Self-DNA Inhibition in Drosophila melanogaster Development: Metabolomic Evidence of the Molecular Determinants
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Library
2.1.1. Generation of a Drosophila melanogaster Genomic Library in Yeast
2.1.2. Amplification of the Self-DNA Library
2.1.3. Preparative Growth of Yeast for Administration to Flies
2.2. Fly Bioassays
2.2.1. Drosophila Culture Conditions
2.2.2. Toxicity Assays
2.2.3. Time-Course of Larval Development
2.2.4. Fecundity and Fertility Tests
2.3. Metabolomic Analysis
2.3.1. Metabolomic Extraction
2.3.2. NMR Analysis
2.3.3. LC-MS Analysis
2.3.4. Molecular Network Analysis
2.4. Data Analysis
3. Results
3.1. Inhibitory Effects of Self-DNA on Larval Growth
3.2. NMR Metabolite Profiling after Self-DNA Exposure
3.3. LC-MS and Molecular Network
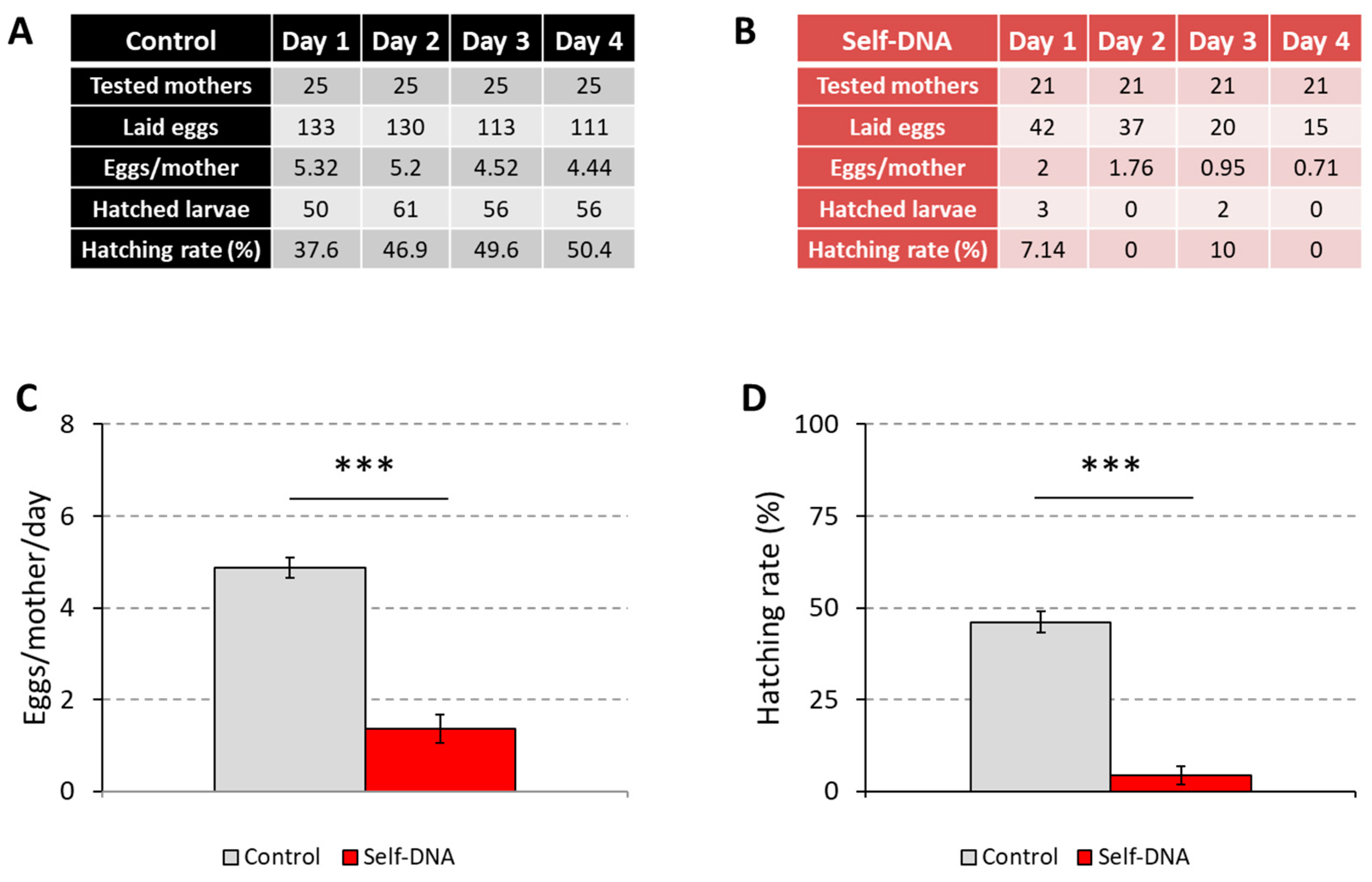
3.4. Inhibitory Effects of Self-DNA on Adult Reproduction
4. Discussion
4.1. Biological Assays Highlight Target Processes of Self-DNA Inhibition
4.2. Metabolomics Clearly Indicates Molecular Agents of Self-DNA Inhibition
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazzoleni, S.; Bonanomi, G.; Incerti, G.; Chiusano, M.L.; Termolino, P.; Mingo, A.; Senatore, M.; Giannino, F.; Cartenì, F.; Rietkerk, M.; et al. Inhibitory and toxic effects of extracellular self-DNA in litter: A mechanism for negative plant-soil feedbacks? New Phytol. 2015, 205, 1195–1210. [Google Scholar] [CrossRef] [PubMed]
- Mazzoleni, S.; Cartenì, F.; Bonanomi, G.; Senatore, M.; Termolino, P.; Giannino, F.; Incerti, G.; Rietkerk, M.; Lanzotti, V.; Chiusano, M.L. Inhibitory effects of extracellular self-DNA: A general biological process? New Phytol. 2015, 206, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Palomba, E.; Chiaiese, P.; Termolino, P.; Paparo, R.; Filippone, E.; Mazzoleni, S.; Chiusano, M.L. Effects of extracellular self- and nonself-DNA on the freshwater microalga Chlamydomonas reinhardtii and on the marine microalga Nannochloropsis gaditana. Plants 2022, 11, 1436. [Google Scholar] [CrossRef] [PubMed]
- Chiusano, M.L.; Incerti, G.; Colantuono, C.; Termolino, P.; Palomba, E.; Monticolo, F.; Benvenuto, G.; Foscari, A.; Esposito, A.; Marti, L.; et al. Arabidopsis thaliana Response to Extracellular DNA: Self Versus Nonself Exposure. Plants 2021, 10, 1744. [Google Scholar] [CrossRef] [PubMed]
