Characterization of Rabbit Mesenchymal Stem/Stromal Cells after Cryopreservation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
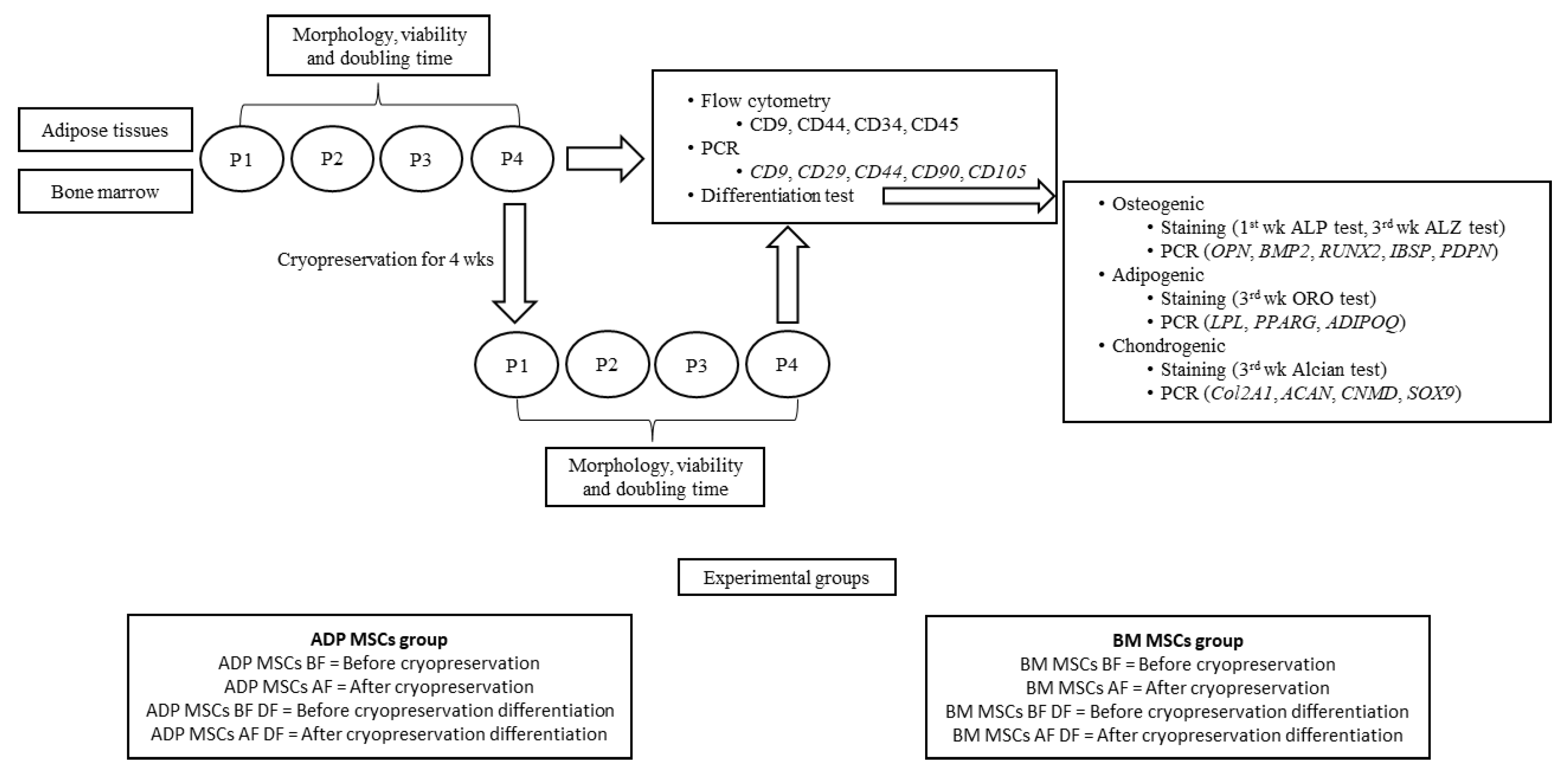
2.1. Experimental Animals and Experimental Groups
2.2. Isolation and Culture of MSCs from ADP and Bone Marrow
2.3. Cryopreservation and Re-Culture of MSCs
2.4. Cell Morphological and Viability Analysis
2.5. Evaluation of Immunophenotypic Characterization on MSCs
2.6. Evaluation of the Differentiation Ability
2.6.1. Osteogenic Differentiation
Alkaline Phosphatase (ALP) Activity
Alizarin Red (ALZ) Staining
Gene Expression in Osteogenic Cells
2.6.2. Adipogenic Differentiation
Oil Red O Staining
Gene Expression in Adipogenic Cells
2.6.3. Chondrogenic Differentiation
Alcian Blue Staining
Gene Expression in Chondrogenic Cells
2.7. Statistical Analysis
3. Results
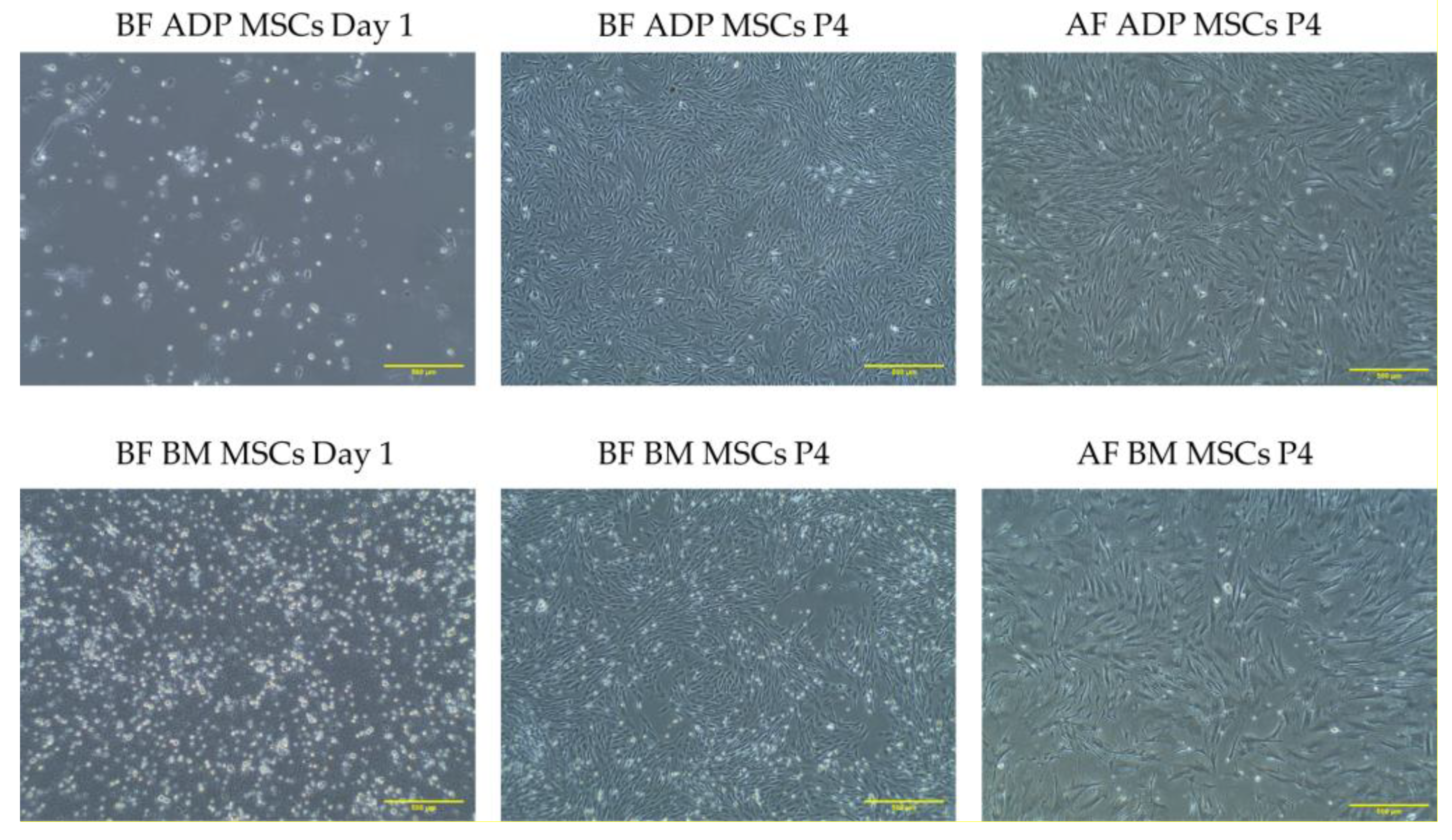
3.1. Isolation and Morphology of Rabbit MSCs
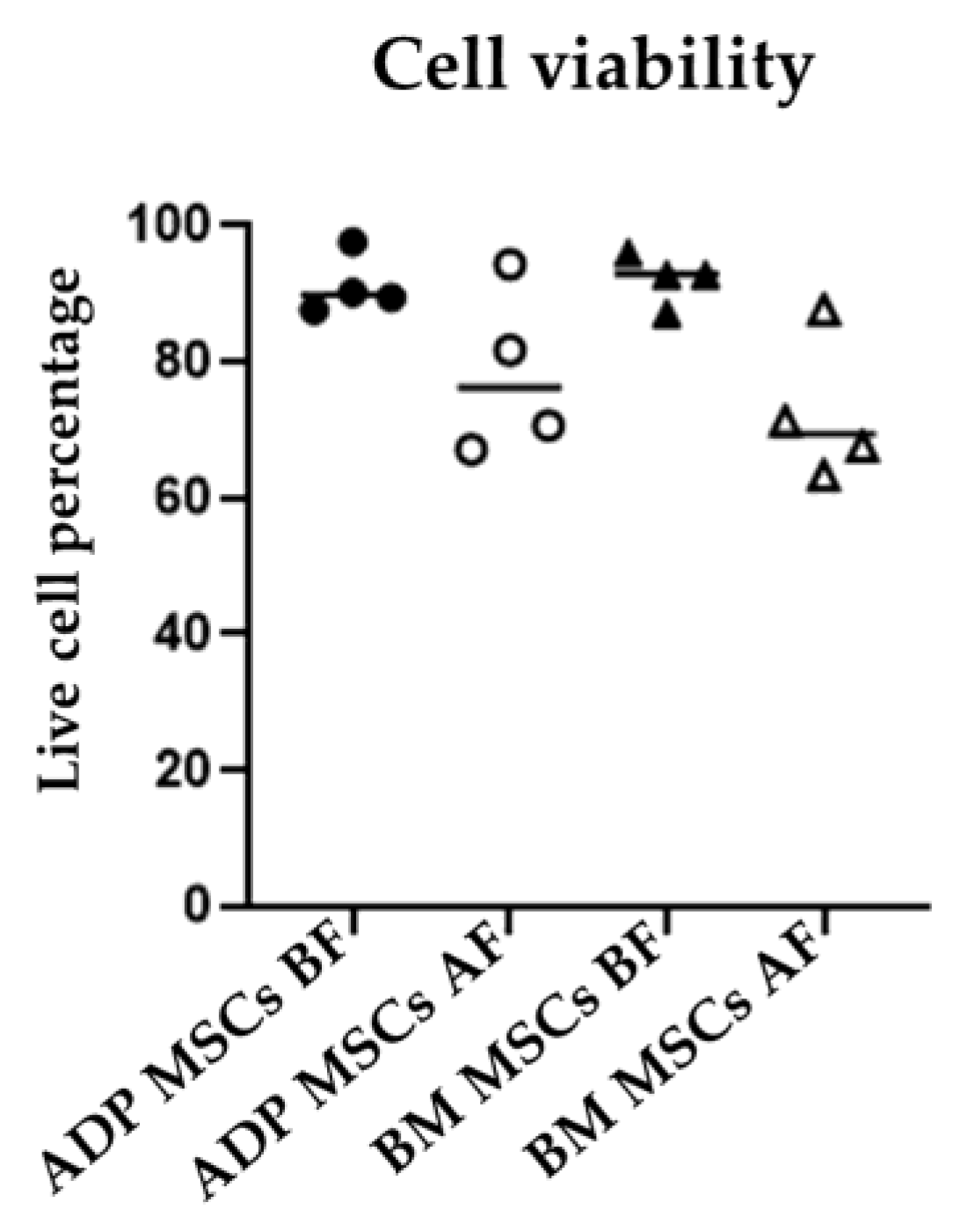
3.2. Viability and Doubling Time
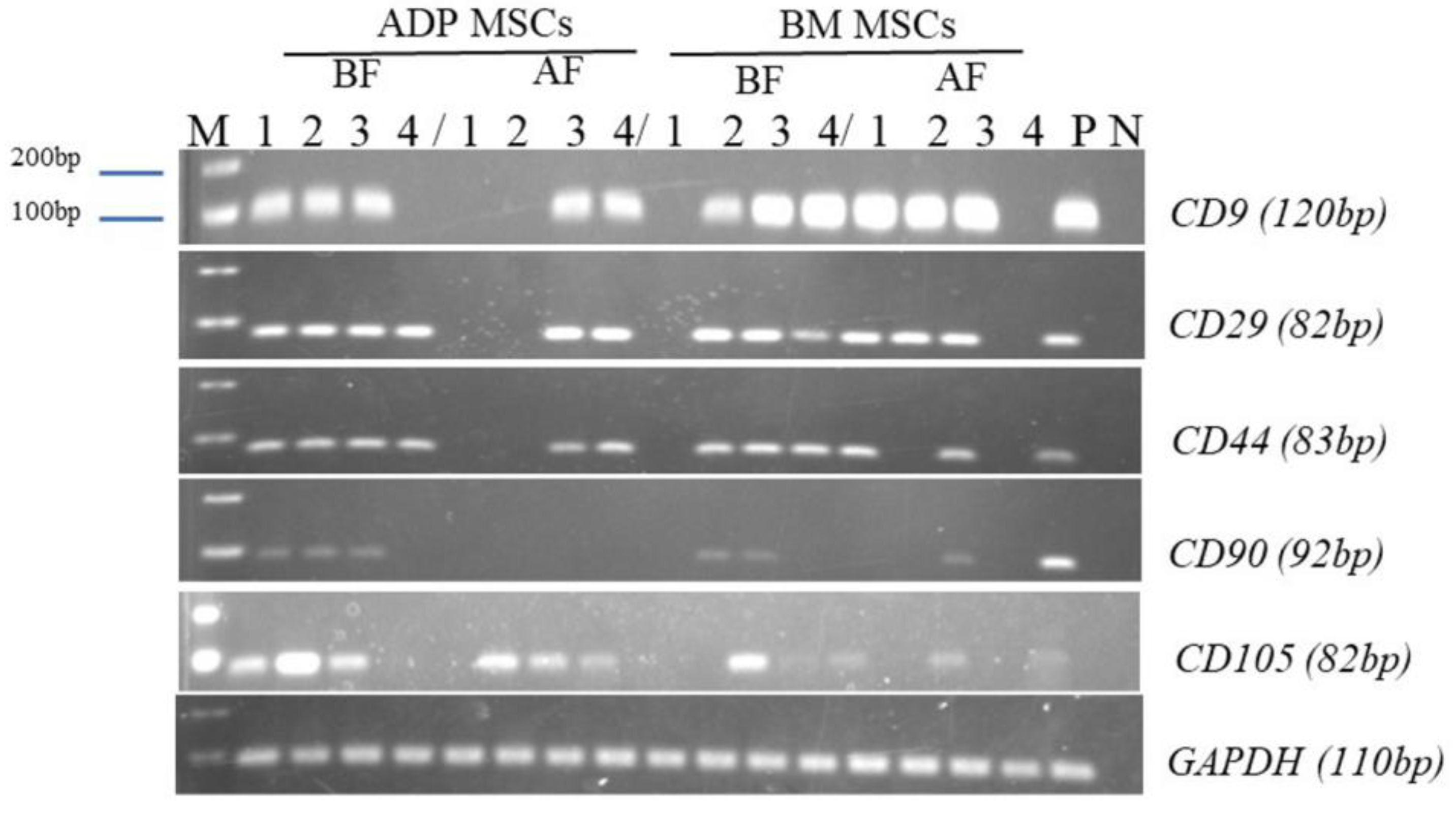
3.3. Surface Marker Expression of MSCs Determined with Flow Cytometry and RT-PCR
3.4. Differentiation Potential of Rabbit MSCs
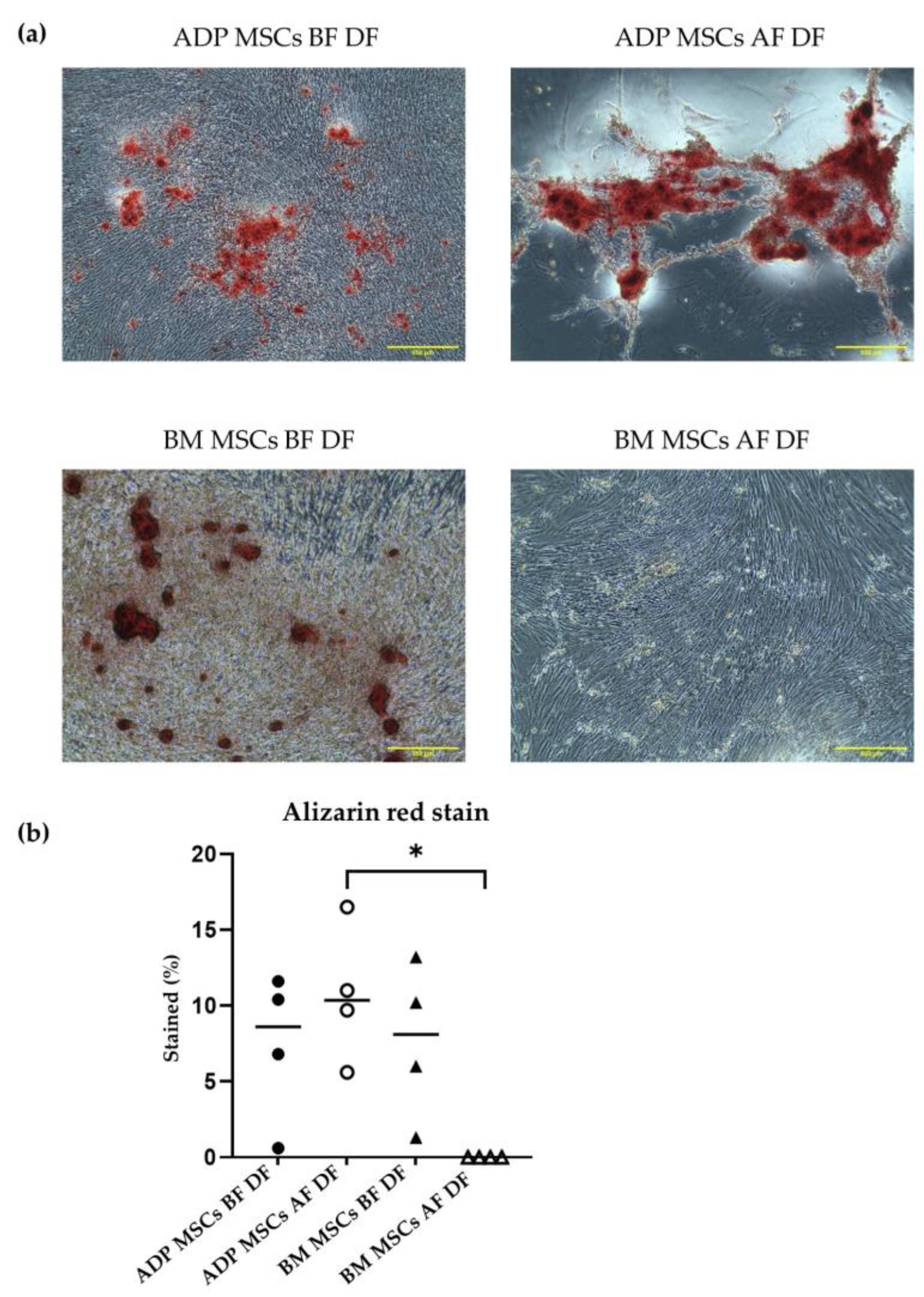
3.4.1. Osteogenic Differentiation
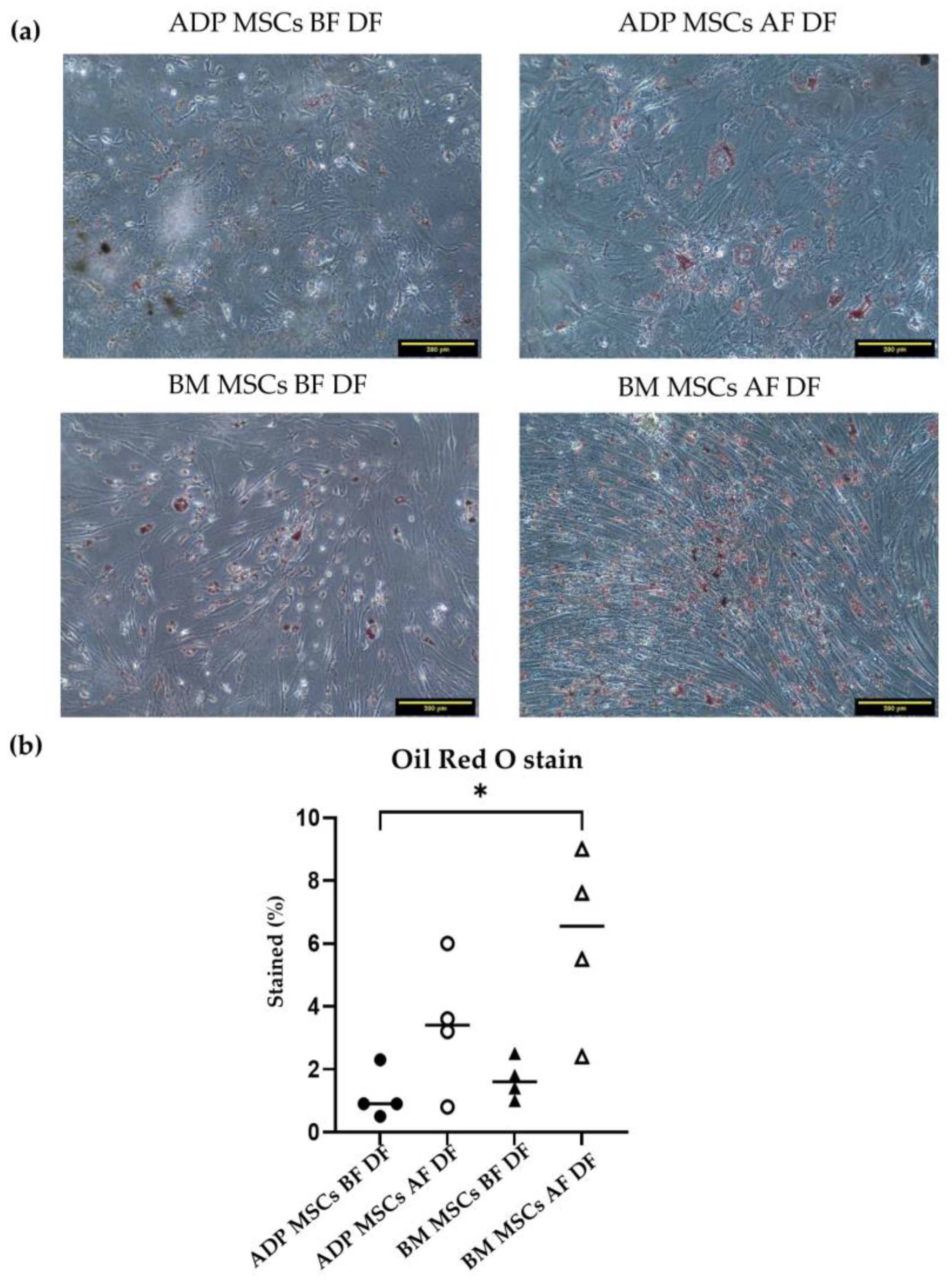
3.4.2. Adipogenic Differentiation Confirmed by Oil Red O Staining
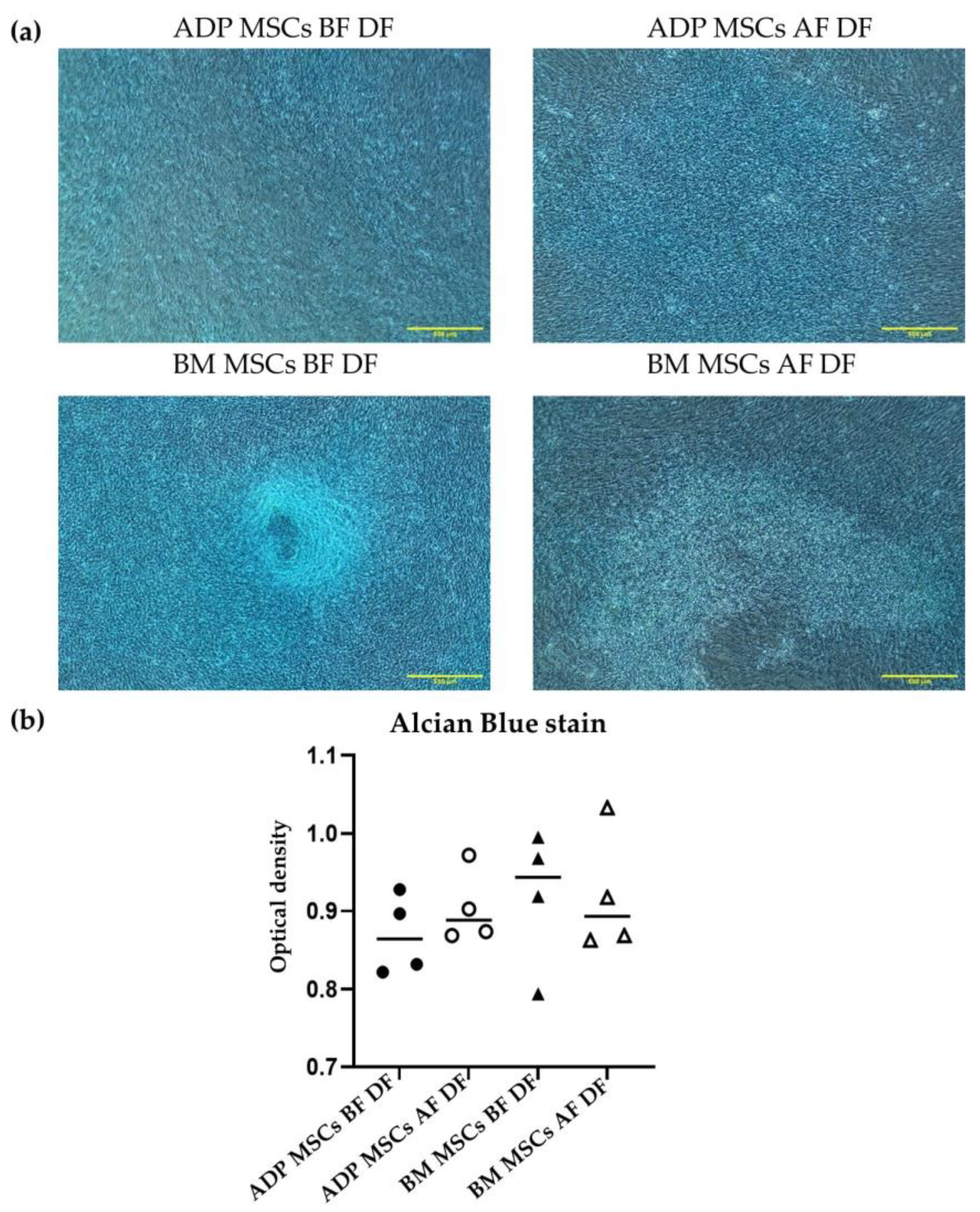
3.4.3. Chondrogenic Differentiation Confirmed by Alcian Blue Stain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bianco, P.; Gehron Robey, P. Marrow stromal stem cells. J. Clin. Investig. 2000, 105, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Bruder, S.P.; Fink, D.J.; Caplan, A.I. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J. Cell. Biochem. 1994, 56, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Owen, M. Marrow stromal stem cells. J. Cell. Sci. Suppl. 1988, 10, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Vašíček, J.; Kováč, M.; Baláži, A.; Kulíková, B.; Tomková, M.; Olexiková, L.; Čurlej, J.; Bauer, M.; Schnabl, S.; Hilgarth, M.; et al. Combined approach for characterization and quality assessment of rabbit bone marrow-derived mesenchymal stem cells intended for gene banking. New Biotechnol. 2020, 54, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.W.; Choi, J.R.; Wan Safwani, W.K. Biobanking of Human Mesenchymal Stem Cells: Future Strategy to Facilitate Clinical Applications. Adv. Exp. Med. Biol. 2016, 951, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Rojewski, M.T.; Weber, B.M.; Schrezenmeier, H. Phenotypic Characterization of Mesenchymal Stem Cells from Various Tissues. Transfus. Med. Hemother. 2008, 35, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Bain, B.J. Bone marrow biopsy morbidity and mortality. Br. J. Haematol. 2003, 121, 949–951. [Google Scholar] [CrossRef]
