Cardiovascular Activity of Ginkgo biloba—An Insight from Healthy Subjects
Abstract
Simple Summary
Abstract
1. Introduction
2. Characterization of the Main Extracts of Ginkgo Biloba Leaves
3. Characterization of Bioactive Compounds (Ginkgolides, Bilobalide) in Ginkgo Biloba Leaves
4. Cardiovascular Activity of Ginkgo Biloba and Its Main Compounds
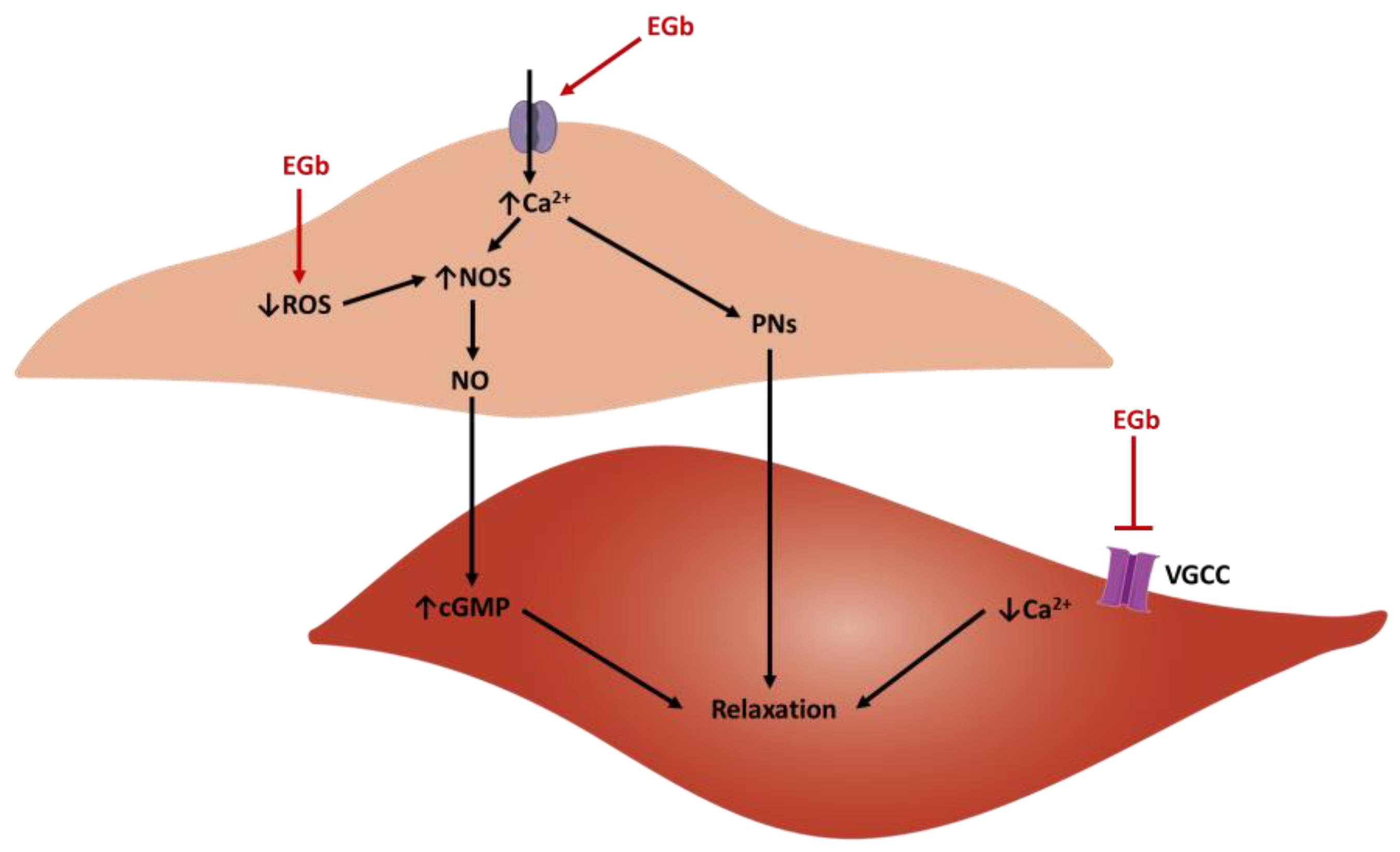
4.1. Antioxidant Activity
4.2. Cardiac Activity In Vitro and Ex Vivo
4.3. Vasorelaxant Activity Ex Vivo
4.4. Vasoconstrictor Activity Ex Vivo
4.5. Anti-Hypertensive Activity of EGb In Vivo—Animal Models
4.6. Effects of Ginkgo biloba on Blood Pressure and Heart Rate in Healthy Subjects—In Vivo Studies
4.7. Effects of Ginkgo biloba on Organ Perfusion in Healthy Subjects—In Vivo Studies
4.7.1. Effect on Skin Perfusion
4.7.2. Effect on Coronary Perfusion
4.7.3. Effect on Cerebral Perfusion
4.7.4. Effect on Ocular Perfusion
4.7.5. Effect on Cochlear Perfusion
5. Adverse Reactions from Ginkgo biloba
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics’2017 Update: A Report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Giedrimiene, D.; King, R. Burden of cardiovascular disease (CVD) on economic cost. Comparison of outcomes in US and Europe. Circ. Cardiovasc. Qual. Outcomes 2017, 10, A207. [Google Scholar] [CrossRef]
- Shad, B.; Ashouri, A.; Hasandokht, T.; Rajati, F.; Salari, A.; Naghshbandi, M.; Mirbolouk, F. Effect of multimorbidity on quality of life in adult with cardiovascular disease: A cross-sectional study. Health Qual. Life Outcomes 2017, 15, 240. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Thuan, D.T.B.; Phu, H.T.; Nguyen, T.H.D.; Hasan, H.; Halabi, S.; Abdelhady, S.; Nasrallah, G.K.; Eid, A.H.; Pintus, G. Herbal Medicine for Cardiovascular Diseases: Efficacy, Mechanisms, and Safety. Front. Pharmacol. 2020, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Gopal, N.M.; Tejaswini, J.; Mantry, S.; Kumar, S.A. International standards of medicinal plants. Int. J. Innov. Pharm. Sci. Res. 2014, 2, 2498–2532. [Google Scholar]
- Bishop, F.L.; Yardley, L.; Lewith, G.T. A systematic review of beliefs involved in the use of complementary and alternative medicine. J. Health Psychol. 2007, 12, 851–867. [Google Scholar] [CrossRef]
- Barnes, P.M.; Bloom, B.; Nahin, R.L. Complementary and alternative medicine use among adults and children: United States, 2007. Natl. Health Stat. Report. 2008, 12, 15188733. [Google Scholar]
- Singh, B.; Kaur, P.; Gopichand; Singh, R.D.; Ahuja, P.S. Biology and chemistry of Ginkgo biloba. Fitoterapia 2008, 79, 401–418. [Google Scholar] [CrossRef]
- Coombes, A.J. The Hamlyn Dictionary of Plant Names; Coombes, A.J., Ed.; Hamlyn: London, UK, 1994. [Google Scholar]
- Santamour, F.; He, S.; Ewert, T. Growth, Survival, and Sex Expression in Ginkgo. Arboric. Urban For. 1983, 9, 170–171. [Google Scholar] [CrossRef]
- Jacobs, B.P.; Browner, W.S. Ginkgo biloba: A living fossil. Am. J. Med. 2000, 108, 341–342. [Google Scholar] [CrossRef]
- Bedard, M. Ginkgo biloba. Can. Pharm. J. 2001, 134, 25–26. [Google Scholar]
- Chan, P.C.; Xia, Q.; Fu, P.P. Ginkgo biloba leave extract: Biological, medicinal, and toxicological effects. J. Environ. Sci. Health-Part C Environ. Carcinog. Ecotoxicol. Rev. 2007, 25, 211–244. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Hussain, S.A.; Shahen, M.; Wang, H.; Alagawany, M.; Abd El-Hac, M.E.; Ali Kalhor, S.; Rashid, M.; Ali Shar, P. Pharmacological Uses of Ginkgo biloba Extracts for Cardiovascular Disease and Coronary Heart Diseases. Int. J. Pharmacol. 2018, 15, 1–9. [Google Scholar] [CrossRef]
- Mahady, G.B. Ginkgo biloba for the prevention and treatment of cardiovascular disease: A review of the literature. J. Cardiovasc. Nurs. 2002, 16, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Hoerr, R.; Noeldner, M.; Koch, E. Ginkgo biloba Extract EGb 761: From an Ancient Asian Plant to a Modern European Herbal Medicinal Product. In Evidence and Rational Based Research on Chinese Drugs; Wagner, H., Ulrich-Merzenich, G., Eds.; Springer-Verlag: Wien, Austria, 2013; pp. 431–470. ISBN 9783709104422. [Google Scholar]
- International Agency for Research on Cancer. Some Drugs and Herbal Products; International Agency for Research on Cancer: Lyon, France, 2015; Volume 108. [Google Scholar]
- Xiong, X.J.; Liu, W.; Yang, X.C.; Feng, B.; Zhang, Y.Q.; Li, S.J.; Li, X.K.; Wang, J. Ginkgo biloba extract for essential hypertension: A systemic review. Phytomedicine 2014, 21, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Rejali, D.; Sivakumar, A.; Balaji, N. Ginkgo biloba does not benefit patients with tinnitus: A randomized placebo-controlled double-blind trial and meta-analysis of randomized trials. Clin. Otolaryngol. Allied Sci. 2004, 29, 226–231. [Google Scholar] [CrossRef]
- Sereda, M.; Xia, J.; Scutt, P.; Hilton, M.P.; El Refaie, A.; Hoare, D.J. Ginkgo biloba for tinnitus. Cochrane Database Syst. Rev. 2013, 1–20. [Google Scholar] [CrossRef]
- Spiegel, R.; Kalla, R.; Mantokoudis, G.; Maire, R.; Mueller, H.; Hoerr, R.; Ihl, R. Ginkgo biloba extract EGb 761® alleviates neurosensory symptoms in patients with dementia: A meta-analysis of treatment effects on tinnitus and dizziness in randomized, placebo-controlled trials. Clin. Interv. Aging 2018, 13, 1121–1127. [Google Scholar] [CrossRef]
