Genes and Pathway Reactions Related to Carotenoid Biosynthesis in Purple Bacteria
Abstract
Simple Summary
Abstract
1. Introduction
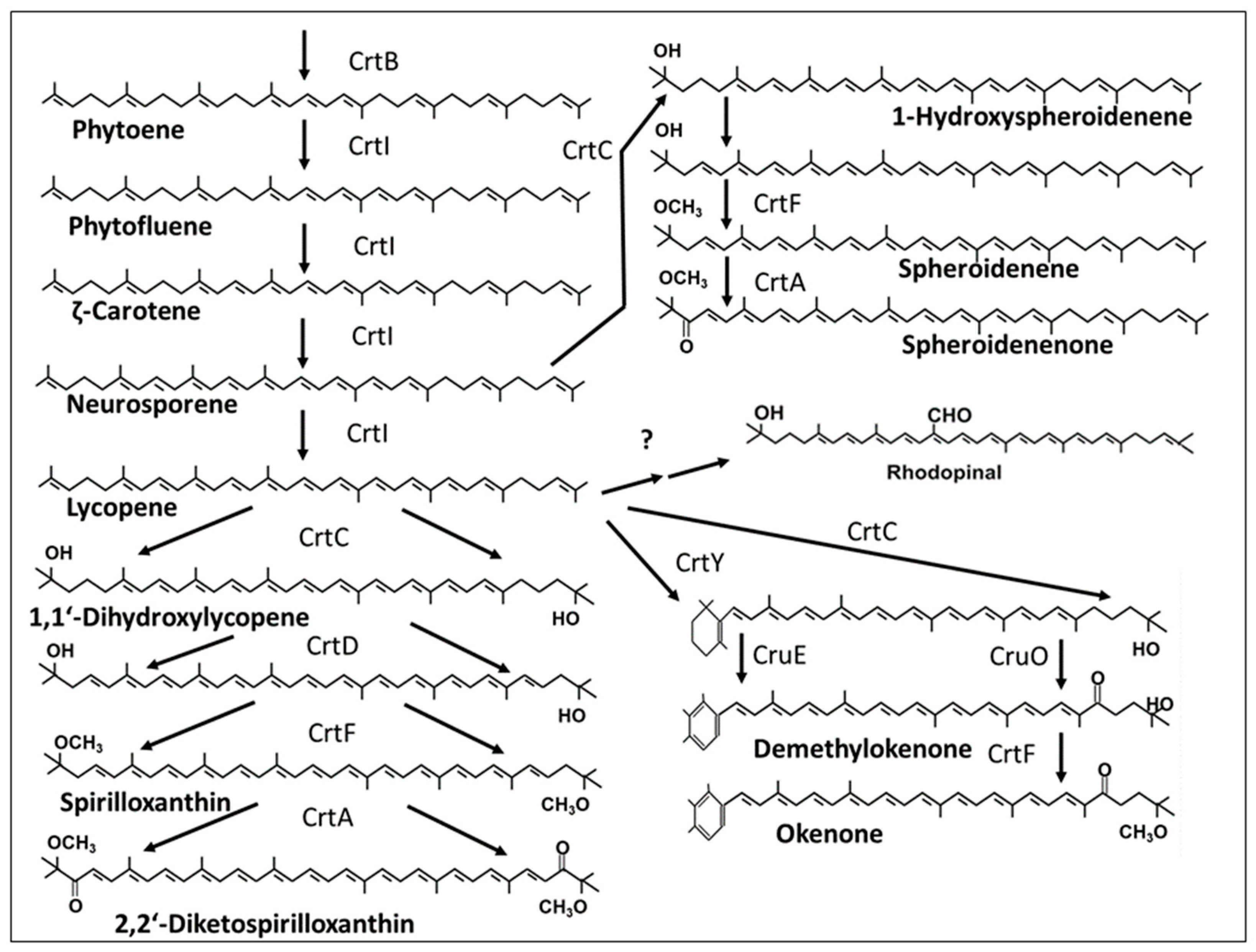
2. Carotenogenic Pathways with Related Genes
3. Reactions and Properties of Enzymes of the Carotenogenic Pathways of Purple Bacteria
3.1. CrtE and CrtB, Comparison to Other Bacteria
3.2. CrtI Determines the Pathways towards the End Products
| Enzymes | Reactions | References | |
|---|---|---|---|
| a. Crtl-related enzymes | Formations of carbocation: |  | [54] |
| Stabilisation reactions: | |||
| Crtl | 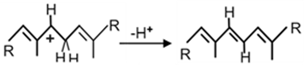 | [54] | |
| CrtD | 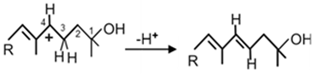 | [54] | |
| CruH (CrtO a) | 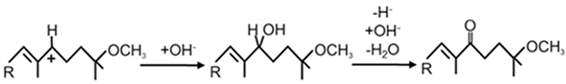 | [63] | |
| b. CrtY | 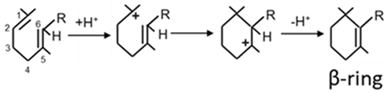 | [64] | |
| c. CruE-CrtU |  | [64,65] | |
| d. CrtA |  | [59] | |
3.3. CrtC for Anaerobic Formation of a Tertiary Alcohol Group
3.4. CrtD, a 3,4-Desaturase of the CrtI-Family
3.5. CrtF, a Conserved Methyltransferase
3.6. CrtY, the Dominating Lycopene Cyclase in Bacteria
3.7. CruE versus CrtU for the Formation of Different Aryl End Groups
3.8. CrtA and CruO: Oxygen-Dependent and Oxygen-Independent Ketolases
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madigan, M.T.; Jung, D.O. An Overview of purple bacteria: Systematics, physiology, and habitats. In The Purple Phototrophic Bacteria; Hunter, C.N., Daldal, F., Thurnauer, M.C., Beatty, J.T., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 1–15. [Google Scholar]
- McEwan, A.G. Photosynthetic electron transport and anaerobic metabolism in purple non-sulfur phototrophic bacteria. Antonie Van Leeuwenhoek 1994, 66, 151–164. [Google Scholar] [CrossRef]
- Frank, H.A.; Cogdell, R.J. Carotenoids in photosynthesis. Photochem. Photobiol. 1996, 63, 257–264. [Google Scholar] [CrossRef]
- Cogdell, R.J.; Howard, T.D.; Bittl, R.; Schlodder, E.; Geisenheimer, I.; Lubitz, W. How carotenoids protect bacterial photosynthesis. Phil. Trans. R. Soc. Lond. B 2000, 355, 1345–1349. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids and carotenogenesis in anoxygenic photosynthetic bacteria. In The Photochemistry of Carotenoids; Frank, H.A., Young, A.J., Britton, G., Cogdell, R.J., Eds.; Kluwer: Dordrecht, The Netherlands, 1999; pp. 39–69. [Google Scholar]
- Imhoff, J.F.; Trüper, H.G.; Pfennig, N. Rearrangement of the species and genera of the phototrophic purple non sulfur bacteria. Int. J. Syst. Bacteriol. 1984, 34, 340–343. [Google Scholar] [CrossRef]
- Schmidt, K. Biosynthesis of carotenoids. In The Photosynthetic Bacteria; Clayton, R.K., Sistrom, W.R., Eds.; Plenum Press: New York, NY, USA, 1978; pp. 729–750. [Google Scholar]
- Schmidt, K. Carotenoids of purple nonsulfur bacteria: Composition and biosynthesis of the carotenoids of some strains of Rhodopseudomonas acidophila, Rhodospirillum tenue, and Rhodocyclus purpureus. Arch. Mikrobiol. 1971, 77, 231–238. [Google Scholar] [CrossRef]
