Essential Trace Elements Status in Portuguese Pregnant Women and Their Association with Maternal and Neonatal Outcomes: A Prospective Study from the IoMum Cohort
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
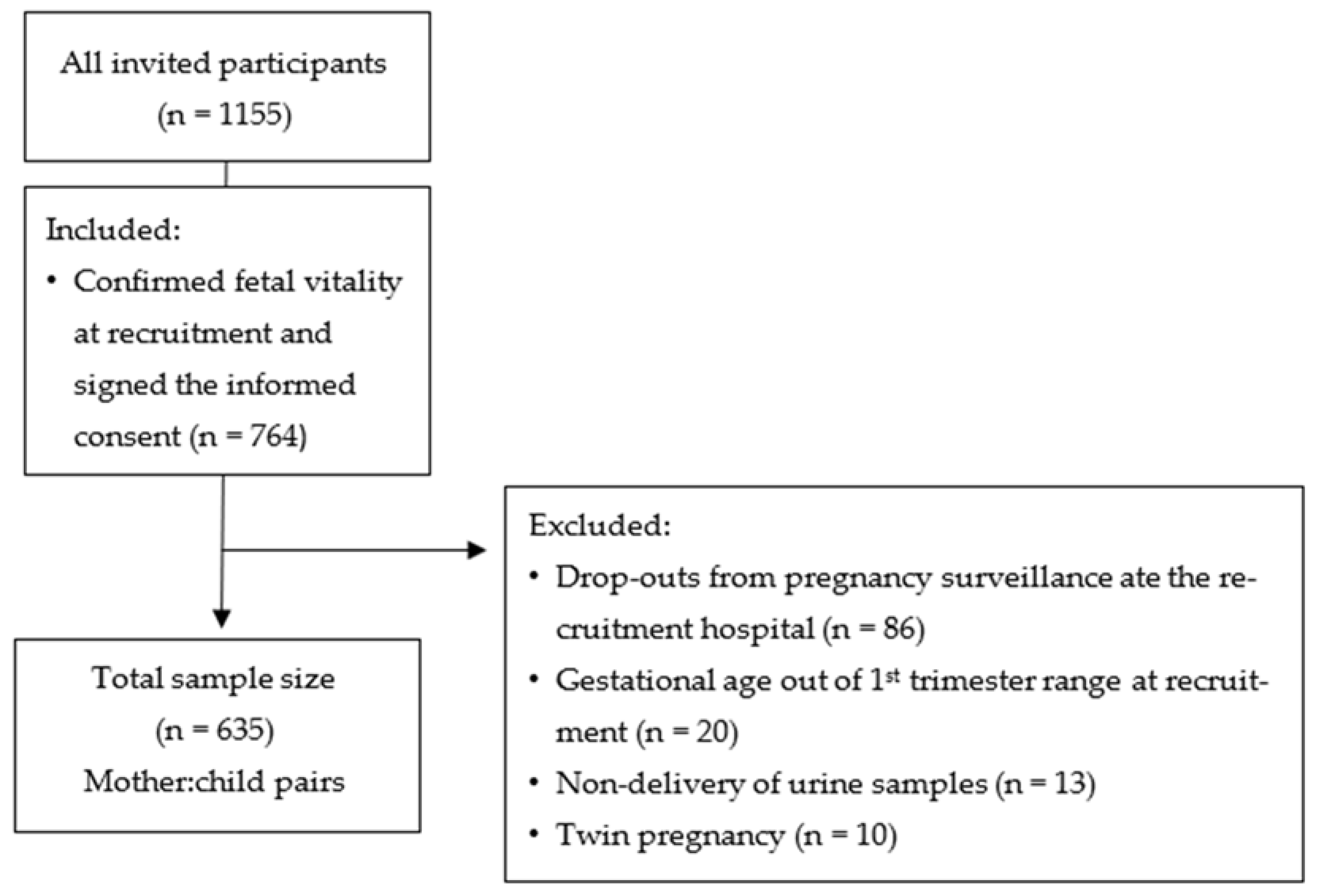
2.2. Study Design and Population
2.3. Biochemical Analyses
Creatinine Determination
2.4. Variables
2.4.1. Categorization of Anthropometric Percentiles at Birth
2.4.2. Classification of Gestational Weight Variation
2.4.3. Categorization of Supplementation Use
2.5. Statistical Analysis
3. Results
3.1. Study Sample Characterization
3.2. ETEs Levels and Association with Sociodemographic Characteristics
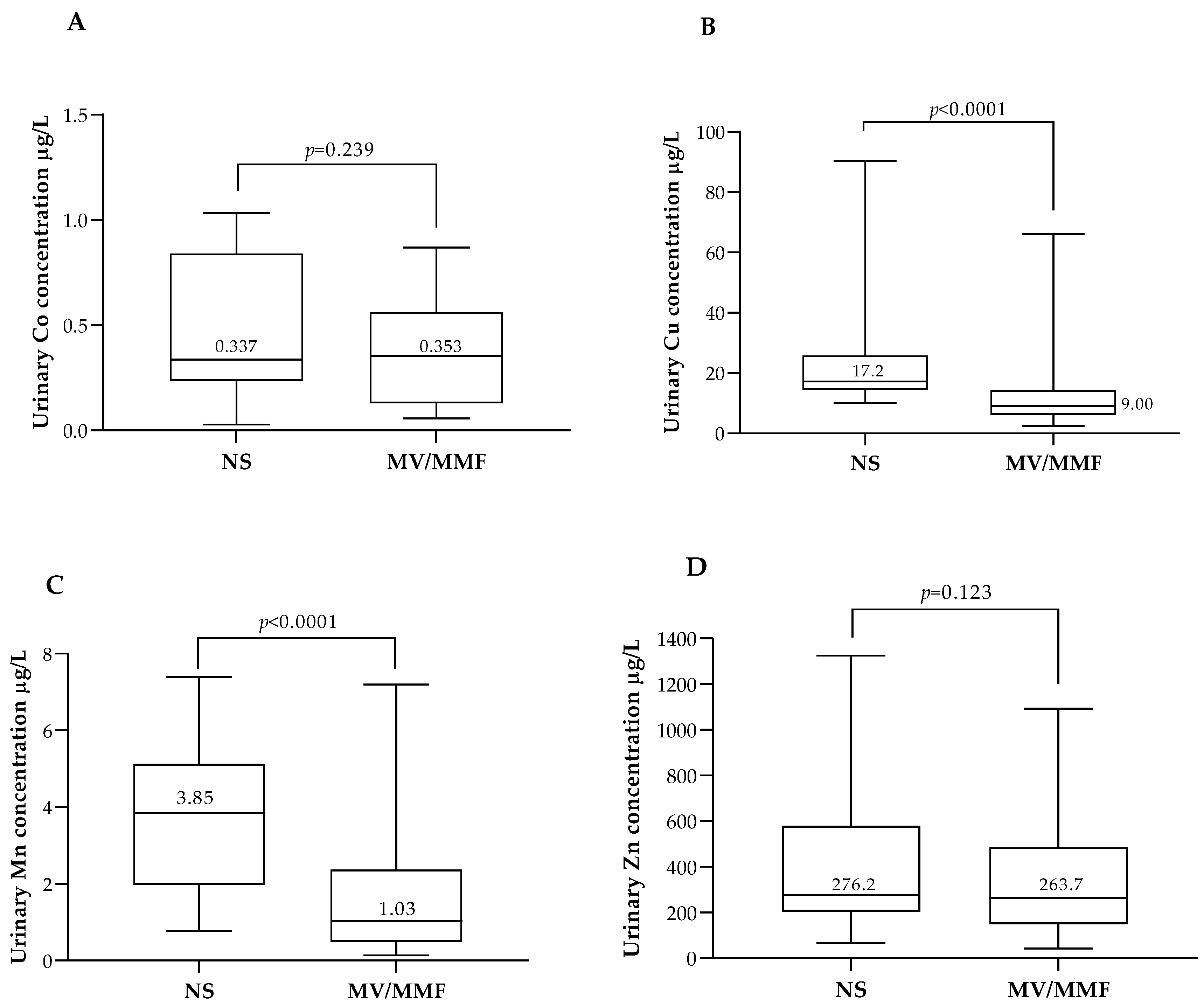
3.3. ETEs Status in Association with Food Intake and Food Supplementation
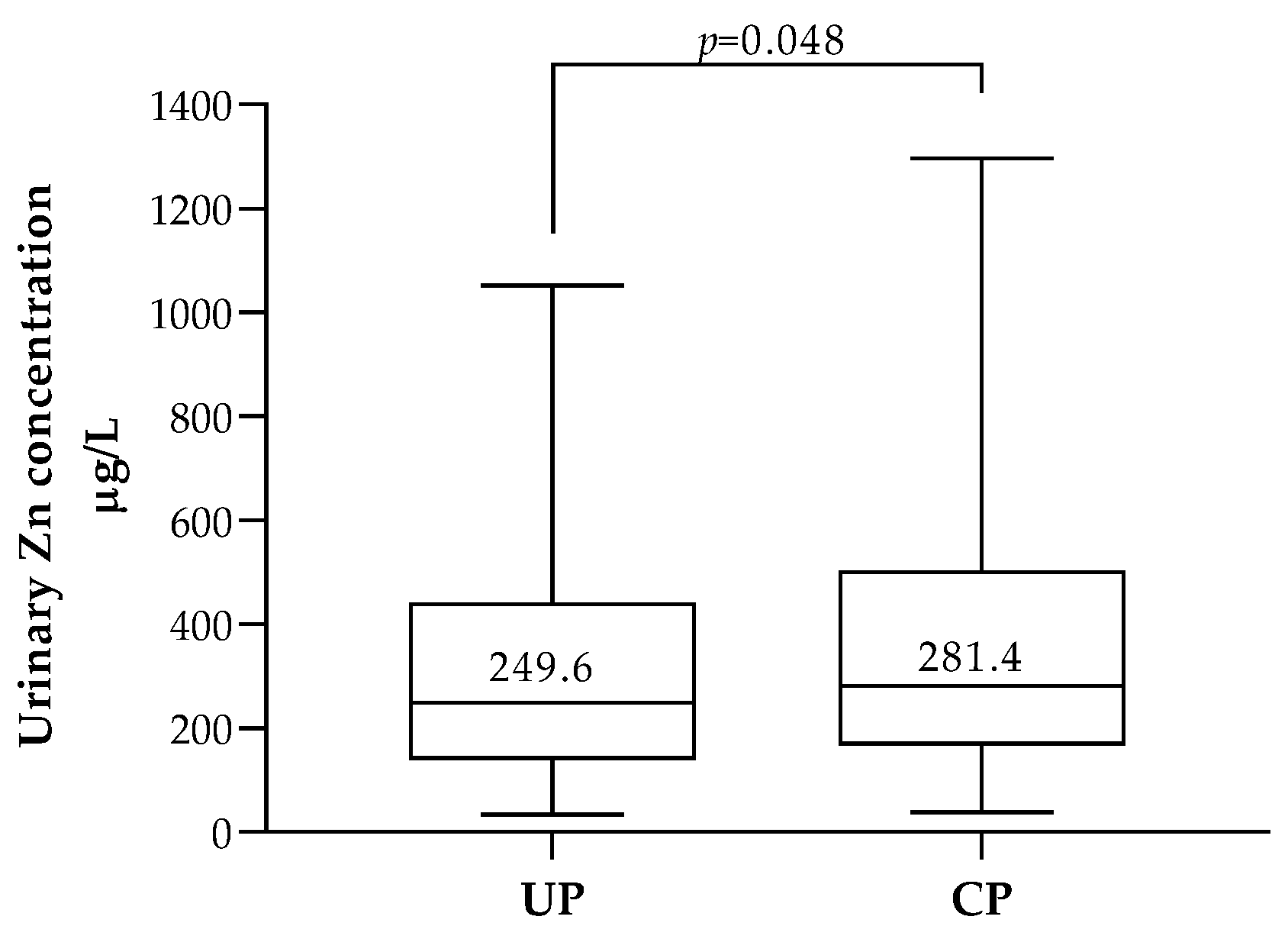
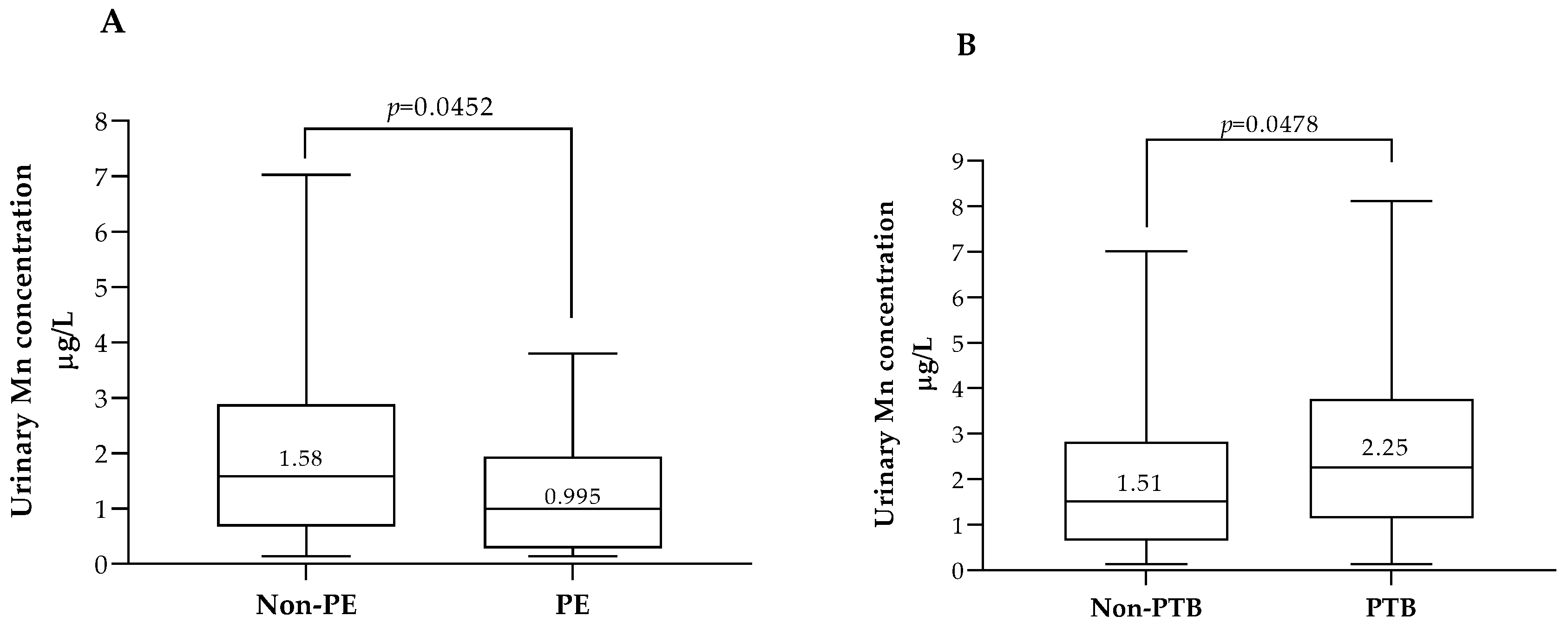
3.4. Association between ETEs, Pregnancy Outcomes and Neonatal Anthropometric Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P., Jr.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chauhan, S.P.; Ward, T.C.; Mori, N.; Gass, E.T.; Cisler, R.A. Aberrant fetal growth and early, late, and postneonatal mortality: An analysis of Milwaukee births, 1996-2007. Am. J. Obstet. Gynecol. 2011, 204, 261.e1–261.e10. [Google Scholar] [CrossRef] [PubMed]
- Frieden, E. New perspectives on the essential trace elements. J. Chem. Educ. 1985, 62, 917. [Google Scholar] [CrossRef]
- World Health Organization; Food Agriculture Organization of the United Nations. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Lewicka, I.; Kocyłowski, R.; Grzesiak, M.; Gaj, Z.; Oszukowski, P.; Suliburska, J. Selected trace elements concentrations in pregnancy and their possible role - literature review. Ginekol. Polska 2017, 88, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Finley, B.L.; Monnot, A.D.; Paustenbach, D.J.; Gaffney, S.H. Derivation of a chronic oral reference dose for cobalt. Regul. Toxicol. Pharmacol. 2012, 64, 491–503. [Google Scholar] [CrossRef]
- Kim, J.H.; Gibb, H.J.; Howe, P.D.; World Health Organization; International Programme on Chemical Safety. Cobalt and Inorganic Cobalt Compounds; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Schrauzer, G.N. Cobalt. In Elements and Their Compounds in the Environment, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; pp. 825–839. [Google Scholar]
