Economical Production of Phenazine-1-carboxylic Acid from Glycerol by Pseudomonas chlororaphis Using Cost-Effective Minimal Medium
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Basic Culture Conditions
2.2. Determination of Cell Growth and Phenazine Compound Titer
2.3. Statistical Analysis
3. Results
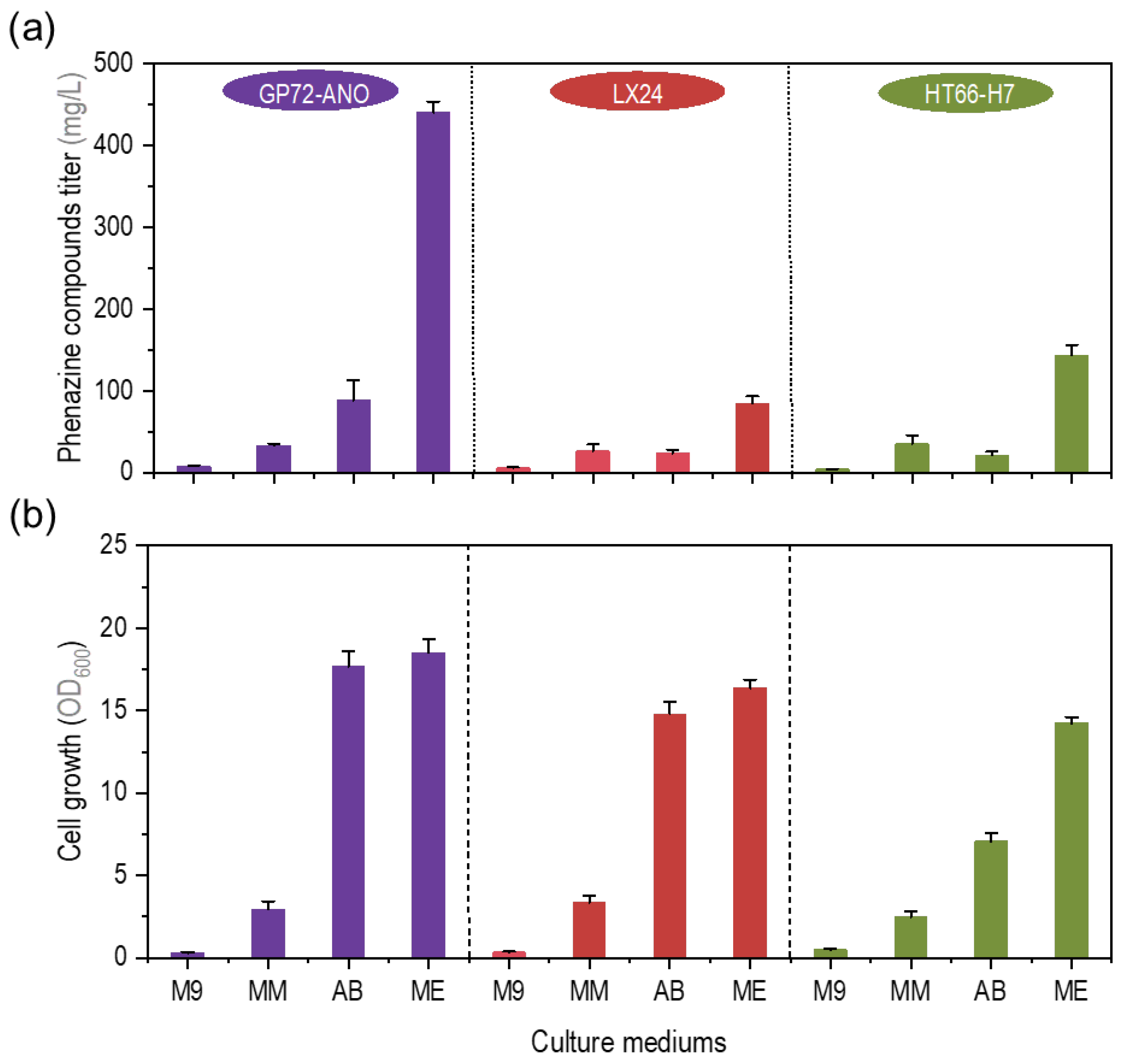
3.1. Comparison of Fermentation Performance of P. chlororaphis in Different Minimal Medium
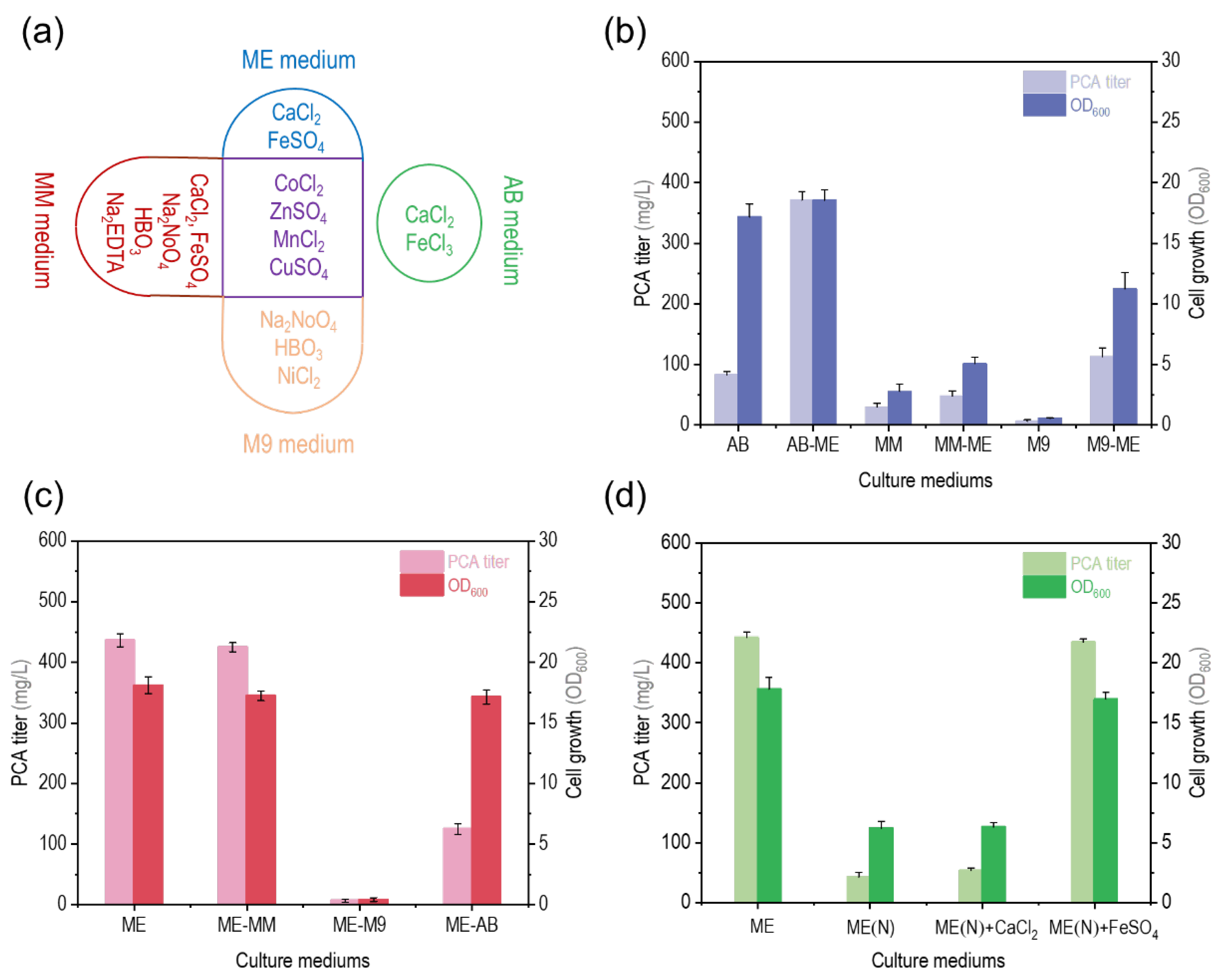
3.2. Effect of Trace Elements in ME Medium on PCA Titer by P. chlororaphis GP72-ANO
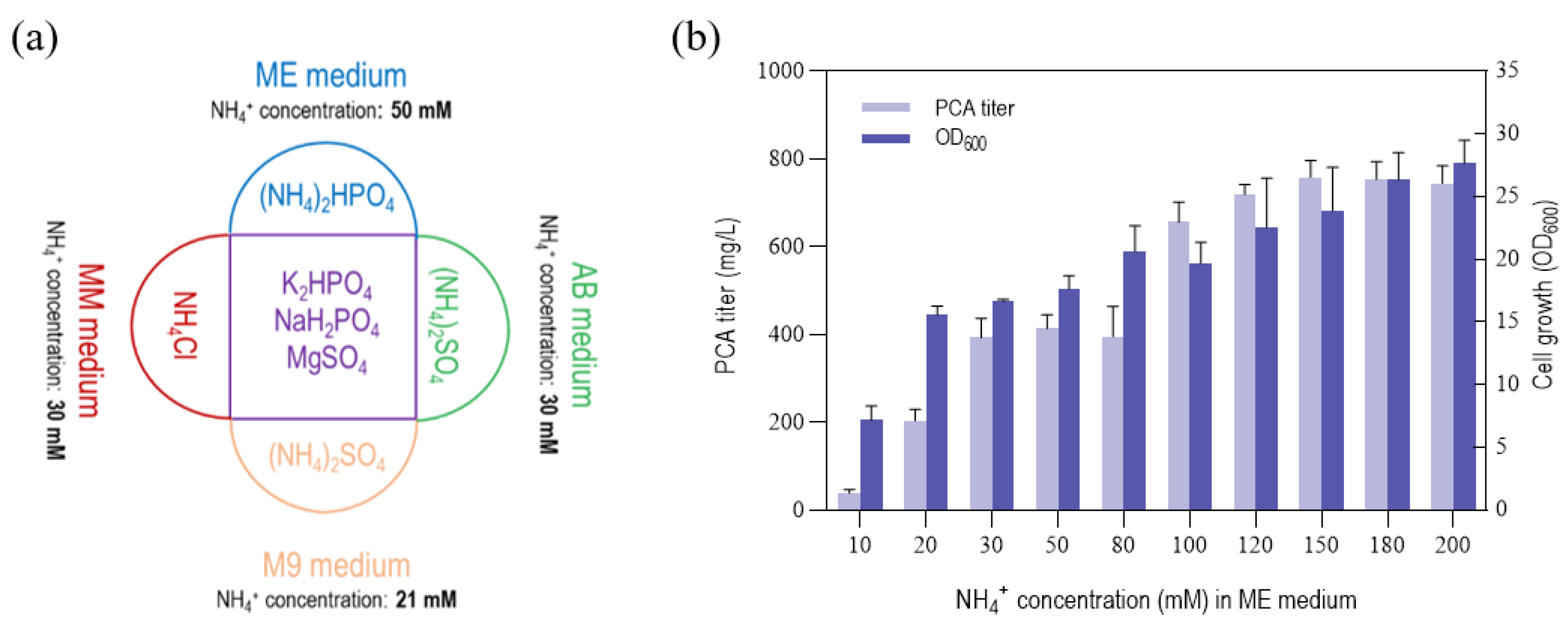
3.3. Effect of NH4+ and Iron Concentration on PCA Titer by P. chlororaphis GP72-ANO
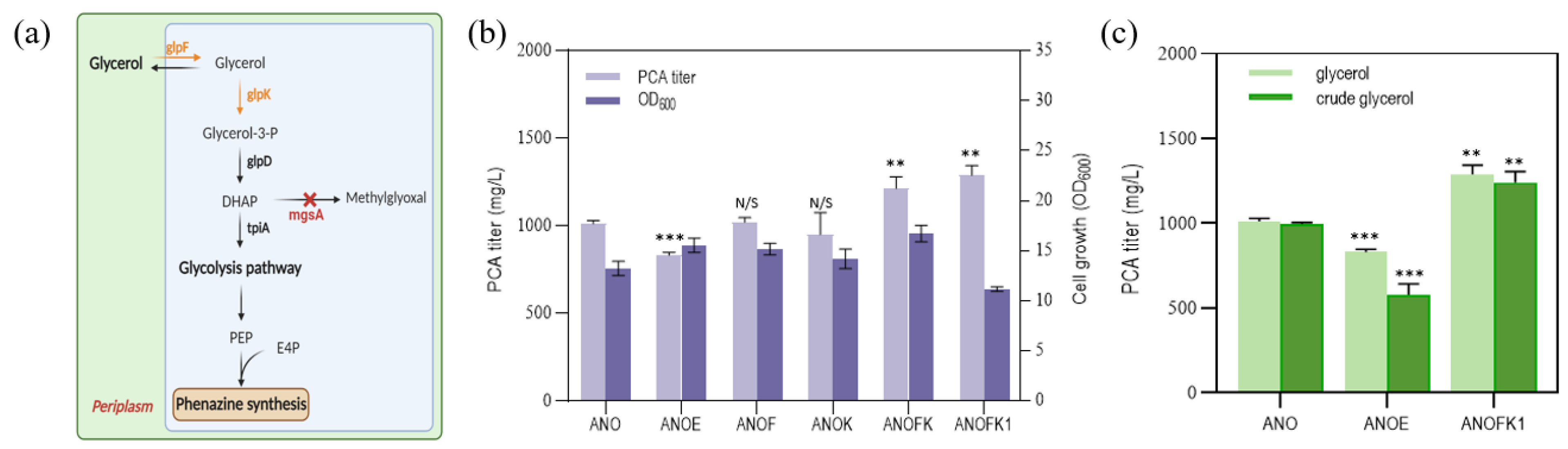
3.4. Engineering Glycerol Metabolic Pathway for More Economical PCA Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vivek, N.; Sindhu, R.; Madhavan, A.; Anju, A.J.; Castro, E.; Faraco, V.; Pandey, A.; Binod, P. Recent advances in the production of value added chemicals and lipids utilizing biodiesel industry generated crude glycerol as a substrate—Metabolic aspects, challenges and possibilities: An overview. Bioresour. Technol. 2017, 239, 507–517. [Google Scholar] [CrossRef]
- Chu, H.S.; Ahn, J.H.; Yun, J.; Choi, I.S.; Nam, T.W.; Cho, K.M. Direct fermentation route for the production of acrylic acid. Metab. Eng. 2015, 32, 23–29. [Google Scholar] [CrossRef]