- Schwerzmann, R.U.; Bachofen, R. Carotenoid profiles in pigment-protein complexes of Rhodospirillum rubrum. Plant Cell Physiol. 1989, 30, 497–504. [Google Scholar] [CrossRef]
- Harada, J.; Nagashima, K.V.; Takaichi, S.; Misawa, N.; Matsuura, K.; Shimada, K. Phytoene desaturase, CrtI, of the purple photosynthetic bacterium, Rubrivivax gelatinosus, produces both neurosporene and lycopene. Plant Cell Physiol. 2001, 42, 1112–1118. [Google Scholar] [CrossRef]
- Steiger, S.; Astier, C.; Sandmann, G. Substrate specificity of the expressed 3,4-carotenoid desaturase from Rubrivivax gelatinosus reveals the detailed reaction sequence to spheroidene and spirilloxanthin. Biochem. J. 2000, 349, 635–640. [Google Scholar] [CrossRef]
- Takaichi, S.; Shimada, K. Pigment composition of two pigment-protein complexes derived from anaerobically and semi-aerobically grown Rubrivivax gelatinosus, and identification of a new keto-carotenoid. Plant Cell Physiol. 1999, 40, 613–617. [Google Scholar] [CrossRef][Green Version]
- Vogl, K.; Bryant, D.A. Elucidation of the biosynthetic pathway for okenone in Thiodictyon sp. CAD16 leads to the discovery of two novel carotene ketolases. J. Biol. Chem. 2011, 286, 38521–38532. [Google Scholar] [CrossRef]
- Pfennig, N.; Markham, M.C.; Liaaen-Jensen, S. Carotenoids of Thiorhodaceae: Isolation and characterization of a Thiothece, Lamprocystis and Thiodictyon strain and their carotenoid pigments. Arch. Microbio. 1968, 62, 178–191. [Google Scholar] [CrossRef]
- Schmidt, K.; Pfennig, N.; Liaaen-Jensen, S. Carotenoids of Thiorhodaceae. IV. The carotenoid composition of 25 pure isolates. Arch. Mikrobiol. 1965, 52, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S. Distribution and Biosynthesis of Carotenoids. In The Purple Phototrophic Bacteria; Hunter, C.N., Daldal, F., Thurnauer, M.C., Beatty, J.T., Eds.; Springer: New York, NY, USA, 2009; pp. 97–117. [Google Scholar]
- Herbert, R.A.; Gall, A.; Maoka, T.; Cogdell, R.J.; Robert, B.; Takaichi, S.; Schwabe, S. Phototrophic purple sulfur bacteria as heat engines in the South Andros Black Hole. Photosynth. Res. 2008, 95, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Swingley, W.D.; Blankenship, R.E.; Raymond, J. Evolutionary relationships among purple photosynthetic bacteria and the origin of proteobacterial photosynthetic systems. In The Purple Phototrophic Bacteria; Hunter, C.N., Daldal, F., Thurnauer, M.C., Beatty, J.T., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 17–29. [Google Scholar]
- Yen, H.-C.; Marrs, B. Map of genes for carotenoid and bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata. J. Bacteriol. 1976, 126, 619–629. [Google Scholar] [CrossRef]
- Scolnik, P.; Walker, A.M.A.; Marrs, B.L. Biosynthesis of carotenoid derivatives derived from neurosporene in Rhodopseudomonas capsulata. J. Biol. Chem. 1980, 255, 2427–2432. [Google Scholar] [CrossRef]
- Giuliano, G.; Pollock, D.; Scolnik, P.A. The gene crtI mediates the conversion of phytoene into colored carotenoids in Rhodopseudomonas capsulata. J. Biol. Chem. 1986, 261, 12925–12929. [Google Scholar] [CrossRef]