- Institute of Medicine (IOM). Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B 6, Folate, Vitamin B 12, Pantothenic Acid, Biotin, and Choline. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B(6), Folate, Vitamin B(12), Pantothenic Acid, Biotin, and Choline; National Academies Press (US): Washington, DC, USA, 1998. [Google Scholar]
- Khayat, S.; Fanaei, H.; Ghanbarzehi, A. Minerals in Pregnancy and Lactation: A Review Article. J. Clin. Diagn. Res. 2017, 11, Qe01–Qe05. [Google Scholar] [CrossRef]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef]
- National Institutes of Health. Copper-Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Copper-HealthProfessional/#en1 (accessed on 11 June 2022).
- Cetin, I.; Berti, C.; Calabrese, S. Role of micronutrients in the periconceptional period. Hum. Reprod. Update 2010, 16, 80–95. [Google Scholar] [CrossRef]
- Spencer, B. Essentiality of Trace Element Micronutrition in Human Pregnancy: A Systematic Review. J. Pregnancy Child Health 2015, 2, 3073009. [Google Scholar] [CrossRef]
- Grzeszczak, K.; Kwiatkowski, S.; Kosik-Bogacka, D. The Role of Fe, Zn, and Cu in Pregnancy. Biomolecules 2020, 10, 1176. [Google Scholar] [CrossRef]
- Institute of Medicine (IOM). Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; The National Academies Press: Washington, DC, USA, 2001; p. 800. [Google Scholar]
- Aschner, J.L.; Aschner, M. Nutritional aspects of manganese homeostasis. Mol. Asp. Med. 2005, 26, 353–362. [Google Scholar] [CrossRef]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxidative Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture Agricultural Research Service. Nutrient Lists from Standard Reference Legacy-Manganese; US. Department of Agriculture Agricultural Research Service: Washington, DC, USA, 2018.
- Novotny, J.A. Molybdenum Nutriture in Humans. J. Evid.-Based Complement. Altern. Med. 2011, 16, 164–168. [Google Scholar] [CrossRef]
- Novotny, J.A.; Peterson, C.A. Molybdenum. Adv. Nutr. 2018, 9, 272–273. [Google Scholar] [CrossRef]
- Barceloux, D.G. Zinc. J. Toxicol. Clin. Toxicol. 1999, 37, 279–292. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Zinc; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2005.
