Differential Cold Tolerance on Immature Stages of Geographically Divergent Ceratitis capitata Populations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Populations
2.2. Insect Rearing
2.3. Assessment of LT50 at Subfreezing Temperatures
2.4. Effect of Subfreezing Temperatures on the Eggs
2.5. Effect of Subfreezing Temperatures on the L3 Instar Wandering Larvae
2.6. Effect of Subfreezing Temperatures on 4-Day-Old Pupae
2.7. Acute Cold Tolerance Assessment
2.8. Data Analysis
3. Results
3.1. Effect of Subfreezing Temperatures on the Eggs
3.2. Effect of Subfreezing Temperatures on the L3 Instar Wandering Larvae
3.3. Effect of Subfreezing Temperatures on 4 Days Old Pupae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mutamiswa, R.; Tarusikirwa, V.; Nyamukondiwa, C.; Chidawanyika, F. Fluctuating environments impact thermal tolerance in an invasive insect species Bactrocera dorsalis (Diptera: Tephritidae). J. Appl. Entomol. 2020, 144, 885–896. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Zhang, Z.; Huang, J.; Zhang, J.; Hafeez, M.; Wang, L.; Guo, W.; Lu, Y. Supercooling capacity and cold tolerance of the South American tomato pinworm, Tuta absoluta, a newly invaded pest in China. J. Pest Sci. 2021, 94, 845–858. [Google Scholar] [CrossRef]
- Zhang, W.; Rudolf, V.H.; Ma, C.S. Stage-specific heat effects: Timing and duration of heat waves alter demographic rates of a global insect pest. Oecologia 2015, 179, 947–957. [Google Scholar] [CrossRef]
- Berger, D.; Walters, R.; Gotthard, K. What limits insect fecundity? Body size-and temperature-dependent egg maturation and oviposition in a butterfly. Funct. Ecol. 2008, 22, 523–529. [Google Scholar] [CrossRef]
- Forster, J.; Hirst, A.G. The temperature-size rule emerges from ontogenetic differences between growth and development rates. Funct. Ecol. 2012, 26, 483–492. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Chown, S.L.; Clusella-Trullas, S. Upper thermal limits in terrestrial ectotherms: How constrained are they? Funct. Ecol. 2013, 27, 934–949. [Google Scholar] [CrossRef]
- Rwomushana, I.; Ekesi, S.; Ogol, C.; Gordon, I. Effect of temperature on development and survival of immature stages of Bactrocera invadens (Diptera: Tephritidae). J. Appl. Entomol. 2008, 132, 832–839. [Google Scholar] [CrossRef]
- Malacrida, A.R.; Gomulski, L.M.; Bonizzoni, M.; Bertin, S.; Gasperi, G.; Guglielmino, C.R. Globalization and fruitfly invasion and expansion: The medfly paradigm. Genetica 2007, 131, 1–9. [Google Scholar] [CrossRef]
- Ben-Yosef, M.; Verykouki, E.; Altman, Y.; Nemni-Lavi, E.; Papadopoulos, N.T.; Nestel, D. Effects of thermal acclimation on the tolerance of Bactrocera zonata (Diptera: Tephritidae) to hydric stress. Front. Physiol. 2021, 12, 686424. [Google Scholar] [CrossRef]
- Gray, E.M. Thermal acclimation in a complex life cycle: The effects of larval and adult thermal conditions on metabolic rate and heat resistance in Culex pipiens (Diptera: Culicidae). J. Insect Physiol. 2013, 59, 1001–1007. [Google Scholar] [CrossRef]
- Liebhold, A.M. Invasion by exotic forest pests: A threat to forest ecosystems. For. Sci. 1995, 30 (Suppl. S1), 1–49. [Google Scholar] [CrossRef]
- Musolin, D.L. Insects in a warmer world: Ecological, physiological and life-history responses of true bugs (Heteroptera) to climate change. Glob. Chang. Biol. 2007, 13, 1565–1585. [Google Scholar] [CrossRef]
- Papadogiorgou, G.D.; Moraiti, C.A.; Nestel, D.; Terblanche, J.S.; Verykouki, E.; Papadopoulos, N.T. Acute cold stress and supercooling capacity of Mediterranean fruit fly populations across the Northern Hemisphere (Middle East and Europe). J. Insect Physiol. 2023, 147, 104519. [Google Scholar] [CrossRef] [PubMed]
- Skendžić, S.; Zovko, M.; Pajač Živković, I.; Lešić, V.; Lemić, D. Effect of climate change on introduced and native agricultural invasive insect pests in Europe. Insects 2021, 12, 985. [Google Scholar] [CrossRef] [PubMed]
- Angilletta, M.J. Adaptation to anthropogenic climate change. In Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford Biology; Oxford University Press: New York, NY, USA, 2009; pp. 214–236. [Google Scholar]
- Bowler, K.; Terblanche, J.S. Insect thermal tolerance: What is the role of ontogeny, ageing and senescence? Biol. Rev. 2008, 83, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Chown, S.; Nicolson, S. Insect Physiological Ecology: Mechanisms and Patterns; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Chown, S.L.; Terblanche, J.S. Physiological diversity in insects: Ecological and evolutionary contexts. Adv. Insect Physiol. 2006, 33, 50–152. [Google Scholar]
- Sinclair, B.J.; Alvarado, L.E.C.; Ferguson, L.V. An invitation to measure insect cold tolerance: Methods, approaches, and workflow. J. Therm. Biol. 2015, 53, 180–197. [Google Scholar] [CrossRef]
- Scolari, F.; Valerio, F.; Benelli, G.; Papadopoulos, N.T.; Vaníčková, L. Tephritid fruit fly semiochemicals: Current Knowledge and Future Perspectives. Insects 2021, 12, 408. [Google Scholar] [CrossRef]
- Christenson, L.D.; Foote, R.H. Biology of fruit flies. Annu. Rev. Entomol. 1960, 5, 171–192. [Google Scholar] [CrossRef]
- Fimiani, P. Mediterranean Region. In Fruit Flies: Their Biology, Natural Enemies and Control; Robinson, A.S., Hooper, G., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; Volume 3A, pp. 39–50. [Google Scholar]