- Raisig, A.; Bartley, G.; Scolnik, P.; Sandmann, G. Purification in an active state and properties of the 3-step phytoene desaturase from Rhodobacter capsulatus after overexpression in Escherichia coli. J. Biochem. 1996, 119, 559–564. [Google Scholar] [CrossRef]
- Armstrong, G.A.; Alberti, M.; Leach, F.; Hearst, J.E. Nucleotide sequence, organization and nature of the protein products of the carotenoid biosynthesis gene cluster of Rhodobacter capsulatus. Mol. Gen. Genet. 1989, 216, 254–268. [Google Scholar] [CrossRef]
- Armstrong, G.A.; Alberti, M.; Hearst, J.E. Conserved enzymes mediate the early reactions of carotenoid biosynthesis in nonphotosynthetic and photosynthetic prokaryotes. Proc. Natl. Acad. Sci. USA 1990, 87, 9975–9979. [Google Scholar] [CrossRef]
- Sandmann, G.; Misawa, N. New functional assignment of the carotenogenic gene crtB and crtE with constructs of these genes from Erwinia species. FEMS Microbiol. Lett. 1992, 90, 253–258. [Google Scholar] [CrossRef]
- Steiger, S.; Mazet, A.; Sandmann, G. Heterologous expression, purification, and enzymatic characterization of the acyclic carotenoid 1,2-hydratase from Rubrivivax gelatinosus. Arch. Biochem. Biophys. 2003, 414, 51–58. [Google Scholar] [CrossRef]
- Kovacs, A.T.; Rakhely, G.; Kovacs, K.L. Genes involved in the biosynthesis of photosynthetic pigments in the purple sulfur photosynthetic bacterium Thiocapsa roseopersicina. Appl. Environ. Microbiol. 2003, 69, 3093–3102. [Google Scholar] [CrossRef]
- Shneur, E.A. Carotenoid pigment conversion in Rhodopseudomonas spheroides. Biochim. Biophy. Acta 1962, 62, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.P.; Cogdell, R.J.; Gardiner, A.T.; Hunter, C.N. Early steps in carotenoid biosynthesis: Sequences and transcriptional analysis of the crtI and crtB genes of Rhodobacter sphaeroides and overexpression and reactivation of crtI in Escherichia coli and Rhodobacter sphaeroides. J. Bacteriol. 1994, 17, 3859–3869. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Licht, M.K.; Nuss, A.M.; Volk, M.; Konzer, A.; Beckstette, M.; Berghoff, B.A.; Klug, G. Adaptation to photooxidative stress: Common and special strategies of the alphaproteobacteria Rhodobacter sphaeroides and Rhodobacter capsulatus. Microorganisms 2020, 8, 283. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Hearst, J.E. Regulation of expression of genes for light-harvesting antenna proteins LH-I and LH-II; reaction center polypeptides RC-L, RC-M, and RC-H; and enzymes of bacteriochlorophyll and carotenoid biosynthesis in Rhodobacter capsulatus by light and oxygen. Proc. Natl. Acad. Sci. USA 1986, 83, 7613–7617. [Google Scholar] [CrossRef] [PubMed]
- Yeliseev, A.A.; Kaplan, S. A sensory transducer homologous to the mammalian peripheraltype benzodiazepine receptor regulates photosynthetic membrane complex formation in Rhodobacter sphaeroides 2.4.1. J. Bio. Chem. 1995, 270, 21167–21175. [Google Scholar] [CrossRef]
