Estimating Effects of Sea Level Rise on Benthic Biodiversity and Ecosystem Functioning in a Large Meso-Tidal Coastal Lagoon
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
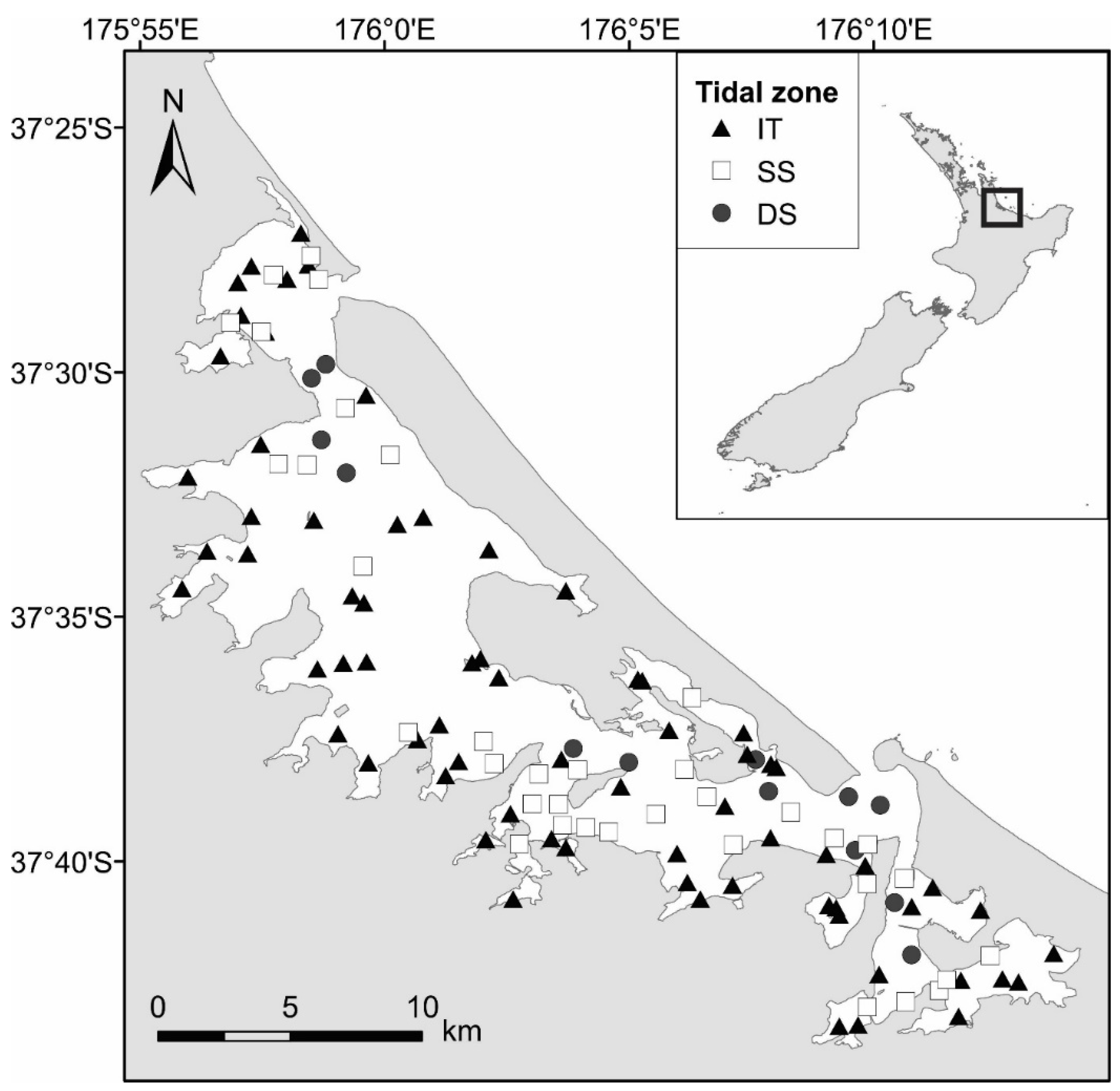
2.1. Study Area
2.2. Data Acquisition
2.3. Environmental Variables
2.4. Macrofauna Data
2.5. Functional Group Assignment
2.6. Statistical Analyses
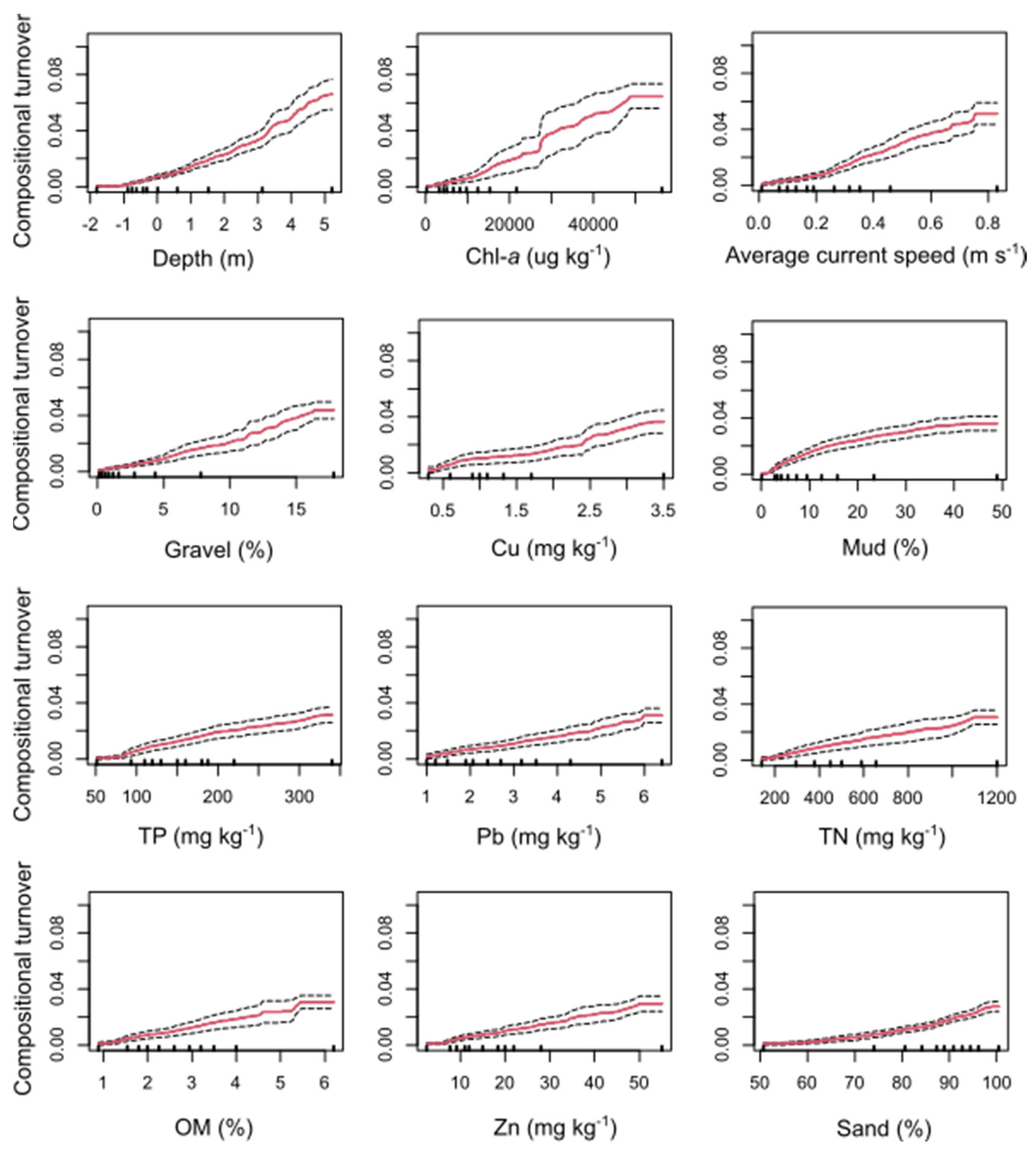
2.6.1. Determining Critical Points of Compositional Turnover along Key Environmental Gradients (Gradient Forest Modelling)
2.6.2. Benthic Macrofauna–Defining Tidal Zones
2.6.3. Functional Group Analysis–Implications for Ecosystem Functioning
3. Results
3.1. Relative Importance of Environmental Gradients for Predicting Compositional Turnover
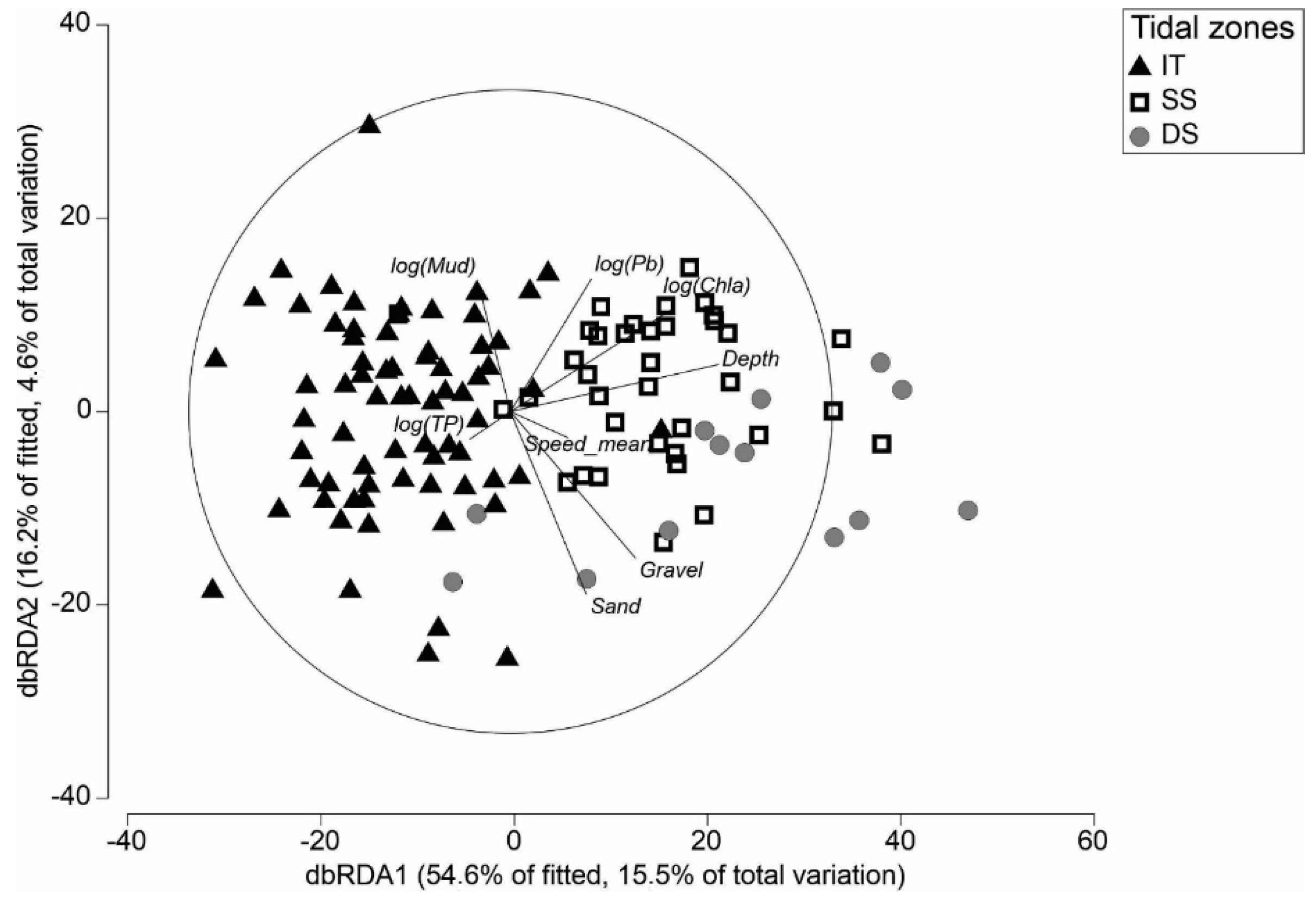
3.2. Definition of Tidal Zones Based on Community and Environmental Data
3.3. Differences in Macrofauna Communities between Tidal Zones
3.4. Functional Group Analysis
4. Discussion
4.1. Environmental Drivers of Macroinvertebrate Community Structure and Compositional Turnover
4.2. Comparisons across Tidal Zones and Implications of Reduced Intertidal Area
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costanza, R.; de Groot, R.; Sutton, P.; van der Ploeg, S.; Anderson, S.J.; Kubiszewski, I.; Farber, S.; Turner, R.K. Changes in the global value of ecosystem services. Glob. Environ. Chang. 2014, 26, 152–158. [Google Scholar] [CrossRef]
- Levin, L.A.; Boesch, D.F.; Covich, A.; Dahm, C.; Erséus, C.; Ewel, K.C.; Kneib, R.T.; Moldenke, A.; Palmer, M.A.; Snelgrove, P.; et al. The Function of Marine Critical Transition Zones and the Importance of Sediment Biodiversity. Ecosystems 2001, 4, 430–451. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 2013, 3, 961–968. [Google Scholar] [CrossRef]
- Shepard, C.C.; Crain, C.M.; Beck, M.W. The Protective Role of Coastal Marshes: A Systematic Review and Meta-analysis. PLoS ONE 2011, 6, e27374. [Google Scholar] [CrossRef]
- Christianen, M.J.A.; Middelburg, J.J.; Holthuijsen, S.J.; Jouta, J.; Compton, T.J.; van der Heide, T.; Piersma, T.; Sinninghe Damsté, J.S.; van der Veer, H.W.; Schouten, S.; et al. Benthic primary producers are key to sustain the Wadden Sea food web: Stable carbon isotope analysis at landscape scale. Ecology 2017, 98, 1498–1512. [Google Scholar] [CrossRef]
