.; Roghani, M. Rutin, a quercetin glycoside, alleviates acute endotoxemic kidney injury in C57BL/6 mice via suppression of inflammation and up-regulation of antioxidants and SIRT1. Eur. J. Pharm. 2018, 833, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, C.; Kandemir, F.M.; Yildirim, S.; Kucukler, S.; Eser, G. Rutin protects mercuric chloride-induced nephrotoxicity via targeting of aquaporin 1 level, oxidative stress, apoptosis and inflammation in rats. J. Trace Elem. Med. Biol. 2019, 54, 69–78. [Google Scholar] [CrossRef]
- Cai, C.; Liu, C.; Zhao, L.; Liu, H.; Li, W.; Guan, H.; Zhaou, L.; Xiao, J. Effects of Taxifolin on osteoclastogenesis in vitro and in vivo. Front. Pharmacol. 2018, 9, 1286. [Google Scholar] [CrossRef]
- Pan, S.; Zhao, X.; Ji, N.; Shao, C.; Fu, B.; Zhang, Z.; Wang, R.; Qiu, Y.; Jin, M.; Kong, D. Inhibitory effect of taxifolin on mast cell activation and mast cell-mediated allergic inflammatory response. Int. Immunopharmacol. 2019, 71, 205–214. [Google Scholar] [CrossRef]
- Herrera, C.L.; Alvear, M.; Barrientos, L.; Montenegro, G.; Salazar, L.A. The antifungal effect of six commercial extracts of Chilean propolis on Candida spp. Cienc. Investig. Agrar. 2010, 37, 75–84. [Google Scholar] [CrossRef]
- Yousefbeyk, F.; Gohari, A.R.; Hashemighahderijani, Z.; Ostad, S.N.; Salehi Sourmaghi, M.H.; Amini, M.; Golfakhrabadi, F.; Jamalifar, H.; Amin, G. Bioactive terpenoids and flavonoids from Daucus littoralis Smith subsp. hyrcanicus Rech.f, an endemic species of Iran. DARU J. Pharm. Sci. 2014, 22, 12. [Google Scholar] [CrossRef]
- Gao, M.; Wang, H.; Zhu, L. Quercetin assists fluconazole to inhibit biofilm formations of fluconazole-resistant Candida albicans in in vitro and in vivo antifungal managements of vulvovaginal candidiasis. Cell. Physiol. Biochem. 2016, 40, 727–742. [Google Scholar] [CrossRef]
- Yoon, T.M.; Kim, J.W.; Kim, J.G.; Kim, W.G.; Suh, J.W. Talosins A and B: New isoflavonol glycosides with potent antifungal activity from Kitasatospora kifunensis MJM341 I. Taxonomy, fermentation, isolation, and biological activities. J. Antibiot. 2006, 59, 633–639. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taveira, M.; Silva, L.R.; Vale-Silva, L.A.; Pinto, E.; Valentão, P.; Ferreres, F.; Guedes De Pinho, P.; Andrade, P.B. Lycopersicon esculentum seeds: An industrial byproduct as an antimicrobial agent. J. Agric. Food Chem. 2010, 58, 9529–9536. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zuo, G.Y.; Hao, X.Y.; Wang, G.C.; Li, Z.S. Antibacterial and synergy of a flavanonol rhamnoside with antibiotics against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA). Phytomedicine 2011, 18, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Asmi, K.S.; Lakshmi, T.; Balusamy, S.R.; Parameswari, R. Therapeutic aspects of taxifolin—An update. J. Adv. Pharm. Educ. Res. 2017, 7, 187–189. [Google Scholar]
- Ahamed, S.; Lakshmi, T. Antibacterial activity of Taxifolin isolated from Acacia catechu leaf extract—An in vitro study. Indian J. Public Health Res. Dev. 2018, 7, 133–137. [Google Scholar]
- Cushnie, T.P.T.; Lamb, A. J Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Mahboubi, M.; Haghi, G. Antimicrobial activity and chemical composition of Mentha pulegium L. essential oil. J. Ethnopharmacol. 2008, 119, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Morteza-Semnani, K.; Saeedi, M.; Akbarzadeh, M. Chemical composition and antimicrobial activity of the essential oil of Mentha pulegium L. J. Essent. Oil-Bear. Plants 2011, 14, 208–213. [Google Scholar] [CrossRef]
