Simple Summary
A growing number of studies have shown that mushroom polysaccharides could exert anti-diabetes, anti-intestinal inflammation and antitumor effects by regulating gut microbiota. Thus, the relationship between mushroom polysaccharides and gut microbiota was comprehensively summarized in this review. The vital role of gut microbiota in disease was also emphasized.
Abstract
Mushroom polysaccharides are a kind of biological macromolecule extracted from the fruiting body, mycelium or fermentation liquid of edible fungi. In recent years, the research on mushroom polysaccharides for alleviating metabolic diseases, inflammatory bowel diseases, cancers and other symptoms by changing the intestinal microenvironment has been increasing. Mushroom polysaccharides could promote human health by regulating gut microbiota, increasing the production of short-chain fatty acids, improving intestinal mucosal barrier, regulating lipid metabolism and activating specific signaling pathways. Notably, these biological activities are closely related to the molecular weight, monosaccharide composition and type of the glycosidic bond of mushroom polysaccharide. This review aims to summarize the latest studies: (1) Regulatory effects of mushroom polysaccharides on gut microbiota; (2) The effect of mushroom polysaccharide structure on gut microbiota; (3) Metabolism of mushroom polysaccharides by gut microbiota; and (4) Effects of mushroom polysaccharides on gut microbe-mediated diseases. It provides a theoretical basis for further exploring the mechanism of mushroom polysaccharides for regulating gut microbiota and gives a reference for developing and utilizing mushroom polysaccharides as promising prebiotics in the future.
1. Introduction
The gut microbiota is a complex ecosystem, with an estimated 100 trillion gut microbes in adults [1]. Gut microbiota can be divided into six categories: Bacteroidetes, Firmicutes, Proteobacteria, Actinobacteria, Fusobacteria and Verrucomicrobia. Among them, the dominant phyla Bacteroidetes and Firmicutes account for more than 90% of the gut microbiota [2,3]. Gut microbiota is an important bridge between diet and host health, and plays a crucial role in maintaining homeostasis [4,5]. Meanwhile, gut microbiota is closely related to host metabolism, immune regulation, energy consumption and other physiological processes [6]. A healthy gut microbiota shows diverse species, stable microbiota structure and balanced microecology. Studies have shown that dyshomeostasis of gut microbiota is associated with diseases such as inflammatory bowel disease (IBD), obesity, diabetes mellitus (DM), non-alcoholic fatty liver disease (NAFLD) and carcinomas [7,8,9,10].
Mushrooms are a kind of large fungi, which mainly grow in tropical and humid environments, and have been observed in Asian countries for more than two thousand years. Mushrooms are an important source of natural bioactive ingredients that mainly contain polysaccharides, proteins, vitamins, minerals and dietary fibers [11]. Currently, commonly reported mushrooms mainly include Ganoderma lucidum (G. lucidum), Lentinula edodes (L. edodes), Hericium erinaceus (H. erinaceus), Auricularia auricular, Grifola frondosa (G. frondosa) and Pleurotus eryngii (P. eryngii). Polysaccharides are the main active ingredients in mushrooms. In recent decades, many studies have shown that mushroom polysaccharides have anti-tumor, anti-inflammatory, antioxidant, anti-diabetes, anti-obesity and other biological activities [12,13,14]. In addition to the above-mentioned biological activities that have been widely reported, the regulation of gut microbiota by mushroom polysaccharides by stimulating the growth of beneficial bacteria has also received extensive attention [15,16,17]. This paper reviews recent developments regarding the regulatory effects of various mushroom polysaccharides on gut microbiota and summarizes the potential mechanism of mushroom polysaccharides to prevent and control diseases through gut microbiota. Additionally, as the biological activity of mushroom polysaccharides is largely affected by their complex structure, it is of great significance to explore the relationship between the structure of mushroom polysaccharides and the regulatory activity of gut microbiota. Consequently, the potential relationship between the structure of mushroom polysaccharides and intestinal flora has been summarized in this paper, which aimed to provide a reference for the development of mushroom polysaccharides in intestinal flora-related diseases.
2. Effects of the Structural Characteristics of Mushroom Polysaccharides on Gut Microbiota Diversity
Mushrooms polysaccharides are a natural polymer connected by polyhydroxyaldehyde or polyhydroxyketone through the glycosidic bond; they exert notable bioactivities such as anti-tumor, antioxidant, anti-diabetes, immunomodulation and anti-inflammatory effects [18,19,20,21,22]. Besides, mushroom polysaccharides exert an essential role in regulating the abundance and proportion of gut microbiota closely related to various diseases [23]. Many studies indicated that mushroom polysaccharides such as Wild morchella polysaccharides, Inonotus obliquus polysaccharides, Flammulina velutipes (F. velutipes) polysaccharides and Lentinan could elevate the relative abundance of Bacteroidetes and reduce the relative abundance of Firmicutes in the intestinal tract [24,25,26,27]. Supplementation with Pleurotus ostreatus polysaccharides could decrease the relative abundance of Proteobacteria, which regulated the innate immunity on Apostichopus japonicus [28]. G. lucidum polysaccharide increased levels of short-chain fatty acids (SCFAs)-producing bacteria such as Ruminococcus_1, Paraprevotella and Fusicatenibacter, and decreased levels of Escherichia-Shigella, Ruminococcaceae, Corynebacterium_1 and Sutterella, thereby reducing the animal disease activity index [29]. In conclusion, all these facts implied that mushroom polysaccharides could benefit the composition and metabolism of gut microbiota.
The biological activity of mushroom polysaccharides is largely affected by its monosaccharide composition, molecular weight and the type of glycosidic bond [30]. Therefore, it is of great significance to explore the relationship between the structure of mushroom polysaccharides and the regulatory activity of gut microbiota for the treatment of diseases. Mushroom polysaccharides that have been reported usually exhibit the biological activity of specific structures. Due to the complex and diverse structure of polysaccharides, it is difficult to draw conclusions about the structure-activity relationship of polysaccharides from “special” to “universal”. This paper displays the typical structure of five mushroom polysaccharides (Figure 1) and summarizes the common structural characteristics of mushroom polysaccharides with gut microbiota regulation activity (Table 1).
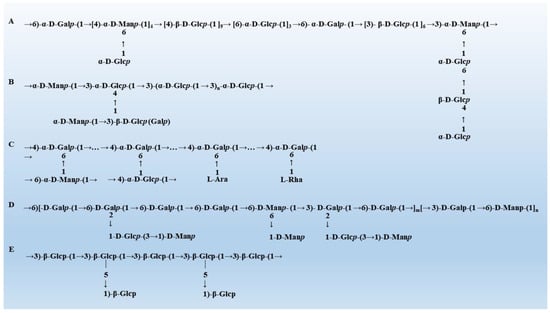
Figure 1.
The typical structure of mushroom polysaccharides. (A) The structure of Grifola frondosa [31]. (B) The structure of Hericium erinaceus [32]. (C) The structure of G. lucidum [33]. (D) The structure of Pleurotus eryngii [34]. (E) The structure of Lentinula edodes [35].

Table 1.
Effects of the source and structural features of mushroom polysaccharide on the regulation of gut microbiota.
2.1. Molecular Weight
The molecular weight is a key parameter for the activity of polysaccharides. The molecular weight of most polysaccharides was 10–800 kDa, but some polysaccharides had a molecular weight as high as 100,000 kDa or as low as 4 kDa. The molecular weight of Ramaria flava polysaccharide was 101.68 kDa, which could promote the production of acetic acid and propionic acid and stimulate the growth of Lactobacillus rhamnosus [38]. Tian et al. isolated three kinds of polysaccharides with different molecular weights from H. erinaceus. It was found that the polysaccharide with a molecular weight of 4 kDa was easily decomposed by gut microbiota, while the polysaccharide with a molecular weight of 823 kDa was hydrolyzed with difficultly [39]. Therefore, polysaccharides with a molecular weight that is either too large or too small are not conducive for the regulation of gut microbiota. However, there are exceptions to this inference. Helvella leucopus polysaccharide with an average molecular weight of 39.14 × 108 Da could significantly improve the abundance of Lactobacillus and decrease the abundance of Lachnospiraceae_NK4A136 and Lachnospiraceae_unclassified in DSS-induced colitis mice [40].
2.2. Monosaccharide Composition
On the basis of the monosaccharide composition, mushroom polysaccharides can be divided into glucan and heterosaccharide. Glucan is an isotype polysaccharide that is formed from glucose units by a glycosidic bond. It can be categorized as α- and β-glucan according to the type of glucoside bond [63]. Heterosaccharide is a polysaccharide composed of two or more different monosaccharides (glucose, galactose, mannose, xylose, etc.) [64]. Mushrooms rich in β-glucan have immunomodulatory activities [65]. Recent studies have shown that β-glucan could be fermented by the gut microbiome into SCFAs with immunomodulatory activity [66]. In addition, β-glucan can increase the number of beneficial bacteria such as Bifidobacterium and Lactobacillus, playing an important role in maintaining the balance of the gut microbiome [67]. Based on this conclusion, Saxami et al. emphasized that β-glucan-rich P. eryngii promoted the production of SCFAs and protected the integrity of the intestinal barrier [68]. The β-glucan in L. edodes has been shown to prevent cognitive impairment caused by a high-fat diet by improving the colon-brain axis [41]. For heterosaccharides, glucose, galactose and mannose are the most common monosaccharide residues, and they may play a pivotal role in the regulation of gut microbiota. Intestinal microorganisms can utilize P. eryngii polysaccharides that are rich in glucose (78.32%), galactose (8.47%) and mannose (9.43%) to produce acetic acid and propionic acid, while increasing the relative abundance of Firmicutes and decreasing the relative abundance of Proteobacteria and Bacteroidetes [43]. H. erinaceus polysaccharide, which consists of fructose, mannose, glucose and galactose, could increase the abundance of SCFAs-producing bacteria [39]. To date, no definitive conclusion has been drawn on the relationship between the regulation function of gut microbiota and monosaccharide composition. Notably, the activity of the regulating gut microbiota is higher when the monosaccharide composition of polysaccharides is more complex [69]. F. velutipes polysaccharide significantly promoted the proliferation of Bifidobacteriaceae, Bacteroidaceae, Lachnospiraceae and Enterococcaceae. However, G. lucidum polysaccharide only reduced the levels of Oscillospira and Desulfovibrionaceae, while Poria cocos sclerotium polysaccharide only increased the levels of Lachnospiracea and Clostridium [70,71]. The reason may be that Flammulina velatus polysaccharide is composed of glucose, mannose, xylose, fucose and galactose, while the monosaccharide of G. lucidum polysaccharide and Poria cocos sclerotium polysaccharide is mainly glucose.
2.3. Glycosidic Bonds
The relationship between the types and configurations of mushroom glucans glycosidic bonds and their immunomodulatory and antitumor activities has been proposed in many reports. The G. frondosa polysaccharides extracted by different methods had different structures, including α-1,6-, α-1,4-, β-1,6- and β-1, 3-glycosidic bonds, while (1→3, 1→6)-β-D-glucan is the main component with immunomodulatory and antitumor activities [72]. Both Ganoderma sinense glucan with main chains of (1→4)- and (1→6) -Glcp and Ganoderma leucocontextum glucan with main chains of →4)-α-D-Glcp-(1→4,6)-β-D-Glcp-(1→ have immunity-boosting effects [73,74]. L. edodes polysaccharide is a typical β-glucan with 1→3 link as the main chain and 1→6 link as the branch chain, which has significant immunomodulatory activity [75]. In addition, studies on the relationship between the types and configurations of heterosaccharides glycosidic bonds and the regulatory activity of gut microbiota have been reported. Xu et al. obtained a heteropolysaccharide (L2) with a molecular weight of 26 kDa from L. edodes, which was mainly composed of glucose and galactose, linked by 1→3 or 1→6 glycosidic bonds [36]. L2 could markedly increase the relative abundance of Proteobacteria, Bacteroides acidifaciens, Alistipes and Helicobacter suncus [27]. Furthermore, L2 could also reduce the abundance of age-related intestinal bacteria such as Bacilli, Betaproteobacteria, the Firmicutes/Bacteroidetes ratio and Lactobacillaceae [37]. The main chains of Sparassis crispa heteropolysaccharide (SCP-1) were (1→6)-α-D-Galp, (1→6)-β-D-Glcp and (1→3)-β-D-Glcp, and the side chains were (1→4)-β-D-Glcp, (1→3)-β-D-Glcp, T-α-L-Fucp and T-β-D-Glcp [45]. The SCP-1 has been shown to promote the production of SCFAs such as acetic acid, propionic acid and butyrate; elevate the levels of beneficial bacteria such as Dialister and Megasphaera; and inhibit the proliferation of harmful bacteria such as Escherichia/Shigella [44].
In conclusion, the complex structure of mushroom polysaccharides makes the relationship between mushroom polysaccharides and gut microbiota still in the preliminary stage. Although some studies have reported the relationship between the chemical structure of mushroom polysaccharides and the regulation of gut microbiota by mushroom polysaccharides, no general conclusions can be drawn. This paper suggests that the regulation effect of mushroom polysaccharides on gut microbiota is closely related to its molecular weight, monosaccharide composition and the type of glycosidic bond. It can be found that glucose, galactose, mannose and fucose were the monosaccharides that occurred frequently among the polysaccharides that possessed the regulatory function of gut microbiota, and the (1→3) and (1→6) linkage appeared frequently. Firmicutes, Lactobacillus and Bacteroides were the main regulated gut bacteria. However, the exact structure or the monosaccharides that stimulate specific gut bacteria have not yet been determined. Therefore, the relationship between them needs to be further studied.
3. Metabolism of Polysaccharides by Gut Microbiota
Polysaccharides are incapable of being decomposed and digested by saliva and under gastric and small intestinal conditions; hence, they are difficult to be absorbed by the body [76]. The reason is that only 17 polysaccharide digestive enzymes are encoded by the human genome; the remaining polysaccharide digestive enzymes are encoded by the microbes and their genomes in the human gut [77,78]. Polysaccharide digestive enzymes are responsible for the degradation and modification of polysaccharides, collectively known as carbohydrate-activated enzymes (CAZymes). According to the different catalytic mechanisms, CAZymes can be divided into six categories: glycoside hydrolases (GHs), polysaccharide lyases (PLs), carbohydrate esterases (CEs), glycosyltransferases (GTs), auxiliary activities (AAs) and carbohydrate-binding modules (CBMs) [79,80,81]. GHs and PLs are two types of enzymes that degrade glycosidic bonds, and contain 153 and 28 families, respectively. GHs breaks the glycosidic bonds between two or more carbohydrates and between carbohydrates and non-carbohydrates by inserting water molecules to degrade the main chain of carbohydrates. In contrast, PLs degrades the long chain of polysaccharides containing uronic acids through the β exclusion mechanism [82,83]. CEs remove polysaccharide ester groups and participate in carbohydrate side chain degradation [84]. CAZymes convert polysaccharides into a series of small-molecules that are easily absorbed, including SCFAs (acetic acid, propionic acid, butyric acid and iso-valerate acid), lipopolysaccharides and carbon monoxide [85]. The degradation of complex polysaccharides requires the cooperation of gut microbes, for example, the small molecule products formed by the degradation of inulin by Bacteroidetes ovatus can be further metabolized and utilized by Bacteroidetes vulgatus [86].