- Germoglio, M.; Adamo, A.; Incerti, G.; Cartenì, F.; Gigliotti, S.; Storlazzi, A.; Mazzoleni, S. Self-DNA exposure induces developmental defects and germline DNA damage response in Caenorhabditis elegans. Biology 2022, 11, 262. [Google Scholar] [CrossRef]
- De Alteriis, E.; Chiusano, M.L.; Lanzotti, V.; Mazzoleni, S. Extracellular DNA accumulating in yeast fed-batch cultures is metabolism-specific and inhibits cell proliferation: Evidence from a multidisciplinary study. In Proceedings of the 31st International Conference on Yeast Genetics and Molecular Biology (ICYGMB31), Florence, Italy, 20–25 August 2023. [Google Scholar]
- Ronchi, A.; Foscari, A.; Zaina, G.; De Paoli, E.; Incerti, G. Self-DNA early exposure in cultivated and weedy Setaria triggers ROS degradation signaling pathways and root growth inhibition. Plants 2023, 12, 1288. [Google Scholar] [CrossRef]
- Barbero, F.; Guglielmotto, M.; Capuzzo, A.; Maffei, M.E. Extracellular Self-DNA (esDNA), but Not Heterologous Plant or Insect DNA (etDNA), Induces Plasma Membrane Depolarization and Calcium Signaling in Lima Bean (Phaseolus lunatus) and Maize (Zea mays). Int. J. Mol. Sci. 2016, 17, 1659. [Google Scholar] [CrossRef]
- Duran-Flores, D.; Heil, M. Extracellular self-DNA as a damage-associated molecular pattern (DAMP) that triggers self-specific immunity induction in plants. Brain Behav. Immun. 2018, 72, 78–88. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, H.; Zhang, X.; Khashi u Rahman, M.; Mazzoleni, S.; Du, M.; Wu, F. Plant extracellular self-DNA inhibits growth and induces immunity via the jasmonate signaling pathway. Plant Physiol. 2023, 192, 2475–2491. [Google Scholar] [CrossRef]
- Lanzotti, V.; Grauso, L.; Mangoni, A.; Termolino, P.; Palomba, E.; Anzano, A.; Incerti, G.; Mazzoleni, S. Metabolomics and molecular networking analyses in Arabidopsis thaliana show that extracellular self-DNA affects nucleoside/nucleotide cycles with accumulation of cAMP, cGMP and N6-methyl-AMP. Phytochemistry 2022, 204, 113453. [Google Scholar] [CrossRef]
- Demerec, M. Biology of Drosophila; Wiley: New York, NY, USA, 1950. [Google Scholar]
- Scarpato, S.; Teta, R.; Della Sala, G.; Pawlik, J.R.; Costantino, V.; Mangoni, A. New Tricks with an Old Sponge: Feature-Based Molecular Networking Led to Fast Identification of New Stylissamide L from Stylissa caribica. Mar. Drugs 2020, 18, 443. [Google Scholar] [CrossRef] [PubMed]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Starmer, W.T. A comparison of Drosophila habitats according to the physiological attributes of the associated yeast communities. Evolution 1981, 35, 38–52. [Google Scholar] [CrossRef]
