Simple Summary
Caffeine and ethanol are among the most commonly consumed legal psychoactive substances worldwide. They are primarily administered in beverages for arguably opposite physiological effects—caffeine is consumed as a stimulant, and ethanol is consumed as a depressant/sedative. Both caffeine and ethanol influence many biochemical pathways including adenosine receptor-mediated signaling. Caffeine is an antagonist of adenosine receptors and ethanol elevates adenosine levels, which promotes sleep. It is important to study the interaction between caffeine and alcohol because both are easily accessible and are frequently consumed together, especially by young adults. Studies on humans have found that simultaneous intake of caffeinated drinks and alcohol increases the likelihood of alcohol consumption. In this study, using fruit flies Drosophila melanogaster as a model, we show that flies raised on a caffeine-supplemented diet for as little as one day or with mutation in adenosine receptor take longer to sedate when exposed to ethanol vapors. Further research in flies, which is an excellent model for behavioral studies, on the interaction between caffeine and ethanol will improve our understanding of the biochemical effect of pharmacological and psychoactive substances on behaviors associated with alcohol use disorders.
Abstract
Caffeine and ethanol are among the most widely available and commonly consumed psychoactive substances. Both interact with adenosine receptor-mediated signaling which regulates numerous neurological processes including sleep and waking behaviors. In mammals, caffeine is an adenosine receptor antagonist and thus acts as a stimulant. Conversely, ethanol is a sedative because it promotes GABAergic neurotransmission, inhibits glutamatergic neurotransmission, and increases the amount of adenosine in the brain. Despite seemingly overlapping interactions, not much is known about the effect of caffeine on ethanol-induced sedation in Drosophila. In this study, using Drosophila melanogaster as a model, we show that caffeine supplementation in food delays the onset of ethanol-induced sedation in males and females of different strains. The resistance to sedation reverses upon caffeine withdrawal. Heterozygous adenosine receptor mutant flies are resistant to sedation. These findings suggest that caffeine and adenosine receptors modulate the sedative effects of ethanol in Drosophila.
1. Introduction
Caffeine is a widely consumed natural stimulant found in coffee, tea, and chocolate [1,2]. In the United States, individuals consume an estimated daily average of 165–170 mg of caffeine [3,4] and an estimated 10 billion kg of coffee was consumed worldwide in 2020–21 [5]. Caffeine (1,3,7-trimethylxanthine) is an alkaloid with structural similarities to purine nucleic acids. Neurally, caffeine has multiple proposed mechanisms of action, including intracellular calcium mobilization, cAMP phosphodiesterase inhibition, and adenosine receptor antagonism [2,6]. Caffeine is an antagonist of all four subtypes of mammalian adenosine receptors (A1, A2A, A2B, and A3) but the antagonism of the A1 and A2A receptors is most well characterized [7,8]. Drosophila has a single adenosine receptor subtype which is most similar to the mammalian A2A subtype of adenosine receptor. Drosophila adenosine receptor is expressed in all developmental stages with the highest expression observed in the adult head [9]. Adenosine receptors are G-protein coupled receptors (GPCRs) and primarily function through cAMP and PKA signaling pathways for downstream functionality [9].
Adenosine is a nucleoside produced during the breakdown of ATP [10], which acts as a neuromodulator performing multiple functions related to reducing neuronal excitability in the central nervous system [11]. Adenosine signaling promotes sleep by inhibiting wakefulness-promoting neurons [12,13]. Neuronal production of adenosine is also higher during waking hours than during sleep, allowing the promotion of wakefulness as morning approaches [12,14].
Ethanol is another widely consumed psychoactive substance that acts as a depressant and promotes sleep [15]. Ethanol has multiple mechanisms of action, contributing to these behavioral effects, which include promoting GABA signaling and inhibiting glutamatergic signaling [16]. Additionally, ethanol interacts with adenosine neurotransmission to exert somnogenic effects, potentially by increasing the amount of adenosine available to target A1 receptors [16,17].
Drosophila melanogaster serves as an ideal animal model for studying the effects of psychoactive compounds on behavior [18,19]. Upon exposure to ethanol, Drosophila exhibits analogous responses to humans including biphasic hyperactivity and sedation, tolerance, and preference for alcohol using similar biochemical pathways [20,21]. Furthermore, Drosophila and vertebrates share many of the metabolic pathways implicated in the behavioral response to ethanol [21]. To date, no studies have characterized the effect of caffeine and adenosine receptors on ethanol-induced sedation in flies. Here, we demonstrate that caffeine and heterozygous adenosine receptor mutation delay the onset of ethanol-induced sedation in Drosophila.