- Liquido, N.J.; Cunningham, R.T.; Nakagawa, S. Host plants of Mediterranean fruit fly (Diptera: Tephritidae) on the island of Hawaii (1949–1985 Survey). J. Econ. Entomol. 1990, 83, 1863–1878. [Google Scholar] [CrossRef]
- Papadopoulos, N.T. Fruit fly invasion: Historical, biological, economic aspects and management. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies; Shelly, T., Epsky, N., Jang, E.B., Reyes-Flores, J., Vargas, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 219–252. [Google Scholar] [CrossRef]
- Papadopoulos, N.T.; Carey, J.R.; Katsoyannos, B.I.; Kouloussis, N.A. Overwintering of the Mediterranean fruit fly (Diptera: Tephritidae) in Northern Greece. Ann. Entomol. Soc. Am. 1996, 89, 526–534. [Google Scholar] [CrossRef]
- White, I.M.; Elson-Harris, M.M. Fruit flies of economic significance: Their identification and bionomics. In Fruit Flies of Economic Significance: Their Identification and Bionomics; CAB International: Wallingford, UK, 1992. [Google Scholar]
- Bonizzoni, M.; Malacrida, A.R.; Guglielmino, C.R.; Gomulski, L.M.; Gasperi, G.; Zheng, L. Microsatellite polymorphism in the Mediterranean fruit fly, Ceratitis capitata. Insect Mol. Biol. 2000, 9, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, G.; Bonizzoni, M.; Gomulski, L.M.; Murelli, V.; Torti, C.; Malacrida, A.R.; Guglielmino, C.R. Genetic differentiation, gene flow and the origin of infestations of the medfly, Ceratitis capitata. Genetica 2002, 116, 125–135. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, M.; Copeland, R.S.; Wharton, R.A.; McPheron, B.A.; Barnes, B.N. On the geographic origin of the medfly Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). In Proceedings of the 6th International Fruit Fly Symposium, Stellenbosch, South Africa, 6–10 May 2002; pp. 45–53. [Google Scholar]
- De Breme, F. Note sur le genre Ceratitis de M. Mac Heay (Diptera). Ann. Soc. Entomol. Fr. 1842, 11, 183–190. [Google Scholar]
- Gallo, D.; Nakano, O.; Wiendl, F.; Silveira Neto, S.; Carvalho, R. PL Manual de Entomologia Agrícola; Agronomica “Ceres”: Sao Paolo, Brazil, 1970. [Google Scholar]
- Bonizzoni, M.; Guglielmino, C.R.; Smallridge, C.J.; Gomulski, M.; Malacrida, A.R.; Gasperi, G. On the origins of medfly invasion and expansion in Australia: Medfly invasion into Australia. Mol. Ecol. 2004, 13, 3845–3855. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C. A Serious fruit pest. The fruit or peach maggot. J. Bur. Agric. 1897, 4, 1147–1148. [Google Scholar]
- Cayol, J.P.; Causse, R. Mediterranean fruit fly Ceratitis Capitata Wiedemann (Dipt., Trypetidae) back in Southern France. J. Appl. Entomol. 1993, 116, 94–100. [Google Scholar] [CrossRef]
- Rigamonti, I.E. La Ceratitis capitata in Lombardia; Universita Degli Studi Di Milano: Milano, Italy, 2005. [Google Scholar]
- Rigamonti, I.E. Contributions to the knowledge of Ceratitis capitata Wied. (Diptera, Tephritidae) in Northern Italy. I. Observations on the biology. Boll. Zool. Agrar. E Bachic. 2004, 36, 89–100. [Google Scholar]
- Rigamonti, I.E.; Agosti, M.; Malacrida, A.R. Distribuzione e danni della mosca Mediterranea della frutta Ceratitis capitata Wied. (Diptera Tephritidae) in Lombardia (Italia Settentrionale). In Proceedings of the XIX Congresso Nazionale Italiano di Entomologia, Catania, Italy, 10–15 June 2002; pp. 581–587. [Google Scholar]
- Zanoni, S. Study of the Bio-Ethology of Ceratitis capitata Wied. In Trentino and Development of Sustainable Strategies for Population Control. Ph.D. Thesis, Universita Degli Studi Di Milano, Milano, Italy, 2017. [Google Scholar]
- Zanoni, S.; Baldessari, M.; De Cristofaro, A.; Angeli, G.; Ioriatti, C. Susceptibility of selected apple cultivars to the Mediterranean fruit fly. J. Appl. Entomol. 2019, 143, 744–753. [Google Scholar] [CrossRef]
- Žežlina, J.; Rot, M.; Trdan, S. Monitoring of Mediterranean fruit fly (Ceratitis capitata [Wiedemann]) in Primorska region in the period of 2016–2018. In Proceedings of the Zbornik Predavanj in Referatov, 14 Slovensko Posvetovanje O Varstvu Rastlin Z Mednarodno Udeležbo, Maribor, Slovenia, 5–6 March 2019; pp. 142–148. [Google Scholar]
- Egartner, A.; Lethmayer, C.; Gottsberger, R.A.; Blümel, S. Recent records of the Mediterranean fruit fly, Ceratitis capitata (Tephritidae, Diptera), in Austria. In Proceedings of the Joint Meeting of the IOBC-WPRS Working Groups “Pheromones and Other Semiochemicals in Integrated Production” & “Integrated Protection of Fruit Crops”, Lisbon, Portugal, 20–25 January 2019; Volume 146, pp. 143–152. [Google Scholar]
- König, S.; Steinmöller, S.; Baufeld, P. Origin and potential for overwintering of Ceratitis capitata (Wiedemann) captured in an official survey in Germany. J. Plant Dis. Prot. 2022, 129, 1201–1215. [Google Scholar] [CrossRef]
- Gilioli, G.; Sperandio, G.; Colturato, M.; Pasquali, S.; Gervasio, P.; Wilstermann, A.; Dominic, A.R.; Schrader, G. Non-Linear physiological responses to climate change: The case of Ceratitis capitata distribution and abundance in Europe. Biol. Invasions 2022, 24, 261–279. [Google Scholar] [CrossRef]
- Rigamonti, I.E. Contributions to the knowledge of Ceratitis capitata Wied. (Diptera, Tephritidae) in Northern Italy. II. Overwintering in Lombardy. Boll. Zool. Agrar. E Bachic. 2004, 36, 101–116. [Google Scholar]
