Fecundity, Length at First Sexual Maturity and Gonadal Development of Lepidorhombus boscii in the Eastern Adriatic Sea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
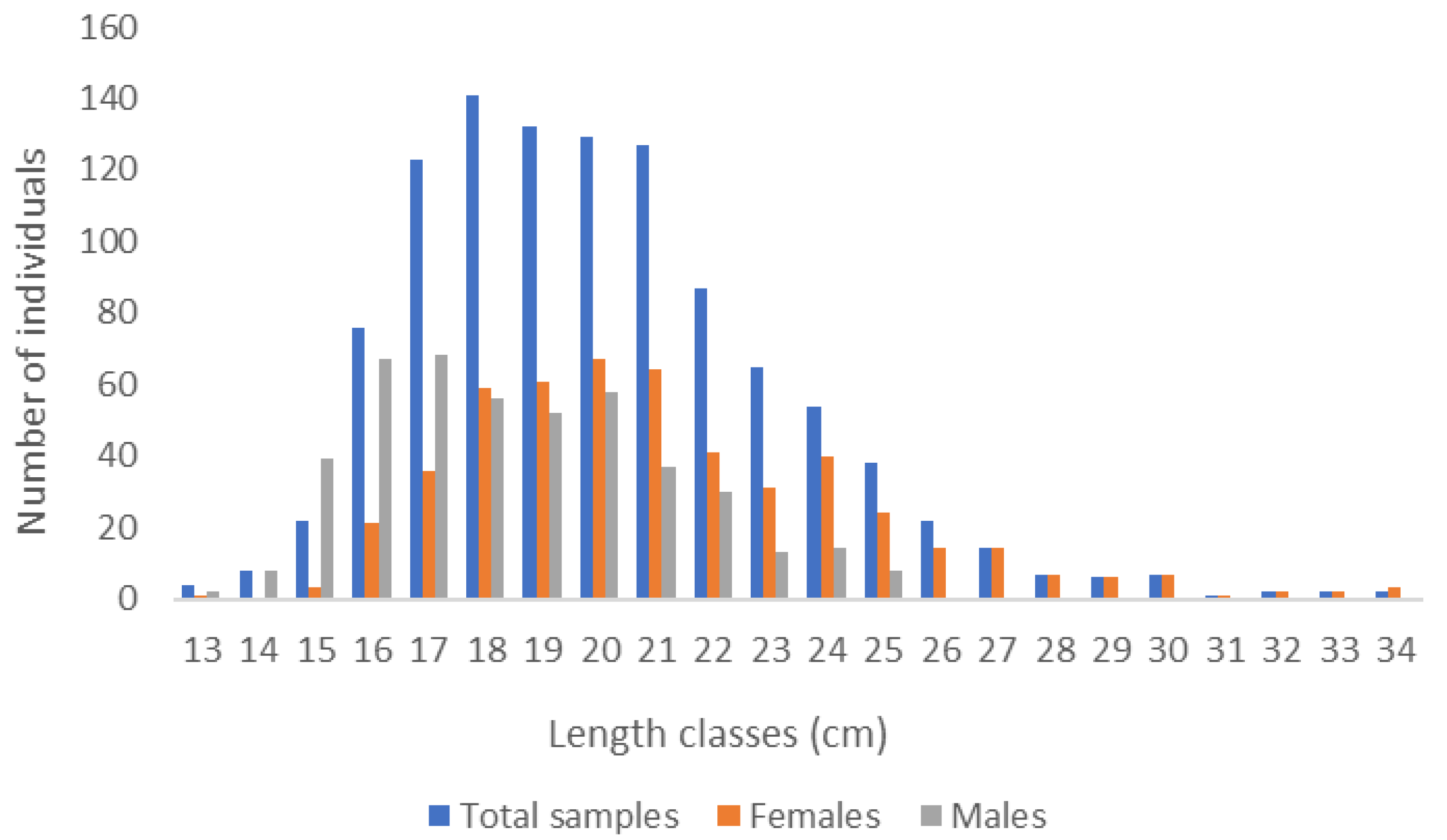
3.1. Size Structure and Sex Ratio
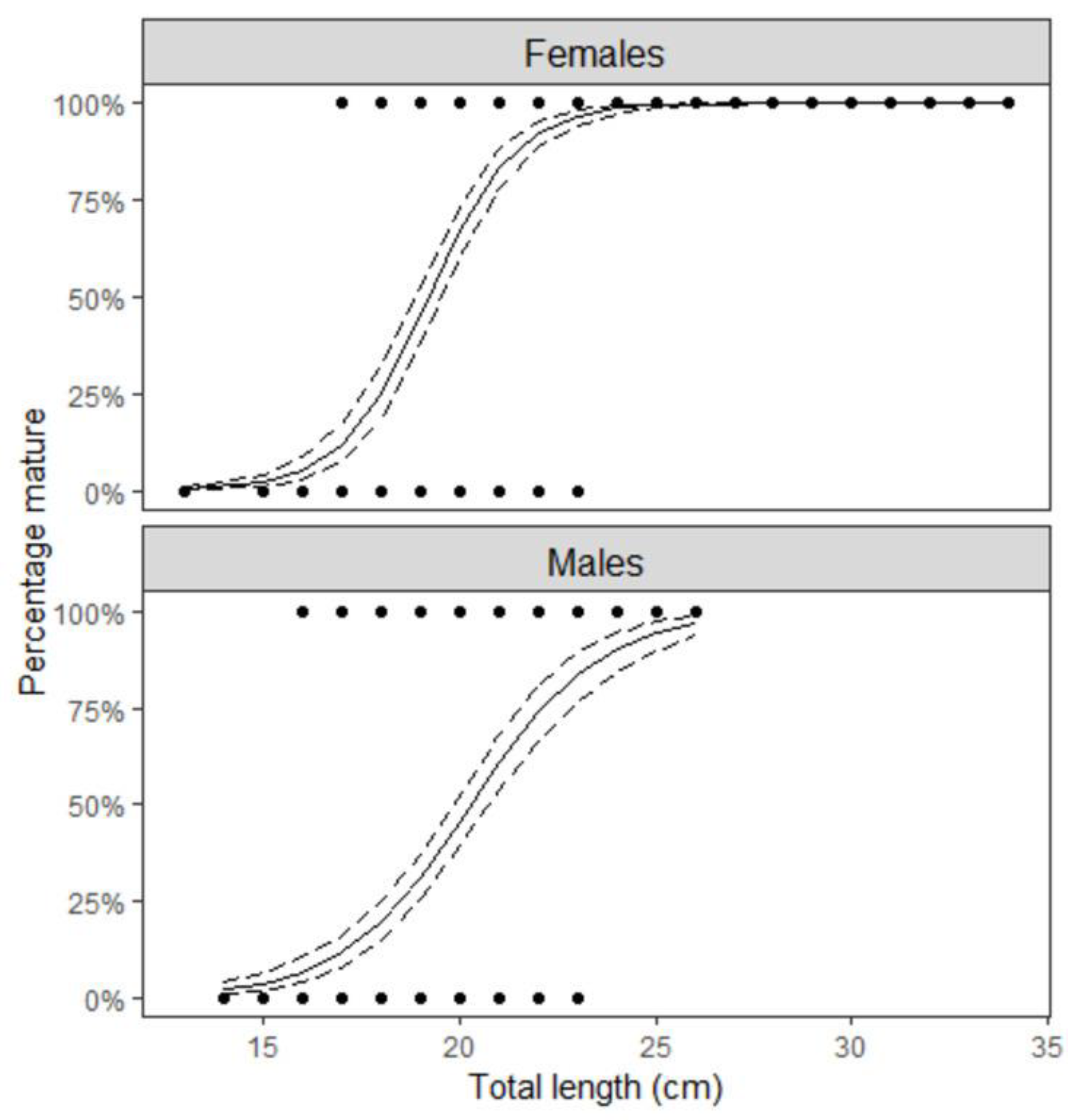
3.2. Maturation
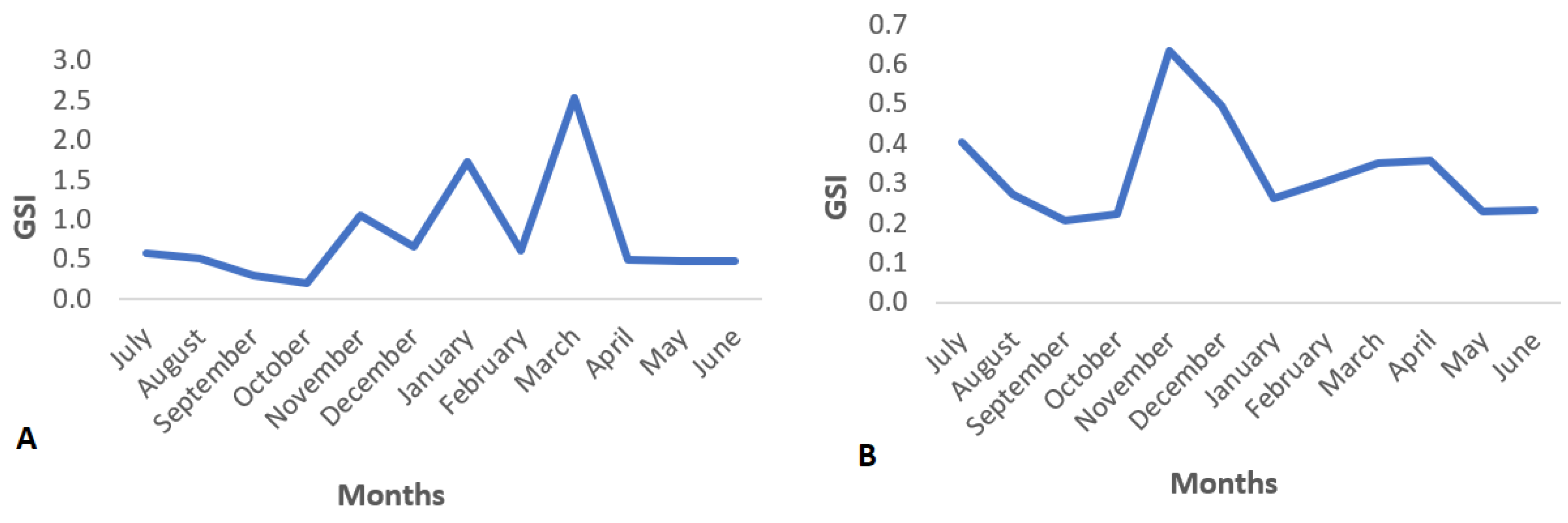
3.3. Gonadogenesis and Spawning Season
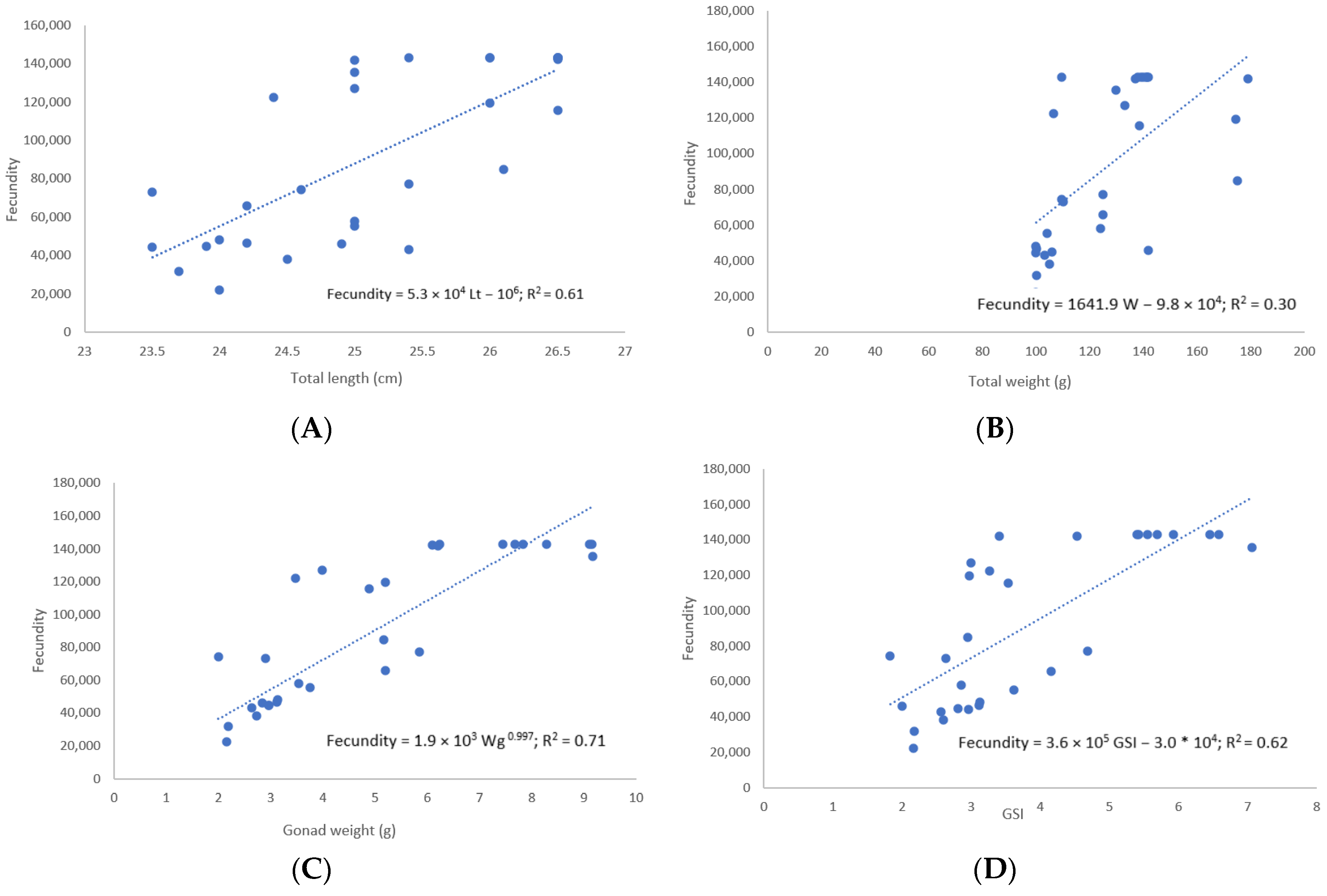
3.4. Fecundity
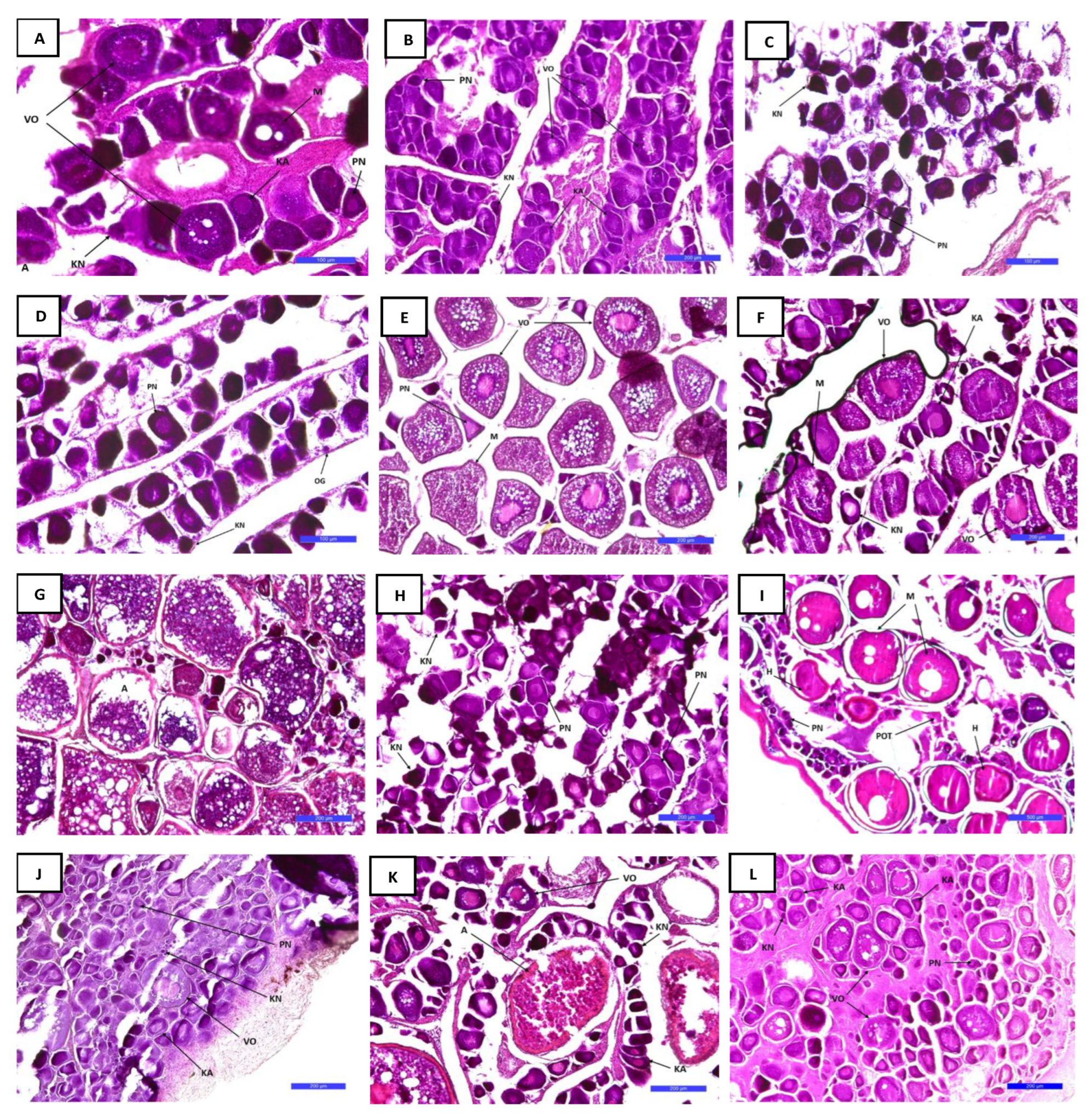
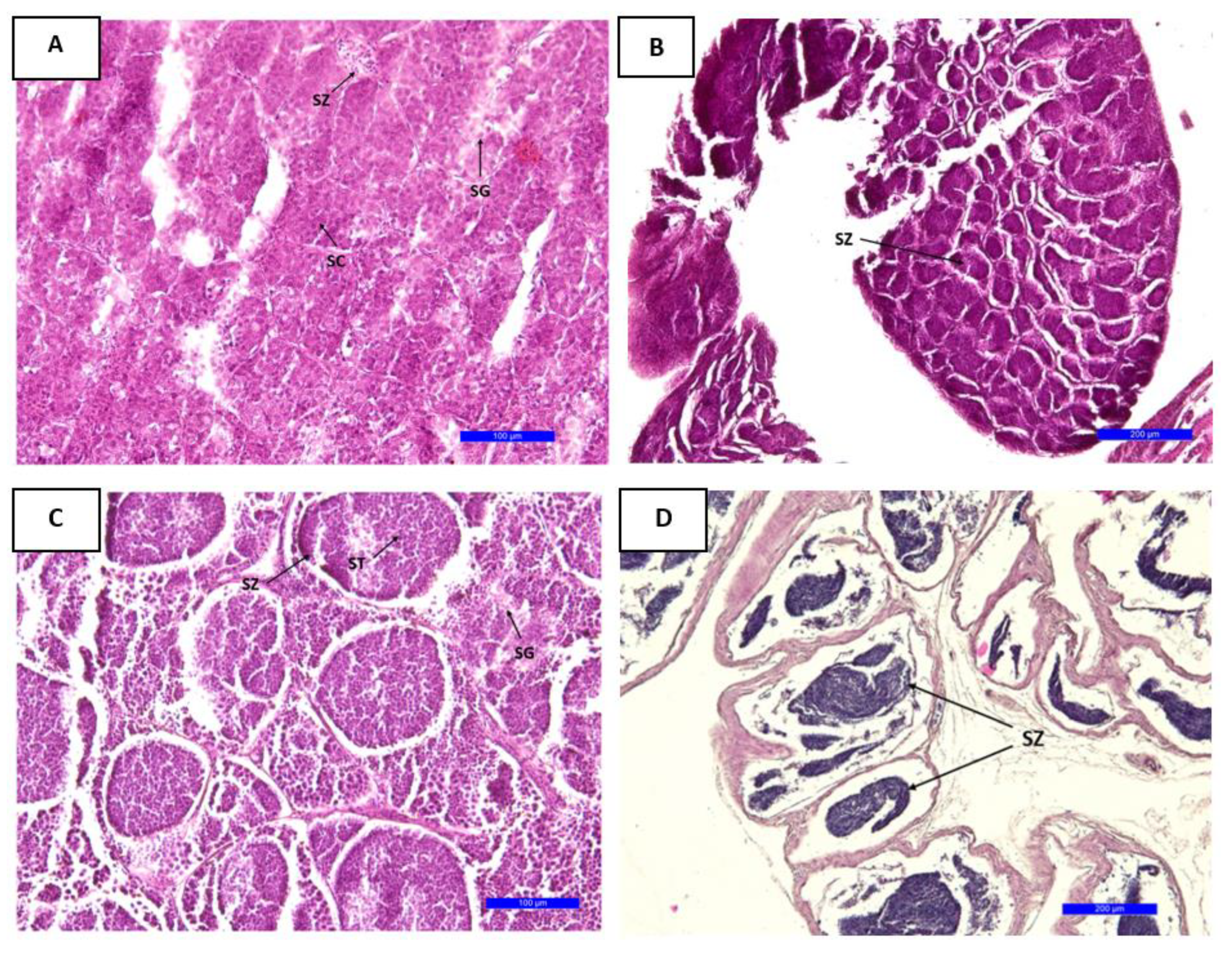
3.5. Histology
3.5.1. Gonadal Development in Females
3.5.2. Gonadal Development in Males
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cengiz, O.; Ismen, A.; Ozekinci, U. Reproductive biology of the spotted flounder, Citharus linguatula (Actinopterygii: Pleuronectiformes: Citharidae), from Saros Bay (Northern Aegean Sea, Turkey). Acta Ichthyol. Piscat. 2014, 44, 123–129. [Google Scholar] [CrossRef]
- Norman, J.R. A Systematic Monograph of the Flatfishes (Heterosomata). Br. Mus. (Nat. Hist.) 1934, 1, 459. [Google Scholar]
- Nielsen, J.G. Scophthalmidae. In Fishes of the North-Eastern Atlantic and the Mediterranean (FNAM); Whitehead, P.J.P., Bauchot, M.-L., Hureau, J.-C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1989; pp. 1286–1293. [Google Scholar]
- Sartor, P.; Sbrana, M.; Ungaro, N.; Marano, C.A.; Piccinetti, C.; Manfrin, G.P. Distribution and abundance of Citharus linguatula, Lepidorhombus boscii and Solea vulgaris (Osteichthyes: Pleuronectiformes) in the Mediterranean Sea. Sci. Mar. 2002, 66, 83–102. [Google Scholar] [CrossRef]
