Simple Summary
Infertility is a global issue and the currently available treatments for infertility are known to pose some risks and are lacking in solving infertility problems that are closely related to genetic disorders. In this review, we summarized the use of autologous mesenchymal stem cells (MSCs) as the cell-based treatment for infertility and the future treatments of infertility using various methods.
Abstract
Infertility could be associated with a few factors including problems with physical and mental health, hormonal imbalances, lifestyles, and genetic factors. Given that there is a concern about the rise of infertility globally, increased focus has been given to its treatment for the last several decades. Traditional assisted reproductive technology (ART) has been the prime option for many years in solving various cases of infertility; however, it contains significant risks and does not solve the fundamental problem of infertility such as genetic disorders. Attention toward the utilization of MSCs has been widely regarded as a promising option in the development of stem-cell-based infertility treatments. This narrative review briefly presents the challenges in the current ART treatment of infertility and the various potential applications of autologous MSCs in the treatment of these reproductive diseases.
1. Introduction
In clinical practice, infertility is defined as a disease of the male or female reproductive system in failure of conceiving within 12 months of regular, unprotected sexual intercourse in women less than 35 years old or within 6 months in women 35 years and older [1]. According to the latest data from World Health Organization, this condition affects 48 million reproductive-age couples worldwide and is categorized into primary and secondary infertility. Primary infertility is when a person has never achieved a successful pregnancy while secondary infertility is when a person is having difficulty getting pregnant after a prior successful pregnancy [2]. The general perception of infertility is predominantly caused by women; however, the causes are equally distributed among men and women. Men and women each contributed 40% as a sole or a contributing cause of infertility, and in the remaining 20%, it is the male and the female partner or unidentifiable cause termed unexplained infertility [3].
An increasing number of infertile couples are turning to ART as the most effective way in treating infertility in humans. Nonetheless, the presence of a few limitations within this method has diverted scientists’ attention toward stem cells, particularly germ-like stem cells in the treatment of infertility [4,5,6,7,8]. Research on MSCs is intriguing compared to ESCs that involve the destruction of a human embryo which sparked some serious ethical issues debate [9,10]. Therefore, spindle-shaped cells of MSCs with the ability to differentiate into multiple germ layers coupled with the unique characteristics of MSCs have been suggested as an ideal candidate for regenerative medicine [11]. Different reports have presented solid evidence on the role of MSCs in the recovery of fertility and the future outlook of infertility treatment [8,12,13]. However, due to the immunogenicity and the risk of anti-donor immune response with allogeneic MSCs application, this present review specifically focuses on the advantage of only using autologous MSCs as a safer treatment of infertility, which was not discussed by previous reports.
2. Causes and Mechanisms Leading to Infertility
Couples that fail to conceive within a year will usually undergo initial evaluation for the standard clinical diagnosis that includes semen analysis, assessment of ovulation, and tubal patency test [14]. In 20% of the cases, this standard fertility evaluation fails to identify any abnormalities, termed unexplained infertility [15]. In known causes among infertile couples, there are various environmental and internal identifiable factors that play a role including ovulatory dysfunction, tubal diseases, male infertility factors, lifestyle, and environmental factors such as smoking and obesity that can adversely affect fertility (Table 1) [16,17,18,19]. Most of these factors impacted several biological systems such as the neuroendocrine systems, the reproductive organs, and the immature and growing follicles or sperm. The most known common factors in infertility include infection [20,21,22], congenital defects [23,24], and gonadotoxins [25,26,27,28].

Table 1.
Common causes of infertility in male and female.
3. Limitations in The Current Conception Treatment
The treatments received by couples depend on the cause of the problems. Couples with presenting fertility issues will either receive medication, surgical procedures, and/or in combination with assisted conception such as intrauterine insemination (IUI), in vitro fertilization (IVF), or intracytoplasmic sperm injection (ICSI) [14]. Medications that are commonly used to invoke ovulation include clomiphene, letrozole, tamoxifen, metformin, and gonadotropins [29]. On the other hand, women usually undergo surgical procedures on fallopian tubes with a presentation of fibroid, polycystic ovarian syndromes (PCOS), or endometriosis issues. In men with varicocele [30], a corrective surgical procedure will be performed and sperm retrieval will be done on the block epididymal [31]. On the other hand, the gold standard treatment for cancer and non-cancerous patients that need to undergo chemotherapy treatments involves cryopreservation of the gonadal tissues [32]. Many known complications are associated with ART despite it being envisioned as the solution for infertile couples.
3.1. Multifetal Gestations and The Effects
ART is a known risk for conceiving higher multifetal gestations due to the transferring of more than one embryo. From a total of 1.8% of infants born using ART in the United States, ART procedures resulted in 16.4% of multiple gestations with 16.2% of twins and 19.4% of triplets or higher order pregnancies [33]. Some of these ART procedures are accompanied by clomiphene, letrozole, and gonadotrophin as oral ovarian induction agents, which show to possess a risk of multiple gestations based on a review [34]. Multifetal gestation also leads to various maternal complications such as birth defects [35] and stillbirths [36], which imposed serious effects on the fetus. Besides that, maternal complications are also present in singleton births conceived via ART. A comparison of singleton births between naturally conceived and ART revealed a significantly low birth weight and preterm birth [37] with 1.1% extreme preterm birth (20–27 gestational weeks) and 2.2% very preterm birth (28–32 gestational weeks) in ART conceived babies [38]. The double risk of cardiovascular, genitourinary, and musculoskeletal systems on the fetus after a cycle of ICSI has been reviewed [39]. This is a worrying fact since it consists of 70% of ART treatments worldwide [40].
3.2. Ectopic Pregnancy
Ectopic pregnancy following infertility treatments is one of the risk factors of ART [41], which has been also associated with significant morbidity and mortality [42]. It was reported that the incidence is <5% in ART pregnancies [43]. Tubal factor infertility is suggested to contribute to the highest percentage of ectopic pregnancy up to 11% among infertile patients [44,45] and the highest rate is after tubal reconstructive surgery [46]. Hormonal changes that happen after infertility treatments could also affect the expression of signaling molecules that are needed for the interaction between the embryo, Fallopian tube, and endometrium [47,48]. Evidence also exists on the increased risk of ectopic pregnancy due to the immunological changes after embryo transfer [49].
3.3. Ovarian Hyperstimulation Syndrome (OHSS)
The development of OHSS during ART is an iatrogenic side effect of using high-dose ovary stimulatory agents, mainly gonadotrophin [50], and can be fatal. This often resulted in multi-follicular growth characterized by abdominal tenderness, nausea, vomiting, renal failure, and swelling due to increased ovarian volume. After decades, an alternative stimulation to triggering final oocyte maturation has eliminated the risk of severe OHS, nonetheless, it reduces the probability of achieving pregnancy [51].
3.4. Birth Defects
Major congenital malformations, aneuploidy, and mosaicism are examples of worrying issues in IVF and ICSI [52,53,54]. The high prevalence of this occurrence could be due to the transferring of poor embryos during the procedure [55] and the most well-documented evidence is the twinning effects or higher-order pregnancies [56]. Much debate also revolves around the possible factor of ovulation induction [56,57] and the micromanipulation of the techniques themselves [58], which resulted in injuries to the internal structure of oocytes leading to deleterious consequences such as aneuploidy and chromosomal abnormalities.
3.5. Cryopreservation of Testes and Ovary
Cryopreservation of testicular or ovarian tissue is one method of fertility preservation in childhood cancer patients [59,60]. Thus far, all ovary transplanted studies reported freezing and ischemic injury limitations. Transplantation resulted in far greater ovarian follicle damage compared to cryopreservation due to ischemic and oxygen deficit [61]. Using a sheep model, the survival of the primordial follicles and the formation of secondary follicles on different sites have been successfully established [62]. However, according to the study, a longer experimental study evaluating hormone recovery, follicular maturation, and successful pregnancy are critical for clinical reference.
4. MSCs and Their Mechanism in The Treatment of Infertility
With the shortcomings of the ART treatments, the heightened pathological immune response following infection and inflammation leading to deleterious effects on fertility, and gonadal disorders that are seen to impede normal pregnancy necessitates a good balance between immune activation and immune suppression, and tissue replenishment. A long search for an alternative treatment in correcting limitations in ART or conditions that are untreatable through ART has set stem cells as an appealing new treatment with the possession of several biological characteristics that qualify them to be used for cellular therapy and new hope in infertility treatments.
Stem cells have the ability to renew themselves for long periods without significant changes in their general properties. Under certain physiological or environmental conditions, these cells are able to differentiate into various specialized cells. Despite the research excitement on embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), they possess ethical concerns, immune rejection after transplantation, and teratoma formation issues. This exposes MSCs and tissue-specific stem cells as an area of great interest [63] since they can be expanded and manipulated ex vivo. MSCs are a subset of non-hematopoietic adult stem cells that originate from the mesoderm and possess self-renewal abilities and multilineage differentiation. MSCs can differentiate into mesoderm lineages [64,65,66] and are harvested from multiple adult tissues including the following: skeletal muscle, cervical tissue [67], menstrual blood [68], bone marrow, adipose tissue [69], umbilical cord [70], umbilical cord blood [71], amnion [72], placenta [73], and fetal tissues such as blood, liver, and bone marrow [74]. While tissue-specific stem cells are derived from reproductive organs known as germline stem cells such as from the testis [75] and ovary [76] for the continuous production of sperm and oocytes. The precise mechanisms of MSCs are still not fully understood. However, in relation to infertility possible actions, the four most prominent are their biological characteristics of differentiation, secretory capacity, mitochondrial transfer, and immunomodulatory and anti-inflammatory capacity (Figure 1):
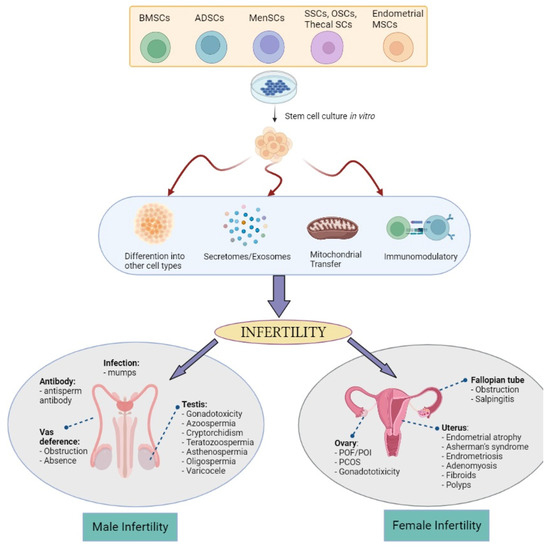
Figure 1.
