Selection of Nest Material and Summer Nest Location by the Hazel Dormouse (Muscardinus avellanarius) in the Bidstrup Forests, Denmark
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
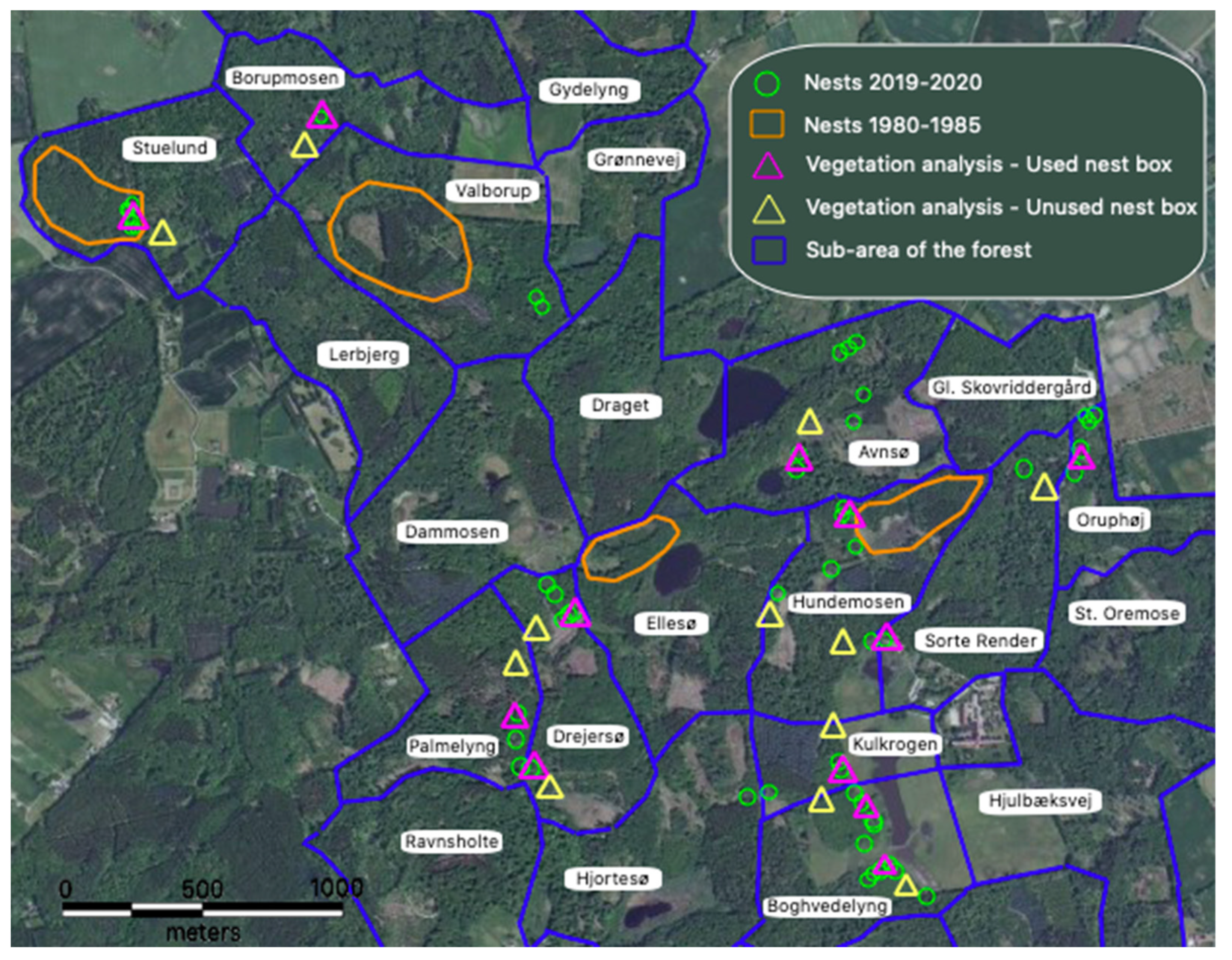
2.1. Study Site
2.2. Nest Data
2.3. Vegetation Data
2.4. Data Analysis
3. Results
3.1. Nest Materials
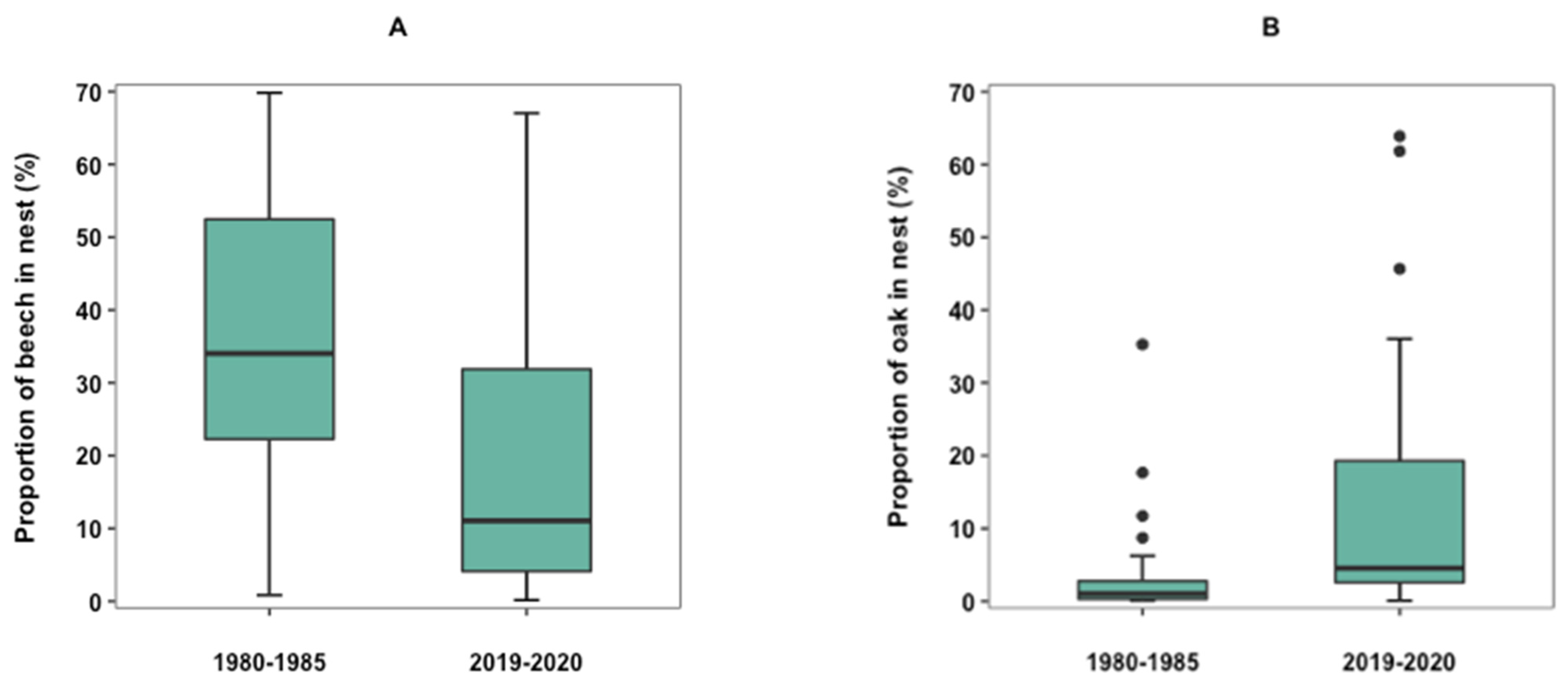
3.2. Changes in Selection of Nest Materials over Time
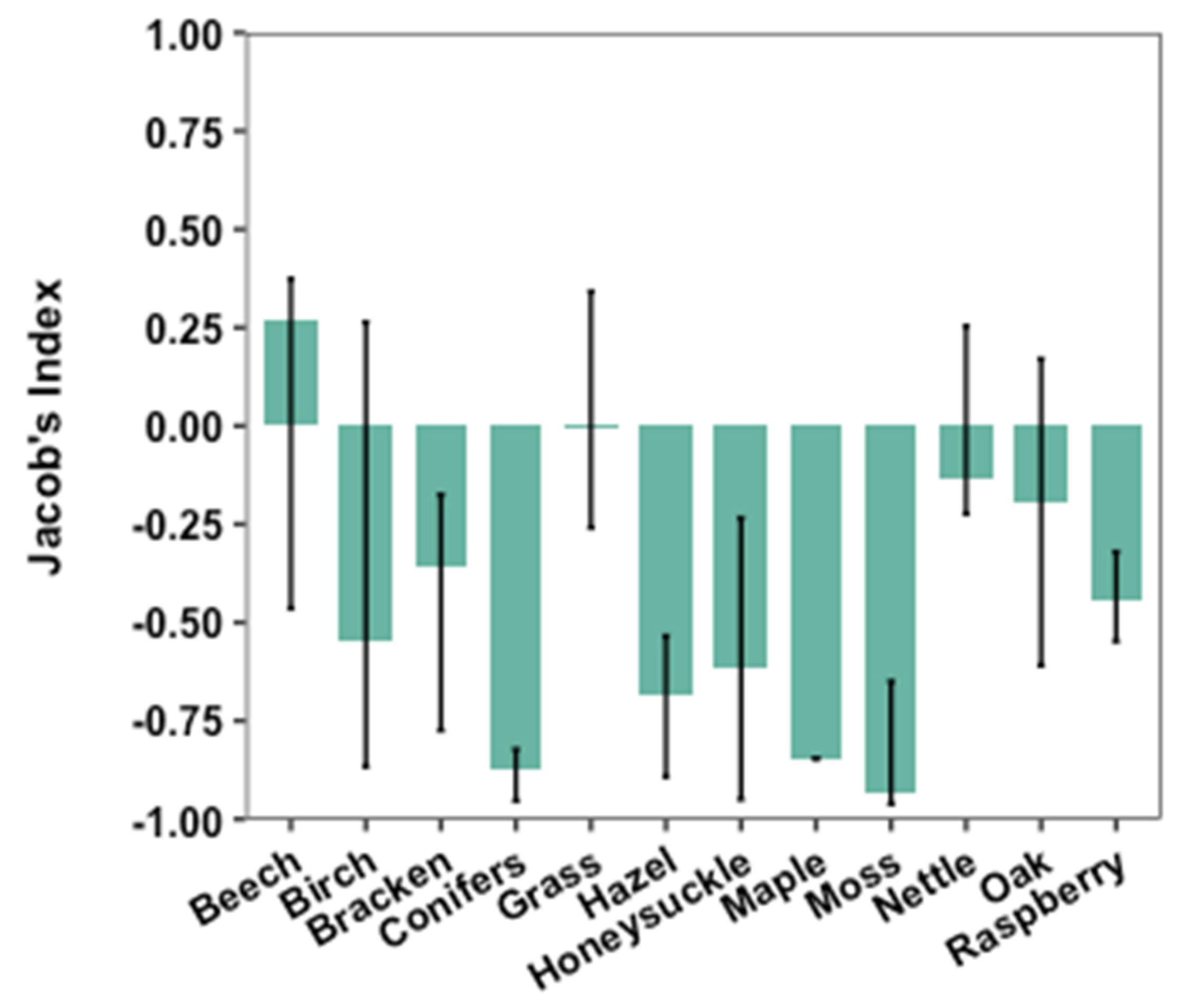
3.3. Selection of Nest Materials
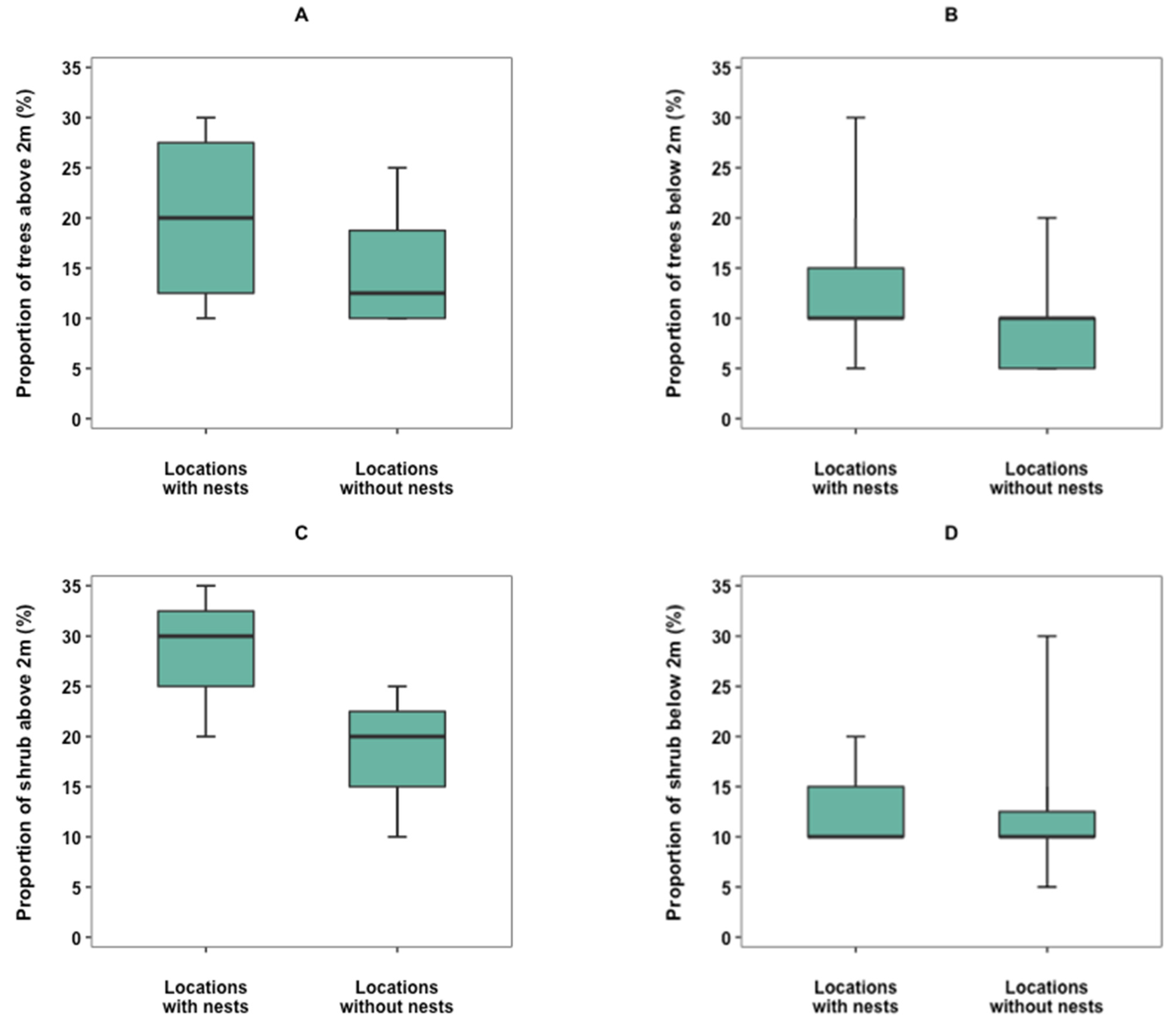