- Morte, S.; Redon, M.J.; Sanz-Brau, A. Feeding ecology of two megrims Lepidorhombus boscii and Lepidorhombus whffiagonis in the western Mediterranean (Gulf of Valencia, Spain). J. Mar. Biol. Assoc. UK 1999, 79, 161–169. [Google Scholar] [CrossRef]
- Dulčić, J.; Kovačić, M. Ihtiofauna Jadranskog Mora; Golden marketing—Tehnička knjiga; Institut za Oceanografiju i Ribarstvo: Split, Croatia, 2020; pp. 468–470. (In Croatian) [Google Scholar]
- Santos, P.T. Growth and reproduction of the population of the four- spot megrim (Lepidorhombus boscii Risso) off the Portuguese coast. Neth. J. Sea Res. 1994, 32, 379–383. [Google Scholar] [CrossRef]
- Santos, P.T. Growth, Mortality and Maturation of Lepidorhombus boscii in Portuguese Waters; Demersal Fish Committee CM: Braunschweig, Germany, 1995; p. 38. [Google Scholar]
- Landa, J.; Perez, N.; Pineiro, C. Growth patterns of the four-spot megrim (Lepidorhombus boscii) in the northeast Atlantic. Fish. Res. 2002, 55, 141–152. [Google Scholar] [CrossRef]
- Castilho, R.; Dinis, M.T.; Erzini, K. Age and growth of megrim Lepidorhombus boscii, Risso of the Portuguese continental coast. Fish. Res. 1993, 16, 339–346. [Google Scholar] [CrossRef]
- Cengiz, Ö.; Ozekinci, U.; Ismen, A.; Oztekin, A. Age and growth of the four-spotted megrim (Lepidorhombus boscii Risso, 1810) from Saros Bay (Northern Aegean Sea, Turkey). Med. Mar. Sci. 2013, 14, 36–44. [Google Scholar] [CrossRef]
- Vassilopoulou, V.; Haralabous, J. Effects of sexual maturity and feeding on condition of a deep-sea flatfish, Lepidorhombus boscii, in north-eastern Mediterranean waters. J. Nat. Hist. 2008, 42, 695–720. [Google Scholar] [CrossRef]