- Stenderup, K.; Justesen, J.; Clausen, C.; Kassem, M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 2003, 33, 919–926. [Google Scholar] [CrossRef]
- Wexler, S.A.; Donaldson, C.; Denning-Kendall, P.; Rice, C.; Bradley, B.; Hows, J.M. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br. J. Haematol. 2003, 121, 368–374. [Google Scholar] [CrossRef]
- Zuk, P.A. The adipose-derived stem cell: Looking back and looking ahead. Mol. Biol. Cell 2010, 21, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Fossett, E.; Khan, W.S. Optimising human mesenchymal stem cell numbers for clinical application: A literature review. Stem Cells Int. 2012, 2012, 465259. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Lee, B.J.; Park, H.Y.; Song, J.S.; Shin, S.C.; Lee, J.C.; Wang, S.G.; Jung, J.S. Effects of donor age, long-term passage culture, and cryopreservation on tonsil-derived mesenchymal stem cells. Cell Physiol. Biochem. 2015, 36, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, K.A.; Cury, C.C.; Oliveira, L.; Cattaned, R.I.; Malvezzi, M.; Francisco, J.C.; Pachalok, A.; Olandoski, M.; Faria-Neto, J.R.; Guarita-Souza, L.C. Evaluation of bone marrow mesenchymal stem cell standard cryopreservation procedure efficiency. Transplant. Proc. 2008, 40, 839–841. [Google Scholar] [CrossRef] [PubMed]
- Davies, O.G.; Smith, A.J.; Cooper, P.R.; Shelton, R.M.; Scheven, B.A. The effects of cryopreservation on cells isolated from adipose, bone marrow and dental pulp tissues. Cryobiology 2014, 69, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, F.; Lei, L.; Li, Y.; Xiao, Y. Application of autologous cryopreserved bone marrow mesenchymal stem cells for periodontal regeneration in dogs. Cells Tissues Organs 2009, 190, 94–101. [Google Scholar] [CrossRef]
- Zhu, X.; Yuan, F.; Li, H.; Zheng, Y.; Xiao, Y.; Yan, F. Evaluation of canine bone marrow-derived mesenchymal stem cells after long-term cryopreservation. Zool. Sci. 2013, 30, 1032–1037. [Google Scholar] [CrossRef]
- Thirumala, S.; Gimble, J.M.; Devireddy, R.V. Evaluation of methylcellulose and dimethyl sulfoxide as the cryoprotectants in a serum-free freezing media for cryopreservation of adipose-derived adult stem cells. Stem Cells Dev. 2010, 19, 513–522. [Google Scholar] [CrossRef]