- Kuspradini, H.; Mitsunaga, T.; Ohashi, H. Antimicrobial activity against Streptococcus sobrinus and glucosyltransferase inhibitory activity of taxifolin and some flavanonol rhamnosides from kempas (Koompassia malaccensis) extracts. J. Wood Sci. 2009, 55, 308–313. [Google Scholar] [CrossRef]
- Thabrew, M.I.; Hughes, R.D.; McFarlane, I.G. Screening of hepatoprotective plant components using a HepG2 cell cytotoxicity assay. J. Pharm. Pharmacol. 2011, 49, 1132–1135. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Nguyen, T.H.N.; Tran, T.N.M.; Vu, N.B.D.; Tran, T.T. Comparison of the nematode-controlling effectiveness of 10 different essential oil-encapsulated lipid nanoemulsions. Arch. Phytopathol. Plant Prot. 2022, 55, 420–432. [Google Scholar] [CrossRef]
- Ismail, M.; Fayyaz, S.; Kowsar, A.; Javed, S.; Ali, I.; Ali, S.; Ali, S.; Hussain, F.; Ali, H. Evaluation of nematocidal effects of some medicinal plant extracts against root-knot nematodes (Meloidogyne incognita). Ital. J. Agron. 2020, 15, 63–69. [Google Scholar] [CrossRef]
- Oka, Y.; Nacar, S.; Putievsky, E.; Ravid, U.; Yaniv, Z.; Spiegel, Y. Nematicidal activity of essential oils and their components against the root-knot nematode. Phytopathology 2000, 90, 710–715. [Google Scholar] [CrossRef]
- Kostyukovsky, M.; Chen, B.; Atsmi, S.; Shaaya, E. Biological activity of two juvenoids and two ecdysteroids against three stored product insects. Insect Biochem. Mol. Biol. 2000, 30, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Priestley, C.M.; Williamson, E.M.; Wafford, K.A.; Sattelle, D.B. Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABAA receptors and a homo-oligomeric GABA receptor from Drosophila melanogaster. Br. J. Pharmacol. 2003, 140, 1363–1372. [Google Scholar] [CrossRef]
- Lei, J.; Leser, M.; Enan, E. Nematicidal activity of two monoterpenoids and SER-2 tyramine receptor of Caenorhabditis elegans. Biochem. Pharmacol. 2010, 79, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H. Biphasic membrane effects of capsaicin, an active component in Capsicum species. J. Ethnopharmacol. 2001, 75, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Rana, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Citronellol disrupts membrane integrity by inducing free radical generation. Z. Naturforschung C 2011, 66, 260–266. [Google Scholar] [CrossRef]
- Isman, M.B.; Miresmailli, S.; Machial, C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem. Rev. 2011, 10, 197–204. [Google Scholar] [CrossRef]
- Holzer, P.; Maggi, C.A. Synergistic role of muscarinic acetylcholine and tachykinin NK-2 receptors in intestinal peristalsis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1994, 349, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Nakajo, K.; Egashira, Y.; Yamamoto, Y.; Kitada, K.; Taniguchi, K.; Kawai, M.; Tomiyama, H.; Kawakami, K.; Uchiyama, K.; et al. Gastrointestinal neurons expressing HCN4 regulate retrograde peristalsis. Cell Rep. 2020, 30, 2879–2888. [Google Scholar] [CrossRef]
- Hirota, C.L.; McKay, D.M. Cholinergic regulation of epithelial ion transport in the mammalian intestine. Br. J. Pharmacol. 2006, 149, 463–479. [Google Scholar] [CrossRef]
- Falé, P.L.; Ascensão, L.; Serralheiro, M.L.; Haris, P.I. Interaction between Plectranthus barbatus herbal tea components and acetylcholinesterase: Binding and activity studies. Food Funct. 2012, 3, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Hasumi, K. Biochemical aspect of HMG CoA reductase inhibitors. Adv. Enzyme Regul. 1989, 28, 53–64. [Google Scholar] [CrossRef]
- Sato, R. Sterol metabolism and SREBP activation. Arch. Biochem. Biophys. 2010, 501, 177–181. [Google Scholar] [CrossRef]