- Teixeira, C.M.; Batisat, M.I.; Cabral, H.N. Diet, growth and reproduction of four flatfishes on the Portuguese coast. Sci. Mar. 2010, 74, 223–233. [Google Scholar] [CrossRef]
- Cengiz, Ö.; Ozekinci, U.; Ismen, A.; Oztekin, A. Some Reproductive Characteristics of Four-spotted megrim (Lepidorhombus boscii Risso, 1810) from Saros Bay (Northern Aegean Sea, Turkey). J. Agric. Sci. 2015, 21, 270–278. [Google Scholar] [CrossRef]
- Taylan, B.; Uluturk, E. Determination of fecundity in the four-spot megrim Lepidorhombus boscii (Risso, 1810) (Pisces: Scophthalmidae) from the Aegean Sea. CBM Cah. Biol. Mar. 2017, 58, 213–217. [Google Scholar] [CrossRef]
- Holden, M.J.; Raitt, D.F.S. Manual of Fisheries Science. Part 2: Methods of Resource Investigation and Their Application; FAO Fisheries Technical Paper (115Rev.1); FAO: Rome, Italy, 1974. [Google Scholar]
- Sparre, P.; Venema, S.C. Introduction to Tropical Fish Stock Assessment. Part 1. Manual; FAO Fisheries Technical Paper No. 306/1, Rev.2; FAO: Rome, Italy, 1998; p. 407. [Google Scholar]
- Laevast, T. Section 4. Research of fish stocks. In Manual of Methods in Fisherries Biology; FAO Manual on Fishery Science; FAO: Rome, Italy, 1965; Volume 1, pp. 1–20. [Google Scholar]
- Vassilopoulou, V.; Ondrias, I. Age and growth of the four-spotted megrim (Lepidorhombus boscii) in eastern Mediterranean waters. J. Mar. Biol. Assoc. UK 1999, 79, 171–178. [Google Scholar] [CrossRef]