Figure 2 shows three polysaccharide degradation mechanisms of gut microbiota. The starch utilization system (Sus), ABC transport system and multienzyme complexes system are the main mechanisms for the degradation of polysaccharides by gut microbiota [87,88]. The Sus is an important way for Bacteroides to degrade polysaccharides. Sus R, as a transmembrane regulator, can detect the decomposition of polysaccharides. SusE and SusF proteins can recognize the polysaccharides and collect them at the cell surface. GHs decompose the polysaccharides into several oligosaccharides, which are bound by SusD protein and transported from the outer membrane into the periplasm by SusC protein. These oligosaccharides are further degraded into smaller oligosaccharides by GHs or PLs and further degraded into glucose by linking specific Sus A and Sus B before entering the cytoplasm [89]. Although Firmicutes have fewer genes encoding CAZymes, they encode more ABC transporters, phosphoenolpyruvate, carbohydrate PTS transporters, major facilitator superfamily transporters and glycoside-pentoside-hexuronide transporters to transport carbohydrates [90]. The multienzyme complexes system can efficiently degrade cellulose or resistant starch by interacting with dockerins and cohesins domains [91]. This system has been found in Ruminococcus champanellensis in human feces; dockerin is a small domain found in enzyme components and cohesin is a β-sandwich domain found in non-catalytic scaffold proteins that provides carbohydrate binding and/or cell wall anchoring functions [92]. These findings show that at least some Firmicutes in the microbiome have evolved to use cellulose in the human gut.
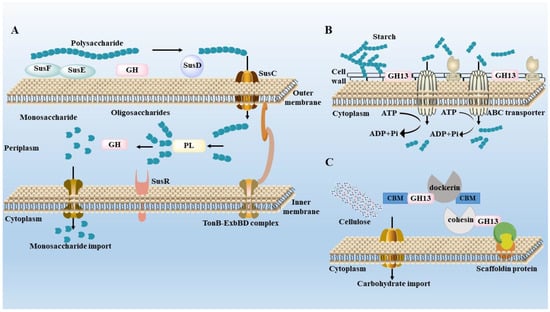
Figure 2.
Degradation mechanisms of gut microbiota. (A) Starch utilization system (Sus) in Bacteroides thetaiotaomicron. (B) ATP-binding cassette (A–C) transport system in Eubacterium rectale. (C) The multi-enzyme complexes system in Ruminococcus champanellensis.
4. Effects of Mushroom Polysaccharides on Gut Microbe-Mediated Diseases
4.1. Improvement of Lipid and Glucose Metabolism Disorders
4.1.1. T2DM
Figure 3 shows the mechanism of mushroom polysaccharides on common diseases including T2DM. T2DM accounts for more than 95% of diabetes cases, mainly manifested as abnormal levels of glucose and lipids and low-intensity inflammation in both the blood and the liver [93]. The pathogeny of T2DM is due to dietary issues, physical inactivity, being overweight and being a smoker [94]. The common treatments for T2DM include insulin injections, oral sulfonylureas and biguanides drugs, and operative treatment [95]. However, these therapies may cause different degrees of side effects that greatly reduce the quality of life.
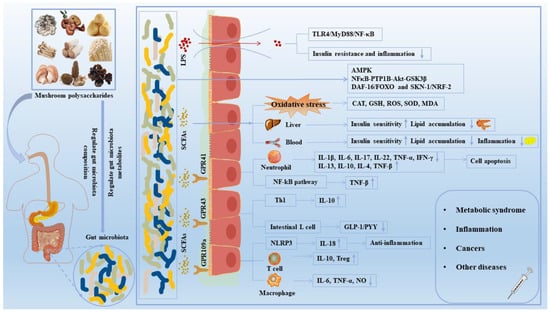
Figure 3.
The effect of mushroom polysaccharides on human diseases by the regulation of gut microbiota. Mushroom polysaccharides could regulate the composition and relative abundance of gut microbiota and promote the production of SCFAs. SCFAs bond to receptors such as GPR41, GPR43 and GPR109A, activating downstream NF-κB, MAPK and other signaling pathways, thereby improving the intestinal mucosal barrier and maintaining intestinal homeostasis. In addition, SCFAs exerted an anti-diabetic and anti-obesity role by alleviating oxidative stress damage and regulating liver and blood biochemical indexes. Notably, harmful gut microbiota induced inflammation and insulin resistance by stimulating LPS secretion and activating TLR4/MyD88/NF-κB pathways. ↑, increased; ↓, decreased; CAT, catalase; GLP-1, glucagon-like peptide-1; GPR 109a, G protein-coupled receptor 109A; GPR 43, G protein-coupled receptor 43; GPR 41, G protein-coupled receptor 41; GSK3β, glycogen synthase kinase 3β; GSH, glutathione; IFN-γ, interferon-γ; IL, interleukin; LPS, lipopolysaccharide; MDA, malondialdehyde; MyD88, myeloid differentiation factor 88; NF-κB, nuclear factor-κB; NO, nitrogen monoxide; PYY, peptide YY; ROS, reactive oxygen species; SCFAs, short-chain fatty acids; SOD, superoxide dismutase; Th, helper T cell; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor-α.
Mushroom polysaccharides could improve insulin resistance and promote gastrointestinal health by promoting the abundance of healthy bacteria and suppressing the abundance of harmful bacteria [96]. Table 2 summarizes the regulatory effects of typical mushroom polysaccharides on gut microbiota composition in chronic diseases, including T2DM. Akkermansia muciniphila accounts for 0.5–5% of the human intestinal tract, but it accounts for a lower proportion in obese and diabetic patients. This decrease results in damage to the intestinal barrier function and causes intestinal inflammation [97]. G. frondosa polysaccharides could reverse the level of Akkermansia in T2DM mice to inhibit the development of inflammation [60]. Moreover, Armillariella tabescens polysaccharides up-regulated the levels of Lactobacillus and Ackermanella to reduce lipopolysaccharide (LPS) content and improve intestinal barrier function, thereby reducing systemic inflammation in T2DM mice [98]. G. lucidum F31 increased the Bacteroidetes/Firmicutes ratio to improve glucose metabolism disorder and enriched SCFAs-producing bacteria including Lactobacillus, Bacteroides and Ruminococcaceae to improve the integrity of the intestinal barrier [99,100]. Thus, G. lucidum F31 could alleviate insulin resistance and inflammation by reducing the release of LPS from the gut into circulation [101]. Resistant starch encapsulated G. lucidum spores resulted in a synergistic hypoglycemic effect by enhancing glycolipid metabolism, insulin secretion and glycogen synthesis to reduce adipogenesis [102]. Moreover, G. lucidum polysaccharide-Chromium (III) complex (900 mg/kg day) supplements decreased the relative abundance of Streptococcus, Enterococcus and Alistipes and increased the relative abundance of Enterorhabdus, Coriobacteriaceae and Micrococcaceae, improving serum biochemical parameters, insulin sensitivity and glucose tolerance [103].
SCFAs were closely associated with the occurrence and development of T2DM and related metabolic diseases, which could protect the intestinal mucosal barrier, reduce inflammation levels and stimulate gastrointestinal movement [104]. SCFAs are mainly produced by specific gut microbiota through fermenting dietary fiber, including acetic acid, propionic acid, butyric acid and valeric acid [105]. The hypoglycemic mechanism of Astragalus polysaccharide was to decrease acetic acid and propionate concentration and increase butyric acid concentration [106]. G. lucidum sporoderm-broken spore polysaccharide increased the production of butyrate and acetate in high-fat-diet (HFD) mice [107]. The elevated intake of dietary fibers can increase the numbers of beneficial bacteria and then induce the production of SCFAs to activate G-protein coupled receptor (GPR43), comprehensively improving the blood glucose homeostasis of patients with T2DM [108]. Fomitopsis castaneus Imaz exopolysaccharides increased the contents of SCFAs in the digestive system of children, especially for butyric acid.
Impaired glucose tolerance and impaired fasting glucose referred to an intermediate transition state between the normal condition and diabetes, with a high risk of T2DM. Phellinus linteus polysaccharides significantly reduced fasting blood glucose levels and improved oral glucose tolerance by increasing the ratio of phosphatidylcholine to phosphatidyl ethanolamine and the ratio of S-adenosylmethionine to S-adenosylhomocysteine [109]. Oral G. frondosa polysaccharides reduced serum fasting blood glucose, oral glucose tolerance, cholesterol, triglyceride, and low-density lipoprotein cholesterol levels, as well as levels of cholesterol, triglyceride, and free fatty acids in the liver. The relative abundance of Streptococcus, Enterococcus, Staphylococcus and Pneumococcus decreased with high concentration of G. frondosa polysaccharides [20]. These results indicated that Streptococcus, Enterococcus, Staphylococcus, and Pneumococcus were positively correlated with serum and liver lipid biochemical parameters, further proving a close relationship between metabolic parameters and intestinal microbiota. Auricularia auricula polysaccharides alleviated insulin resistance and improved blood glucose stability through restoring amino acid metabolism, lipid metabolism, bile acid synthesis and glycerophospholipid pathways [110].
Many studies revealed that mushroom polysaccharides regulated glucose and lipid metabolism by activating related protein expression in metabolic pathways. Auricularia auricula-judae polysaccharides improved glucose-lipid metabolism disorders by activating protein kinase B (AKT) and adenosine monophosphate-activated protein kinase (AMPK) signaling pathways in T2DM mice [111]. Using 55% ethanol extraction for G. frondose inhibited the liver stearoyl-Coenzyme A (CoA) desaturase 1, sterol regulatory element-binding transcription factor-1c and acetyl CoA carboxylase signaling pathways by increasing the levels of AKT1, glucokinase, AMPK-α and cholesterol 7-α hydroxylase, thereby improving glucose and lipid metabolism disorders [112]. Therefore, regulation of specific signaling pathways could reverse blood glucose, blood lipids and the liver index as well as improve the gut microbiota, thus resolving metabolic disorders of the liver.

Table 2.
The modulation of gut microbiota in metabolic disorders by mushroom polysaccharides.
Table 2.
The modulation of gut microbiota in metabolic disorders by mushroom polysaccharides.
| Disease | Mushroom | Model | Gut Microbiota Regulation | Effects on Hosts & Functional Mechanisms | Ref. |
|---|---|---|---|---|---|
| T2DM | Grifola frondosa | STZ-induced KM mice | Alistipes↑ Streptococcus, Enterococcus, Staphylococcus and Aerococcus↓ | Reduced the serum levels of FBG, OGT, TC, TG and LDL-C, and decreased the hepatic levels of TC, TG and FFA; increased mRNA expression of CYP7A1 and BSEP. | [20] |
| T2DM | Ganoderma lucidum | STZ-induced mice | Blautia, Dehalobacterium, Parabacteroides and Bacteroides↑ Aerococcus, Ruminococcus, Corynebactrium and Proteus↓ | Decreased the levels of fasting blood glucose and insulin; restored the amino acids metabolism, carbohydrates metabolism, inflammatory substances metabolism. | [51] |
| Hyperlipidemia | Grifola frondosa | HFD-induced Wistar rats | Helicobater, Intestinimonas, Parasutterella, Ruminococcus and Flavonifracter↑ Clostridium-XVIII, Butyricicoccus and Turicibacter↓ | Through decreasing the serum TG, TC, and FFA levels, and increasing the serum HDL-C level; increased the mRNA levels of BSEP, CYP7A1, Acox1 and hepatic GS. | [59] |
| T2DM | Grifola frondosa | STZ-induced ICR mice | Porphyromonas gingivalis, Akkermansia muciniphila, Lactobacillus acidophilus, Bacteroides acidifaciens↑ Firmicutes/Bacteroidetes ratio and Proteobacteria↓ | Decreased the fasting blood glucose level, improved oral glucose tolerance, alleviated insulin resistance; activated IRS1, PI3K, and GLUT4, inhibited JNK1/2; regulated the IRS1/PI3K and JNK signaling. | [60] |
| T2DM | Ganoderma lucidum | HFD-induced and STZ-induced KM mice | Ruminococcaceae, Prevotellaceae and Peptococcaceae↑ Lachnospiraceae, Desulfovibrionaceae and Lactobacillaceae↓ | Repaired islet cells and increased insulin secretion, improved insulin resistance, and improved carbohydrate metabolism, amino acid metabolism and lipid metabolism. | [101] |
| T2DM | Ganoderma lucidum | STZ-induced SD rats | Lactobacillus↑ Proteobacteria↓ | Promoted the expression of GS2, GYG1, Insig1, Insig2, ACC; elevated the level of HDL-C and reduced levels of TC and TG. | [102] |
| T2DM | Grifola frondosa | STZ-induced KM mice | Alistipes↑ Streptococcus, Enterococcus, Staphylococcus and Aerococcus↓ | Improved abnormal serum biochemical indicators TG, TC, LDL-C and glucose, inhibited lipid accumulation and steatosis; downregulated CD36 and SREBP-1C, upregulated CYP7A1. | [103] |
| Obesity | Ganoderma lucidum | HFD-induced C57BL/6J mice | Akkermansia, Bifidobacterium, Turicibacter, Parabacteroides↑ Blautia, Rikenella, Ruminiclostridium_UGC-009 and Lachnospiraceae↓ | Inhibited fat accumulation and body weight, hyperlipidemia; reduced LPS level; decreased levels of TNF-α and IL-1β; increased acetate and butyrate production; inhibited LPS/TLR4/NF-κB signaling pathway. | [107] |
| T2DM | Grifola frondosa | STZ-induced ICR mice | Lactobacillus, Desulfovibrio, Helicobacter, Lactobacillus and Bacteroides↑ Verrucomicrobia, Ruminococcus and Prevotella↓ | Improved the composition of gut microbiota and promoted the proliferation of beneficial bacteria. | [113] |
| Obesity | Agrocybe cylindracea | HFD-induced C57BL/6J mice | Bacteroides, Parabacteroides, Butyricimonas and Dubosiella↑ Desulfovibrio and Oscillibacter↓ | Reduced the levels of obesity-related TNF-α and IL-6, reduced fasting glucose and insulin levels. | [114] |
| T2DM | Sanghuang- porous vaninii | high-fat and high-sucrose ICR mice | Akkermansia, Dubosiella, Bacteroides and Parabacteroides↑ Lactobacillus, Flavonifractor, Odoribacter and Desulfovibrio↓ | Improved body weight, glycolipid metabolism, and inflammation-related parameters; ameliorated pancreas and jejunum injuries; enriched insulin signaling pathway and PI3K-Akt signaling pathway. | [115] |
| Obesity | Grifola frondosa | HFD-induced C57BL/6JNju mice | Mucispirillum, Bilophila and Dehalobacterium, Sutterella↑ Coprococcus and Ruminococcus↓ | Controlled the body weight, blood glucose and related organ indices, counteracted hyperlipidemia and IR triggered; regulated AST and ALT; down-regulated TLR4/NF-κB signaling. | [116] |
| NAFLD | Lentinan | HFD-induced C57BL/6J mice | Bifidobacterium, Streptococcaceae and Enterococcaceae genus, Streptococcus, Enterococcus, Ruminococcaceae↑ Helicobacteraceae and Helicobacter↓ | Restored intestinal redox balance, and reduced serum LPS; altered inflammation-insulin (NFκB-PTP1B- Akt-GSK3β) signaling molecules. | [117] |
| NAFLD | Grifola frondosa | HFD-induced Wistar rats | Bacteroides, Bifidobacterium, Blautia, Coprococcus, Phascolarctobacterium, Prevotella↑ Alistipes, Flavonifractor, Paraprevotella and Oscillibacter↓ | Modulated the expression of specific gene related to lipid synthesis and conversion, CYP4A1, ACC, TNF-α, SOCS2 and CYP7A1; reduced hepatocyte steatosis and liver cell injury. | [118] |
| Hyperlipidemia | Auricularia auricular | HFD-induced SD rats | Firmicutes, Roseburia, Flavonifractor and Clostridium IV↑ Bacteroidetes↓ | Reduced the levels of TC and LDL-C; induced the significant growth of SCFA-producing bacteria and the accumulation of SCFAs concentrations. | [119] |
| Obesity | Pleurotus eryngiion | HFD-induced C57BL/6J mice | Lactococcus↑ Roseburia↓ | Suppressed fat accumulation; decreased LDL-C; increased fecal bile acids; increased the concentration of SCFAs. | [120] |
| T2DM | G. frondosa | HFD-induced SD rats | Bacteroidetes/Firmicutes, Lactobacillus and Turicibacter↑ Prevotella and Bifidobacterium↓ | Decreased the expression levels of TNF-α, IL-1β and IL-6; alleviated inflammation by the TLR4/MyD88/NF-κB pathway. | [121] |
| Obesity | Ganoderma lucidum | HFD-induced C57BL/6 mice | Bacteroides spp., Anaerotruncus colihominis, Clostridium↑ Enterococcus spp., Lactococcus lactis and Oscillibacter valericigenes↓ | Improved gut barrier integrity, reduced endotoxemia, decreased TLR4 signal and inflammation; reduced the number of macrophages. | [122] |
| Hypercholesterolemia | Ganoderma lucidum | HCD-induced C57BL/6 mice | Faecalibacterium prausnitzii, Lactobacillus and Prevotella↑ Bacteroides acidifaciens, Mucispirillum schaedleri and Parabacteroides distasonis↓ | Prevented FA synthesis and accumulation through down-modulating genes involved in lipogenesis, elongation and desaturation; activation of PPARs, fatty acid oxidation and bile acid conversion. | [123] |
| Hyperlipidemia | Ganoderma lucidum | HFD-induced Syrian golden hamsters | Ruminococcus, Oscillibacter, Bifidobacterium, Prevotella and Alistipes↑ Desulfovibrio, Clostridium↓ | Alleviated the serum levels of TG, TC, and LDL-C; decreased the serum levels of AST; increased beneficial bacteria and reduced harmful bacteria. | [124] |
Note: ↑, increased; ↓, decreased; ACC: acetyl CoA carboxylase; Acox1: acyl-Coenzyme A oxidase 1; AKT, protein kinase B; ALT: alpha-alanine aminotransferase; AST: aspartate aminotransferase; BSEP: bile salt export pump; CYP4A1: cholesterol 4 alpha-hydroxylase; CYP7A1: cholesterol 7 alpha-hydroxylase; FBG: fasting blood glucose; FFA: free fatty acids; GLUT4: glucose transporter 4; GSK3β, glycogen synthase kinase 3β; GS2: glycogen synthase 2; GYG1: glycogenin-1; HDL-C: high-density lipoprotein cholesterol; HFD: high-fat diet; IL-6: interleukin-6; IL-1β: interleukin-1β; IR: insulin resistance; IRS1: insulin receptor substrate 1; JNK, c-Jun N-terminal kinase; KM, kunming; LDL-C: low-density lipoprotein cholesterol; LPS: lipopolysaccharide; MyD88: myeloid differentiation factor 88; NF-κB: nuclear factor-κB; OGT: oral glucose tolerance; PI3K: phosphatidylinositol 3 kinase; PPAR: peroxisome proliferator-activated receptors; Ref: reference; SCFA: short-chain fatty acid; SOCS2: suppressors of cytokine signaling 2; SREBP-1c: sterol regulatory element-binding protein-1c; STZ: streptozocin; T2DM, type 2 diabetic mellitus; TC: total cholesterol; TG: triglyceride; TLR4: toll-like receptor 4; TNF-α: tumor necrosis factor-α.