3.4. Nest Site Selection
4. Discussion
4.1. Nest Materials
4.2. Selection of Nest Materials
4.3. Changes in the Selection of Nest Materials and Nest Type over Time
4.4. Nest Site Selection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juškaitis, R. The Common Dormouse Muscardinus avellanarius: Ecology, Population Structure and Dynamics, 2nd ed.; Nature Research Centre Publishers: Vilnius, Lithuania, 2014. [Google Scholar]
- Hutterer, R.; Kryštufek, B.; Yigit, N.; Mitsainas, G.; Meinig, H.; Juškaitis, R. Muscardinus avellanarius (Amended Version of 2016 Assessment). The IUCN Red List of Threatened Species. 2021. Available online: https://www.iucnredlist.org/species/13992/197519168 (accessed on 30 March 2022).
- Goodwin, C.E.D.; Suggitt, A.J.; Bennie, J.; Silk, M.J.; Duffy, J.P.; Al-Fulaij, N.; Bailey, S.; Hodgson, D.J.; Mcdonald, R.A. Climate, Landscape, Habitat, and Woodland Management Associations with Hazel Dormouse Muscardinus avellanarius Population Status. Mammal Rev. 2017, 48, 209–223. [Google Scholar] [CrossRef]
- Den Danske Rødliste. Hasselmus. 2019. Available online: https://ecos.au.dk/forskningraadgivning/temasider/redlistframe/soeg-en-art#22661 (accessed on 3 March 2022).
- Vilhelmsen, H. Bevar Hasselmusen—En Håndbog i Bevarelse af Hasselmusen og dens Levesteder; Foreningen til Dyrenes Beskyttelse i Danmark, Skov- og Naturstyrelsen, Miljøministeriet: Copenhagen, Denmark, 1992. [Google Scholar]
- EEC. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. 1992. Available online: https://eur-lex.europa.eu/LexUriServ.do?uri=CONSLEG:1992L0043:20070101:EN:PDF (accessed on 20 April 2022).