- Nikolsky, G.V. The Ecology of Fishes; Academic Press: New York, NY, USA, 1963; 352p. [Google Scholar]
- Arendt, J.D. Size-fecundity relationships, growth trajectories, and the temperature-size rule for ectotherms. Evolution 2011, 65, 43–51. [Google Scholar] [CrossRef]
- Angilletta, M.J.; Steury, T.D.; Sears, M.W. Temperature, Growth Rate, and Body Size in Ectotherms: Fitting Pieces of a Life-History Puzzle. Integr. Comp. Biol. 2004, 44, 498–509. [Google Scholar] [CrossRef]
- Petrakis, G.; Stergiou, K.I. Size selectivity of diamond and square mesh codends for four commercial Mediterranean fish species. ICES J. Mar. Sci. 1997, 54, 13–23. [Google Scholar] [CrossRef]
- Anderson, C.N.K.; Hsieh, C.-H.; Sandin, S.A.; Hewitt, R.; Hollowed, A.; Beddington, J.; May, R.M.; Sugihara, G. Why fishing magnifies fluctuations in fish abundance. Nature 2008, 452, 835–839. [Google Scholar] [CrossRef]
- Krstulović Šifner, S.; Vrgoč, N. Reproductive cycle and sexual maturation of the musky octopus Eledone moschata (Cephalopoda: Octopodidae) in the northern and central Adriatic Sea. Sci. Mar. 2009, 73, 439–447. [Google Scholar] [CrossRef]
- Ungaro, N.; Martino, M. Lepidorhombus boscii (Risso,1810): Biologia della specie e demografia della popolazione suifondi strascicabili dell’adriatico pugliese. Biol. Mar. Medit. 1998, 5, 192–200. [Google Scholar]
- Robson, S.M. Age, Growth, Reproductive Biology and Population Dynamics of the Common Megrim Lepidorhombus whiffiagonis (Walbaum, 1792) from Off the West Coast of Ireland. Ph.D. Thesis, Galway-Mayo Institute of Technology, Galway, Ireland, 2004. [Google Scholar]