- Becher, P.G.; Flick, G.; Rozpędowska, E.; Schmidt, A.; Hagman, A.; Lebreton, S.; Larsson, M.C.; Hansson, B.S.; Piškur, J.; Witzgall, P.; et al. Yeast, not fruit volatiles mediate D rosophila melanogaster attraction, oviposition and development. Funct. Ecol. 2012, 26, 822–828. [Google Scholar] [CrossRef]
- Palanca, L.; Gaskett, A.C.; Günther, C.S.; Newcomb, R.D.; Goddard, M.R. Quantifying variation in the ability of yeasts to attract Drosophila melanogaster. PLoS ONE 2013, 8, e75332. [Google Scholar] [CrossRef]
- Murphy, K.A.; Tabuloc, C.A.; Cervantes, K.R.; Chiu, J.C. Ingestion of genetically modified yeast symbiont reduces fitness of an insect pest via RNA interference. Sci. Rep. 2016, 6, 22587. [Google Scholar] [CrossRef]
- Olcott, M.H.; Henkels, M.D.; Rosen, K.L.; Walker, F.; Sneh, B.; Loper, J.E.; Taylor, B.J. Lethality and developmental delay in Drosophila melanogaster larvae after ingestion of selected Pseudomonas fluorescens strains. PLoS ONE 2010, 5, e12504. [Google Scholar] [CrossRef][Green Version]
- Rand, M.D.; Tennessen, J.M.; Mackay, T.F.; Anholt, R.R. Perspectives on the Drosophila melanogaster Model for Advances in Toxicological Science. Curr. Protoc. 2023, 3, e870. [Google Scholar] [CrossRef]
- Tennessen, J.M.; Baker, K.D.; Lam, G.; Evans, J.; Thummel, C.S. The Drosophila estrogen-related receptor directs a metabolic switch that supports developmental growth. Cell Metab. 2011, 13, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Terhzaz, S.; Naseem, M.T.; Nagy, S.; Rewitz, K.; Dow, J.A.; Davies, S.A.; Halberg, K.V. A nutrient-responsive hormonal circuit mediates an inter-tissue program regulating metabolic homeostasis in adult Drosophila. Nat. Commun. 2021, 12, 5178. [Google Scholar] [CrossRef] [PubMed]
- Texada, M.J.; Koyama, T.; Rewitz, K. Regulation of body size and growth control. Genetics 2020, 216, 269–313. [Google Scholar] [CrossRef] [PubMed]
- Colombani, J.; Raisin, S.; Pantalacci, S.; Radimerski, T.; Montagne, J.; Léopold, P. A nutrient sensor mechanism controls Drosophila growth. Cell 2003, 114, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Nishimura, T. Signaling from glia and cholinergic neurons controls nutrient-dependent production of an insulin-like peptide for Drosophila body growth. Dev. Cell 2015, 35, 295–310. [Google Scholar] [CrossRef]