- Chin, S.P.; Poey, A.C.; Wong, C.Y.; Chang, S.K.; Teh, W.; Mohr, T.J.; Cheong, S.K. Cryopreserved mesenchymal stromal cell treatment is safe and feasible for severe dilated ischemic cardiomyopathy. Cytotherapy 2010, 12, 31–37. [Google Scholar] [CrossRef]
- Ock, S.A.; Rho, G.J. Effect of dimethyl sulfoxide (DMSO) on cryopreservation of porcine mesenchymal stem cells (pMSCs). Cell Transplant. 2011, 20, 1231–1239. [Google Scholar] [CrossRef]
- Agata, H.; Sumita, Y.; Hidaka, T.; Iwatake, M.; Kagami, H.; Asahina, I. Intra-Bone Marrow Administration of Mesenchymal Stem/Stromal Cells Is a Promising Approach for Treating Osteoporosis. Stem Cells Int. 2019, 2019, 4214281. [Google Scholar] [CrossRef] [PubMed]
- Bagi, C.M.; Berryman, E.; Moalli, M.R. Comparative bone anatomy of commonly used laboratory animals: Implications for drug discovery. Comp. Med. 2011, 61, 76–85. [Google Scholar] [PubMed]
- Calle, A.; Zamora-Ceballos, M.; Bárcena, J.; Blanco, E.; Ramírez, M. Comparison of Biological Features of Wild European Rabbit Mesenchymal Stem Cells Derived from Different Tissues. Int. J. Mol. Sci. 2022, 23, 6420. [Google Scholar] [CrossRef] [PubMed]
- Mapara, M.; Thomas, B.S.; Bhat, K.M. Rabbit as an animal model for experimental research. Dent. Res. J. 2012, 9, 111–118. [Google Scholar] [CrossRef]
- Nunamaker, D.M. Experimental models of fracture repair. Clin. Orthop. Relat. Res. 1998, 355S, S56–S65. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.H.; Murray, I.R. Osteoporotic fracture models. Curr. Osteoporos. Rep. 2015, 13, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Karaoz, E.; Aksoy, A.; Ayhan, S.; Sariboyaci, A.E.; Kaymaz, F.; Kasap, M. Characterization of mesenchymal stem cells from rat bone marrow: Ultrastructural properties, differentiation potential and immunophenotypic markers. Histochem. Cell Biol. 2009, 132, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Lapi, S.; Nocchi, F.; Lamanna, R.; Passeri, S.; Iorio, M.; Paolicchi, A.; Urciuoli, P.; Coli, A.; Abramo, F.; Miragliotta, V.; et al. Different media and supplements modulate the clonogenic and expansion properties of rabbit bone marrow mesenchymal stem cells. BMC Res. Notes 2008, 1, 53. [Google Scholar] [CrossRef]
- Lee, T.H.; Huang, Y.H.; Chang, N.K.; Lin, W.C.; Chien, P.W.; Su, T.M.; Hsieh, D.J.; Lee, T.C. Characterization and spinal fusion effect of rabbit mesenchymal stem cells. BMC Res. Notes 2013, 6, 528. [Google Scholar] [CrossRef]
- Martínez-Lorenzo, M.J.; Royo-Cañas, M.; Alegre-Aguarón, E.; Desportes, P.; Castiella, T.; García-Alvarez, F.; Larrad, L. Phenotype and chondrogenic differentiation of mesenchymal cells from adipose tissue of different species. J. Orthop. Res. 2009, 27, 1499–1507. [Google Scholar] [CrossRef]