- Izadi, H.; Mohammadzadeh, M.; Mehrabian, M. Changes in biochemical contents and survival rates of two stored product moths under different thermal regimes. J. Therm. Biol. 2019, 80, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.E., Jr. Principles of Insect Low Temperature Tolerance. In Insects at Low Temperature; Springer: Berlin/Heidelberg, Germany, 1991; pp. 17–46. [Google Scholar]
- Morris, G.J.; Watson, P.F. Cold shock injury—A comprehensive bibliography. Cryo-Lett. 1984, 5, 352–372. [Google Scholar]
- Wang, H.-S.; Kang, L. Effect of cooling rates on the cold hardiness and cryoprotectant profiles of locust eggs. Cryobiology 2005, 51, 220–229. [Google Scholar] [CrossRef]
- Salt, R.W. Principles of insect cold-hardiness. Annu. Rev. Entomol. 1961, 6, 55–74. [Google Scholar] [CrossRef]
- Lee, R.E. A Primer on Insect Cold-Tolerance. In Low Temperature Biology of Insects; Denlinger, D.L., Lee, R.E., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 3–34. [Google Scholar]
- Toxopeus, J.; Sinclair, B.J. Mechanisms underlying insect freeze tolerance. Biol. Rev. 2018, 93, 1891–1914. [Google Scholar] [CrossRef]
- Toxopeus, J.; McKinnon, A.H.; Štětina, T.; Turnbull, K.F.; Sinclair, B.J. Laboratory acclimation to autumn-like conditions induces freeze tolerance in the spring field cricket Gryllus veletis (Orthoptera: Gryllidae). J. Insect Physiol. 2019, 113, 9–16. [Google Scholar] [CrossRef]
- Toxopeus, J.; Lebenzon, J.E.; McKinnon, A.H.; Sinclair, B.J. Freeze tolerance of Cyphoderris monstrosa (Orthoptera: Prophalangopsidae). Can. Entomol. 2016, 148, 668–672. [Google Scholar] [CrossRef]
- Roberts, K.T.; Rank, N.E.; Dahlhoff, E.P.; Stillman, J.H.; Williams, C.M. Snow modulates winter energy use and cold exposure across an elevation gradient in a montane ectotherm. Glob. Chang. Biol. 2021, 27, 6103–6116. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, W.; Terblanche, J.S.; Addison, P. Do thermal tolerances and rapid thermal responses contribute to the invasion potential of Bactrocera dorsalis (Diptera: Tephritidae)? J. Insect Physiol. 2017, 98, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yosef, M.; Altman, Y.; Nemni-Lavi, E.; Papadopoulos, N.T.; Nestel, D. Larval nutritional-stress and tolerance to extreme temperatures in the peach fruit fly, Bactrocera zonata (Diptera: Tephritidae). Fly. 2023, 17, 2157161. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.D.J.; Jamieson, L.E.; Laidlaw, W.G.; De Silva, N.; Waddell, B.C. Decay of thermal tolerance in Queensland fruit fly eggs (Bactrocera tryoni, Diptera: Tephritidae) following non-lethal heat hardening. J. Econ. Entomol. 2020, 113, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Meats, A. Rapid acclimatization to low temperature in the Queensland fruit fly, Dacus tryoni. J. Insect Physiol. 1973, 19, 1903–1911. [Google Scholar] [CrossRef]
- Koveos, D.S. Rapid cold hardening in the olive fruit fly Bactrocera oleae under laboratory and field conditions. Entomol. Exp. Appl. 2001, 101, 257–263. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Chen, Z.; Lu, Y. Characterization of cold and heat tolerance of Bactrocera tau (Walker). Insects 2022, 13, 329. [Google Scholar] [CrossRef]
- Pullock, D.A.; Malod, K.; Manrakhan, A.; Weldon, C.W. Larval and adult diet affect phenotypic plasticity in thermal tolerance of the marula Fly, Ceratitis cosyra (Walker) (Diptera: Tephritidae). Front. Insect Sci. 2023, 3, 1122161. [Google Scholar] [CrossRef]
- Nyamukondiwa, C.; Terblanche, J.S. Thermal tolerance in adult Mediterranean and natal fruit flies (Ceratitis capitata and Ceratitis rosa): Effects of age, gender and feeding status. J. Therm. Biol. 2009, 34, 406–414. [Google Scholar] [CrossRef]
- Tanga, C.M.; Khamis, F.M.; Tonnang, H.E.; Rwomushana, I.; Mosomtai, G.; Mohamed, S.A.; Ekesi, S. Risk assessment and spread of the potentially invasive Ceratitis rosa Karsch and Ceratitis quilicii De Meyer, Mwatawala & Virgilio Sp. Nov. Using life-cycle simulation models: Implications for phytosanitary measures and management. PLoS ONE 2018, 13, e0189138. [Google Scholar]
- Bale, J.S.; Hansen, T.N.; Nishino, M.; Baust, J.G. Effect of cooling rate on the survival of larvae, pupariation, and adult emergence of the gallfly Eurosta solidaginis. Cryobiology 1989, 26, 285–289. [Google Scholar] [CrossRef]
- Irwin, J.T.; Lee Jr, R.E. Mild winter temperatures reduce survival and potential fecundity of the goldenrod gall fly, Eurosta solidaginis (Diptera: Tephritidae). J. Insect Physiol. 2000, 46, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Lee Jr, R.E.; Dommel, R.A.; Joplin, K.H.; Denlinger, D.L. Cryobiology of the freeze-tolerant gall fly Eurosta solidaginis: Overwintering energetics and heat shock proteins. Clim. Res. 1995, 5, 61–67. [Google Scholar]
- Lee Jr, R.E.; McGrath, J.J.; Morason, R.T.; Taddeo, R.M. Survival of intracellular freezing, lipid coalescence and osmotic fragility in fat body cells of the freeze-tolerant gall fly Eurosta solidaginis. J. Insect Physiol. 1993, 39, 445–450. [Google Scholar]
- Moraiti, C.A.; Verykouki, E.; Papadopoulos, N.T. Fitness cost of Rhagoletis cerasi (Diptera: Tephritidae) adults emerged from pupae with different dormancy regimes: The case of prolonged chilling. Bull. Entomol. Res. 2023, 113, 11–20. [Google Scholar] [CrossRef]