- Mistry, H.D.; Williams, P.J. The importance of antioxidant micronutrients in pregnancy. Oxid. Med. Cell. Longev. 2011, 2011, 841749. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Sun, J.; Yoo, H.; Kim, S.; Cho, Y.Y.; Kim, H.J.; Kim, S.W.; Chung, J.H.; Oh, S.Y.; Lee, S.Y. A prospective study of serum trace elements in healthy Korean pregnant women. Nutrients 2016, 8, 749. [Google Scholar] [CrossRef]
- Lewandowska, M.; Więeeckowska, B.; Sajdak, S.; Lubiński, J. First trimester microelements and their relationships with pregnancy outcomes and complications. Nutrients 2020, 12, 1108. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Wang, J.; Xia, X.; Wang, Q.; Li, Z.; Tao, R.; Tao, Y.; Xiang, H.; Tong, S.; Tao, F. Serum cobalt status during pregnancy and the risks of pregnancy-induced hypertension syndrome: A prospective birth cohort study. J. Trace Elem. Med. Biol. 2018, 46, 39–45. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, L.; Chen, X.; Zhou, J.; Liu, J.; Chen, J. Association between zinc level and the risk of preeclampsia: A meta-analysis. Arch. Gynecol. Obstet. 2016, 293, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Atazadegan, M.A.; Heidari-Beni, M.; Riahi, R.; Kelishadi, R. Association of selenium, zinc and copper concentrations during pregnancy with birth weight: A systematic review and meta-analysis. J. Trace Elem. Med. Biol. 2022, 69, 126903. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, C.; Weisskopf, M.; Williams, P.L.; Parsons, P.J.; Palmer, C.D.; Buck Louis, G.M.; James-Todd, T. A Prospective Study of Early Pregnancy Essential Metal(loid)s and Glucose Levels Late in the Second Trimester. J. Clin. Endocrinol. Metab. 2019, 104, 4295–4303. [Google Scholar] [CrossRef] [PubMed]
- Vigeh, M.; Yokoyama, K.; Ramezanzadeh, F.; Dahaghin, M.; Fakhriazad, E.; Seyedaghamiri, Z.; Araki, S. Blood manganese concentrations and intrauterine growth restriction. Reprod. Toxicol. 2008, 25, 219–223. [Google Scholar] [CrossRef]
- Watson, C.V.; Lewin, M.; Ragin-Wilson, A.; Jones, R.; Jarrett, J.M.; Wallon, K.; Ward, C.; Hilliard, N.; Irvin-Barnwell, E. Characterization of trace elements exposure in pregnant women in the United States, NHANES 1999-2016. Environ. Res. 2020, 183, 109208. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Human Biomonitoring: Facts and Figures; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Laboratory Procedure Manual: Urine Multi-Element ICP-DRC-MS. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/UHM_G_met_heavy_metals.pdf (accessed on 11 May 2022).