- Peister, A.; Mellad, J.A.; Larson, B.L.; Hall, B.M.; Gibson, L.F.; Prockop, D.J. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 2004, 103, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Divya, M.S.; Roshin, G.E.; Divya, T.S.; Rasheed, V.A.; Santhoshkumar, T.R.; Elizabeth, K.E.; James, J.; Pillai, R.M. Umbilical cord blood-derived mesenchymal stem cells consist of a unique population of progenitors co-expressing mesenchymal stem cell and neuronal markers capable of instantaneous neuronal differentiation. Stem Cell Res. Ther. 2012, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Differential analysis of peripheral blood- and bone marrow-derived endothelial progenitor cells for enhanced vascularization in bone tissue engineering. J. Orthop. Res. 2012, 30, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Lee, T.Q. Contributions of the different rabbit models to our understanding of rotator cuff pathology. J. Shoulder Elbow Surg. 2007, 16, S149–S157. [Google Scholar] [CrossRef] [PubMed]
- Warden, S.J. Animal models for the study of tendinopathy. Br. J. Sports Med. 2007, 41, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Kirihara, Y.; Takechi, M.; Kurosaki, K.; Matsuo, H.; Kajitani, N.; Saito, Y. Effects of an anesthetic mixture of medetomidine, midazolam, and butorphanol and antagonism by atipamezole in rabbits. Exp. Anim. 2019, 68, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Semyari, H.; Rajipour, M.; Bastami, F.; Semyari, H. Isolation and Culture of Mesenchymal Stem Cells from Rabbit Scapular Subcutaneous Adipose Tissue and Their Ability to Differentiate into Osteoblasts. Avicenna J. Dent. Res. 2015, 7, 8. [Google Scholar] [CrossRef]
- Løken, S.; Jakobsen, R.B.; Arøen, A.; Heir, S.; Shahdadfar, A.; Brinchmann, J.E.; Engebretsen, L.; Reinholt, F.P. Bone marrow mesenchymal stem cells in a hyaluronan scaffold for treatment of an osteochondral defect in a rabbit model. Knee Surg. Sports Traumatol. Arthrosc. 2008, 16, 896–903. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, F.; Shi, H.; Tan, R.; Han, S.; Ye, G.; Pan, S.; Sun, F.; Liu, X. Comparisons of rabbit bone marrow mesenchymal stem cell isolation and culture methods in vitro. PLoS ONE 2014, 9, e88794. [Google Scholar] [CrossRef]