2. Materials and Methods
2.1. Drosophila Stocks and Maintenance
Drosophila stocks w1118 (provided by Joshua Kavaler, Colby College, Waterville, ME, USA), OR-R (Bloomington stock #5), and AdoRMB04401 (Bloomington stock #24699) were obtained from the Bloomington Drosophila Stock Center (Bloomington, IN, USA). w1118 flies were used as control flies because the adenosine receptor mutant allele was generated in the w1118 background. The fly stocks were maintained on standard Nutri-FlyTM Bloomington Formulation (Genesee Scientific, San Diego, CA, USA). In all trials, light conditions, (12 h light/dark cycle), environmental temperature (25 °C), food type, and time of day of sedation were kept constant unless otherwise stated to minimize the chance of conflicting variables. Sedation assays were carried out between ZT6 and ZT10.
2.2. Food Preparation
Each food vial contained 1.5 g of Nutri-FlyTM Instant Formulation (Genesee Scientific, San Diego, CA, USA) instant food (Genesee Scientific) and 7.5 mL distilled water, which swells to 10 mL volume. Caffeine (Sigma, St. Louis, MO, USA) was dissolved in distilled water and mixed with instant food to make supplemented food (caffeine—0.25 mg/mL, 0.5 mg/mL, and 1.0 mg/mL).
2.3. Caffeine Supplementation
Flies (1–3 days old) were raised on caffeine-supplemented food for the specified experimental timeline followed by ethanol-induced sedation assay.
For dose-dependency experiments (both caffeine and ethanol) and withdrawal experiments, flies were raised on caffeine-supplemented food for three days.
For variable exposure length studies, time on caffeine-supplemented food ranged from one to five days.
For sustainability of effect (withdrawal) experiments, flies were placed in vials containing non-caffeinated food for a designated period of time ranging from one to three days. Flies were age-matched such that they were all the same age at the time of the sedation assays.
2.4. Caffeine-Induced Mortality
The surviving flies were counted after the full period of supplementation with caffeine (three or five days) to calculate mortality rates.
2.5. Ethanol-Induced Sedation Assay
A modified ethanol sedation assay from Maples and Rothenfluh was followed as previously reported [22,23]. Male or female flies (8–10) were sedated using CO2 and sorted into empty vials. Flies were allowed to recover from the effects of CO2 anesthesia for 0.5–8 h before sedation assay. To determine time of sedation, the cotton plug of each vial was replaced with a new plug soaked with 500 μL of the appropriate dose (25%, 50%, 75%, and 100% v/v) of 200-proof ethanol (Fisher Scientific). After one minute, each vial was tapped once on the table to relocate the flies to the bottom of the vial. After ten seconds, the number of flies exhibiting loss-of-righting reflex were recorded. This procedure was repeated after every minute of ethanol exposure for each vial until at least half of the flies in the vial were sedated. Loss of righting reflex includes flies on their backs, stationary flies, stationary flies with rapid wing movement, and spinning in one location. The number of minutes it took for half the flies in the vial to sedate was recorded for each vial as the median sedation time (ST50). Linear interpolation was used to determine ST50 when there was an odd number of flies in the trial or when 50% sedation was reached in between intervals.
2.6. Statistical Analysis
Multiple two-tailed independent samples t-tests and one-way ANOVAs with Tukey HSD post hoc tests were conducted using RStudio.
3. Results
3.1. Caffeine Delays Onset of Ethanol-Induced Sedation in Wild Type Flies
To determine the effect of caffeine on ethanol-induced sedation, we performed sedation assays (with 100% ethanol), which rely on loss-of-righting reflex, on 1–3 day-old male and female w1118 flies, a widely used control strain, after exposing them to caffeine-supplemented food for three days.
Male flies raised on 0.5 mg/mL caffeine-supplemented food had a significantly higher median sedation time (ST50) compared to control flies with no caffeine supplementation (Figure 1; average ST50caff = 6.49 ± 0.23 min, ST50cont = 5.15 ± 0.28 min, n = 16 biological replicates, one-way ANOVA-Tukey, p = 0.001). Flies exposed to other doses of caffeine (0.25 mg/mL, n = 15 biological replicates or 1.0 mg/mL caffeine, n = 19 biological replicates) did not have a statistically significant different ST50 than control flies. However, we observed a trend for higher ST50 in lower doses of caffeine compared to control flies (Figure 1). The delay in onset of sedation at 0.5 mg/mL caffeine dose was more pronounced when sedation assays were performed with 50% and 75% ethanol (Table 1 and Figure S1).