- Schulz, C.; Wilhelm, M.; Heudorf, U.; Kolossa-Gehring, M. Update of the reference and HBM values derived by the German Human Biomonitoring Commission. Int. J. Hyg. Environ. Health 2011, 215, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Cheikh Ismail, L.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Chauhan, S.P.; Rice, M.M.; Grobman, W.A.; Bailit, J.; Reddy, U.M.; Wapner, R.J.; Varner, M.W.; Thorp, J.M.; Leveno, K.J.; Caritis, S.N.; et al. Neonatal Morbidity of Small- and Large-for-Gestational-Age Neonates Born at Term in Uncomplicated Pregnancies. Obstet. Gynecol. 2017, 130, 511–519. [Google Scholar] [CrossRef]
- Medicine, I.o.; Council, N.R. Weight Gain During Pregnancy: Reexamining the Guidelines; The National Academies Press: Washington, DC, USA, 2009; p. 868. [Google Scholar]
- ARUP Lab Laboratories. Available online: https://www.aruplab.com/ (accessed on 12 July 2022).
- Bocca, B.; Ruggieri, F.; Pino, A.; Rovira, J.; Calamandrei, G.; Martínez, M.Á.; Domingo, J.L.; Alimonti, A.; Schuhmacher, M. Human biomonitoring to evaluate exposure to toxic and essential trace elements during pregnancy. Part A. concentrations in maternal blood, urine and cord blood. Environ. Res. 2019, 177, 108599. [Google Scholar] [CrossRef]
- Fort, M.; Grimalt, J.O.; Casas, M.; Sunyer, J. Interdependence between urinary cobalt concentrations and hemoglobin levels in pregnant women. Environ. Res. 2015, 136, 148–154. [Google Scholar] [CrossRef]
- Vázquez-Salas, R.A.; López-Carrillo, L.; Menezes-Filho, J.A.; Rothenberg, S.J.; Cebrián, M.E.; Schnaas, L.; Freitas de Souza Viana, G.; Torres-Sánchez, L. Prenatal molybdenum exposure and infant neurodevelopment in Mexican children. Nutr. Neurosci. 2014, 17, 72–80. [Google Scholar] [CrossRef]
- Hoet, P.; Jacquerye, C.; Deumer, G.; Lison, D.; Haufroid, V. Reference values and upper reference limits for 26 trace elements in the urine of adults living in Belgium. Clin. Chem. Lab. Med. 2013, 51, 839–849. [Google Scholar] [CrossRef]
- Goullé, J.P.; Mahieu, L.; Castermant, J.; Neveu, N.; Bonneau, L.; Lainé, G.; Bouige, D.; Lacroix, C. Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair: Reference values. Forensic Sci. Int. 2005, 153, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Heitland, P.; Köster, H.D. Biomonitoring of 30 trace elements in urine of children and adults by ICP-MS. Clin. Chim. Acta 2006, 365, 310–318. [Google Scholar] [CrossRef]
- Ohashi, F.; Fukui, Y.; Takada, S.; Moriguchi, J.; Ezaki, T.; Ikeda, M. Reference values for cobalt, copper, manganese, and nickel in urine among women of the general population in Japan. Int. Arch. Occup. Environ. Health 2006, 80, 117–126. [Google Scholar] [CrossRef]
- Amorim, M.; Melo, A.N. Revisiting head circumference of Brazilian newborns in public and private maternity hospitals. Arq. Neuropsiquiatr. 2017, 75, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Hackman, D.A.; Farah, M.J.; Meaney, M.J. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nat. Rev. Neurosci. 2010, 11, 651–659. [Google Scholar] [CrossRef]
- Matta Coelho, C.; Guimarães, J.; Bracchi, I.; Xavier Moreira, N.; Pinheiro, C.; Ferreira, P.; Pestana, D.; Barreiros Mota, I.; Cortez, A.; Prucha, C.; et al. Noncompliance to iodine supplementation recommendation is a risk factor for iodine insufficiency in Portuguese pregnant women: Results from the IoMum cohort. J. Endocrinol. Investig. 2022, 45, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Samson, L.; Hircsu, I.; Katko, M.; Bodor, M.; Gazdag, A.; Gazso, A.A.; Kovacs, B.; Posta, J.; Balogh, E.; Mocsary, P.; et al. Lower educational status interferes with maternal iodine intake during both pregnancy and lactation. Endocr. Connect. 2021, 10, 742–749. [Google Scholar] [CrossRef]
- Bocca, B.; Ruggieri, F.; Pino, A.; Rovira, J.; Calamandrei, G.; Mirabella, F.; Martínez, M.Á.; Domingo, J.L.; Alimonti, A.; Schuhmacher, M. Human biomonitoring to evaluate exposure to toxic and essential trace elements during pregnancy. Part B: Predictors of exposure. Environ. Res. 2020, 182, 109108. [Google Scholar] [CrossRef]
- Arredondo, M.; Martínez, R.; Núñez, M.T.; Ruz, M.; Olivares, M. Inhibition of iron and copper uptake by iron, copper and zinc. Biol. Res. 2006, 39, 95–102. [Google Scholar] [CrossRef]
- Illing, A.C.; Shawki, A.; Cunningham, C.L.; Mackenzie, B. Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. J. Biol. Chem. 2012, 287, 30485–30496. [Google Scholar] [CrossRef]
- Chen, P.; Bornhorst, J.; Aschner, M. Manganese metabolism in humans. Front. Biosci. (Landmark Ed.) 2018, 23, 1655–1679. [Google Scholar] [CrossRef] [PubMed]
- Thirumoorthy, N.; Manisenthil Kumar, K.T.; Shyam Sundar, A.; Panayappan, L.; Chatterjee, M. Metallothionein: An overview. World J. Gastroenterol. 2007, 13, 993–996. [Google Scholar] [CrossRef]
- Pinto, E.; Cruz, M.; Ramos, P.; Santos, A.; Almeida, A. Metals transfer from tobacco to cigarette smoke: Evidences in smokers’ lung tissue. J. Hazard. Mater. 2017, 325, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, D.; Rossmann, A.; Wick, G. Metals in cigarette smoke. Iubmb Life 2005, 57, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Hutter, H.P.; Wallner, P.; Moshammer, H.; Marsh, G. Dust and Cobalt Levels in the Austrian Tungsten Industry: Workplace and Human Biomonitoring Data. Int. J. Environ. Res. Public Health 2016, 13, 931. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Manganese; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2012.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Copper (Draft for Public Comment); U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2022.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Cobalt; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2004.
