Chemical- and Drug-Induced Allergic, Inflammatory, and Autoimmune Diseases Via Haptenation
Abstract
Simple Summary
Abstract
1. Introduction
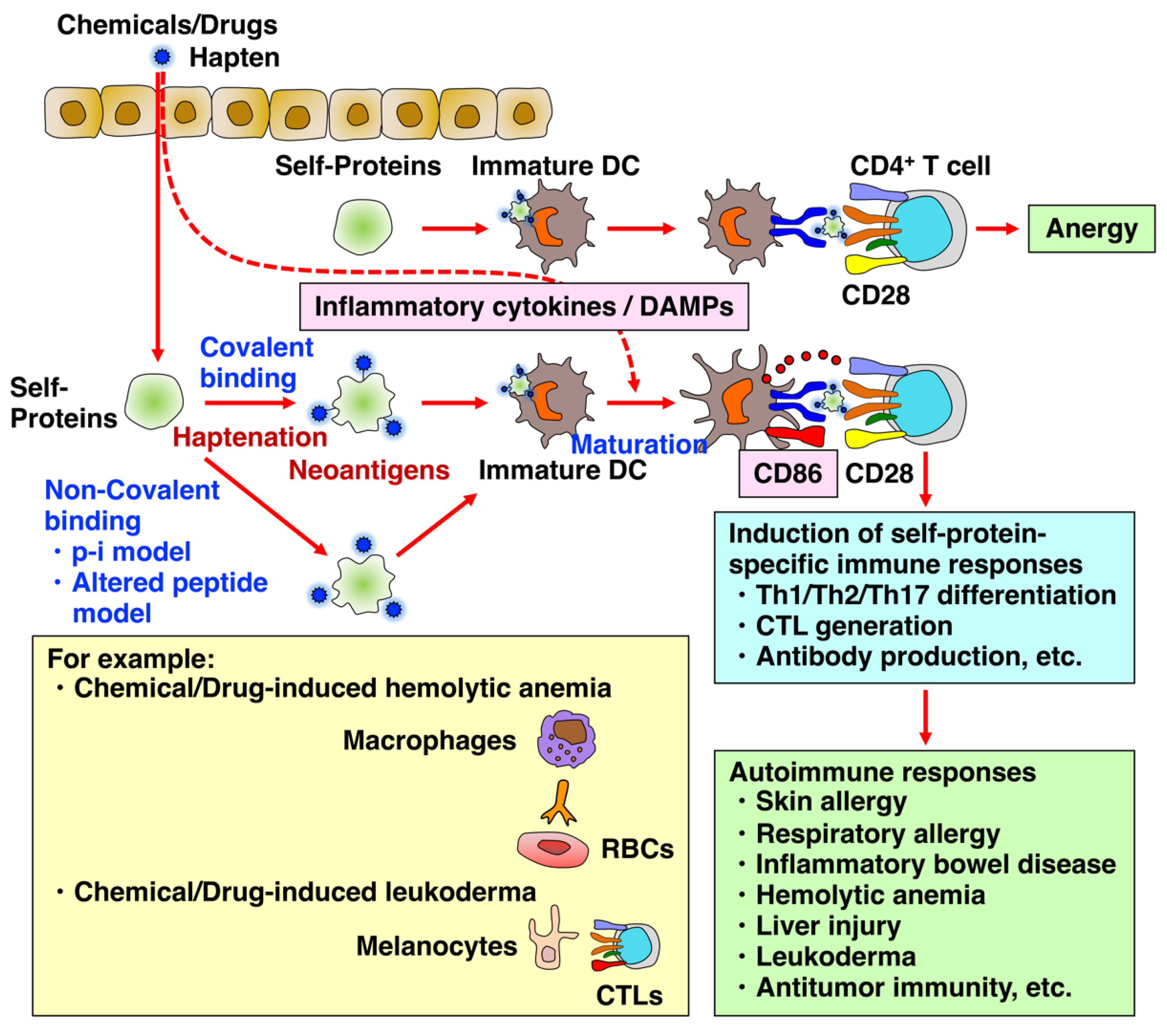
2. Chemical- and Drug-Induced Allery, Inflammatory, and Autoimmune Diseases
2.1. Chemical- and Drug-Induced Allergy
2.2. Chemical- and Drug-Induced IBD
2.3. Chemical- and Drug-Induced Autoimmune Hemolytic Anemia
2.4. Chemical- and Drug-Induced Liver Injury
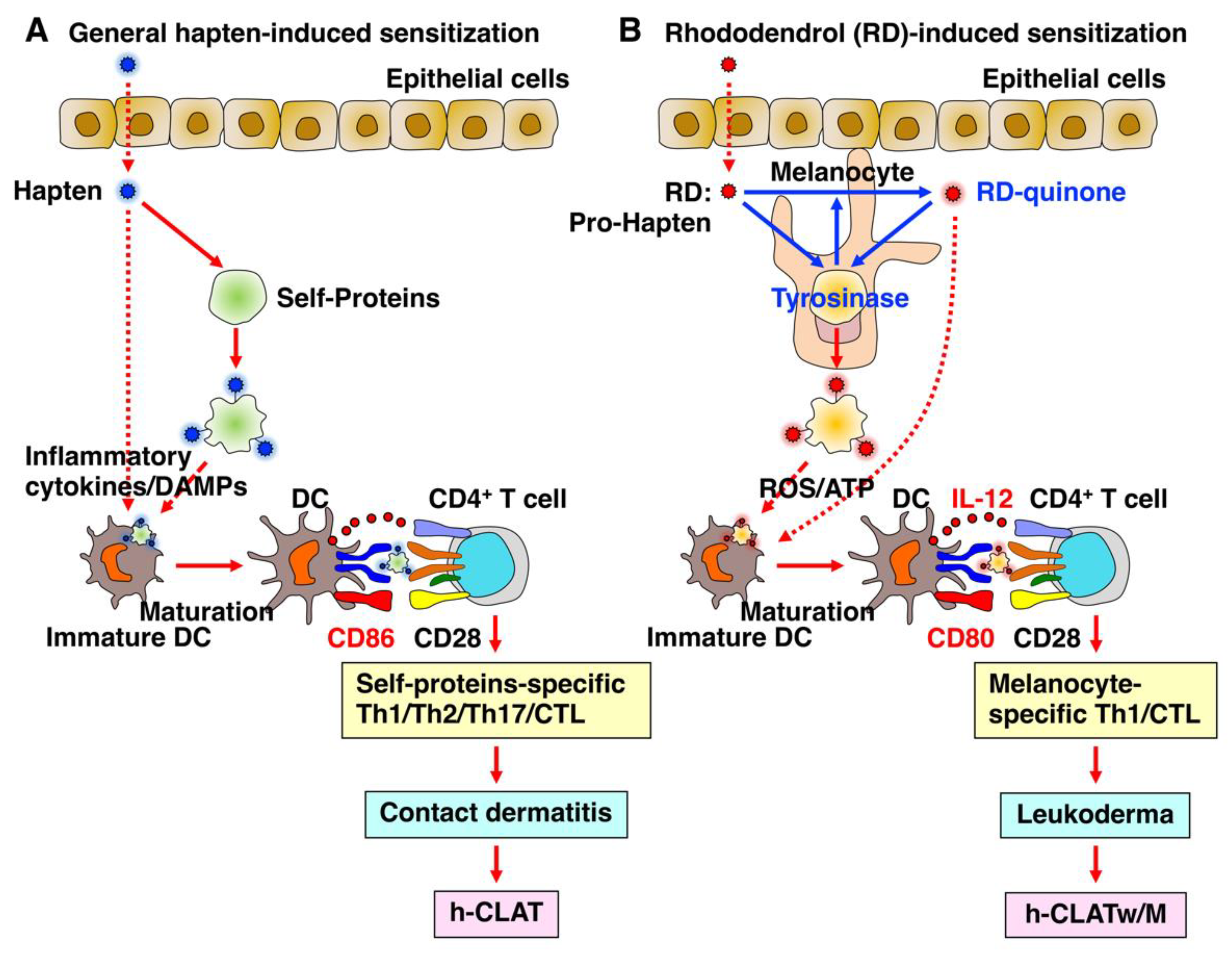
2.5. Chemical- and Drug-Induced Leukoderma
2.6. Chemical- and Drug-Induced Antitumor Immunity
2.7. Chemical- and Drug-Induced Hapten Inhibition
3. In Vitro Cell Culture System to Predict Whether Chemicals and Drugs May Cause Autoimmune Responses in Advance
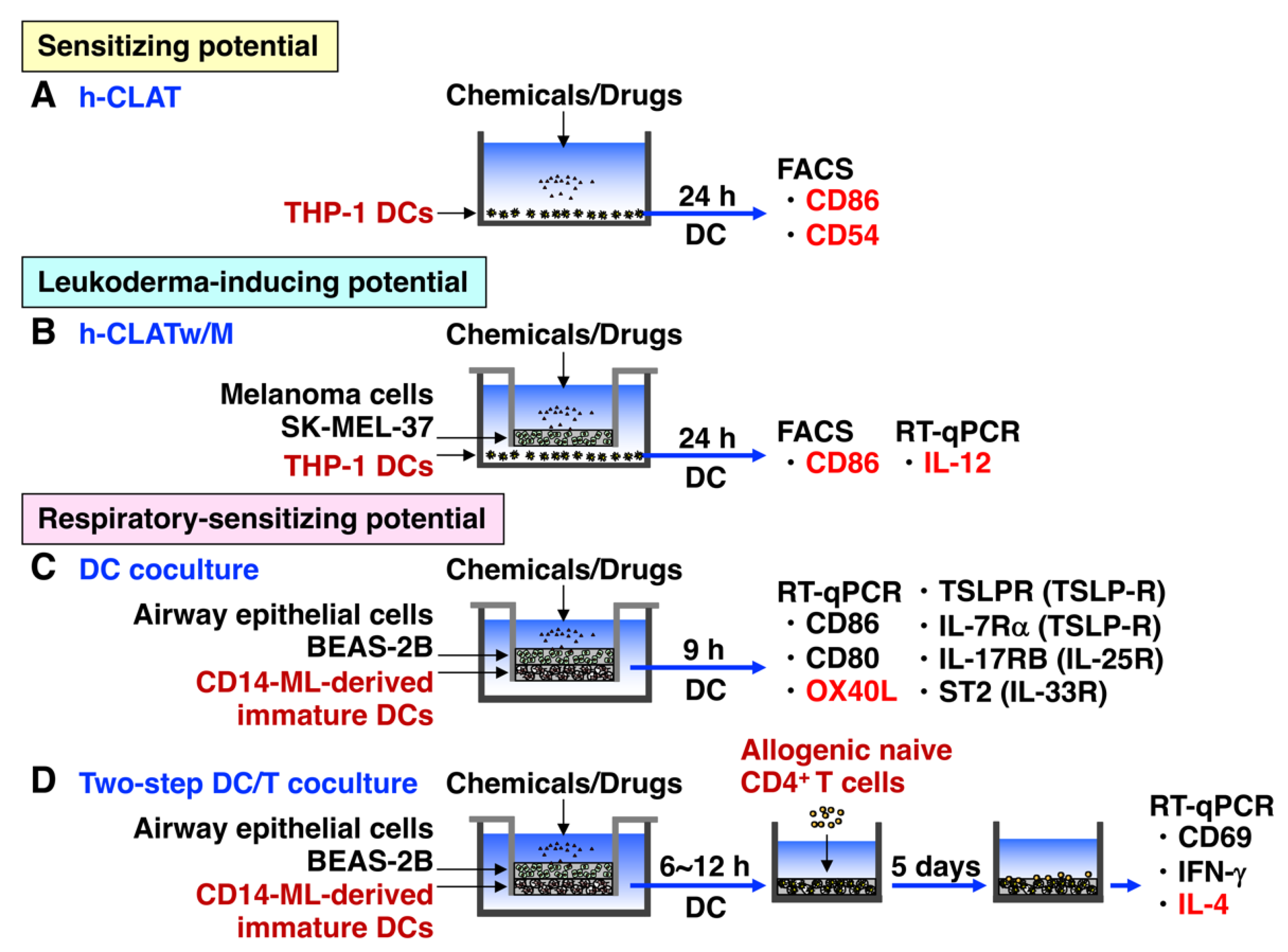
3.1. h-CLAT
3.2. h-CLATw/M
3.3. h-CLAT with Other Cells
3.4. DC Coculture
3.5. DC/T Coculture
3.6. DC/T/B Coculture
4. New Concepts of Chemical- and Drug-Induced T Cell Activation
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Landsteiner, K.; Jacobs, J. Studies on the Sensitization of Animals with Simple Chemical Compounds. J. Exp. Med. 1936, 64, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Aptula, A.O.; Roberts, D.W.; Pease, C.K. Haptens, prohaptens and prehaptens, or electrophiles and proelectrophiles. Contact Dermat. 2007, 56, 54–56. [Google Scholar] [CrossRef]
- Gell, P.G.H.; Coombs, R.R.A. The classification of allergic reactions underlying disease. In Clinical Aspects of Immunology; Gell, P.G.H., Coombs, R.R.A., Eds.; Blackwell Science: Hoboken, NJ, USA, 1963. [Google Scholar]
- Vocanson, M.; Hennino, A.; Rozieres, A.; Poyet, G.; Nicolas, J.F. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy 2009, 64, 1699–1714. [Google Scholar] [CrossRef] [PubMed]
- Erkes, D.A.; Selvan, S.R. Hapten-induced contact hypersensitivity, autoimmune reactions, and tumor regression: Plausibility of mediating antitumor immunity. J. Immunol. Res. 2014, 2014, 175265. [Google Scholar] [CrossRef] [PubMed]
- Esser, P.R.; Wolfle, U.; Durr, C.; von Loewenich, F.D.; Schempp, C.M.; Freudenberg, M.A.; Jakob, T.; Martin, S.F. Contact sensitizers induce skin inflammation via ROS production and hyaluronic acid degradation. PLoS ONE 2012, 7, e41340. [Google Scholar] [CrossRef]
- Martin, S.F.; Dudda, J.C.; Bachtanian, E.; Lembo, A.; Liller, S.; Durr, C.; Heimesaat, M.M.; Bereswill, S.; Fejer, G.; Vassileva, R.; et al. Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. J. Exp. Med. 2008, 205, 2151–2162. [Google Scholar] [CrossRef]
- Schmidt, M.; Raghavan, B.; Muller, V.; Vogl, T.; Fejer, G.; Tchaptchet, S.; Keck, S.; Kalis, C.; Nielsen, P.J.; Galanos, C.; et al. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat. Immunol. 2010, 11, 814–819. [Google Scholar] [CrossRef]
- Weber, F.C.; Esser, P.R.; Muller, T.; Ganesan, J.; Pellegatti, P.; Simon, M.M.; Zeiser, R.; Idzko, M.; Jakob, T.; Martin, S.F. Lack of the purinergic receptor P2X(7) results in resistance to contact hypersensitivity. J. Exp. Med. 2010, 207, 2609–2619. [Google Scholar] [CrossRef]
- Nagaoka, M.; Radi, Z.A. Pharmacologic efficacy in inflammatory bowel disease models. Front. Biosci. 2012, 4, 1295–1314. [Google Scholar] [CrossRef]
- Matsunaga, K.; Suzuki, K.; Ito, A.; Tanemura, A.; Abe, Y.; Suzuki, T.; Yoshikawa, M.; Sumikawa, Y.; Yagami, A.; Masui, Y.; et al. Rhododendrol-induced leukoderma update I: Clinical findings and treatment. J. Dermatol. 2021, 48, 961–968. [Google Scholar] [CrossRef]
- Inoue, S.; Katayama, I.; Suzuki, T.; Tanemura, A.; Ito, S.; Abe, Y.; Sumikawa, Y.; Yoshikawa, M.; Suzuki, K.; Yagami, A.; et al. Rhododendrol-induced leukoderma update II: Pathophysiology, mechanisms, risk evaluation, and possible mechanism-based treatments in comparison with vitiligo. J. Dermatol. 2021, 48, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Chipinda, I.; Hettick, J.M.; Siegel, P.D. Haptenation: Chemical reactivity and protein binding. J. Allergy 2011, 2011, 839682. [Google Scholar] [CrossRef]