- Neven, L.G. Reduction of optimal thermal range in aging Western cherry fruit flies (Diptera: Tephritidae). J. Insect Sci. 2015, 15, 77. [Google Scholar] [CrossRef]
- Andersen, J.L.; Manenti, T.; Sørensen, J.G.; MacMillan, H.A.; Loeschcke, V.; Overgaard, J. How to assess Drosophila cold tolerance: Chill coma temperature and lower lethal temperature are the best predictors of cold distribution limits. Funct. Ecol. 2015, 29, 55–65. [Google Scholar] [CrossRef]
- Nyamukondiwa, C.; Kleynhans, E.; Terblanche, J.S. Phenotypic plasticity of thermal tolerance contributes to the invasion potential of Mediterranean fruit flies (Ceratitis capitata). Ecol. Entomol. 2010, 35, 565–575. [Google Scholar] [CrossRef]
- Nyamukondiwa, C.; Terblanche, J.S. Within-generation variation of Critical Thermal Limits in adult Mediterranean and natal fruit flies Ceratitis capitata and Ceratitis rosa: Thermal history affects short-term responses to temperature. Physiol. Entomol. 2010, 35, 255–264. [Google Scholar] [CrossRef]
- Steyn, V.M. Causes and Consequences of Dispersal in the Mediterranean Fruit Fly, Ceratitis capitata. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2015. [Google Scholar]
- Steyn, V.M.; Mitchell, K.A.; Nyamukondiwa, C.; Terblanche, J.S. Understanding costs and benefits of thermal plasticity for pest management: Insights from the integration of laboratory, Semi-field and field assessments of Ceratitis capitata (Diptera: Tephritidae). Bull. Entomol. Res. 2022, 112, 458–468. [Google Scholar] [CrossRef]
- Weldon, C.W.; Nyamukondiwa, C.; Karsten, M.; Chown, S.L.; Terblanche, J.S. Geographic variation and plasticity in climate stress resistance among Southern African populations of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Sci. Rep. 2018, 8, 9849. [Google Scholar] [CrossRef]
- Basson, C.H.; Nyamukondiwa, C.; Terblanche, J.S. Fitness costs of rapid cold-hardening in Ceratitis capitata. Evolution 2012, 66, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Moraiti, C.A.; Verykouki, E.; Papadopoulos, N.T. Chill coma recovery of Ceratitis capitata adults across the Northern Hemisphere. Sci. Rep. 2022, 12, 17555. [Google Scholar] [CrossRef] [PubMed]
- Pujol-Lereis, L.M.; Fagali, N.S.; Rabossi, A.; Catalá, Á.; Quesada-Allué, L.A. Chill-coma recovery time, age and sex determine lipid profiles in Ceratitis capitata tissues. J. Insect Physiol. 2016, 87, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Pujol-Lereis, L.M.; Rabossi, A.; Quesada-Allué, L.A. Analysis of survival, gene expression and behavior following chill-coma in the medfly Ceratitis capitata: Effects of population heterogeneity and age. J. Insect Physiol. 2014, 71, 156–163. [Google Scholar] [CrossRef]
- Nyamukondiwa, C.; Weldon, C.W.; Chown, S.L.; Le Roux, P.C.; Terblanche, J.S. Thermal biology, population fluctuations and implications of temperature extremes for the management of two globally significant insect pests. J. Insect Physiol. 2013, 59, 1199–1211. [Google Scholar] [CrossRef]
- Esterhuizen, N.; Clusella-Trullas, S.; Van Daalen, C.E.; Schoombie, R.E.; Boardman, L.; Terblanche, J.S. Effects of within-generation thermal history on the flight performance of Ceratitis capitata: Colder Is Better. J. Exp. Biol. 2014, 217, 3545–3556. [Google Scholar] [CrossRef]
- Al-Behadili, F.J.; Bilgi, V.; Li, J.; Wang, P.; Taniguchi, M.; Agarwal, M.; Ren, Y.; Xu, W. Cold response of the Mediterranean fruit fly (Ceratitis capitata) on a lab diet. Insects 2019, 10, 48. [Google Scholar] [CrossRef]
- Cavalloro, C.V. Fruit Flies of Economic Importance 84; CRC Press: Boca Raton, FL, USA, 1986; Volume 9647. [Google Scholar]
- Dionysopoulou, N.K.; Papanastasiou, S.A.; Kyritsis, G.A.; Papadopoulos, N.T. Effect of host fruit, temperature and Wolbachia infection on survival and development of Ceratitis capitata immature stages. PLoS ONE 2020, 15, e0229727. [Google Scholar] [CrossRef]
- Duyck, P.F.; Quilici, S. Survival and development of different life stages of three Ceratitis spp. (Diptera: Tephritidae) reared at five constant temperatures. Bull. Entomol. Res. 2002, 92, 461–469. [Google Scholar] [CrossRef]
- Grout, T.G.; Stoltz, K.C. Developmental rates at constant temperatures of three economically important Ceratitis spp.(Diptera: Tephritidae) from Southern Africa. Environ. Entomol. 2014, 36, 1310–1317. [Google Scholar] [CrossRef]
- Manrakhan, A.; Daneel, J.H.; Stephen, P.R.; Hattingh, V. Cold tolerance of immature stages of Ceratitis capitata and Bactrocera dorsalis (Diptera: Tephritidae). J. Econ. Entomol. 2022, 115, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Ricalde, M.P.; Nava, D.E.; Loeck, A.E.; Donatti, M.G. Temperature-dependent development and survival of Brazilian populations of the Mediterranean fruit fly, Ceratitis capitata, from tropical, subtropical and temperate regions. J. Insect Sci. 2012, 12, 33. [Google Scholar] [PubMed]
- Shoukry, A.; Hafez, M. Studies on the biology of the Mediterranean fruit fly Ceratitis capitata. Entomol. Exp. Appl. 1979, 26, 33–39. [Google Scholar] [CrossRef]
- Vargas, R.I.; Walsh, W.A.; Jang, E.B.; Armstrong, J.W.; Kanehisa, D.T. Survival and development of immature stages of four Hawaiian fruit flies (Diptera: Tephritidae) reared at five constant temperatures. Ann. Entomol. Soc. Am. 1996, 89, 64–69. [Google Scholar] [CrossRef]
- Climate-Data.Org. Available online: https://en.climate-data.org/ (accessed on 23 May 2023).