- Vilhelmsen, H. Status of Dormice (Muscardinus avellanarius) in Denmark. Acta Zool. Acad. Sci. Hung. 2003, 49 (Suppl. S1), 139–145. [Google Scholar]
- Kjær, C.; Nygaard, B.; Terkelsen, O.R.; Elmeros, M.; Bladt, J.; Mikkelsen, P. Arter 2019; Videnskabelig rapport nr. 421; Aarhus Universitet, DCE—Nationalt Center for Miljø og Energi: Roskilde, Denmark, 2021; p. 40. [Google Scholar]
- Bright, P.W.; Morris, P.A. Habitat Requirements of Dormice Muscardinus avellanarius in Relation to Woodland Management in Southwest England. Biol. Conserv. 1990, 54, 307–326. [Google Scholar] [CrossRef]
- Panchetti, F.; Sorace, A.; Amori, G.; Carpaneto, G.M. Nest Site Preference of Common Dormouse (Muscardinus avellanarius) in Two Different Habitat Types of Central Italy. Ital. J. Zool. 2007, 74, 363–369. [Google Scholar] [CrossRef]
- Goodwin, C.E.D.; Hodgson, D.J.; Al-Fulaij, N.; Bailey, S.; Langton, S.; Mcdonald, R.A. Voluntary Recording Scheme Reveals Ongoing Decline in the United Kingdom Hazel Dormouse Muscardinus avellanarius Population. Mammal Rev. 2018, 47, 183–197. [Google Scholar] [CrossRef]
- Richards, C.G.J.; White, A.C.; Hurrell, E.; Price, F.E.F. The Food of the Common Dormouse, Muscardinus avellanarius, in South Devon. Mammal Rev. 1984, 14, 19–28. [Google Scholar] [CrossRef]
- Bright, P.W.; Morris, P.A. Foraging Behaviour of Dormice Muscardinus avellanarius in Two Contrasting Habitats. J. Zool. 1992, 230, 69–85. [Google Scholar] [CrossRef]
- Juškaitis, R. Feeding by the Common Dormouse (Muscardinus avellanarius): A Review. Acta Zool. Litu. 2007, 17, 151–159. [Google Scholar] [CrossRef]
- Bright, P.W.; Morris, P.A. Why are Dormice Rare? A Case Study in Conservation Biology. Mammal Rev. 1996, 26, 157–187. [Google Scholar]
- Juškaitis, R. Long-Term Common Dormouse Monitoring: Effects of Forest Management on Abundance. Biodivers. Conserv. 2008, 17, 3559–3565. [Google Scholar] [CrossRef]
- Mortelliti, A.; Amori, G.; Capizzi, D.; Cervone, C.; Fagiani, S.; Pollini, B.; Boitani, L. Indipendent Effects of Habitat Loss, Habitat Fragmentation and Structural Connectivity on the Distribution of Two Arboreal Rodents. J. Appl. Ecol. 2011, 48, 153–162. [Google Scholar] [CrossRef]
- Rondinini, C.; Rodrigues, A.S.L.; Boitani, L. The Key Elements of a Comprehensive Global Mammal Conservation Strategy. Philos. Trans. R. Soc. Biol. Sci. 2011, 366, 2591–2597. [Google Scholar] [CrossRef] [PubMed]
- Law, B.A.; Dickman, C.R. The use of Habitat Mosaics by Terrestrial Vertebrate Fauna: Implications for Conservation and Management. Biodivers. Conserv. 1998, 7, 323–333. [Google Scholar] [CrossRef]
- Burns, K.J.; Hackett, S.J. Nest and Nest-Site Characteristics of a Western Population of Fox Sparrow (Passerekka iliaca). Southwest. Nat. 1993, 38, 277–279. [Google Scholar] [CrossRef]
- Roubik, D.W. Stingless Bee Nesting Biology. Apidologie 2006, 37, 124–143. [Google Scholar] [CrossRef]
- Cosarinsky, M.I.; Roces, F. The Construction of Turrets for Nest Ventilation in the Grass-Cutting Ant Atta vollenweideri: Import and Assembly of Building Materials. J. Insect Behav. 2012, 25, 222–241. [Google Scholar] [CrossRef]
- Koops, K.; McGrew, W.C.; Matsuzawa, T.; Knapp, L.A. Terrestrial Nest-Building by Wild Chimpanzees (Pan troglodytes): Implications for the Tree-to-Ground Sleep Transition in Early Hominins. Am. J. Phys. Anthropol. 2012, 148, 351–361. [Google Scholar] [CrossRef]
- Wachtendorf, W. Beiträge zur Ökologie der Haselmaus (Muscardinus avellanarius) im Alpenvorland. Zool. Jahrbūcher Syst. 1951, 80, 189–204. [Google Scholar]
- Zaytseva, H. Nest Material of the Common Dormouse (Muscardinus avellanarius L.) Used in Nestboxes, Podilla (West Ukraine). Pol. J. Ecol. 2006, 54, 397–401. [Google Scholar]
- Čanády, A. Factors Predicting Summer Nest Construction of Muscardinus avellanarius in Deciduous Woodland Edges in Slovakia. Biologia 2015, 70, 132–140. [Google Scholar] [CrossRef]
- Gubert, L.; McDonald, R.A.; Wilson, R.J.; Chanin, P.; Bennie, J.J.; Mathews, F. The Elusive Winter Engineers: Structure and Materials of Hazel Dormouse Hibernation Nests. J. Zool. 2022, 316, 81–91. [Google Scholar] [CrossRef]
- Naturstyrelsen. Historie—Bidstrup Skovene. 2022. Available online: https://naturstyrelsen.dk/naturoplevelser/naturguider/bidstrup-skovene/historie/ (accessed on 20 May 2022).