- Pittler, M.H.; Ernst, E. Complementary therapies for peripheral arterial disease: Systematic review. Atherosclerosis 2005, 181, 1–7. [Google Scholar] [CrossRef]
- Pittler, M.H.; Ernst, E. Ginkgo Biloba extract for the treatment of intermittent claudication: A meta-analysis of randomized trials. Am. J. Med. 2000, 108, 276–281. [Google Scholar] [CrossRef]
- Muir, A.H.; Robb, R.; McLaren, M.; Daly, F.; Belch, J.J.F. The use of Ginkgo biloba in Raynaud’s disease: A double-blind placebo-controlled trial. Vasc. Med. 2002, 7, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Bredie, S.J.H.; Jong, M.C. No significant effect of ginkgo biloba special extract EGb 761 in the treatment of primary raynaud phenomenon: A randomized controlled trial. J. Cardiovasc. Pharmacol. 2012, 59, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.S.; Choi, C.J.; Kim, K.S.; Lee, J.H.; Song, C.H.; Chung, J.H.; Ock, S.M.; Lee, J.B.; Kim, C.M. To compare the efficacy and safety of nifedipine sustained release with Ginkgo biloba extract to treat patients with primary Raynaud’s phenomenon in South Korea; Korean Raynaud study (KOARA study). Clin. Rheumatol. 2009, 28, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Malenfant, D.; Catton, M.; Pope, J.E. The efficacy of complementary and alternative medicine in the treatment of Raynaud’s phenomenon: A literature review and meta-analysis. Rheumatology 2009, 48, 791–795. [Google Scholar] [CrossRef][Green Version]
- Liu, H.; Ye, M.; Guo, H. An Updated Review of Randomized Clinical Trials Testing the Improvement of Cognitive Function of Ginkgo biloba Extract in Healthy People and Alzheimer’s Patients. Front. Pharmacol. 2020, 10, 1688. [Google Scholar] [CrossRef]
- Tan, M.S.; Yu, J.T.; Tan, C.C.; Wang, H.F.; Meng, X.F.; Wang, C.; Jiang, T.; Zhu, X.C.; Tan, L. Efficacy and adverse effects of Ginkgo Biloba for cognitive impairment and dementia: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2015, 43, 589–603. [Google Scholar] [CrossRef]
- Demarin, V.; Kes, V.B.; Trkanjec, Z.; Budišić, M.; Pašić, M.B.; Črnac, P.; Budinčević, H. Efficacy and safety of Ginkgo biloba standardized extract in the treatment of vascular cognitive impairment: A randomized, double-blind, placebo-controlled clinical trial. Neuropsychiatr. Dis. Treat. 2017, 13, 483–490. [Google Scholar] [CrossRef]
- Wheatley, D. Triple-blind, placebo-controlled trial of Gingko biloba in sexual dysfunction due to antidepressant drugs. Hum. Psychopharmacol. 2004, 19, 545–548. [Google Scholar] [CrossRef]
- Kang, B.J.; Lee, S.J.; Kim, M.D.; Cho, M.J. A placebo-controlled, double-blind trial of Ginkgo biloba for antidepressant-induced sexual dysfunction. Hum. Psychopharmacol. 2002, 17, 279–284. [Google Scholar] [CrossRef]
- Elsabagh, S.; Hartley, D.E.; Ali, O.; Williamson, E.M.; File, S.E. Differential cognitive effects of Ginkgo biloba after acute and chronic treatment in healthy young volunteers. Psychopharmacology 2005, 179, 437–446. [Google Scholar] [CrossRef]
- Burns, N.R.; Bryan, J.; Nettelbeck, T. Ginkgo biloba: No robust effect on cognitive abilities or mood in healthy young or older adults. Hum. Psychopharmacol. 2006, 21, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Scholey, A.B.; Wesnes, K.A. The dose-dependent cognitive effects of acute administration of Ginkgo biloba to healthy young volunteers. Psychopharmacology 2000, 151, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Stough, C.; Silberstein, R.B.; Pipingas, A.; Song, J.; Camfield, D.A.; Nathan, P.J. Examining brain-cognition effects of ginkgo biloba extract: Brain activation in the left temporal and left prefrontal cortex in an object working memory task. Evid.-Based Complement. Altern. Med. 2011, 2011, 164139. [Google Scholar] [CrossRef]
- Canter, P.; Ernst, E. Ginkgo biloba is not a smart drug: An updated systematic review of randomised clinical trials testing the nootropic effects of G. biloba extracts in healthy people. Hum. Psychopharmacol. 2007, 22, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Laws, K.R.; Sweetnam, H.; Kondel, T.K. Is Ginkgo biloba a cognitive enhancer in healthy individuals? A meta-analysis. Hum. Psychopharmacol. 2012, 27, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Guirguis, A.; Fergus, S.; Schifano, F. The use and impact of cognitive enhancers among university students: A systematic review. Brain Sci. 2021, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Ponnet, K.; Tholen, R.; De Bruyn, S.; Wouters, E.; Van Ouytsel, J.; Walrave, M.; Van Hal, G. Students’ stimulant use for cognitive enhancement: A deliberate choice rather than an emotional response to a given situation. Drug Alcohol. Depend. 2021, 218, 108410. [Google Scholar] [CrossRef]
- Mattioli, A.V.; Pennella, S.; Farinetti, A.; Manenti, A. Energy Drinks and atrial fibrillation in young adults. Clin. Nutr. 2018, 37, 1073–1074. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Y.; Chen, K. Ginkgo biloba Extract in Vascular Protection: Molecular Mechanisms and Clinical Applications. Curr. Vasc. Pharmacol. 2017, 15, 532–548. [Google Scholar] [CrossRef]
- Nuhu, A.A. Ginkgo biloba: A ‘living fossil’ with modern day phytomedicinal applications. J. Appl. Pharm. Sci. 2014, 4, 96–103. [Google Scholar] [CrossRef]
- Mota, A.H. A review of medicinal plants used in therapy of cardiovascular diseases. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 572–591. [Google Scholar]