- Willett, J.; Smart, J.L.; Bauer, C.E. RegA control of bacteriochlorophyll and carotenoid synthesis in Rhodobacter capsulatus. J. Bacteriol. 2007, 189, 7765–7773. [Google Scholar] [CrossRef]
- O’Gara, J.P.; Kaplan, S. Evidence for the role of redox carriers in photosynthesis gene expression and carotenoid biosynthesis in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 1997, 179, 1951–1961. [Google Scholar] [CrossRef][Green Version]
- Munk, C.; Copeland, A.; Lucas, S.; Lapidus, A.; Del Rio, T.G.; Barry, K.; Detter, J.C.; Hammon, N.; Israni, S.; Pitluck, S.; et al. Complete genome sequence of Rhodospirillum rubrum type strain (S1T). Stand. Genom. Sci. 2011, 4, 293–302. [Google Scholar] [CrossRef]
- Goodwin, T.W.; Osman, H.G. Studies in carotenogenesis. 10. Spirilloxanthin synthesis by washed cells of Rhodospirillum rubrum. Biochem. J. 1954, 56, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Kamimura, A.; Shimizu, T.; Nakamura-Isaki, S.; Aono, E.; Sakamoto, K.; Ichikawa, N.; Nakazawa, H.; Sekine, M.; Yamazaki, S.; et al. Complete genome sequence of phototrophic betaproteobacterium Rubrivivax gelatinosus IL144. J. Bacteriol. 2012, 19, 3541–3542. [Google Scholar] [CrossRef]
- Suresh, G.; Lodha, T.D.; Indu, B.; Sasikala, C.; Ramana, C.V. Taxogenomics resolves conflict in the genus Rhodobacter: A two and half decades pending thought to reclassify the genus Rhodobacter. Front. Microbiol. 2019, 10, 2480. [Google Scholar] [CrossRef] [PubMed]
- Ouchane, S.; Picaud, M.; Vernotte, C.; Reiss-Husson, F.; Astier, C. Pleiotropic effects of puf interposon mutagenesis on carotenoid biosynthesis in Rubrivivax gelatinosus. J. Biol. Chem. 1997, 272, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, K.V.P.; Hiraishi, A.; Shimada, K.; Matsuura, K. Horizontal transfer of genes coding for the photosynthetic reaction centers of purple bacteria. J. Molec. Evol. 1997, 45, 131–136. [Google Scholar] [CrossRef]
- Igarashi, N.; Harada, J.; Nagashima, S.; Matsuura, K.; Shimada, K.; Nagashima, K.V.P. Horizontal transfer of the photosynthesis gene cluster and operon rearrangement in purple bacteria. J. Molec. Evol. 2001, 52, 333–341. [Google Scholar] [CrossRef]
- Krügel, H.; Krubasik, P.; Weber, K.; Saluz, H.P.; Sandmann, G. Functional analysis of genes from Streptomyces griseus involved in the synthesis of isorenieratene, a carotenoid with aromatic end groups, revealed a novel type of carotenoid desaturase. Biochim. Biophys. Acta 1999, 1439, 57–64. [Google Scholar] [CrossRef]
- Graham, J.E.; Bryant, D.A. The biosynthetic pathway for synechoxanthin, an aromatic carotenoid synthesized by the euryhaline, unicellular cyanobacterium Synechococcus sp. strain PCC 7002. J. Bacteriol. 2008, 190, 7966–7974. [Google Scholar] [CrossRef]
- Orsi, E.; Beekwilder, J.; Eggink, G.; Kengen, S.W.M.; Weusthuis, R.A. The transition of Rhodobacter sphaeroides into a microbial cell factory. Biotechnol. Biogene. 2021, 118, 531–541. [Google Scholar] [CrossRef]