- Hamaoka, T.; Fujiwara, H.; Teshima, K.; Aoki, H.; Yamamoto, H.; Kitagawa, M. Regulatory functions of hapten-reactive helper and suppressor T lymphocytes. III. Amplification of a generation of tumor-specific killer T-lymphocyte activities by suppressor T-cell-depleted hapten-reactive T lymphocytes. J. Exp. Med. 1979, 149, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Moriyama, Y.; Suda, T.; Tsuchida, T.; Shearer, G.M.; Hamaoka, T. Enhanced TNP-reactive helper T cell activity and its utilization in the induction of amplified tumor immunity that results in tumor regression. J. Immunol. 1984, 132, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Berd, D.; Sato, T.; Maguire, H.C., Jr.; Kairys, J.; Mastrangelo, M.J. Immunopharmacologic analysis of an autologous, hapten-modified human melanoma vaccine. J. Clin. Oncol. 2004, 22, 403–415. [Google Scholar] [CrossRef]
- Lu, Y.; Low, P.S. Folate targeting of haptens to cancer cell surfaces mediates immunotherapy of syngeneic murine tumors. Cancer Immunol. Immunother. 2002, 51, 153–162. [Google Scholar] [CrossRef]
- Martin, S.F.; Esser, P.R.; Weber, F.C.; Jakob, T.; Freudenberg, M.A.; Schmidt, M.; Goebeler, M. Mechanisms of chemical-induced innate immunity in allergic contact dermatitis. Allergy 2011, 66, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Roggen, E.L. In vitro approaches for detection of chemical sensitization. Basic Clin. Pharmacol. Toxicol. 2014, 115, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Arts, J.H.; de Jong, W.H.; van Triel, J.J.; Schijf, M.A.; de Klerk, A.; van Loveren, H.; Kuper, C.F. The respiratory local lymph node assay as a tool to study respiratory sensitizers. Toxicol. Sci. 2008, 106, 423–434. [Google Scholar] [CrossRef]
- Dearman, R.J.; Basketter, D.A.; Kimber, I. Differential cytokine production following chronic exposure of mice to chemical respiratory and contact allergens. Immunology 1995, 86, 545–550. [Google Scholar]
- Vandebriel, R.J.; De Jong, W.H.; Spiekstra, S.W.; Van Dijk, M.; Fluitman, A.; Garssen, J.; Van Loveren, H. Assessment of preferential T-helper 1 or T-helper 2 induction by low molecular weight compounds using the local lymph node assay in conjunction with RT-PCR and ELISA for interferon-gamma and interleukin-4. Toxicol. Appl. Pharmacol. 2000, 162, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Goutet, M.; Pepin, E.; Langonne, I.; Huguet, N.; Ban, M. Identification of contact and respiratory sensitizers according to IL-4 receptor alpha expression and IL-2 production. Toxicol. Appl. Pharmacol. 2012, 260, 95–104. [Google Scholar] [CrossRef]
- Adenuga, D.; Woolhiser, M.R.; Gollapudi, B.B.; Boverhof, D.R. Differential gene expression responses distinguish contact and respiratory sensitizers and nonsensitizing irritants in the local lymph node assay. Toxicol. Sci. 2012, 126, 413–425. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Arts, J.H.; De Klerk, A.; Schijf, M.A.; Ezendam, J.; Kuper, C.F.; Van Loveren, H. Contact and respiratory sensitizers can be identified by cytokine profiles following inhalation exposure. Toxicology 2009, 261, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Kimber, I.; Dearman, R.J. Chemical respiratory allergy: Role of IgE antibody and relevance of route of exposure. Toxicology 2002, 181–182, 311–315. [Google Scholar] [CrossRef]
- Kimber, I.; Dearman, R.J.; Basketter, D.A.; Boverhof, D.R. Chemical respiratory allergy: Reverse engineering an adverse outcome pathway. Toxicology 2014, 318, 32–39. [Google Scholar] [CrossRef]
- Savage, J.H.; Matsui, E.C.; Wood, R.A.; Keet, C.A. Urinary levels of triclosan and parabens are associated with aeroallergen and food sensitization. J. Allergy Clin. Immunol. 2012, 130, 453–460.e457. [Google Scholar] [CrossRef]
- Spanier, A.J.; Fausnight, T.; Camacho, T.F.; Braun, J.M. The associations of triclosan and paraben exposure with allergen sensitization and wheeze in children. Allergy Asthma Proc. 2014, 35, 475–481. [Google Scholar] [CrossRef]