- Lee, T.C.; Lee, T.H.; Huang, Y.H.; Chang, N.K.; Lin, Y.J.; Chien, P.W.; Yang, W.H.; Lin, M.H. Comparison of surface markers between human and rabbit mesenchymal stem cells. PLoS ONE 2014, 9, e111390. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wei, L.K.; Jian, X.F.; Huang, J.; Zou, H.; Zhang, S.Z.; Yuan, G.H. Isolation, culture and induced differentiation of rabbit mesenchymal stem cells into osteoblasts. Exp. Ther. Med. 2018, 15, 3715–3724. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.L.; Ahmad, T.S.; Selvaratnam, L.; Kamarul, T. Isolation, characterization and the multi-lineage differentiation potential of rabbit bone marrow-derived mesenchymal stem cells. J. Anat. 2013, 222, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Hsu, S.C.; Yao, M.; Huang, D.M. CD9 Upregulation-Decreased CCL21 Secretion in Mesenchymal Stem Cells Reduces Cancer Cell Migration. Int. J. Mol. Sci. 2021, 22, 1738. [Google Scholar] [CrossRef] [PubMed]
- González Hernández, Y.; Fischer, R.W. Serum-free culturing of mammalian cells--adaptation to and cryopreservation in fully defined media. Altex 2007, 24, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Bamba, K.; Ozawa, M.; Tamai, M.; Shimbo, E.; Shindo, H.; Ota, T.; Ahn, S.; Kohara, A.; Tagawa, Y.-I. Human serum albumin-containing xeno-free freezing medium optimized for regenerative medicine. Transl. Regul. Sci. 2021, 3, 77–82. [Google Scholar] [CrossRef]
- Bahsoun, S.; Coopman, K.; Akam, E.C. The impact of cryopreservation on bone marrow-derived mesenchymal stem cells: A systematic review. J. Transl. Med. 2019, 17, 397. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Borghesi, J.; Mario, L.C.; Carreira, A.C.; Miglino, M.A.; Favaron, P.O. Phenotype and multipotency of rabbit (Oryctolagus cuniculus) amniotic stem cells. Stem Cell Res. Ther. 2017, 8, 27. [Google Scholar] [CrossRef]
- Tirpáková, M.; Vašíček, J.; Svoradová, A.; Baláži, A.; Tomka, M.; Bauer, M.; Makarevich, A.; Chrenek, P. Phenotypical Characterization and Neurogenic Differentiation of Rabbit Adipose Tissue-Derived Mesenchymal Stem Cells. Genes 2021, 12, 431. [Google Scholar] [CrossRef]
- Izadpanah, R.; Trygg, C.; Patel, B.; Kriedt, C.; Dufour, J.; Gimble, J.M.; Bunnell, B.A. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J. Cell. Biochem. 2006, 99, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Lechanteur, C.; Briquet, A.; Giet, O.; Delloye, O.; Baudoux, E.; Beguin, Y. Clinical-scale expansion of mesenchymal stromal cells: A large banking experience. J. Transl. Med. 2016, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Mosna, F.; Sensebé, L.; Krampera, M. Human bone marrow and adipose tissue mesenchymal stem cells: A user’s guide. Stem Cells Dev. 2010, 19, 1449–1470. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Prockop, D.J. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair--current views. Stem Cells 2007, 25, 2896–2902. [Google Scholar] [CrossRef] [PubMed]