- Xu, Y.; Wei, Y.; Long, T.; Wang, R.; Li, Z.; Yu, C.; Wu, T.; He, M. Association between urinary metals levels and metabolic phenotypes in overweight and obese individuals. Chemosphere 2020, 254, 126763. [Google Scholar] [CrossRef]
- Gu, K.; Li, X.; Xiang, W.; Jiang, X. The Relationship Between Serum Copper and Overweight/Obesity: A Meta-analysis. Biol. Trace Elem. Res. 2020, 194, 336–347. [Google Scholar] [CrossRef]
- Sánchez, C.; López-Jurado, M.; Aranda, P.; Llopis, J. Plasma levels of copper, manganese and selenium in an adult population in southern Spain: Influence of age, obesity and lifestyle factors. Sci. Total Environ. 2010, 408, 1014–1020. [Google Scholar] [CrossRef]
- Stewart, L. Mineral Supplements for Beef Cattle; University of Georgia: Athens, GA, USA, 2010. [Google Scholar]
- Pinto, C.; Viana, J.; Aranha, P. Carências em Oligoelementos em Bovinos dos Açores. Available online: http://aasm-cua.com.pt/aDefInfTec.asp?ID=84 (accessed on 22 July 2022).
- Arthington, J.D.; Swensont, C.K. Effects of Trace Mineral Source and Feeding Method on the Productivity of Grazing Braford Cows1. Prof. Anim. Sci. 2004, 20, 155–161. [Google Scholar] [CrossRef]
- van den Berghe, P.V.E.; Klomp, L.W.J. New developments in the regulation of intestinal copper absorption. Nutr. Rev. 2009, 67, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Saúde Doutor Ricardo Jorge. Tabela da Composição dos Alimentos. Available online: https://portfir-insa.min-saude.pt/ (accessed on 10 June 2023).
- Sandström, B. Micronutrient interactions: Effects on absorption and bioavailability. Br. J. Nutr. 2001, 85 (Suppl. 2), S181–S185. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Hu, C.; Zheng, Y. Maternal serum zinc level is associated with risk of preeclampsia: A systematic review and meta-analysis. Front. Public Health 2022, 10, 968045. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Zhou, Y.; Zheng, T.; Zhang, B.; Bassig, B.A.; Li, Y.; Wise, J.P., Sr.; Zhou, A.; Wan, Y.; Wang, Y.; et al. Maternal urinary manganese and risk of low birth weight: A case-control study. BMC Public Health 2016, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; O’Callaghan, F.J.; Godfrey, K.M.; Law, C.M.; Martyn, C.N. Critical periods of brain growth and cognitive function in children. Brain 2004, 127, 321–329. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Taneja, S. Zinc and cognitive development. Br. J. Nutr. 2001, 85 (Suppl. 2), S139–S145. [Google Scholar] [CrossRef]
- Jędrusik, P.; Symonides, B.; Gaciong, Z. Performance of 24-hour urinary creatinine excretion-estimating equations in relation to measured 24-hour urinary creatinine excretion in hospitalized hypertensive patients. Sci. Rep. 2019, 9, 3593. [Google Scholar] [CrossRef]
- Chang, A.R.; Zafar, W.; Grams, M.E. Kidney Function in Obesity-Challenges in Indexing and Estimation. Adv. Chronic Kidney Dis. 2018, 25, 31–40. [Google Scholar] [CrossRef]
- Fotheringham, J.; Weatherley, N.; Kawar, B.; Fogarty, D.G.; Ellam, T. The body composition and excretory burden of lean, obese, and severely obese individuals has implications for the assessment of chronic kidney disease. Kidney Int. 2014, 86, 1221–1228. [Google Scholar] [CrossRef]
| ETE | Enzymes Using ETE as Cofactors | Biological Functions | Food Sources | RDA for Pregnant Women | Refs |
|---|---|---|---|---|---|
| Co | Methionine synthase L-methyl-malonylCoA mutase | Component of Vitamin B12 (Vit B12, cobalamin); metabolism of folates and purines;synthesis of methionine | Fresh cereals and green vegetables (0.2–0.6 µg Co/g dry mass) | Not available for Co; Vit B12: 2.6 µg/day, which corresponds to about 0.1 µg of Co | [6,7,8,9] |
| Cu | Oxireductases (e.g., copper/zinc superoxide dismutase and cytochrome oxidase) | Cellular respiration; antioxidant defense; neuropeptide synthesis | Shellfish, nuts, sunflower seeds, cocoa, bran cereals, whole grain products, beef liver and other organ meats | 1000 µg/day | [10,11,12,13,14,15,16] |
| Mn | Metalloenzymes (e.g., arginase, glutaminase synthetase and phosphoenolpyruvate decarboxylase) Oxireductases (manganese superoxide dismutase) | Carbohydrate, protein, amino acid and lipid metabolism; antioxidant defense | Whole grains, legumes, nuts, seeds, tea, wheat, brown rice, and spinach | Not available | [16,17,18,19] |
| Mo | Sulfite oxidase, xanthine oxidoreductase, aldehyde oxidase, and mitochondrial amidoxime-reducing component | Metabolism of sulfur amino acids and heterocyclic compounds (e.g., purines and pyridines) Production of uric acid and in detoxification | Beans, oat, rice, nuts, and dark-leafy vegetables | 50 μg/day | [16,20,21] |
| Zn | Over 300 metalloenzymes (e.g., carbonic anhydrase and alkaline phosphatases and) Oxireductases (e.g., copper/zinc superoxide dismutase) | Protein synthesis; antioxidant defense | Meat, seafood, dairy products, eggs, seeds, and nuts | 11 mg/day | [16,22,23,24] |