The possible mechanism of actions of bone-marrow stem cells (BMSCs), adipose tissue stem cells (ADSCs), menstrual stem cells (Men-SCs), endometrial MSCs, and germline-derived stem cells: spermatogonial stem cells (SSCs), oogonial stem cells (OSCs), and thecal stem cells in treating various male and female infertility problems (Created with Biorender.com).
Differentiative capacity. MSCs are capable of differentiating, albeit limited to the mesoderm layer, into various cell types such as epithelial, stromal, and endothelial cells [77]. This highlighted the potential of MSCs for advanced tissue repair treatments for infertile couples with damaged endometrial [78], restoring endometrial function [77], or ovarian tissues [79]. Although according to some studies that MSCs improve and help ovarian function recovery, the number of differentiated and functionally integrated MSCs is too small to observe improvements in ovarian function. However, it remains unclear on the mechanism of MSCs differentiation into target cells such as oocytes or supporting cells after migrating to the injured tissues to improve and correct ovarian dysfunction [80]. A new hypothesis also emerges by indicating that rather than assuming the engraftment and differentiation of MSC in diseased tissues or organs, an alternative mode of rescue and repair could enhance the cell viability and proliferation responses of MSC, which is discussed in the point below.
Secretory capacity. At present, this new hypothesis is widely accepted among researchers in which MSCs’ effect on reproductive treatment thus far is linked to various bioactive secretome factors such as insulin-like growth factor (IGF), vascular endothelial growth factor (VEGF), cytokines, and other growth factors [81,82]. Nevertheless, the importance of the paracrine effects of MSCs and their secretomes in restoring the cellular composition of tissues via regulating the immune response, stimulating angiogenesis, and maintaining the viability of the microenvironment [83] is highlighted by several groups. Some evidence includes the paracrine activity mechanisms driving the improved ovarian function [84,85] and endometrial reserve [86,87] than its stimulatory effects on cell growth and differentiation. For example, the paracrine activity of VEGF, as a strong proangiogenic factor in ovary vascularization [88], secreted by MSCs has been shown to enhance ovarian function by restoring its structure [89]. Apart from the secretomes, some miRNAs and exosomes carried by the MSCs have been found to be useful biomarkers in targeting infertility issues. miRNA-644-5p and miRNA-144-5p carried by bone marrow-derived mesenchymal stem cells (BMSCs)-derived exosomes have been shown to promote the recovery of ovarian function in chemotherapy-induced POI in a rat model [90,91]. As well in men, MSC-derived exosomes are able to induce spermatogenesis in the testes of infertile azoospermic mice models [92]. These findings underpin the role of the gene expression regulated by miRNA in MSC-based therapy outcomes and its importance in regulating stem cells self-renewal and differentiation by repressing selected mRNA translation [93].
Mitochondrial transfer. In reproduction, mitochondria are transmitted to the offspring exclusively by the oocytes from the mother. This organelle is important for optimal oocyte quality, proper fertilization, and embryo development [94]. Therefore, the mitochondrial transfer (MT) technique is seen as an utmost strategy for improving oocyte quality in women with a history of poor oocyte quality, advanced maternal age women, and patients with previous IVF failures which shared defects at the oocyte level. MT can be accomplished heterologously (using a donor oocyte) or autologously from ovarian stem cells or granulosa cells [95]. However, heterologous MT introduces a third source of DNA [96] while the existence of ovarian stem cells remains questionable [97] and granulosa cells undergo an aging process along with the oocyte [98]. In an animal model of aged mice, autologous adipose-derived stem cells MT rescues the oocyte quality with a significant mitochondrial oocyte difference between the young and aged mice [98], while another study found no advantage of MT [99]. This difference in the observation could be explained by the different genetic backgrounds of the mice. There is also a growing body of evidence on the ability of Sertoli cells, which is now believed to possess some MSCs characteristic [100], to exhibit the properties of MT [101]. Acknowledging the lack of studies on MSCs MT in infertility, a review study highlighted few studies on the importance of MT from MSCs in restoring normal physiological function and disease recovery [102]. Therefore, with extensive further research using in vivo models and human studies, MT could provide a valid treatment strategy for low fertility or infertility in women and men. While MSCs can give some effect of cell-to-cell interaction via MT, their secreted properties of MSCs via exosome can help to modulate the immune system.
Immunomodulatory and anti-inflammatory capacity. MSCs, via their exosomes, have been shown to possess broad immunoregulatory abilities by influencing both adaptive and innate immune responses. Findings showed that MSCs can inhibit T cell proliferation and conversion to regulatory T cells (Tregs) through the reprogramming of M1 macrophage cells to M2 phenotype, leading to tissue repair and healing [103,104]. PCOS, as one of the most common endocrine–reproductive–metabolic disorders in women [105], exhibited higher Th1 inflammatory responses [106,107]. Therefore, the capability of MSCs to suppress Th1 may influence the internal PCOS inflammatory environment hence restoring ovarian function via the cessation of the autoimmune reaction and the regression of the endocrine disease [108]. Bacterial infection-induced pelvic inflammatory animal models showed a promising immunomodulatory role of MSCs in partially restoring fertility, in which the MSCs repair the tubal epithelium structure subjected to chronic inflammation, decreasing the inflammatory factors, and restoring the oviductal glycoprotein secretion level [109,110]. In the same study, inducing E. coli in rabbits showed decreased inflammatory factors and increased oviductal glycoprotein expression, reflecting improved sperm fertilization capacity [109]. Improved pregnancy rate, ovary morphology, and apoptotic oocytes were also observed in inflammation-induced ovary mice [111]. Antisperm antibody (ASA) that is naturally present in the fertile population or due to traumatic testis rupture [112] resulted in autoimmunization against spermatozoa in the form of a humoral immune response. An in vivo mice model study revealed that MSCs are able to suppress the production of ASA by modulating the humoral immune response [113] thus increasing sperm concentration and motility. Considering that COVID-19 virus infection affected male fertility by reducing sperm count and motility [114], studies on MSCs could be advantageous in COVID-19-induced infection and inflammation [115] and semen quality.
5. Potential Usage of MSCs in Infertility
ART is undoubtedly a well-recognized method for achieving pregnancy for infertile couples. Despite that, it presents challenges to public health as evidenced by the high rate of multifetal gestation, preterm delivery, and low birth weight of infants [116]. Exhaustion of traditional treatments and genetic defects that lead to gamete deficiency have led to mounting preclinical studies on animals and clinical studies on humans using stem cells one of which is using MSCs. This MSCs therapy-based technology could generate auxiliary factors in improving the functional roles of various cell types in the reproductive systems. Manipulation of BMSCs, umbilical cord stem cells, amniotic fluid mesenchymal stem cells, menstrual stem cells (MenSCs), adipose-derived stem cells (ADSCs), and endometrial MSCs with the options using autologous or allogeneic treatments proved the effectiveness of MSCs in infertility. The success of these interventions in pre-clinical and clinical studies has brought huge hope in improving female and male reproductive health [12]. Nonetheless, autologous treatment is favorable among researchers for several reasons. Following MSCs therapy, studies reported the potential of immunogenicity after allogeneic applications [117,118,119]. In one of the studies, in the effort of preserving infarcted heart function using allogeneic MSCs, a biphasic immune response was seen after 5 months of implantation suggesting the MSCs transition from immunoprivilege to an immunogenic state [120]. Due to the potential risk of anti-donor immune responses, several strategies were suggested in a systematic review including the use of immunosuppressive drugs in combination with MSCs therapy [121]. To date, the risks and limitations of both autologous and allogeneic therapeutic applications are highly debated such as in terms of the potential impact of donor-donor heterogeneity. Since there is a lack of review studies on the autologous applications of MSCs in infertility treatment, in this section, we focus on the available autologous MSCs treatments for infertility issues, completed or ongoing, in both animal models and human clinical trials.
5.1. Preclinical Studies
Current in vitro studies involve using MSCs alone or in combination with other drugs or stimulants for the potential application of ovarian dysfunction and endometrial disorders in females and spermatogenesis in males. ADSCs are a type of MSCs that can be easily isolated and collected in a minimally invasive procedure and an abundant in quantities. It is also safe to be transplanted autologously into a host [122]. Apart from the whole cells, mitochondria from ADSCs have also been used to correct aging as a predisposing factor to fertility health using an animal study [98]. The microinjection of ADSCs mitochondria promotes oocyte quality, embryonic development, and fertility in elderly mice thus promising an exciting strategy for elderly women.
Since the concept of pluripotent stem cells could differentiate into functional gametes, few germline stem cells have been associated with the recruitment and stimulation or conversion into functional gametes [123]. These extragonadal sources of gametes could aid in the hampered spermatogenesis by testicular damage or oogenesis in producing healthy eggs. An earlier study on autologous transplantation of spermatogonial stem cells (SSC) done on monkeys showed positive results on the structure of the testes [124], yet no evidence on sperm quality. A later study using macaque supported the finding with the same increase in the size of the testes and evidence of the successful production of healthy sperm [125]. Table 2 shows a list of autologous MSCs stem cell transplantation for various infertility issues using animal models.

Table 2.
Animal models of autologous stem cell treatment for infertility issues.
5.2. Clinical Studies
Translating the fundamental approach of preclinical studies to human studies is challenging. Most clinical trials have yet to acquire full regulatory approval thus hindering the promotion of stem cells into clinical practice. Despite that, there is encouraging news that could pave a way for the use of MSCs in infertility treatments in the future. One successful intervention was the birth of a baby after transplanting autologous BMSCs into an ovary of a POI woman [128]. However, there is a lack of data on serum hormone levels, MSC preparation details, and imaging data. A pilot result from a clinical trial fulfilled these void data and presented a report showing a 50% increase in ovarian volume compared to the atrophic contralateral ovary and a 150% increase in estrogen level. This trial showed enhanced fertility health with a resumption of menses and diminished menopausal symptoms [129]. Successful transplantation of BMSCs using various methods in intrauterine adhesion (IUA) or known as Asherman’s syndrome showed positive outcomes for clinical practice [130,131,132,133,134]. Subendometrial transplantation of BMSCs resulted in menstruation restoration in 5 of 6 IUA cases [132]. In addition, BMSCs also could be a promising therapy for advanced reproductive-age women with ovarian reserve issues [135]. Loss of the anti-Mullerian hormone and antral follicle count that is associated with aging is restored with BMSCs potentially via the homing ability of stem cells. Together with other multiple paracrine factors, these cells differentiate into a variety of cells to facilitate ovarian recovery [136]. In azoospermic condition, a promising result was obtained in a pilot clinical study [137] with various clinical trials being performed or are underway on the injection of BMSCs to the rete testis to assess the hormonal level, testicular size, and sexual potency (Table 3).