- Vilhelmsen, H. Hasselmusen, Muscardinus avellanarius L. 1758. Master’s Thesis, Zoologisk Museum, Copenhagen, Denmark, 1989. [Google Scholar]
- Vilhelmsen, H. Forvaltningsplan—Beskyttelse og Forvaltning af Hasselmusen, Muscardinus avellanarius og dens Levesteder i Danmark; Naturstyrelsen, Miljøministeriet: Copenhagen, Denmark, 2011.
- Frederiksen, S.; Rasmussen, F.N.; Seberg, O. Dansk Flora, 3rd ed.; Gyldendal: Copenhagen, Denmark, 2019. [Google Scholar]
- Fredshavn, J.; Nielsen, K.E.; Ejrnæs, R.; Nygaard, B. Overvågning af Terrestriske Naturtyper—Teknisk Anvisning; Fagdatacenter for Biodiversitet og Terrestrisk Natur, DCA, Aarhus Universitet: Copenhagen, Denmark, 2019. [Google Scholar]
- Quintans, D. Package ‘Electivity’. 2019. Available online: https://github.com/DesiQuintans/electivity (accessed on 23 May 2022).
- Morris, P.A.; Bright, P.W.; Woods, D. Use of Nestboxes by the Dormouse Muscardinus avellanarius. Biol. Conserv. 1990, 51, 1–13. [Google Scholar] [CrossRef]
- Bracewell, M.; Downs, N.C. Hazel Dormouse (Muscardinus avellanarius) Nest Material Preferences and Collection Distances in Southern England. Mammal Commun. 2017, 3, 1–10. [Google Scholar]
- Verbeylen, G.; Andre, A.; Desmet, A.; Manzanares, L.; Mels, B.; Pulles, R.; Swinnen, K.; Vanseuningen, I.; Vermeiren, M. Nest Site Selection and Use of Other Habitats by the Hazel Dormouse Muscardinus avellanarius in Voeren (Flanders); Report Natuur.studie2017/3; Natuurpunt Research Department (Mammal Working Group): Mechelen, Belgium, 2017. [Google Scholar]
- Juškaitis, R. Use of Nestboxes by the Common Dormouse (Muscardinus avellanarius L.) in Lithuania. Nat. Croat. 1997, 6, 177–188. [Google Scholar]
- Biddle, L.E.; Broughton, R.E.; Goodman, A.M.; Deeming, D.C. Composition of Bird Nests is a Species-Specific Characteristic. Avian Biol. Res. 2018, 11, 132–153. [Google Scholar] [CrossRef]
- Naturstyrelsen. Hasselmusprojekt i Bidstrupskovene. 2022. Available online: https://naturstyrelsen.dk/naturbeskyttelse/naturprojekter/hasselmusprojekt-i-bidstrupskovene/ (accessed on 29 April 2022).