- Papanastasiou, S.A.; Carey, J.R.; Papadopoulos, N.T. Effects of early-life protein starvation on longevity and sexual performance of male medfly. PLoS ONE 2019, 14, e0219518. [Google Scholar] [CrossRef] [PubMed]
- Diamantidis, A.D.; Papadopoulos, N.T.; Carey, J.R. Medfly populations differ in diel and age patterns of sexual signalling. Entomol. Exp. Appl. 2008, 128, 389–397. [Google Scholar] [CrossRef]
- Boller, E.F. Rhagoletis cerasi and Ceratitis capitata. Handb. Insect Rearing. 1985, 2, 135–144. [Google Scholar]
- Little, H.F.; Kobayashi, R.M.; Ozaki, E.T.; Cunningham, R.T. Irreversible damage to flight muscles resulting from disturbance of pupae during rearing of the Mediterranean fruit fly, Ceratitis capitata. Ann. Entomol. Soc. Am. 1981, 74, 24–26. [Google Scholar] [CrossRef]
- Rabossi, A.; Wappner, P.; Quesada-Allué, L.A. Larva to pharate adult transformation in the medfly Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Can. Entomol. 1992, 124, 1139–1147. [Google Scholar] [CrossRef]
- Chan Jr, H.T.; Hansen, J.D.; Tam, S.Y. Larval diets from different protein sources for Mediterranean fruit flies (Diptera: Tephritidae). J. Econ. Entomol. 1990, 83, 1954–1958. [Google Scholar] [CrossRef]
- Churchill-Stanland, C.; Stanland, R.; Wong, T.T.; Tanaka, N.; McInnis, D.O.; Dowell, R.V. Size as a factor in the mating propensity of Mediterranean fruit flies, Ceratitis capitata (Diptera: Tephritidae), in the laboratory. J. Econ. Entomol. 1986, 79, 614–619. [Google Scholar] [CrossRef]
- Enkerlin, W.R.; Gutiérrez Ruelas, J.M.; Pantaleon, R.; Soto Litera, C.; Villaseñor Cortés, A.; Zavala López, J.L.; Orozco Dávila, D.; Montoya Gerardo, P.; Silva Villarreal, L.; Cotoc Roldán, E. The Moscamed regional programme: Review of a success story of area-wide Sterile Insect Technique application. Entomol. Exp. Appl. 2017, 164, 188–203. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. 1925. J. Am. Mosq. Control Assoc. 1987, 3, 302–303. [Google Scholar] [PubMed]
- World Health Organization. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes, 2nd ed.; World Health Organization: Geneva, Switzerland, 2016; pp. 26–33. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer-Verlag: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A. ggpubr: “ggplot2” Based Publication Ready Plots; R Package Version 0.4.0. 2023. Available online: https://cran.r-project.org/web/packages/ggpubr/index.html (accessed on 23 May 2023).
- Addo-Bediako, A.; Chown, S.L.; Gaston, K.J. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Nation Sr, J.L. Reproduction. In Insect Physiology and Biochemistry, 4th ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 447–469. [Google Scholar]
- Tufail, M.; Takeda, M. Molecular characteristics of insect vitellogenins. J. Insect Physiol. 2008, 54, 1447–1458. [Google Scholar] [CrossRef]
- Margaritis, L.H.; Kafatos, F.C.; Petri, W.H. The eggshell of Drosophila melanogaster: I. Fine structure of the layers and regions of the wild-type eggshell. J. Cell Sci. 1980, 43, 1–35. [Google Scholar] [CrossRef]