| Characteristics | Total | Porto | Lisbon | p |
|---|---|---|---|---|
| (n = 635) | (n = 337) | (n = 298) | ||
| Age, n | 631 | 336 | 295 | |
| Mean ± SD, years | 33 ± 5 | 32 ± 5 | 34 ± 5 | <0.001 a |
| Gestational age at recruitment, n | 635 | 337 | 298 | |
| Median (P25; P75), weeks | 12 (12; 13) | 12 (12; 13) | 12 (12; 12) | 0.265 b |
| Gestational age at delivery, n | 602 | 336 | 298 | |
| Median (P25; P75), weeks | 39 (38; 40) | 39 (38; 40) | 39 (38; 40) | 0.763 b |
| Sub-cohort, n (%) | ||||
| Porto | 337 (53) | nd | nd | nd |
| Lisbon | 298 (47) | |||
| Education level, n (%) | ||||
| Low (≤ 9 years) | 155 (25) | 154 (48) | 1 (0) | <0.001 c |
| Medium (10 to 12 years) | 181 (29) | 122 (38) | 59 (20) | |
| University (≥ 13 years) | 282 (46) | 47 (15) | 235 (80) | |
| Smoking habits, n (%) | ||||
| Non-smoker | 443 (70) | 232 (70) | 211 (71) | 0.001 c |
| Former smoker | 132 (21) | 60 (18) | 72 (24) | |
| Smoker | 56 (9) | 42 (13) | 14 (5) | |
| Use of nutritional supplements, n (%) | ||||
| NS | 23 (4) | 20 (7) | 3 (1) | <0.001 c |
| MV/MMF | 249 (46) | 88 (33) | 161 (59) | |
| SIF | 269 (50) | 160 (60) | 109 (40) | |
| Pre-pregnancy BMI, n (%) | ||||
| Underweight | 35 (6) | 20 (6) | 15 (5) | 0.846 c |
| Normal weight | 400 (63) | 209 (62) | 191 (64) | |
| Overweight | 114 (18) | 59 (18) | 55 (19) | |
| Obese | 85 (14) | 48 (14) | 37 (12) | |
| Total weight variation, n (%) * | ||||
| below adequacy | 96 (20) | 58 (21) | 38 (19) | 0.841 c |
| adequate | 163 (34) | 93 (33) | 70 (36) | |
| above adequacy | 217 (46) | 129 (46) | 88 (45) | |
| 1st trimester weight variation, n (%) # | ||||
| Below adequacy | 132 (21) | 72 (22) | 60 (21) | 0.809 c |
| Adequate | 189 (31) | 97 (30) | 92 (32) | |
| Above adequacy | 293 (48) | 158 (48) | 135 (47) | |
| Pregnancy complications, n (%) | ||||
| No | 476 (80) | 248 (83) | 228 (77) | 0.041 c |
| Yes | 120 (20) | 50 (17) | 70 (24) | |
| Preeclampsia | 16 (3) | 6 (2) | 10 (3) | 0.314 c |
| Gestational diabetes | 56 (9) | 23 (8) | 33 (11) | 0.164 c |
| Fetal growth restriction | 13 (2) | 11 (4) | 2 (1) | 0.011 c |
| Infection | 3 (1) | 1 (0) | 2 (1) | 1.000 d |
| Preterm birth | 25 (4) | 18 (6) | 7 (2) | 0.025 c |
| Parity, n (%) | ||||
| Nulliparous | 314 (51) | 168 (52) | 146 (49) | 0.671 c |
| Primiparous | 256 (41) | 132 (41) | 124 (42) | |
| Multiparous | 49 (8) | 23 (7) | 26 (9) | |
| Sex, n (%) | ||||
| Male | 313 (49) | 164 (49) | 149 (50) | 0.737 c |
| Female | 322 (51) | 173 (51) | 149 (50) | |
| Birth weight, n | 635 | 337 | 298 | |
| Mean ± SD, grams | 3207 ± 433 | 3158 ± 460 | 3263 ± 394 | 0.002 a |
| SGA, n (%) | 34 (5) | 24 (7) | 10 (3) | 0.088 c |
| AGA, n (%) | 580 (92) | 303 (90) | 277 (93) | |
| LGA, n (%) | 20 (3) | 9 (3) | 11 (4) | |
| Birth head circumference, n | 612 | 326 | 286 | |
| Median (P25; P75), cm | 34.0 (33.5; 35.0) | 34.0 (33.0; 35.0) | 34.5 (33.7; 35.5) | <0.001 b |
| SGA, n (%) | 32 (5) | 25 (8) | 7 (2) | 0.002 c |
| AGA, n (%) | 523 (86) | 278 (86) | 245 (86) | |
| LGA, n (%) | 56 (9) | 22 (7) | 34 (12) | |
| Birth length, n | 624 | 337 | 287 | |
| Mean ± SD, cm | 49.0 ± 2.0 | 49.1 ± 2.2 | 48.8 ± 1.7 | 0.029 a |
| SGA, n (%) | 49 (8) | 20 (6) | 29 (10) | 0.031 c |
| AGA, n (%) | 570 (92) | 312 (93) | 258 (90) | |
| LGA, n (%) | 4 (1) | 4 (1) | 0 (0) |
| ETEs (μg/L) | <LOD * | Min | P5 | P25 | Median | P75 | P95 | Max | |
|---|---|---|---|---|---|---|---|---|---|
| n | % | ||||||||
| Co | 113 | 18 | 0.03 | 0.03 | 0.13 | 0.31 | 0.53 | 1.08 | 3.92 |
| Cu | 0 | 0 | 1.01 | 3.70 | 7.00 | 11.1 | 17.8 | 59.6 | 328.9 |
| Mn | 48 | 8 | 0.14 | 0.14 | 0.71 | 1.67 | 3.05 | 5.95 | 18.6 |
| Mo | 0 | 0 | 3.22 | 8.41 | 22.1 | 39.3 | 63.6 | 107.7 | 468.7 |
| Zn | 1 | 0 | 8.57 | 53.0 | 145.8 | 256.9 | 446.8 | 839.4 | 3620.9 |
| Characteristics | n | % | Co (μg/L) | Cu (μg/L) | Mn (μg/L) | Mo (μg/L) | Zn (μg/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | (P25; P75) | p | Median | (P25; P75) | p | Median | (P25; P75) | p | Median | (P25; P75) | p | Median | (P25; P75) | p | |||
| Sub-cohort | |||||||||||||||||
| Porto | 337 | 53 | 0.27 | (0.08; 0.51) | <0.001 a | 15.5 | (10.0; 26.3) | <0.001 a | 2.83 | (1.89; 4.30) | <0.001 a | 36.8 | (21.1; 58.5) | 0.004 a | 258.9 | (148.8; 446.0) | 0.799 a |
| Lisbon | 298 | 47 | 0.35 | (0.17; 0.55) | 8.36 | (5.81; 11.5) | 0.73 | (0.33; 1.27) | 45.3 | (23.1; 68.0) | 253.5 | (141.2; 452.8) | |||||
| Education level | |||||||||||||||||
| Low (≤9 years) | 155 | 25 | 0.26 | (0.12; 0.48) | 0.158 b | 17.0 | (9.90; 28.1) | <0.001 b | 2.99 | (1.9; 4.31) | <0.001 b | 36.3 | (20.5; 51.0) | 0.007 b | 276.2 | (149.3; 487.9) | 0.207 b |
| Medium (10 to 12 years) | 181 | 29 | 0.32 | (0.10; 0.56) | 12.0 | (7.89; 18.8) | 2.03 | (1.00; 3.62) | 37.8 | (21.1; 59.8) | 275.9 | (163.2; 439.6) | |||||