- Krutz, N.L.; Kimber, I.; Ryan, C.A.; Kern, P.S.; Gerberick, G.F. Critical Evaluation of Low-Molecular Weight Respiratory Sensitizers and Their Protein Reactivity Potential Toward Lysine Residues. Toxicol. Sci. 2021, 182, 346–354. [Google Scholar] [CrossRef]
- Sadekar, N.; Boisleve, F.; Dekant, W.; Fryer, A.D.; Gerberick, G.F.; Griem, P.; Hickey, C.; Krutz, N.L.; Lemke, O.; Mignatelli, C.; et al. Identifying a reference list of respiratory sensitizers for the evaluation of novel approaches to study respiratory sensitization. Crit. Rev. Toxicol. 2021, 51, 792–804. [Google Scholar] [CrossRef]
- Kim, Y.; Flamm, A.; ElSohly, M.A.; Kaplan, D.H.; Hage, R.J., Jr.; Hamann, C.P.; Marks, J.G., Jr. Poison Ivy, Oak, and Sumac Dermatitis: What Is Known and What Is New? Dermatitis 2019, 30, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Watchmaker, L.; Reeder, M.; Atwater, A.R. Plant Dermatitis: More Than Just Poison Ivy. Cutis 2021, 108, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Lofgran, T.; Mahabal, G.D. Toxicodendron Toxicity. In StatPearls; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Pacheco, R.; Quezada, S.A.; Kalergis, A.M.; Becker, M.I.; Ferreira, J.; De Ioannes, A.E. Allergens of the urushiol family promote mitochondrial dysfunction by inhibiting the electron transport at the level of cytochromes b and chemically modify cytochrome c(1). Biol. Res. 2021, 54, 35. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.B.; Kalergis, A.M.; Becker, M.I.; Garbarino, J.A.; De Ioannes, A.E. CD8+ T cells are the effectors of the contact dermatitis induced by urushiol in mice and are regulated by CD4+ T cells. Int. Arch. Allergy Immunol. 1998, 117, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Neurath, M.F.; Fuss, I.; Kelsall, B.L.; Stuber, E.; Strober, W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J. Exp. Med. 1995, 182, 1281–1290. [Google Scholar] [CrossRef]
- Katsandegwaza, B.; Horsnell, W.; Smith, K. Inflammatory Bowel Disease: A Review of Pre-Clinical Murine Models of Human Disease. Int. J. Mol. Sci. 2022, 23, 9344. [Google Scholar] [CrossRef]
- Silva, I.; Pinto, R.; Mateus, V. Preclinical Study in Vivo for New Pharmacological Approaches in Inflammatory Bowel Disease: A Systematic Review of Chronic Model of TNBS-Induced Colitis. J. Clin. Med. 2019, 8, 1574. [Google Scholar] [CrossRef]
- Silva, I.; Solas, J.; Pinto, R.; Mateus, V. Chronic Experimental Model of TNBS-Induced Colitis to Study Inflammatory Bowel Disease. Int. J. Mol. Sci. 2022, 23, 4739. [Google Scholar] [CrossRef]
- Boirivant, M.; Fuss, I.J.; Chu, A.; Strober, W. Oxazolone colitis: A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J. Exp. Med. 1998, 188, 1929–1939. [Google Scholar] [CrossRef]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Heller, F.; Fuss, I.J.; Nieuwenhuis, E.E.; Blumberg, R.S.; Strober, W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity 2002, 17, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, W.; Panes, J.; Khurana, S.; Toth, G.; Hua, F.; Comer, G.M.; Hinz, M.; Page, K.; O’Toole, M.; Moorehead, T.M.; et al. Anrukinzumab, an anti-interleukin 13 monoclonal antibody, in active UC: Efficacy and safety from a phase IIa randomised multicentre study. Gut 2015, 64, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Rudzinski, J.; Brandt, W.; Dupas, J.L.; Peyrin-Biroulet, L.; Bouhnik, Y.; Kleczkowski, D.; Uebel, P.; Lukas, M.; Knutsson, M.; et al. Tralokinumab for moderate-to-severe UC: A randomised, double-blind, placebo-controlled, phase IIa study. Gut 2015, 64, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Weltzien, H.U.; Padovan, E. Molecular features of penicillin allergy. J. Investig. Dermatol. 1998, 110, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.J.R.; Tuomisto, J.E.E.; Purcell, A.W.; Mifsud, N.A.; Illing, P.T. The complexity of T cell-mediated penicillin hypersensitivity reactions. Allergy 2021, 76, 150–167. [Google Scholar] [CrossRef]