- Ginis, I.; Grinblat, B.; Shirvan, M.H. Evaluation of bone marrow-derived mesenchymal stem cells after cryopreservation and hypothermic storage in clinically safe medium. Tissue Eng. Part C Methods 2012, 18, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.F.; Gagnon, J. Neuronal cell Thy-1 glycoprotein: Homology with immunoglobulin. Science 1982, 216, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Moraes, D.A.; Sibov, T.T.; Pavon, L.F.; Alvim, P.Q.; Bonadio, R.S.; Da Silva, J.R.; Pic-Taylor, A.; Toledo, O.A.; Marti, L.C.; Azevedo, R.B.; et al. A reduction in CD90 (THY-1) expression results in increased differentiation of mesenchymal stromal cells. Stem Cell Res. Ther. 2016, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Oyarzo, R.; Valderrama, X.; Valenzuela, F.; Bahamonde, J. Bovine Fetal Mesenchymal Stem Cells Obtained from Omental Adipose Tissue and Placenta Are More Resistant to Cryoprotectant Exposure Than Those from Bone Marrow. Front. Vet. Sci. 2021, 8, 708972. [Google Scholar] [CrossRef]
- Renzi, S.; Lombardo, T.; Dotti, S.; Dessì, S.S.; De Blasio, P.; Ferrari, M. Mesenchymal Stromal Cell Cryopreservation. Biopreserv. Biobank. 2012, 10, 276–281. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, M.; Zhou, L.; Chen, X.; Chen, J.; Wu, D. Promoting effect of rapamycin on osteogenic differentiation of maxillary sinus membrane stem cells. PeerJ 2021, 9, e11513. [Google Scholar] [CrossRef]
- Komori, T. Regulation of osteoblast differentiation by Runx2. Adv. Exp. Med. Biol. 2010, 658, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Hasegawa, T.; Yimin; Yamamoto, T.; Hongo, H.; Abe, M.; Yoshida, T.; Yokoyama, A.; de Freitas, P.H.L.; Li, M.; et al. Immunocytochemical assessment of cell differentiation of podoplanin-positive osteoblasts into osteocytes in murine bone. Histochem. Cell Biol. 2021, 155, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.B.; Stein, G.S. Development of the osteoblast phenotype: Molecular mechanisms mediating osteoblast growth and differentiation. Iowa Orthop J. 1995, 15, 118–140. [Google Scholar] [PubMed]
- Abuna, R.P.; De Oliveira, F.S.; Santos Tde, S.; Guerra, T.R.; Rosa, A.L.; Beloti, M.M. Participation of TNF-α in Inhibitory Effects of Adipocytes on Osteoblast Differentiation. J. Cell Physiol. 2016, 231, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.M.; Guo, Y.; Wang, Q.; Xu, Y.; Wang, M.; Chen, H.N.; Shen, W.M. Over-expression of PPAR-γ2 gene enhances the adipogenic differentiation of hemangioma-derived mesenchymal stem cells in vitro and in vivo. Oncotarget 2017, 8, 115817–115828. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.; Stöve, J. Collagens—Major component of the physiological cartilage matrix, major target of cartilage degeneration, major tool in cartilage repair. Adv. Drug Deliv. Rev. 2003, 55, 1569–1593. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Tummala, P.; King, A.; Lee, B.; Kraus, M.; Tse, V.; Jacobs, C.R. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng. Part C Methods 2009, 15, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.-C.; Du, L.-X.; Zhou, W.; Hu, Y.-Q.; Feng, Y.; Liang, H.-F.; Sang, L.; Qi, M.; Zhai, L.-J.; Wang, Z.-Q. Proteomic analysis of chondromodulin-I-induced differentiation of mesenchymal stem cells into chondrocytes. J. Proteom. 2017, 159, 1–18. [Google Scholar] [CrossRef]
- Bi, W.; Deng, J.M.; Zhang, Z.; Behringer, R.R.; de Crombrugghe, B. Sox9 is required for cartilage formation. Nat. Genet. 1999, 22, 85–89. [Google Scholar] [CrossRef]
- Jo, A.; Denduluri, S.; Zhang, B.; Wang, Z.; Yin, L.; Yan, Z.; Kang, R.; Shi, L.L.; Mok, J.; Lee, M.J.; et al. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis. 2014, 1, 149–161. [Google Scholar] [CrossRef]
- Mutlu, L.; Manavella, D.D.; Gullo, G.; McNamara, B.; Santin, A.D.; Patrizio, P. Endometrial Cancer in Reproductive Age: Fertility-Sparing Approach and Reproductive Outcomes. Cancers 2022, 14, 5187. [Google Scholar] [CrossRef]




| Gene Name | Direction | Primer Sequences (5′–3′) | |
|---|---|---|---|
| Surface marker genes | CD9 | Forward | GGCTCCGATTCGACTCTCAG |
| Reverse | AACCCACCAGCATCATGAGG | ||
| CD29 | Forward | AGAACCCTTGCACGAGTGAG | |
| Reverse | TCCTCCCCTCTGTCTGTGAG | ||
| CD44 | Forward | TTGGCGATTTCCTGGGTCTC | |
| Reverse | TGGTTGTGTTGTGGCTGTCT | ||
| CD90 | Forward | CTTCTCTGAGGCTGCTGACC | |
| Reverse | GCAGCACTGGGGTTCCTTAT | ||
| CD105 | Forward | CCACCGGCGAATACTCTCTC | |
| Reverse | AACAGGCCTTGGATGGTGTC | ||
| Osteogenic genes | OPN | Forward | AGACCCTCCCGAGTAAGTCC |
| Reverse | CGGCATCGTCGGATTCATTG | ||
| IBSP | Forward | TTCTGACATCACCTTGGCCG | |
| Reverse | TCTGCTCTCCCACTCACTCA | ||
| BMP2 | Forward | GGAAACGCCTCAAATCCAGC | |
| Reverse | TAAAAGGCGTGATACCCCGG | ||
| RUNX2 | Forward | CTTCAAGGTGGTAGCCCTCG | |
| Reverse | CCGGCCCACAAATCTCAGAT | ||
| PDPN | Forward | ATGAGCCGCAGAAAACTCCA | |
| Reverse | CTTAGAGGAGGGAGCCGAGT | ||
| Adipogenic genes | LPL | Forward | CGACTGGGAACGTGTGTGTA |
| Reverse | CCACACACAACCCTCTCTCC | ||
| PPARG | Forward | CAAGTACGGCGTCCATGAGA | |
| Reverse | CGTCATGAAGCCTTGTCCCT | ||
| ADIPOQ | Forward | GGTGCCTATGTCTACCGCTC | |
| Reverse | CGTGGTGCTGTCATAGTGGT | ||
| Chondrogenic genes | COL2A1 | Forward | GGATAGACCCCAACCAAGGC |