- Oliveira, M.R.; Silva, N.B.; Yamamoto, M.E.; Chellappa, S. Gonad development and reproduction of the ballyhoo half beak, Hemiramphus brasiliensis from the coastal waters of Rio Grande do Norte, Brazil. Braz. J. Biol. 2015, 75, 324–330. [Google Scholar] [CrossRef]
- Duarte, C.M.; Alcaraz, M. To produce many small or few large eggs: A size independent reproductive tactic of fish. Oecologia 1989, 80, 401–404. [Google Scholar] [CrossRef]
- Tomkiewicz, J.; Tybjerg, L.; Jespersen, A. Micro—And macroscopic characteristics to stage gonadal maturation of female Baltic cod. J. Fish Biol. 2003, 62, 253–275. [Google Scholar] [CrossRef]
- Luckenbach, J.A.; Iliev, D.B.; Goetz, F.W.; Swanson, P. Identification of differentially expressed ovarian genes during primary and early secondary oocyte growth in coho salmon, Oncorhynchus kisutch. Reprod. Biol. Endocrinol. 2008, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Alba Serrat, A.; Saborido-Rey, F.; Garcia-Fernandez, C.; Muñoz, M.; Lloret, J.; Thorsen, A.; Kjesbu, O.S. New insights in oocyte dynamics shed light on the complexities associated with fish reproductive strategies. Sci. Rep. 2019, 9, 18411. [Google Scholar] [CrossRef] [PubMed]
| Stage | State | Description |
|---|---|---|
| I | Immature | Ovary and testis about 1/3rd length of body cavity. Ovaries pinkish, translucent; testis whitish. Eggs not visible to naked eye. |
| II | Maturing virgin and recovering spent | Ovary and testis about 1/2 length of body cavity. Ovary pinkish, translucent; testis whitish, symmetrical. Eggs not visible to naked eye. |