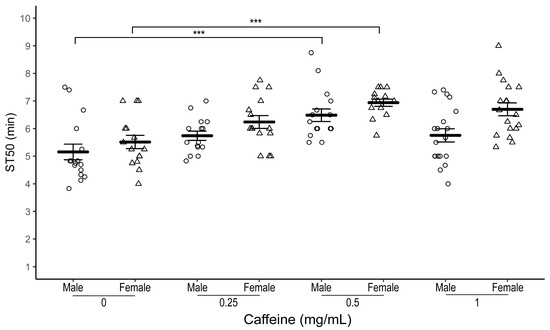
Figure 1.
Effect of caffeine supplementation on ethanol-induced sedation in w1118 flies. Comparison of ST50 when exposed to 100% ethanol of 1–3 day old male (circles) and female (triangles) w1118 flies raised for three days at different dosages (0.25, 0.5, and 1 mg/mL) of caffeine-supplemented food. Both male and female w1118 flies raised on 0.5 mg/mL caffeine-supplemented food have a significantly higher ST50 than control flies. Horizontal bars represent the mean ST50 ± standard error of 15–19 biological replicates. For each sedation assay 8–10 male flies were used. *** p = 0.001; ANOVA, post hoc Tukey HSD.

Table 1.
Effect of caffeine on sedation at different ethanol concentrations.
Similarly, female w1118 flies showed the most robust increase in ST50 at 0.5 mg/mL caffeine concentration (Figure 1; average ST50caff = 6.94 ± 0.13 min, ST50cont = 5.51 ± 0.24 min, n = 14–15 biological replicates, one-way ANOVA-Tukey HSD, p = 0.0001).
The mortality was not statistically significant for any caffeine dose in either males or females. However, we observed a trend of increased mortality in both males and females in a dose- and duration of exposure-dependent manner, with the highest mortality occurring in flies exposed to 1.0 mg/mL caffeine for five days (Supplementary Table S1).
We then tested the effect of caffeine on ethanol-induced sedation in OR-R, a commonly used wildtype strain of Drosophila. Both male and female OR-R flies raised on caffeine-supplemented food (0.25 and 0.5 mg/mL) for three days had a higher ST50 at all caffeine doses with the most robustly elevated ST50 observed at 0.5 mg/mL dose compared to control flies (Figure 2; Males—average ST50caff = 5.64 ± 0.18 min, ST50cont = 4.5 ± 0.20 min, n = 7–12 biological replicates, one-way ANOVA-Tukey HSD, p = 0.04 and Females—average ST50caff = 5.0 ± 0.29 min, ST50cont = 4.21 ± 0.19 min, n = 7–12 biological replicates, one-way ANOVA-Tukey HSD, p = 0.06).
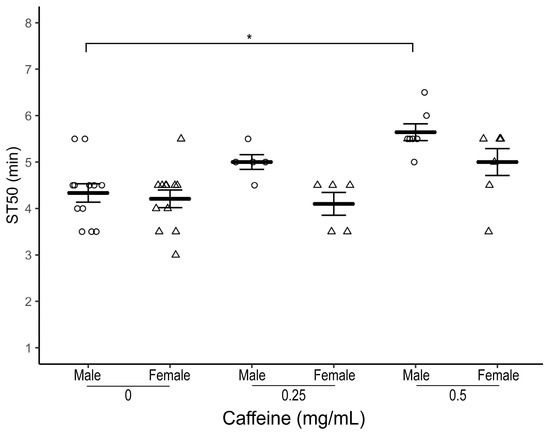
Figure 2.
Effect of caffeine supplementation on ethanol-induced sedation in wildtype (OR-R) flies. Comparison of ST50 when exposed to 100% ethanol of 1–3 day old male (circles) and female (triangles) OR-R flies raised for three days at different dosages (0.25 and 0.5 mg/mL) of caffeine-supplemented food. Both male and female flies raised on 0.5 mg/mL caffeine-supplemented food for three days have the most elevated ST50 than control flies (p = 0.04 (males), p = 0.06 (females)). Horizontal bars represent the mean ST50 ± standard error of 5–12 biological replicates. For each ST50 8–10 male and female flies were used. * p < 0.05; ANOVA, post hoc Tukey HSD.