- Kuller, L.H.; Ives, D.G.; Fitzpatrick, A.L.; Carlson, M.C.; Mercado, C.; Lopez, O.L.; Burke, G.L.; Furberg, C.D.; DeKosky, S.T. Does ginkgo biloba reduce the risk of cardiovascular events? Circ. Cardiovasc. Qual. Outcomes 2010, 3, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Shankar, P.; Upadhyaya, D.; Deshpande, V. Ginkgo biloba - an appraisal. Kathmandu Univ. Med. J. 2003, 2, 225–229. [Google Scholar]
- van Beek, T.A.; Montoro, P. Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals. J. Chromatogr. A 2009, 1216, 2002–2032. [Google Scholar] [CrossRef]
- Ude, C.; Schubert-Zsilavecz, M.; Wurglics, M. Ginkgo biloba extracts: A review of the pharmacokinetics of the active ingredients. Clin. Pharmacokinet. 2013, 52, 727–749. [Google Scholar] [CrossRef]
- Del Tredici, P. The Evolution, ecology, and cultivation of Ginkgo biloba. In Ginkgo Biloba; van Beek, T.A., Ed.; Harwood Academic Publishers: London, UK, 2000. [Google Scholar]
- Leistner, E.; Drewke, C. Ginkgo biloba and Ginkgotoxin. J. Nat. Prod. 2010, 73, 86–92. [Google Scholar] [CrossRef]
- Gafner, B.S. Ginkgo Leaf Extract—Laboratory Guidance Document; ABC-AHP- NCNPR Botanical Adulterants Prevention Program: Austin, TX, USA, 2022. [Google Scholar]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and Antioxidant Effect of Ginkgo biloba Extract Against AD and Other Neurological Disorders. Neurotherapeutics 2019, 16, 666–674. [Google Scholar] [CrossRef]
- World Health Organization. Folium Ginkgo. WHO Monogr. Sel. Med. Plants 1999, 1, 154–167. [Google Scholar]
- Carrier, D.J.; Van Beek, T.A.; Van Der Heijden, R.; Verpoorte, R. Distribution of ginkgolides and terpenoid biosynthetic activity in Ginkgo biloba. Phytochemistry 1998, 48, 89–92. [Google Scholar] [CrossRef]
- Christen, Y.; Maixent, J.M. What is Ginkgo biloba extract EGb 761? An overview--from molecular biology to clinical medicine. Cell. Mol. Biol. (Noisy-le-grand) 2002, 48, 601–611. [Google Scholar]
- Alves-Silva, J.M.; Zuzarte, M.; Marques, C.; Salgueiro, L.; Girao, H. Protective Effects of Terpenes on the Cardiovascular System: Current Advances and Future Perspectives. Curr. Med. Chem. 2016, 23, 4559–4600. [Google Scholar] [CrossRef] [PubMed]
- Badal, S.; Delgoda, R. Pharmacognosy; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128021040. [Google Scholar]
- Van Beek, T.A. Ginkgolides and bilobalide: Their physical, chromatographic and spectroscopic properties. Bioorganic Med. Chem. 2005, 13, 5001–5012. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Quispe, C.; Jamaddar, S.; Hossain, R.; Ray, P.; Mondal, M.; Abdulwanis Mohamed, Z.; Sani Jaafaru, M.; Salehi, B.; Islam, M.T.; et al. Therapeutic promises of ginkgolide A: A literature-based review. Biomed. Pharmacother. 2020, 132, 110908. [Google Scholar] [CrossRef]
- Xia, S.; Fang, D. Pharmacological action and mechanisms of ginkgolide B. Chin. Med. J. (Engl) 2007, 120, 922–928. [Google Scholar] [CrossRef]
- Dziwenka, M.; Coppock, R.W. Ginkgo biloba. Nutraceuticals Effic. Saf. Toxic. 2016, 49, 681–691. [Google Scholar] [CrossRef]
- Liu, X.W.; Yang, J.L.; Niu, W.; Jia, W.W.; Olaleye, O.E.; Wen, Q.; Duan, X.N.; Huang, Y.H.; Wang, F.Q.; Du, F.F.; et al. Human pharmacokinetics of ginkgo terpene lactones and impact of carboxylation in blood on their platelet-activating factor antagonistic activity. Acta Pharmacol. Sin. 2018, 39, 1935–1946. [Google Scholar] [CrossRef]
- Mohutsky, M.A.; Anderson, G.D.; Miller, J.W.; Elmer, G.W. Ginkgo biloba: Evaluation of CYP2C9 drug interactions in vitro and in vivo. Am. J. Ther. 2006, 13, 24–31. [Google Scholar] [CrossRef]
- Markowitz, J.S.; Donovan, J.L.; DeVane, C.L.; Sipkes, L.; Chavin, K.D. Multiple-Dose Administration of Ginkgo biloba Did Not Affect Cytochrome P-450 2D6 or 3A4 Activity in Normal Volunteers. J. Clin. Psychopharmacol. 2003, 23, 576–581. [Google Scholar] [CrossRef]
- Eckert, A.; Keil, U.; Scherping, I.; Hauptmann, S.; Müller, W.E. Stabilization of mitochondrial membrane potential and improvement of neuronal energy metabolism by Ginkgo biloba extract EGb 761. Ann. N. Y. Acad. Sci. 2005, 1056, 474–485. [Google Scholar] [CrossRef]
- Liu, C.S.; Cheng, Y.; Hu, J.F.; Zhang, W.; Chen, N.H.; Zhang, J.T. Comparison of antioxidant activities between salvianolic acid B and Ginkgo biloba extract (EGb 761). Acta Pharmacol. Sin. 2006, 27, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Qi, Y.; Chen, T. Long-term pre-treatment of antioxidant Ginkgo biloba extract EGb-761 attenuates cerebral-ischemia-induced neuronal damage in aged mice. Biomed. Pharmacother. 2017, 85, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Ren, S.M.; Dong, J.Z.; Qiu, C.G.; Chen, Y.W.; Tao, H.L. Ginkgo biloba extract-761 protects myocardium by regulating Akt/Nrf2 signal pathway. Drug Des. Dev. Ther. 2019, 13, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jin, Z.; Xu, Z.; Yang, H.; Li, L.; Li, G.; Li, F.; Gu, S.; Zong, S.; Zhou, J.; et al. Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones 2019, 24, 441–452. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, G.; Yao, L.; Xue, P.; Yu, D.; Xu, R.; Shi, W.; Yao, X.; Yan, Z.; Duan, J.A. Protective effect of Ginkgo biloba leaves extract, EGb761, on myocardium injury in ischemia reperfusion rats via regulation of TLR-4/NF-κB signaling pathway. Oncotarget 2017, 8, 86671–86680. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Geng, T. Ginkgetin aglycone attenuates the apoptosis and inflammation response through nuclear factor-kB signaling pathway in ischemic-reperfusion injury. J. Cell. Biochem. 2019, 120, 8078–8085. [Google Scholar] [CrossRef]