- Sadri, H.; Goodarzi, M.T.; Salemi, Z.; Seifi, M. Antioxidant effects of Biochanin A in streptozotocin induced diabetic rats. Braz. Arch. Biol. Technol. 2017, 60. [Google Scholar] [CrossRef]
| Authors | [17] | [23] | [9] | [15] | [20] | [24] | [27] | [32] | [35] | [24] | [6] | [22] | [15] | [35] | [43] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extraction method | Methanolic | Aqueous | Ethanolic | NA | ||||||||||||||||||
| Part plant used | Crude | flowers | Stems and leaves | Flowers | Aerial parts | In vitro culture | Leaves + Flowers | NA | Flowers | Flowers | ||||||||||||
| Sampling localization | Vila Real | Serra da Estrela | Serra da Estrela | Cinfães | Montesinho | Herbal Shop—DIÉTICA ® | Malcata | Gardunha | Cinfães | Herbal Shop—Ervital | Viseu | Malcata | Gardunha | Malcata | Gardunha | Herbal shop—Ervital | Herbal Shop—DIÉTICA ® | Montesinho | Viseu | Herbal shop—Ervital | ||
| Sampling period | NA | Spring | NA | May | May | NA | Spring 2012 | NA | February | May | February | May | NA | Spring 2019 | NA | Spring 2012 | ||||||
| 2019 | ||||||||||||||||||||||
| 5,5′-Dihydroxy-3′-methoxy-isoflavone-7-O-β-glucoside | ND | ND | ND | X | ND | ND | X | X | ND | X | ND | X | X | X | X | X | X | ND | ND | ND | ND | X |
| 5,5′-Dihydroxi-3′-methoxyisoflavone | ND | ND | ND | ND | X | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND |
| 7-Methylorobol | ND | ND | ND | ND | ND | ND | X | ND | X | X | ND | ND | ND | X | X | X | ND | ND | ND | ND | ND | X |
| Apigenin 5,7-dimethyl | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Apigenin 5,7-dimethyl ether 4′galactoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND |
| Biochanin A | X | ND | ND | ND | ND | X | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | X |
| Biochanin A O-acetylhexoside-O-hexoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X |
| Biochanin A O-hexoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X |
| Biochanin A O-hexoside-O-hexoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X |
| Biochanin A-glucoside | X | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND |
| Caffeic acid | ND | X | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Chlorogenic acid | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Citric acid | ND | ND | ND | ND | ND | ND | X | X | ND | ND | ND | ND | X | X | X | X | X | ND | ND | ND | ND | ND |
| Dihydroquercetin 6-C-hesoxide | ND | ND | ND | ND | X | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | X |
| Ellagic acid | ND | X | X | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND |
| Ferulic acid | ND | X | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Genistein-8-C-glucoside | ND | ND | ND | ND | ND | X | X | X | ND | X | ND | X | X | X | X | X | X | ND | X | ND | ND | X |
| Ginestein | X | ND | ND | ND | ND | ND | X | X | X | X | X | X | ND | ND | X | X | ND | ND | ND | ND | X | X |
| Ginestein derivatives | X | ND | ND | ND | X | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | X | X | ND |
| Ginestin | X | ND | ND | X | ND | ND | ND | ND | X | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X |
| Isoquercitrin | ND | ND | ND | X | ND | X | X | X | X | X | X | ND | X | ND | X | X | X | ND | X | ND | X | X |
| Isorhamnetin-O-hexoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND |
| Luteolin-O-(O-acetyl)-glucuronide | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND |
| Luteolin-O-glucuronide | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND |
| Methylbiochanin A/methylprunetin | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X |
| Methylbiochanin A/methylprunetin derivative | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X |