3.2. Exposure to Caffeine for One Day Is Sufficient to Increase Ethanol-Induced Sedation Time
To test if the effects of caffeine on sedation time depend on the duration of its exposure, we did a time-course of caffeine exposure by raising 1–3 day-old w1118 flies on 0.5 mg/mL caffeine-supplemented food for one and five days. Male and female flies exposed to caffeine for both one day and five days had a significantly higher ST50 than corresponding control males and females, respectively (Figure 3; 1-day: Males—average ST50caff = 6.63 ± 0.12 min, ST50cont = 5.38 min ± 0.31, n = 4 biological replicates, two-tailed independent samples t-test, p = 0.022 and Females (Figure S2)—average ST50caff = 6.13 ± 0.24 min, ST50cont = 5.00 ± 0.35 min, n = 4 biological replicates, two-tailed independent samples t-test, p = 0.044. 5-day: Males—average ST50caff = 6.05 ± 0.21 min, ST50cont = 4.71 ± 0.30 min, n = 6–18 biological replicates, two-tailed independent samples t-test, p = 0.002 and Females—average ST50caff = 6.50 ± 0.2 min, ST50cont = 4.88 ± 0.28 min, n = 6–20 biological replicates, two-tailed independent samples t-test, p < 0.001).
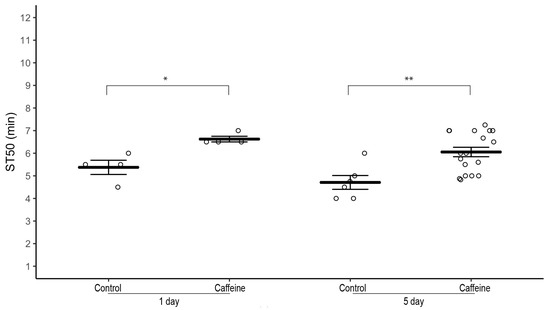
Figure 3.
Effect of time course of caffeine supplementation on ethanol-induced sedation in w1118 flies. Male w1118 flies (1–3 day old) raised on 0.5 mg/mL caffeine-supplemented food for one day and five days have a significantly higher ST50 when exposed to 100% ethanol than control flies. Horizontal bars represent the mean ST50 ± standard error of 4–18 biological replicates. For each sedation assay 8–10 male flies were used (circles). * p < 0.05, ** p < 0.01; two-tailed independent samples t-test.
Unless specified otherwise, for all subsequent experiments data from 1–3 day-old male w1118 flies raised on 0.5 mg/mL caffeine-supplemented food for three days is presented.
3.3. The Effect of Caffeine on Ethanol-Induced Sedation Reverses after Caffeine Withdrawal
To determine the lasting effect of caffeine-mediated resistance to sedation upon cessation of exposure to caffeine, 1–3 day-old male w1118 flies were raised on 0.5 mg/mL caffeine-supplemented food for three days and subsequently transferred to non-caffeinated food for one day or three days.
At one day post-transfer to regular food, both male (Figure 4) and female (not shown) flies had a significantly higher ST50 compared to control male and female flies, respectively (average ST50caff = 6.39 ± 0.19 min, ST50cont = 4.74 ± 0.16 min, n = 16 biological replicates, two-tailed independent samples t-test, p < 0.01).
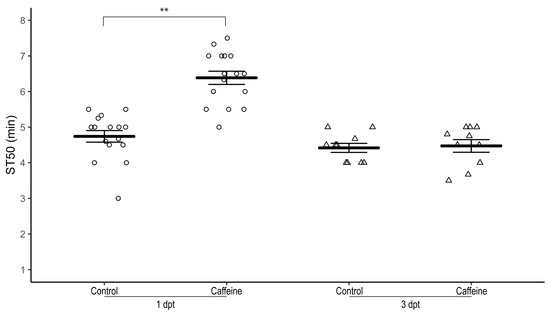
Figure 4.
Sustainability of caffeine-mediated effect on ethanol-induced sedation after caffeine withdrawal. Male w1118 flies raised on 0.5 mg/mL caffeine-supplemented food for three days have a significantly higher ST50 when exposed to 100% ethanol than control flies (p < 0.001) one day post caffeine withdrawal (circles) but the ST50 three days post caffeine withdrawal (triangles) is not significantly different (p = 0.804). Horizontal bars represent the mean ST50 ± standard error of 10–16 biological replicates. For each sedation assay 8–10 male flies were used. dpt—days post-transfer to control food; ** two-tailed independent samples t-test.
At three days post-transfer to regular food, ST50 of male (Figure 4) and female (not shown) flies was not significantly different from control flies (average ST50caff = 4.47 ± 0.13 min, ST50cont = 4.42 ± 0.18 min, n = 10 biological replicates, two-tailed independent samples t-test, p = 0.804).
Taken together, these data show that caffeine delays onset of sedation in male and female wild type flies that can initiate after exposure to caffeine for one day and lasts up to three days after caffeine withdrawal.
3.4. Adenosine Receptor Mutation Delays Onset of Ethanol-Induced Sedation
In mammals, caffeine is an adenosine receptor antagonist [6,7,24]. Therefore, we wondered if adenosine receptor mutant flies are resistant to sedation. Indeed, heterozygous adenosine receptor mutant flies (AdoRMB04401/TM6C, Sb) raised on normal food showed a significantly higher ST50 compared to w1118 flies (Figure 5; average ST50AdoR+/− = 6.86 ± 0.18 min, ST50w1118 = 4.95 ± 0.17 min, n = 7–10 biological replicates, two-tailed independent samples t-test, p < 0.001).