ADSCs clinical trials have been performed in POI [138], IUA [139,140], azoospermia, and post-prostate cancer treatment for erectile dysfunction [141]. Subendometrial injection in women with thin endometrium lining is associated with a total of 13 pregnancies and 9 live births [140], while subendometrial transplantation increases endometrium lining to 7 mm in 3 out of 6 cases [139]. This small cohort of patients granted replication and expansion before drawing any conclusion since these trials have not resulted in complete positive outcomes for most of the participants. In POI treatments, each of the patients in the trial experienced variable ovarian volume, anti-Mullerian hormone, and antral follicular count after transplantation. Although menstruation was resumed in two patients, the other patients showed no improvement, while all patients demonstrated inconsistent FSH levels [138]. Meanwhile, only 47% of men showed recovered erectile dysfunction and were able to accomplish sexual intercourse after stem cell transplantation [141].
Another promising trial is the transplantation of autologous MenSCs for severe IUA syndrome patients. Two independent research studies demonstrated improved endometrial thickness in women with thin endometrium. One study reported increased endometrial thickness to 7 mm and an improved pregnancy rate between 47% [142] to 50% [143]. These data open new hope for infertility female patients caused by IUA with the ease of sample collection and less invasive method compared to other sources of MSCs. As reported in a reviewed study, almost 130 cryopreservation have been conducted worldwide. However, frozen ovarian tissue cryopreservation transplantation showed evidence of relapse or re-introduction of cancer cells [144] and needs further improvement in pregnancy rates [145].
Clinical use of endometrium MSCs in a case presentation of a woman with multiple failed ART cycles successfully increases the endometrium receptivity of the woman. Ultrasound examination prior to embryo transfer showed that the endometrium thickness improves 2.15-fold after endometrial MSCs transplant [146] and the patient achieved a live successful pregnancy. Despite the increase in the endometrium thickness not within the optimal range, a cocktail of growth factors, cytokines, and hormones may assist in the endometrial receptivity for pregnancy [147].

Table 3.
Clinical trials of autologous stem cells treatment for infertility issues.
Table 3.
Clinical trials of autologous stem cells treatment for infertility issues.
| Cell Source | Disease | Mode of Treatment | Outcome | Reference/ NCT ID |
|---|---|---|---|---|
| BMSCs | IUA (Asherman’s syndrome) | Transplantation in the endometrial cavity | Restoration of the menstrual cycle | [132] |
| Increased endometrium thickness and good vascularity | [130] | |||
| IUA (Asherman’s syndrome) | Intra-arterial to the uterus |
| [131] | |
| IUA (Asherman’s syndrome) | BMSCs-loaded collagen scaffold |
| [133] | |
| IUA (Asherman’s syndrome) | Transplantation to the uterine cavity | Recovered endometrium | [148] | |
| POI | Laparoscopic instillation into ovaries |
| [149] | |
| Injection into the ovary |
| [128] | ||
| [129] | |||
| NCT03069209 | |||
| NCT02043743 | |||
| NCT02062931 | |||
| Injection via peripheral vein | No results posted (unknown status) | NCT02779374 | ||
| Azoospermia | Injection into rete testis |
| [137] | |
| Azoospermia | Intra-testicular transplantation | No results posted (recruiting) | NCT02641769 | |
| Azoospermia (Klinefelter Syndrome) | Injection into testicular tubules and artery | No results posted (recruiting) | NCT02414295 | |
| Non-obstructive azoospermia | Injection into testis | No results posted (recruiting) | NCT02041910 | |
| Non-obstructive azoospermia | Injection into testis | No results posted (recruiting) | NCT02008799 | |
| Ovarian reserve | Intra-ovarian artery injection |
| [135] | |
| ADSCs | Thin endometrium syndrome | Subendometrial injection |
| [140] |
| IUA (Asherman’s syndrome) | Transcervical instillation |
| [139] | |
| Azoospermia and oligozoospermia | Injection into testis | No results posted (Enrolling by invitation) | NCT03762967 | |
| POI | Intra ovarian transplantation |
| [138] | |
| POI and Ovarian Ageing | Injection into ovary |
| [150] | |
| Post-cancer surgical removal of erectile dysfunction | Single intracavernous injection | Improved erectile function | [141] | |
| MenSCs | IUA (Asherman’s syndrome) | Transplanted into uterus | Regenerating the endometrium, prolonging menstrual duration, and increasing the rate of pregnancy | [142] |
| Transplantation |
| [143] | ||
| Endometrial-MSCs | Thin endometrium syndrome | Submucosal injection |
| [146] |
6. New Strategies and Future Perspectives
There are hundreds of registered clinical trials trying to explore multipotent MSCs in imaginable infertility treatments for clinical application. However, these clinical-stage MSCs therapies are unable to meet the primary efficacy endpoints as their administration in humans is not as robust as demonstrated in preclinical studies. Meanwhile, the translation of cell-based therapy is impaired by the biological differences between normal and stem cells from the same tissues (heterogeneity population) and it is not a straightforward application. Therefore, leveraging other possible perspectives are needed to achieve more potent and versatile autologous therapies as proposed in Figure 2.
6.1. Cell-Free Therapy
Although autologous stem cell treatments have been widely used in reproductive medicine due to their promising properties, extensive clinical application is impeded by its safety, high cost, low quality, and manufacturing. In recent years, mounting evidence has emerged linking the secretion of extracellular vesicles (EVs) such as exosomes from MSCs as the main driver of the mechanism of action. Although the isolation methods involve differential centrifugation [151], extracting these exosomes from low-immunogenic MSCs could solve challenges associated with autologous or allogeneic stem cell treatments. As such, this has attracted the attention of researchers in making use of these cell-derived EVs with a concept known as cell-free therapy.
6.1.1. MSCs-Derived Exosomes/miRNA
In understanding the potential use of the miRNA-derived exosomes, miRNA-21 was overexpressed in the MSCs. Upregulation of the miRNA-21 increases the estrogen, decreases the FSH level, and decreases granulosa cells apoptosis by downregulating the programmed cell death protein 4 (PDCD4) and phosphatase and tensin homolog (PTEN) [152]. The full potential of the exosomes derived from MSCs is reflected in multiple studies. Using the POI animal model, the ovarian function was restored by the downregulation of PDCD4 and PTEN and regulation of reproductive hormone levels via miRNA-155-5p [91] while miRNA-644-5p acted by targeting the p53 [90]. In a model of IUA, miRNA-29a [153] and miRNA-340 [154] have been shown to inhibit fibrosis during an endometrial repair. Although the exact mechanism is unknown, it is speculated that the exosomes reverse the epithelial-mesenchymal transition (EMT) and promote endometrial repair via TGF-β1/Smad signaling pathway [155] and this was further proved in cutaneous wound healing [156]. In addition to possessing a repair mechanism, the exosomes exhibit a protective effect against sperm genomic integrity injuries (such as cell membrane injury and DNA damage) and ROS [157], which raises the possibility of exosome therapy for asthenozoospermia.
6.1.2. ADSCs-Derived Exosomes/miRNA
Few studies also explored the outcomes of exosomes derived from ADSCs in a few infertility issues. One study demonstrated that ADSC-exosome promoted endometrial regeneration and receptivity thus restoring fertility [158]. Transplantation of the exosomes in the tissue grafts of the POI model, on the other hand, showed decreased apoptosis by downregulating the Fas ligand and increased the SMAD5 expression [159]. Both notions hold great promise in addressing implantation and pregnancy facilitation in infertile patients. Using a PCOS model, miRNA-323-3p extracted from modified ADSCs provided new insight into developing new strategies for PCOS patients since the miRNA promoted cell proliferation and inhibit cumulus cell apoptosis [134]. The ADSCs-derived exosomes were also explored in male infertility whereby in a diabetic rat model, transplantation of the exosomes ameliorates erectile function [160], suggesting possible erectile correction in human infertile males.
6.1.3. Menstrual SCs-Derived Exosomes/miRNA
Results from the transplantation of MenSCs on the POI animal model were successfully replicated in the in vivo and in vitro MenSCs-derived exosomes study. Exosome exposure inhibits follicle apoptosis and promotes the proliferation of granulosa cells while in vivo models presented with promoted follicle development, restored estrous cycle and serum hormone levels, and improved live birth [161]. This exciting outcome suggests a desirable cell-free bioresource in infertility treatments. Applying exosomes to living tissues has grabbed the focus as the future therapeutic effects since it does not induce inflammation, teratomas, and degraded by enzymes. Many potentials of the exosome’s effects on tissue engineering, regenerative, and reproductive medicine [162] have been depicted, which could be the answer to filling the gap in clinical trials-bedside of infertility treatment.
6.2. Very Small Embryonic-like Stem Cells (VSELs)
Over a decade, a small population of small, early-development stem cells known as VSELs was identified as pluripotent stem cells based on their primitive morphology and gene expression profiles [163,164]. Researchers proposed that these cells originate from the germ line, are deposited in the developing organs during embryogenesis, and play a crucial role as a backup population for monopotent tissue-committed stem cells. VSELs are highly quiescent when residing in the adult tissues due to the erasure of the regulatory sequences for certain imprinted genes [165] and they possess large nuclei containing euchromatin and a thin rim of cytoplasm enriched in spherical mitochondria. During stress situations or induced to proliferate, they are activated and released into circulation (as reviewed in [166]). Although these cells are evolutionarily conserved in mammals (reviewed in [167]) and demonstrated in a small number, a recent study has successfully expanded the cells ex vivo without feeder layer cells while preserving their capacity to differentiate into organ-specific cells [168]. Convincingly as well, these cells can be isolated from the testes [169] and ovarian surface epithelium of young and postmenopausal women [170], which can differentiate into oocyte-like cells in response to sperm cells and release zona pellucida [171]. The existence of these cells in azoospermic testicular biopsies of an adult male cancer survivor [172] and busulfan and cyclophosphamide ovaries-treated mouse model [173] is anticipated to open a new avenue for fertility restoration in cancer survivors. Despite the very existence of these cells being highly questionable among the experts in the field, it is hopeful that these cells can be the new hope for the collection of autologous pluripotent stem cells treatments in infertility issues such as delaying menopause and most importantly enable aged mothers to have better egg quality.
6.3. Regenerative Therapy
Furthermore, the use of microfluidic chip devices and BMSCs and ADMSCs has been able to promote stem cell maintenance and differentiation into functional organ models [174]. Although this novel study of stem cells and microfluidic chip-based model was performed on a bone, this has created an interesting area to be explored and manipulated on reproductive organs to understand their physiological function and disease modeling before transplantation. This chip-based model in turn could provide a unique opportunity for infertile patients with impaired gametogenesis caused by congenital disorders in sex development or cancer survivors [175] that is lacking in the current infertility treatments. One recent study has taken a step forward in generating regenerative therapies in the treatment of type 1 diabetes using scalable GMP-grade human pancreas organoids [176]. A similar approach could be devised in infertility treatments by developing endometrium organoids from small endometrial biopsies and transplanting autologously to restore damaged epithelium hence would avoid allogeneic immune responses.