- Rulifson, E.J.; Kim, S.K.; Nusse, R. Ablation of insulin-producing neurons in flies: Growth and diabetic phenotypes. Science 2002, 296, 1118–1120. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Li, C.R.; Momen, B.; Kohanski, R.A.; Pick, L. Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities. Proc. Natl. Acad. Sci. USA 2009, 106, 19617–19622. [Google Scholar] [CrossRef]
- Tennessen, J.M.; Bertagnolli, N.M.; Evans, J.; Sieber, M.H.; Cox, J.; Thummel, C.S. Coordinated metabolic transitions during Drosophila embryogenesis and the onset of aerobic glycolysis. G3 Genes. Genom. Genet. 2014, 4, 839–850. [Google Scholar] [CrossRef]
- Puri, S.; Sohal, S.K. Antibiosis effect of phloroglucinol on the larvae of herbivorous insect Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae). Int. J. Adv. Res. 2017, 6, 999–1006. [Google Scholar] [CrossRef]
- Kang, M.H.; Kim, I.H.; Nam, T.J. Phloroglucinol induces apoptosis via apoptotic signaling pathways in HT-29 colon cancer cells. Oncol. Rep. 2014, 32, 1341–1346. [Google Scholar] [CrossRef]
- Caldwell, P.E.; Walkiewicz, M.; Stern, M. Ras activity in the Drosophila prothoracic gland regulates body size and developmental rate via ecdysone release. Curr. Biol. 2005, 15, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Mirth, C.; Truman, J.W.; Riddiford, L.M. The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr. Biol. 2005, 15, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Boulan, L.; Martín, D.; Milán, M. bantam miRNA promotes systemic growth by connecting insulin signaling and ecdysone production. Curr. Biol. 2013, 23, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Moeller, M.E.; Nagy, S.; Gerlach, S.U.; Soegaard, K.C.; Danielsen, E.T.; Texada, M.J.; Rewitz, K.F. Warts signaling controls organ and body growth through regulation of ecdysone. Curr. Biol. 2017, 27, 1652–1659. [Google Scholar] [CrossRef]
- Bourgin, R.C.; Krumins, R.; Quastler, H. Radiation-induced delay of pupation in Drosophila. Radiat. Res. 1956, 5, 657–673. [Google Scholar] [CrossRef]
- Simpson, P.; Berreur, P.; Berreur-Bonnenfant, J. The initiation of pupariation in Drosophila: Dependence on growth of the imaginal discs. Development 1980, 57, 155–165. [Google Scholar] [CrossRef]
- Smith-Bolton, R.K.; Worley, M.I.; Kanda, H.; Hariharan, I.K. Regenerative growth in Drosophila imaginal discs is regulated by Wingless and Myc. Dev. Cell 2009, 16, 797–809. [Google Scholar] [CrossRef]
- Garelli, A.; Gontijo, A.M.; Miguela, V.; Caparros, E.; Dominguez, M. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science 2012, 336, 579–582. [Google Scholar] [CrossRef]