- Sasaki, Y.; Yoshikuni, Y. Metabolic engineering for valorization of macroalgae biomass. Metab. Eng. 2022, 71, 42–61. [Google Scholar] [CrossRef]
- Krishnaiah, M.; de Almeida, N.R.; Udumula, V.; Song, Z.; Chhonker, Y.S.; Abdelmoaty, M.M.; Do, N.V.; Murry, D.J.; Conda-Sheridan, M. Synthesis, biological evaluation, and metabolic stability of phenazine derivatives as antibacterial agents. Eur. J. Med. Chem. 2018, 143, 936–947. [Google Scholar] [CrossRef]
- Wu, S.; Liang, X.; Luo, F.; Liu, H.; Shen, L.; Yang, X.; Huang, Y.; Xu, H.; Wu, N.; Zhang, Q.; et al. Synthesis, crystal structure and bioactivity of phenazine-1-carboxylic acylhydrazone derivatives. Molecules 2021, 26, 5320. [Google Scholar] [CrossRef]
- Bitzenhofer, N.L.; Höfel, C.; Thies, S.; Weiler, A.J.; Eberlein, C.; Heipieper, H.J.; Batra-Safferling, R.; Sundermeyer, P.; Heidler, T.; Sachse, C.; et al. Exploring engineered vesiculation by Pseudomonas putida KT2440 for natural product biosynthesis. Microb. Biotechnol. 2023, 1–18. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, F.X.; Liu, C.; Wang, L.; Qi, Y.; Cao, M.; Guo, X.; Li, J.; Huang, X.; Yang, J.; et al. Isolation and Biosynthesis of Phenazine–Polyketide Hybrids from Streptomyces sp. KIB-H483. J. Nat. Prod. 2022, 85, 1324–1331. [Google Scholar] [CrossRef]