- Sheaves, M.; Baker, R.; Nagelkerken, I.; Connolly, R.M. True Value of Estuarine and Coastal Nurseries for Fish: Incorporating Complexity and Dynamics. Estuaries Coasts 2015, 38, 401–414. [Google Scholar] [CrossRef]
- Galbraith, H.; Jones, R.; Park, R.; Clough, J.; Herrod-Julius, S.; Harrington, B.; Page, G. Global Climate Change and Sea Level Rise: Potential Losses of Intertidal Habitat for Shorebirds. Waterbirds 2002, 25, 173–183. [Google Scholar] [CrossRef]
- Snelgrove, P.V.R.; Thrush, S.F.; Wall, D.H.; Norkko, A. Real world biodiversity–ecosystem functioning: A seafloor perspective. Trends Ecol. Evol. 2014, 29, 398–405. [Google Scholar] [CrossRef]
- Jones, H.F.E.; Pilditch, C.A.; Hamilton, D.P.; Bryan, K.R. Impacts of a bivalve mass mortality event on an estuarine food web and bivalve grazing pressure. N. Z. J. Mar. Freshw. Res. 2017, 51, 370–392. [Google Scholar] [CrossRef]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef]
- Jaffe, B.E.; Smith, R.E.; Foxgrover, A.C. Anthropogenic influence on sedimentation and intertidal mudflat change in San Pablo Bay, California: 1856–1983. Estuar. Coast. Shelf Sci. 2007, 73, 175–187. [Google Scholar] [CrossRef]
- Bonsdorff, E.; Blomqvist, E.M.; Mattila, J.; Norkko, A. Coastal eutrophication: Causes, consequences and perspectives in the Archipelago areas of the northern Baltic Sea. Estuar. Coast. Shelf Sci. 1997, 44, 63–72. [Google Scholar] [CrossRef]
- Thrush, S.; Hewitt, J.; Cummings, V.; Ellis, J.; Hatton, C.; Lohrer, A.; Norkko, A. Muddy waters: Elevating sediment input to coastal and estuarine habitats. Front. Ecol. Environ. 2004, 2, 299–306. [Google Scholar] [CrossRef]
- Douglas, E.J.; Pilditch, C.A.; Kraan, C.; Schipper, L.A.; Lohrer, A.M.; Thrush, S.F. Macrofaunal Functional Diversity Provides Resilience to Nutrient Enrichment in Coastal Sediments. Ecosystems 2017, 20, 1324–1336. [Google Scholar] [CrossRef]
- Ellis, J.I.; Clark, D.; Atalah, J.; Jiang, W.; Taiapa, C.; Patterson, M.; Sinner, J.; Hewitt, J. Multiple stressor effects on marine infauna: Responses of estuarine taxa and functional traits to sedimentation, nutrient and metal loading. Sci. Rep. 2017, 7, 12013. [Google Scholar] [CrossRef]