| III | Ripening | Ovary and testis are about 2/3rds length of body cavity. Ovary pinkish-yellow color with granular appearance, testis whitish to creamy. No transparent or translucent eggs visible. |
| IV | Ripe | Ovary and testis from 2/3rds to full length of body cavity. Ovary orange-pink in color with conspicuous superficial blood vessels. Large transparent, ripe eggs visible. Testis whitish-creamy, soft. |
| V | Spent | Ovary and testis shrunken to about 1/2 length of body cavity. Walls loose. Ovary may contain remnants of disintegrating opaque and ripe eggs, darkened or translucent. Testis bloodshot and flabby. |
| Months | N | Sex Ratio | Lt Range (cm) | W Range (g) | ||||
|---|---|---|---|---|---|---|---|---|
| Total | Males | Females | Males | Females | Males | Females | ||
| July | 99 | 36 | 63 | 1:1.75 * | 16.0–25.0 | 14.5–34.0 | 22.6–126.2 | 20.4–288.2 |
| August | 86 | 44 | 39 | 1:1.12 * | 15.9–22.0 | 16.9–30.1 | 26.6–75.5 | 29.1–210.5 |
| September | 50 | 15 | 26 | 1:0.57 | 16.9–21.9 | 16.1–21.3 | 29.4–69.2 | 24.8–60.3 |
| October | 86 | 37 | 49 | 1:0.75 | 13.6–21.9 | 16.6–20.5 | 14.4–74.1 | 27.9–59.6 |
| November | 94 | 41 | 44 | 1:0.93 | 17.0–26.1 | 16.7–27.5 | 30.7–117.0 | 27.5–177.3 |
| December | 76 | 37 | 37 | 1:1 | 15.8–23.4 | 16.2–21.6 | 23.5–79.3 | 25.6–62.0 |
| January | 104 | 43 | 39 | 1:1.10 * | 16.0–25.7 | 17.9–26.5 | 26.3–113.8 | 34.6–175.1 |
| February | 80 | 27 | 45 | 1:0.6 * | 16.3–22.9 | 16.0–23.5 | 27.5–85.2 | 25.0–103.8 |
| March | 108 | 45 | 40 | 1:1.12 * | 14.9–25.5 | 18.4–26.6 | 22.3–112.9 | 38.6–139.2 |