- Mouzaki, D.G.; Margaritis, L.H. Choriogenesis in the medfly Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Int. J. Insect Morphol. Embryol. 1991, 20, 51–68. [Google Scholar] [CrossRef]
- Koštál, V.; Korbelová, J.; Poupardin, R.; Moos, M.; Šimek, P. Arginine and proline applied as food additives stimulate high freeze tolerance in larvae of Drosophila melanogaster. J. Exp. Biol. 2016, 219, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Mollaei, M.; Hoseini, S.A.; Karimi, M.; Hekmat, Z. Impact of the amino acid proline on the cold hardiness of honey bee, Apis mellifera L. Span. J. Agric. Res. 2013, 11, 714–717. [Google Scholar] [CrossRef]
- Zarani, F.E.; Margaritis, L.H. Fine structure and morphogenesis of the micropylar apparatus in the medfly Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Int. J. Insect Morphol. Embryol. 1991, 20, 127–139. [Google Scholar] [CrossRef]
- Margaritis, L.H. Structure and physiology of the eggshell. Compr. Insect Physiol. Biochem. Pharmacol. Embryog. Reprod. 1985, 1, 153–230. [Google Scholar]
- Waring, G.L. Morphogenesis of the eggshells in Drosophila. Int. Rev. Cytol. 2000, 198, 67–108. [Google Scholar] [PubMed]
- Agrell, I.P.; Lundquist, A.M. Physiological and biochemical changes during insect development. In The Physiology of Insecta; Elsevier: Amsterdam, The Netherlands, 1973; pp. 159–247. [Google Scholar]
- Langley, P.A. Physiology of the Mediterranean fruit fly in relation to the Sterile-Male Technique. In Sterile-Male Technique for Control of Fruit Flies; International Atomic Energy Agency: Vienna, Austria, 1970. [Google Scholar]
- Nestel, D.; Tolmasky, D.; Rabossi, A.; Quesada-Allué, L.A. Lipid, Carbohydrates and protein patterns during metamorphosis of the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2003, 96, 237–244. [Google Scholar] [CrossRef]
- Papadopoulos, N.T.; Katsoyannos, B.I.; Carey, J.R. Temporal changes in the composition of the overwintering larval population of the Mediterranean fruit fly (Diptera: Tephritidae) in Northern Greece. Ann. Entomol. Soc. Am. 1998, 91, 430–434. [Google Scholar] [CrossRef]
- Jessup, A.J.; De Lima, C.P.F.; Hood, C.W.; Sloggett, R.F.; Harris, A.M.; Beckingham, M. Quarantine disinfestation of lemons against Bactrocera tryoni and Ceratitis capitata (Diptera: Tephritidae) using cold storage. J. Econ. Entomol. 1993, 86, 798–802. [Google Scholar] [CrossRef]
- Colinet, H.; Hance, T.; Vernon, P. Water Relations, Fat reserves, survival, and longevity of a cold-exposed parasitic wasp Aphidius colemani (Hymenoptera: Aphidiinae). Environ. Entomol. 2006, 35, 228–236. [Google Scholar] [CrossRef]
- Terblanche, J.S.; Clusella-Trullas, S.; Deere, J.A.; Chown, S.L. Thermal tolerance in a South-East African population of the tsetse fly Glossina pallidipes (Diptera, Glossinidae): Implications for forecasting climate change impacts. J. Insect Physiol. 2008, 54, 114–127. [Google Scholar] [CrossRef]