- Bernardino, A.F.; Sanders, C.J.; Bissoli, L.B.; Gomes, L.E.d.O.; Kauffman, J.B.; Ferreira, T.O. Land use impacts on benthic bioturbation potential and carbon burial in Brazilian mangrove ecosystems. Limnol. Oceanogr. 2020, 65, 2366–2376. [Google Scholar] [CrossRef]
- Mangan, S.; Bryan, K.R.; Thrush, S.F.; Gladstone-Gallagher, R.V.; Lohrer, A.M.; Pilditch, C.A. Shady business: The darkening of estuaries constrains benthic ecosystem function. Mar. Ecol. Prog. Ser. 2020, 647, 33–48. [Google Scholar] [CrossRef]
- Fujii, T.; Raffaelli, D. Sea-level rise, expected environmental changes, and responses of intertidal benthic macrofauna in the Humber estuary, UK. Mar. Ecol. Prog. Ser. 2008, 371, 23–35. [Google Scholar] [CrossRef]
- Rullens, V.; Mangan, S.; Stephenson, F.; Clark, D.E.; Bulmer, R.H.; Berthelsen, A.; Crawshaw, J.; Gladstone-Gallagher, R.V.; Thomas, S.; Ellis, J.I.; et al. Understanding the consequences of sea level rise: The ecological implications of losing intertidal habitat. N. Z. J. Mar. Freshw. Res. 2022, 1–18. [Google Scholar] [CrossRef]
- Beukema, J.J. Expected changes in the benthic fauna of Wadden Sea tidal flats as a result of sea-level rise or bottom subsidence. J. Sea Res. 2002, 47, 25–39. [Google Scholar] [CrossRef]
- Yamanaka, T.; Raffaelli, D.; White, P.C.L. Non-Linear Interactions Determine the Impact of Sea-Level Rise on Estuarine Benthic Biodiversity and Ecosystem Processes. PLoS ONE 2013, 8, e68160. [Google Scholar] [CrossRef] [PubMed]
- Flemming, B.W. Siliciclastic Back-Barrier Tidal Flats. In Principles of Tidal Sedimentology; Davis, R.A., Jr., Dalrymple, R.W., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 231–267. [Google Scholar]
- Sweet, W.V.; Hamlington, B.D.; Kopp, R.E.; Weaver, C.P.; Barnard, P.L.; Bekaert, D.; Brooks, W.; Craghan, M.; Dusek, G.; Frederikse, T.; et al. Global and Regional Sea Level Rise Scenarios for the United States: Updated Mean Projections and Extreme Water Level Probabilities Along U.S. Coastlines; NOAA Technical Report NOS 01; National Oceanic and Atmospheric Administration, National Ocean Service: Silver Spring, MD, USA, 2022; p. 111.