- Berg, L.; Berg, Å. Nest Site Selection by the Dormouse Muscardinus avellanarius in Two Different Landscapes. Ann. Zool. Fenn. 1998, 35, 115–122. [Google Scholar]
- Juškaitis, R.; Balčiauskas, L.; Šiožinytė, V. Nest Site Selection by the Hazel Dormouse Muscardinus avellanarius: Is Safety More Important Than Food? Zool. Stud. 2013, 52, 53. [Google Scholar] [CrossRef]
- Mortensen, R.M.; Fuller, M.F.; Dalby, L.; Berg, T.B.; Sunde, P. Hazel Dormouse in Managed Woodland Select for Young, Dense, and Species-Rich Tree Stands. For. Ecol. Manag. 2022, 519, 120348. [Google Scholar] [CrossRef]
- Juškaitis, R.; Baltrünaité, L. Feeding on the Edge: The Diet of the Hazel Dormouse Muscardinus avellanarius (Linnaeus 1758) on the Northern Periphery of its Distributional Range. Mammalia 2013, 77, 149–155. [Google Scholar] [CrossRef]
- Goodwin, C.E.D.; Swan, G.J.F.; Hodgson, D.J.; Bailey, S.; Chanin, P.; McDonals, R.A. Effects of Food Availability on the Trophic Niche of the Hazel Dormouse Muscardinus avellanarius. For. Ecol. Manag. 2020, 118215, 470–471. [Google Scholar] [CrossRef]
- Williams, R.L.; Goodenough, A.E.; Hart, A.G.; Stafford, R. Using Long-Term Volunteer Records to Examine Dormouse (Muscardinus avellanarius) Nestbox Selection. PLoS ONE 2013, 8, e67986. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.C.W.; Morris, P.A. The Use of Dormouse Muscardinus avellanarius Nest Boxes by Two Species of Apodemus in Britain. Acta Theriol. 2000, 45, 443–453. [Google Scholar] [CrossRef]
- Goodwin, C.E.D.; Hodgson, D.J.; Bailey, S.; Bennie, J.; McDonald, R.A. Habitat Preferences of Hazel Dormice Muscardinus avellanarius and the Effects of Tree-Felling on Their Movement. For. Ecol. Manag. 2018, 427, 190–199. [Google Scholar] [CrossRef]

| Nest Material (No. of Nests with Material) | 1980–1985 | 2019–2020 | Difference in Median Proportion of Nest Material between Periods | ||||
|---|---|---|---|---|---|---|---|
| Proportion of Nests with Material (%) | Median % of Material in Nests (IQ Range) | Proportion of Nests with Material as Heaviest Component (%) | Proportion of Nests with Material (%) | Median % of Material in Nests (IQ Range) | Proportion of Nests with Material as Heaviest Component (%) | ||
| Alder, Alnus glutinosa (n = 9) | - | - | - | 13.43 | 6.96 (1.5–9.3) | 4.48 | - |
| Anthropogenic (n = 5) | 6.10 | 0.29 (0.3–0.4) | - | 1.49 | 1.42 (1.4–1.4) | - | W = 5, p = 0.24 |
| Bark (n = 55) | 67.07 | 8.82 (2.6–26.7) | 14.63 | 43.28 | 15.32 (5.7–26.5) | 7.46 | W = 931, p = 0.21 |
| Beech, Fagus sylvatica (n = 72) | 87.80 | 40.06 (25.8-57.4) | 51.22 | 86.57 | 18.40 (5.8–49.7) | 38.81 | W = 1521, p = 0.0053 * |
| Birch, Betula spp. (n = 5) | 6.10 | 1.17 (1.0–3.1) | - | 10.45 | 1.22 (0.5–8.6) | 1.49 | W = 18, p = 1 |
| Blackberry, Rubus fruticosus (n = 1) | 1.22 | 16.36 (16.4–16.4) | - | - | - | - | - |
| Bracken, Pteridium aquilinum (n = 20) | 24.39 | 1.02 (0.2–19.5) | - | 11.94 | 7.72 (2.4–16.0) | - | W = 109, p = 0.15 |
| Branches (n = 41) | 50.00 | 0.89 (0.5–1.9) | - | 16.42 | 1.23 (0.6–1.4) | - | W = 232, p = 0.89 |