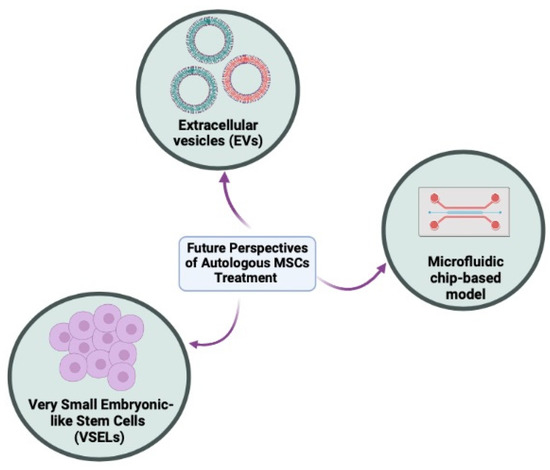
Figure 2.
The future autologous methods in tackling infertility issues in men and women. These methods include cell-free therapy using extracellular vesicles (EVs), very small embryonic-like stem cells (VSELs), and organoids or functional organ-on-a-chip (OOAC) models (Created with Biorender.com).
7. Conclusion
In summary, stem cells provide an exciting opportunity in developing potential new treatments for infertility in both men and women. Both allogeneic and autologous MSCs are the key player in cell-based therapy; however, autologous stem cell treatment is regarded as safer and immunoprivileged. The combined effects of MSCs transplantation and the secretomes or exosomes predominantly play an important role in the recovery of failing reproductive tissues or organs. Considering that many MSCs are in the preclinical investigational stage, the progress in using these potential MSCs as stem cell therapy requires further long-term planning with strict evaluation and supervision to ensure accuracy, quality, and safety before implementing these approaches at the bedside. The emergence of invaluable cell sources such as VSELs, EVs, and the cutting-edge technology of organoids could offer promise in the quest to overcome human infertility.
Author Contributions
Conceptualization, F.N. and W.S.W.K.Z.; writing—original draft preparation, Z.B.M.R., Y.-F.T., and W.S.W.K.Z.; writing—review and editing, Z.B.M.R., F.N., W.S.W.K.Z., Y.-F.T., and N.H.A.A.; visualization, Z.B.M.R., F.N., W.S.W.K.Z., Y.-F.T., and N.H.A.A.; supervision, F.N. and W.S.W.K.Z.; project administration, Y.-F.T. and N.H.A.A.; funding acquisition, F.N. and N.H.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Geran Universiti Penyelidikan (GUP) for the awarded grant GUP-2020-024.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study was conducted at the Centre for Tissue Engineering and Regenerative Medicine, Universiti Kebangsaan, Malaysia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: A committee opinion. Fertil. Steril. 2020, 113, 533–535. [Google Scholar] [CrossRef]
- World Health Organization. Infertility. Available online: https://www.who.int/health-topics/infertility#tab=tab_1 (accessed on 14 April 2022).
- Stevenson, E.L.; Hershberger, P.E.; Bergh, P.A. Evidence-Based Care for Couples with Infertility. J. Obstet. Gynecol. Neonatal. Nurs. 2016, 45, 100–110; quiz e101–e102. [Google Scholar] [CrossRef] [PubMed]
- Nayernia, K.; Lee, J.H.; Drusenheimer, N.; Nolte, J.; Wulf, G.; Dressel, R.; Gromoll, J.; Engel, W. Derivation of male germ cells from bone marrow stem cells. Lab. Investig. 2006, 86, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh-Hasankolaei, M.; Sedighi-Gilani, M.A.; Eslaminejad, M.B. Induction of ram bone marrow mesenchymal stem cells into germ cell lineage using transforming growth factor-beta superfamily growth factors. Reprod. Domest. Anim. 2014, 49, 588–598. [Google Scholar] [CrossRef]
- Cakici, C.; Buyrukcu, B.; Duruksu, G.; Haliloglu, A.H.; Aksoy, A.; Isik, A.; Uludag, O.; Ustun, H.; Subasi, C.; Karaoz, E. Recovery of fertility in azoospermia rats after injection of adipose-tissue-derived mesenchymal stem cells: The sperm generation. Biomed. Res. Int. 2013, 2013, 529589. [Google Scholar] [CrossRef] [PubMed]
- Tamadon, A.; Mehrabani, D.; Rahmanifar, F.; Jahromi, A.R.; Panahi, M.; Zare, S.; Khodabandeh, Z.; Jahromi, I.R.; Tanideh, N.; Dianatpour, M.; et al. Induction of Spermatogenesis by Bone Marrow-derived Mesenchymal Stem Cells in Busulfan-induced Azoospermia in Hamster. Int. J. Stem Cells 2015, 8, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Saeed, Y.; Liu, X. Mesenchymal stem cells to treat female infertility; future perspective and challenges: A review. Int. J. Reprod. Biomed. 2022, 20, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Wood, A. Ethics and embryonic stem cell research. Stem Cell Rev. 2005, 1, 317–324. [Google Scholar] [CrossRef]
- Hyun, I. The bioethics of stem cell research and therapy. J. Clin. Investig. 2010, 120, 71–75. [Google Scholar] [CrossRef]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transpl. 2014, 23, 1045–1059. [Google Scholar] [CrossRef]
- Fazeli, Z.; Abedindo, A.; Omrani, M.D.; Ghaderian, S.M.H. Mesenchymal Stem Cells (MSCs) Therapy for Recovery of Fertility: A Systematic Review. Stem Cell Rev. Rep. 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Saha, S.; Roy, P.; Corbitt, C.; Kakar, S.S. Application of Stem Cell Therapy for Infertility. Cells 2021, 10, 1613. [Google Scholar] [CrossRef] [PubMed]
- Garolla, A.; Pizzol, D.; Carosso, A.R.; Borini, A.; Ubaldi, F.M.; Calogero, A.E.; Ferlin, A.; Lanzone, A.; Tomei, F.; Engl, B.; et al. Practical Clinical and Diagnostic Pathway for the Investigation of the Infertile Couple. Front. Endocrinol. 2020, 11, 591837. [Google Scholar] [CrossRef] [PubMed]
- Gelbaya, T.A.; Potdar, N.; Jeve, Y.B.; Nardo, L.G. Definition and epidemiology of unexplained infertility. Obstet. Gynecol. Surv. 2014, 69, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, M.; Juul, A.; Feldt-Rasmussen, U.; Jorgensen, N. Semen quality in patients with pituitary disease and adult-onset hypogonadotropic hypogonadism. Endocr. Connect. 2018, 7, 523–533. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, F.; Wang, Q.; Liu, C.; Lan, Y.; Wang, S.; Xin, Z.; Yang, X. Salpingectomy may decrease antral follicle count but not live birth rate for IVF-ET patients aged 35–39 years: A retrospective study. J. Ovarian Res. 2020, 13, 80. [Google Scholar] [CrossRef]
- Dreisler, E.; Kjer, J.J. Asherman’s syndrome: Current perspectives on diagnosis and management. Int. J. Womens Health 2019, 11, 191–198. [Google Scholar] [CrossRef]
- Egbe, T.O.; Nana-Njamen, T.; Elong, F.; Tchounzou, R.; Simo, A.G.; Nzeuga, G.P.; Njamen Nana, C.; Manka’a, E.; Tchente Nguefack, C.; Halle-Ekane, G.E. Risk factors of tubal infertility in a tertiary hospital in a low-resource setting: A case-control study. Fertil. Res. Pract. 2020, 6, 3. [Google Scholar] [CrossRef]
- Tao, X.; Ge, S.Q.; Chen, L.; Cai, L.S.; Hwang, M.F.; Wang, C.L. Relationships between female infertility and female genital infections and pelvic inflammatory disease: A population-based nested controlled study. Clinics 2018, 73, e364. [Google Scholar] [CrossRef]
- Liu, L.; Li, C.; Sun, X.; Liu, J.; Zheng, H.; Yang, B.; Tang, W.; Wang, C. Chlamydia infection, PID, and infertility: Further evidence from a case-control study in China. BMC Womens Health 2022, 22, 294. [Google Scholar] [CrossRef]
- Aitken, R.J. COVID-19 and human spermatozoa-Potential risks for infertility and sexual transmission? Andrology 2021, 9, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Dukhovny, S.; Wilkins-Haug, L. Genetic Basis of Female Infertility. In Clinical Genomics: Practical Applications in Adult Patient Care; Murray, M.F., Babyatsky, M.W., Giovanni, M.A., Alkuraya, F.S., Stewart, D.R., Eds.; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Wikstrom, A.M.; Hoei-Hansen, C.E.; Dunkel, L.; Rajpert-De Meyts, E. Immunoexpression of androgen receptor and nine markers of maturation in the testes of adolescent boys with Klinefelter syndrome: Evidence for degeneration of germ cells at the onset of meiosis. J. Clin. Endocrinol. Metab. 2007, 92, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Poganitsch-Korhonen, M.; Masliukaite, I.; Nurmio, M.; Lahteenmaki, P.; van Wely, M.; van Pelt, A.M.M.; Jahnukainen, K.; Stukenborg, J.B. Decreased spermatogonial quantity in prepubertal boys with leukaemia treated with alkylating agents. Leukemia 2017, 31, 1460–1463. [Google Scholar] [CrossRef]
- Bar-Shira Maymon, B.; Yogev, L.; Marks, A.; Hauser, R.; Botchan, A.; Yavetz, H. Sertoli cell inactivation by cytotoxic damage to the human testis after cancer chemotherapy. Fertil. Steril. 2004, 81, 1391–1394. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.; Bath, L.E.; Wallace, W.H. Radiation damage to the uterus -- review of the effects of treatment of childhood cancer. Hum. Fertil. 2002, 5, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Sklar, C.A.; Mertens, A.C.; Mitby, P.; Whitton, J.; Stovall, M.; Kasper, C.; Mulder, J.; Green, D.; Nicholson, H.S.; Yasui, Y.; et al. Premature menopause in survivors of childhood cancer: A report from the childhood cancer survivor study. J. Natl. Cancer Inst. 2006, 98, 890–896. [Google Scholar] [CrossRef]
- Sharma, M.; Balasundaram, P. Ovulation Induction Techniques. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kroese, A.C.; de Lange, N.M.; Collins, J.; Evers, J.L. Surgery or embolization for varicoceles in subfertile men. Cochrane Database Syst. Rev. 2012, 10, CD000479. [Google Scholar] [CrossRef]
- Chen, X.F.; Chen, B.; Liu, W.; Huang, Y.P.; Wang, H.X.; Huang, Y.R.; Ping, P. Microsurgical vasoepididymostomy for patients with infectious obstructive azoospermia: Cause, outcome, and associated factors. Asian J. Androl. 2016, 18, 759–762. [Google Scholar] [CrossRef]
- Rives, N.; Courbiere, B.; Almont, T.; Kassab, D.; Berger, C.; Grynberg, M.; Papaxanthos, A.; Decanter, C.; Elefant, E.; Dhedin, N.; et al. What should be done in terms of fertility preservation for patients with cancer? The French 2021 guidelines. Eur. J. Cancer 2022, 173, 146–166. [Google Scholar] [CrossRef]
- Sunderam, S.; Kissin, D.M.; Zhang, Y.; Folger, S.G.; Boulet, S.L.; Warner, L.; Callaghan, W.M.; Barfield, W.D. Assisted Reproductive Technology Surveillance—United States, 2016. MMWR Surveill. Summ. 2019, 68, 1–23. [Google Scholar] [CrossRef]