- Oppenheimer, M.; Glavovic, B.; Hinkel, J.; van de Wal, R.; Magnan, A.K.; Abd-Elgawad, A.; Cai, R.; Cifuentes-Jara, M.; Deconto, R.M.; Ghosh, T.; et al. Sea Level Rise and Implications for Low Lying Islands, Coasts and Communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Portner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegria, A., Nicolai, M., Okem, A., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019; pp. 321–445. [Google Scholar] [CrossRef]
- Crooks, S. The effect of sea-level rise on coastal geomorphology. Ibis 2004, 146, 18–20. [Google Scholar] [CrossRef]
- Pethick, J.S.; Crooks, S. Development of a coastal vulnerability index: A geomorphological perspective. Environ. Conserv. 2000, 27, 359–367. [Google Scholar] [CrossRef]
- Alpar, B. Vulnerability of Turkish coasts to accelerated sea-level rise. Geomorphology 2009, 107, 58–63. [Google Scholar] [CrossRef]
- Mazaris, A.D.; Matsinos, G.; Pantis, J.D. Evaluating the impacts of coastal squeeze on sea turtle nesting. Ocean. Coast. Manag. 2009, 52, 139–145. [Google Scholar] [CrossRef]
- Welsh, D.T. It’s a dirty job but someone has to do it: The role of marine benthic macrofauna in organic matter turnover and nutrient recycling to the water column. Chem. Ecol. 2003, 19, 321–342. [Google Scholar] [CrossRef]
- Lohrer, A.M.; Townsend, M.; Hailes, S.F.; Rodil, I.F.; Cartner, K.; Pratt, D.R.; Hewitt, J.E. Influence of New Zealand cockles (Austrovenus stutchburyi) on primary productivity in sandflat-seagrass (Zostera muelleri) ecotones. Estuar. Coast. Shelf Sci. 2016, 181, 238–248. [Google Scholar] [CrossRef]
- Schenone, S.; Thrush, S.F. Unraveling ecosystem functioning in intertidal soft sediments: The role of density-driven interactions. Sci. Rep. 2020, 10, 11909. [Google Scholar] [CrossRef]
- Snelgrove, P.V.R. The biodiversity of macrofaunal organisms in marine sediments. Biodivers. Conserv. 1998, 7, 1123–1132. [Google Scholar] [CrossRef]
- Lohrer, A.M.; Thrush, S.F.; Gibbs, M.M. Bioturbators enhance ecosystem function through complex biogeochemical interactions. Nature 2004, 431, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Lohrer, A.M.; Halliday, N.J.; Thrush, S.F.; Hewitt, J.E.; Rodil, I.F. Ecosystem functioning in a disturbance-recovery context: Contribution of macrofauna to primary production and nutrient release on intertidal sandflats. J. Exp. Mar. Biol. Ecol. 2010, 390, 6–13. [Google Scholar] [CrossRef]
- Thrush, S.F.; Hewitt, J.E.; Gibbs, M.; Lundquist, C.; Norkko, A. Functional Role of Large Organisms in Intertidal Communities: Community Effects and Ecosystem Function. Ecosystems 2006, 9, 1029–1040. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Waldbusser, G.G.; Marinelli, R.L.; Whitlatch, R.B.; Visscher, P.T. The effects of infaunal biodiversity on biogeochemistry of coastal marine sediments. Limnol. Oceanogr. 2004, 49, 1482–1492. [Google Scholar] [CrossRef]
- Foster, S.Q.; Fulweiler, R.W. Estuarine Sediments Exhibit Dynamic and Variable Biogeochemical Responses to Hypoxia. J. Geophys. Res. Biogeosciences 2019, 124, 737–758. [Google Scholar] [CrossRef]
- Rosenfeld, J.S. Functional redundancy in ecology and conservation. Oikos 2002, 98, 156–162. [Google Scholar] [CrossRef]
- O’Gorman, E.J.; Yearsley, J.M.; Crowe, T.P.; Emmerson, M.C.; Jacob, U.; Petchey, O.L. Loss of functionally unique species may gradually undermine ecosystems. Proc. R. Soc. B: Biol. Sci. 2011, 278, 1886–1893. [Google Scholar] [CrossRef]
- Ellingsen, K.E.; Hewitt, J.E.; Thrush, S.F. Rare species, habitat diversity and functional redundancy in marine benthos. J. Sea Res. 2007, 58, 291–301. [Google Scholar] [CrossRef]
- Naeem, S. Species Redundancy and Ecosystem Reliability. Conserv. Biol. 1998, 12, 39–45. [Google Scholar] [CrossRef]
- Pickett, S.T.A. Space-for-Time Substitution as an Alternative to Long-Term Studies. In Long-Term Studies in Ecology: Approaches and Alternatives; Likens, G.E., Ed.; Springer: New York, NY, USA, 1989; pp. 110–135. [Google Scholar]
- Inglis, G.; Gust, N.; Fitridge, I.; Floerl, O.; Woods, C.; Kospartov, M.; Hayden, B.J.; Fenwick, G.D.; Staff, M.B.N.Z.; Directorate, M.B.N.Z.P.-b. Port of Tauranga, Second Baseline Survey for Non-Indigenous Marine Species: (Research Project ZBS2000/04); New Zealand Government-Ministry of Agriculture & Forestry-MAF Biosecurity New Zealand: Wellington, New Zealand, 2008.