4.1.2. Obesity
Obesity is a serious public health issue affecting everyone, characterized by metabolic disorders accompanied by changes in gut microbiome composition and diversity [125]. It is defined as abnormal or excessive fat accumulation, mainly caused by a sedentary lifestyle and an imbalance between energy intake and consumption. Being obese or overweight is associated with an increased risk of many chronic diseases such as T2DM, hypertension, cardiovascular disease and hyperlipidemia, so it is crucial to elucidate how to prevent obesity in modern medical research [126]. Notably, improving diet and lifestyle or pharmacotherapy may be the options for treating obesity, but these methods may not have the desired anti-obese effect. Surgical treatment still has certain limitations and risks [127].
Changes in gut microbiota composition were closely associated with the development of obesity and related metabolic diseases [128]. A large number of clinical and experimental studies showed that gut microbiota plays a key role in the occurrence and development of obesity by regulating the host’s energy metabolism, substrate metabolism and inflammatory response. The content of LPS-producing Proteobacteria and the proportions of Firmicutes and Bacteroidetes in HFD-induced mice were significantly higher than those of normal-fed mice [129]. Among them, Bacteroidetes and Firmicutes have a mutually reinforcing symbiotic relationship that can jointly promote host intestinal energy absorption or storage. Thus, gut microbiota is a promising strategy for improving obesity and related metabolic disorders.
Polysaccharides could reduce weight by inhibiting the growth of obese gut microbiome and increasing the level of microbiota-derived metabolites [130]. The effects of Agrocybe cylindracea polysaccharide on adipose accumulation and weight loss in HFD-induced mice have been reported, and it was found that Desulfovibrio was decreased and Parabacteroides was increased, which markedly reduced the levels of obesity-related tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) [114]. Sanghuangporous vaninii ethanol extract observably improved body weight and glucose and lipid metabolism by elevating the relative abundance of Akkermansia, Dubosiella, Bacteroides and Parabacteroides and reducing the relative abundance of Lactobacillus, Flavonifractor, Odoribacter and Desulfovibrio. Furthermore, it enriched glycerolipid metabolism, insulin signaling pathway and fatty acid degradation in T2DM-induced mice [115]. G. lucidum sporoderm-broken spore polysaccharides significantly inhibited weight in HFD mice after treatment for 9 weeks. Its underlying mechanisms were increased levels of SCFAs, improved intestinal barrier function, reduced endotoxemia and increased production of beneficial bacteria. The regulation of gut microbiota was mainly manifested by G. lucidum sporoderm-broken spore polysaccharide that reversed the relative abundance of many bacteria in HFD-fed mice, particularly some potential probiotics including Allobaculum and Bifidobacterium, which showed a positive correlation with anti-obesity [107]. Bifidobacterium, Lactobacillus and Akkermansia could promote the production of SCFAs and inhibit the abundance of Clostridiaceae, Desulfovibrio and Enterococcus, which would help reduce body weight and lipid accumulation [131]. Butyric acid in SCFAs may alleviate diet-induced obesity, and another study showed that adding butyric acid to the diet can prevent diet-induced obesity by promoting the transition from adipogenesis to lipid oxidation [132]. Inconsistently, one study showed that butyric acid can promote energy absorption and thus contribute to the development of obesity [133].
Ganoderma applanatum polysaccharides significantly reduced total cholesterol, triglycerides, low-density lipoprotein cholesterol levels and atherosclerotic index in obese rats, improving the symptoms of dyslipidemia [134]. The water-soluble polysaccharide components of P. eryngii reduced adiposity and cholesterol via inhibiting the intestinal circulation of bile acids and up-regulating the mRNA levels of sterol-regulatory element binding proteins 2 and its target gene low-density lipoprotein receptor in the liver [135]. Both G. lucidum polysaccharide and G. frondosa polysaccharides could significantly improve blood glucose and organ indexes of HFD mice and reverse the changes in gut microbiota caused by obesity. G. frondosa polysaccharides promoted lipid metabolism and homeostasis by activating Decay Accelerating Factor16/Forkhead Box O and SKN-1/nuclear factor erythroid 2-related factor 2 (Nrf2) signal pathways or inhibiting Toll-like receptor 4 (TLR4)/NF-κB signal transduction, all of which are associated with inflammation and insulin resistance [116,136]. G. lucidum polysaccharides inhibited HFD-induced splenic lymphocyte apoptosis by reducing the ratio of Bax (Bcl2-associated X protein)/Bcl-2 (B-cell lymphoma-2) and inhibiting the activation of caspase-3 [19]. Astragalus polysaccharides regulated Cux1 by Mir-1258-5p to promote the differentiation of C3H10T 1/2 cells into brown adipocytes, increasing energy consumption; this may be used as a treatment for obesity [137]. Volvariella volvacea polysaccharides reduced fat accumulation by regulating the AAK-2/NHR-49 mediated fatty acid synthase pathway and the ACS-2 mediated fatty acid oxidation pathway [138].
4.1.3. NAFLD
NAFLD is the most common chronic liver disease and is generally considered a common metabolic disorder closely related to obesity. Previous research even indicated that overweight or obese subjects lost at least 5% of their body weight was the only effective strategy for treating NAFLD and non-alcoholic steatohepatitis [139]. The pathogenesis of NAFLD was described as a “two-hit” hypothesis. Firstly, insulin resistance promotes excessive hepatic fat accumulation, leading to liver sensitivity. The secondary pathogenic damage of oxidative stress is that it induces inflammation and cell death that are also caused by gut microbiota changes [140]. An imbalance in the relative abundance of Bacteroidetes and Firmicutes has been found in NAFLD patients, which leads to an increase in microbial metabolites such as SCFAs, lipopolysaccharides and secondary bile acids that can cross the intestinal barrier and reach the liver, contributing to inflammation and disease progression [141,142].
Mushrooms could promote intestinal health by decreasing intestinal lipid uptake and influencing intestinal microbiota. Agaricus bisporus mushroom positively affected human intestinal health by increasing the abundance of Bacteroidetes and decreasing the abundance of Firmicutes [143]. Lentinan increased levels of phylum Actinobacteria and decreased the levels of phylum Proteobacteria and Epsilonbacteraeota, alleviating intestinal microbiota disorder in HFD mice. Moreover, it also improved NFκB-PTP1B-Akt-GSK3β (inflammation-insulin) signaling pathways to prevent hepatic steatohepatitis [117]. G. frondosa polysaccharide significantly increased the composition of beneficial bacteria, especially Alistipes, Flavonifractor and Oscillibacter, which may play an important role in preventing NAFLD [118]. In addition to the above-mentioned edible fungi, the H1 component of the Hirsutella sinensis polysaccharide reversed the dysbacteriosis caused by HFD, and promoted the number of neomycin-sensitive bacteria such as Parabacteroides. goldsteinii mycelium, which indirectly improved glycolipid metabolism disorders [144]. Cauliflower mushroom extract combined with the fermented fungus JS can alleviate the lipid metabolism disorders of rats treated with ovarian resection; the changes of intestinal flora and menopausal symptoms caused by estrogen deficiency, such as tail skin temperature increase, visceral fat volume increase, dyslipidemia and glucose intolerance, all of which were similar effects compared with positive drugs [145].
Mitochondria play a central role in the progression of NAFLD and mainly control cell death signaling. A chronic imbalance between lipid anabolism and catabolic processes in the liver leads to mitochondrial dysfunction [146]. Mushroom ingestion may alleviate the metabolic burden of liver mitochondria through reducing insulin resistance and hepatic steatosis. Bletilla striata polysaccharide showed great potential in treating NAFLD, which markedly regulated the liver metabolism of fatty acids, arachidonic acid and other related metabolites in HFD-fed mice, and reduced lipid accumulation and fibrosis in liver tissues [147]. Antrodia cinnamomea alleviated oxidative stress and inflammation to inhibit fat production by up-regulating aldehyde dehydrogenase 2 activity and accelerating the elimination of reactive oxygen species [148].
4.2. Immunoregulation Effects
Changes in gut microbiota are closely related to the immune system and other inflammatory conditions. There is a growing awareness that the microbiome is crucial for regulating the immune system. IBD is a chronic, multifactorial, high-incidence gastrointestinal inflammatory disease that includes ulcerative colitis and Crohn’s disease [149]. The etiology of IBD may be microbial symbiosis and immunity, intestinal barrier rupture, oxidative stress and DNA damage [150,151]. Various factors such as dietary patterns, environmental changes, genetic predispositions and adaptive immune responses can contribute to IBD [152]. A high-fat diet or lack of dietary polysaccharides in the diet promoted an imbalanced gut microbiome, which degraded host mucosal polysaccharides, destroyed the intestinal mucosal barrier and increased the secretion of LPS. LPS can enter the circulatory system through the mesenteric veins and act on target organs and tissues, causing intestinal inflammation or inducing colorectal cancer [153]. Current pharmacologic treatments for IBD mainly include aminosalicylates, glucocorticoids, immunosuppressants, biologics and novel small molecule drugs, but they all have certain limitations in terms of efficacy and safety [154]. Thus, treatment of IBD with safe, low-toxicant and efficient mushroom polysaccharides has been widely explored.
Table 3 exhibits the regulatory effects of typical mushroom polysaccharides on gut microbiota composition in IBD and other inflammatory models. Previous studies found that Lactobacillus spp. could alleviate DSS-induced colitis by modulating gut microbiota composition and stimulating natural killer (NK) cells, macrophages and T lymphocytes [155]. Bifidobacterium breve relieved DSS-induced colitis by reducing the levels of TNF-α, interleukin-1β (IL-1β), IL-6 and partially restoring the unbalanced gut microbiome [156]. Other studies also verified that anti-inflammatory microorganisms such as Lactobacillus spp., Bacteroides, Bifidobacterium and Prevoella were significantly reduced in trinitro-benzene-sulfonic acid-induced rat colon, while pro-inflammatory bacteria such as Corynebacteriums, Staphylococcus and Rumencoccus were enriched [157]. However, a water-soluble G. lucidum mycelium extract could reverse this gut microbiota imbalance [158]. Some other studies also indicated that G. lucidum polysaccharides improved the composition of intestinal microbiota in DSS-induced mice by reducing the abundance of Escherichia/Shigella, Enterococcus and Staphylococcus [159].

Table 3.
The modulation of the gut microbiota in immunological diseases by mushroom polysaccharides.
Different mushroom polysaccharides showed different immunomodulatory pathways in IBD. G. lucidum polysaccharides attenuated inflammatory factors in LPS-activated macrophages in mice; this anti-inflammatory effect was mediated by inhibiting NF-κB and MAPK signaling pathways [167]. Another study used Lentinan-loaded Budesonide (LNT/BuD-NPS) to treat ulcerative colitis and significantly alleviated inflammation by inhibiting the TLR4/MyD88/NF-κB signaling pathway [168]. SCFAs possessed the function of improving the intestinal mucosal barrier and stimulating the production of immunosuppressive cytokines [152]. The immunomodulatory effects should be attributed to SCFAs, which acted as signaling molecules to regulate and maintain the host’s immune system [169]. Bacteroides spp. fermented polysaccharides could produce SCFAs such as acetic acid, propionic acid and butyric acid that inhibited the production of pro-inflammatory cytokines, enhanced the expression of interleukin-10 (IL-10) and activated Treg cells, all of which were important in improving chronic inflammatory diseases and promoting colon cell health [170]. Acetic acid and butyric acid in SCFAs can activate GPR41 and GPR43 and inhibit histone deacetylase activity to exert anti-inflammatory effects [171].
In addition to intestinal inflammation, other inflammatory diseases also affect the microbiome homeostasis in the intestinal tract. Increasing the activity of antioxidant enzymes can protect oxidative damage to the pancreas caused by the proliferation of Helicobacter pylori [172]. Thus, increased oxidative stress was thought to be the pathogenesis of pancreatitis. Selenium-lentinan intake could enhance the activity of superoxide dismutase and glutathione peroxidase to alleviate oxidative stress [173]. G. lucidum strain polysaccharides alleviated oxidative stress by inhibiting the overgrowth of Bacteroides, Prevotalles and Helicobacter in mice induced by diethyldithiocarbamate [160]. Bacteroides were negatively correlated with lipase and trypsin, while Firmicutes were negatively correlated with glutathione peroxidase. Inonotus obliquus polysaccharides increased the relative abundance of Bacteroides and decreased the abundance of Firmicutes in pancreatitis mice, thereby reducing levels of serum pro-inflammatory cytokines [25].