| Reverse | TCCACCAGTTCTTCTTGGGC | ||
| ACAN | Forward | GGAACATCACTGAGGGCGAA | |
| Reverse | CTTCAGTCCCGTTCTCCACC | ||
| CNMD | Forward | AGGAGGCTCTAGTCTGGGTG | |
| Reverse | TCGCCGCAGAGTTCTAAGAC | ||
| SOX9 | Forward | GCCCAGAAGAGCCTCAAAGT | |
| Reverse | TAAGAGAGGTGGGGAGGGTG | ||
| Housekeeping gene | GAPDH | Forward | AGCTGGTCATCAACGGGAAG |
| Reverse | GAAGACGCCAGTGGATTCCA | ||
| Antibodies | ADP MSCs BF | ADP MSCs AF | BM MSCs BF | BM MSCs AF |
|---|---|---|---|---|
| CD9 | 92 (90–95) | 41 (27–50) * | 83 (22–95) | 77 (41–82) |
| CD44 | 50 (32–66) | 17 (10–37) | 51 (29–93) | 31 (22–41) |
| CD34 | 2.5 (0.8–10) | 2.1 (0.7–4.0) | 1.8 (0.3–6.0) | 7 (4–11) |
| CD45 | 2.4 (0.6–3.0) | 0.8 (0–0.9) | 0.5 (0.02–1) | 0.2 (0.05–0.8) |
| Surface Marker Genes | ADP MSCs BF | ADP MSCs AF | BM MSCs BF | BM MSCs AF |
|---|---|---|---|---|
| CD9 | 3 | 2 | 3 | 3 |
| CD29 | 4 | 2 | 3 | 3 |
| CD44 | 4 | 2 | 3 | 2 |
| CD90 | 3 | 0 | 2 | 1 |
| CD105 | 3 | 2 | 3 | 2 |
| Gene | ADP MSCs | BM MSCs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BF | BF DF | AF | AF DF | BF | BF DF | AF | AF DF | ||
| Osteogenic gene | OPN | 2 | 0 | 1 | 2 | 1 | 2 | 3 | 1 |
| IBSP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| BMP2 | 2 | 2 | 0 | 2 | 2 | 0 | 1 | 1 | |
| RUNX2 | 4 | 4 | 2 | 4 | 3 | 3 | 2 | 3 | |
| PDPN | 4 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | |
| Adipogenic gene | LPL | 2 | 4 | 1 | 2 | 2 | 0 | 2 | 2 |
| PPARG | 3 | 0 | 0 | 2 | 2 | 3 | 0 | 0 | |
| ADIPOQ | 0 | 4 | 0 | 2 | 0 | 0 | 0 | 0 | |
| Chondrogenic gene | COL2A1 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 |
| ACAN | 0 | 3 | 0 | 0 | 0 | 4 | 0 | 1 | |
| CNMD | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 1 | |
| SOX9 | 3 | 2 | 2 | 4 | 2 | 4 | 1 | 4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koung Ngeun, S.; Shimizu, M.; Kaneda, M. Characterization of Rabbit Mesenchymal Stem/Stromal Cells after Cryopreservation. Biology 2023, 12, 1312. https://doi.org/10.3390/biology12101312
Koung Ngeun S, Shimizu M, Kaneda M. Characterization of Rabbit Mesenchymal Stem/Stromal Cells after Cryopreservation. Biology. 2023; 12(10):1312. https://doi.org/10.3390/biology12101312
Chicago/Turabian StyleKoung Ngeun, Sai, Miki Shimizu, and Masahiro Kaneda. 2023. "Characterization of Rabbit Mesenchymal Stem/Stromal Cells after Cryopreservation" Biology 12, no. 10: 1312. https://doi.org/10.3390/biology12101312
APA StyleKoung Ngeun, S., Shimizu, M., & Kaneda, M. (2023). Characterization of Rabbit Mesenchymal Stem/Stromal Cells after Cryopreservation. Biology, 12(10), 1312. https://doi.org/10.3390/biology12101312