- Rodriguez-Pena, R.; Antunez, C.; Martin, E.; Blanca-Lopez, N.; Mayorga, C.; Torres, M.J. Allergic reactions to beta-lactams. Expert Opin. Drug Saf. 2006, 5, 31–48. [Google Scholar] [CrossRef]
- Garratty, G. Drug-induced immune hemolytic anemia. Hematol. Am. Soc. Hematol. Educ. Program. 2009, 8, 73–79. [Google Scholar] [CrossRef]
- Arndt, P.A. Drug-induced immune hemolytic anemia: The last 30 years of changes. Immunohematology 2014, 30, 44–54. [Google Scholar] [CrossRef]
- Branch, D.R. Drug-induced immune haemolytic anemias. ISBT Sci. Ser. 2019, 14, 49–52. [Google Scholar] [CrossRef]
- Fisher, K.; Vuppalanchi, R.; Saxena, R. Drug-Induced Liver Injury. Arch. Pathol. Lab. Med. 2015, 139, 876–887. [Google Scholar] [CrossRef]
- Katarey, D.; Verma, S. Drug-induced liver injury. Clin. Med. 2016, 16, s104–s109. [Google Scholar] [CrossRef] [PubMed]
- Kuna, L.; Bozic, I.; Kizivat, T.; Bojanic, K.; Mrso, M.; Kralj, E.; Smolic, R.; Wu, G.Y.; Smolic, M. Models of Drug Induced Liver Injury (DILI)—Current Issues and Future Perspectives. Curr. Drug Metab. 2018, 19, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Habibollahi, P.; Mahboobi, N.; Esmaeili, S.; Safari, S.; Dabbagh, A.; Alavian, S.M. Halothane-induced hepatitis: A forgotten issue in developing countries: Halothane-induced hepatitis. Hepat. Mon. 2011, 11, 3–6. [Google Scholar] [PubMed]
- Kenna, J.G.; Knight, T.L.; van Pelt, F.N. Immunity to halothane metabolite-modified proteins in halothane hepatitis. Ann. N. Y. Acad. Sci. 1993, 685, 646–661. [Google Scholar] [CrossRef]
- Kurth, M.J.; Yokoi, T.; Gershwin, M.E. Halothane-induced hepatitis: Paradigm or paradox for drug-induced liver injury. Hepatology 2014, 60, 1473–1475. [Google Scholar] [CrossRef]
- Kenna, J.G.; Neuberger, J.; Williams, R. Evidence for expression in human liver of halothane-induced neoantigens recognized by antibodies in sera from patients with halothane hepatitis. Hepatology 1988, 8, 1635–1641. [Google Scholar] [CrossRef]
- Kenna, J.G.; Martin, J.L.; Satoh, H.; Pohl, L.R. Factors affecting the expression of trifluoroacetylated liver microsomal protein neoantigens in rats treated with halothane. Drug Metab. Dispos. 1990, 18, 788–793. [Google Scholar]
- Harris, J.E. Chemical-Induced Vitiligo. Dermatol. Clin. 2017, 35, 151–161. [Google Scholar] [CrossRef]
- Tokura, Y.; Fujiyama, T.; Ikeya, S.; Tatsuno, K.; Aoshima, M.; Kasuya, A.; Ito, T. Biochemical, cytological, and immunological mechanisms of rhododendrol-induced leukoderma. J. Dermatol. Sci. 2015, 77, 146–149. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Biochemical Mechanism of Rhododendrol-Induced Leukoderma. Int. J. Mol. Sci. 2018, 19, 552. [Google Scholar] [CrossRef] [PubMed]
- van den Boorn, J.G.; Picavet, D.I.; van Swieten, P.F.; van Veen, H.A.; Konijnenberg, D.; van Veelen, P.A.; van Capel, T.; Jong, E.C.; Reits, E.A.; Drijfhout, J.W.; et al. Skin-depigmenting agent monobenzone induces potent T-cell autoimmunity toward pigmented cells by tyrosinase haptenation and melanosome autophagy. J. Investig. Dermatol. 2011, 131, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Nishigaki, A.; Ishii-Osai, Y.; Ojika, M.; Wakamatsu, K.; Yamashita, T.; Tamura, Y.; Ito, A.; Honda, H.; Nakayama, E.; et al. Mechanism of putative neo-antigen formation from N-propionyl-4-S-cysteaminylphenol, a tyrosinase substrate, in melanoma models. Biochem. Pharmacol. 2012, 84, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Westerhof, W.; Manini, P.; Napolitano, A.; d’Ischia, M. The haptenation theory of vitiligo and melanoma rejection: A close-up. Exp. Dermatol. 2011, 20, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Zeng, H.; Takahashi, T.; Maeda, K. In vitro methods for predicting chemical leukoderma caused by quasi-drug cosmetics. Cosmetics 2017, 4, 31. [Google Scholar] [CrossRef]