Polysaccharides affect the number of intestinal immune cells by regulating the composition of gut microbiota, and then affect the secretion of cytokines such as immunoglobulin interleukin-1α (IL-1α) and interleukin-2 (IL-2), which play a crucial role in the mucosal immune system. G. lucidum polysaccharides significantly reduced the proportion of Firmicuteum and Bacteroidetes, and increased the levels of immune interferon-γ (IFN-γ), IL-2, interleukin-4 (IL-4) and other serum cytokines that strengthen the intestinal barrier [174]. Lentinan can increase the diversity of gut microbiota; reduce the ratio between Firmicutes and Bacteroidetes; promote the proliferation of splenic lymphocytes in vitro; increase the levels of TNF-α, IL-1α and IL-2; and enhance the immunity of elderly mice [37]. Poria cocos polysaccharides reduced chronic prostatitis by regulating the levels of anti-inflammatory and pro-inflammatory factors [175].
4.3. Antitumor Effects
Appropriate regulation of the immune response could reduce the risk of pathogen invasion caused by inflammatory responses and thus maintain a healthy gastrointestinal system [176]. However, excessive immune regulation can disrupt intestinal homeostasis and promote the metastasis of normal cells to malignant cells [177]. Since gut microbiota is closely associated with the inflammatory response, gut microbiota may also be indirectly involved in carcinogenesis by regulating local and systemic immune responses. Currently, mainstream cancer therapeutic approaches include chemotherapy, radiation therapy and surgery [178]. These traditional treatments cause side effects, but their safety and efficacy are still questionable.
Changes in gut microbiota have been reported to affect the incidence of cancer [179]. Some gut microbiota can also be used as predictive biomarkers for the early detection of cancers. Many well-defined species that promoted the development of colorectal cancer include Fusobacterium spp., Streptococcus bovis, Bacteroides fragilis, superoxide-producing Enterococcus. faecalis, Streptococcus gallolyticus, Peptostreptococcus spp., and Porphyromonas spp. [180]. Helicobacter pylori has been identified as a Class I carcinogen for stomach cancer [181]. Enterobacteriaceae was associated with human colon carcinogenesis [182]. Ingestion of a probiotic Lactobacillus Scidophilus could inhibit mouse tumor growth [183]. Bifidobacteria has beneficial effects on the host and improves tumor-specific immunity by enhancing dendritic cell functions [184].
In recent years, increasing research has focused on the antitumor effect of mushroom polysaccharides. However, only a few studies have focused on the interaction between polysaccharides and cancer based on gut microbiota which mainly reported the treatment of breast cancer and colon cancer with G. lucidum polysaccharides [185]. G. lucidum spore extract (ESG) reshaped the intestinal microbiota in 4T1 tumor-bearing mice: there was an increase in the relative abundance of Firmicutes and Proteobacteria and a decrease in the relative abundance of Actinobacteria, Bacteroidetes and Cyanobacteria [186]. In the same year, another study showed similar conclusions that G. lucidum polysaccharides combined with paclitaxel exerted an antitumor effect on 4T1 breast tumor-bearing mice. The combined treatment significantly enriched five genera such as Bacteroidetes and Ruminococcus and reduced the abundance of Desulfovibrios and Odoribacter, which balanced intestinal flora and inhibited tumor metabolism [187]. G. lucidum polysaccharides regulated the relative abundance of beneficial bacteria such as Lactobacillus and Bifidobacterium to induce the production of SCFAs, improving intestinal barrier damage and inhibiting the TLR4/MyD88/NF-κB signal pathway, thereby reducing the risk of colitis and carcinogenesis [161]. Gynostemma pentaphyllum combined with G. lucidum polysaccharides markedly promoted the abundance of SCFAs-producing bacteria, elevated butyrate and iso-butyrate levels and suppressed the abundance of sulfate-reducing bacteria [188]. The abundance of Oscillospira was higher in colorectal cancer mice, G. lucidum polysaccharides could reverse the abundance of this bacterium and also reduce the number of Desulfovibrionaceae. In addition, four cancer-related genes including Acaa1b, Fabp4, Mgll and stearoyl-CoA desaturase 1 were down-regulated [70]. Thus, reducing specific bacteria, rather than ensuring recovery of the overall microbial diversity and regulation of cancer-associated genes, was the mainspring for alleviating colorectal cancer. Carboxymethylated Poria alleviated colon injury induced by 5-fluorouracil in CT26 tumor-bearing mice. The diversity of gut microbiota was restored by increasing the proportion of Bacteroidetes, Lactobacillus and butyric acid- and acetic acid-producing bacteria. In addition, Carboxymethylated Poria also increased the levels of Nrf2 and Bcl-2 and decreased the levels of NF-κB, P-P38 and Bax [189]. In conclusion, mushroom polysaccharides remodeled the gut microbiota and inhibited tumor growth, possibly due to the secondary metabolites produced by gut microbiota selectively fermented mushroom polysaccharides.
SCFAs are important signaling molecules and the end-products of metabolic reactions that could reduce the risk of colorectal cancer by regulating the immune system and improving the integrity of the intestinal barrier [190]. Targeted changes in the proportion of SCFAs will affect the relationship between gut microbiota and host health [191]. Degradation of macromolecular carbohydrates to produce SCFAs is one of the main mechanisms of the gut microbiota for inhibiting tumor growth [192]. Indigestible mushroom polysaccharides that are fermented by gut microbiota could produce SCFAs and further regulate the intestinal microenvironment. It had been reported that propionate and n-butyrate possessed anti-tumor properties [193]. F. velutipes polysaccharides increased the content of bacterial metabolites such as acetic acid, butyric acid and propionic acid that promoted the development and maintenance of the immune system, thus reducing intestinal inflammation and the occurrence of tumors [26]. One study suggested the antitumor effects of polysaccharides from G. lucidum and G. Sinense, which had similar effects and mechanisms in inducing macrophage phagocytosis, producing cytokines and inhibiting the activity and migration of breast cancer cells. Their anti-tumor activity was verified by enriching Alistipes that produced SCFAs, which activated the TLR4-related MAPK/NF-κB signaling pathway [194]. However, the anti-tumor mechanisms of polysaccharides still need further research.
4.4. Other Beneficial Effects
With increasing research on the gut microbiota, dysregulation of the gut microbiota affected not only intestinal diseases, metabolic diseases and cancers, but also induced psychiatric disorders such as Parkinson’s disease (PD) and Alzheimer’s disease (AD) [195]. Dysregulation of the gut microbiome could increase intestinal permeability and systemic inflammation, which may induce learning, memory and other cognitive impairments through the “microbio-gut-brain” axis [196]. A recent study found significant differences in the abundance of gut microbiota in AD patients at the phylum level compared to those with healthy gut microbiota, with a decrease in Firmicutes and an increase in Bacteroides [197]. Zhang et al. showed that Sparassis crispa polysaccharide may play an anti-AD role by reshaping the gut microbiota composition and inhibiting inflammation. In terms of regulating the gut microbiota, the relative abundance of ventriosum group, Lachnospiraceae_UCG_010, and Lachnospiraceae_UCG_001 was increased, and the growth of Escherichia/Shigella was inhibited [198]. Gao et al. proposed that Cistanche deserticola polysaccharide improved cognitive function in D-galactose-induced aging mouse models by restoring gut microbial homeostasis, thereby reducing oxidative stress and peripheral inflammation [199]. Another study showed that a galactoglucan isolated from Cistanche deserticola could promote the growth of Bacteroides and Lactobacillus [200]. F. velutipes polysaccharides enhanced scopolamine-induced learning and memory impairment by mediating intestinal flora and inhibiting inflammation. The results showed that F. velutipes polysaccharides-fed mice significantly increased the number of platform crossings and swimming distances in the probe test. The relative abundance of Bacteroidia, Erysipelotrichia and Actinobacteria were increased, while the relative abundance of Clostridia and Bacilli were decreased. Inflammatory factors such as IL-1β, TNF-α, IL-6 and IL-10 were also suppressed [79]. The SCFAs produced by the degradation of polysaccharides by the gut microbiota can not only maintain the intestinal barrier function and intestinal homeostasis, but also directly or indirectly affect brain function. Studies have shown that butyrate intake can regulate cytoneurotrophic factors, thereby improving cognitive function and having neuroprotective effects [201]. Additionally, existing research has found that mushroom polysaccharides such as Amanita caesarea polysaccharide, Hericium erinaceus polysaccharide and Armillaria mellea polysaccharide could reduce AD through an antioxidant effect, which is mainly manifested as enhancing the Nrf2 cascade reaction; increasing the contents of endogenous antioxidant enzymes GSH-Px, CAT and SOD; and reducing oxidation indexes such as MDA and 4-Hydroxynonenal [202,203,204].
PD is the second most serious neurodegenerative disease affecting the elderly, resulting in symptoms such as salivation, difficulty in swallowing and delayed gastric emptying that seriously affect patients’ quality of life. The unique changes in the gut microbiota of PD patients can be used as markers for early disease judgment, for example, the increase in the abundance of Enterobacter, Enterococcus, Verrucomicrobium, Streptococcus and Ruminococcus and the decrease in the abundance of Prevotella, Blautia, Faecalibacterium, Roseburia and Lachnospira [205,206,207]. Another study reported an increased abundance of Verrucomicrobiaceae and Firmicutes and a decreased abundance of Prevotellaceae and Erysipelotrichaceae in PD patients [208]. The imbalance of these PD-related gut microbiota promoted the progression of PD by increasing intestinal permeability, aggravating neuroinflammation, increasing oxidative stress and reducing neurotransmitter production [209]. Castelli et al. verified that oral compound preparation of Bifidobacterium and Lactobacillus could effectively protect dopaminergic neurons in PD mouse model induced by 6-hydroxydopamine as well as improve movement disorders [210]. In contrast, the imbalance of gut microbiota may also lead to the impairment of SCFAs production, lipid metabolism, immune regulatory function and intestinal permeability, thus leading to PD [211]. G. lucidum extract can regulate mitochondrial function, autophagy and apoptosis by activating AMPK/mTOR and PINK1/Parkin signaling pathways, thus improving the pathological status of PD [212]. Morchella esculenta polysaccharides possessed inhibitory activities against acetylcholinesterase and butanoyl cholinesterase, which may be useful for the treatment of PD [213]. However, no studies have evaluated the use of mushroom polysaccharides through regulating gut microbiota for the treatment of PD.
Age-related changes in the gut microbiota can cause brain aging and age-related neurodegenerative diseases. Aging is one of the key factors of a weakened immune system, so maintaining a healthy gut microbiota is crucial to anti-aging. Lentinan ameliorated the increase of Bacteroidetes and the decrease of Lactobacillus and alkali-producing bacteria in the intestinal tract of elderly mice, and partially reversed the composition of age-induced intestinal microbiota by increasing the cytokine levels in the peripheral blood to restore hypoimmunity induced by age [37]. The relative abundance of Lactobacillus and Bifidobacterium decreased with age [214]. The ratio of Firmicutes to Bacteroides decreased in older adults in Ireland and France, but this phenomenon was not observed in Italy [215]. Akkermansia was more abundant in the gut microbiota of the elderly, suggesting that Akkermansia may promote human health and longevity [216].
Excessive fatigue increases the accumulation of harmful metabolites and is a critical health problem [217]. The regulatory effects of Tuber indicum on fatigued mice were accompanied by improved intestinal integrity and increased SCFAs. It could decrease the ratio of Firmicutes to Proteobacteria and the ratio of Firmicutes to Bacteroidetes in the fatigue group, and elevate the relative abundance of the Bacteroidetes and Actinobacteria [218].
5. Conclusions and Future Perspectives
Polysaccharides are an important active ingredient of mushrooms, which are not absorbed by the gastrointestinal tract and can only be fermented by gut microbiota in the large intestine. Mushroom polysaccharides can also specifically change the composition and abundance of gut microbiota and maintain intestinal microecological balance. It is well known that numerous biological activities of polysaccharides are affected by their complex structure. Therefore, the authors investigated the relationship between the structure of mushroom polysaccharides and its corresponding regulation function of gut microbiota. It was concluded that mushroom polysaccharides containing glucose, galactose, mannose and fucose, as well as (1→3) and (1→6) linkages exerted better regulatory effects of gut microbiota. Firmicutes, Lactobacillus and Bacteroides were the main regulated gut microbiota. However, the exact structure or monosaccharide composition that stimulates specific gut bacteria has not yet been determined. The complex structure and the wide range sources of polysaccharides, and the great differences in the regulation of gut microbiota are the main factors hindering further research in this area. Therefore, it is necessary to strengthen the research on the regulation effect of mushroom polysaccharides specifically targeting gut microbiota.
In addition, the interactions between mushroom polysaccharides and gut microbiota and their effects on human health were reviewed. It was found that mushroom polysaccharides can promote human health by regulating gut microbiota, promoting the production of SCFAs, improving intestinal mucosal barrier, regulating lipid metabolism and activating specific signaling pathways. Although numerous animal studies have revealed the mechanism of mushroom polysaccharides on disease through the regulation of gut microbiota, further validation and exploration of its potential mechanisms are needed through metabolomics and metagenomics methods and higher-quality clinical trials. Notably, the role of microbial metabolites produced by mushroom polysaccharides in promoting host health is also a major concern to explore, so as to fully understand the multiple benefits of mushroom polysaccharides on human health.