| Methylbiochanin A/methylprunetin O-hexoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X |
| Myricetin-6-C-glucoside | ND | ND | ND | ND | X | X | X | X | X | X | ND | X | X | X | X | X | X | ND | X | X | - | X |
| p-Coumaric acid | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Pentahydroxy-flavonol-di-O-glucoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND |
| Prunetin | ND | ND | ND | X | ND | ND | X | ND | X | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | X |
| Quercetin | ND | X | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Quercetin 3-O-galactoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | X | ND | ND | ND | ND | ND | ND | ND |
| Quercetin deoxyhexosyl-hexoside | ND | ND | ND | ND | X | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | X |
| Quercetin O-hexoside | ND | ND | ND | ND | X | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | X |
| Quercetin-3-O-rutinoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | X |
| Quercetin derivates | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | X | X | ND |
| Quinic acid | ND | ND | ND | ND | ND | ND | X | X | ND | ND | ND | X | X | X | X | X | X | ND | ND | ND | ND | ND |
| Rosmarinic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND |
| Rutin | ND | ND | ND | ND | ND | ND | X | X | X | ND | ND | X | X | X | X | X | X | ND | ND | ND | ND | ND |
| Sissotrin | ND | ND | ND | X | ND | ND | X | X | X | X | ND | ND | ND | X | X | ND | ND | ND | ND | ND | ND | X |
| Syringic acid | ND | X | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Taxifolin | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Taxifolin-6-C-glucoside | ND | ND | ND | ND | ND | X | X | X | ND | ND | ND | X | X | X | X | ND | ND | ND | X | ND | ND | ND |
| Vanillic acid | ND | X | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Components | Flowers | Leaves + Stems | Aerial Parts | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | [11] | [29] | [11] | ||||||||
| RI | AMF02 | AMF03 | Herbal Shop | AML02 | AML03 | PAPN | PFSPNa | PFSPNb | SCB | MCSB | |
| trans-2-Hexenal | 866 | 1.6 | 0.5 | 0.1 | ND | 1.6 | ND | ND | 1.7 | 3.2 | ND |
| cis-3-Hexen-1-ol | 868 | 1.6 | 1.2 | ND | ND | 5.3 | ND | ND | 0.8 | 3 | ND |
| cis-2-Hexen-1-ol | 882 | 1.5 | 1.2 | ND | ND | 0.8 | ND | ND | 0.6 | 1.2 | ND |
| n-Hexanol | 882 | 0.5 | 1.6 | ND | ND | 1.1 | ND | ND | 1.1 | 0.7 | ND |
| n-Heptanal | 897 | 11.8 | 4.8 | 0.9 | ND | 0.5 | 0.8 | ND | ND | 0.3 | ND |
| n-Nonane | 900 | ND | ND | ND | ND | 0.2 | ND | ND | 2.3 | 0.2 | ND |
| Benzaldehyde | 927 | 0.5 | 0.8 | 0.3 | ND | 0.6 | 1 | ND | 0.6 | 0.1 | ND |
| α-Pinene | 930 | ND | 0.3 | 0.3 | ND | 0.8 | ND | ND | 0.5 | 0.1 | ND |
| n-Heptanol | 952 | 0.5 | 1.6 | ND | ND | 1.5 | ND | ND | ND | ND | 1.3 |
| 1-Octen-3-ol | 961 | 10.7 | 21 | 9.2 | 11.5 | 22.6 | 1.7 | 29.7 | 15 | 25.8 | 36.8 |
| 2-Pentyl furan | 972 | 2.4 | 1.3 | 0.8 | 2.5 | 0.5 | ND | ND | 0.7 | 2.1 | 1.4 |
| n-octanal | 973 | ND | ND | 0.6 | ND | ND | ND | ND | ND | ND | ND |
| 3-Octanol | 974 | 1.4 | 1.5 | ND | 1.9 | ND | ND | ND | 1.9 | 0.3 | 1.5 |
| Benzyl alcohol | 996 | ND | ND | ND | 0.3 | 0.4 | ND | ND | ND | 0.3 | ND |
| Benzene acetaldehyde | 1002 | 1.8 | 1.8 | ND | 0.3 | 1.2 | ND | ND | 0.4 | 1.4 | 0.6 |
| ρ-Cymene | 1003 | ND | ND | 0.3 | ND | ND | ND | ND | ND | ND | ND |
| 1,8-Cineole | 1005 | 0.9 | 1 | 0.7 | 1.1 | 0.2 | ND | ND | ND | ND | ND |
| Limonene | 1009 | 0.9 | 1 | ND | 1.1 | 0.2 | ND | ND | 0.3 | ND | ND |
| Acetophenone | 1017 | ND | 1.4 | ND | 2.1 | 0.5 | ND | ND | ND | ND | ND |
| n-Octanol | 1045 | 0.5 | 0.4 | 0.7 | 2.1 | 0.3 | 0.6 | ND | ND | ND | ND |