- McClamrock, H.D.; Jones, H.W., Jr.; Adashi, E.Y. Ovarian stimulation and intrauterine insemination at the quarter centennial: Implications for the multiple births epidemic. Fertil. Steril. 2012, 97, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Kurinczuk, J.J.; Bower, C.; Webb, S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N. Engl. J. Med. 2002, 346, 725–730. [Google Scholar] [CrossRef]
- Wisborg, K.; Ingerslev, H.J.; Henriksen, T.B. IVF and stillbirth: A prospective follow-up study. Hum. Reprod. 2010, 25, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Rahu, K.; Allvee, K.; Karro, H.; Rahu, M. Singleton pregnancies after in vitro fertilization in Estonia: A register-based study of complications and adverse outcomes in relation to the maternal socio-demographic background. BMC Pregnancy Childbirth 2019, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE); Wyns, C.; Bergh, C.; Calhaz-Jorge, C.; De Geyter, C.; Kupka, M.S.; Motrenko, T.; Rugescu, I.; Smeenk, J. ART in Europe, 2016: Results generated from European registries by ESHRE. Hum. Reprod. Open 2020, 2020, hoaa032. [Google Scholar] [CrossRef]
- von Wolff, M.; Haaf, T. In Vitro Fertilization Technology and Child Health. Dtsch. Arztebl. Int. 2020, 117, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.D.; Neri, Q.V.; Takeuchi, T.; Rosenwaks, Z. ICSI: Where we have been and where we are going. Semin. Reprod. Med. 2009, 27, 191–201. [Google Scholar] [CrossRef]
- Assisted reproductive technology in the United States: 1996 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry. Fertil. Steril. 1999, 71, 798–807. [CrossRef]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gulmezoglu, A.M.; Van Look, P.F. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef]
- Clayton, H.B.; Schieve, L.A.; Peterson, H.B.; Jamieson, D.J.; Reynolds, M.A.; Wright, V.C. Ectopic pregnancy risk with assisted reproductive technology procedures. Obstet Gynecol. 2006, 107, 595–604. [Google Scholar] [CrossRef]
- Li, C.; Zhao, W.H.; Zhu, Q.; Cao, S.J.; Ping, H.; Xi, X.; Qin, G.J.; Yan, M.X.; Zhang, D.; Qiu, J.; et al. Risk factors for ectopic pregnancy: A multi-center case-control study. BMC Pregnancy Childbirth 2015, 15, 187. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, J.B.; Aubriot, F.X.; Mathieu, L.; Foulot, H.; Mandelbrot, L.; de Joliere, J.B. Risk factors for ectopic pregnancy in 556 pregnancies after in vitro fertilization: Implications for preventive management. Fertil. Steril. 1991, 56, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Audebert, A.; Pouly, J.L.; Bonifacie, B.; Yazbeck, C. Laparoscopic surgery for distal tubal occlusions: Lessons learned from a historical series of 434 cases. Fertil. Steril. 2014, 102, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Jia-Rong, Z.; Shuang-Di, L.; Xiao-Ping, W. Eutopic or ectopic pregnancy: A competition between signals derived from the endometrium and the fallopian tube for blastocyst implantation. Placenta 2009, 30, 835–839. [Google Scholar] [CrossRef]
- Shao, R.; Nutu, M.; Weijdegard, B.; Egecioglu, E.; Fernandez-Rodriguez, J.; Karlsson-Lindahl, L.; Gemzell-Danielsson, K.; Bergh, C.; Billig, H. Clomiphene citrate causes aberrant tubal apoptosis and estrogen receptor activation in rat fallopian tube: Implications for tubal ectopic pregnancy. Biol. Reprod. 2009, 80, 1262–1271. [Google Scholar] [CrossRef]
- Lekovich, J.; Witkin, S.S.; Doulaveris, G.; Orfanelli, T.; Shulman, B.; Pereira, N.; Rosenwaks, Z.; Spandorfer, S.D. Elevated serum interleukin-1beta levels and interleukin-1beta-to-interleukin-1 receptor antagonist ratio 1 week after embryo transfer are associated with ectopic pregnancy. Fertil. Steril. 2015, 104, 1190–1194. [Google Scholar] [CrossRef]
- Papanikolaou, E.G.; Pozzobon, C.; Kolibianakis, E.M.; Camus, M.; Tournaye, H.; Fatemi, H.M.; Van Steirteghem, A.; Devroey, P. Incidence and prediction of ovarian hyperstimulation syndrome in women undergoing gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil. Steril. 2006, 85, 112–120. [Google Scholar] [CrossRef]
- Griesinger, G.; Diedrich, K.; Devroey, P.; Kolibianakis, E.M. GnRH agonist for triggering final oocyte maturation in the GnRH antagonist ovarian hyperstimulation protocol: A systematic review and meta-analysis. Hum. Reprod. Update 2006, 12, 159–168. [Google Scholar] [CrossRef]
- Palermo, G.D.; Schlegel, P.N.; Hariprashad, J.J.; Ergun, B.; Mielnik, A.; Zaninovic, N.; Veeck, L.L.; Rosenwaks, Z. Fertilization and pregnancy outcome with intracytoplasmic sperm injection for azoospermic men. Hum. Reprod. 1999, 14, 741–748. [Google Scholar] [CrossRef]
- Ponjaert-Kristoffersen, I.; Bonduelle, M.; Barnes, J.; Nekkebroeck, J.; Loft, A.; Wennerholm, U.B.; Tarlatzis, B.C.; Peters, C.; Hagberg, B.S.; Berner, A.; et al. International collaborative study of intracytoplasmic sperm injection-conceived, in vitro fertilization-conceived, and naturally conceived 5-year-old child outcomes: Cognitive and motor assessments. Pediatrics 2005, 115, e283–e289. [Google Scholar] [CrossRef]
- Woldringh, G.H.; Horvers, M.; Janssen, A.J.; Reuser, J.J.; de Groot, S.A.; Steiner, K.; D’Hauwers, K.W.; Wetzels, A.M.; Kremer, J.A. Follow-up of children born after ICSI with epididymal spermatozoa. Hum. Reprod. 2011, 26, 1759–1767. [Google Scholar] [CrossRef]
- Abel, K.; Healey, M.; Finch, S.; Osianlis, T.; Vollenhoven, B. Associations between embryo grading and congenital malformations in IVF/ICSI pregnancies. Reprod. Biomed. Online 2019, 39, 981–989. [Google Scholar] [CrossRef]
- Lv, H.; Diao, F.; Du, J.; Chen, T.; Meng, Q.; Ling, X.; Li, H.; Song, C.; Xi, Q.; Jiang, Y.; et al. Assisted reproductive technology and birth defects in a Chinese birth cohort study. Lancet Reg. Health West Pac. 2021, 7, 100090. [Google Scholar] [CrossRef]
- El-Chaar, D.; Yang, Q.; Gao, J.; Bottomley, J.; Leader, A.; Wen, S.W.; Walker, M. Risk of birth defects increased in pregnancies conceived by assisted human reproduction. Fertil. Steril. 2009, 92, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Bonduelle, M.; Van Assche, E.; Joris, H.; Keymolen, K.; Devroey, P.; Van Steirteghem, A.; Liebaers, I. Prenatal testing in ICSI pregnancies: Incidence of chromosomal anomalies in 1586 karyotypes and relation to sperm parameters. Hum. Reprod. 2002, 17, 2600–2614. [Google Scholar] [CrossRef]
- Braye, A.; Tournaye, H.; Goossens, E. Setting Up a Cryopreservation Programme for Immature Testicular Tissue: Lessons Learned After More Than 15 Years of Experience. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119886342. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Lee, S.; Kim, T. Ovarian tissue cryopreservation and transplantation in patients with cancer. Obstet Gynecol. Sci. 2018, 61, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Nottola, S.A.; Camboni, A.; Van Langendonckt, A.; Demylle, D.; Macchiarelli, G.; Dolmans, M.M.; Martinez-Madrid, B.; Correr, S.; Donnez, J. Cryopreservation and xenotransplantation of human ovarian tissue: An ultrastructural study. Fertil. Steril. 2008, 90, 23–32. [Google Scholar] [CrossRef]
- Cheng, H.; Ye, X.; Zhu, H.; Li, Y.; Xudong, l.; Chang, X.; Cui, H. An experimental study on autologous transplantation of fresh ovarian tissue in sheep. Gynecol. Obstet. Clin. Med. 2021, 1, 87–93. [Google Scholar] [CrossRef]
- Blum, B.; Benvenisty, N. The tumorigenicity of human embryonic stem cells. Adv. Cancer Res. 2008, 100, 133–158. [Google Scholar] [CrossRef]
- Dezawa, M.; Ishikawa, H.; Itokazu, Y.; Yoshihara, T.; Hoshino, M.; Takeda, S.; Ide, C.; Nabeshima, Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 2005, 309, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.K.; Thiemermann, C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells 2010, 28, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Orciani, M.; Caffarini, M.; Lazzarini, R.; Delli Carpini, G.; Tsiroglou, D.; Di Primio, R.; Ciavattini, A. Mesenchymal Stem Cells from Cervix and Age: New Insights into CIN Regression Rate. Oxid. Med. Cell. Longev. 2018, 2018, 1545784. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qu, J.; Xiang, C. The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res. Ther. 2019, 10, 1. [Google Scholar] [CrossRef]
- Palumbo, P.; Lombardi, F.; Siragusa, G.; Cifone, M.G.; Cinque, B.; Giuliani, M. Methods of Isolation, Characterization and Expansion of Human Adipose-Derived Stem Cells (ASCs): An Overview. Int. J. Mol. Sci. 2018, 19, 1897. [Google Scholar] [CrossRef]
- Lee, O.K.; Kuo, T.K.; Chen, W.M.; Lee, K.D.; Hsieh, S.L.; Chen, T.H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004, 103, 1669–1675. [Google Scholar] [CrossRef]
- Amati, E.; Sella, S.; Perbellini, O.; Alghisi, A.; Bernardi, M.; Chieregato, K.; Lievore, C.; Peserico, D.; Rigno, M.; Zilio, A.; et al. Generation of mesenchymal stromal cells from cord blood: Evaluation of in vitro quality parameters prior to clinical use. Stem Cell Res. Ther. 2017, 8, 14. [Google Scholar] [CrossRef]
- Roberts, E.G.; Piekarski, B.L.; Huang, K.; Emani, S.; Wong, J.Y.; Emani, S.M. Evaluation of Placental Mesenchymal Stem Cell Sheets for Myocardial Repair and Regeneration. Tissue Eng. Part A 2019, 25, 867–877. [Google Scholar] [CrossRef]
- In‘t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; de Groot-Swings, G.M.; Claas, F.H.; Fibbe, W.E.; Kanhai, H.H. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef]