- Wójcicki, J.; Samochowiec, L.; Juźwiak, S.; Gonet, B.; Syryński, W.; Barcew-wiszniewska, B.; Roźewicka, L.; Tustanowski, S.; Ceglecka, M.; Juzyszyn, Z.; et al. Ginkgo biloba extract inhibits the development of experimental atherosclerosis in rabbits. Phytomedicine 1994, 1, 33–38. [Google Scholar] [CrossRef]
- Abdel-Zaher, A.O.; Farghaly, H.S.M.; El-Refaiy, A.E.M.; Abd-Eldayem, A.M. Protective effect of the standardized extract of ginkgo biloba (EGb761) against hypertension with hypercholesterolemia-induced renal injury in rats: Insights in the underlying mechanisms. Biomed. Pharmacother. 2017, 95, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.A.; Hussain, S.A.; Mahwi, T.O.; Ahmed, Z.A. Efficacy and safety of Ginkgo biloba extract as an “add-on” treatment to metformin for patients with metabolic syndrome: A pilot clinical study. Ther. Clin. Risk Manag. 2018, 14, 1219–1226. [Google Scholar] [CrossRef]
- Hirata, B.K.S.; Banin, R.M.; Dornellas, A.P.S.; De Andrade, I.S.; Zemdegs, J.C.S.; Caperuto, L.C.; Oyama, L.M.; Ribeiro, E.B.; Telles, M.M. Ginkgo biloba extract improves insulin signaling and attenuates inflammation in retroperitoneal adipose tissue depot of obese rats. Mediat. Inflamm. 2015, 2015, 419106. [Google Scholar] [CrossRef]
- Siegel, G.; Ermilov, E.; Knes, O.; Rodríguez, M. Combined lowering of low grade systemic inflammation and insulin resistance in metabolic syndrome patients treated with Ginkgo biloba. Atherosclerosis 2014, 237, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Fazal, H.; Abbasi, B.H.; Farooq, S. Efficient free radical scavenging activity of Ginkgo biloba, stevia rebaudiana and Parthenium hysterophorous leaves through DPPH (2, 2-diphenyl-1-picrylhydrazyl). Int. J. Phytomedicine 2010, 2, 231–239. [Google Scholar] [CrossRef]
- Tsai, T.N.; Lin, W.S.; Wu, C.H.; Lin, W.Y.; Chu, K.M.; Cheng, C.C.; Hsu, C.H.; Tsai, W.C.; Cheng, S.M.; Yang, S.P. Activation of krüppel-like factor 2 with Ginkgo biloba extract induces eNOS expression and increases NO production in cultured human umbilical endothelial cells. Acta Cardiol. Sin. 2014, 30, 215–222. [Google Scholar]
- Chen, J.Z.; Wang, X.X.; Zhu, J.H.; Shang, Y.P.; Guo, X.G.; Sun, J. Effects of Ginkgo Biloba Extract on Number and Activity of Endothelial Progenitor Cells from Peripheral Blood. J. Cardiovasc. Pharmacol. 2004, 43, 347–352. [Google Scholar] [CrossRef]
- Campos-Toimil, M.; Lugnier, C. Inhibition of Type 4 Phosphodiesterase by Rolipram and Ginkgo biloba Extract (EGb 761) Decreases Agonist-Induced Rises in Internal Calcium in Human Endothelial Cells. Arter. Thromb. Vasc. Biol. 2000, 20, 34–40. [Google Scholar] [CrossRef]
- O’Riordan, E.; Chen, J.; Brodsky, S.V.; Smirnova, I.; Li, H.; Goligorsky, M.S. Endothelial cell dysfunction: The syndrome in making. Kidney Int. 2005, 67, 1654–1658. [Google Scholar] [CrossRef]
- Satoh, H. Effects of Ginkgo biloba extract and bilobalide, a main constituent, on the ionic currents in guinea pig ventricular cardiomyocytes. Arzneim.-Forsch./Drug Res. 2003, 53, 407–413. [Google Scholar] [CrossRef]
- Satoh, H. Suppression of pacemaker activity by Ginkgo biloba extract and its main constituent, bilobalide in rat sino-atrial nodal cells. Life Sci. 2005, 78, 67–73. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Yang, J.; Wu, P.; Wang, X.; Huang, C. Effect of Ginkgo biloba extract on pacemaker channels encoded by HCN gene. Herz 2021, 46, 255–261. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Chen, X.; Guo, C.; Guo, Y.; Wang, H. Ginkgo biloba extract EGB761 protects against aging-associated diastolic dysfunction in cardiomyocytes of D-galactose-induced aging rat. Oxid. Med. Cell. Longev. 2012, 2012, 418748. [Google Scholar] [CrossRef]
- Santos, A.C.O.; Souza, J.A.S.; Conde-Garcia, E.A.; Souza, A.A.; Menezes-Filho, J.E.R.; Oliveira, E.D.; Vasconcelos, C.M.L. Electrocardiographic profile of guinea pig heart submitted to Ginkgo biloba extract and its terpenoids. Rev. Bras. Plantas Med. 2014, 16, 819–825. [Google Scholar] [CrossRef]
- Gokbas, C.; Duman, I. Vascular Effects of Ginkgo Biloba Extract (EGb-761) on Isolated Human Umbilical Artery Contraction Responses. Selcuk Tip Derg. 2021, 2, 158–165. [Google Scholar] [CrossRef]
- Nishida, S.; Satoh, H. Mechanisms for the vasodilations induced by Ginkgo biloba extract and its main constituent, bilobalide, in rat aorta. Life Sci. 2003, 72, 2659–2667. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Satoh, H. Comparative vasodilating actions among terpenoids and flavonoids contained in Ginkgo biloba extract. Clin. Chim. Acta 2004, 339, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Satoh, H. Age-related changes in the vasodilating actions of Ginkgo biloba extract and its main constituent, bilobalide, in rat aorta. Clin. Chim. Acta 2005, 354, 141–146. [Google Scholar] [CrossRef]
- Laukeviciene, A. Reduction of small arteries contractility with improving the relaxation properties by Ginkgo biloba extract. J. Med. Plants Res. 2012, 6, 4785–4789. [Google Scholar] [CrossRef]