| University (≥13 years) | 282 | 46 | 0.33 | (0.15; 0.54) | 9.02 | (6.18; 12.9) | 0.90 | (0.44; 1.72) | 45.6 | (23.2; 70.4) | 245.0 | (132.3; 441.6) | |||||
| Smoking habits | |||||||||||||||||
| Non-smoker | 443 | 70 | 0.29 | (0.10; 0.52) | 0.076 b | 11.0 | (6.84; 18.0) | 0.047 b | 1.61 | (0.68; 2.92) | <0.001 b | 40.1 | (21.2; 64.6) | 0.582 b | 256.9 | (139.8; 437.1) | 0.502 b |
| Former smoker | 132 | 21 | 0.34 | (0.17; 0.55) | 10.0 | (6.86; 16.7) | 1.45 | (0.68; 2.60) | 39.5 | (26.2; 63.0) | 257.9 | (162.1; 481.1) | |||||
| Smoker | 56 | 9 | 0.35 | (0.19; 0.53) | 12.8 | (9.84; 24.4) | 3.13 | (1.11; 4.38) | 36.7 | (22.8; 50.9) | 278.9 | (145.2; 532.5) | |||||
| Pre-pregnancy BMI | |||||||||||||||||
| Underweight | 35 | 6 | 0.23 | (0.15; 0.40 | 0.336 b | 9.26 | (6.73; 14.5) | 0.027 b | 2.33 | (0.59; 3.09) | 0.349 b | 33.2 | (20.8; 59.8) | 0.236 b | 231.8 | (161.0; 385.8) | 0.062 b |
| Normal weight | 400 | 63 | 0.32 | (0.13; 0.54) | 10.7 | (6.77; 17.3) | 1.66 | (0.69; 3.01) | 40.1 | (22.4; 64.7) | 252.2 | (139.3; 436.4) | |||||
| Overweight | 114 | 18 | 0.27 | (0.09; 0.50) | 11.6 | (7.44; 17.0) | 1.44 | (0.63; 2.63) | 35.5 | (19.8; 53.5) | 256.6 | (138.6; 495.7) | |||||
| Obese | 85 | 13 | 0.33 | (0.18; 0.57) | 14.3 | (8.37; 25.0) | 1.72 | (1.01; 3.58) | 41.1 | (23.7; 65.3) | 313.8 | (188.4; 531.7) | |||||
| 1st trimester weight variation | |||||||||||||||||
| Below adequacy | 132 | 21 | 0.27 | (0.10; 0.45) | 0.020 b | 13.2 | (7.08; 23.5) | 0.008 b | 1.70 | (0.75; 2.82) | 0.485 b | 32.5 | (20.2; 64.6) | 0.057 b | 250.0 | (149.2; 455.8) | 0.219 b |
| Adequate | 189 | 31 | 0.28 | (0.09; 0.49) | 9.82 | (6.37; 15.7) | 1.52 | (0.64; 2.99) | 35.2 | (21.0; 58.6) | 241.0 | (139.4; 410.1) | |||||
| Above adequacy | 293 | 48 | 0.33 | (0.17; 0.57) | 11.2 | (7.65; 17.3) | 1.76 | (0.75; 3.42) | 44.0 | (24.8; 64.5) | 267.7 | (148.2; 472.0) | |||||
| Food Intake | n | % | Co (μg/L) | Cu (μg/L) | Mn (μg/L) | Mo (μg/L) | Zn (μg/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | (P25; P75) | p | Median | (P25; P75) | p | Median | (P25; P75) | p | Median | (P25; P75) | p | Median | (P25; P75) | p | |||
| Milk | |||||||||||||||||
| <3 times a month | 178 | 28.4 | 0.31 | (0.10; 0.52) | 0.677 a | 10.1 | (7.14; 17.5) | 0.686 a | 1.26 | (0.53; 2.58) | 0.002 a | 39.6 | (23.3; 60.9) | 0.584 a | 266.19 | (153.9; 463.7) | 0.570 a |
| 1 a 6 times a week | 157 | 25.0 | 0.32 | (0.15; 0.52) | 11.6 | (6.84; 17.1) | 1.56 | (0.77; 2.72) | 44.8 | (20.8; 65.5) | 263.87 | (145.0; 504.2) | |||||
| 1 time a day | 216 | 34.4 | 0.33 | (0.14; 0.56) | 11.6 | (7.65; 18.1) | 1.77 | (0.75; 3.29) | 38.7 | (22.6; 64.37) | 253.46 | (160.4; 407.4) | |||||
| ≥2 times a day | 76 | 12.1 | 0.28 | (0.12; 0.49) | 10.3 | (6.01; 19.8) | 2.39 | (0.97; 4.00) | 34.3 | (21.4; 58.4) | 225.38 | (119.3; 466.3) | |||||
| Eggs | |||||||||||||||||
| <3 times a month | 126 | 20.2 | 0.32 | (0.15; 0.53) | 0.993 a | 11.7 | (7.87; 20.5) | 0.015 a | 2.11 | (1.03; 3.68) | <0.001 a | 39.6 | (22.5; 59.8) | 0.721 a | 257.01 | (141.8; 470.2) | 0.665 a |
| 1 a 3 times a week | 410 | 65.6 | 0.31 | (0.13; 0.53) | 11.5 | (7.15; 17.5) | 1.67 | (0.73; 2.99) | 40.2 | (22.3; 65.3) | 263.81 | (153.2; 443.9) | |||||
| ≥4 times a week | 89 | 14.2 | 0.32 | (0.12; 0.56) | 9.47 | (6.15; 15.3) | 1.02 | (0.47; 2.06) | 38.7 | (22.6; 59.8) | 242.44 | (137.6; 472.1) | |||||
| Fish | |||||||||||||||||
| <3 times a month | 71 | 11.6 | 0.32 | (0.14; 0.55) | 0.709 a | 12.6 | (8.46; 19.8) | 0.041 a | 1.83 | (0.94; 3.47) | 0.005 a | 41.1 | (20.1; 61.5) | 0.722 a | 281.99 | (181.0; 479.7) | 0.313 a |
| 1 a 3 times a week | 407 | 66.6 | 0.31 | (0.12; 0.52) | 11.0 | (7.00; 17.6) | 1.67 | (0.75; 2.85) | 39.0 | (22.2; 63.6) | 249.44 | (139.4; 436.7) | |||||
| ≥4 times a week | 133 | 21.8 | 0.33 | (0.16; 0.56) | 9.61 | (6.57; 15.5) | 1.04 | (0.48; 2.68) | 40.2 | (22.7; 65.1) | 266.29 | (156.3; 471.6) | |||||
| Seafood | |||||||||||||||||
| do not eat Seafood at all | 180 | 60.8 | 0.35 | (0.17; 0.56) | 0.597 b | 8.32 | (5.87; 11.7) | 0.630 b | 0.75 | (0.38; 1.28) | 0.181 b | 49.2 | (24.7; 74.3) | 0.029 b | 264.49 | (154.9; 467.6) | 0.086 b |
| ≥1 time a month | 116 | 39.2 | 0.33 | (0.16; 0.52) | 8.44 | (5.72; 11.1) | 0.66 | (0.31; 1.18) | 40.0 | (20.3; 61.3) | 242.13 | (132.0; 411.6) | |||||
| Offal | |||||||||||||||||
| do not eat Offal at all | 228 | 80.0 | 0.35 | (0.17; 0.56) | 0.092 b | 8.46 | (5.98; 11.7) | 0.067 b | 0.76 | (0.36; 1.31) | 0.153 b | 47.2 | (24.3; 67.7) | 0.046 b | 267.97 | (148.7; 489.9) | 0.047 b |
| ≥1 time a month | 57 | 20.0 | 0.27 | (0.12; 0.48) | 7.52 | (5.35; 9.92) | 0.60 | (0.31; 1.05) | 33.8 | (15.3; 64.2) | 219.76 | (123.9; 355.4) | |||||
| n | Pregnancy Complications a | PE | PTB | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| aOR b | (95% CI) | p | aOR b | (95% CI) | p | aOR b | (95% CI) | p | ||
| Zinc, μg/L | ||||||||||