| Conifer Needle, Pinophyta spp. (n = 55) | 67.07 | 0.47 (0.2–1.2) | - | 11.94 | 0.36 (0.1–1.0) | - | W = 176, p = 0.37 |
| Cypress, Cupressus sempervirens (n = 2) | 2.44 | 0.35 (0.2–0.5) | - | - | - | - | - |
| Elm, Ulmus glabra (n = 1) | 1.22 | 12.14 (12.1–12.1) | - | - | - | - | - |
| Field Maple, Acer campestre (n = 9) | - | - | - | 26.87 | 0.96 (0.8–6.0) | 2.99 | - |
| Grass, Poaceae (n = 59) | 71.95 | 26.86 (5.8–64.1) | 30.49 | 52.24 | 14.12 (5.3–38.4) | 17.91 | W = 883, p = 0.24 |
| Hawthorn, Crataegus monogyna (n = 1) | 1.22 | 0.74 (0.7–0.7) | - | 5.97 | 1.85 (1.3–3.7) | - | W = 4, p = 0.29 |
| Hazel, Corylus avellana (n = 6) | - | - | - | 8.96 | 3.91 (1.9–5.6) | - | - |
| Honeysuckle, Lonicera spp. (n = 8) | - | - | - | 11.94 | 7.55 (1.3–8.9) | 1.49 | - |
| Hornbeam, Carpinus betulus (n = 9) | - | - | - | 13.43 | 8.54 (2.8–17.5) | 2.99 | - |
| Linden, Tilia cordata (n = 5) | - | - | - | 7.46 | 10.59 (4.2–12.7) | - | - |
| Maple, Acer pseudoplatanus (n = 1) | 1.22 | 12.87 (12.9–12.9) | - | 1.49 | 2.91 (2.9–2.9) | - | W = 0, p = 1 |
| Moss, Bryophyta (n = 39) | 47.56 | 0.75 (0.3–5.0) | 2.44 | 34.33 | 0.52 (0.3–3.2) | 4.48 | W = 452, p = 0.97 |
| Nettle, Urtica dioica (n = 12) | 14.63 | 4.30 (1.6–13.0) | - | 7.46 | 10.17 (2.3–14.6) | 1.49 | W = 36, p = 0.56 |
| Oak, Quercus spp. (n = 30) | 36.59 | 0.99 (0.3–2.8) | 1.22 | 80.60 | 4.52 (2.6–19.3) | 11.94 | W = 1304, p = 1.08 × 10−5 * |
| Raspberry, Rubus idaeus (n = 3) | 3.66 | 1.19 (0.7–5.4) | - | 5.97 | 4.78 (2.9–6.8) | - | W = 8, p = 0.60 |
| Rowan, Sorbus aucuparia (n = 3) | 3.66 | 1.31 (0.9–13.9) | - | 25.37 | 5.44 (1.3–16.1) | 2.99 | W = 32, p = 0.53 |
| Seed Coma (n = 3) | - | - | - | 4.48 | 0.06 (0.04–0.3) | - | - |
| Nest Type | % of Nests from 1980 to 1985 | % of Nests from 2019 to 2020 |
|---|---|---|
| Mixed (M) | 26.83 | 49.25 |
| Foliar (F) | 29.27 | 20.90 |
| Layered (L) | 28.05 | 20.90 |
| Grass (G) | 15.85 | 8.96 |
| Chi-squared (X2) | X2 = 10.6, df = 3, p < 0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, H.H.; Bertelsen, S.S.; Pertoldi, C.; Pagh, S.; Vilhelmsen, H. Selection of Nest Material and Summer Nest Location by the Hazel Dormouse (Muscardinus avellanarius) in the Bidstrup Forests, Denmark. Biology 2023, 12, 139. https://doi.org/10.3390/biology12010139
Hansen HH, Bertelsen SS, Pertoldi C, Pagh S, Vilhelmsen H. Selection of Nest Material and Summer Nest Location by the Hazel Dormouse (Muscardinus avellanarius) in the Bidstrup Forests, Denmark. Biology. 2023; 12(1):139. https://doi.org/10.3390/biology12010139
Chicago/Turabian StyleHansen, Heidi Holm, Sara Sofie Bertelsen, Cino Pertoldi, Sussie Pagh, and Helle Vilhelmsen. 2023. "Selection of Nest Material and Summer Nest Location by the Hazel Dormouse (Muscardinus avellanarius) in the Bidstrup Forests, Denmark" Biology 12, no. 1: 139. https://doi.org/10.3390/biology12010139
APA StyleHansen, H. H., Bertelsen, S. S., Pertoldi, C., Pagh, S., & Vilhelmsen, H. (2023). Selection of Nest Material and Summer Nest Location by the Hazel Dormouse (Muscardinus avellanarius) in the Bidstrup Forests, Denmark. Biology, 12(1), 139. https://doi.org/10.3390/biology12010139