- Murakami, E.; Deppenmeier, U.; Ragsdale, S.W. Characterization of the intramolecular electron transfer pathway from 2-hydroxyphenazine to the heterodisulfide reductase from Methanosarcina thermophila. J. Biol. Chem. 2001, 276, 2432–2439. [Google Scholar] [CrossRef]
- Guttenberger, N.; Blankenfeldt, W.; Breinbauer, R. Recent developments in the isolation, biological function, biosynthesis, and synthesis of phenazine natural products. Bioorg. Med. Chem. 2017, 25, 6149–6166. [Google Scholar] [CrossRef]
- Agaronyan, K.; Sharma, L.; Vaidyanathan, B.; Glenn, K.; Yu, S.; Annicelli, C.; Wiggen, T.D.; Penningroth, M.R.; Hunter, R.C.; Dela, C.C.; et al. Tissue remodeling by an opportunistic pathogen triggers allergic inflammation. Immunity 2022, 55, 895–911. [Google Scholar] [CrossRef]
- Song, C.; Yue, S.J.; Liu, W.H.; Zheng, Y.F.; Zhang, C.H.; Feng, T.T.; Hu, H.B.; Wang, W.; Zhang, X.H. Engineering of glycerol utilization in Pseudomonas chlororaphis GP72 for enhancing phenazine-1-carboxylic acid production. World J. Microb. Biot. 2020, 36, 49. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Urgel, M.; Ramos-González, M.I. Becoming settlers: Elements and mechanisms for surface colonization by Pseudomonas putida. Environ. Microbiol. 2023, 25, 1575–1593. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Chen, T.; Jiang, Y.; Lu, J.; Dong, W.; Zhang, W.; Ma, J.; Zhang, M.; Jiang, M. Enhanced biobutanol production with high yield from crude glycerol by acetone uncoupled clostridium sp. Strain CT7. Bioresour. Technol. 2017, 244 Pt 1, 575–581. [Google Scholar] [CrossRef]