- Naylor, G.W.; Addlesee, H.A.; Gibson, L.C.D.; Hunter, C.N. The photosynthesis gene cluster of Rhodobacter sphaeroides. Photosynth. Res. 1999, 62, 121–139. [Google Scholar] [CrossRef]
- Garcia-Asua, G.; Cogdell, R.J.; Hunter, C.N. Functional assembly of the foreign carotenoid lycopene into the photosynthetic apparatus of Rhodobacter sphaeroides achieved by replacement of the native 3-step phytoene desaturase with its 4-step counterpart from Erwinia herbicola. Mol. Microbiol. 2002, 44, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Su, A.; Chi, S.; Li, Y.; Tan, S.; Qiang, S.; Chen, Z.; Meng, Y. Metabolic redesign of Rhodobacter sphaeroides for lycopene production. J. Agric. Food Chem. 2018, 66, 5879–5885. [Google Scholar] [CrossRef] [PubMed]
- Qiang, S.; Su, A.P.; Li, Y.; Chen, Z.; Hu, C.Y.; Meng, Y.H. Elevated β-carotene synthesis by the engineered Rhodobacter sphaeroides. J. Agric. Food Chem. 2019, 67, 9560–9568. [Google Scholar] [CrossRef] [PubMed]
- Math, S.K.; Hearst, J.E.; Poulter, C.D. The crtE gene in Erwinia herbicola encodes geranylgeranyl diphosphate synthase. Proc. Natl. Acad. Sci. USA 1992, 89, 6761–6764. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, M.; Misawa, N.; Sandmann, G. Purification and enzymatic characterization of the geranylgeranyl pyrophosphate synthase from Erwinia uredovora after expression in Escherichia coli. Arch. Biochem. Biophys. 1993, 306, 152–157. [Google Scholar] [CrossRef]
- Kandutsch, A.A.; Paulus, H.; Levin, E.; Bloch, K. Purification of geranylgeranyl pyrophosphate synthetase from Micrococcus lysodeikticus. J. Biol. Chem. 1964, 239, 2507–2515. [Google Scholar] [CrossRef]
- Heider, S.A.E.; Peters-Wendisch, P.; Beekwilder, J.; Wendisch, V.F. IdsA is the major geranylgeranyl pyrophosphate synthase involved in carotenogenesis in Corynebacterium glutamicum. FEBS J. 2014, 281, 4906–4920. [Google Scholar] [CrossRef]
- Neudert, U.; Martinez-Ferez, I.; Fraser, P.D.; Sandmann, G. Expression of an active phytoene synthase from Erwinia uredovora and biochemical properties of the enzyme. Biochim. Biophys. Acta 1998, 1392, 51–58. [Google Scholar] [CrossRef]
- Sandmann, G. Evolution of carotene desaturation: The complication of a simple pathway. Arch. Biochem. Bio. Phys. 2009, 483, 169–174. [Google Scholar] [CrossRef]
- Wang, G.-S.; Grammel, H.; Abou-Aisha, K.; Sägesser, R.; Ghosh, R. High-level production of the industrial product lycopene by the photosynthetic bacterium Rhodospirillum rubrum. Appl. Environ. Microbiol. 2012, 78, 7205–7215. [Google Scholar] [CrossRef]
- Fraser, P.D.; Misawa, N.; Linden, H.; Yamano, S.; Kobayashi, K.; Sandmann, G. Expression in Escherichia coli, purification and reactivation of the recombinant Erwinia uredovora phytoene desaturase. J. Biol. Chem. 1992, 267, 19891–19895. [Google Scholar] [CrossRef] [PubMed]