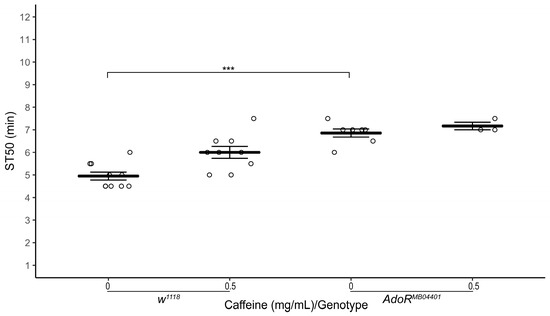
Figure 5.
Effect of adenosine receptor mutation on ethanol-induced sedation. Comparison of ST50 when exposed to 100% ethanol of 1–3 day old male adenosine receptor mutant flies (AdoRMB04401/TMC, Sb) and w1118 flies raised for three days on (0 mg/mL and 0.5 mg/mL) caffeine-supplemented food. Heterozygous AdoR mutant flies (AdoRMB04401/TM6C, Sb) raised on control food and 0.5 mg/mL caffeine-supplemented food have a significantly higher ST50 than w1118 flies raised on control (p < 0.001) and 0.5 mg/mL caffeine-supplemented food (p = 0.004), respectively. Horizontal bars represent the mean ST50 ± standard error of 3–9 biological replicates. For each sedation assay 8–10 male flies were used (circles). *** p < 0.001; two-tailed independent samples t-test.
4. Discussion
Drosophila is an excellent model to study the effects of caffeine, adenosine receptor signaling, and alcohol on a variety of behaviors such as circadian rhythm, locomotion, and cognition [9,13,22,25]. This study, to the best of our knowledge, is the first to examine interplay among caffeine, Drosophila adenosine receptor, and ethanol-induced sedation in Drosophila. Our results demonstrate that exposure to caffeine prolongs the onset of sedation in both male and female w1118 and OR-R flies, commonly used control and wild type Drosophila strains, respectively. Sex differences in ethanol-induced sedation time are observed in both vertebrates and invertebrates including Drosophila. Female flies are reported to have a shorter sedation time than males [26]. However, in w1118 sexual dimorphism of sedation does not resolve when exposed to 100% ethanol [27]. We observed that w1118 females showed a trend (not statistically significant) for a higher ST50 than males in control at all concentrations of caffeine tested.
The effect of caffeine on sedation time when sedated with 100% ethanol was most robust and statistically significant at 0.5 mg/mL dose and did not hold at 1 mg/mL dose. The effect of caffeine on sedation time was more prominent with 50% and 75% ethanol possibly because the effect of caffeine manifests more effectively when the rate of onset of sedation is less drastic at lower doses of ethanol. Other studies in flies have examined the effects of caffeine, at a similar dose range, on adenosine receptor expression, fecundity, egg laying, and life span [28,29]. It is possible that at higher doses, flies are generally weaker or less viable, as caffeine appears to begin to confer mortality in flies at 1 mg/mL concentration. We observed significant widespread mortality at 3 mg/mL and 5 mg/mL dose after one-week exposure (data not shown). At higher caffeine doses, a weakened state is possible either because flies are avoiding consuming highly caffeinated food due to its bitter taste [30,31] and/or due to the direct physiological effects of caffeine. There is support for a direct effect of caffeine on physiological functions leading to mortality in flies as shown by the correlation between caffeine-induced mortality and reduced transcript levels of neuromodulators and adenosine receptors [28,32]. The effect of caffeine on sedation is reversible and wears off within three days of caffeine withdrawal, likely due to its metabolic clearance. Caffeine is metabolized by the Cytochrome P-450 enzymes in both Drosophila and humans [33,34]. Caffeine metabolites (theophylline, theobromine, paraxanthine) are also detected within 3 h of caffeine ingestion in flies [33]. The half-life of caffeine in humans is 3–7 h [34].