- Yang, F.; Hanna, M.A.; Sun, R. Value-added uses for crude glycerol—A byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Du, S.; Yan, Y.; Pan, F.; Wang, R.; Li, S. Systematic engineering of Bacillus amyloliquefaciens for efficient production of poly-γ-glutamic acid from crude glycerol. Bioresour. Technol. 2022, 359, 127382. [Google Scholar] [CrossRef]
- Liu, K.; Hu, H.; Wang, W.; Zhang, X. Genetic engineering of Pseudomonas chlororaphis GP72 for the enhanced production of 2-hydroxyphenazine. Microb. Cell Fact. 2016, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Mcrose, D.L.; Newman, D.K. Redox-active antibiotics enhance phosphorus bioavailability. Science 2021, 371, 1033–1037. [Google Scholar] [CrossRef]
- Wang, D.; Lee, S.H.; Seeve, C.; Yu, J.M.; Pierson, L.R.; Pierson, E.A. Roles of the Gac-Rsm pathway in the regulation of phenazine biosynthesis in Pseudomonas chlororaphis 30–84. Microbiologyopen 2013, 2, 505–524. [Google Scholar] [CrossRef]
- Selin, C.; Fernando, W.; de Kievit, T. The phzI/phzR quorum-sensing system is required for pyrrolnitrin and phenazine production, and exhibits cross-regulation with rpoS in Pseudomonas chlororaphis PA23. Microbiology 2012, 158 Pt 4, 896–907. [Google Scholar] [CrossRef]