- O’Donoghue, K.; Fisk, N.M. Fetal stem cells. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 853–875. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Brinster, R.L. Spermatogonial stem cells. Biol. Reprod. 2018, 99, 52–74. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, D.; Sharma, D. Ovary does harbor stem cells—Size of the cells matter! J. Ovarian Res. 2020, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Rungsiwiwut, R.; Virutamasen, P.; Pruksananonda, K. Mesenchymal stem cells for restoring endometrial function: An infertility perspective. Reprod. Med. Biol. 2021, 20, 13–19. [Google Scholar] [CrossRef]
- Xin, L.; Lin, X.; Pan, Y.; Zheng, X.; Shi, L.; Zhang, Y.; Ma, L.; Gao, C.; Zhang, S. A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater. 2019, 92, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Ding, L.; Wang, L.; Cao, Y.; Zhu, H.; Lu, J.; Li, X.; Song, T.; Hu, Y.; Dai, J. Umbilical cord-derived mesenchymal stem cells on scaffolds facilitate collagen degradation via upregulation of MMP-9 in rat uterine scars. Stem Cell Res. Ther. 2017, 8, 84. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Chen, S.R.; Su, P.P.; Huang, F.H.; Shi, Y.C.; Shi, Q.Y.; Lin, S. Using Mesenchymal Stem Cells to Treat Female Infertility: An Update on Female Reproductive Diseases. Stem Cells Int. 2019, 2019, 9071720. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef]
- Ding, C.; Zou, Q.; Wang, F.; Wu, H.; Wang, W.; Li, H.; Huang, B. HGF and BFGF Secretion by Human Adipose-Derived Stem Cells Improves Ovarian Function During Natural Aging via Activation of the SIRT1/FOXO1 Signaling Pathway. Cell. Physiol. Biochem. 2018, 45, 1316–1332. [Google Scholar] [CrossRef]
- Sagaradze, G.D.; Basalova, N.A.; Efimenko, A.Y.; Tkachuk, V.A. Mesenchymal Stromal Cells as Critical Contributors to Tissue Regeneration. Front. Cell. Dev. Biol. 2020, 8, 576176. [Google Scholar] [CrossRef]
- Feng, X.; Ling, L.; Zhang, W.; Liu, X.; Wang, Y.; Luo, Y.; Xiong, Z. Effects of Human Amnion-Derived Mesenchymal Stem Cell (hAD-MSC) Transplantation In Situ on Primary Ovarian Insufficiency in SD Rats. Reprod. Sci. 2020, 27, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Feng, X.; Wei, T.; Wang, Y.; Wang, Y.; Wang, Z.; Tang, D.; Luo, Y.; Xiong, Z. Human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation improves ovarian function in rats with premature ovarian insufficiency (POI) at least partly through a paracrine mechanism. Stem Cell Res. Ther. 2019, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Cervello, I.; Gil-Sanchis, C.; Santamaria, X.; Cabanillas, S.; Diaz, A.; Faus, A.; Pellicer, A.; Simon, C. Human CD133(+) bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome. Fertil. Steril. 2015, 104, 1552–1560.e1551–1553. [Google Scholar] [CrossRef] [PubMed]
- Kilic, S.; Yuksel, B.; Pinarli, F.; Albayrak, A.; Boztok, B.; Delibasi, T. Effect of stem cell application on Asherman syndrome, an experimental rat model. J. Assist. Reprod. Genet. 2014, 31, 975–982. [Google Scholar] [CrossRef]
- Zimmermann, R.C.; Xiao, E.; Husami, N.; Sauer, M.V.; Lobo, R.; Kitajewski, J.; Ferin, M. Short-term administration of antivascular endothelial growth factor antibody in the late follicular phase delays follicular development in the rhesus monkey. J. Clin. Endocrinol. Metab. 2001, 86, 768–772. [Google Scholar] [CrossRef]
- Cho, J.; Kim, T.H.; Seok, J.; Jun, J.H.; Park, H.; Kweon, M.; Lim, J.Y.; Kim, G.J. Vascular remodeling by placenta-derived mesenchymal stem cells restores ovarian function in ovariectomized rat model via the VEGF pathway. Lab. Investig. 2021, 101, 304–317. [Google Scholar] [CrossRef]
- Sun, B.; Ma, Y.; Wang, F.; Hu, L.; Sun, Y. miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res. Ther. 2019, 10, 360. [Google Scholar] [CrossRef]
- Yang, M.; Lin, L.; Sha, C.; Li, T.; Zhao, D.; Wei, H.; Chen, Q.; Liu, Y.; Chen, X.; Xu, W.; et al. Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. Lab. Investig. 2020, 100, 342–352. [Google Scholar] [CrossRef]
- Deng, C.; Xie, Y.; Zhang, C.; Ouyang, B.; Chen, H.; Lv, L.; Yao, J.; Liang, X.; Zhang, Y.; Sun, X.; et al. Urine-Derived Stem Cells Facilitate Endogenous Spermatogenesis Restoration of Busulfan-Induced Nonobstructive Azoospermic Mice by Paracrine Exosomes. Stem Cells Dev. 2019, 28, 1322–1333. [Google Scholar] [CrossRef]
- Gangaraju, V.K.; Lin, H. MicroRNAs: Key regulators of stem cells. Nat. Rev. Mol. Cell Biol. 2009, 10, 116–125. [Google Scholar] [CrossRef]
- Kim, K.; Kenigsberg, S.; Jurisicova, A.; Bentov, Y. The Role of Mitochondria in Oocyte and Early Embryo Health. OBM Genet. 2019, 3, 1. [Google Scholar] [CrossRef]
- Rodriguez-Varela, C.; Labarta, E. Role of Mitochondria Transfer in Infertility: A Commentary. Cells 2022, 11, 1867. [Google Scholar] [CrossRef] [PubMed]
- Costa-Borges, N.; Nikitos, E.; Spath, K.; Rink, K.; Kostaras, K.; Zervomanolakis, I.; Kontopoulos, G.; Polyzos, P.; Grigorakis, S.; Prokopakis, T.; et al. First registered pilot trial to validate the safety and effectiveness of maternal spindle transfer to overcome infertility associated with poor oocyte quality. Fertil. Steril. 2020, 114, E71–E72. [Google Scholar] [CrossRef]
- Zhang, H.; Panula, S.; Petropoulos, S.; Edsgard, D.; Busayavalasa, K.; Liu, L.; Li, X.; Risal, S.; Shen, Y.; Shao, J.; et al. Adult human and mouse ovaries lack DDX4-expressing functional oogonial stem cells. Nat. Med. 2015, 21, 1116–1118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Hao, J.X.; Meng, T.G.; Guo, L.; Dong, M.Z.; Fan, L.H.; Ouyang, Y.C.; Wang, G.; Sun, Q.Y.; Ou, X.H.; et al. Transfer of autologous mitochondria from adipose tissue-derived stem cells rescues oocyte quality and infertility in aged mice. Aging 2017, 9, 2480–2488. [Google Scholar] [CrossRef]
- Sheng, X.; Yang, Y.; Zhou, J.; Yan, G.; Liu, M.; Xu, L.; Li, Z.; Jiang, R.; Diao, Z.; Zhen, X.; et al. Mitochondrial transfer from aged adipose-derived stem cells does not improve the quality of aged oocytes in C57BL/6 mice. Mol. Reprod. Dev. 2019, 86, 516–529. [Google Scholar] [CrossRef]
- Gong, D.; Zhang, C.; Li, T.; Zhang, J.; Zhang, N.; Tao, Z.; Zhu, W.; Sun, X. Are Sertoli cells a kind of mesenchymal stem cells? Am. J. Transl. Res. 2017, 9, 1067–1074. [Google Scholar]
- Porubska, B.; Vasek, D.; Somova, V.; Hajkova, M.; Hlaviznova, M.; Tlapakova, T.; Holan, V.; Krulova, M. Sertoli Cells Possess Immunomodulatory Properties and the Ability of Mitochondrial Transfer Similar to Mesenchymal Stromal Cells. Stem Cell Rev. Rep. 2021, 17, 1905–1916. [Google Scholar] [CrossRef]
- Li, C.; Cheung, M.K.H.; Han, S.; Zhang, Z.; Chen, L.; Chen, J.; Zeng, H.; Qiu, J. Mesenchymal stem cells and their mitochondrial transfer: A double-edged sword. Biosci. Rep. 2019, 39, BSR20182417. [Google Scholar] [CrossRef]
- Arabpour, M.; Saghazadeh, A.; Rezaei, N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int. Immunopharmacol. 2021, 97, 107823. [Google Scholar] [CrossRef]
- Xie, M.; Xiong, W.; She, Z.; Wen, Z.; Abdirahman, A.S.; Wan, W.; Wen, C. Immunoregulatory Effects of Stem Cell-Derived Extracellular Vesicles on Immune Cells. Front. Immunol. 2020, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Xu, W.; Li, X.; Meng, W.; Hu, L.; Luo, Z.; Wang, Y.; Luo, S.; Li, S. Differential Expression Profile of Immunological Cytokines in Local Ovary in Patients with Polycystic Ovarian Syndrome: Analysis by Flow Cytometry. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 197, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Su, N.J.; Ma, J.; Feng, D.F.; Zhou, S.; Li, Z.T.; Zhou, W.P.; Deng, H.; Liang, J.Y.; Yang, X.H.; Zhang, Y.M.; et al. The peripheral blood transcriptome identifies dysregulation of inflammatory response genes in polycystic ovary syndrome. Gynecol. Endocrinol. 2018, 34, 584–588. [Google Scholar] [CrossRef]
- Xie, Q.; Xiong, X.; Xiao, N.; He, K.; Chen, M.; Peng, J.; Su, X.; Mei, H.; Dai, Y.; Wei, D.; et al. Mesenchymal Stem Cells Alleviate DHEA-Induced Polycystic Ovary Syndrome (PCOS) by Inhibiting Inflammation in Mice. Stem Cells Int. 2019, 2019, 9782373. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Chen, X.; Zhou, J.; Xiao, X.M. Treatment evaluation of Wharton’s jelly-derived mesenchymal stem cells using a chronic salpingitis model: An animal experiment. Stem Cell Res. Ther. 2017, 8, 232. [Google Scholar] [CrossRef]
- Liao, W.; Tang, X.; Li, X.; Li, T. Therapeutic effect of human umbilical cord mesenchymal stem cells on tubal factor infertility using a chronic salpingitis murine model. Arch. Gynecol. Obstet. 2019, 300, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Volkova, N.; Yukhta, M.; Goltsev, A. Mesenchymal Stem Cells in Restoration of Fertility at Experimental Pelvic Inflammatory Disease. Stem Cells Int. 2017, 2017, 2014132. [Google Scholar] [CrossRef]
- Sreenivasa, G.; Kavitha, P.; Chaithra, P.; Vineeth, V.; Kumar, C.S.; Malini, S.S. Clinical Significance of Antisperm Antibody Analysis in Evaluating Male Infertility of South Karnataka. Bioscan 2011, 6, 125–128. [Google Scholar]
- Aghamir, S.M.; Salavati, A.; Yousefie, R.; Tootian, Z.; Ghazaleh, N.; Jamali, M.; Azimi, P. Does bone marrow-derived mesenchymal stem cell transfusion prevent antisperm antibody production after traumatic testis rupture? Urology 2014, 84, 82–86. [Google Scholar] [CrossRef]
- Hajizadeh Maleki, B.; Tartibian, B. COVID-19 and male reproductive function: A prospective, longitudinal cohort study. Reproduction 2021, 161, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Ozveri, H.; Eren, M.T.; Kirisoglu, C.E.; Sariguzel, N. Atypical presentation of SARS-CoV-2 infection in male genitalia. Urol. Case Rep. 2020, 33, 101349. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention What is Assisted Reproductive Technology? Available online: https://www.cdc.gov/art/whatis.html (accessed on 3 September 2022).