| ρ-Cymenene | 1050 | ND | ND | 0.6 | ND | ND | ND | ND | ND | ND | ND |
| Heptanoic acid | 1056 | 0.5 | 1.2 | ND | ND | 0.4 | ND | ND | ND | ND | 2.1 |
| Phenyl ethyl alcohol | 1064 | 0.7 | 1.2 | ND | 2 | 1.7 | ND | 3.6 | 3.3 | 3.4 | 6.3 |
| n-Nonanal | 1073 | 14.5 | 6.1 | 6.5 | 4.6 | 0.9 | 10.5 | 4.1 | 0.2 | 0.9 | 1 |
| Linalol | 1074 | 2.9 | 0.5 | 7.1 | ND | 2 | ND | 5.2 | ND | 2.3 | 1 |
| cis-Rose oxide | 1083 | 2.9 | 0.5 | ND | ND | ND | 2 | ND | 5.2 | 2.3 | 1 |
| Camphor | 1095 | ND | ND | 0.7 | ND | ND | ND | ND | ND | ND | ND |
| n-Undecane | 1100 | ND | ND | ND | ND | ND | ND | 1 | 2.3 | 0.2 | ND |
| trans-Rose oxide | 1100 | ND | ND | ND | 2.1 | 0.7 | ND | 1 | ND | ND | ND |
| trans-Pinocarveol | 1106 | ND | ND | 0.3 | ND | ND | ND | ND | ND | 0.2 | ND |
| 2- trans,6 cis-Nonadienal | 1106 | 2.1 | 0.3 | 0.2 | ND | ND | ND | ND | ND | ND | ND |
| 2- trans-Nonen-1-al | 1114 | 0.5 | 0.4 | ND | 2.2 | 0.2 | ND | ND | ND | ND | ND |
| Pentyl benzene | 1119 | 1.5 | ND | ND | ND | 0.3 | ND | ND | ND | ND | ND |
| Menthone | 1120 | ND | ND | 0.2 | ND | ND | ND | ND | ND | ND | ND |
| Benzyl acetate | 1123 | ND | ND | 0.2 | ND | ND | ND | ND | ND | ND | ND |
| Borneol | 1134 | ND | ND | 1.1 | ND | ND | ND | ND | ND | ND | ND |
| Lavandulol | 1142 | ND | ND | 0.3 | ND | ND | ND | ND | ND | ND | ND |
| Menthol | 1148 | ND | ND | 0.5 | ND | ND | ND | ND | ND | ND | ND |
| Terpinen-4-ol | 1148 | ND | ND | 0.7 | ND | ND | ND | ND | ND | ND | ND |
| Octanoic acid | 1156 | 0.3 | ND | 0.5 | 0.5 | ND | ND | ND | ND | ND | ND |
| α-Terpineol | 1159 | ND | ND | 1.8 | ND | ND | ND | 1.2 | 0.8 | 0.3 | ND |
| Safranal | 1160 | 1.4 | 0.3 | ND | ND | 0.5 | ND | ND | ND | ND | ND |
| Methyl chavicol (=estragole) | 1163 | ND | ND | 0.9 | ND | ND | ND | ND | ND | ND | ND |
| n-Decanal | 1180 | ND | 0.3 | 0.4 | ND | ND | ND | ND | ND | ND | ND |
| Pulegone | 1210 | ND | ND | 1.4 | ND | ND | ND | ND | ND | ND | ND |
| Geraniol | 1236 | 0.3 | 1.6 | 0.6 | 4 | 9.2 | 3.2 | 1 | - | 1.4 | 2.8 |
| Linalyl acetate | 1245 | ND | ND | 1.4 | ND | ND | ND | ND | ND | ND | ND |
| Trans-Anethole | 1254 | ND | ND | 4.7 | ND | ND | ND | ND | ND | ND | ND |
| n-Decanol | 1259 | 0.3 | 1.6 | 0.6 | 4 | 0.2 | 3.2 | 3.4 | 2.5 | 3.2 | 1.9 |
| 2-Undecanone | 1273 | ND | ND | 2.2 | ND | ND | ND | ND | ND | ND | ND |
| Perilla alcohol | 1274 | ND | ND | ND | ND | 3.4 | ND | ND | ND | 0.6 | ND |
| Nonanoic acid | 1274 | ND | 0.3 | 1.5 | 2.3 | ND | ND | ND | ND | ND | ND |
| cis-Theaspirane | 1279 | 1.6 | 2.2 | ND | 12.7 | 7.1 | 14.2 | 5.3 | 13.2 | 9 | 6.2 |
| 2 trans,4 trans-Decadienal | 1285 | 0.8 | 1.3 | ND | ND | 0.1 | ND | 1.8 | ND | 2 | ND |
| cis-Transpirane | 1286 | ND | ND | 3.2 | ND | ND | ND | ND | ND | ND | ND |
| Carvacrol | 1286 | ND | ND | 0.3 | ND | ND | ND | ND | ND | ND | ND |
| 2-trans-4-trans-Decadienal | 1286 | ND | ND | 1 | ND | ND | ND | ND | ND | ND | ND |
| trans-Theaspirane | 1300 | 2.4 | 1.9 | 3.9 | 12.1 | 6.8 | 17.2 | 6.3 | 13.6 | 10 | 5.5 |
| Hexyl tiglate ester | 1316 | ND | ND | 0.2 | ND | ND | ND | ND | ND | ND | ND |
| Eugenol | 1327 | 1.4 | 1.7 | 0.8 | 3.5 | 2.6 | ND | 3.1 | 3 | 3.2 | 3.6 |
| α-Terpenyl acetate | 1334 | ND | ND | 0.3 | ND | ND | ND | ND | ND | ND | ND |
| α-Longipinene | 1338 | ND | ND | 0.1 | ND | ND | ND | ND | ND | ND | ND |
| Decanoic acid | 1350 | ND | ND | 0.8 | ND | ND | ND | ND | ND | ND | ND |
| trans-β-Dasmascenone | 1356 | ND | ND | 0.8 | ND | ND | ND | ND | ND | ND | ND |
| Geranyl acetate | 1370 | ND | ND | 0.5 | ND | ND | ND | ND | ND | ND | ND |
| α-Copaene | 1375 | ND | ND | ND | ND | ND | ND | 0.9 | ND | ND | ND |
| β-Bourbonene | 1379 | ND | ND | ND | ND | ND | ND | 1.5 | ND | 1.1 | ND |