Author Contributions
Conceptualization, J.Z., Y.H. and C.Q.; writing-original draft, J.Z.; editing, A.Z. and M.H.; project administration, S.L.; funding acquisition, R.H. and P.S.; supervision, R.H. and P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Project of Zhejiang Province (No. 2022C02063).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, C.; Zhou, N.; Du, M.X.; Sun, Y.T.; Wang, K.; Wang, Y.J.; Li, D.H.; Yu, H.Y.; Song, Y.Q.; Bai, B.B.; et al. The Mouse Gut Microbial Biobank expands the coverage of cultured bacteria. Nat. Commun. 2020, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Backhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Levy, M.; Blacher, E.; Elinav, E. Microbiome, metabolites and host immunity. Curr. Opin. Microbiol. 2017, 35, 8–15. [Google Scholar] [CrossRef]
- Schwalm, N.D.; Groisman, E.A. Navigating the Gut Buffet: Control of Polysaccharide Utilization in Bacteroides spp. Trends Microbiol. 2017, 25, 1005–1015. [Google Scholar] [CrossRef]
- Zhu, Y.; Bai, J.; Zhang, Y.; Xiao, X.; Dong, Y. Effects of bitter melon (Momordica charantia L.) on the gut microbiota in high fat diet and low dose streptozocin-induced rats. Int. J. Food Sci. Nutr. 2016, 67, 686–695. [Google Scholar] [CrossRef]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastro Hepat. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Li, H.Y.; Xie, W.C.; Sun, H.H.; Cao, K.W.; Yang, X.H. Effect of the structural characterization of the fungal polysaccharides on their immunomodulatory activity. Int. J. Biol. Macromol. 2020, 164, 3603–3610. [Google Scholar] [CrossRef]
- Seedevi, P.; Ganesan, A.R.; Mohan, K.; Raguraman, V.; Sivakumar, M.; Sivasankar, P.; Loganathan, S.; Rajamalar, P.; Vairamani, S.; Shanmugam, A.; et al. Chemical structure and biological properties of a polysaccharide isolated from Pleurotus sajor-caju. RSC Adv. 2019, 9, 20472–20482. [Google Scholar] [CrossRef]
- Jayachandran, M.; Xiao, J.B.; Xu, B.J. A Critical Review on Health Promoting Benefits of Edible Mushrooms through Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef]
- Li, L.Y.; Guo, Y.N.; Huang, Q.; Shi, X.J.; Liu, Q.Q.; Wang, F.; Liu, Q.F.; Yu, K.; Wang, Z. GPP (composition of Ganoderma lucidum polysaccharides and Polyporus umbellatus polysaccharides) protects against DSS-induced murine colitis by enhancing immune function and regulating intestinal flora. Food Sci. Hum. Wellness 2022, 11, 795–805. [Google Scholar] [CrossRef]
- Zhang, S.S.; Liu, X.Q.; Yan, L.H.; Zhang, Q.W.; Zhu, J.J.; Huang, N.; Wang, Z.M. Chemical Compositions and Antioxidant Activities of Polysaccharides from the Sporophores and Cultured Products of Armillaria mellea. Molecules 2015, 20, 5680–5697. [Google Scholar] [CrossRef]
- Cao, J.P.; Tang, D.D.; Wang, Y.; Li, X.; Hong, L.; Sun, C.D. Characteristics and immune-enhancing activity of pectic polysaccharides from sweet cherry (Prunus avium). Food Chem. 2018, 254, 47–54. [Google Scholar] [CrossRef]
- Chu, Q.; Zhang, Y.R.; Chen, W.; Jia, R.Y.; Yu, X.; Wang, Y.X.; Li, Y.L.; Liu, Y.Y.; Ye, X.; Yu, L.S.; et al. Apios americana Medik flowers polysaccharide (AFP) alleviate Cyclophosphamide-induced immunosuppression in ICR mice. Int. J. Biol. Macromol. 2020, 144, 829–836. [Google Scholar] [CrossRef]
- Cao, Q.Z.; Lin, Z.B. Antitumor and anti-angiogenic activity of Ganoderma lucidum polysaccharides peptide. Acta Pharmacol. Sin. 2004, 25, 833–838. [Google Scholar]
- Liang, Z.E.N.; Yuan, Z.H.; Li, G.Y.; Fu, F.H.; Shan, Y. Hypolipidemic, Antioxidant, and Antiapoptotic Effects of Polysaccharides Extracted from Reishi Mushroom, Ganoderma lucidum (Leysser: Fr) Karst, in Mice Fed a High-Fat Diet. J. Med. Food 2018, 21, 1218–1227. [Google Scholar] [CrossRef]
- Guo, W.L.; Deng, J.C.; Pan, Y.Y.; Xu, J.X.; Hong, J.L.; Shi, F.F.; Liu, G.L.; Qian, M.; Bai, W.D.; Zhang, W.; et al. Hypoglycemic and hypolipidemic activities of Grifola frondosa polysaccharides and their relationships with the modulation of intestinal microflora in diabetic mice induced by high-fat diet and streptozotocin. Int. J. Biol. Macromol. 2020, 153, 1231–1240. [Google Scholar] [CrossRef]
- Chen, X.P.; Chen, Y.; Li, S.B.; Chen, Y.G.; Lan, J.Y.; Liu, L.P. Free radical scavenging of Ganoderma lucidum polysaccharides and its effect on antioxidant enzymes and immunity activities in cervical carcinoma rats. Carbohydr. Polym. 2009, 77, 389–393. [Google Scholar]
- Guo, Y.J.; Deng, G.F.; Xu, X.R.; Wu, S.; Li, S.; Xia, E.Q.; Li, F.; Chen, F.; Ling, W.H.; Li, H.B.; et al. Antioxidant capacities, phenolic compounds and polysaccharide contents of 49 edible macro-fungi. Food Funct. 2012, 3, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.; Chopra, H.; Baig, A.A.; Avula, S.K.; Kumari, S.; Mohanta, T.K.; Saravanan, M.; Mishra, A.K.; Sharma, N.; Mohanta, Y.K.; et al. Edible Mushrooms as Novel Myco-Therapeutics: Effects on Lipid Level, Obesity, and BMI. J. Fungi 2022, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.Y.; Qi, P.; Cui, L.J.; Zhang, L.G.; Dai, L.; Liu, Y.; Hu, S.Y.; Feng, Z.P.; Qiao, T.; Li, J.Z.; et al. Polysaccharide from wild morels alters the spatial structure of gut microbiota and the production of short-chain fatty acids in mice. Biosci. Microbiota Food Health 2020, 39, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Teng, C.Y.; Yu, S.M.; Wang, X.; Liang, J.S.; Bai, X.; Dong, L.Y.; Song, T.; Yu, M.; Qu, J.J.; et al. Inonotus obliquus polysaccharide regulates gut microbiota of chronic pancreatitis in mice. AMB Express 2017, 7, 39. [Google Scholar] [CrossRef]
- Zhao, R.Q.; Hu, Q.H.; Ma, G.X.; Su, A.X.; Xie, M.H.; Li, X.F.; Chen, G.T.; Zhao, L.Y. Effects of Flammulina velutipes polysaccharide on immune response and intestinal microbiota in mice. J. Funct. Foods 2019, 56, 255–264. [Google Scholar] [CrossRef]
- Xu, X.F.; Zhang, X.W. Lentinula edodes-Derived Polysaccharide Alters the Spatial Structure of Gut Microbiota in Mice. PLoS ONE 2015, 10, e0115037. [Google Scholar] [CrossRef]
- Song, X.J.; Feng, Z.F.; Tan, J.B.; Wang, Z.Y.; Zhu, W. Dietary administration of Pleurotus ostreatus polysaccharides (POPS) modulates the non-specific immune response and gut microbiota diversity of Apostichopus japonicus. Aquac. Rep. 2021, 19, 100578. [Google Scholar] [CrossRef]
- Xie, J.L.; Liu, Y.X.; Chen, B.H.; Zhang, G.W.; Ou, S.Y.; Luo, J.M.; Peng, X.C. Ganoderma lucidum polysaccharide improves rat DSS-induced colitis by altering cecal microbiota and gene expression of colonic epithelial cells. Food Nutr. Res. 2019, 63, 1559. [Google Scholar] [CrossRef]
- Zhang, S.; Lei, L.; Zhou, Y.; Ye, F.Y.; Zhao, G.H. Roles of mushroom polysaccharides in chronic disease management. J. Integr. Agric. 2022, 21, 1839–1866. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, F.; Chen, G.; Chen, Y.; Zhang, W.; Mao, G.; Zhao, T.; Zhang, M.; Yang, L.; Wu, X.; et al. Purification, characterization and immunomodulatory activity of a novel polysaccharide from Grifola frondosa. Int. J. Biol. Macromol. 2018, 111, 1293–1303. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Y.; Xu, D.; Gao, Q. A polysaccharide from cultured mycelium of Hericium erinaceus and its anti-chronic atrophic gastritis activity. Int. J. Biol. Macromol. 2015, 81, 656–661. [Google Scholar] [CrossRef]
- Li, Y.Q.; Fang, L.; Zhang, K.C. Structure and bioactivities of a galactose rich extracellular polysaccharide from submergedly cultured Ganoderma lucidum. Carbohydr. Polym. 2007, 68, 323–328. [Google Scholar] [CrossRef]
- Xu, D.D.; Wang, H.Y.; Zheng, W.; Gao, Y.; Wang, M.X.; Zhang, Y.Q.; Gao, Q.P. Charaterization and immunomodulatory activities of polysaccharide isolated from Pleurotus eryngii. Int. J. Biol. Macromol. 2016, 92, 30–36. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Xu, X.J.; Zhang, L. Gel formation and low-temperature intramolecular conformation transition of a triple-helical polysaccharide lentinan in water. Biopolymers 2008, 89, 852–861. [Google Scholar] [CrossRef]
- Xu, X.F.; Yan, H.D.; Zhang, X.W. Structure and Immuno-Stimulating Activities of a New Heteropolysaccharide from Lentinula edodes. J. Agric. Food Chem. 2012, 60, 11560–11566. [Google Scholar] [CrossRef]
- Xu, X.F.; Yang, J.G.; Ning, Z.X.; Zhang, X.W. Lentinula edodes-derived polysaccharide rejuvenates mice in terms of immune responses and gut microbiota. Food Funct. 2015, 6, 2653–2663. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, Y.T.; Li, C.; Jia, S.T.; Shi, Y.A.; Tang, Y.F.; Li, Y.Q. A preliminary study on preparation, characterization, and prebiotic activity of a polysaccharide from the edible mushroom Ramaria flava. J. Food Biochem. 2022, 46, e14371. [Google Scholar] [CrossRef]
- Tian, B.M.; Geng, Y.; Xu, T.R.; Zou, X.G.; Mao, R.L.; Pi, X.G.; Wu, W.C.; Huang, L.S.; Yang, K.; Zeng, X.X.; et al. Digestive Characteristics of Hericium erinaceus Polysaccharides and Their Positive Effects on Fecal Microbiota of Male and Female Volunteers During in vitro Fermentation. Front. Nutr. 2022, 9, 858585. [Google Scholar] [CrossRef]
- Abdureyim, Z.; Wang, L.; Tao, Q.; Xu, J.; Yimiti, D.; Gao, Q. Bachu Mushroom Polysaccharide Alleviates Colonic Injury by Modulating the Gut Microbiota. Comput. Math. Methods Med. 2022, 2022, 1353724. [Google Scholar] [CrossRef]
- Pan, W.; Jiang, P.F.; Zhao, J.X.; Shi, H.L.; Zhang, P.; Yang, X.Y.; Biazik, J.; Hu, M.M.; Hua, H.; Ge, X.; et al. β-Glucan from Lentinula edodes prevents cognitive impairments in high-fat diet-induced obese mice: Involvement of colon-brain axis. J. Transl. Med. 2021, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.U.; Siddiqui, N.Z.; Farooqui, N.A.; Alam, G.; Gul, A.; Ahmad, B.; Asim, M.; Khan, A.I.; Xin, Y.; Zexu, W.; et al. Morchella esculenta mushroom polysaccharide attenuates diabetes and modulates intestinal permeability and gut microbiota in a type 2 diabetic mice model. Front. Nutr. 2022, 9, 984695. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.X.; Xu, Q.; Du, H.J.; Kimatu, B.M.; Su, A.X.; Yang, W.J.; Hu, Q.H.; Xiao, H. Characterization of polysaccharide from Pleurotus eryngii during simulated gastrointestinal digestion and fermentation. Food Chem. 2022, 370, 131303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Hu, B.; Liu, C.; Hua, H.Y.; Guo, Y.H.; Cheng, Y.L.; Yao, W.R.; Qian, H. Comprehensive analysis of Sparassis crispa polysaccharide characteristics during the in vitro digestion and fermentation model. Food Res. Int. 2022, 154, 111005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Hu, B.; Han, M.; Guo, Y.H.; Cheng, Y.L.; Qian, H. Purification, structural characterization and neuroprotective effect of a neutral polysaccharide from Sparassis crispa. Int. J. Biol. Macromol. 2022, 201, 389–399. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Xie, L.Y.; Zhang, Z.Y.; Zhang, W.W.; Tang, J.; He, X.L.; Zhou, J.; Peng, W.H. Tremella fuciformis Polysaccharides Inhibited Colonic Inflammation in Dextran Sulfate Sodium-Treated Mice via Foxp3+T Cells, Gut Microbiota, and Bacterial Metabolites. Front. Immunol. 2021, 12, 648162. [Google Scholar] [CrossRef]
- Wang, T.; Han, J.J.; Dai, H.Q.; Sun, J.Z.; Ren, J.W.; Wang, W.Z.; Qiao, S.S.; Liu, C.; Sun, L.; Liu, S.J.; et al. Polysaccharides from Lyophyllum decastes reduce obesity by altering gut microbiota and increasing energy expenditure. Carbohydr. Polym. 2022, 295, 119862. [Google Scholar] [CrossRef]
- Yang, L.; Kang, X.C.; Dong, W.J.; Wang, L.; Liu, S.F.; Zhong, X.H.; Liu, D.B. Prebiotic properties of Ganoderma lucidum polysaccharides with special enrichment of Bacteroides ovatus and B. uniformis in vitro. J. Funct. Foods 2022, 92, 105069. [Google Scholar] [CrossRef]
- Pan, M.C.; Kong, F.G.; Xing, L.; Yao, L.; Li, Y.; Liu, Y.; Li, C.T.; Li, L.Z. The Structural Characterization and Immunomodulatory Activity of Polysaccharides from Pleurotus abieticola Fruiting Bodies. Nutrients 2022, 14, 4410. [Google Scholar] [CrossRef]
- Huo, W.Y.; Feng, Z.P.; Hu, S.Y.; Cui, L.J.; Qiao, T.; Dai, L.; Qi, P.; Zhang, L.G.; Liu, Y.; Li, J.Z.; et al. Effects of polysaccharides from wild morels on immune response and gut microbiota composition in non-treated and cyclophosphamide-treated mice. Food Funct. 2020, 11, 4291–4303. [Google Scholar] [CrossRef]
- Chen, M.Y.; Xiao, D.; Liu, W.; Song, Y.F.; Zou, B.R.; Li, L.; Li, P.; Cai, Y.; Liu, D.L.; Liao, Q.F.; et al. Intake of Ganoderma lucidum polysaccharides reverses the disturbed gut microbiota and metabolism in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 155, 890–902. [Google Scholar] [CrossRef]
- Zhao, D.; Dai, W.; Tao, H.; Zhuang, W.; Qu, M.; Chang, Y.N. Polysaccharide isolated from Auricularia auricular-judae (Bull.) prevents dextran sulfate sodium-induced colitis in mice through modulating the composition of the gut microbiota. J. Food Sci. 2020, 85, 2943–2951. [Google Scholar] [CrossRef]
- Kanwal, S.; Aliya, S.; Xin, Y. Anti-Obesity Effect of Dictyophora indusiata Mushroom Polysaccharide (DIP) in High Fat Diet-Induced Obesity via Regulating Inflammatory Cascades and Intestinal Microbiome. Front. Endocrinol. 2020, 11, 558874. [Google Scholar] [CrossRef]
- Zhang, R.; Yuan, S.; Ye, J.; Wang, X.; Zhang, X.; Shen, J.; Yuan, M.; Liao, W. Polysaccharide from Flammuliana velutipes improves colitis via regulation of colonic microbial dysbiosis and inflammatory responses. Int. J. Biol. Macromol. 2020, 149, 1252–1261. [Google Scholar] [CrossRef]