- Garelli, A.; Heredia, F.; Casimiro, A.P.; Macedo, A.; Nunes, C.; Garcez, M.; Dias, A.R.M.; Volonte, Y.A.; Uhlmann, T.; Caparros, E.; et al. Dilp8 requires the neuronal relaxin receptor Lgr3 to couple growth to developmental timing. Nat. Commun. 2015, 6, 8732. [Google Scholar] [CrossRef]
- Colombani, J.; Andersen, D.S.; Léopold, P. Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science 2012, 336, 582–585. [Google Scholar] [CrossRef]
- Colombani, J.; Andersen, D.S.; Boulan, L.; Boone, E.; Romero, N.; Virolle, V.; Texada, M.; Léopold, P. Drosophila Lgr3 couples organ growth with maturation and ensures developmental stability. Curr. Biol. 2015, 25, 2723–2729. [Google Scholar] [CrossRef]
- Vallejo, D.M.; Juarez-Carreño, S.; Bolivar, J.; Morante, J.; Dominguez, M. A brain circuit that synchronizes growth and maturation revealed through Dilp8 binding to Lgr3. Science 2015, 350, aac6767. [Google Scholar] [CrossRef]
- Parker, N.F.; Shingleton, A.W. The coordination of growth among Drosophila organs in response to localized growth-perturbation. Dev. Biol. 2011, 357, 318–325. [Google Scholar] [CrossRef]
- Maniscalco, M.; Fuschillo, S.; Paris, D.; Cutignano, A.; Sanduzzi, A.; Motta, A. Clinical metabolomics of exhaled breath condensate in chronic respiratory diseases. Adv. Clin. Chem. 2019, 88, 121–149. [Google Scholar] [PubMed]
- Castellano, I.; Merlino, A. γ-Glutamyltranspeptidases: Sequence, structure, biochemical properties, and biotechnological applications. Cell. Mol. Life Sci. 2012, 69, 3381–3394. [Google Scholar] [CrossRef] [PubMed]
- Del Bello, B.; Paolicchi, A.; Comporti, M.; Pompella, A.; Maellaro, E. Hydrogen peroxide produced during gamma-glutamyl transpeptidase activity is involved in prevention of apoptosis and maintainance of proliferation in U937 cells. FASEB J. 1999, 13, 69–79. [Google Scholar] [CrossRef]
- Falabella, P.; Riviello, L.; Caccialupi, P.; Rossodivita, T.; Teresa Valente, M.; Luisa De Stradis, M.; Tranfaglia, A.; Varricchio, P.; Gigliotti, S.; Graziani, F.; et al. A gamma-glutamyl transpeptidase of Aphidius ervi venom induces apoptosis in the ovaries of host aphids. Insect Biochem. Mol. Biol. 2007, 37, 453–465. [Google Scholar] [CrossRef]
- Russo, E.; Di Lelio, I.; Becchimanzi, A.; Pennacchio, F. Aphidius ervi venom regulates Buchnera contribution to host nutritional suitability. J. Insect Physiol. 2023, 147, 104506. [Google Scholar] [CrossRef] [PubMed]