- Gabe, Y.; Miyaji, A.; Kohno, M.; Hachiya, A.; Moriwaki, S.; Baba, T. Substantial evidence for the rhododendrol-induced generation of hydroxyl radicals that causes melanocyte cytotoxicity and induces chemical leukoderma. J. Dermatol. Sci. 2018, 91, 311–316. [Google Scholar] [CrossRef]
- Katahira, Y.; Sakamoto, E.; Watanabe, A.; Furusaka, Y.; Inoue, S.; Hasegawa, H.; Mizoguchi, I.; Yo, K.; Yamaji, F.; Toyoda, A.; et al. Upregulation of CD86 and IL-12 by rhododendrol in THP-1 cells cocultured with melanocytes through ROS and ATP. J Dermatol Sci. 2022. [Google Scholar] [CrossRef]
- Takagi, R.; Kawano, M.; Nakamura, K.; Tsuchida, T.; Matsushita, S. T-Cell Responses to Tyrosinase-Derived Self-Peptides in Patients with Leukoderma Induced by Rhododendrol: Implications for Immunotherapy Targeting Melanoma. Dermatology 2016, 232, 44–49. [Google Scholar] [CrossRef]
- Schrand, B.; Clark, E.; Levay, A.; Capote, A.R.; Martinez, O.; Brenneman, R.; Castro, I.; Gilboa, E. Hapten-mediated recruitment of polyclonal antibodies to tumors engenders antitumor immunity. Nat. Commun. 2018, 9, 3348. [Google Scholar] [CrossRef]
- Ljungstrom, K.G.; Willman, B.; Hedin, H. Hapten inhibition of dextran anaphylaxis. Nine years of post-marketing surveillance of dextran 1. Ann. Françaises D’anesthésie Réanimation 1993, 12, 219–222. [Google Scholar] [CrossRef]
- Hanzek, I.; Tonkovic, D.; Margaretic Piljek, N.; Palian, M.; Mihaljevic, D.; Penavic, A.; Mihaljevic, S. Allergic reactions to colloid fluids in anesthesia. Psychiatr. Danub. 2020, 32, 429–431. [Google Scholar] [PubMed]
- Lack, G. Epidemiologic risks for food allergy. J. Allergy Clin. Immunol. 2008, 121, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- MacKay, C.; Davies, M.; Summerfield, V.; Maxwell, G. From pathways to people: Applying the adverse outcome pathway (AOP) for skin sensitization to risk assessment. ALTEX 2013, 30, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.M.; Enoch, S.J.; Ezendam, J.; Sewald, K.; Roggen, E.L.; Cochrane, S. An Adverse Outcome Pathway for Sensitization of the Respiratory Tract by Low-Molecular-Weight Chemicals: Building Evidence to Support the Utility of In Vitro and In Silico Methods in a Regulatory Context. Appl. Vitr. Toxicol. 2017, 3, 213–226. [Google Scholar] [CrossRef]
- Kimber, I.; Poole, A.; Basketter, D.A. Skin and respiratory chemical allergy: Confluence and divergence in a hybrid adverse outcome pathway. Toxicol. Res. 2018, 7, 586–605. [Google Scholar] [CrossRef] [PubMed]
- Hoper, T.; Mussotter, F.; Haase, A.; Luch, A.; Tralau, T. Application of proteomics in the elucidation of chemical-mediated allergic contact dermatitis. Toxicol. Res. 2017, 6, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Weninger, W.; von Andrian, U.H. Chemokine regulation of naive T cell traffic in health and disease. Semin. Immunol. 2003, 15, 257–270. [Google Scholar] [CrossRef]
- Salomon, B.; Bluestone, J.A. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Ann. Rev. Immunol. 2001, 19, 225–252. [Google Scholar] [CrossRef]
- Wahlberg, J.E.; Boman, A. Guinea pig maximization test. Curr. Probl. Dermatol. 1985, 14, 59–106. [Google Scholar]
- Robinson, M.K.; Nusair, T.L.; Fletcher, E.R.; Ritz, H.L. A review of the Buehler guinea pig skin sensitization test and its use in a risk assessment process for human skin sensitization. Toxicology 1990, 61, 91–107. [Google Scholar] [CrossRef]
- Kimber, I.; Dearman, R.J.; Scholes, E.W.; Basketter, D.A. The local lymph node assay: Developments and applications. Toxicology 1994, 93, 13–31. [Google Scholar] [CrossRef]
- Kimber, I.; Dearman, R.J.; Basketter, D.A.; Ryan, C.A.; Gerberick, G.F. The local lymph node assay: Past, present and future. Contact Dermat. 2002, 47, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Tornqvist, E.; Annas, A.; Granath, B.; Jalkesten, E.; Cotgreave, I.; Oberg, M. Strategic focus on 3R principles reveals major reductions in the use of animals in pharmaceutical toxicity testing. PLoS ONE 2014, 9, e101638. [Google Scholar] [CrossRef] [PubMed]