| ≤P50 (256.9 μg/L) | 227 | 1 | 1 | 1 | ||||||
| >P50 (256.9 μg/L) | 229 | 1.72 | (1.03–2.88) | 0.040 | 5.35 | (1.04–27.42) | 0.044 | 2.06 | (0.615–6.89) | 0.241 |
| Mn, μg/L | ||||||||||
| ≤P35 (1.02 μg/L) | 154 | nd | 1.86 | (0.397–8.72) | 0.430 | nd | ||||
| >P35 (1.02 μg/L) | 302 | nd | 1 | nd | ||||||
| ≤P60 (2.11 μg/L) | 270 | nd | nd | 1 | ||||||
| >P60 (2.11 μg/L) | 186 | nd | nd | 0.958 | (0.263–3.48) | 0.948 | ||||
| Neonatal Outcomes | n | % | Co (μg/L) | Cu (μg/L) | Mn (μg/L) | Mo (μg/L) | Zn (μg/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | (P25; P75) | p | Median | (P25; P75) | p | Median | (P25; P75) | p | Median | (P25; P75) | p | Median | (P25; P75) | p | |||
| Birth weight adequacy | |||||||||||||||||
| SGA | 34 | 5 | 0.30 | (0.12; 0.45) | 0.150 a | 10.2 | (6.91; 14.7) | 0.602 a | 2.26 | (1.12; 3.30) | 0.071 a | 35.6 | (22.8; 55.7) | 0.816 a | 209.8 | (111.9; 352.8) | 0.189 a |
| AGA | 580 | 91 | 0.32 | (0.14; 0.55) | 11.2 | (7.00; 18.3) | 1.66 | (0.69; 3.06) | 39.8 | (21.9; 64.2) | 259.0 | (148.4; 458.7) | |||||
| LGA | 20 | 3 | 0.24 | (0.06; 0.45) | 10.1 | (6.42; 15.7) | 1.06 | (0.51; 2.26) | 37.4 | (22.8; 53.8) | 239.8 | (149.5; 360.3) | |||||
| Birth head circumference adequacy | |||||||||||||||||
| SGA | 32 | 5 | 0.25 | (0.08; 0.48) | 0.371 a | 9.4 | (6.74; 14.7) | 0.312 a | 2.12 | (1.35; 3.05) | 0.053 a | 37.0 | (19.3; 60.7) | 0.239 a | 195.2 | (115.7; 314.5) | 0.088 a |
| AGA | 523 | 86 | 0.32 | (0.14; 0.54) | 11.4 | (7.02; 18.6) | 1.68 | (0.72; 3.08) | 38.7 | (22.3; 62.0) | 259.5 | (148.5; 458.2) | |||||
| LGA | 56 | 9 | 0.35 | (0.14; 0.52) | 10.9 | (6.72; 17.0) | 1.09 | (0.48; 2.67) | 46.4 | (28.0; 72.2) | 281.7 | (170.8; 470.9) | |||||
| Birth length adequacy | |||||||||||||||||
| SGA | 49 | 8 | 0.36 | (0.16; 0.51) | 0.582 b | 11.8 | (8.73; 17.5) | 0.945 b | 1.71 | (0.64; 3.36) | 0.885 b | 45.8 | (25.7; 65.3) | 0.210 b | 316.8 | (216.6; 481.2) | 0.152 b |
| AGA | 570 | 91 | 0.31 | (0.13; 0.54) | 11.2 | (6.91; 18.1) | 1.67 | (0.74; 3.03) | 38.7 | (21.7; 62.5) | 252.3 | (146.0; 443.8) | |||||
| LGA | 4 | 1 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | |||||
| BW SGA | BW LGA | BHC SGA | BHC LGA | BL SGA | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | aOR a | (95% CI) | p | n | aOR a | (95% CI) | p | n | aOR a | (95% CI) | p | n | aOR a | (95% CI) | p | n | aOR a | (95% CI) | p | |
| Zinc, μg/L | ||||||||||||||||||||
| ≤P50 (256.86 μg/L) | nd | nd | 202 | 1 | 205 | 1 | 219 | 1 | ||||||||||||
| >P50 (256.86 μg/L) | nd | nd | 202 | 0.315 | 0.113–0.883 | 0.028 | 219 | 1.06 | 0.528–2.12 | 0.871 | 225 | 2.53 | 1.06–6.06 | 0.037 | ||||||
| Mn, μg/L | ||||||||||||||||||||
| ≤P35 (1.02 μg/L) | nd | 150 | 0.331 | 0.061–1.793 | 0.200 | nd | 143 | 0.992 | 0.431–2.28 | 0.985 | nd | |||||||||
| >P35 (1.02 μg/L) | nd | 283 | 1 | nd | 281 | 1 | nd | |||||||||||||
| ≤P60 (2.11 μg/L) | 261 | 1 | nd | 231 | 1 | nd | nd | |||||||||||||
| >P60 (2.11 μg/L) | 190 | 0.551 | 0.181–1.68 | 0.295 | nd | 173 | 1.27 | 0.438–3.70 | 0.658 | nd | nd | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bracchi, I.; Guimarães, J.; Rodrigues, C.; Azevedo, R.; Coelho, C.M.; Pinheiro, C.; Morais, J.; Barreiros-Mota, I.; Fernandes, V.C.; Delerue-Matos, C.; et al. Essential Trace Elements Status in Portuguese Pregnant Women and Their Association with Maternal and Neonatal Outcomes: A Prospective Study from the IoMum Cohort. Biology 2023, 12, 1351. https://doi.org/10.3390/biology12101351
Bracchi I, Guimarães J, Rodrigues C, Azevedo R, Coelho CM, Pinheiro C, Morais J, Barreiros-Mota I, Fernandes VC, Delerue-Matos C, et al. Essential Trace Elements Status in Portuguese Pregnant Women and Their Association with Maternal and Neonatal Outcomes: A Prospective Study from the IoMum Cohort. Biology. 2023; 12(10):1351. https://doi.org/10.3390/biology12101351
Chicago/Turabian StyleBracchi, Isabella, Juliana Guimarães, Catarina Rodrigues, Rui Azevedo, Cláudia Matta Coelho, Cátia Pinheiro, Juliana Morais, Inês Barreiros-Mota, Virgínia Cruz Fernandes, Cristina Delerue-Matos, and et al. 2023. "Essential Trace Elements Status in Portuguese Pregnant Women and Their Association with Maternal and Neonatal Outcomes: A Prospective Study from the IoMum Cohort" Biology 12, no. 10: 1351. https://doi.org/10.3390/biology12101351
APA StyleBracchi, I., Guimarães, J., Rodrigues, C., Azevedo, R., Coelho, C. M., Pinheiro, C., Morais, J., Barreiros-Mota, I., Fernandes, V. C., Delerue-Matos, C., Pinto, E., Moreira-Rosário, A., de Azevedo, L. F. R., Dias, C. C., Lima, J., Sapinho, I., Ramalho, C., Calhau, C., Leite, J. C., ... Keating, E. (2023). Essential Trace Elements Status in Portuguese Pregnant Women and Their Association with Maternal and Neonatal Outcomes: A Prospective Study from the IoMum Cohort. Biology, 12(10), 1351. https://doi.org/10.3390/biology12101351