- Zhou, W.; Chai, H.; Courson, A.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. Ginkgolide A attenuates homocysteine-induced endothelial dysfunction in porcine coronary arteries. J. Vasc. Surg. 2006, 44, 853–862. [Google Scholar] [CrossRef]
- Chen, X.; Salwinski, S.; Lee, T.J.F. Extracts of Ginkgo biloba and ginsenosides exert cerebral vasorelaxation via a nitric oxide pathway. Clin. Exp. Pharmacol. Physiol. 1997, 24, 958–959. [Google Scholar] [CrossRef]
- Kim, J.J.; Han, D.H.; Lim, S.H.; Kim, T.H.; Chae, M.R.; Chung, K.J.; Kam, S.C.; Jeon, J.H.; Park, J.K.; Lee, S.W. Effects of Ginkgo biloba extracts with mirodenafil on the relaxation of corpus cavernosal smooth muscle and the potassium channel activity of corporal smooth muscle cells. Asian J. Androl. 2011, 13, 742–746. [Google Scholar] [CrossRef]
- Kubota, Y.; Tanaka, N.; Kagota, S.; Nakamura, K.; Kunitomo, M.; Shinozuka, K.; Umegaki, K. Effects of Ginkgo biloba extract on blood pressure and vascular endothelial response by acetylcholine in spontaneously hypertensive rats. J. Pharm. Pharmacol. 2006, 58, 243–249. [Google Scholar] [CrossRef]
- Kubota, Y.; Kagota, S.; Tada, Y.; Nejime, N.; Nakamura, K.; Kunitomo, M.; Umegaki, K.; Shinozuka, K. Ginkgo biloba extract causes decrease in heart rate in aged spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2007, 34, 49–50. [Google Scholar] [CrossRef]
- Mansour, S.M.; Bahgat, A.K.; El-Khatib, A.S.; Khayyal, M.T. Ginkgo biloba extract (EGb 761) normalizes hypertension in 2K, 1C hypertensive rats: Role of antioxidant mechanisms, ACE inhibiting activity and improvement of endothelial dysfunction. Phytomedicine 2011, 18, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Koltermann, A.; Hartkorn, A.; Koch, E.; Fürst, R.; Vollmar, A.M.; Zahler, S. Ginkgo biloba extract EGb® 761 increases endothelial nitric oxide production in vitro and in vivo. Cell. Mol. Life Sci. 2007, 64, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Auguet, M.; DeFeudis, F.V.; Clostre, F. Effects of Ginkgo biloba on arterial smooth muscle responses to vasoactive stimuli. Gen. Pharmacol. 1982, 13, 169–171. [Google Scholar] [CrossRef] [PubMed]
- White, H.L.; Scates, P.W.; Cooper, B.R. Extracts of Ginkgo biloba leaves inhibit monoamine oxidase. Life Sci. 1996, 58, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Sloley, B.; Urichuk, L.; Morley, P.; Durkin, J.; Shan, J.; Pang, P.; Coutts, R. Identification of Kaempferol as a Monoamine Oxidase Inhibitor and Potential Neuroprotectant in Extracts of Ginkgo Biloba Leaves. J. Pharm. Pharmacol. 2000, 52, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Hellegouarch, A.; Clostre, F.; Drieu, K. Comparison of the Contractile Effects of an Extract of Ginkgo Biloba and Some Neurotransmitters on Rabbit Isolated Vena Cava. Gen. Pharmacol. 1985, 16, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Abd-Eldayem, A.; Farghaly, H.M.; Abdel-Zaher, A. The nephroprotective effects of ginkgo biloba extract (EGb761) against l-N G -nitroarginine methyl ester-induced hypertension in rats: Role of oxidative stress and inflammatory markers. J. Curr. Med. Res. Pract. 2016, 1, 79. [Google Scholar] [CrossRef]
- Umegaki, K.; Shinozuka, K.; Watarai, K.; Takenaka, H.; Yoshimura, M.; Daohua, P.; Esashi, T. Ginkgo biloba extract attenuates the development of hypertension in deoxycorticosterone acetate-salt hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2000, 27, 277–282. [Google Scholar] [CrossRef]
- Sasaki, Y.; Noguchi, T.; Yamamoto, E.; Giddings, J.C.; Ikeda, K.; Yamori, Y.; Yamamoto, J. Effects of Ginkgo biloba extract (EGb 761) on cerebral thrombosis and blood pressure in stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2002, 29, 963–967. [Google Scholar] [CrossRef]
- Wimpissinger, B.; Berisha, F.; Garhoefer, G.; Polak, K.; Schmetterer, L. Influence of Ginkgo biloba on ocular blood flow. Acta Ophthalmol. Scand. 2007, 85, 445–449. [Google Scholar] [CrossRef]
- Kudolo, G.B. The Effect of3-Month Ingestion ofGinkgo biloba Extract on Pancreatic B-Cell Function in Response to Glucose Loading in Normal Glucose Tolerant Individuals. J. Clin. Pharmacol. 2000, 40, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Harris, A.; Kristinsson, J.K.; Ciulla, T.A.; Kagemann, C.; Ritch, R. Ginkgo biloba extract increases ocular blood flow velocity. J. Ocul. Pharmacol. Ther. 1999, 15, 233–240. [Google Scholar] [CrossRef]
- Moulton, P.L.; Boyko, L.N.; Fitzpatrick, J.L.; Petros, T.V. The effect of Ginkgo biloba on memory in healthy male volunteers. Physiol. Behav. 2001, 73, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Kalus, J.S.; Piotrowski, A.A.; Fortier, C.R.; Liu, X.; Kluger, J.; White, C.M.; Monsanto, H.A.; Martineau, P. Hemodynamic and electrocardiographic effects of short-term Ginkgo biloba. Ann. Pharmacother. 2003, 37, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Meendering, J.R.; Torgrimson, B.N.; Houghton, B.L.; Halliwill, J.R.; Minson, C.T. Menstrual cycle and sex affect hemodynamic responses to combined orthostatic and heat stress. Am. J. Physiol.-Heart Circ. Physiol. 2005, 289, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Jezova, D.; Duncko, R.; Lassanova, M.; Kriska, M.; Moncek, F.; Faculty, M. Redution of Rise in Blood Pressure and Cortisol. J. Physiol. Pharmacol. 2002, 53, 337–348. [Google Scholar]