- Liaaen-Jensen, S.; Cohen-Bazire, G.; Stanier, R.Y. Biosynthesis of carotenoids in purple bacteria: A re-evaluation based on consideration of chemical structure. Nature 1961, 192, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Badenhop, F.; Steiger, S.; Sandmann, M.; Sandmann, G. Expression and biochemical characterization of the 1-HO-carotenoid methylase CrtF from Rhodobacter capsulatus. FEMS Microbiol. Lett. 2003, 222, 237–242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gerjets, T.; Steiger, S.; Sandmann, G. Catalytic properties of the expressed acyclic carotenoid 2-ketolases from Rhodobacter capsulatus and Rubrivivax gelatinosus. Biochim. Biophys. Acta 2009, 1791, 125–131. [Google Scholar] [CrossRef]
- Bartley, G.S.; Schmidhauser, T.J.; Yanofsky, C.; Scolnik, P.A. Carotenoid desaturases from Rhodobacter capsulatus and Neurospora crassa are structurally and functionally conserved and contain domains homologous to flavoprotein disulfide oxidoreductases. J. Biol. Chem. 1990, 265, 16020–16024. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Liao, J.C. Alteration of product specificity of Rhodobacter sphaeroides phytoene desaturase by directed evolution. J. Biol. Chem. 2001, 276, 41161–41164. [Google Scholar] [CrossRef]
- Stickforth, P.; Sandmann, G. Structural and kinetics properties of a mutated phytoene desaturase from Rubrivivax gelatinosus with modified product specificity. Arch. Biochem. Biophys. 2011, 505, 118–122. [Google Scholar] [CrossRef]
- Breitenbach, J.; Gerjets, T.; Sandmann, G. Catalytic properties and reaction mechanism of the CrtO carotenoid ketolase from the cyanobacterium Synechocystis sp. PCC 6803. Arch. Biochem. Biophys. 2013, 529, 86–91. [Google Scholar] [CrossRef]
- Goodwin, T.W. The Biochemistry of the Carotenoids; Chapter 2.4.3; Chapman and Hall: London, UK, 1980; Volume 1. [Google Scholar]
- Liaaen-Jensen, S. Selected examples of structure determination of natural carotenoids. Pure Appl. Chem. 1969, 20, 421–448. [Google Scholar] [CrossRef][Green Version]
- Klassen, J.L. Pathway evolution by horizontal transfer and positive selection is accommodated by relaxed negative selection upon upstream pathway genes in purple bacterial carotenoid biosynthesis. J. Bacteriol. 2009, 191, 7500–7508. [Google Scholar] [CrossRef]
- Šlouf, V.; Chábera, P.; Olsen, J.D.; Martin, E.C.; Qian, P.; Hunter, C.N.; Polívka, T. Photoprotection in a purple phototrophic bacterium mediated by oxygen-dependent alteration of carotenoid excited-state properties. Proc. Natl. Acad. Sci. USA 2012, 109, 8570–8575. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.E.; Bryant, D.A. The biosynthetic pathway for myxol-2′ fucoside (Myxoxanthophyll) in the cyanobacterium Synechococcus sp. strain PCC 7002. J. Bacteriol. 2009, 191, 3292–3300. [Google Scholar] [CrossRef] [PubMed]
- Yeliseev, A.A.; Kaplan, S. Anaerobic carotenoid biosynthesis in Rhodobacter sphaeroides 2.4.1: H2O is a source of oxygen for the 1-methoxy group of spheroidene but not for the 2-oxo group of spheroidenone. FEBS Lett. 1997, 403, 10–14. [Google Scholar] [CrossRef]