- Hwang, J.W.; Lee, N.K.; Yang, J.H.; Son, H.J.; Bang, S.I.; Chang, J.W.; Na, D.L. A Comparison of Immune Responses Exerted Following Syngeneic, Allogeneic, and Xenogeneic Transplantation of Mesenchymal Stem Cells into the Mouse Brain. Int. J. Mol. Sci. 2020, 21, 3052. [Google Scholar] [CrossRef] [PubMed]
- Joswig, A.J.; Mitchell, A.; Cummings, K.J.; Levine, G.J.; Gregory, C.A.; Smith, R., 3rd; Watts, A.E. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res. Ther. 2017, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Berglund, A.K.; Fortier, L.A.; Antczak, D.F.; Schnabel, L.V. Immunoprivileged no more: Measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 288. [Google Scholar] [CrossRef]
- Huang, X.P.; Sun, Z.; Miyagi, Y.; McDonald Kinkaid, H.; Zhang, L.; Weisel, R.D.; Li, R.K. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation 2010, 122, 2419–2429. [Google Scholar] [CrossRef]
- Lohan, P.; Treacy, O.; Griffin, M.D.; Ritter, T.; Ryan, A.E. Anti-Donor Immune Responses Elicited by Allogeneic Mesenchymal Stem Cells and Their Extracellular Vesicles: Are We Still Learning? Front. Immunol. 2017, 8, 1626. [Google Scholar] [CrossRef] [PubMed]
- Anderi, R.; Makdissy, N.; Azar, A.; Rizk, F.; Hamade, A. Cellular therapy with human autologous adipose-derived adult cells of stromal vascular fraction for alopecia areata. Stem Cell Res. Ther. 2018, 9, 141. [Google Scholar] [CrossRef]
- Izadyar, F.; Den Ouden, K.; Stout, T.A.; Stout, J.; Coret, J.; Lankveld, D.P.; Spoormakers, T.J.; Colenbrander, B.; Oldenbroek, J.K.; Van der Ploeg, K.D.; et al. Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction 2003, 126, 765–774. [Google Scholar] [CrossRef]
- Jahnukainen, K.; Ehmcke, J.; Quader, M.A.; Saiful Huq, M.; Epperly, M.W.; Hergenrother, S.; Nurmio, M.; Schlatt, S. Testicular recovery after irradiation differs in prepubertal and pubertal non-human primates, and can be enhanced by autologous germ cell transplantation. Hum. Reprod. 2011, 26, 1945–1954. [Google Scholar] [CrossRef]
- Hermann, B.P.; Sukhwani, M.; Winkler, F.; Pascarella, J.N.; Peters, K.A.; Sheng, Y.; Valli, H.; Rodriguez, M.; Ezzelarab, M.; Dargo, G.; et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell 2012, 11, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.Y.; Chen, S.R.; Zhao, Y.X.; Chen, J.M.; Chen, W.H.; Lin, S.; Shi, Q.Y. Melatonin enhances autologous adipose-derived stem cells to improve mouse ovarian function in relation to the SIRT6/NF-kappaB pathway. Stem Cell Res. Ther. 2022, 13, 399. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh-Hasankolaei, M.; Batavani, R.; Eslaminejad, M.B.; Sayahpour, F. Transplantation of Autologous Bone Marrow Mesenchymal Stem Cells into the Testes of Infertile Male Rats and New Germ Cell Formation. Int. J. Stem Cells 2016, 9, 250–263. [Google Scholar] [CrossRef]
- Edessy, M.; Hosni, H.; Shady, Y.; Waf, Y.; Bakr, S.; Kamel, M. Autologous stem cells therapy, The first baby of idiopathic premature ovarian failure. Acta Med. Int. 2016, 3, 19–23. [Google Scholar] [CrossRef]
- Igboeli, P.; El Andaloussi, A.; Sheikh, U.; Takala, H.; ElSharoud, A.; McHugh, A.; Gavrilova-Jordan, L.; Levy, S.; Al-Hendy, A. Intraovarian injection of autologous human mesenchymal stem cells increases estrogen production and reduces menopausal symptoms in women with premature ovarian failure: Two case reports and a review of the literature. J. Med. Case Rep. 2020, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Nagori, C.B.; Panchal, S.Y.; Patel, H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman’s syndrome. J. Hum. Reprod. Sci. 2011, 4, 43–48. [Google Scholar] [CrossRef]
- Santamaria, X.; Cabanillas, S.; Cervello, I.; Arbona, C.; Raga, F.; Ferro, J.; Palmero, J.; Remohi, J.; Pellicer, A.; Simon, C. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: A pilot cohort study. Hum. Reprod. 2016, 31, 1087–1096. [Google Scholar] [CrossRef]
- Singh, N.; Mohanty, S.; Seth, T.; Shankar, M.; Bhaskaran, S.; Dharmendra, S. Autologous stem cell transplantation in refractory Asherman’s syndrome: A novel cell based therapy. J. Hum. Reprod. Sci. 2014, 7, 93–98. [Google Scholar] [CrossRef]
- Zhao, G.; Cao, Y.; Zhu, X.; Tang, X.; Ding, L.; Sun, H.; Li, J.; Li, X.; Dai, C.; Ru, T.; et al. Transplantation of collagen scaffold with autologous bone marrow mononuclear cells promotes functional endometrium reconstruction via downregulating DeltaNp63 expression in Asherman’s syndrome. Sci. China Life Sci. 2017, 60, 404–416. [Google Scholar] [CrossRef]
- Zhao, Y.; Tao, M.; Wei, M.; Du, S.; Wang, H.; Wang, X. Mesenchymal stem cells derived exosomal miR-323-3p promotes proliferation and inhibits apoptosis of cumulus cells in polycystic ovary syndrome (PCOS). Artif. Cells Nanomed. Biotechnol. 2019, 47, 3804–3813. [Google Scholar] [CrossRef]
- Herraiz, S.; Romeu, M.; Buigues, A.; Martinez, S.; Diaz-Garcia, C.; Gomez-Segui, I.; Martinez, J.; Pellicer, N.; Pellicer, A. Autologous stem cell ovarian transplantation to increase reproductive potential in patients who are poor responders. Fertil. Steril. 2018, 110, 496–505.e491. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, D.; Yang, L.; Hou, Q.; Ma, H.; Xu, X. The therapeutic potential of bone marrow mesenchymal stem cells in premature ovarian failure. Stem Cell Res. Ther. 2018, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Gabr, H.; Elkheir, W.A. Autologous MSC therapy for azoospermia: A pilot clinical. Cytotherapy 2015, 17, S51. [Google Scholar] [CrossRef]
- Mashayekhi, M.; Mirzadeh, E.; Chekini, Z.; Ahmadi, F.; Eftekhari-Yazdi, P.; Vesali, S.; Madani, T.; Aghdami, N. Evaluation of safety, feasibility and efficacy of intra-ovarian transplantation of autologous adipose derived mesenchymal stromal cells in idiopathic premature ovarian failure patients: Non-randomized clinical trial, phase I, first in human. J. Ovarian Res. 2021, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Shin, J.E.; Kwon, H.; Choi, D.H.; Kim, J.H. Effect of Autologous Adipose-Derived Stromal Vascular Fraction Transplantation on Endometrial Regeneration in Patients of Asherman’s Syndrome: A Pilot Study. Reprod. Sci. 2020, 27, 561–568. [Google Scholar] [CrossRef]
- Sudoma, I.; Pylyp, L.; Kremenska, Y.; Goncharova, Y. Application of autologous adipose-derived stem cells for thin endometrium treatment in patients with failed ART programs. J. Stem Cell Ther. Transpl. 2019, 3, 1–8. [Google Scholar] [CrossRef]
- Haahr, M.K.; Jensen, C.H.; Toyserkani, N.M.; Andersen, D.C.; Damkier, P.; Sorensen, J.A.; Lund, L.; Sheikh, S.P. Safety and Potential Effect of a Single Intracavernous Injection of Autologous Adipose-Derived Regenerative Cells in Patients with Erectile Dysfunction Following Radical Prostatectomy: An Open-Label Phase I Clinical Trial. EBioMedicine 2016, 5, 204–210. [Google Scholar] [CrossRef]
- Ma, H.; Liu, M.; Li, Y.; Wang, W.; Yang, K.; Lu, L.; He, M.; Deng, T.; Li, M.; Wu, D. Intrauterine transplantation of autologous menstrual blood stem cells increases endometrial thickness and pregnancy potential in patients with refractory intrauterine adhesion. J. Obstet. Gynaecol. Res. 2020, 46, 2347–2355. [Google Scholar] [CrossRef]
- Tan, J.; Li, P.; Wang, Q.; Li, Y.; Li, X.; Zhao, D.; Xu, X.; Kong, L. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman’s syndrome. Hum. Reprod. 2016, 31, 2723–2729. [Google Scholar] [CrossRef]
- Gellert, S.E.; Pors, S.E.; Kristensen, S.G.; Bay-Bjorn, A.M.; Ernst, E.; Yding Andersen, C. Transplantation of frozen-thawed ovarian tissue: An update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J. Assist. Reprod. Genet 2018, 35, 561–570. [Google Scholar] [CrossRef]
- Pacheco, F.; Marin, L.; Oktay, K.H. Autologous cryopreserved ovarian tissue transplantation (acott): An update from our previous meta-analytical dataset as of march 2021. Fertil. Steril. 2021, 116, e217. [Google Scholar] [CrossRef]