- Winther, K.; Randløv, C.; Rein, E.; Mehlsen, J. Effects of Ginkgo biloba extract on cognitive function and blood pressure in elderly subjects. Curr. Ther. Res.-Clin. Exp. 1998, 59, 881–888. [Google Scholar] [CrossRef]
- Keheyan, G.; Dunn, L.A.; Hall, W.L. Acute Effects of Ginkgo Biloba Extract on Vascular Function and Blood Pressure. Plant Foods Hum. Nutr. 2011, 66, 209–211. [Google Scholar] [CrossRef]
- Mehlsen, J.; Drabæk, H.; Wiinberg, N.; Winther, K. Effects of a Ginkgo biloba extract on forearm haemodynamics in healthy volunteers. Clin. Physiol. Funct. Imaging 2002, 22, 375–378. [Google Scholar] [CrossRef]
- Boelsma, E.; Lamers, R.J.A.N.; Hendriks, H.F.J.; Van Nesselrooij, J.H.J.; Roza, L. Evidence of the regulatory effect of Ginkgo biloba extract on skin blood flow and study of its effects on urinary metabolites in healthy humans. Planta Med. 2004, 70, 1052–1057. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Cui, W.; Zu, X.; Du, J.; Wang, F. Ginkgo biloba extract improves coronary blood flow in healthy elderly adults: Role of endothelium-dependent vasodilation. Phytomedicine 2008, 15, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Mashayekh, A.; Pham, D.L.; Yousem, D.M.; Dizon, M.; Barker, P.B.; Lin, D.D.M. Effects of Ginkgo biloba on cerebral blood flow assessed by quantitative MR perfusion imaging: A pilot study. Neuroradiology 2011, 53, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Didier, A.; Droy-Lefaix, M.-T.; Aurousseau, C.; Cazals, Y. Effects of Ginkgo biloba extract (EGb 761) on cochlear vasculature in the guinea pig: Morphometric measurements and laser Doppler flowmetry. Eur Arch Otorhinolaryngol 1996, 253, 25–30. [Google Scholar] [CrossRef]
- Jang, C.H.; Cho, Y.B.; Kim, J.S.; Cho, S.W.; Yang, H.C.; Jung, K.H.; Kim, J.Y.; Choi, C.H.; Lim, Y.; Park, H.; et al. Effect of Ginkgo biloba extract on endotoxin-induced labyrinthitis. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.; Gross, J.; Moore, N.; Do, T.; Huang, A.; Gama, W.; Siesky, B. The effects of antioxidants on ocular blood flow in patients with glaucoma. Acta Ophthalmol. 2018, 96, e237–e241. [Google Scholar] [CrossRef]
- Park, J.W.; Kwon, H.J.; Chung, W.S.; Kim, C.Y.; Seong, G.J. Short-term effects of Ginkgo biloba extract on peripapillary retinal blood flow in normal tension glaucoma. Korean J. Ophthalmol. 2011, 25, 323–328. [Google Scholar] [CrossRef]
- Miman, M.C.; Ozturan, O.; Iraz, M.; Erdem, T.; Olmez, E. Amikacin ototoxicity enhanced by Ginkgo biloba extract (EGb 761). Hear. Res. 2002, 169, 121–129. [Google Scholar] [CrossRef]
- Yang, T.H.; Young, Y.H.; Liu, S.H. EGb 761 (Ginkgo biloba) protects cochlear hair cells against ototoxicity induced by gentamicin via reducing reactive oxygen species and nitric oxide-related apoptosis. J. Nutr. Biochem. 2011, 22, 886–894. [Google Scholar] [CrossRef]
- Huth, M.E.; Ricci, A.J.; Cheng, A.G. Mechanisms of Aminoglycoside Ototoxicity and Targets of Hair Cell Protection. Int. J. Otolaryngol. 2011, 1–19. [Google Scholar] [CrossRef]
- Tziridis, K.; Korn, S.; Ahlf, S.; Schulze, H. Protective effects of ginkgo biloba extract EGb 761 against noise trauma-induced hearing loss and tinnitus development. Neural Plast. 2014, 2014, 427298. [Google Scholar] [CrossRef]
- Krauss, P.; Tziridis, K.; Buerbank, S.; Schilling, A.; Schulze, H. Therapeutic value of ginkgo biloba extract EGb 761® in an animal model (meriones unguiculatus) for noise trauma induced hearing loss and tinnitus. PLoS ONE 2016, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dogan, R.; Sjostrand, A.P.; Yenıgun, A.; Karatas, E.; Kocyigit, A.; Ozturan, O. Influence of Ginkgo Biloba extract (EGb 761) on expression of IL-1 Beta, IL-6, TNF-alfa, HSP-70, HSF-1 and COX-2 after noise exposure in the rat cochlea. Auris Nasus Larynx 2018, 45, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.; Yuan, H.; Wang, X.; Sha, S.H. Noise-induced loss of hair cells and cochlear synaptopathy are mediated by the activation of AMPK. J. Neurosci. 2016, 36, 7497–7510. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.Q.; Zheng, H.W.; Hill, K.; Sha, S.H. Traumatic noise activates Rho-family GTPases through transient cellular energy depletion. J. Neurosci. 2012, 32, 12421–12430. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Miller, J.M.; Tucker, K.L.; Hu, H.; Park, S.K. Antioxidant vitamins and magnesium and the risk of hearing loss in the US general population. Am. J. Clin. Nutr. 2014, 99, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Le Prell, C.G.; Gagnon, P.M.; Bennett, D.C.; Ohlemiller, K.K. Nutrient-enhanced diet reduces noise-induced damage to the inner ear and hearing loss. Transl. Res. 2011, 158, 38–53. [Google Scholar] [CrossRef]
- Cianfrocca, C.; Pelliccia, F.; Auriti, A.; Santini, M. Gingko biloba-induced frequent ventricular arrythmia. Ital. Hear. J. 2002, 3, 689–691. [Google Scholar]
- Russo, V. Ginkgo biloba First report of paroxysmal atrial fibrillation: Case report. Reactions 2011, 1382, 17. [Google Scholar]
- Shaw, D.; Leon, C.; Kolev, S.; Murray, V. Traditional Remedies and Food Supplements. Drug Saf. 1997, 17, 342–356. [Google Scholar] [CrossRef]
- Granger, A.S. Ginkgo biloba precipitating epileptic seizures. Age Ageing 2001, 30, 523–525. [Google Scholar] [CrossRef]