Caffeine primarily acts as a stimulant in mammals because of its antagonism of adenosine receptor signaling, which promotes sleep [6,7,24]. In this study, we addressed the relationship between adenosine receptor gene dosage and sedation in flies. We found that heterozygous adenosine receptor mutant flies are resistant to sedation. We were unable to conduct sedation assays on homozygous mutant flies for the AdoRMB04401 allele because they generally do not survive to adulthood. This finding is in accordance with a previous study in which adenosine A2A receptor knockout mice were shown to be resistant to ethanol-induced hypnotic effects [35]. Mammalian adenosine A2A receptor has the highest homology to the only adenosine receptor isoform in Drosophila [13]. However, in Drosophila, caffeine may also act through additional pathways, such as the dopamine receptor-mediated signaling. The fly dopamine/ecdysteroid receptor (DopEcR) mediates ethanol-induced sedation [36]. The Drosophila dopamine receptor (dDA1) [37] and dopamine signaling [38] have been independently shown to modulate the wake-promoting effect of caffeine, suggesting a potential adenosine receptor-independent mechanism of action for caffeine in flies. Additionally, the effects of caffeine on sleep does not depend on adenosine activity [13]. Further, caffeine was unable to inhibit adenosine receptor-mediated signaling in Drosophila neuroblast cell line in vitro [39]. Therefore, it appears that caffeine and adenosine receptor either through coordination and/or independently play a role in ethanol-induced sedation.
Our data suggests that pharmacological (caffeine exposure) and genetic (adenosine receptor mutation) disruption of adenosine receptor function delays ethanol sedation. Therefore, it can be hypothesized that agonists of the adenosine receptor, especially the endogenous ligand, adenosine, will promote sedation. Ethanol elevates extracellular adenosine levels which in turn activate the adenosine receptors [40]. In mammalian systems, the ethanol-mediated elevation of adenosine modulates ethanol-induced behaviors primarily through adenosine A1 and A2 receptors [11,35,40]. In future studies, it will be interesting to determine effects of adenosine receptor agonists on sedation and the cellular and molecular characteristics of signaling pathways downstream of adenosine receptors that mediate ethanol-induced sedation in flies.
5. Conclusions
Broadly, this study further supports the use of Drosophila as a model to study complex human behavior and to examine the colloquial notion of the negative implications of mixing caffeine with alcohol. Human correlational studies have found that individuals concurrently consuming caffeinated energy drinks and alcohol are more likely to consume more alcohol and have more severe negative consequences due to alcohol consumption [41]. When both substances are consumed in conjunction, caffeine reduces perceived inebriation, which leads to further consumption, thereby promoting binge drinking and risky behavior such as driving under the influence of alcohol [42]. Further studies on the interaction between caffeine and ethanol will improve our understanding of the biochemical and behavioral consequences of their consumption and aid in creating awareness of this public health crisis, especially for adolescents and young adults [43,44].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology12010063/s1. Figure S1. Effect on caffeine on sedation at different ethanol concentrations. Figure S2. Effect of time course of caffeine supplementation on ethanol-induced sedation in w1118 flies. Table S1. Effect of caffeine on mortality in flies.
Author Contributions
S.B., S.D. and S.T.A. initially designed the project; S.T., Y.Z., A.Y., K.C., A.M., S.B. and S.D. performed the experiments; S.T., Y.Z. and S.T.A. analyzed the data; S.T., Y.Z. and S.T.A. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM0103423 to Colby College/STA, Natural Science Division Grant, Colby College (STA).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We like to thank Joshua Kavaler for fly strains and discussion and Ahmad lab members for suggestions on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Ashihara, H.; Suzuki, T. Distribution and biosynthesis of caffeine in plants. Front. Biosci. 2004, 9, 1864–1876. [Google Scholar] [CrossRef] [PubMed]