| 2-Pentadecanone | 1390 | ND | ND | 0.8 | ND | ND | ND | ND | ND | ND | ND |
| Longifolene | 1399 | ND | ND | ND | ND | ND | ND | 1.4 | ND | ND | ND |
| β-Caryophyllene | 1414 | ND | 0.4 | 1.2 | ND | ND | ND | 2.7 | ND | 2 | 0.9 |
| Geranyl acetonea | 1434 | ND | 3.6 | 0.7 | ND | ND | ND | 1.2 | ND | 0.6 | ND |
| allo-Aromadendrene | 1456 | ND | ND | 0.7 | ND | ND | ND | ND | ND | ND | ND |
| trans-β-Ionone | 1456 | ND | ND | 1.1 | ND | ND | ND | ND | ND | ND | ND |
| Germacrene-D | 1474 | ND | 0.2 | ND | ND | ND | 9.7 | 3.3 | ND | 0.7 | ND |
| α-Curcumene | 1475 | ND | ND | 0.5 | ND | ND | ND | ND | ND | ND | ND |
| ƴ-Cadinene | 1500 | ND | 3.3 | ND | ND | ND | ND | 1.2 | ND | 1.1 | 1.9 |
| σ-Cadinene | 1505 | ND | 2.4 | ND | ND | ND | ND | 1.6 | ND | 2 | 1.9 |
| Dodecanoic acid | 1551 | 3.5 | 2.1 | 5.3 | 2.6 | 0.3 | 15 | ND | ND | 0.9 | 1.1 |
| β-Caryophyllene oxide | 1561 | ND | ND | ND | ND | ND | ND | 1.3 | ND | 1.2 | 2.9 |
| n-Tetradecanal | 1596 | ND | ND | ND | ND | ND | ND | 1.1 | ND | 2.7 | 1.5 |
| n-Pentadecanal; | 1688 | ND | ND | ND | ND | ND | ND | ND | ND | 0.8 | ND |
| Tetradecanoic acid | 1734 | ND | ND | 0.2 | ND | ND | ND | ND | ND | ND | ND |
| Hexadecanoic acid | 1779 | ND | ND | 0.7 | ND | ND | ND | ND | ND | ND | ND |
| 9,12-Octadecadienoic acid | 1820 | ND | ND | 0.4 | ND | ND | ND | ND | ND | ND | ND |
| % of identified components | 71.8 | 75.1 | 71.8 | 78.4 | 76.8 | 77.1 | 82.9 | 64.8 | 88.5 | 82.2 | |
| Grouped components | |||||||||||
| Monoterpene hydrocarbons | 0.9 | 1.3 | 0.6 | 1.1 | 1 | ND | ND | 0.8 | 0.1 | ND | |
| Oxygen-containing monoterpenes | 6.2 | 7 | 18.6 | 10.6 | 17.5 | 3.2 | 9.6 | 0.8 | 5.4 | 3.8 | |
| Sesquiterpene hydrocarbons | ND | 6.3 | 2.5 | ND | ND | 9.7 | 12.6 | ND | 6.9 | 4.7 | |
| Oxygen-containing sesquiterpenes | ND | ND | 7.1 | ND | ND | ND | 1.3 | ND | 1.2 | 2.9 | |
| Phenylpropanoids | 1.4 | 1.7 | 6.4 | 3.5 | 2.6 | ND | 3.1 | 3 | 3.2 | 3.6 | |
| Oil yield (v/w) | <0.05% | <0.05% | 0.01% | <0.05% | <0.05% | <0.05% | <0.05% | <0.05% | <0.05% | <0.05% | |
| Authors | Extraction | Part Plant Used | Localization | Sampling Period | DPPH IC50 | Lipidic Peroxidation (TBARS) | Total Phenol Content (mg GAEg−1 dw) | Total Flavonoids Content (QE mg/g dw) |
|---|---|---|---|---|---|---|---|---|
| [17] | Methanolic | Crude | Vila Real. Pt | NA | NA | NA | 3.664 ± 0.04 mg g−1 dw | NA |
| [12] | Flowers | Trás-os-Montes. Pt | Spring 2010 (Freeze-drying) | NA | 0.12 ± 0.02 mg/mL | 523.42 ± 36.09 mg ClAE/g ext | 58.12 ± 5.78 | |
| Trás-os-Montes. Pt | Spring 2010 (Shade-drying) | NA | 0.13 ± 0.04 mg/mL | 519.81 ± 40.24 mg ClAE/g ext | 85.58 ± 5.60 | |||
| [23] | Serra da Estrela. Pt | NA | 26.1 ± 1.3 mg/L | NR | 171.4 ± 0.7 | NR | ||
| [36] | Herbal Shop—Ervital | Spring 2012 | NR | 1.18 ± 0.06 mg/mL | NR | NR | ||
| [23] | Stems and leaves | Serra da Estrela. Pt | NA | 69.7 ± 11.9 mg/L | NR | 113.6 ± 1.5 | NR | |
| [13] | Aqueous | Crude | Herbal Shop—Ervital | NA | NR | NR | 0.308 ± 0.004 (g L−1 GAE) | NR |
| [5] | Flowers | Orvalho Mountain. Pt | May | 3.6 ± 0.03 mMT/Kg dw | NR | 402.9 ± 17.07 | NR | |
| Gardunha Mountain. Pt | 3.2 ± 0.14 mMT/Kg dw | NR | 337.7 ± 50.83 | NR | ||||
| Malcata Mountain. Pt | 3.5 ± 0.03 mMT/Kg dw | NR | 309.5 ± 19.82 | NR | ||||
| [15] | Montesinho. Pt | Spring 2019 | NR | IC50 (μg/mL): 5.3 ± 0.1 | NR | NR | ||
| [20] | Herbal Shop—Dietética | NA | IC50 (μg/mL): 18.6 ± 0.7 | NR | NR | NR | ||
| [34] | Herbal Shop—Ervital | NA | NR | 8.4 ± 0.2 μg/mL | 107 ± 2 (mg/g) | 107 ± 2 (mg/g) | ||
| [35] | Viseu. Pt | NA | IC50 (μg/mL): 158 ± 1.45 | IC50 (μg/mL): 83.48 ± 6.17 | 34.80 mg/g of dried extract | NR | ||