- Hiseni, A.; Arends, I.W.C.E.; Otten, L.G. Biochemical characterization of the carotenoid 1,2-hydratases (CrtC) from Rubrivivax gelatinosus and Thiocapsa roseopersicina. Appl. Microbiol. Biotechnol. 2011, 91, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.H.; Than, A. Monohydroxycarotenoids from diphenylamine-inhibited cultures of Rhodospirillum rubrum. Phytochemistry 1974, 13, 209–219. [Google Scholar] [CrossRef]
- Hiseni, A.; Otten, L.G.; Arends, I.W.C.E. Identification of catalytically important residues of the carotenoid 1,2-hydratases from Rubrivivax gelatinosus and Thiocapsa roseopersicina. Appl. Microbiol. Biotechnol. 2016, 100, 1275–1284. [Google Scholar] [CrossRef]
- Gari, E.; Toledo, J.C.; Gibert, I.; Barbe, J. Nucleotide sequence of the methoxyneurosporene dehydrogenase gene from Rhodobacter sphaeroides: Comparison with other bacterial carotenoid dehydrogenases. FEMS Microbiol. Lett. 1992, 93, 103–108. [Google Scholar] [CrossRef]
- Albrecht, M.; Ruther, A.; Sandmann, G. Purification and biochemical characterization of a hydroxyneursporene desaturase involved in the biosynthetic pathway of the carotenoid spheroidene in Rhodobacter spheroides. J. Bacteriol. 1997, 179, 7462–7467. [Google Scholar] [CrossRef][Green Version]
- Lang, H.P.; Cogdell, R.J.; Takaichi, S.; Hunter, C.N.; Lang, H.P.; Cogdell, R.J.; Gardiner, A.T.; Hunter, C.N. Complete DNA sequence, specific Tn5 insertion map, and gene assignment of the carotenoid biosynthesis pathway of Rhodobacter sphaeroides. J. Bacteriol. 1995, 177, 2064–2073. [Google Scholar] [CrossRef][Green Version]
- Singh, R.K.; Britton, G.; Goodwin, T.W. Carotenoid biosynthesis in Rhodopseudomonas spheroides: S-adenosylmethionine as the methylating agent in the biosynthesis of spheroidene and spheroidenone. Biochem J. 1973, 136, 413–419. [Google Scholar] [CrossRef][Green Version]
- Maresca, J.A.; Graham, J.E.; Wu, M.; Eisen, J.A.; Bryant, D.A. Identification of a fourth family of lycopene cyclases in photosynthetic bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 11784–11789. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, T.W. Development in carotenoid biochemistry over 40 years. Biochem. Soc. Trans. 1983, 11, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.; Lockley, W.J.S.; Patel, N.J.; Goodwin, T.W.; Englert, G. Use of deuterium labelling to elucidate the stereochemistry of the initial step of the cyclization reaction in zeaxanthin biosynthesis in a Flavobacterium. J. Chem. Soc., Chem. Commun. 1977, 655–656. [Google Scholar] [CrossRef]
- Williams, R.J.H.; Britton, G.; Goodwin, T.W. The biosynthesis of cyclic carotenes. Biochem J. 1967, 105, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Vogl, K.; Bryant, D.A. Biosynthesis of the biomarker okenone: χ-ring formation. Geobiology 2012, 10, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Liu, X.L.; Shen, G.; Ma, J.; Husain, F.; Rocher, D.; Zumberge, J.E.; Bryant, D.A.; Summons, R.E. Niche expansion for phototrophic sulfur bacteria at the Proterozoic-Phanerozoic transition. Proc. Natl. Acad. Sci. USA 2020, 117, 17599–17606. [Google Scholar] [CrossRef]