- Gerberick, G.F.; Vassallo, J.D.; Bailey, R.E.; Chaney, J.G.; Morrall, S.W.; Lepoittevin, J.P. Development of a peptide reactivity assay for screening contact allergens. Toxicol. Sci. 2004, 81, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Natsch, A.; Emter, R. Nrf2 activation as a key event triggered by skin sensitisers: The development of the stable KeratinoSens reporter gene assay. Altern. Lab. Anim. 2016, 44, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Ashikaga, T.; Yoshida, Y.; Hirota, M.; Yoneyama, K.; Itagaki, H.; Sakaguchi, H.; Miyazawa, M.; Ito, Y.; Suzuki, H.; Toyoda, H. Development of an in vitro skin sensitization test using human cell lines: The human Cell Line Activation Test (h-CLAT). I. Optimization of the h-CLAT protocol. Toxicol. Vitr. 2006, 20, 767–773. [Google Scholar] [CrossRef]
- Kimura, Y.; Fujimura, C.; Ito, Y.; Takahashi, T.; Nakajima, Y.; Ohmiya, Y.; Aiba, S. Optimization of the IL-8 Luc assay as an in vitro test for skin sensitization. Toxicol. Vitr. 2015, 29, 1816–1830. [Google Scholar] [CrossRef]
- Forreryd, A.; Gradin, R.; Humfrey, C.; Sweet, L.; Johansson, H. Exploration of the GARDskin applicability domain: Indirectly acting haptens, hydrophobic substances and UVCBs. ALTEX 2022. [Google Scholar] [CrossRef]
- Gradin, R.; Forreryd, A.; Mattson, U.; Jerre, A.; Johansson, H. Quantitative assessment of sensitizing potency using a dose-response adaptation of GARDskin. Sci. Rep. 2021, 11, 18904. [Google Scholar] [CrossRef]
- OECD. Test No. 442E: In Vitro Skin Sensitisation: In Vitro Skin Sensitisation assays addressing the Key Event on activation of dendritic cells on the Adverse Outcome Pathway for Skin Sensitisation. OECD Guidel. Test. Chem. 2018, 4, 21. [Google Scholar] [CrossRef]
- Croft, M.; Dubey, C. Accessory Molecule and Costimulation Requirements for CD4 T Cell Response. Crit. Rev. Immunol. 2017, 37, 261–290. [Google Scholar] [CrossRef]
- Ashikaga, T.; Hoya, M.; Itagaki, H.; Katsumura, Y.; Aiba, S. Evaluation of CD86 expression and MHC class II molecule internalization in THP-1 human monocyte cells as predictive endpoints for contact sensitizers. Toxicol. Vitr. 2002, 16, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Sebastiao, A.I.; Ferreira, I.; Brites, G.; Silva, A.; Neves, B.M.; Teresa Cruz, M. NLRP3 Inflammasome and Allergic Contact Dermatitis: A Connection to Demystify. Pharmaceutics 2020, 12, 867. [Google Scholar] [CrossRef]
- OECD. OECD Guidance Document on the Reporting of Defined Approaches and Individual Information Sources to Be Used within Integrated Approaches to Testing and Assessment (IATA) for Skin Sensitization; Organisation for Economic Cooperation and Development: Paris, France, 2016. [Google Scholar]
- Eskes, C.; Hennen, J.; Schellenberger, M.T.; Hoffmann, S.; Frey, S.; Goldinger-Oggier, D.; Peter, N.; Van Vliet, E.; Blomeke, B. The HaCaT/THP-1 Cocultured Activation Test (COCAT) for skin sensitization: A study of intra-lab reproducibility and predictivity. ALTEX 2019, 36, 613–622. [Google Scholar] [CrossRef]
- Chipinda, I.; Ruwona, T.B.; Templeton, S.P.; Siegel, P.D. Use of the human monocytic leukemia THP-1 cell line and co-incubation with microsomes to identify and differentiate hapten and prohapten sensitizers. Toxicology 2011, 280, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Aramaki, Y.; Yotsumoto, S.; Watanabe, H.; Tsuchiya, S. NADPH-oxidase may contribute to IL-12 production in macrophages stimulated with CpG phosphorothioate oligodeoxynucleotides. Biol. Pharm. Bull. 2002, 25, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, S.; Miyake, M.; Fujii, R.; Konishi, Y. MyD88 associated ROS generation is crucial for Lactobacillus induced IL-12 production in macrophage. PLoS ONE 2012, 7, e35880. [Google Scholar] [CrossRef]
- Schnurr, M.; Then, F.; Galambos, P.; Scholz, C.; Siegmund, B.; Endres, S.; Eigler, A. Extracellular ATP and TNF-alpha synergize in the activation and maturation of human dendritic cells. J. Immunol. 2000, 165, 4704–4709. [Google Scholar] [CrossRef]