- Hume, T.M.; Snelder, T.; Weatherhead, M.; Liefting, R. A controlling factor approach to estuary classification. Ocean. Coast. Manag. 2007, 50, 905–929. [Google Scholar] [CrossRef]
- LINZ. Standard Port Tidal Levels. Available online: https://www.linz.govt.nz/guidance/marine-information/tide-prediction-guidance/standard-port-tidal-levels (accessed on 15 May 2022).
- Tay, H.W.; Bryan, K.R.; de Lange, W.P.; Pilditch, C.A. The hydrodynamics of the southern basin of Tauranga Harbour. N. Z. J. Mar. Freshw. Res. 2013, 47, 249–274. [Google Scholar] [CrossRef]
- Bell, R.; Hannah, J.; Andrews, C. Update to 2020 of the Annual Mean Sea Level Series and Trends Around New Zealand. Prepared for Ministry for the Environment; NIWA Client Report No: 2021236HN; NIWA: Newmarket, New Zealand, 2022. Available online: https://environment.govt.nz/publications/update-to-2020-of-the-annual-mean-sea-level-series-and-trends-around-new-zealand (accessed on 2 January 2023).
- NZSeaRise. Sea-Level Rise and Vertical land Movement, Map Portal. Available online: https://www.searise.nz/maps-2 (accessed on 27 December 2022).
- Ellis, J.; Clark, D.; Hewitt, J.E.; Taiapa, C.; Sinner, J.; Patterson, M.; Hardy, D.; Park, S.; Gardner, B.; Morrison, A.; et al. Ecological Survey of Tauranga Harbour; Prepared for Manaaki Taha Moana, Manaaki Taha Moana Research Report No. 13. Cawthron Report No. 2321; Cawthron Institute: Nelson, New Zealand, 2013. [Google Scholar]
- Clark, D.; Taiapa, C.; Sinner, J.; Taikato, V.; Culliford, D.; Battershill, C.N.; Ellis, J.I.; Hewitt, J.E.; Gower, F.; Borges, H.; et al. 2016 Subtidal Ecological Survey of Tauranga Harbour and Development of Benthic Health Models; OTOT Research Report No. 4; Massey University: Palmerston, New Zealand, 2018; pp. 1–72. [Google Scholar]
- Robertson, B.M.; Gillespie, P.A.; Asher, R.; Frisk, S.; Keeley, N.B.; Hopkins, G.A.; Thompson, S.J.; Tuckey, B.J. Estuarine Environmental Assessment and Monitoring: A National Protocol. Part A. Development, Part B. Appendices, and Part C. Application; Prepared for supporting Councils and the Ministry for the Environment, Sustainable Management Fund Contract No. 5096; Cawthron Institute: Nelson, New Zealand, 2002; p. Part A. 93p. Part B. 159p. Part C. 140p plus field sheets. [Google Scholar]
- Ellis, J.I.; Hewitt, J.E.; Clark, D.; Taiapa, C.; Patterson, M.; Sinner, J.; Hardy, D.; Thrush, S.F. Assessing ecological community health in coastal estuarine systems impacted by multiple stressors. J. Exp. Mar. Biol. Ecol. 2015, 473, 176–187. [Google Scholar] [CrossRef]
- Knight, B.R. Estuary Transport Module for Tauranga Harbour. Prepared for Oranga Taiao Oranga Tāngata Research Programme; Cawthron Report No. 3381. 31 p. plus appendices; Cawthron Institute: Nelson, New Zealand, 2019. [Google Scholar]
- de Ruiter, P.J.; Mullarney, J.C.; Bryan, K.R.; Winter, C. The links between entrance geometry, hypsometry and hydrodynamics in shallow tidally dominated basins. Earth Surf. Process. Landf. 2019, 44, 1957–1972. [Google Scholar] [CrossRef]
- Cook, S.D.C. (Ed.) New Zealand Coastal Marine Invertebrates; Canterbury University Press: Christchurch, New Zealand, 2010. [Google Scholar]
- NIWA. NIWA Invertebrate Collection. v1.1. The National Institute of Water and Atmospheric Research (NIWA). Dataset/Occurrence. 2018. Available online: https://nzobisipt.niwa.co.nz/resource?r=obisspecify&v=1.1. (accessed on 2 January 2023).