| [16] | Aerial Parts | Algarve. Pt | Spring 2012 (Cold) | NR | NR | 314.89 ± 47.49 (μmol GAE gdw−1) | NR | |
| Spring 2012 (Hot) | NR | NR | 529.35 ± 3.01 (μmol GAE gdw−1) | NR | ||||
| [5] | Stems (dormancy period) | Orvalho Mountain. Pt | January | 3.6 ± 0.07 mMT/Kg dw | NR | 331.7 ± 35.05 | NR | |
| Gardunha Mountain. Pt | 3.2 ± 0.07 mMT/Kg dw | NR | 394 ± 74.5 | NR | ||||
| Malcata Mountain. Pt | 3.5 ± 0.08 mMT/Kg dw | NR | 320 ± 70.23 | NR | ||||
| Stems (flowering period) | Orvalho mountain. Pt | May | 3.6 ± 0.01 mMT/Kg dw | NR | 335.9 ± 34.59 | NR | ||
| Gardunha Mountain. Pt | 3.2 ± 0.07 mMT/Kg dw | NR | 270.7 ± 70.8 | NR | ||||
| Malcata Mountain. Pt | 3.6 ± 0.05 mMT/Kg dw | NR | 315.8 ± 73.5 | NR | ||||
| [13] | Leaves | Herbal Shop—Ervital | NA | NR | NR | 0.130 ± 0.026 (g L−1 GAE) | NR | |
| [23] | NA | Serra da Estrela. Pt | NA | NR | NR | 222.69 ± 5.12 | NR | |
| [28] | Serra da Estrela. Pt | NA | 42.97 ± 1.69 (IC50 mg/L) | NR | - | NR | ||
| [30] | Herbal Shop—Ervital | NA | NR | NR | 44.1 ± 0.6 (CAE. mg g−1 dw) | NR | ||
| [32] | Herbal Shop—Ervital | Spring 2012 | NR | NR | NR | 33.40 ± 0.28 mg/g | ||
| [15] | Ethanolic | Flowers | Montesinho. Pt | Spring 2019 | NR | IC50 (μg/mL): 3.19 ± 0.02 | NR | NR |
| [35] | Viseu. Pt | NA | IC50 (μg/mL): 115 ± 0.70 | IC50 (μg/mL): 113 ± 15.53 | 42.84 mg/g of dried extract | NR | ||
| [6] | Herbal Shop—Ervital | NA | NA | NR | NR | 15.5 ± 16.5 | NR | |
| [28] | NA | Serra da Estrela. Pt | NA | 60.39 ± 1.78 (IC50 mg/L) | NR | 196.61 ± 3.94 | NR |
| Authors | Extraction | Part Plant Used | Species | Strains | Method | Results |
|---|---|---|---|---|---|---|
| [37] | Methanolic | Flowers | Candida albicans | ATCC90028 | Disc diffusion assay | IZ: 10 mm |
| 575541 | - | |||||
| 557834 | IZ: 10 mm | |||||
| 558234 | IZ: 9 mm | |||||
| Candida galabrata | ATCC2001 | IZ: 11 mm | ||||
| D1 | IZ: 11 mm | |||||
| 513100 | IZ: 9 mm | |||||
| Candida parapsilosis | ATCC22019 | ++ | ||||
| AM2 | ++ | |||||
| AD | - | |||||
| 491861 | - | |||||
| 513143 | - | |||||
| Candida tropicalis | ATCC750 | + | ||||
| AG1 | +++ | |||||
| 75 | - | |||||
| 12 | - | |||||
| 544123 | - | |||||
| 519468 | - | |||||
| T2.2 | - | |||||
| [34] | Aqueous | Flowers | Aspergillus niger | ATCC 6275 | Microdilution method | MIC: 8 mg/mL |
| Aspergillus versicolor | ATCC 11730 | MIC: 0.5 mg/mL | ||||
| Penicillium funiculosum | ATCC 36839 | MIC: 0.5 mg/mL | ||||
| Penicillium verrucosum | Food isolates | MIC: 0.5 mg/mL | ||||
| [15] | Aspergillus niger | ATCC 6275 | MIC: 0.5 mg/mL | |||
| Aspergillus versicolor | ATCC 11730 | MIC: 0.5 mg/mL | ||||
| Aspergillus fumigatus | Human isolate | MIC: 1 mg/mL | ||||
| Penicillium funiculosum | ATCC 26839 | MIC: 0.5 mg/mL | ||||
| Penicillium aurantiogriseum | ATCC 58604 | MIC: 0.5 mg/mL | ||||
| Ethanolic | Flowers | Aspergillus niger | ATCC 6275 | MIC: 0.25 mg/mL | ||
| Aspergillus versicolor | ATCC 11730 | MIC: 0.25 mg/mL | ||||
| Aspergillus fumigatus | Human isolate | MIC: 0.25 mg/mL | ||||
| Penicillium funiculosum | ATCC 26839 | MIC: 0.25 mg/mL | ||||
| Penicillium aurantiogriseum | ATCC 58604 | MIC: 0.5 mg/mL | ||||
| [17] | Methanolic | Crude | Staphylococcus aureus | ATCC 13565 | Microdilution method | MIC: 312.5 µg.mL−1 (moderate) |
| MJMC021 | MIC: 78.1 µg.mL−1 (strong) | |||||
| MJMC024 | MIC: 78.1 µg.mL−1 (strong) | |||||
| MJMC026 | MIC: 78.1 µg.mL−1 (strong) | |||||
| MJMC025 | MIC: 39.1 µg.mL−1 (strong) | |||||
| MJMC027 | MIC: 39.1 µg.mL−1 (strong) | |||||
| MJMC029 | MIC: 39.1 µg.mL−1 (strong) | |||||
| [38] | S. aureus CECT 97 | Disc diffusion test | MIC < 4 g/L−1 (indifferent) | |||