- Xue, Z.; Ma, Q.; Chen, Y.; Lu, Y.; Wang, Y.; Jia, Y.; Zhang, M.; Chen, H. Structure characterization of soluble dietary fiber fractions from mushroom Lentinula edodes (Berk.) Pegler and the effects on fermentation and human gut microbiota in vitro. Food Res. Int. 2020, 129, 108870. [Google Scholar] [CrossRef]
- Zhao, R.Q.; Fang, D.G.; Ji, Y.; Chen, X.; Ma, G.X.; Su, A.X.; Xie, M.H.; Zhao, L.Y.; Hu, Q.H. In vitro and in vivo functional characterization of an immune activation Flammulina velutipes polysaccharide based on gut microbiota regulation. Food Agric. Immunol. 2020, 31, 667–686. [Google Scholar] [CrossRef]
- Kanwal, S.; Joseph, T.P.; Aliya, S.; Song, S.Y.; Saleem, M.Z.; Nisar, M.A.; Wang, Y.; Meyiah, A.; Ma, Y.F.; Xin, Y.; et al. Attenuation of DSS induced colitis by Dictyophora indusiata polysaccharide (DIP) via modulation of gut microbiota and inflammatory related signaling pathways. J. Funct. Foods 2020, 64, 103641. [Google Scholar] [CrossRef]
- Zhao, R.Q.; Cheng, N.H.; Nakata, P.A.; Zhao, L.Y.; Hu, Q.H. Consumption of polysaccharides from Auricularia auricular modulates the intestinal microbiota in mice. Food Res. Int. 2019, 123, 383–392. [Google Scholar] [CrossRef]
- Li, L.; Guo, W.L.; Zhang, W.; Xu, J.X.; Qian, M.; Bai, W.D.; Zhang, Y.Y.; Rao, P.F.; Ni, L.; Lv, X.C. Grifola frondosa polysaccharides ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet fed rats. Food Funct. 2019, 10, 2560–2572. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Liu, D.; Wang, D.Y.; Lai, S.S.; Zhong, R.T.; Liu, Y.Y.; Yang, C.F.; Liu, B.; Sarker, M.R.; Zhao, C.; et al. Hypoglycemic activity and gut microbiota regulation of a novel polysaccharide from Grifola frondosa in type 2 diabetic mice. Food Chem. Toxicol. 2019, 126, 295–302. [Google Scholar] [CrossRef]
- Kanwal, S.; Joseph, T.P.; Owusu, L.; Ren, X.M.; Li, M.Q.; Xin, Y. A Polysaccharide Isolated from Dictyophora indusiata Promotes Recovery from Antibiotic-Driven Intestinal Dysbiosis and Improves Gut Epithelial Barrier Function in a Mouse Model. Nutrients 2018, 10, 1003. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.L.; Geng, Y.; Du, Y.; Li, W.; Lu, Z.M.; Xu, H.Y.; Xu, G.H.; Shi, J.S.; Xu, Z.H. Polysaccharide of Hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J. Nutr. Biochem. 2018, 57, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Mallard, B.; Leach, D.N.; Wohlmuth, H.; Tiralongo, J. Synergistic immuno-modulatory activity in human macrophages of a medicinal mushroom formulation consisting of Reishi, Shiitake and Maitake. PLoS ONE 2019, 14, e0224740. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods 2016, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Cerletti, C.; Esposito, S.; Iacoviello, L. Edible Mushrooms and Beta-Glucans: Impact on Human Health. Nutrients 2021, 13, 2195. [Google Scholar] [CrossRef]
- Ranjbar, R.; Vahdati, S.N.; Tavakoli, S.; Khodaie, R.; Behboudi, H. Immunomodulatory roles of microbiota-derived short-chain fatty acids in bacterial infections. Biomed. Pharmacother. 2021, 141, 111817. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chen, J.L.; Chung, S.S.M.; Xu, B.J. A critical review on the impacts of beta-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef]
- Saxami, G.; Kerezoudi, E.N.; Mitsou, E.K.; Koutrotsios, G.; Zervakis, G.I.; Pletsa, V.; Kyriacou, A. Fermentation Supernatants of Pleurotus eryngii Mushroom Ameliorate Intestinal Epithelial Barrier Dysfunction in Lipopolysaccharide-Induced Caco-2 Cells via Upregulation of Tight Junctions. Microorganisms 2021, 9, 2071. [Google Scholar] [CrossRef]
- Wang, G.L.; Zhang, Y.L.; Zhang, R.G.; Pan, J.L.; Qi, D.F.; Wang, J.; Yang, X.Y. The protective effects of walnut green husk polysaccharide on liver injury, vascular endothelial dysfunction and disorder of gut microbiota in high fructose-induced mice. Int. J. Biol. Macromol. 2020, 162, 92–106. [Google Scholar] [CrossRef]
- Luo, J.M.; Zhang, C.; Liu, R.; Gao, L.J.; Ou, S.Y.; Liu, L.; Peng, X.C. Ganoderma lucidum polysaccharide alleviating colorectal cancer by alteration of special gut bacteria and regulation of gene expression of colonic epithelial cells. J. Funct. Foods 2018, 47, 127–135. [Google Scholar] [CrossRef]
- Sun, S.S.; Wang, K.; Ma, K.; Bao, L.; Liu, H.W. An insoluble polysaccharide from the sclerotium of Poria cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in ob/ob mice via modulation of gut microbiota. Chin. J. Nat. Med. 2019, 17, 3–14. [Google Scholar] [CrossRef]
- He, X.R.; Wang, X.X.; Fang, J.C.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.H.; Huang, X.Q.; Zhao, Z.F. Polysaccharides in Grifola frondosa mushroom and their health promoting properties: A review. Int. J. Biol. Macromol. 2017, 101, 910–921. [Google Scholar] [CrossRef]
- Han, X.Q.; Yue, G.L.; Yue, R.Q.; Dong, C.X.; Chan, C.L.; Ko, C.H.; Cheung, W.S.; Luo, K.W.; Dai, H.; Wong, C.K.; et al. Structure Elucidation and Immunomodulatory Activity of A Beta Glucan from the Fruiting Bodies of Ganoderma Sinense. PLoS ONE 2014, 9, 100380. [Google Scholar] [CrossRef]
- Gao, X.; Qi, J.Y.; Ho, C.T.; Li, B.; Mu, J.J.; Zhang, Y.T.; Hu, H.P.; Mo, W.P.; Chen, Z.Z.; Xie, Y.Z.; et al. Structural characterization and immunomodulatory activity of a water-soluble polysaccharide from Ganoderma leucocontextum fruiting bodies. Carbohydr. Polym. 2020, 249, 116874. [Google Scholar] [CrossRef]
- Xu, X.F.; Yan, H.D.; Tang, J.; Chen, J.; Zhang, X.W. Polysaccharides in Lentinus edodes: Isolation, Structure, Immunomodulating Activity and Future Prospective. Crit. Rev. Food Sci. Nutr. 2014, 54, 474–487. [Google Scholar] [CrossRef]
- Su, A.X.; Ma, G.X.; Xie, M.H.; Ji, Y.; Li, X.F.; Zhao, L.Y.; Hu, Q.H. Characteristic of polysaccharides from Flammulina velutipes in vitro digestion under salivary, simulated gastric and small intestinal conditions and fermentation by human gut microbiota. J. Food Sci. Technol. 2019, 54, 2277–2287. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Lombard, V.; Henrissat, B. Complex Carbohydrate Utilization by the Healthy Human Microbiome. PLoS ONE 2012, 7, e28742. [Google Scholar] [CrossRef]
- Salyers, A.A.; Vercellotti, J.R.; West, S.E.; Wilkins, T.D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl. Environ. Microbiol. 1977, 33, 319–322. [Google Scholar] [CrossRef]
- Su, A.X.; Yang, W.J.; Zhao, L.Y.; Pei, F.; Yuan, B.; Zhong, L.; Ma, G.X.; Hu, Q.H. Flammulina velutipes polysaccharides improve scopolamine-induced learning and memory impairment in mice by modulating gut microbiota composition. Food Funct. 2018, 9, 1424–1432. [Google Scholar] [CrossRef]
- Ravcheev, D.A.; Godzik, A.; Osterman, A.L.; Rodionov, D.A. Polysaccharides utilization in human gut bacterium Bacteroides thetaiotaomicron: Comparative genomics reconstruction of metabolic and regulatory networks. BMC Genom. 2013, 14, 873. [Google Scholar] [CrossRef]
- Wardman, J.F.; Bains, R.K.; Rahfeld, P.; Withers, S.G. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat. Rev. Microbiol. 2022, 20, 542–556. [Google Scholar] [CrossRef]
- White, B.A.; Lamed, R.; Bayer, E.A.; Flint, H.J. Biomass utilization by gut microbiomes. Annu. Rev. Microbiol. 2014, 68, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Bernard, T.; Rancurel, C.; Brumer, H.; Coutinho, P.M.; Henrissat, B. A hierarchical classification of polysaccharide lyases for glycogenomics. Biochem. J. 2010, 432, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Biely, P. Microbial carbohydrate esterases deacetylating plant polysaccharides. Biotechnol. Adv. 2012, 30, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- El Kaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef]
- Rakoff-Nahoum, S.; Foster, K.R.; Comstock, L.E. The evolution of cooperation within the gut microbiota. Nature 2016, 533, 255–259. [Google Scholar] [CrossRef]
- Yonit, B.D.; Dassa, B.; Borovok, I.; Lamed, R.; Koropatkin, N.M.; Martens, E.C.; White, B.A.; Bernalier-Donadille, A.; Duncan, S.H.; Flint, H.J.; et al. Ruminococcal cellulosome systems from rumen to human. Environ. Microbiol. 2015, 17, 3407–3426. [Google Scholar]
- Cockburn, D.W.; Orlovsky, N.I.; Foley, M.H.; Kwiatkowski, K.J.; Bahr, C.M.; Maynard, M.; Demeler, B.; Koropatkin, N.M. Molecular details of a starch utilization pathway in the human gut symbiont Eubacterium rectale. Mol. Microbiol. 2015, 95, 209–230. [Google Scholar] [CrossRef]
- Singh, R.P. Glycan utilisation system in Bacteroides and Bifidobacteria and their roles in gut stability and health. Appl. Microbiol. Biotechnol. 2019, 103, 7287–7315. [Google Scholar] [CrossRef]
- Turroni, F.; Milani, C.; Duranti, S.; Mahony, J.; van Sinderen, D.; Ventura, M. Glycan Utilization and Cross-Feeding Activities by Bifidobacteria. Trends Microbiol. 2018, 26, 339–350. [Google Scholar] [CrossRef]
- Zhang, T.H.; Yang, Y.; Liang, Y.; Jiao, X.; Zhao, C.H. Beneficial Effect of Intestinal Fermentation of Natural Polysaccharides. Nutrients 2018, 10, 1055. [Google Scholar] [CrossRef]
- Fierobe, H.P.; Bayer, E.A.; Tardif, C.; Czjzek, M.; Mechaly, A.; Belaich, A.; Lamed, R.; Shoham, Y.; Belaich, J.P. Degradation of cellulose substrates by cellulosome chimeras-Substrate targeting versus proximity of enzyme components. J. Biol. Chem. 2002, 277, 49621–49630. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, X.Y.; Shi, F.L.; Liu, Y. Comparison of antidiabetic effects of saponins and polysaccharides from Momordica charantia L. in STZ-induced type 2 diabetic mice. Biomed. Pharmacother. 2019, 109, 744–750. [Google Scholar] [CrossRef]
- Songer, T.J.; Zimmet, P.Z. Epidemiology of type II diabetes: An international perspective. Pharmacoeconomics 1995, 8, 1–11. [Google Scholar] [CrossRef]
- Nasr, N.E.; Sadek, K.M. Role and mechanism(s) of incretin-dependent therapies for treating diabetes mellitus. Environ. Sci. Pollut. Res. 2022, 29, 18408–18422. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A. Therapeutic potential of mushrooms in diabetes mellitus: Role of polysaccharides. Int. J. Biol. Macromol. 2020, 164, 1194–1205. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Yang, R.; Li, Y.D.; Mehmood, S.; Yan, C.C.; Huang, Y.Z.; Cai, J.J.; Ji, J.Q.; Pan, W.J.; Zhang, W.N.; Chen, Y.; et al. Polysaccharides from Armillariella tabescens mycelia ameliorate renal damage in type 2 diabetic mice. Int. J. Biol. Macromol. 2020, 162, 1682–1691. [Google Scholar] [CrossRef]
- Yi, H.B.; Wang, L.; Xiong, Y.X.; Wang, Z.L.; Qiu, Y.Q.; Wen, X.L.; Jiang, Z.Y.; Yang, X.F.; Ma, X.Y. Lactobacillus reuteri LR1 Improved Expression of Genes of Tight Junction Proteins via the MLCK Pathway in IPEC-1 Cells during Infection with Enterotoxigenic Escherichia coli K88. Mediat. Inflamm. 2018, 2018, 6434910. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, W.K.; Han, D.H.; Lee, K.; Ko, G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes 2019, 10, 696–711. [Google Scholar] [CrossRef]
- Shao, W.M.; Xiao, C.; Yong, T.Q.; Zhang, Y.F.; Hu, H.P.; Xie, T.; Liu, R.J.; Huang, L.H.; Li, X.M.; Xie, Y.Z.; et al. A polysaccharide isolated from Ganoderma lucidum ameliorates hyperglycemia through modulating gut microbiota in type 2 diabetic mice. Int. J. Biol. Macromol. 2022, 197, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.M.; Zhang, N.; Zhou, W.; Zhou, Z.K.; Bai, Y.; Strappe, P.; Blanchard, C. Manipulations of glucose/lipid metabolism and gut microbiota of resistant starch encapsulated Ganoderma lucidum spores in T2DM rats. Food Sci. Biotechnol. 2021, 30, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.L.; Chen, M.; Pan, W.L.; Zhang, Q.; Xu, J.X.; Lin, Y.C.; Li, L.; Liu, B.; Bai, W.D.; Zhang, Y.Y.; et al. Hypoglycemic and hypolipidemic mechanism of organic chromium derived from chelation of Grifola frondosa polysaccharide-chromium (III) and its modulation of intestinal microflora in high fat-diet and STZ-induced diabetic mice. Int. J. Biol. Macromol. 2020, 145, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Mori, K.; Ouchi, K.; Kushida, M.; Tsuduki, T. Effects of Dietary Intake of Japanese Mushrooms on Visceral Fat Accumulation and Gut Microbiota in Mice. Nutrients 2018, 10, 610. [Google Scholar] [CrossRef] [PubMed]
- Kilner, J.; Corfe, B.M.; McAuley, M.T.; Wilkinson, S.J. A deterministic oscillatory model of microtubule growth and shrinkage for differential actions of short chain fatty acids. Mol. BioSystems 2016, 12, 93–101. [Google Scholar] [CrossRef]
- Liu, Y.M.; Liu, W.; Li, J.; Tang, S.; Wang, M.J.; Huang, W.H.; Yao, W.B.; Gao, X.D. A polysaccharide extracted from Astragalus membranaceus residue improves cognitive dysfunction by altering gut microbiota in diabetic mice. Carbohydr. Polym. 2019, 205, 500–512. [Google Scholar] [CrossRef]
- Sang, T.T.; Guo, C.J.; Guo, D.D.; Wu, J.J.; Wang, Y.J.; Wang, Y.; Chen, J.J.; Chen, C.J.; Wu, K.K.; Na, K.; et al. Suppression of obesity and inflammation by polysaccharide from sporoderm-broken spore of Ganoderma lucidum via gut microbiota regulation. Carbohydr. Polym. 2021, 256, 117594. [Google Scholar] [CrossRef]
- Waddell, I.S.; Orfila, C. Dietary fiber in the prevention of obesity and obesity-related chronic diseases: From epidemiological evidence to potential molecular mechanisms. Crit. Rev. Food Sci. Nutr. 2022, 26, 1–16. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, S.J.; Wan, J.M.F.; Gui, L.F.; Ruan, M.C.; Li, N.; Zhang, H.Y.; Liu, Z.G.; Wang, H.L. Polysaccharides extracted from Phellinus linteus ameliorate high-fat high-fructose diet induced insulin resistance in mice. Carbohydr. Polym. 2018, 200, 144–153. [Google Scholar] [CrossRef]