| April | 99 | 56 | 28 | 0.5:1 * | 14.6–21.0 | 14.5–22.0 | 21.9–63.8 | 21.4–77.5 |
| May | 117 | 43 | 61 | 1:0.70 * | 15.5–26.0 | 15.8–34.0 | 26.6–129.7 | 26.4–280.0 |
| June | 70 | 31 | 37 | 1:0.83 | 14.2–25.7 | 12.5–24.8 | 20.4–126.3 | 12.1–120.1 |
| Females | Males | |||||
|---|---|---|---|---|---|---|
| Month | N | Range | ± SD | N | Range | ± SD |
| July | 63 | 0.02–2.92 | 0.58 ± 0.56 | 36 | 0.10–2.00 | 0.40 ± 0.39 |
| August | 38 | 0.09–1.81 | 0.51 ± 0.43 | 44 | 0.17–1.93 | 0.27 ± 0.28 |
| September | 26 | 0.09–0.59 | 0.30 ± 0.14 | 15 | 0.08–0.62 | 0.20 ± 0.14 |
| October | 49 | 0.09–0.45 | 0.20 ± 0.07 | 36 | 0.05–1.31 | 0.22 ± 0.21 |
| November | 44 | 0.03–3.27 | 1.05 ± 0.82 | 41 | 0.07–1.82 | 0.63 ± 0.57 |
| December | 37 | 0.09–3.29 | 0.66 ± 0.60 | 36 | 0.11–2.31 | 0.49 ± 0.44 |
| January | 39 | 0.08–7.06 | 1.73 ± 2.01 | 43 | 0.04–1.05 | 0.26 ± 0.17 |
| February | 45 | 0.50–5.19 | 0.60 ± 1.21 | 27 | 0.10–2.47 | 0.30 ± 0.48 |
| March | 40 | 0.09–7.46 | 2.53 ± 1.88 | 43 | 0.09–2.33 | 0.35 ± 0.47 |
| April | 28 | 0.11–2.05 | 0.49 ± 0.46 | 52 | 0.09–2.19 | 0.35 ± 0.40 |
| May | 61 | 0.10–1.07 | 0.48 ± 0.22 | 42 | 0.03–0.73 | 0.23 ± 0.16 |
| June | 37 | 0.18–1.99 | 0.47 ± 0.31 | 29 | 0.05–0.61 | 0.23 ± 0.16 |
| Length Class (cm) | W (g) | Ovary Weight (g) | GSI | Fecundity (F) | N |
|---|---|---|---|---|---|
| 24 | 105.24 ± 8.25 | 3.12 ± 0.88 | 2.93 ± 0.60 | 55,479 ± 29,355 | 9 |
| 25 | 121.76 ± 16.57 | 4.50± 2.54 | 3.60 ± 1.75 | 81,695 ± 45 | 9 |
| 26 | 148.18 ± 27.94 | 5.76 ± 1.19 | 4.03 ± 1.26 | 119,420 ± 27 | 7 |
| 27 | 139.32 ± 1.29 | 7.77± 1.75 | 5.57 ± 1.23 | 137,359 ± 122,273 | 5 |