- Shreve, S.M.; Yi, S.-X.; Lee, R.E. Increased dietary cholesterol enhances cold tolerance in Drosophila melanogaster. Cryoletters. 2007, 28, 33–37. [Google Scholar]
- Enriquez, T.; Teets, N.M. Lipid metabolism in response to cold. EcoEvoRxiv 2023. [Google Scholar] [CrossRef]
- Tanaka, K.; Udagawa, T. Cold adaptation of the terrestrial isopod, Porcellio scaber, to subnivean environments. J. Comp. Physiol. B 1993, 163, 439–444. [Google Scholar] [CrossRef]
- Renault, D.; Salin, C.; Vannier, G.; Vernon, P. Survival at low temperatures in insects: What is the ecological significance of the supercooling point? CryoLetters 2002, 23, 217–228. [Google Scholar] [PubMed]
- Andreadis, S.S.; Athanassiou, C.G. A review of insect cold hardiness and its potential in stored product insect control. Crop Prot. 2017, 91, 93–99. [Google Scholar] [CrossRef]
- Mohammadzadeh, M.; Izadi, H. Enzyme activity, cold hardiness, and supercooling point in developmental stages of Acrosternum arabicum (Hemiptera: Pentatomidae). J. Insect Sci. 2016, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, J.; Sørensen, J.G.; Petersen, S.O.; Loeschcke, V.; Holmstrup, M. Changes in membrane lipid composition following rapid cold hardening in Drosophila melanogaster. J. Insect Physiol. 2005, 51, 1173–1182. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Biochemistry of Cryoprotectants. In Insects at Low Temperature; Springer: Berlin/Heidelberg, Germany, 1991; pp. 64–93. [Google Scholar]
- Bemani, M.; Izadi, H.; Mahdian, K.; Khani, A. Study on the physiology of diapause, cold hardiness and supercooling point of overwintering pupae of the pistachio fruit hull borer, Arimania comaroffi. J. Insect Physiol. 2012, 58, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Heydari, M.; Izadi, H. Effects of seasonal acclimation on cold tolerance and biochemical status of the carob moth, Ectomyelois ceratoniae Zeller, last instar larvae. Bull. Entomol. Res. 2014, 104, 592–600. [Google Scholar] [CrossRef]
- Holmstrup, M.; Bayley, M.; Ramløv, H. Supercool or dehydrate? An experimental analysis of overwintering strategies in small permeable arctic invertebrates. Proc. Natl. Acad. Sci. USA 2002, 99, 5716–5720. [Google Scholar] [CrossRef]
- Storey, K.B. Organic solutes in freezing tolerance. Comp. Biochem. Physiol. A Physiol. 1997, 117, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, D.L. Regulation of diapause. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef]
- Argyriou, A.V.; Sarris, A.; Teeuw, R.M. Using geoinformatics and geomorphometrics to quantify the geodiversity of Crete, Greece. Int. J. Appl. Earth Obs. Geoinf. 2016, 51, 47–59. [Google Scholar] [CrossRef]
- Mahajan-Miklos, S.; Cooley, L. Intercellular cytoplasm transport during Drosophila oogenesis. Dev. Biol. 1994, 165, 336–351. [Google Scholar] [CrossRef] [PubMed]