- Liu, N.N.; Chen, X.F.; Song, J.N.; Chen, M.Y.; Gong, P.; Jia, W.; Li, G.L. Hypoglycemic effects of Auricularia auricula polysaccharides on high fat diet and streptozotocin-induced diabetic mice using metabolomics analysis. Food Funct. 2021, 12, 9994–10007. [Google Scholar] [CrossRef]
- Xu, N.; Zhou, Y.J.; Lu, X.Y.; Chang, Y.N. Auricularia auricula-judae (Bull.) polysaccharides improve type 2 diabetes in HFD/STZ-induced mice by regulating the AKT/AMPK signaling pathways and the gut microbiota. J. Food Sci. 2021, 86, 5479–5494. [Google Scholar] [CrossRef]
- Wang, C.E.; Zeng, F.; Liu, Y.L.; Pan, Y.Y.; Xu, J.X.; Ge, X.D.; Zheng, H.P.; Pang, J.; Liu, B.; Huang, Y.; et al. Coumarin-rich Grifola frondosa ethanol extract alleviate lipid metabolism disorders and modulates intestinal flora compositions of high-fat diet rats. J. Funct. Foods 2021, 85, 104649. [Google Scholar] [CrossRef]
- Gao, X.X.; Liu, D.; Gao, L.Y.; Ouyang, Y.Z.; Wen, Y.X.; Ai, C.; Chen, Y.Q.; Zhao, C. Health benefits of Grifola frondosa polysaccharide on intestinal microbiota in type 2 diabetic mice. Food Sci. Hum. Wellness 2022, 11, 68–73. [Google Scholar] [CrossRef]
- Zhu, Z.J.; Huang, R.; Huang, A.H.; Wang, J.; Liu, W.; Wu, S.J.; Chen, M.F.; Chen, M.T.; Xie, Y.Z.; Jiao, C.W.; et al. Polysaccharide from Agrocybe cylindracea prevents diet-induced obesity through inhibiting inflammation mediated by gut microbiota and associated metabolites. Int. J. Biol. Macromol. 2022, 209, 1430–1438. [Google Scholar] [CrossRef]
- Huang, Z.R.; Zhao, L.Y.; Zhu, F.R.; Liu, Y.; Xiao, J.Y.; Chen, Z.C.; Lv, X.C.; Huang, Y.; Liu, B. Anti-Diabetic Effects of Ethanol Extract from Sanghuangporous vaninii in High-Fat/Sucrose Diet and Streptozotocin-Induced Diabetic Mice by Modulating Gut Microbiota. Foods 2022, 11, 974. [Google Scholar] [CrossRef]
- Jiang, X.; Hao, J.; Liu, Z.J.; Ma, X.T.; Feng, Y.X.; Teng, L.R.; Li, Y.; Wang, D. Anti-obesity effects of Grifola frondosa through the modulation of lipid metabolism via ceramide in mice fed a high-fat diet. Food Funct. 2021, 12, 6725–6739. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zheng, M.X.; Zhou, M.L.; Zhou, L.M.; Ge, X.; Pang, N.; Li, H.C.; Li, X.Y.; Li, M.D.; Zhang, J.; et al. Lentinan Supplementation Protects the Gut-Liver Axis and Prevents Steatohepatitis: The Role of Gut Microbiota Involved. Front. Nutr. 2022, 8, 803691. [Google Scholar] [CrossRef]
- Li, X.; Zeng, F.; Huang, Y.F.; Liu, B. The Positive Effects of Grifola frondosa Heteropolysaccharide on NAFLD and Regulation of the Gut Microbiota. Int. J. Mol. Sci. 2019, 20, 5302. [Google Scholar] [CrossRef]
- Zhang, T.T.; Zhao, W.Y.; Xie, B.Z.; Liu, H. Effects of Auricularia auricula and its polysaccharide on diet-induced hyperlipidemia rats by modulating gut microbiota. J. Funct. Foods 2020, 72, 104038. [Google Scholar] [CrossRef]
- Nakahara, D.; Nan, C.; Mori, K.; Hanayama, M.; Kikuchi, H.; Hirai, S.; Egashira, Y. Effect of mushroom polysaccharides from Pleurotus eryngiion obesity and gut microbiota in mice fed a high-fat diet. Eur. J. Nutr. 2020, 59, 3231–3244. [Google Scholar] [CrossRef]
- Xiao, C.; Jiao, C.W.; Xie, Y.Z.; Ye, L.H.; Li, Q.Q.; Wu, Q.P. Grifola frondosa GF5000 improves insulin resistance by modulation the composition of gut microbiota in diabetic rats. J. Funct. Foods 2021, 77, 104313. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cordoba, S.L.; Salido-Guadarrama, I.; Meneses, M.E.; Cosentino, G.; Iorio, M.V.; Tagliabue, E.; Torres, N.; Sanchez-Tapia, M.; Bonilla, M.; Castillo, I.; et al. Mexican Ganoderma Lucidum Extracts Decrease Lipogenesis Modulating Transcriptional Metabolic Networks and Gut Microbiota in C57BL/6 Mice Fed with a High-Cholesterol Diet. Nutrients 2021, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.J.; Hu, R.K.; Wu, L.X.; Lv, X.C.; Li, X.; Zhao, L.N.; Liu, B. Ganoderma polysaccharide and chitosan synergistically ameliorate lipid metabolic disorders and modulate gut microbiota composition in high fat diet-fed golden hamsters. J. Food Biochem. 2020, 44, e13109. [Google Scholar] [CrossRef] [PubMed]
- Pung, H.C.; Lin, W.S.; Lo, Y.C.; Hsu, C.C.; Ho, C.T.; Pan, M.H. Ulva prolifera polysaccharide exerts anti-obesity effects via upregulation of adiponectin expression and gut microbiota modulation in high-fat diet-fed C57BL/6 mice. J. Food Drug Anal. 2022, 30, 46–61. [Google Scholar] [CrossRef]
- Gurevich-Panigrahi, T.; Panigrahi, S.; Wiechec, E.; Los, M. Obesity: Pathophysiology and Clinical Management. Curr. Med. Chem. 2009, 16, 506–521. [Google Scholar] [CrossRef]
- Hale, C.; Veniant, M.M. Growth differentiation factor 15 as a potential therapeutic for treating obesity. Mol. Metab. 2021, 46, 101117. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.Y.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Brun, P.; Castagliuolo, I.; di Leo, V.; Buda, A.; Pinzani, M.; Palu, G.; Martines, D. Increased intestinal permeability in obese mice: New evidence in the pathogenesis of nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G518–G525. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Backhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Li, R.L.; Xue, Z.H.; Li, S.Q.; Zhou, J.N.; Liu, J.Y.; Zhang, M.; Panichayupakaranant, P.; Chen, H.X. Mulberry leaf polysaccharides ameliorate obesity through activation of brown adipose tissue and modulation of the gut microbiota in high-fat diet fed mice. Food Funct. 2022, 13, 561–573. [Google Scholar] [CrossRef]
- Den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPAR-Dependent Switch from Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.L.; Li, D.F.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef]
- Mfopa, A.; Mediesse, F.K.; Mvongo, C.; Nkoubatchoundjwen, S.; Lum, A.A.; Sobngwi, E.; Kamgang, R.; Boudjeko, T. Antidyslipidemic Potential of Water-Soluble Polysaccharides of Ganoderma applanatum in MACAPOS-2-Induced Obese Rats. Evid. Based Complement. Altern. Med. 2021, 18, 2452057. [Google Scholar] [CrossRef]
- Ma, G.X.; Kimatu, B.M.; Zhao, L.Y.; Yang, W.J.; Pei, F.; Hu, Q.H. In vivo fermentation of a Pleurotus eryngii polysaccharide and its effects on fecal microbiota composition and immune response. Food Funct. 2017, 8, 1810–1821. [Google Scholar] [CrossRef]
- Aranaz, P.; Pena, A.; Vettorazzi, A.; Fabra, M.J.; Martinez-Abad, A.; Lopez-Rubio, A.; Pera, J.; Parlade, J.; Castellari, M.; Milagro, F.I.; et al. Grifola frondosa (Maitake) Extract Reduces Fat Accumulation and Improves Health Span in C. elegans through the DAF-16/FOXO and SKN-1/NRF2 Signalling Pathways. Nutrients 2021, 13, 3968. [Google Scholar] [CrossRef]
- Cao, Y.X.; Deng, B.H.; Zhang, S.H.; Gao, H.M.; Song, P.K.; Zhang, J.X.; Zhao, J.X. Astragalus polysaccharide regulates brown adipogenic differentiation through miR-1258-5p-modulated cut-like homeobox 1 expression. Acta Biochim. Biophys. Sin. 2021, 53, 1713–1722. [Google Scholar] [CrossRef]
- Bai, J.; Li, J.; Pan, R.R.; Zhu, Y.; Xiao, X.; Li, Y.; Li, C.T. Polysaccharides from Volvariella volvacea inhibit fat accumulation in C. elegans dependent on the aak-2/nhr-49-mediated pathway. J. Food Biochem. 2021, 45, e13912. [Google Scholar] [CrossRef]
- Ferro, D.; Baratta, F.; Pastori, D.; Cocomello, N.; Colantoni, A.; Angelico, F.; Del Ben, M. New Insights into the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Gut-Derived Lipopolysaccharides and Oxidative Stress. Nutrients 2020, 12, 2762. [Google Scholar] [CrossRef]
- Abu-Shanab, A.; Quigley, E.M.M. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 691–701. [Google Scholar] [CrossRef]
- Ghetti, F.D.; Oliveira, D.G.; de Oliveira, J.M.; de Castro Ferreira, L.E.V.V.; Cesar, D.E.; Moreira, A.P.B. Influence of gut microbiota on the development and progression of nonalcoholic steatohepatitis. Eur. J. Nutr. 2018, 57, 861–876. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.L.; He, J.; Zheng, P.; Mao, X.B.; Huang, Z.Q.; Yan, H.; Luo, Y.H.; Yu, J.; Luo, J.Q.; Yu, B.; et al. Prebiotic inulin as a treatment of obesity related nonalcoholic fatty liver disease through gut microbiota: A critical review. Crit. Rev. Food Sci. 2021, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Wang, Q.; Gould, T.; Slavin, J. Impact of Agaricus bisporus Mushroom Consumption on Gut Health Markers in Healthy Adults. Nutrients 2018, 10, 1402. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.R.; Lin, C.S.; Chang, C.J.; Lin, T.L.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Lu, C.C.; Young, J.D.; Lai, H.C.; et al. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 2019, 68, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Kang, S.; Hua, C.S.; Ting, Z.; Park, S. Synbiotic effects of beta-glucans from cauliflower mushroom and Lactobacillus fermentum on metabolic changes and gut microbiome in estrogen-deficient rats. Genes Nutr. 2017, 12, 31. [Google Scholar] [CrossRef]
- Fontes, A.; Alemany-Pages, M.; Oliveira, P.J.; Ramalho-Santos, J.; Zischka, H.; Azul, A.M. Antioxidant Versus Pro-Apoptotic Effects of Mushroom-Enriched Diets on Mitochondria in Liver Disease. Int. J. Mol. Sci. 2019, 20, 3987. [Google Scholar] [CrossRef]
- Hu, B.F.; Yang, H.B.; Chen, G.M.; Sun, X.J.; Zou, X.J.; Ma, J.; Yao, X.W.; Liang, Q.; Liu, H.T. Structural characterization and preventive effect on non-alcoholic fatty liver disease of oligosaccharides from Bletilla striata. Food Funct. 2022, 13, 4757–4769. [Google Scholar] [CrossRef]
- Cao, Y.N.; Yue, S.S.; Wang, A.Y.; Xu, L.; Hu, Y.T.; Qiao, X.; Wu, T.Y.; Ye, M.; Wu, Y.C.; Qi, R.; et al. Antrodia cinnamomea and its compound dehydroeburicoic acid attenuate nonalcoholic fatty liver disease by upregulating ALDH2 activity. J. Ethnopharmacol. 2022, 292, 115146. [Google Scholar] [CrossRef]
- Wu, X.F.; Wu, X.X.; Guo, W.J.; Luo, Q.; Gu, Y.H.; Shen, Y.; Tan, R.X.; Sun, Y.; Xu, Q.; Cerebroside, D.; et al. A glycoceramide compound, improves experimental colitis in mice with multiple targets against activated T lymphocytes. Toxicol. Appl. Pharm. 2012, 263, 296–302. [Google Scholar] [CrossRef]
- Rashvand, S.; Behrooz, M.; Samsamikor, M.; Jacobson, K.; Hekmatdoost, A. Dietary patterns and risk of ulcerative colitis: A case—Control study. J. Hum. Nutr. Diet. 2018, 31, 408–412. [Google Scholar] [CrossRef]
- Pereira, C.; Gracio, D.; Teixeira, J.P.; Magro, F. Oxidative Stress and DNA Damage: Implications in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2403–2417. [Google Scholar] [CrossRef]
- Wasilewski, A.; Zielinska, M.; Storr, M.; Fichna, J. Beneficial Effects of Probiotics, Prebiotics, Synbiotics, and Psychobiotics in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 1674–1682. [Google Scholar] [CrossRef]
- De Waal, G.M.; de Villiers, W.J.S.; Forgan, T.; Roberts, T.; Pretorius, E. Colorectal cancer is associated with increased circulating lipopolysaccharide, inflammation and hypercoagulability. Sci. Rep. 2020, 10, 8777. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Chen, D.; Wang, F.; Li, X.W.; Xue, X.M.; Jiang, M.Z.; Xu, B.; Chu, Y.; Wang, W.J.; Wu, K.C.; et al. Comparison of the efficiency of different enemas on patients with distal ulcerative colitis. Cell Prolif. 2019, 52, e12559. [Google Scholar] [CrossRef]
- Park, J.S.; Choi, J.; Jhun, J.; Kwon, J.Y.; Lee, B.I.; Yang, C.W.; Park, S.H.; Cho, M.L. Lactobacillus acidophilus Improves Intestinal Inflammation in an Acute Colitis Mouse Model by Regulation of Th17 and Treg Cell Balance and Fibrosis Development. J. Med. Food 2018, 21, 215–224. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, Y.; Stanton, C.; Ross, R.P.; Zhao, J.X.; Zhan, H.; Yang, B.; Chen, W. Alleviation effects of Bifidobacterium breve on DSS-induced colitis depends on intestinal tract barrier maintenance and gut microbiota modulation. Eur. J. Nutr. 2021, 60, 369–387. [Google Scholar] [CrossRef]
- Hoffmann, T.W.; Pham, H.P.; Bridonneau, C.; Aubry, C.; Lamas, B.; Martin-Gallausiaux, C.; Moroldo, M.; Rainteau, D.; Lapaque, N.; Six, A.; et al. Microorganisms linked to inflammatory bowel disease-associated dysbiosis differentially impact host physiology in gnotobiotic mice. ISME J. 2016, 10, 460–477. [Google Scholar] [CrossRef]
- Hanaoka, R.; Ueno, Y.; Tanaka, S.; Nagai, K.; Onitake, T.; Yoshioka, K.; Chayama, K. The Water-Soluble Extract from Cultured Medium of Ganoderma lucidum (Reishi) Mycelia (Designated as MAK) Ameliorates Murine Colitis Induced by Trinitrobenzene Sulphonic Acid. Scand. J. Immunol. 2011, 74, 454–462. [Google Scholar] [CrossRef]
- Li, M.Y.; Yu, L.L.; Zhai, Q.X.; Liu, B.S.; Zhao, J.X.; Zhang, H.; Chen, W.; Tian, F.W. Ganoderma applanatum polysaccharides and ethanol extracts promote the recovery of colitis through intestinal barrier protection and gut microbiota modulations. Food Funct. 2022, 13, 688–701. [Google Scholar] [CrossRef]
- Li, K.K.; Zhuo, C.; Teng, C.Y.; Yu, S.M.; Wang, X.; Hu, Y.; Ren, G.M.; Yu, M.; Qu, J.J. Effects of Ganoderma lucidum polysaccharides on chronic pancreatitis and intestinal microbiota in mice. Int. J. Biol. Macromol. 2016, 93, 904–912. [Google Scholar] [CrossRef]
- Guo, C.L.; Guo, D.D.; Fang, L.; Sang, T.T.; Wu, J.J.; Guo, C.J.; Wang, Y.J.; Wang, Y.; Chen, C.J.; Chen, J.J.; et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef]