- Greenfield, B.L.; Kraan, C.; Pilditch, C.A.; Thrush, S.F. Mapping functional groups can provide insight into ecosystem functioning and potential resilience of intertidal sandflats. Mar. Ecol. Prog. Ser. 2016, 548, 1–10. [Google Scholar] [CrossRef]
- Ellis, N.; Smith, S.J.; Pitcher, C.R. Gradient forests: Calculating importance gradients on physical predictors. Ecology 2012, 93, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Pitcher, R.C.; Lawton, P.; Ellis, N.; Smith, S.J.; Incze, L.S.; Wei, C.-L.; Greenlaw, M.E.; Wolff, N.H.; Sameoto, J.A.; Snelgrove, P.V.R. Exploring the role of environmental variables in shaping patterns of seabed biodiversity composition in regional-scale ecosystems. J. Appl. Ecol. 2012, 49, 670–679. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Clarke, K.R.; Gorley, R.N.; Somerfield, P.J.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; PRIMER-E: Plymouth, UK, 2014. [Google Scholar]
- Anderson, M.; Gorley, R.; Clarke, K. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Mindel, B.L.; Neat, F.C.; Trueman, C.N.; Webb, T.J.; Blanchard, J.L. Functional, size and taxonomic diversity of fish along a depth gradient in the deep sea. PeerJ 2016, 4, e2387. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Tamir, R.; Eyal, G.; Loya, Y. Coral Morphology Portrays the Spatial Distribution and Population Size-Structure Along a 5–100 m Depth Gradient. Front. Mar. Sci. 2020, 7, 615. [Google Scholar] [CrossRef]
- Villalobos, V.I.; Valdivia, N.; Försterra, G.; Ballyram, S.; Espinoza, J.P.; Wadham, J.L.; Burgos-Andrade, K.; Häussermann, V. Depth-Dependent Diversity Patterns of Rocky Subtidal Macrobenthic Communities Along a Temperate Fjord in Northern Chilean Patagonia. Front. Mar. Sci. 2021, 8, 635855. [Google Scholar] [CrossRef]
- Gogina, M.; Zettler, M.L. Diversity and distribution of benthic macrofauna in the Baltic Sea: Data inventory and its use for species distribution modelling and prediction. J. Sea Res. 2010, 64, 313–321. [Google Scholar] [CrossRef]
- Flach, E.; de Bruin, W. Diversity patterns in macrobenthos across a continental slope in the NE Atlantic. J. Sea Res. 1999, 42, 303–323. [Google Scholar] [CrossRef]
- Holleman, R.C.; Stacey, M.T. Coupling of Sea Level Rise, Tidal Amplification, and Inundation. J. Phys. Oceanogr. 2014, 44, 1439–1455. [Google Scholar] [CrossRef]
- Rullens, V.; Stephenson, F.; Lohrer, A.M.; Townsend, M.; Pilditch, C.A. Combined species occurrence and density predictions to improve marine spatial management. Ocean. Coast. Manag. 2021, 209, 105697. [Google Scholar] [CrossRef]
- Kraan, C.; Aarts, G.; van der Meer, J.; Piersma, T. The role of environmental variables in structuring landscape-scale species distributions in seafloor habitats. Ecology 2010, 91, 1583–1590. [Google Scholar] [CrossRef]
- Puls, W.; van Bernem, K.H.; Eppel, D.; Kapitza, H.; Pleskachevsky, A.; Riethmüller, R.; Vaessen, B. Prediction of benthic community structure from environmental variables in a soft-sediment tidal basin (North Sea). Helgol. Mar. Res. 2012, 66, 345–361. [Google Scholar] [CrossRef]
- Ysebaert, T.; Herman, P.M.J.; Meire, P.; Craeymeersch, J.; Verbeek, H.; Heip, C.H.R. Large-scale spatial patterns in estuaries: Estuarine macrobenthic communities in the Schelde estuary, NW Europe. Estuar. Coast. Shelf Sci. 2003, 57, 335–355. [Google Scholar] [CrossRef]
- Liao, Y.; Shou, L.; Jiang, Z.; Gao, A.; Zeng, J.; Chen, Q.; Yan, X. Benthic macrofaunal communities along an estuarine gradient in the Jiaojiang River estuary, China. Aquat. Ecosyst. Health Manag. 2016, 19, 314–325. [Google Scholar] [CrossRef]
- Denis-Roy, L.; Ling, S.D.; Fraser, K.M.; Edgar, G.J. Relationships between invertebrate benthos, environmental drivers and pollutants at a subcontinental scale. Mar. Pollut. Bull. 2020, 157, 111316. [Google Scholar] [CrossRef]
- Engle, V.D.; Summers, J.K. Latitudinal gradients in benthic community composition in Western Atlantic estuaries. J. Biogeogr. 1999, 26, 1007–1023. [Google Scholar] [CrossRef]
- Snelgrove, P.V.R. Diversity of marine species. In Encyclopedia of Ocean Sciences; Steele, J.H., Ed.; Academic Press: Oxford, UK, 2001; pp. 748–757. [Google Scholar]
- Lee, J.; Valle-Levinson, A. Influence of bathymetry on hydrography and circulation at the region between an estuary mouth and the adjacent continental shelf. Cont. Shelf Res. 2012, 41, 77–91. [Google Scholar] [CrossRef]
- Conroy, T.; Sutherland, D.A.; Ralston, D.K. Estuarine Exchange Flow Variability in a Seasonal, Segmented Estuary. J. Phys. Oceanogr. 2020, 50, 595–613. [Google Scholar] [CrossRef]
- Chadwick, D.B.; Largier, J.L. Tidal exchange at the bay-ocean boundary. J. Geophys. Res. Ocean. 1999, 104, 29901–29924. [Google Scholar] [CrossRef]
- Bolaños, R.; Brown, J.M.; Amoudry, L.O.; Souza, A.J. Tidal, Riverine, and Wind Influences on the Circulation of a Macrotidal Estuary. J. Phys. Oceanogr. 2013, 43, 29–50. [Google Scholar] [CrossRef]
- Norkko, J.; Hewitt, J.E.; Thrush, S.F. Effects of increased sedimentation on the physiology of two estuarine soft-sediment bivalves, Austrovenus stutchburyi and Paphies australis. J. Exp. Mar. Biol. Ecol. 2006, 333, 12–26. [Google Scholar] [CrossRef]