- Osz, B.E.; Jitca, G.; Stefanescu, R.E.; Puscas, A.; Tero-Vescan, A.; Vari, C.E. Caffeine and its antioxidant properties-it is all about dose and source. Int. J. Mol. Sci. 2022, 23, 13074. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R.; Agarwal, S.; Fulgoni, V.L., 3rd. Daily patterns of caffeine intake and the association of intake with multiple sociodemographic and lifestyle factors in us adults based on the nhanes 2007–2012 surveys. J. Acad. Nutr. Diet. 2019, 119, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.C.; Knight, C.A.; Hockenberry, J.; Teplansky, R.; Hartman, T.J. Beverage caffeine intakes in the U.S. Food Chem. Toxicol. 2014, 63, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, M.; Calvello, R.; Porro, C.; Messina, G.; Cianciulli, A.; Panaro, M.A. Neurodegenerative diseases: Can caffeine be a powerful ally to weaken neuroinflammation? Int. J. Mol. Sci. 2022, 23, 12958. [Google Scholar] [CrossRef]
- Nehlig, A.; Daval, J.L.; Debry, G. Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Rev. 1992, 17, 139–170. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Battig, K.; Holmen, J.; Nehlig, A.; Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar]
- Ribeiro, J.A.; Sebastiao, A.M. Caffeine and adenosine. J. Alzheimer’s Dis. 2010, 20 (Suppl. 1), S3–S15. [Google Scholar] [CrossRef]
- Dolezelova, E.; Nothacker, H.P.; Civelli, O.; Bryant, P.J.; Zurovec, M. A drosophila adenosine receptor activates camp and calcium signaling. Insect Biochem. Mol. Biol. 2007, 37, 318–329. [Google Scholar] [CrossRef]
- Sperlagh, B.; Vizi, E.S. The role of extracellular adenosine in chemical neurotransmission in the hippocampus and basal ganglia: Pharmacological and clinical aspects. Curr. Top. Med. Chem. 2011, 11, 1034–1046. [Google Scholar] [CrossRef]
- Ruby, C.L.; Adams, C.A.; Knight, E.J.; Nam, H.W.; Choi, D.S. An essential role for adenosine signaling in alcohol abuse. Curr. Drug Abus. Rev. 2010, 3, 163–174. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reichert, C.F.; Deboer, T.; Landolt, H.P. Adenosine, caffeine, and sleep-wake regulation: State of the science and perspectives. J. Sleep Res. 2022, 31, e13597. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.N.; Ho, K.; Crocker, A.; Yue, Z.; Koh, K.; Sehgal, A. The effects of caffeine on sleep in drosophila require pka activity, but not the adenosine receptor. J. Neurosci. 2009, 29, 11029–11037. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Kumar, S.; Rai, S.; Methippara, M.; Szymusiak, R.; McGinty, D. Role of adenosine a(1) receptor in the perifornical-lateral hypothalamic area in sleep-wake regulation in rats. Brain Res. 2009, 1304, 96–104. [Google Scholar] [CrossRef]
- Sharma, R.; Parikh, M.; Mishra, V.; Zuniga, A.; Sahota, P.; Thakkar, M. Sleep, sleep homeostasis and arousal disturbances in alcoholism. Brain Res. Bull. 2022, 182, 30–43. [Google Scholar] [CrossRef]
- Ferre, S.; O’Brien, M.C. Alcohol and caffeine: The perfect storm. J. Caffeine Res. 2011, 1, 153–162. [Google Scholar] [CrossRef]
- Erickson, E.K.; DaCosta, A.J.; Mason, S.C.; Blednov, Y.A.; Mayfield, R.D.; Harris, R.A. Cortical astrocytes regulate ethanol consumption and intoxication in mice. Neuropsychopharmacology 2021, 46, 500–508. [Google Scholar] [CrossRef]
- Kaun, K.R.; Devineni, A.V.; Heberlein, U. Drosophila melanogaster as a model to study drug addiction. Hum. Genet. 2012, 131, 959–975. [Google Scholar] [CrossRef]
- Philyaw, T.J.; Rothenfluh, A.; Titos, I. The use of drosophila to understand psychostimulant responses. Biomedicines 2022, 10, 119. [Google Scholar] [CrossRef]
- Devineni, A.V.; Heberlein, U. The evolution of drosophila melanogaster as a model for alcohol research. Annu. Rev. Neurosci. 2013, 36, 121–138. [Google Scholar] [CrossRef]
- Rodan, A.R.; Rothenfluh, A. The genetics of behavioral alcohol responses in drosophila. Int. Rev. Neurobiol. 2010, 91, 25–51. [Google Scholar] [PubMed]
- Liao, J.; Seggio, J.A.; Ahmad, S.T. Mutations in the circadian gene period alter behavioral and biochemical responses to ethanol in drosophila. Behav. Brain Res. 2016, 302, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Maples, T.; Rothenfluh, A. A simple way to measure ethanol sensitivity in flies. J. Vis. Exp. 2011, 48, e2541. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, M.; Shen, H.Y.; Cherasse, Y.; Qu, W.M.; Huang, Z.L.; Bass, C.E.; Winsky-Sommerer, R.; Semba, K.; Fredholm, B.B.; Boison, D.; et al. Arousal effect of caffeine depends on adenosine a2a receptors in the shell of the nucleus accumbens. J. Neurosci. 2011, 31, 10067–10075. [Google Scholar] [CrossRef] [PubMed]