- Ren, G.; Xu, L.; Lu, T.; Zhang, Y.; Wang, Y.; Yin, J. Protective effects of lentinan on lipopolysaccharide induced inflammatory response in intestine of juvenile taimen (Hucho taimen, Pallas). Int. J. Biol. Macromol. 2019, 121, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.L.; Yang, X.; Zheng, C.Q.; Yang, J.; Tang, X.C.; Chen, J.; Shuai, O.; Xie, Y.Z. Extracts from Hericium erinaceus relieve inflammatory bowel disease by regulating immunity and gut microbiota. Oncotarget 2017, 8, 85838–85857. [Google Scholar]
- Tsopmejio, I.S.N.; Ding, M.; Wei, J.L.; Zhao, C.; Jiang, Y.; Li, Y.T.; Song, H. Auricularia polytricha and Flammulina velutipes ameliorate inflammation and modulate the gut microbiota via regulation of NF-kappa B and Keap1/Nrf2 signaling pathways on DSS-induced inflammatory bowel disease. Food Biosci. 2022, 47, 101426. [Google Scholar] [CrossRef]
- Lu, C.L.; Li, H.X.; Zhu, X.Y.; Luo, Z.S.; Rao, S.Q.; Yang, Z.Q. Regulatory effect of intracellular polysaccharides from Antrodia cinnamomea on the intestinal microbiota of mice with antibiotic-associated diarrhea. Qual. Assur. Saf. Crops Foods 2022, 14, 124–134. [Google Scholar] [CrossRef]
- Hu, Q.; Yuan, B.; Wu, X.; Du, H.; Gu, M.; Han, Y.; Yang, W.; Song, M.; Xiao, H. Dietary Intake of Pleurotus eryngii Ameliorated Dextran-Sodium-Sulfate-Induced Colitis in Mice. Mol. Nutr. Food Res. 2019, 63, 1801265. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Pervin, M.; Cha, K.M.; Kim, S.K.; Lim, B.O. Anti-inflammatory activity on mice of extract of Ganoderma lucidum grown on rice via modulation of MAPK and NF-kappaB pathways. Phytochemistry 2015, 114, 125–136. [Google Scholar] [CrossRef]
- Lin, M.; Dong, L.; Chen, Q.; Xu, H.; Han, X.; Luo, R.; Pu, X.; Qi, S.; Nie, W.; Ma, M.; et al. Lentinan-Based Oral Nanoparticle Loaded Budesonide with Macrophage-Targeting Ability for Treatment of Ulcerative Colitis. Front. Bioeng. Biotechnol. 2021, 9, 702173. [Google Scholar] [CrossRef]
- Yin, C.M.; Noratto, G.D.; Fan, X.Z.; Chen, Z.Y.; Yao, F.; Shi, D.F.; Gao, H. The Impact of Mushroom Polysaccharides on Gut Microbiota and Its Beneficial Effects to Host: A Review. Carbohydr. Polym. 2020, 250, 116942. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Shao, S.; Wang, D.; Zheng, W.; Li, X.; Zhang, H.; Zhao, D.; Wang, M. A unique polysaccharide from Hericium erinaceus mycelium ameliorates acetic acid-induced ulcerative colitis rats by modulating the composition of the gut microbiota, short chain fatty acids levels and GPR41/43 respectors. Int. Immunopharmacol. 2019, 71, 411–422. [Google Scholar] [CrossRef]
- Grigsby, B.; Rodriguez-Rilo, H.; Khan, K. Antioxidants and Chronic Pancreatitis: Theory of Oxidative Stress and Trials of Antioxidant Therapy. Digest. Dis. Sci. 2012, 57, 835–841. [Google Scholar] [CrossRef]
- Ren, G.M.; Yu, M.; Li, K.K.; Hu, Y.; Wang, Y.; Xu, X.H.; Qu, J.J. Seleno-lentinan prevents chronic pancreatitis development and modulates gut microbiota in mice. J. Funct. Foods 2016, 22, 177–188. [Google Scholar] [CrossRef]
- Jin, M.L.; Zhu, Y.M.; Shao, D.Y.; Zhao, K.; Xu, C.L.; Li, Q.; Yang, H.; Huang, Q.S.; Shi, J.L. Effects of polysaccharide from mycelia of Ganoderma lucidum on intestinal barrier functions of rats. Int. J. Biol. Macromol. 2017, 94, 1–9. [Google Scholar] [CrossRef]
- Liu, J.S.; Liu, L.; Zhang, G.W.; Peng, X.C. Poria cocos polysaccharides attenuate chronic nonbacterial prostatitis by targeting the gut microbiota: Comparative study of Poria cocos polysaccharides and finasteride in treating chronic prostatitis. Int. J. Biol. Macromol. 2021, 189, 346–355. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Lu, S.M.; Li, Z.F. Extrahepatic factors in hepatic immune regulation. Front. Immunol. 2022, 13, 941721. [Google Scholar] [CrossRef]
- Huang, Q.X.; Gao, S.; Yao, Y.; Wang, Y.S.; Li, J.; Chen, J.J.; Guo, C.; Zhao, D.Q.; Li, X.Y. Innate immunity and immunotherapy for hemorrhagic shock. Front. Immunol. 2022, 13, 918380. [Google Scholar] [CrossRef]
- Wyatt, J.; Muller, M.M.; Tavassoli, M. Cancer Treatment Goes Viral: Using Viral Proteins to Induce Tumour-Specific Cell Death. Cancers 2019, 11, 1975. [Google Scholar] [CrossRef]
- Isik, A.; Soyturk, M.; Suleyman, S.; Firat, D.; Peker, K.; Yilmaz, I.; Celebi, F. Correlation of Bowel Wall Thickening Seen Using Computerized Tomography with Colonoscopies: A Preliminary Study. Surg. Laparosc. Endosc. Percutaneous Tech. 2017, 27, 154–157. [Google Scholar] [CrossRef]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef]
- Wang, F.; Meng, W.B.; Wang, B.Y.; Qiao, L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014, 345, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Mira-Pascual, L.; Cabrera-Rubio, R.; Ocon, S.; Costales, P.; Parra, A.; Suarez, A.; Moris, F.; Rodrigo, L.; Mira, A.; Collado, M.C.; et al. Microbial mucosal colonic shifts associated with the development of colorectal cancer reveal the presence of different bacterial and archaeal biomarkers. J. Gastroenterol. 2015, 50, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Lin, W.C.; Kong, M.S.; Shi, H.N.; Walker, W.A.; Lin, C.Y.; Huang, C.T.; Lin, Y.C.; Jung, S.M.; Lin, T.Y.; et al. Oral inoculation of probiotics Lactobacillus acidophilus NCFM suppresses tumour growth both in segmental orthotopic colon cancer and extra-intestinal tissue. Br. J. Nutr. 2012, 107, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; Delgado, S.; Ruas-Madiedo, P.; Sanchez, B.; Margolles, A. Bifidobacteria and Their Molecular Communication with the Immune System. Front. Microbiol. 2017, 8, 2345. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Li, K.; Sang, T.; Wu, K.; Wang, Y.; Wang, X. Anticarcinogenic effects of water extract of sporoderm-broken spores of Ganoderma lucidum on colorectal cancer in vitro and in vivo. Int. J. Oncol. 2017, 50, 1541–1554. [Google Scholar] [CrossRef]
- Su, J.Y.; Su, L.; Li, D.; Shuai, O.; Zhang, Y.F.; Liang, H.J.; Jiao, C.W.; Xu, Z.C.; Lai, Y.; Xie, Y.Z.; et al. Antitumor Activity of Extract from the Sporoderm-Breaking Spore of Ganoderma lucidum: Restoration on Exhausted Cytotoxic T Cell with Gut Microbiota Remodeling. Front. Immunol. 2018, 9, 1765. [Google Scholar] [CrossRef]
- Su, J.Y.; Li, D.; Chen, Q.J.; Li, M.X.; Su, L.; Luo, T.; Liang, D.L.; Lai, G.X.; Shuai, O.; Jiao, C.W.; et al. Anti-breast Cancer Enhancement of a Polysaccharide from Spore of Ganoderma lucidum With Paclitaxel: Suppression on Tumor Metabolism with Gut Microbiota Reshaping. Front. Microbiol. 2019, 10, 3099. [Google Scholar] [CrossRef]
- Khan, I.; Huang, G.X.; Li, X.A.; Liao, W.L.; Leong, W.K.; Xia, W.R.; Bian, X.Q.; Wu, J.L.; Hsiao, W.L.W. Mushroom polysaccharides and jiaogulan saponins exert cancer preventive effects by shaping the gut microbiota and microenvironment in Apc(Min/+) mice. Pharmacol. Res. 2019, 148, 104448. [Google Scholar] [CrossRef]
- Wang, C.H.; Yang, S.X.; Gao, L.; Wang, L.L.; Cao, L. Carboxymethyl pachyman (CMP) reduces intestinal mucositis and regulates the intestinal microflora in 5-fluorouracil-treated CT26 tumour-bearing mice. Food Funct. 2018, 9, 2695–2704. [Google Scholar] [CrossRef]
- Eslami, M.; Yousefi, B.; Kokhaei, P.; Hemati, M.; Nejad, Z.R.; Arabkari, V.; Namdar, A. Importance of probiotics in the prevention and treatment of colorectal cancer. J. Cell Physiol. 2019, 234, 17127–17143. [Google Scholar] [CrossRef]
- Tannock, G.W.; Liu, Y.F. Guided dietary fibre intake as a means of directing short-chain fatty acid production by the gut microbiota. J. R. Soc. N. Z. 2020, 50, 434–455. [Google Scholar] [CrossRef]
- Raman, M.; Ambalam, P.; Kondepudi, K.K.; Pithva, S.; Kothari, C.; Patel, A.T.; Purama, R.K.; Dave, J.M.; Vyas, B.R.M. Potential of probiotics, prebiotics and synbiotics for management of colorectal cancer. Gut Microbes 2013, 4, 181–192. [Google Scholar] [CrossRef]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary Gut Microbial Metabolites, Short-chain Fatty Acids, and Host Metabolic Regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef]
- Li, L.F.; Liu, H.B.; Zhang, Q.W.; Li, Z.P.; Wong, T.L.; Fung, H.Y.; Zhang, J.X.; Bai, S.P.; Lu, A.P.; Han, Q.B. Comprehensive comparison of polysaccharides from Ganoderma lucidum and G-sinense: Chemical, antitumor, immunomodulating and gut-microbiota modulatory properties. Sci. Rep. 2018, 8, 6172. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Lin, C.X.; Zhao, S.; Zhu, Y.L.; Fan, Z.Q.; Wang, J.; Zhang, B.R.; Chen, Y.X. Microbiota-gut-brain axis and toll-like receptors in Alzheimer’s disease. Comput. Struct. Biotechnol. J. 2019, 17, 1309–1317. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.; Cheng, Y.; Yao, W.; Qian, H. Neuroprotective effects of polysaccharide from Sparassis crispa on Alzheimer’s disease-like mice: Involvement of microbiota-gut-brain axis. Int. J. Biol. Macromol. 2022, 225, 974–986. [Google Scholar] [CrossRef]
- Gao, Y.; Li, B.; Liu, H.; Tian, Y.; Gu, C.; Du, X.; Bu, R.; Gao, J.; Liu, Y.; Li, G.; et al. Cistanche deserticola polysaccharides alleviate cognitive decline in aging model mice by restoring the gut microbiota-brain axis. Aging 2021, 13, 15320–15335. [Google Scholar] [CrossRef]
- Newgard, C.B.; Sharpless, N.E. Coming of age: Molecular drivers of aging and therapeutic opportunities. J. Clin. Investig. 2013, 123, 946–950. [Google Scholar] [CrossRef]
- Muturi, E.J.; Ramirez, J.L.; Rooney, A.P.; Kim, C.H. Comparative analysis of gut microbiota of mosquito communities in central Illinois. PLoS Negl. Trop. Dis. 2017, 11, e0005377. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.P.; Chen, X.; Zhang, Y.F.; Liu, X.; Wang, C.Y.; Teng, L.S.; Wang, D. Protective roles of Amanita caesarea polysaccharides against Alzheimer’s disease via Nrf2 pathway. Int. J. Biol. Macromol. 2019, 121, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.R.; Xing, S.L.; Chen, C.; Shen, D.Z.; Chen, J.L. Codonopsis pilosula Polysaccharides Alleviate Abeta (1-40)-Induced PC12 Cells Energy Dysmetabolism via CD38/NAD+ Signaling Pathway. Curr. Alzheimer Res. 2021, 18, 208–221. [Google Scholar] [PubMed]
- An, S.; Lu, W.; Zhang, Y.; Yuan, Q.; Wang, D. Pharmacological Basis for Use of Armillaria mellea Polysaccharides in Alzheimer’s Disease: Antiapoptosis and Antioxidation. Oxidative Med. Cell Longev. 2017, 2017, 4184562. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, P.; Chen, Z.; Sui, X.; Xie, X.; Zhang, J. Alteration of the fecal microbiota in North-Eastern Han Chinese population with sporadic Parkinson’s disease. Neurosci. Lett. 2019, 707, 134297. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Song, Y.; Qin, N.; Chen, S.D.; Xiao, Q. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav. Immun. 2018, 70, 194–202. [Google Scholar] [CrossRef]
- Aho, V.T.E.; Pereira, P.A.B.; Voutilainen, S.; Paulin, L.; Pekkonen, E.; Auvinen, P.; Scheperjans, F. Gut microbiota in Parkinson’s disease: Temporal stability and relations to disease progression. EBioMedicine 2019, 44, 691–707. [Google Scholar] [CrossRef]
- Bedarf, J.R.; Hildebrand, F.; Coelho, L.P.; Sunagawa, S.; Bahram, M.; Goeser, F.; Bork, P.; Wullner, U. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naive Parkinson’s disease patients. Genome Med. 2017, 9, 39. [Google Scholar] [CrossRef]
- Claudino dos Santos, J.C.; Lima, M.P.P.; Brito, G.A.C.; Viana, G.S.B. Role of enteric glia and microbiota-gut-brain axis in parkinson disease pathogenesis. Ageing Res. Rev. 2022, 84, 101812. [Google Scholar] [CrossRef]
- Castelli, V.; d’Angelo, M.; Lombardi, F.; Alfonsetti, M.; Antonosante, A.; Catanesi, M.; Benedetti, E.; Palumbo, P.; Cifone, M.G.; Giordano, A.; et al. Effects of the probiotic formulation SLAB51 in in vitro and in vivo Parkinson’s disease models. Aging 2020, 12, 4641–4659. [Google Scholar] [CrossRef]
- Shen, T.; Yue, Y.M.; He, T.T.; Huang, C.; Qu, B.Y.; Lv, W.; Lai, H.Y. The Association Between the Gut Microbiota and Parkinson’s Disease, a Meta-Analysis. Front. Aging Neurosci. 2021, 13, 636545. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.L.; Wang, C.D.; Wang, T.; Ding, H.; Zhou, M.; Yang, N.; Liu, Y.Y.; Chan, P. Ganoderma lucidum extract ameliorates MPTP-induced parkinsonism and protects dopaminergic neurons from oxidative stress via regulating mitochondrial function, autophagy, and apoptosis. Acta Pharmacol. Sin. 2019, 40, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Badshah, S.L.; Riaz, A.; Muhammad, A.; Cayan, G.T.; Cayan, F.; Duru, M.E.; Ahmad, N.; Emwas, A.H.; Jaremko, M. Isolation, Characterization, and Medicinal Potential of Polysaccharides of Morchella esculenta. Molecules 2021, 26, 1459. [Google Scholar] [CrossRef] [PubMed]
- Hebuterne, X. Gut changes attributed to ageing: Effects on intestinal microflora. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 49–54. [Google Scholar] [CrossRef]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkila, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through Ageing, and Beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut Microbiota and Extreme Longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef]
- Roberts, C.K.; Barnard, R.J. Effects of exercise and diet on chronic disease. J. Appl. Physiol. 2005, 98, 3–30. [Google Scholar] [CrossRef]
- Zhong, L.; Ma, N.; Zheng, H.H.; Ma, G.X.; Zhao, L.Y.; Hu, Q.H. Tuber indicum polysaccharide relieves fatigue by regulating gut microbiota in mice. J. Funct. Foods 2019, 63, 103580. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).