- Sapozhak, I.M.; Gubar, S.; Rodnichenko, A.E.; Zlatska, A.V. Application of autologous endometrial mesenchymal stromal/stem cells increases thin endometrium receptivity: A case report. J. Med. Case Rep. 2020, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Zlatska, A.V.; Rodnichenko, A.E.; Gubar, O.S.; Zubov, D.O.; Novikova, S.N.; Vasyliev, R.G. Endometrial stromal cells: Isolation, expansion, morphological and functional properties. Exp. Oncol. 2017, 39, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, A.; Tang, X.; Li, M.; Yan, L.; Shang, W.; Gao, M. Intrauterine transplantation of autologous bone marrow derived mesenchymal stem cells followed by conception in a patient of severe intrauterine adhesions. Open J. Obstet. Gynecol. 2013, 3, 31293. [Google Scholar] [CrossRef]
- Gupta, S.; Lodha, P.; Karthick, M.S.; Tandulwadkar, S.R. Role of Autologous Bone Marrow-Derived Stem Cell Therapy for Follicular Recruitment in Premature Ovarian Insufficiency: Review of Literature and a Case Report of World’s First Baby with Ovarian Autologous Stem Cell Therapy in a Perimenopausal Woman of Age 45 Year. J. Hum. Reprod. Sci. 2018, 11, 125–130. [Google Scholar] [CrossRef]
- Estuardo, L.I.J.; Carlos, D.M.; Roberto, H.R.; Jesús, Á.P.F.d.; Juan, G.V.J.; Alejandro, K.B.; Daniela, Á.R.; Maruxa, P.F.; Angélica, P.N.M.; Ruiz, G.d.l.R.; et al. Therapeutic Potential of Autologous Adipose Derived Mesenchymal Stem Cells in Human POI and Ovarian Aging. J. Evol. Stem Cell Res. 2021, 1, 5–18. [Google Scholar] [CrossRef]
- Gao, M.; Yu, Z.; Yao, D.; Qian, Y.; Wang, Q.; Jia, R. Mesenchymal stem cells therapy: A promising method for the treatment of uterine scars and premature ovarian failure. Tissue Cell 2022, 74, 101676. [Google Scholar] [CrossRef]
- Fu, X.; He, Y.; Wang, X.; Peng, D.; Chen, X.; Li, X.; Wang, Q. Overexpression of miR-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting PDCD4 and PTEN to inhibit granulosa cell apoptosis. Stem Cell Res. Ther. 2017, 8, 187. [Google Scholar] [CrossRef]
- Tan, Q.; Xia, D.; Ying, X. miR-29a in Exosomes from Bone Marrow Mesenchymal Stem Cells Inhibit Fibrosis during Endometrial Repair of Intrauterine Adhesion. Int. J. Stem Cells 2020, 13, 414–423. [Google Scholar] [CrossRef]
- Xiao, B.; Zhu, Y.; Huang, J.; Wang, T.; Wang, F.; Sun, S. Exosomal transfer of bone marrow mesenchymal stem cell-derived miR-340 attenuates endometrial fibrosis. Biol. Open 2019, 8, bio039958. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, R.; Wang, G.; Zhang, Y.; Liu, F. Exosomes derived from mesenchymal stem cells reverse EMT via TGF-beta1/Smad pathway and promote repair of damaged endometrium. Stem Cell Res. Ther. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, Z.; Sun, J. Human bone marrow mesenchymal stem cell-derived exosomes stimulate cutaneous wound healing mediates through TGF-beta/Smad signaling pathway. Stem Cell Res. Ther. 2020, 11, 198. [Google Scholar] [CrossRef]
- Qamar, A.Y.; Fang, X.; Kim, M.J.; Cho, J. Improved Post-Thaw Quality of Canine Semen after Treatment with Exosomes from Conditioned Medium of Adipose-Derived Mesenchymal Stem Cells. Animals 2019, 9, 865. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Qi, W.; Zheng, J.; Tian, Y.; Qi, X.; Kong, D.; Zhang, J.; Huang, X. Exosomes Derived from Adipose Mesenchymal Stem Cells Restore Functional Endometrium in a Rat Model of Intrauterine Adhesions. Reprod. Sci. 2020, 27, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lu, J.; Ding, C.; Zou, Q.; Wang, W.; Li, H. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res. Ther. 2018, 9, 216. [Google Scholar] [CrossRef]
- Zhu, L.L.; Huang, X.; Yu, W.; Chen, H.; Chen, Y.; Dai, Y.T. Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia 2018, 50, e12871. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, B.; Su, P.; Chang, Q.; Li, P.; Song, A.; Zhao, X.; Yuan, Z.; Tan, J. Concentrated exosomes from menstrual blood-derived stromal cells improves ovarian activity in a rat model of premature ovarian insufficiency. Stem Cell Res. Ther. 2021, 12, 178. [Google Scholar] [CrossRef]
- Claridge, B.; Lozano, J.; Poh, Q.H.; Greening, D.W. Development of Extracellular Vesicle Therapeutics: Challenges, Considerations, and Opportunities. Front. Cell Dev. Biol. 2021, 9, 734720. [Google Scholar] [CrossRef]
- Kucia, M.; Reca, R.; Campbell, F.R.; Zuba-Surma, E.; Majka, M.; Ratajczak, J.; Ratajczak, M.Z. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia 2006, 20, 857–869. [Google Scholar] [CrossRef]
- Shin, D.M.; Liu, R.; Wu, W.; Waigel, S.J.; Zacharias, W.; Ratajczak, M.Z.; Kucia, M. Global gene expression analysis of very small embryonic-like stem cells reveals that the Ezh2-dependent bivalent domain mechanism contributes to their pluripotent state. Stem Cells Dev. 2012, 21, 1639–1652. [Google Scholar] [CrossRef]
- Shin, D.M.; Liu, R.; Klich, I.; Ratajczak, J.; Kucia, M.; Ratajczak, M.Z. Molecular characterization of isolated from murine adult tissues very small embryonic/epiblast like stem cells (VSELs). Mol. Cells 2010, 29, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Ratajczak, J.; Suszynska, M.; Miller, D.M.; Kucia, M.; Shin, D.M. A Novel View of the Adult Stem Cell Compartment From the Perspective of a Quiescent Population of Very Small Embryonic-Like Stem Cells. Circ. Res. 2017, 120, 166–178. [Google Scholar] [CrossRef]
- Bhartiya, D.; Unni, S.; Parte, S.; Anand, S. Very small embryonic-like stem cells: Implications in reproductive biology. Biomed. Res. Int. 2013, 2013, 682326. [Google Scholar] [CrossRef] [PubMed]
- Lahlil, R.; Scrofani, M.; Barbet, R.; Tancredi, C.; Aries, A.; Henon, P. VSELs Maintain their Pluripotency and Competence to Differentiate after Enhanced Ex Vivo Expansion. Stem Cell Rev. Rep. 2018, 14, 510–524. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, D.; Kasiviswanathan, S.; Unni, S.K.; Pethe, P.; Dhabalia, J.V.; Patwardhan, S.; Tongaonkar, H.B. Newer insights into premeiotic development of germ cells in adult human testis using Oct-4 as a stem cell marker. J. Histochem. Cytochem. 2010, 58, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Parte, S.; Bhartiya, D.; Telang, J.; Daithankar, V.; Salvi, V.; Zaveri, K.; Hinduja, I. Detection, characterization, and spontaneous differentiation in vitro of very small embryonic-like putative stem cells in adult mammalian ovary. Stem Cells Dev. 2011, 20, 1451–1464. [Google Scholar] [CrossRef]
- Virant-Klun, I. Functional Testing of Primitive Oocyte-like Cells Developed in Ovarian Surface Epithelium Cell Culture from Small VSEL-like Stem Cells: Can They Be Fertilized One Day? Stem Cell Rev. Rep. 2018, 14, 715–721. [Google Scholar] [CrossRef]
- Kurkure, P.; Prasad, M.; Dhamankar, V.; Bakshi, G. Very small embryonic-like stem cells (VSELs) detected in azoospermic testicular biopsies of adult survivors of childhood cancer. Reprod. Biol. Endocrinol. 2015, 13, 122. [Google Scholar] [CrossRef]
- Sriraman, K.; Bhartiya, D.; Anand, S.; Bhutda, S. Mouse Ovarian Very Small Embryonic-Like Stem Cells Resist Chemotherapy and Retain Ability to Initiate Oocyte-Specific Differentiation. Reprod. Sci. 2015, 22, 884–903. [Google Scholar] [CrossRef]
- Park, S.H.; Sim, W.Y.; Min, B.H.; Yang, S.S.; Khademhosseini, A.; Kaplan, D.L. Chip-based comparison of the osteogenesis of human bone marrow- and adipose tissue-derived mesenchymal stem cells under mechanical stimulation. PLoS ONE 2012, 7, e46689. [Google Scholar] [CrossRef]
- Tahmasbpour Marzouni, E.; Sinclair, A.H.; Stern, C.; Tucker, E.J. Stem cells and organs-on-chips: New promising technologies for human infertility treatment. Endocr. Rev. 2021, 43, 878–906. [Google Scholar] [CrossRef] [PubMed]
- Dossena, M.; Piras, R.; Cherubini, A.; Barilani, M.; Dugnani, E.; Salanitro, F.; Moreth, T.; Pampaloni, F.; Piemonti, L.; Lazzari, L. Standardized GMP-compliant scalable production of human pancreas organoids. Stem Cell Res. Ther. 2020, 11, 94. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).