| Months | Range (μm) | ± SD |
|---|---|---|
| July | 66.0–128.9 | 99.9 ± 16.2 |
| August | 76.8–124.2 | 102.1 ± 12.9 |
| September | 34.0–63.9 | 53.4 ± 7.1 |
| October | 46.5–74.7 | 61.2 ± 7.6 |
| November | 190.2–300.6 | 243.4 ± 31.6 |
| December | 113.8–281.6 | 203.9 ± 48.2 |
| January | 126.2–3687 | 244 ± 74.9 |
| February | 53.2–127.2 | 86.9 ± 18.2 |
| March | 300.6–562.8 | 419.5 ± 71.9 |
| April | 80.0–202.7 | 117.9 ± 34.6 |
| May | 210.7–517.9 | 401.6 ± 101.1 |
| June | 72.9–165.6 | 107.5 ± 25.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugrin, N.; Paladin, A.; Krstulović Šifner, S. Fecundity, Length at First Sexual Maturity and Gonadal Development of Lepidorhombus boscii in the Eastern Adriatic Sea. Biology 2023, 12, 131. https://doi.org/10.3390/biology12010131
Ugrin N, Paladin A, Krstulović Šifner S. Fecundity, Length at First Sexual Maturity and Gonadal Development of Lepidorhombus boscii in the Eastern Adriatic Sea. Biology. 2023; 12(1):131. https://doi.org/10.3390/biology12010131
Chicago/Turabian StyleUgrin, Nika, Antonela Paladin, and Svjetlana Krstulović Šifner. 2023. "Fecundity, Length at First Sexual Maturity and Gonadal Development of Lepidorhombus boscii in the Eastern Adriatic Sea" Biology 12, no. 1: 131. https://doi.org/10.3390/biology12010131
APA StyleUgrin, N., Paladin, A., & Krstulović Šifner, S. (2023). Fecundity, Length at First Sexual Maturity and Gonadal Development of Lepidorhombus boscii in the Eastern Adriatic Sea. Biology, 12(1), 131. https://doi.org/10.3390/biology12010131