- Keramaris, K.E.; Margaritis, L.H.; Zografou, E.N.; Tsiropoulos, G.J. Egg laying suppression in Drosophila melanogaster (Diptera: Drosophilidae) and Dacus (Bactrocera) oleae (Diptera: Tephritidae) by phloroglucinol, a peroxidase inhibitor. Bull. Entomol. Res. 1996, 86, 369–374. [Google Scholar] [CrossRef]
- Gout, R.; Colinet, H. The reproductive cost of Drosophila melanogaster: Effects on cold tolerance. In Proceedings of the ISEPEP9, Rennes, France, 10–14 July 2022. [Google Scholar]
- Drummond-Barbosa, D.; Spradling, A.C. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev. Biol. 2001, 231, 265–278. [Google Scholar] [CrossRef]
- Hsu, H.J.; Drummond-Barbosa, D. Insulin signals control the competence of the Drosophila female germline stem cell niche to respond to Notch ligands. Dev. Biol. 2011, 350, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Badisco, L.; Van Wielendaele, P.; Vanden Broeck, J. Eat to reproduce: A key role for the insulin signaling pathway in adult insects. Front. Physiol. 2013, 4, 202. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.S.; Rybczynski, R.; Wilson, T.G.; Wang, Y.; Wayne, M.L.; Zhou, Y.; Partridge, L.; Harshman, L.G. Insulin signaling is necessary for vitellogenesis in Drosophila melanogaster independent of the roles of juvenile hormone and ecdysteroids: Female sterility of the chico1 insulin signaling mutation is autonomous to the ovary. J. Insect Physiol. 2005, 51, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, D.R.; Carpenter, A.M.; Ospina, S.R.; Merkler, D.J. Probing the chemical mechanism and critical regulatory amino acid residues of Drosophila melanogaster arylalkylamine N-acyltransferase like 2. Insect Biochem. Mol. Biol. 2015, 66, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.O. Insect cuticular sclerotization: A review. Insect Biochem. Mol. Biol. 2010, 40, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Brunet, P.C. The metabolism of the aromatic amino acids concerned in the cross-linking of insect cuticle. Insect Biochem. 1980, 10, 467–500. [Google Scholar] [CrossRef]
- Wright, T.R. The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Adv. Genet. 1987, 24, 27–222. [Google Scholar]
- Kelly, K.P.; Alassaf, M.; Sullivan, C.E.; Brent, A.E.; Goldberg, Z.H.; Poling, M.E.; Dubrulle, J.; Rajan, A. Fat body phospholipid state dictates hunger-driven feeding behavior. eLife 2022, 11, e80282. [Google Scholar] [CrossRef]