- Mustard, J.A. The buzz on caffeine in invertebrates: Effects on behavior and molecular mechanisms. Cell Mol. Life Sci. 2014, 71, 1375–1382. [Google Scholar] [CrossRef]
- Devineni, A.V.; Heberlein, U. Acute ethanol responses in drosophila are sexually dimorphic. Proc. Natl. Acad. Sci. USA 2012, 109, 21087–21092. [Google Scholar] [CrossRef]
- Oyeyinka, A.; Kansal, M.; O’Sullivan, S.M.; Gualtieri, C.; Smith, Z.M.; Vonhoff, F.J. Corazonin neurons contribute to dimorphic ethanol sedation sensitivity in drosophila melanogaster. Front. Neural Circuits 2022, 16, 702901. [Google Scholar] [CrossRef]
- Francikowski, J.; Baran, B.; Płachetka-Bożek, A.; Krzyżowski, M.; Augustyniak, M. Caffeine effects on ador mrna expression in drosophila melanogaster. Open Life Sci. 2016, 11, 244–249. [Google Scholar] [CrossRef]
- Itoyama, M.M.; Bicudo, H.E.M.d.C. Effects of caffeine on fecundity, egg laying capacity, development time and longevity in drosophila prosaltans. Rev. Bras. Genét. 1992, 15, 303–321. [Google Scholar]
- Ebbs, M.L.; Amrein, H. Taste and pheromone perception in the fruit fly drosophila melanogaster. Pflug. Arch. 2007, 454, 735–747. [Google Scholar] [CrossRef]
- Lee, Y.; Moon, S.J.; Montell, C. Multiple gustatory receptors required for the caffeine response in drosophila. Proc. Natl. Acad. Sci. USA 2009, 106, 4495–4500. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.J.; Shin, B.; Han, S.H.; Woo, M.J.; Hong, K.B. Behavioral changes and survival in drosophila melanogaster: Effects of ascorbic acid, taurine, and caffeine. Biol. Pharm. Bull. 2017, 40, 1873–1882. [Google Scholar] [CrossRef]
- Coelho, A.; Fraichard, S.; Le Goff, G.; Faure, P.; Artur, Y.; Ferveur, J.F.; Heydel, J.M. Cytochrome p450-dependent metabolism of caffeine in drosophila melanogaster. PLoS ONE 2015, 10, e0117328. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The safety of ingested caffeine: A comprehensive review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef]
- El Yacoubi, M.; Ledent, C.; Parmentier, M.; Costentin, J.; Vaugeois, J.M. Caffeine reduces hypnotic effects of alcohol through adenosine a2a receptor blockade. Neuropharmacology 2003, 45, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Petruccelli, E.; Li, Q.; Rao, Y.; Kitamoto, T. The unique dopamine/ecdysteroid receptor modulates ethanol-induced sedation in drosophila. J. Neurosci. 2016, 36, 4647–4657. [Google Scholar] [CrossRef]
- Andretic, R.; Kim, Y.C.; Jones, F.S.; Han, K.A.; Greenspan, R.J. Drosophila d1 dopamine receptor mediates caffeine-induced arousal. Proc. Natl. Acad. Sci. USA 2008, 105, 20392–20397. [Google Scholar] [CrossRef]
- Nall, A.H.; Shakhmantsir, I.; Cichewicz, K.; Birman, S.; Hirsh, J.; Sehgal, A. Caffeine promotes wakefulness via dopamine signaling in drosophila. Sci. Rep. 2016, 6, 20938. [Google Scholar] [CrossRef]
- Kucerova, L.; Broz, V.; Fleischmannova, J.; Santruckova, E.; Sidorov, R.; Dolezal, V.; Zurovec, M. Characterization of the drosophila adenosine receptor: The effect of adenosine analogs on camp signaling in drosophila cells and their utility for in vivo experiments. J. Neurochem. 2012, 121, 383–395. [Google Scholar] [CrossRef]
- Mailliard, W.S.; Diamond, I. Recent advances in the neurobiology of alcoholism: The role of adenosine. Pharmacol. Ther. 2004, 101, 39–46. [Google Scholar] [CrossRef]
- Patrick, M.E.; Maggs, J.L. Energy drinks and alcohol: Links to alcohol behaviors and consequences across 56 days. J. Adolesc. Health 2014, 54, 454–459. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perez-Mana, C.; Mateus, J.A.; Diaz-Pellicer, P.; Diaz-Baggerman, A.; Perez, M.; Pujadas, M.; Fonseca, F.; Papaseit, E.; Pujol, J.; Langohr, K.; et al. Effects of mixing energy drinks with alcohol on driving-related skills. Int. J. Neuropsychopharmacol. 2022, 25, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Powers, G.; Berger, L. Alcohol mixed with energy drinks: Expectancies of use and alcohol-related negative consequences among a young adult sample. Addict. Behav. Rep. 2020, 12, 100292. [Google Scholar] [CrossRef] [PubMed]
- Sampasa-Kanyinga, H.; Masengo, L.; Hamilton, H.A.; Chaput, J.P. Energy drink consumption and substance use among middle and high school students. Int. J. Environ. Res. Public Health 2020, 17, 3110. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).