| [39] | Flowers | ATCC 25923 | Disc diffusion test | Inhibitory zone: 5 mm | ||
| [15] | Aqueous | Flowers | ATCC 6538 | Microdilution method | MIC: 0.25 mg/mL | |
| [34] | Escherichia coli | NR | Microdilution method | MIC: 0.5 mg/mL | ||
| Salmonela ryphimurium | NR | MIC: 1 mg/mL | ||||
| Bacillus cereus | NR | MIC: 1 mg/mL | ||||
| Listeria monocytogenes | NR | MIC: 1 mg/mL | ||||
| [15] | Micrococcus flavus | ATCC 10240 | MIC: 2 mg/mL | |||
| Enterobacter cloacae | ATCC 35030 | MIC: 1 mg/mL | ||||
| Bacillus cereus | Clinical isolate | MIC: 1 mg/mL | ||||
| Listeria monocytogenes | NCTC 7973 | MIC: 1 mg/mL | ||||
| Salmonella typhimurium | ATCC 13311 | MIC: 1 mg/mL | ||||
| Ethanolic | Staphylococcus aureus | ATCC 6538 | MIC: 0.25 mg/mL | |||
| Micrococcus flavus | ATCC 10240 | MIC: 1 mg/mL | ||||
| Enterobacter cloacae | ATCC 35030 | MIC: 1 mg/mL | ||||
| Bacillus cereus | Clinical isolate | MIC: 0.5 mg/mL | ||||
| Listeria monocytogenes | NCTC 7973 | MIC: 0.5 mg/mL | ||||
| Salmonella typhimurium | ATCC 13311 | MIC: 0.5 mg/mL |
| References | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | Score | Rating |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [4] | 17 | Strong | ||||||||||||||||||||
| [5] | 16 | Strong | ||||||||||||||||||||
| [6] | 16 | Strong | ||||||||||||||||||||
| [9] | 16 | Strong | ||||||||||||||||||||
| [11] | 16 | Strong | ||||||||||||||||||||
| [12] | 16 | Strong | ||||||||||||||||||||
| [13] | 16 | Strong | ||||||||||||||||||||
| [14] | 13 | Moderate | ||||||||||||||||||||
| [15] | 16 | Strong | ||||||||||||||||||||
| [16] | 16 | Strong | ||||||||||||||||||||
| [17] | 16 | Strong | ||||||||||||||||||||
| [18] | 15 | Moderate | ||||||||||||||||||||
| [19] | 16 | Strong | ||||||||||||||||||||
| [20] | 16 | Strong | ||||||||||||||||||||
| [22] | 14 | Moderate | ||||||||||||||||||||
| [23] | 15 | Moderate | ||||||||||||||||||||
| [24] | 14 | Moderate | ||||||||||||||||||||
| [27] | 13 | Moderate | ||||||||||||||||||||
| [28] | 13 | Moderate | ||||||||||||||||||||
| [29] | 15 | Moderate | ||||||||||||||||||||
| [30] | 16 | Strong | ||||||||||||||||||||
| [31] | 14 | Moderate | ||||||||||||||||||||
| [32] | 15 | Moderate | ||||||||||||||||||||
| [33] | 14 | Moderate | ||||||||||||||||||||
| [34] | 16 | Strong | ||||||||||||||||||||
| [35] | 17 | Strong | ||||||||||||||||||||
| [36] | 15 | Moderate | ||||||||||||||||||||
| [37] | 15 | Moderate | ||||||||||||||||||||
| [38] | 16 | Strong | ||||||||||||||||||||
| [39] | 16 | Strong | ||||||||||||||||||||
| [40] | 15 | Moderate | ||||||||||||||||||||
| [41] | 13 | Moderate | ||||||||||||||||||||
| [42] | 14 | Moderate | ||||||||||||||||||||
| [43] | 15 | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laranjeira, I.M.; Dias, A.C.P.; Pinto-Ribeiro, F.L. Genista tridentata Phytochemical Characterization and Biological Activities: A Systematic Review. Biology 2023, 12, 1387. https://doi.org/10.3390/biology12111387
Laranjeira IM, Dias ACP, Pinto-Ribeiro FL. Genista tridentata Phytochemical Characterization and Biological Activities: A Systematic Review. Biology. 2023; 12(11):1387. https://doi.org/10.3390/biology12111387
Chicago/Turabian StyleLaranjeira, Inês Martins, Alberto Carlos Pires Dias, and Filipa Lacerda Pinto-Ribeiro. 2023. "Genista tridentata Phytochemical Characterization and Biological Activities: A Systematic Review" Biology 12, no. 11: 1387. https://doi.org/10.3390/biology12111387
APA StyleLaranjeira, I. M., Dias, A. C. P., & Pinto-Ribeiro, F. L. (2023). Genista tridentata Phytochemical Characterization and Biological Activities: A Systematic Review. Biology, 12(11), 1387. https://doi.org/10.3390/biology12111387