- Zuberova, M.; Fenckova, M.; Simek, P.; Janeckova, L.; Dolezal, T. Increased extracellular adenosine in Drosophila that are deficient in adenosine deaminase activates a release of energy stores leading to wasting and death. Dis. Model Mech. 2010, 11–12, 773–784. [Google Scholar] [CrossRef]



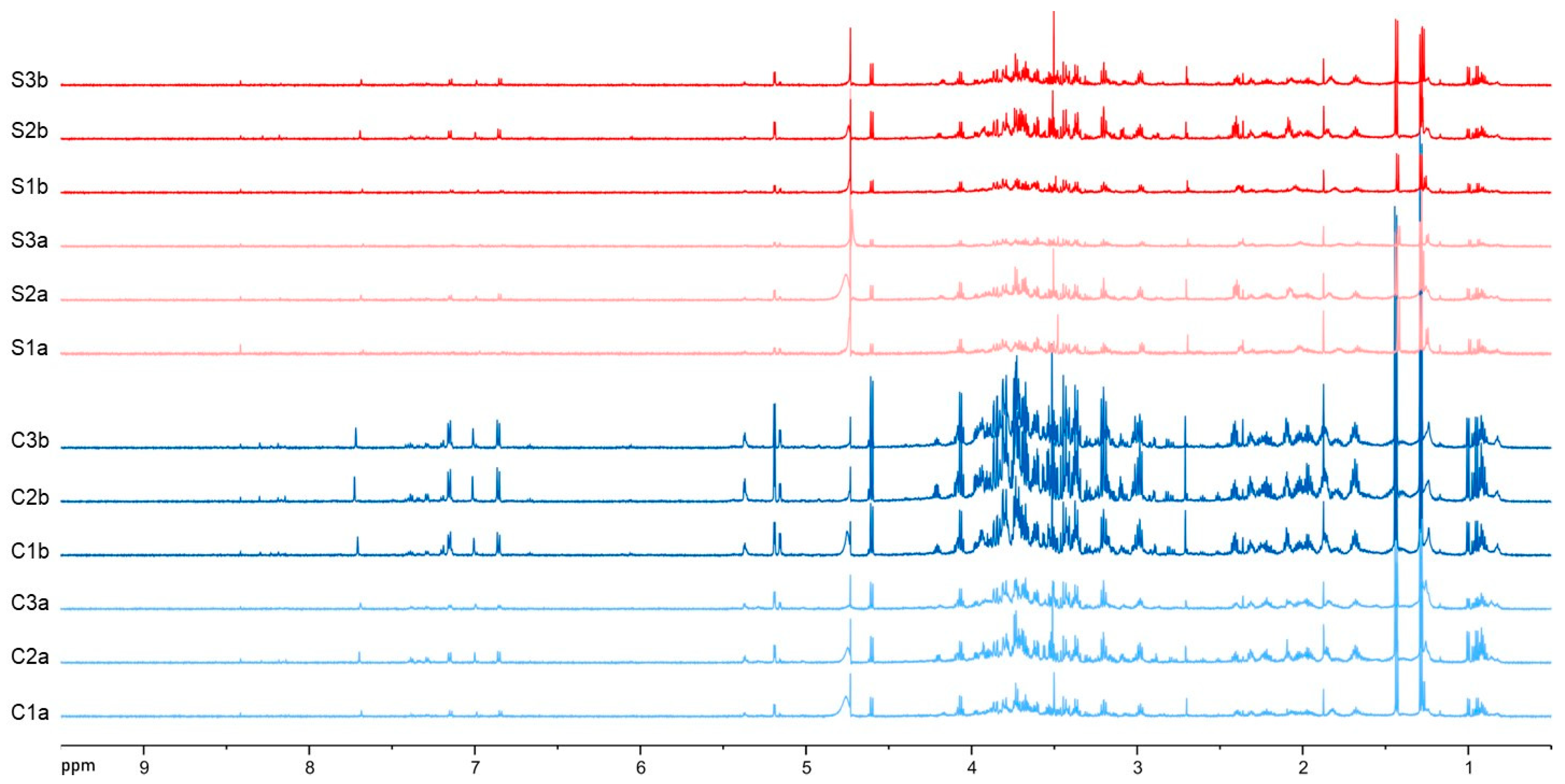
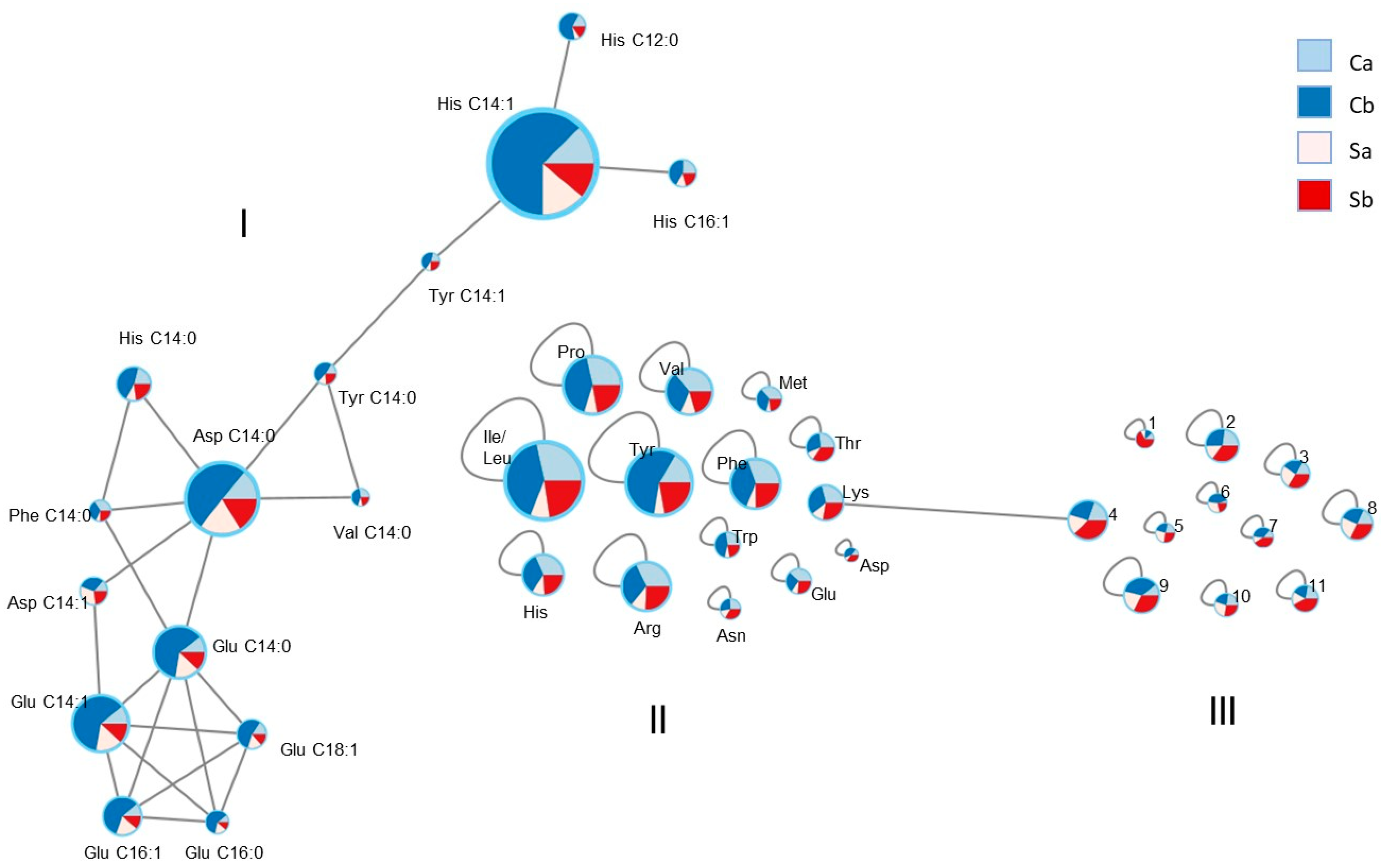
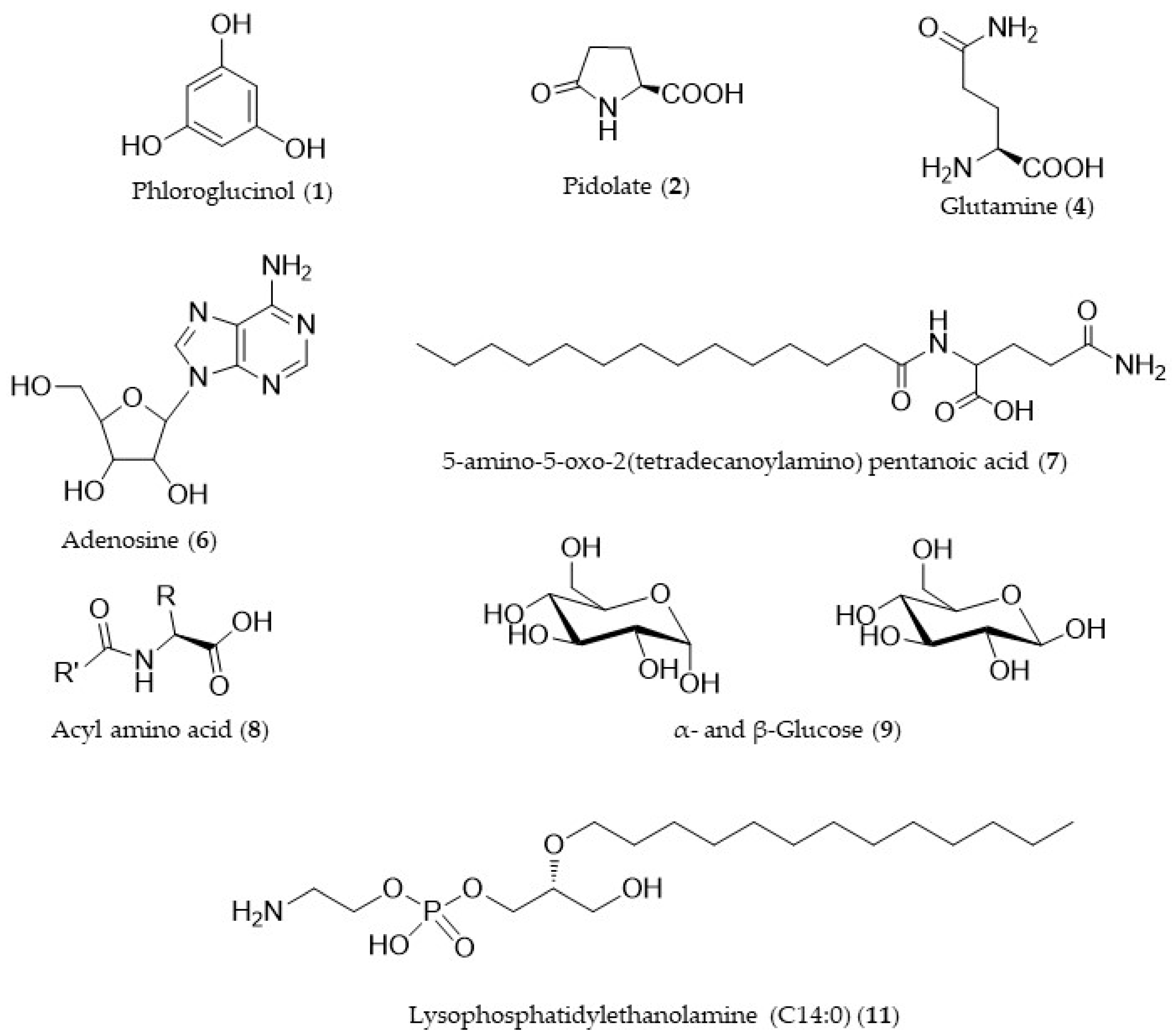
| Abbreviation | m/z | Rt | Ion | Mol. Formula | Putative Identification | Δppm | |
|---|---|---|---|---|---|---|---|
| Group I | His C12:0 | 338.2437 | 29.81 | M + H+ | C18H32O3N3 | Lauryl histidine | −0.350 |
| His C14:0 | 366.2753 | 31.33 | M + H+ | C20H36O3N3 | Myristoyl histidine | −1.002 | |
| His C14:1 | 364.2593 | 31.75 | M + H+ | C20H34O3N3 | Myristoleyl histidine | −0.462 | |
| His C16:1 | 392.2908 | 34.27 | M + H+ | C22H38O3N3 | Palmitoleyl histidine | 0.080 | |
| Tyr C14:1 | 390.2637 | 35.26 | M + H+ | C23H36O4N | Myristoleyl tyrosine | −0.474 | |
| Tyr C14:0 | 392.2797 | 36.86 | M + H+ | C23H38O4N | Myristoyl tyrosine | −0.978 | |
| Val C14:0 | 328.2846 | 38.74 | M + H+ | C19H38O3N | Myristoyl valine | −0.147 | |
| Asp C14:0 | 344.2420 | 36.36 | M + H+ | C18H34O5N | Myristoyl aspartic acid | −3.456 | |
| Asp C14:1 | 342.2274 | 34.62 | M + H+ | C18H32O5N | Myristoleyl aspartic acid | −0.203 | |
| Phe C14:0 | 376.2845 | 39.53 | M + H+ | C23H38O3N | Myristoyl phenylalanine | −0.427 | |
| Glu C14:0 | 358.2588 | 36.42 | M + H+ | C19H36O5N | Myristoyl glutamic acid | −0.027 | |
| Glu C14:1 | 356.2430 | 34.85 | M + H+ | C19H34O5N | Myristoleyl glutamic acid | −0.420 | |
| Glu C16:0 | 386.2905 | 38.82 | M + H+ | C21H40O5N | Palmitoyl glutamic acid | 0.777 | |
| Glu C16:1 | 384.2743 | 37.15 | M + H+ | C21H38O5N | Palmitoleyl glutamic acid | −0.390 | |
| Glu C18:1 | 412.3055 | 39.42 | M + H+ | C23H42O5N | Oleoyl glutamic acid | −0.606 | |
| Group II | Pro | 116.0704 | 0.77 | M + H+ | C5H10O2N | Proline | −1.681 |
| Val | 118.0861 | 0.81 | M + H+ | C5H12O2N | Valine | −1.314 | |
| Thr | 120.0653 | 0.90 | M + H+ | C4H10O3N | Threonine | −1.830 | |
| Ile + Leu | 132.1017 | 1.32 | M + H+ | C6H14O2N | Isoleucine/Leucine | −1.554 | |
| Asn | 133.0606 | 0.79 | M + H+ | C4H9O3N2 | Asparagine | −1.268 | |
| Asp | 134.0302 | 0.83 | M + H+ | C4H8O4N | Aspartic acid | −4.965 | |
| Lys | 147.1127 | 0.67 | M + H+ | C6H15O2N2 | Lysine | −0.709 | |
| Glu | 148.0603 | 0.79 | M + H+ | C5H10O4N | Glutamic acid | −0.907 | |
| Met | 150.0589 | 0.89 | M + H+ | C5H12O2NS | Methionine | −0.329 | |