- Zarzuelo, C.; López-Ruiz, A.; Díez-Minguito, M.; Ortega-Sánchez, M. Tidal and subtidal hydrodynamics and energetics in a constricted estuary. Estuar. Coast. Shelf Sci. 2017, 185, 55–68. [Google Scholar] [CrossRef]
- Schilthuizen, M. Ecotone: Speciation-prone. Trends Ecol. Evol. 2000, 15, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Kark, S.; van Rensburg, B.J. Ecotones: Marginal or Central Areas of Transition? Isr. J. Ecol. Evol. 2006, 52, 29–53. [Google Scholar] [CrossRef]
- Newell, R.C. One-Adaptations to intertidal life. In Adaptation to Environment; Newell, R.C., Ed.; Butterworth-Heinemann: Oxford UK, 1976; pp. 1–82. [Google Scholar]
- Hooper, D.U.; Chapin III, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Byrnes, J.E.K.; Isbell, F.; Gamfeldt, L.; Griffin, J.N.; Eisenhauer, N.; Hensel, M.J.S.; Hector, A.; Cardinale, B.J.; Duffy, J.E. Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nat. Commun. 2015, 6, 6936. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Gambi, C.; Dell’Anno, A.; Corinaldesi, C.; Fraschetti, S.; Vanreusel, A.; Vincx, M.; Gooday, A.J. Exponential Decline of Deep-Sea Ecosystem Functioning Linked to Benthic Biodiversity Loss. Curr. Biol. 2008, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stachowicz, J.J.; Bruno, J.F.; Duffy, J.E. Understanding the Effects of Marine Biodiversity on Communities and Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 739–766. [Google Scholar] [CrossRef]
- Grman, E.; Lau, J.A.; Schoolmaster, D.R., Jr.; Gross, K.L. Mechanisms contributing to stability in ecosystem function depend on the environmental context. Ecol. Lett. 2010, 13, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Hillman, J.R.; Lundquist, C.J.; O’Meara, T.A.; Thrush, S.F. Loss of Large Animals Differentially Influences Nutrient Fluxes Across a Heterogeneous Marine Intertidal Soft-Sediment Ecosystem. Ecosystems 2020, 24, 272–283. [Google Scholar] [CrossRef]
- Luck, G.W.; Harrington, R.; Harrison, P.A.; Kremen, C.; Berry, P.M.; Bugter, R.; Dawson, T.P.; de Bello, F.; Díaz, S.; Feld, C.K.; et al. Quantifying the Contribution of Organisms to the Provision of Ecosystem Services. BioScience 2009, 59, 223–235. [Google Scholar] [CrossRef]
- van der Linden, P.; Marchini, A.; Smith, C.J.; Dolbeth, M.; Simone, L.R.L.; Marques, J.C.; Molozzi, J.; Medeiros, C.R.; Patrício, J. Functional changes in polychaete and mollusc communities in two tropical estuaries. Estuar. Coast. Shelf Sci. 2017, 187, 62–73. [Google Scholar] [CrossRef]
- Wouters, J.M.; Gusmao, J.B.; Mattos, G.; Lana, P. Polychaete functional diversity in shallow habitats: Shelter from the storm. J. Sea Res. 2018, 135, 18–30. [Google Scholar] [CrossRef]
- Jones, H.F.E.; Pilditch, C.A.; Bruesewitz, D.A.; Lohrer, A.M. Sedimentary Environment Influences the Effect of an Infaunal Suspension Feeding Bivalve on Estuarine Ecosystem Function. PLoS ONE 2011, 6, e27065. [Google Scholar] [CrossRef]
- Woodin, S.A.; Volkenborn, N.; Pilditch, C.A.; Lohrer, A.M.; Wethey, D.S.; Hewitt, J.E.; Thrush, S.F. Same pattern, different mechanism: Locking onto the role of key species in seafloor ecosystem process. Sci. Rep. 2016, 6, 26678. [Google Scholar] [CrossRef]
- Elmilady, H.; van der Wegen, M.; Roelvink, D.; Jaffe, B.E. Intertidal Area Disappears Under Sea Level Rise: 250 Years of Morphodynamic Modeling in San Pablo Bay, California. J. Geophys. Res. Earth Surf. 2019, 124, 38–59. [Google Scholar] [CrossRef]
- Rullens, V.; Townsend, M.; Lohrer, A.M.; Stephenson, F.; Pilditch, C.A. Who is contributing where? Predicting ecosystem service multifunctionality for shellfish species through ecological principles. Sci. Total Environ. 2022, 808, 152147. [Google Scholar] [CrossRef]
- Pollard, A.I.; Reed, T. Benthic invertebrate assemblage change following dam removal in a Wisconsin stream. Hydrobiologia 2004, 513, 51–58. [Google Scholar] [CrossRef]
- Tullos, D.D.; Finn, D.S.; Walter, C. Geomorphic and Ecological Disturbance and Recovery from Two Small Dams and Their Removal. PLoS ONE 2014, 9, e108091. [Google Scholar] [CrossRef]
- Bain, D.J.; Green, M.B.; Campbell, J.L.; Chamblee, J.F.; Chaoka, S.; Fraterrigo, J.M.; Kaushal, S.S.; Martin, S.L.; Jordan, T.E.; Parolari, A.J.; et al. Legacy Effects in Material Flux: Structural Catchment Changes Predate Long-Term Studies. BioScience 2012, 62, 575–584. [Google Scholar] [CrossRef]
- Drylie, T.P.; Lohrer, A.M.; Needham, H.R.; Bulmer, R.H.; Pilditch, C.A. Benthic primary production in emerged intertidal habitats provides resilience to high water column turbidity. J. Sea Res. 2018, 142, 101–112. [Google Scholar] [CrossRef]
- Sigleo, A.C. Denitrification Rates Across a Temperate North Pacific Estuary, Yaquina Bay, Oregon. Estuaries Coasts 2019, 42, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Piehler, M.F.; Smyth, A.R. Habitat-specific distinctions in estuarine denitrification affect both ecosystem function and services. Ecosphere 2011, 2, 12. [Google Scholar] [CrossRef]