- Mavrodi, D.V.; Ksenzenko, V.N.; Bonsall, R.F.; Cook, R.J.; Boronin, A.M.; Thomashow, L.S. A seven-gene locus for synthesis of phenazine-1-carboxylic acid by Pseudomonas fluorescens 2-79. J. Bacteriol. 1998, 180, 2541–2548. [Google Scholar] [CrossRef]
- Culbertson, J.E.; Toney, M.D. Expression and characterization of PhzE from P. aeruginosa PAO1: Aminodeoxyisochorismate synthase involved in pyocyanin and phenazine-1-carboxylate production. Biochim. Biophys. Acta 2013, 1834, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Shtark, O.; Shaposhnikov, A.I.; Kravchenko, L.V. The production of antifungal metabolites by Pseudomonas chlororaphis grown on different nutrient sources. Postep. Mikrobiol. 2003, 72, 645–650. [Google Scholar]
- Hu, H.; Li, Y.; Liu, K.; Zhao, J.; Wang, W.; Zhang, X.H. Production of trans-2,3-dihydro-3-hydroxyanthranilic acid by engineered Pseudomonas chlororaphis GP72. Appl. Microbiol. Biotechnol. 2017, 101, 6607–6613. [Google Scholar] [CrossRef] [PubMed]
- Solaiman, D.; Ashby, R.D.; Crocker, N.V. Genetic construction of recombinant Pseudomonas chlororaphis for improved glycerol utilization. Biocatal. Agr.Biotech. 2016, 8, 45–49. [Google Scholar] [CrossRef]