- De Lima, C.P.F.; Jessup, A.J.; Cruickshank, L.; Walsh, C.J.; Mansfield, E.R. Cold disinfestation of citrus (Citrus spp.) for Mediterranean fruit fly (Ceratitis capitata) and Queensland fruit fly (Bactrocera tryoni) (Diptera: Tephritidae). N. Z. J. Crop Hortic. Sci. 2007, 35, 39–50. [Google Scholar] [CrossRef]
- Hallman, G.J.; Wang, L.; Demirbas Uzel, G.; Cancio-Martinez, E.; Cáceres-Barrios, C.E.; Myers, S.W.; Vreysen, M.J. Comparison of populations of Ceratitis capitata (Diptera: Tephritidae) from three continents for susceptibility to cold phytosanitary treatment and implications for generic cold treatments. J. Econ. Entomol. 2019, 112, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.R.; Rigney, C.J.; Sproul, A.N. Cold storage of oranges as a disinfestation treatment against the fruit flies Dacus tryoni (Froggau) and Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). J. Econ. Entomol. 1988, 81, 257–260. [Google Scholar] [CrossRef]
- Willink, E.; Gastaminza, G.; Salvatore, A.; Gramajo, M.C.; Aceñolaza, M.; Avila, R.; Favre, P. Quarantine cold treatments for Ceratitis capitata and Anastrepha fraterculus (Diptera: Tephritidae) for citrus in Argentina: Con-clusions after 10 years of research. In Proceedings of the International Symposium on Fruit Flies of Economic Importance: From Basic to Applied Knowledge, Salvador, Brazil, 10–15 September 2006. [Google Scholar]
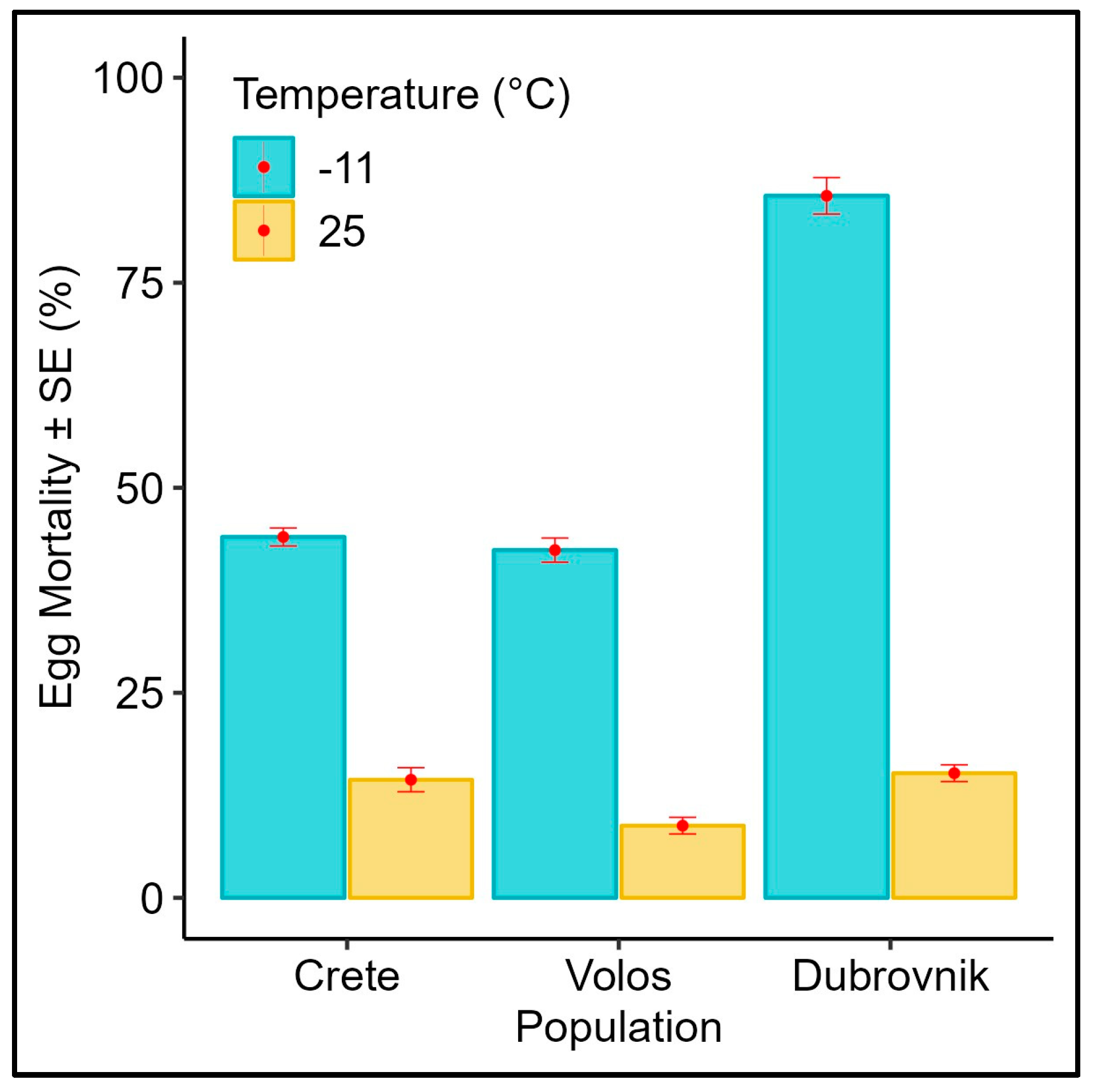
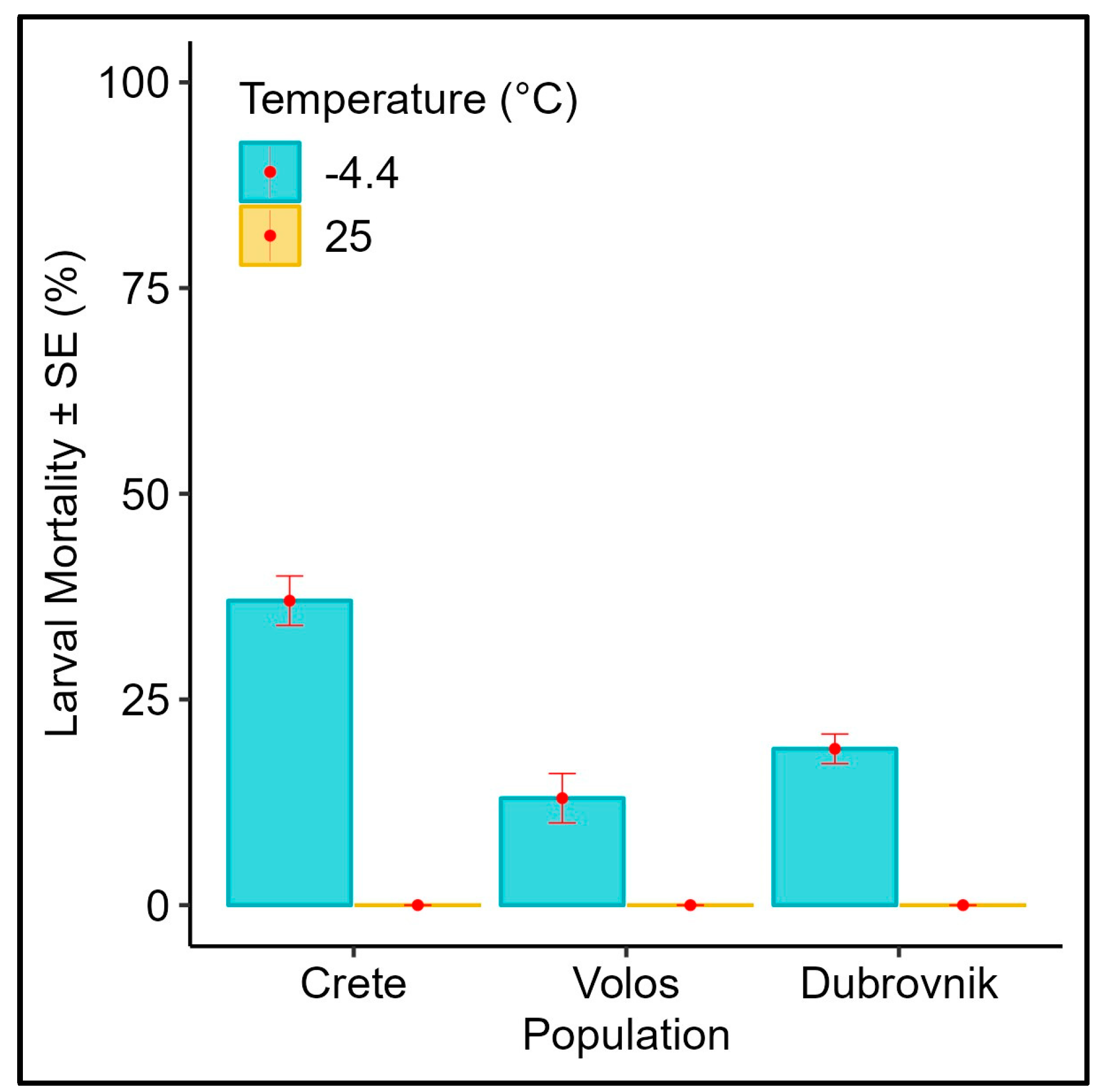
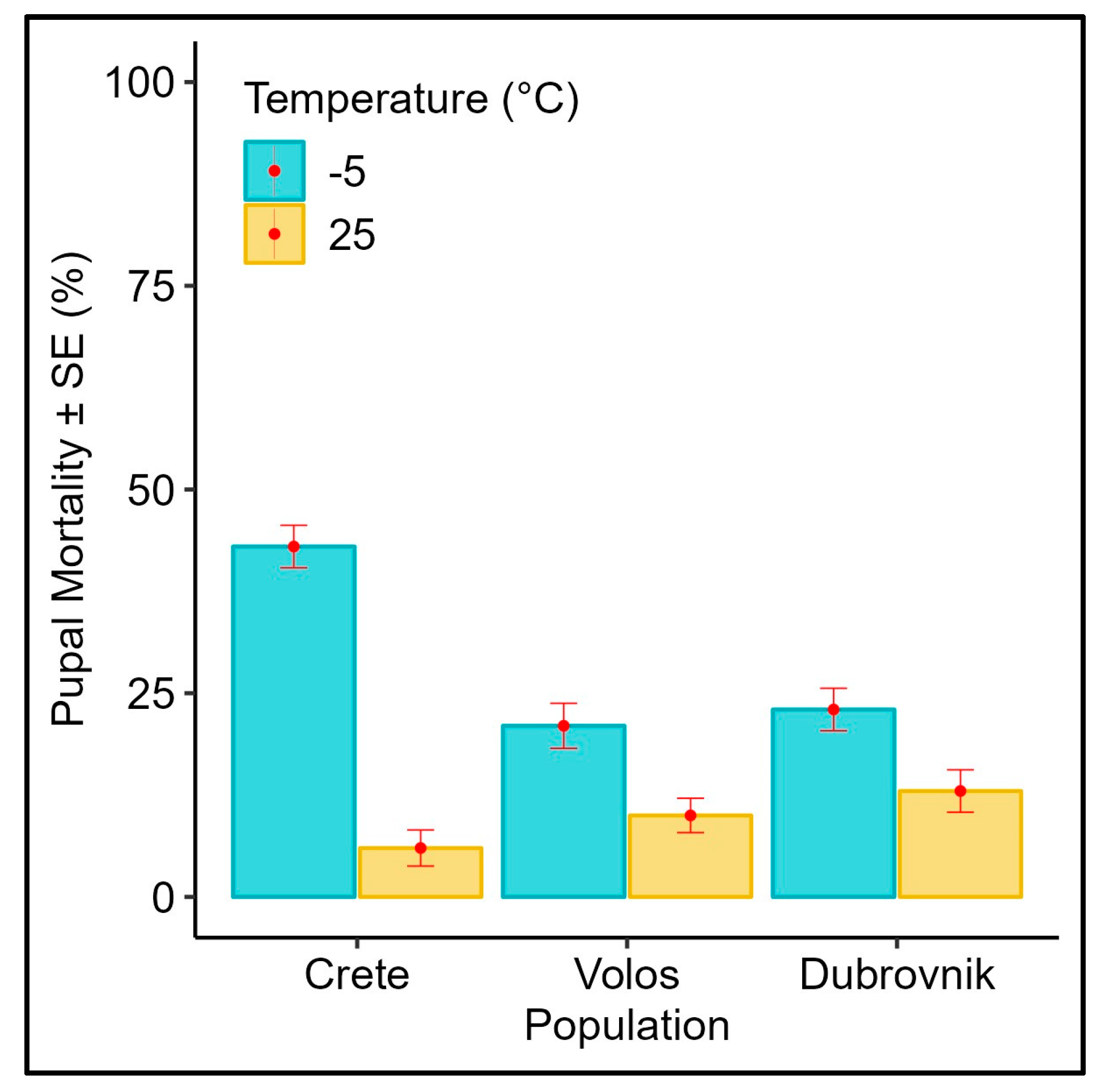
| Source of Variance | Dependent Variable | χ2 | df | p Value |
|---|---|---|---|---|
| (−11 °C) | ||||
| Population | Egg Mortality | 104.24 | 2 | <0.001 |
| (25 °C) | ||||
| Population | Egg Mortality | 5.33 | 2 | ns |
| (−4.4 °C) | ||||
| Population | Larval Mortality | 16.62 | 2 | <0.001 |
| (25 °C) | ||||
| Population | Larval Mortality | * | * | * |
| (−5 °C) | ||||
| Population | Pupal Mortality | 13.92 | 2 | =0.001 |
| (25 °C) | ||||
| Population | Pupal Mortality | 2.72 | 2 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulos, A.G.; Koskinioti, P.; Zarpas, K.D.; Papadopoulos, N.T. Differential Cold Tolerance on Immature Stages of Geographically Divergent Ceratitis capitata Populations. Biology 2023, 12, 1379. https://doi.org/10.3390/biology12111379
Papadopoulos AG, Koskinioti P, Zarpas KD, Papadopoulos NT. Differential Cold Tolerance on Immature Stages of Geographically Divergent Ceratitis capitata Populations. Biology. 2023; 12(11):1379. https://doi.org/10.3390/biology12111379
Chicago/Turabian StylePapadopoulos, Antonis G., Panagiota Koskinioti, Kostas D. Zarpas, and Nikos T. Papadopoulos. 2023. "Differential Cold Tolerance on Immature Stages of Geographically Divergent Ceratitis capitata Populations" Biology 12, no. 11: 1379. https://doi.org/10.3390/biology12111379
APA StylePapadopoulos, A. G., Koskinioti, P., Zarpas, K. D., & Papadopoulos, N. T. (2023). Differential Cold Tolerance on Immature Stages of Geographically Divergent Ceratitis capitata Populations. Biology, 12(11), 1379. https://doi.org/10.3390/biology12111379