- Choi, J.M.; Oh, S.J.; Lee, S.Y.; Im, J.H.; Oh, J.M.; Ryu, C.S.; Kwak, H.C.; Lee, J.Y.; Kang, K.W.; Kim, S.K. HepG2 cells as an in vitro model for evaluation of cytochrome P450 induction by xenobiotics. Arch. Pharm. Res. 2015, 38, 691–704. [Google Scholar] [CrossRef]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P. Epithelial transport of drugs in cell culture. I: A model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells. J. Pharm. Sci. 1990, 79, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Lea, T. Caco-2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., Lopez-Exposito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; pp. 103–111. [Google Scholar]
- Tarlo, S.M.; Lemiere, C. Occupational asthma. N. Engl. J. Med. 2014, 370, 640–649. [Google Scholar] [CrossRef] [PubMed]
- North, C.M.; Ezendam, J.; Hotchkiss, J.A.; Maier, C.; Aoyama, K.; Enoch, S.; Goetz, A.; Graham, C.; Kimber, I.; Karjalainen, A.; et al. Developing a framework for assessing chemical respiratory sensitization: A workshop report. Regul. Toxicol. Pharmacol. 2016, 80, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.E. History of interleukin-4. Cytokine 2015, 75, 3–7. [Google Scholar] [CrossRef]
- Zhu, J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 2015, 75, 14–24. [Google Scholar] [CrossRef]
- Mizoguchi, I.; Ohashi, M.; Chiba, Y.; Hasegawa, H.; Xu, M.; Owaki, T.; Yoshimoto, T. Prediction of Chemical Respiratory and Contact Sensitizers by OX40L Expression in Dendritic Cells Using a Novel 3D Coculture System. Front. Immunol. 2017, 8, 929. [Google Scholar] [CrossRef]
- Ito, T.; Wang, Y.H.; Duramad, O.; Hori, T.; Delespesse, G.J.; Watanabe, N.; Qin, F.X.; Yao, Z.; Cao, W.; Liu, Y.J. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005, 202, 1213–1223. [Google Scholar] [CrossRef]
- Furue, M.; Furue, M. OX40L-OX40 Signaling in Atopic Dermatitis. J. Clin. Med. 2021, 10, 2578. [Google Scholar] [CrossRef]
- Haruta, M.; Tomita, Y.; Imamura, Y.; Matsumura, K.; Ikeda, T.; Takamatsu, K.; Nishimura, Y.; Senju, S. Generation of a large number of functional dendritic cells from human monocytes expanded by forced expression of cMYC plus BMI1. Hum. Immunol. 2013, 74, 1400–1408. [Google Scholar] [CrossRef]
- Mizoguchi, I.; Katahira, Y.; Inoue, S.; Sakamoto, E.; Watanabe, A.; Furusaka, Y.; Irie, A.; Senju, S.; Nishimura, Y.; Mizukami, S.; et al. A novel coculture system for assessing respiratory sensitizing potential by IL-4 in T cells. ALTEX 2022. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, E.; Kuhnl, J.; Goebel, C.; Martinozzi-Teissier, S.; Alepee, N.; Ashikaga, T.; Blomeke, B.; Del Bufalo, A.; Cluzel, M.; Corsini, E.; et al. State-of-the-art and new options to assess T cell activation by skin sensitizers: Cosmetics Europe Workshop. ALTEX 2018, 35, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Lebrec, H.; Molinier, B.; Boverhof, D.; Collinge, M.; Freebern, W.; Henson, K.; Mytych, D.T.; Ochs, H.D.; Wange, R.; Yang, Y.; et al. The T-cell-dependent antibody response assay in nonclinical studies of pharmaceuticals and chemicals: Study design, data analysis, interpretation. Regul. Toxicol. Pharmacol. 2014, 69, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Ladics, G.S. Primary immune response to sheep red blood cells (SRBC) as the conventional T-cell dependent antibody response (TDAR) test. J. Immunotoxicol. 2007, 4, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Ladics, G.S. The Sheep Erythrocyte T-Dependent Antibody Response (TDAR). Methods Mol. Biol. 2018, 1803, 83–94. [Google Scholar] [CrossRef]
- Bretscher, P.A. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc. Natl. Acad. Sci. USA 1999, 96, 185–190. [Google Scholar] [CrossRef]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Alvaro-Benito, M.; Stolzenberg, S.; Noe, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef]