- Kumar, L.; Yellapu, S.K.; Tyagi, R.D.; Zhang, X. A review on variation in crude glycerol composition, bio-valorization of crude and purified glycerol as carbon source for lipid production. Bioresour. Technol. 2019, 293, 122155. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-X.; Yue, S.-J.; Zheng, Y.-F.; Huang, P.; Nie, Y.-F.; Hao, X.-R.; Zhang, H.-Y.; Wang, W.; Hu, H.-B.; Zhang, X.-H. Economical Production of Phenazine-1-carboxylic Acid from Glycerol by Pseudomonas chlororaphis Using Cost-Effective Minimal Medium. Biology 2023, 12, 1292. https://doi.org/10.3390/biology12101292
Li Y-X, Yue S-J, Zheng Y-F, Huang P, Nie Y-F, Hao X-R, Zhang H-Y, Wang W, Hu H-B, Zhang X-H. Economical Production of Phenazine-1-carboxylic Acid from Glycerol by Pseudomonas chlororaphis Using Cost-Effective Minimal Medium. Biology. 2023; 12(10):1292. https://doi.org/10.3390/biology12101292
Chicago/Turabian StyleLi, Yu-Xuan, Sheng-Jie Yue, Yi-Fan Zheng, Peng Huang, Yan-Fang Nie, Xiang-Rui Hao, Hong-Yan Zhang, Wei Wang, Hong-Bo Hu, and Xue-Hong Zhang. 2023. "Economical Production of Phenazine-1-carboxylic Acid from Glycerol by Pseudomonas chlororaphis Using Cost-Effective Minimal Medium" Biology 12, no. 10: 1292. https://doi.org/10.3390/biology12101292
APA StyleLi, Y.-X., Yue, S.-J., Zheng, Y.-F., Huang, P., Nie, Y.-F., Hao, X.-R., Zhang, H.-Y., Wang, W., Hu, H.-B., & Zhang, X.-H. (2023). Economical Production of Phenazine-1-carboxylic Acid from Glycerol by Pseudomonas chlororaphis Using Cost-Effective Minimal Medium. Biology, 12(10), 1292. https://doi.org/10.3390/biology12101292