- Dangles, O.; Malmqvist, B. Species richness–decomposition relationships depend on species dominance. Ecol. Lett. 2004, 7, 395–402. [Google Scholar] [CrossRef]
| Functional Group | Description of Traits | Example Species |
|---|---|---|
| 1 | Calcified, Suspension feeding, Attached | Austrominius modestus (T) |
| 2 | Calcified, Suspension feeding, Top 2 cm, Freely mobile | Austrovenus stutchburyi (B) |
| 3 | Calcified, Suspension feeding, Top 2 cm, Limited mobility | Arthritica bifurca (B) |
| 4 | Calcified, Suspension feeding, Top 2 cm, Sedentary | Arcuatula senhousia (B) |
| 5 | Calcified, Deposit/Pred.Scav/Grazer, Above surface, Freely mobile | Zeacumantus subcarinatus (G) |
| 6 | Calcified, Deposit feeding, Top 2 cm, Limited mobility | Linucula hartvigiana (B) |
| 7 | Calcified, Deposit feeding, Predator/Scavenger, Top 2 cm, Freely mobile | Pisinna zosterophila (G) |
| 8 | Calcified, Deposit feeding, Deep, Limited mobility, No habitat structure, Large | Macomona Liliana (B) |
| 9 | Soft-bodied, Suspension feeding, Attached | Anthopleura aureoradiata (A) |
| 10 | Soft-bodied, Suspension feeding, Tube structure | Euchone sp. (P) |
| 11 | Soft-bodied, Deposit feeding, Top 2 cm, Freely mobile | Spaerodoridae (P) |
| 12 | Soft-bodied, Deposit feeding, Below surface, Freely mobile | Spionidae (P) |
| 13 | Soft-bodied, Deposit feeding, Below surface, Limited mobility | Heteromastus filiformis (P) |
| 14 | Soft-bodied, Deposit feeding, Deep | Hyboscolex longiseta (P) |
| 15 | Soft-bodied, Below surface, Tube structure | Terebellidae (P) |
| 16 | Soft-bodied, Predator/Scavenger, Top 2 cm, Freely mobile | Sigalionidae (P) |
| 17 | Soft-bodied, Predator/Scavenger, Top 2 cm, Limited mobility | Syllidae (P) |
| 18 | Soft-bodied, Predator/Scavenger, Below surface + Deep, Freely mobile, No habitat structure | Perinereis sp. (P) |
| 19 | Soft-bodied, Predator/Scavenger, Below surface, Limited mobility | Oligochaeta |
| 20 | Soft-bodied, Above surface, Top 2 cm, Below surface, Deep, Sedentary, Tube structure | Owenia petersenae (P) |
| 21 | Rigid, Suspension feeding, Top 2 cm | Tanaidacea (M) |
| 22 | Rigid, Deposit feeding, Predator/Scavenger, Top 2 cm, Freely mobile, No habitat structure | Amphipoda (M) |
| 23 | Rigid, Above surface, Freely mobile | Cumacea (M) |
| 24 | Rigid, Above surface, Freely mobile, Large | Ophiuroidea |
| 25 | Rigid, Predator/Scavenger, Attached | No individuals identified |
| 26 | Rigid, Predator/Scavenger, Below surface, Freely mobile, Large burrow former | Hemiplax hirtipes (M) |
| IT | SS | DS | ||
|---|---|---|---|---|
| Environmental Variables | ||||
| Depth (m) | −0.6 (−2.0–3.0) | 1.5 (−1.0–7.9) | 3.0 (−0.2–9.0) | |
| Mud (%) | 13.6 (0.1–76.4) | 9.0 (2.6–25.4) | 3.0 (0.6–5.0) | |
| Sand (%) | 85 (24–100) | 87 (67–96) | 91 (78–99) | |
| Gravel (%) | 1.8 (0.1–14.6) | 4.7 (0.1–15.0) | 5.9 (0.1–17.8) | |
| OM (%) | 2.9 (0.9–10.0) | 2.8 (1.3–6.2) | 1.7 (1.0–3.0) | |
| Chl a (µg/kg) | 6107 (210–16,000) | 16,678 (5900–41,300) | 17,685 (2000–56,300) | |
| TP (mg/kg) | 168 (51–580) | 152 (79–340) | 121 (81–180) | |
| TN (mg/kg) | 484 (140–1900) | 548 (499–1200) | 452 (190–499) | |
| Cu (mg/kg) | 1.3 (1.0–6.1) | 1.1 (0.4–3.5) | 0.7 (0.3–1.0) | |
| Pb (mg/kg) | 2.7 (1.0–13.0) | 3.0 (1.6–6.4) | 2.0 (1.0–3.8) | |
| Zn (mg/kg) | 17.7 (2.5–55.0) | 17.7 (7.7–37.0) | 12.2 (6.4–25.0) | |
| Av. current speed (m/s) | 0.15 (0.01–0.52) | 0.33 (0.01–0.67) | 0.53 (0.23–0.83) | |
| Benthic community | ||||
| S (taxa per core) | 19 (6–31) | 25 (18–37) | 15 (10–21) | |
| N (ind. per core) | 109 (27–329) | 234 (49–744) | 70 (22–183) | |
| Occurrence (% of sites taxa occurs at) | 23 (1–100) | 20 (3–100) | 23 (8–100) | |
| H’ (per core) | 1.92 (0.11–2.71) | 2.02 (0.76–2.74) | 1.72 (0.45–2.55) | |
| Most abundant taxa | Amphipoda (M) Spionidae (P) Heteromastus filiformis (P) Austrovenus stutchburyi (B) Linucula hartvigiana (B) | Spionidae (P) Amphipoda (M) Oligochaeta Aricidea sp. (P) Heteromastus filiformis (P) | Paphies australis (B) Amphipoda (M) Hesionidae (P) Syllidae (P) Magelona sp. (P) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dixon, O.; Gammal, J.; Clark, D.; Ellis, J.I.; Pilditch, C.A. Estimating Effects of Sea Level Rise on Benthic Biodiversity and Ecosystem Functioning in a Large Meso-Tidal Coastal Lagoon. Biology 2023, 12, 105. https://doi.org/10.3390/biology12010105
Dixon O, Gammal J, Clark D, Ellis JI, Pilditch CA. Estimating Effects of Sea Level Rise on Benthic Biodiversity and Ecosystem Functioning in a Large Meso-Tidal Coastal Lagoon. Biology. 2023; 12(1):105. https://doi.org/10.3390/biology12010105
Chicago/Turabian StyleDixon, Olivia, Johanna Gammal, Dana Clark, Joanne I. Ellis, and Conrad A. Pilditch. 2023. "Estimating Effects of Sea Level Rise on Benthic Biodiversity and Ecosystem Functioning in a Large Meso-Tidal Coastal Lagoon" Biology 12, no. 1: 105. https://doi.org/10.3390/biology12010105
APA StyleDixon, O., Gammal, J., Clark, D., Ellis, J. I., & Pilditch, C. A. (2023). Estimating Effects of Sea Level Rise on Benthic Biodiversity and Ecosystem Functioning in a Large Meso-Tidal Coastal Lagoon. Biology, 12(1), 105. https://doi.org/10.3390/biology12010105