- Hayaishi, O. Oxygenases. In Encyclopedia of Biological Chemistry; Academic Press: San Diego, CA, USA, 2004; pp. 178–182. [Google Scholar]
- Guengerich, P. Reactions and significance of cytochrome P-450 enzymes. J. Biol. Chem. 1991, 266, 10019–10022. [Google Scholar] [CrossRef]
- Bugg, T.D.H. Dioxygenase enzymes: Catalytic mechanisms and chemical models. Tetrahedron 2003, 59, 7075–7101. [Google Scholar] [CrossRef]
- Fraser, P.D.; Miura, Y.; Misawa, N. In vitro characterization of astaxanthin biosynthetic enzymes. J. Biol. Chem. 1997, 272, 6128–6135. [Google Scholar]
- Lee, P.C.; Holtzapple, E.; Schmidt-Dannert, C. Novel activity of Rhodobacter sphaeroides spheroidene monooxygenase CrtA expressed in Escherichia coli. Appl. Environ. Microbiol. 2010, 76, 7328–7331. [Google Scholar]
- Takaichi, S.; Jung, D.O.; Madigan, M.T. Accumulation of unusual carotenoids in the spheroidene pathway, demethylspheroidene and demethylspheroidenone, in an alkaliphilic purple nonsulfur bacterium Rhodobaca bogoriensis. Photosynth. Res. 2001, 67, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Klassen, J.L. Phylogenetic and evolutionary patterns in microbial carotenoid biosynthesis are revealed by comparative genomics. PLoS ONE 2010, 5, e11257. [Google Scholar] [CrossRef] [PubMed]


| 1,2-Hydratase CrtC from Rvi. gelatinosus [26]: |
| Neurosporene a, Lycopene a, 1-HO-Neurosporene, 1-HO-Lycopene, |
| Demethylspheroidene, 1-HO-3,4-Didehydrolycopene, Spheroidene |
| 3,4-Desaturase CrtD from Rvi. gelatinosus [11]: |
| 1-HO-ζ-Carotene, 1-HO-γ-Carotene, 1-HO-Neurosporene, |
| 1,1′-(HO)2-Neurosporene, 1-HO-Lycopene, 1,1′-(HO)2-3,4-Didehydrolycopene, |
| 1-HO-3′,4′-Didehydrolycopene, 1-CH3HO-1′-HO-3,4-Didehydrolycopene |
| 1-HO-Methylase CrtF from Rba. capsulatus [58]: |
| 1-HO-Neurosporene, 1,1′-(HO)2-Neurosporene, Spheroidene, 1‘-HO-Demethylspheroidene, |
| 1-HO-Lycopene, 1,1′-(HO)2-Lycopene, 1-HO-3,4-Didehydrolycope, |
| 1,1′-(HO)2-3,4-Didehydrolycope, 1,1′-(HO)2-3,4,3′,4′-Tetradehydrolycope |
| 2-Ketolases CrtA from Rvi. gelatinosus [59]: |
| Spheroidene, 1-HO-Spheroidene, Spirilloxanthin, 2-Ketospirilloxanthin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandmann, G. Genes and Pathway Reactions Related to Carotenoid Biosynthesis in Purple Bacteria. Biology 2023, 12, 1346. https://doi.org/10.3390/biology12101346
Sandmann G. Genes and Pathway Reactions Related to Carotenoid Biosynthesis in Purple Bacteria. Biology. 2023; 12(10):1346. https://doi.org/10.3390/biology12101346
Chicago/Turabian StyleSandmann, Gerhard. 2023. "Genes and Pathway Reactions Related to Carotenoid Biosynthesis in Purple Bacteria" Biology 12, no. 10: 1346. https://doi.org/10.3390/biology12101346
APA StyleSandmann, G. (2023). Genes and Pathway Reactions Related to Carotenoid Biosynthesis in Purple Bacteria. Biology, 12(10), 1346. https://doi.org/10.3390/biology12101346