| His | 156.0768 | 0.79 | M + H+ | C6H12O2N3 | Histydine | −0.084 | |
| Phe | 166.0862 | 2.32 | M + H+ | C9H12O2N | Phenylalanine | −0.332 | |
| Arg | 175,1188 | 0.74 | M + H+ | C6H15O2N4 | Arginine | −0.869 | |
| Tyr | 182.0811 | 1.51 | M + H+ | C4H9O4N6 | Tyrosine | −0.383 | |
| Trp | 205.0942 | 2.77 | M + H+ | C11H13O2N2 | Tryptophan | −0.313 | |
| Group III | 1 | 127.0388 | 0.71 | M + H+ | C6H7O3 | Phloroglucinol | −1.343 |
| 2 | 130.0497 | 0.89 | M + H+ | C5H8O3N | Pidolate | −1.304 | |
| 3 | 245.6156 | 37.18 | M + 2H2+ | unidentified | unidentified | ||
| 4 | 147.0763 | 0.79 | M + H+ | C5H11O3N2 | Glutamine | −0.807 | |
| 5 | 191.1248 | 32.06 | M + 2H2+ | unidentified | unidentified | ||
| 6 | 268.1040 | 1.73 | M + H+ | C10H14O4N5 | Adenosine | −0.113 | |
| 7 | 357.2747 | 35.84 | M + H+ | C19H37O4N2 | 5-amino-5-oxo-2(tetradecanoylamino) pentanoic acid | −0.235 | |
| 8 | 438.3787 | 39.21 | M + NH4+ | C21H50O5N4 | Acyl amino acid | 2.573 | |
| 9 | 203.0525 | 0.78 | M + Na+ | C6H12O6Na | Glucose | −0.193 | |
| 10 | 243.0903 | 30.63 | M + H+ | C10H16O3N2P | unidentified | 4.920 | |
| 11 | 426.2613 | 36.42 | M + H+ | C19H41O7NP | Lysophosphatidylethanolamine (C14:0) | −0.506 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, M.; Grauso, L.; Lanzotti, V.; Incerti, G.; Adamo, A.; Storlazzi, A.; Gigliotti, S.; Mazzoleni, S. Self-DNA Inhibition in Drosophila melanogaster Development: Metabolomic Evidence of the Molecular Determinants. Biology 2023, 12, 1378. https://doi.org/10.3390/biology12111378
Colombo M, Grauso L, Lanzotti V, Incerti G, Adamo A, Storlazzi A, Gigliotti S, Mazzoleni S. Self-DNA Inhibition in Drosophila melanogaster Development: Metabolomic Evidence of the Molecular Determinants. Biology. 2023; 12(11):1378. https://doi.org/10.3390/biology12111378
Chicago/Turabian StyleColombo, Michele, Laura Grauso, Virginia Lanzotti, Guido Incerti, Adele Adamo, Aurora Storlazzi, Silvia Gigliotti, and Stefano Mazzoleni. 2023. "Self-DNA Inhibition in Drosophila melanogaster Development: Metabolomic Evidence of the Molecular Determinants" Biology 12, no. 11: 1378. https://doi.org/10.3390/biology12111378
APA StyleColombo, M., Grauso, L., Lanzotti, V., Incerti, G., Adamo, A., Storlazzi, A., Gigliotti, S., & Mazzoleni, S. (2023). Self-DNA Inhibition in Drosophila melanogaster Development: Metabolomic Evidence of the Molecular Determinants. Biology, 12(11), 1378. https://doi.org/10.3390/biology12111378