- Koch, E. Inhibition of platelet activating factor (PAF)-induced aggregation of human thrombocytes by ginkgolides: Considerations on possible bleeding complications after oral intake of Ginkgo biloba extracts. Phytomedicine 2005, 12, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Rowin, J.; Lewis, S.L. Spontaneous bilateral subdural hematomas associated with chronic ginkgo biloba ingestion. Neurology 1996, 46, 1775–1776. [Google Scholar] [CrossRef]
- Matthews, J.; Lewis, S.L.; Rowin, J. Association of Ginkgo biloba with intracerebral hemorrhage. Neurology 1998, 50, 1933–1934. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, G.J. Ginkgo biloba. Neurology 1997, 48, 1137. [Google Scholar] [CrossRef]
- Vale, S. Subarachnoid haemorrhage associated with Ginkgo biloba. Lancet 1998, 352, 36. [Google Scholar] [CrossRef]
- Dugoua, J.J.; Mills, E.; Perri, D.; Koren, G. Safety and efficacy of ginkgo (ginkgo biloba) during pregnancy and lactation. Can. J. Clin. Pharmacol. 2006, 13, 277–284. [Google Scholar]

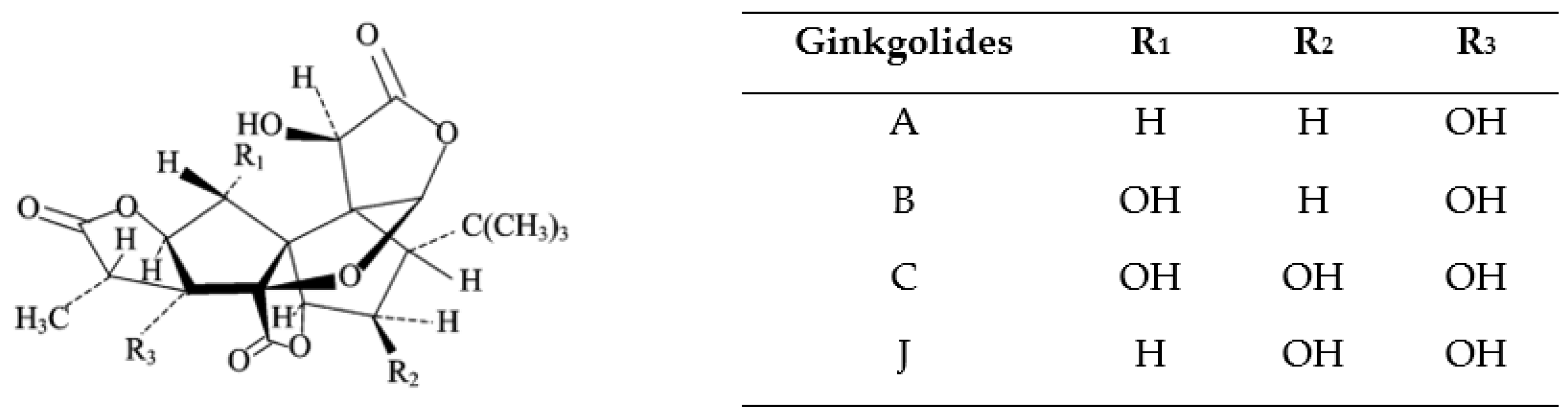

| Compound Class | Percentage (%) |
|---|---|
| Flavonol glycosides | 24 |
| Non-flavonol glycosides | 20 |
| Proanthocyanidins | 7 |
| Terpenes | 6 |
| Catechins | 2 |
| Carboxylic acids | 13 |
| Other compounds (organic, inorganic, unknown) | 28 |
| Authors | Species and Strain | Type of Vessel | Compound and Dose/Concentration | Main Results |
|---|---|---|---|---|
| Nishida and Satoh (2003) [88] | Male Wistar rats (4–10 w.o.; undisclosed weight) | Aorta | EGb (0.3–3 mg/mL) and bilobalide (0.1–100 μmol/L) | EGb and bilobalide relaxed NE-precontracted intact vessels. EGb-mediated relaxation was significantly inhibited by L-NMMA but not by indomethacin or TEA. Bilobalide-mediated relaxation was inhibited in a calcium-free medium or by pretreatment with L-NMMA. |
| Nishida and Satoh (2004) [89] | Male Wistar rats (4–15 w.o.; undisclosed weight) | Aorta | EGb (Ginkgolon-24®, 0.03–3 mg/mL) and its isolated terpenoids and flavonoids (0.1–100 μmol/L) | EGb, terpenoids (ginkgolides A-C, bilobalide), and flavonoids (quercetin, rutin) relaxed NE-precontracted intact vessels. |
| Nishida and Satoh (2005) [90] | Male Wistar rats (5 to 25 w.o.; undisclosed weight) | Aorta | EGb (0.1–3 mg/mL and bilobalide (0.1–100 μmol/L) | Concentration-dependent relaxation of NE-precontracted intact vessels, with response intensity decreasing with the animals’ age. |
| Kubota et al. (2006) [95] | Male WKYRs and SHRs (6 w.o.; undisclosed weight) | Aorta | EGb 0.05–0.5% orally for 30 days | No vascular response in vessels from WKYRs. Dose-dependent potentiation of acetylcholine-mediated vasorelaxation in vessels from SHRs. |
| Koltermann et al. (2007) [98] | Male Sprague-Dawley rats (180–220 g; undisclosed age) | Aorta | EGb 761® 5 mg i.v. | Relaxation of precontracted vessels. |
| Auguet et al. (1982) [99] | Male New Zealand rabbits (1.8–2.6 kg; undisclosed age) | Aorta | EGb 100 μg/mL | Potentiation of NE-induced contraction; no effect on 5-HT- or desipramine-induced contraction. |
| Laukeviciene et al. (2012) [91] | Wistar rats (undisclosed sex, age and weight) | Mesenteric artery | EGb 0.32 mL/kg/day orally for 10 days | Relaxation of KCl- and PE-precontracted vessels; potentiation of SNP-mediated relaxation. |
| Kubota et al. (2007) [96] | Male SHRs (50 w.o.) | Mesenteric artery | EGb 4-week supplementation | No change in PE-precontracted vessels or in ACh-mediated relaxation. |
| Zhou et al. (2006) [92] | Pigs (6–7 m.o.) | Coronary artery | GKA | GKA recovered bradykinin-mediated relaxation in arteries incubated with homocysteine. However, GKA did not modify maximum contraction or relaxation evoked by U46619 or SNP. |
| Chen et al. (1997) [93] | Pigs (undisclosed age and weight) | Basilar artery | EGb from leaves (15–90 μg/mL) and gingenosides fraction (20–120 μg/mL) | Concentration-dependent relaxation of intact and endothelium-denuded arteries, with and without TNS-induced relaxation. The latter was inhibited by N-L-arginine and by TTX but not by SNP. |
| Kim et al. (2011) [94] | New Zealand rabbits (22–26 w.o., 3–4 kg) | Corpus cavernosum | EGb | Relaxation of NE-precontracted tissue by EGb or mirodenafil. This response was inhibited by TEA. |
| Gokbas (2021) [87] | Human subjects (healthy full-term deliveries) | Umbilical artery | EGb 761® (50–500 μg) | Relaxation of 5-HT-precontracted intact vessels. This response was inhibited by indomethacin and L-NAME. |
| Authors | Species and Strain | Compound and Dose/Concentration | Main Results |
|---|---|---|---|
| Koltermann et al. (2007) [98] | Male Sprague-Dawley rats (180–220 g, undisclosed age) | EGb 761® 5 mg i.v. | Significant decrease of systolic blood pressure, inhibited by L-NAME. |
| Abd-Eldayem et al. (2016) [103] | L-NAME-induced hypertensive Wistar rats (undisclosed age and weight) | EGb 761® 100 mg/kg/day orally for 3 weeks | Significant decrease of blood pressure. |
| Abdel-Zaher et al. (2017) [73] | L-NAME-induced hypertensive and hypercholesterolemic Wistar rats (undisclosed age and weight) | EGb 761® 100 mg/kg/day for 3 weeks | Significant decrease of blood pressure. |
| Umegaki et al. (2000) [104] | DOCA-salt hypertensive rats (undisclosed age and weight) | EGb 2% orally for 20 days | Significant decrease of blood pressure and heart rate. |