- Greenfield, E.A.; Nguyen, K.A.; Kuchroo, V.K. CD28/B7 costimulation: A review. Crit. Rev. Immunol. 1998, 18, 389–418. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef]
- Earnshaw, C.J.; Pecaric-Petkovic, T.; Park, B.K.; Naisbitt, D.J. T cell responses to drugs and drug metabolites. Exp. Suppl. 2014, 104, 137–163. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yerly, D.; Naisbitt, D.J. Mechanisms leading to T-cell activation in drug hypersensitivity. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 317–324. [Google Scholar] [CrossRef]
- Adair, K.; Meng, X.; Naisbitt, D.J. Drug hapten-specific T-cell activation: Current status and unanswered questions. Proteomics 2021, 21, e2000267. [Google Scholar] [CrossRef]
- Bechara, R.; Feray, A.; Pallardy, M. Drug and Chemical Allergy: A Role for a Specific Naive T-Cell Repertoire? Front. Immunol. 2021, 12, 653102. [Google Scholar] [CrossRef]
- Han, J.; Pan, C.; Tang, X.; Li, Q.; Zhu, Y.; Zhang, Y.; Liang, A. Hypersensitivity reactions to small molecule drugs. Front. Immunol. 2022, 13, 1016730. [Google Scholar] [CrossRef] [PubMed]
- Pichler, W.J. Pharmacological interaction of drugs with antigen-specific immune receptors: The p-i concept. Curr. Opin. Allergy Clin. Immunol. 2002, 2, 301–305. [Google Scholar] [CrossRef]
- Pichler, W.J.; Adam, J.; Watkins, S.; Wuillemin, N.; Yun, J.; Yerly, D. Drug Hypersensitivity: How Drugs Stimulate T Cells via Pharmacological Interaction with Immune Receptors. Int. Arch. Allergy Immunol. 2015, 168, 13–24. [Google Scholar] [CrossRef]
- Pichler, W.J. The important role of non-covalent drug-protein interactions in drug hypersensitivity reactions. Allergy 2022, 77, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Chessman, D.; Kostenko, L.; Lethborg, T.; Purcell, A.W.; Williamson, N.A.; Chen, Z.; Kjer-Nielsen, L.; Mifsud, N.A.; Tait, B.D.; Holdsworth, R.; et al. Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity 2008, 28, 822–832. [Google Scholar] [CrossRef]
- Illing, P.T.; Vivian, J.P.; Dudek, N.L.; Kostenko, L.; Chen, Z.; Bharadwaj, M.; Miles, J.J.; Kjer-Nielsen, L.; Gras, S.; Williamson, N.A.; et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature 2012, 486, 554–558. [Google Scholar] [CrossRef]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- McFadden, J.P.; White, J.M.; Basketter, D.A.; Kimber, I. Does hapten exposure predispose to atopic disease? The hapten-atopy hypothesis. Trends Immunol. 2009, 30, 67–74. [Google Scholar] [CrossRef] [PubMed]
- McFadden, J.P.; Dearman, R.J.; White, J.M.; Basketter, D.A.; Kimber, I. The Hapten-Atopy hypothesis II: The ‘cutaneous hapten paradox’. Clin. Exp. Allergy 2011, 41, 327–337. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakamoto, E.; Katahira, Y.; Mizoguchi, I.; Watanabe, A.; Furusaka, Y.; Sekine, A.; Yamagishi, M.; Sonoda, J.; Miyakawa, S.; Inoue, S.; et al. Chemical- and Drug-Induced Allergic, Inflammatory, and Autoimmune Diseases Via Haptenation. Biology 2023, 12, 123. https://doi.org/10.3390/biology12010123
Sakamoto E, Katahira Y, Mizoguchi I, Watanabe A, Furusaka Y, Sekine A, Yamagishi M, Sonoda J, Miyakawa S, Inoue S, et al. Chemical- and Drug-Induced Allergic, Inflammatory, and Autoimmune Diseases Via Haptenation. Biology. 2023; 12(1):123. https://doi.org/10.3390/biology12010123
Chicago/Turabian StyleSakamoto, Eri, Yasuhiro Katahira, Izuru Mizoguchi, Aruma Watanabe, Yuma Furusaka, Ami Sekine, Miu Yamagishi, Jukito Sonoda, Satomi Miyakawa, Shinya Inoue, and et al. 2023. "Chemical- and Drug-Induced Allergic, Inflammatory, and Autoimmune Diseases Via Haptenation" Biology 12, no. 1: 123. https://doi.org/10.3390/biology12010123
APA StyleSakamoto, E., Katahira, Y., Mizoguchi, I., Watanabe, A., Furusaka, Y., Sekine, A., Yamagishi, M., Sonoda, J., Miyakawa, S., Inoue, S., Hasegawa, H., Yo, K., Yamaji, F., Toyoda, A., & Yoshimoto, T. (2023). Chemical- and Drug-Induced Allergic, Inflammatory, and Autoimmune Diseases Via Haptenation. Biology, 12(1), 123. https://doi.org/10.3390/biology12010123