| Kubota et al. (2007) [96] | Male SHRs (N = 6, 50 w.o., undisclosed weight) | 0.5% orally for 4 weeks | No change in systolic or diastolic blood pressure; significant reduction in heart rate and blood flow velocity. |
| Sasaki et al. (2002) [105] | SHRSP/Izm rats (6 w.o., undisclosed weight) | EGb 761® (60 and 120 mg/kg) orally for 3 weeks | Significant decrease in blood pressure. |
| Mansour et al. (2011) [97] | Male Wistar albino rats (120–140 g, undisclosed age) | EGb 761® (180 mg/kg/day) orally for 3 weeks) | Significant decrease in blood pressure; no change in heart rate. |
| Authors | Study Sample | EGb Composition | Concentration/Dosage and Duration of Treatment | Main Results |
|---|---|---|---|---|
| Chung et al. (1999) [108] | Healthy (N = 11; 8 females, 3 males; 10–61 y.o., mean 34 ± 3 y.o.) | EGb 761® | 120 mg/day orally for 2 days | No change in heart rate and blood pressure when compared with the placebo. |
| Kudolo et al. (2000) [107] | Healthy (N = 20, 14 females, 6 males, 21–57 y.o.) | EGb 761® | 120 mg/day orally for 3 months | Significant decrease in systolic and diastolic blood pressure. |
| Keheyan et al. (2011) [114] | Healthy (N = 14, males) | EGb 761® | 360 mg orally, single dose | No change in heart rate or blood pressure over a 6 h period when compared with placebo. |
| Moulton et al. (2001) [109] | Healthy (N = 30 males, mean age 20.57 y.o.) | Undisclosed | 120 mg/day orally for 5 days | Significant decrease of heart rate and blood pressure on day 5 when compared to the previous days. |
| Kalus et al. (2003) [110] | Healthy (N = 15; 7 females, 8 males; 20–24 y.o., mean 22.9 ± 1.1 y.o.) | EGb 761® | 240 mg (120 mg twice-daily) orally for 7 days | No change in heart rate, blood pressure, or ECG parameters when compared with the placebo. |
| Jezova et al. (2002) [112] | Healthy (N = 70; 37 females, 33 males; 20–30 y.o.) | EGb 761® (40 mg/mL) | 120 mg orally, single administration | Attenuation of stress-mediated (handgrip and mental stimuli) increase in blood pressure. |
| Winther et al. (1998) [113] | Mild to moderate cognitive cognitive impaired (N = 40, 20 per group; both sexes; 61–88 y.o.; undisclosed cardiovascular status) | Undisclosed composition | 120 (20 subjects) or 240 mg/day (20 subjects) orally for 3 months | Significant decrease of diastolic blood pressure in the subjects treated with 120 mg/day. |
| Wimpissinger et al. (2007) [106] | Healthy male subjects (N = 15; mean 25 ± 3 y.o.) | EGb 761® | 240 mg orally, single administration (57.6 mg ginkgoflavonglycosidesand 14.4 mg terpenlactones) | No change in blood pressure 3 h after administration when compared with the placebo. |
| Authors | Study Sample | Compound Concentration/Dosage and Duration of Treatment | Main Results |
|---|---|---|---|
| Mehlsen et al. (2002) [115] | Healthy subjects (N = 16; both sexes; median 32 y.o.) | 1 tablet of Gibidyl forte® thrice daily per os for 6 weeks | Significant increase in forearm perfusion on weeks 3 and 6 without changing blood pressure. |
| Boelsma et al. (2004) [116] | Healthy middle-aged subjects | 240 mg EGb 761®/day (80 mg thrice daily) orally for 3 weeks | Significant decrease in mean forefoot perfusion, both basal and post-occlusion, when compared to placebo. |
| Wu et al. (2008) [117] | Healthy middle-aged male subjects (N = 30; 54 ± 10 y.o.) | EGb 0.7 mg/min i.v. for 120 min | Significant increase in the perfusion of the left anterior descending coronary artery. |
| Mashayekh et al. (2011) [118] | Healthy middle-aged subjects | EGb 120 mg/day orally (60 mg, twice-daily) for 4 weeks | Significant increase in cerebral perfusion. |
| Chung et al. (1999) [108] | Healthy subjects (N = 11; both sexes, mean 34 ± 3 y.o.) | EGb 40 mg, thrice daily, orally for 2 days | Significant increase in the end-diastolic flow velocity of the ophthalmic artery. |
| Wimpissinger et al. (2007) [106] | Healthy male subjects (N = 15; mean 25 ± 3 y.o.) | EGb 761® 240 mg orally | No signficant vascular change in comparison with the placebo. |
| Didier et al. (1996) [119] | Adult guinea pigs (N = 4, undisclosed age) | 100 mg/kg/day EGb 761® orally for 6 weeks | Attenuation of salicylate-induced decrease in cochlear perfusion; potentiation of hypoxia-mediated cochlear perfusion. |
| Jang et al. (2011) [120] | Adult male guinea pigs (N = 10; 250–300 g) | Ear instillation of 10 mg/kg EGb and of LPS, followed by i.p. administration of EGb 100 mg/kg/day for 3 days | Significant attenuation of LPS-mediated decrease in cochlear perfusion and hair cell damage. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, H.; Martins, F.G. Cardiovascular Activity of Ginkgo biloba—An Insight from Healthy Subjects. Biology 2023, 12, 15. https://doi.org/10.3390/biology12010015
Silva H, Martins FG. Cardiovascular Activity of Ginkgo biloba—An Insight from Healthy Subjects. Biology. 2023; 12(1):15. https://doi.org/10.3390/biology12010015
Chicago/Turabian StyleSilva, Henrique, and Filipe Gazalho Martins. 2023. "Cardiovascular Activity of Ginkgo biloba—An Insight from Healthy Subjects" Biology 12, no. 1: 15. https://doi.org/10.3390/biology12010015
APA StyleSilva, H., & Martins, F. G. (2023). Cardiovascular Activity of Ginkgo biloba—An Insight from Healthy Subjects. Biology, 12(1), 15. https://doi.org/10.3390/biology12010015






