Escape from Cellular Senescence Is Associated with Chromosomal Instability in Oral Pre-Malignancy
Abstract
Simple Summary
Abstract
1. Introduction
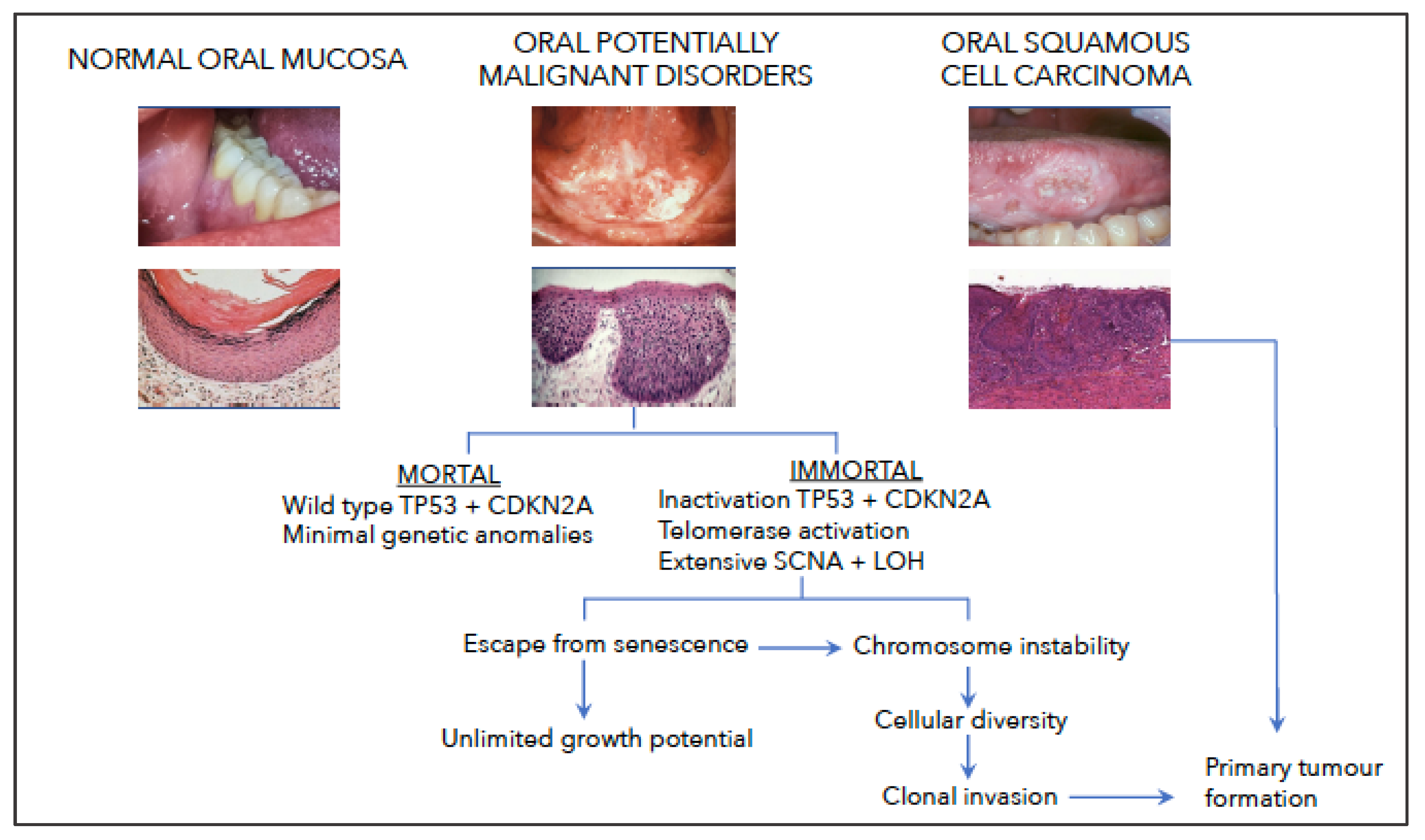
2. Pathogenesis of OPMD
2.1. Clinical Characteristics
2.2. Limitations of Current Models of Progression
2.3. Somatic Copy Number Alterations (SCNA)
2.4. Driver Genes
3. Cellular Senescence
3.1. Senescence in Keratinocytes
3.2. Escape from Cellular Senescence
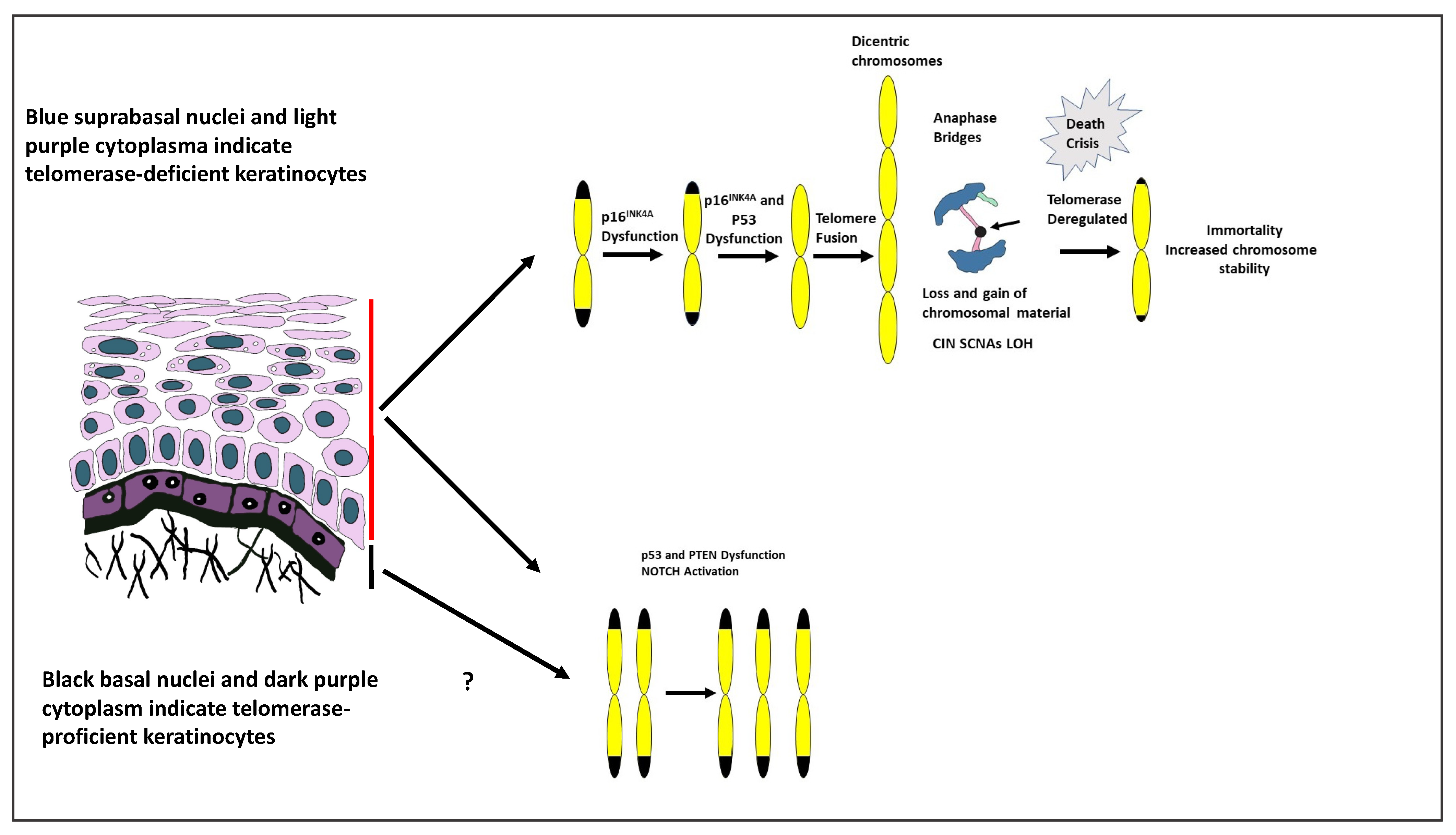
4. Development of Aneuploidy
4.1. Telomeres
4.2. Telomerase Expression in Squamous Epithelium
4.3. Telomerase Expression in Epithelial Malignancy
4.4. Telomere Dysfunction Co-Operates with Inactivation of TP53 and CDKN2A
4.5. CIN and Driver Genes Other than TP53 and CDKN2A
4.6. Spindle Assembly Checkpoints (SAC)
4.7. Anaphase Bridges
5. Function of Aneuploidy
5.1. Tumour Promotion
5.2. Tumour Suppression
6. Does Aneuploidy Initiate Cancer?
7. Keratinocyte Cell Lines from OPMD
8. Hypothesis: The Role of Genetic Instability in the Development of OSCC
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Genome Atlas. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Jia, Y.; Guo, F.; Zhengjun, S.; Shao, Z. Intratumoural heterogeneity and clone evolution of oral squamous cell carcinoma. Mol. Carcinogen. 2021, 60, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Geigl, J.B.; Obenauf, A.C.; Schwarzbraun, T.; Speicher, M.R. Defining chromosomal instability. Trends Genet. 2008, 24, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Endesfelder, D.; Rowan, A.J.; Walther, A.; Birbak, N.J.; Futreal, P.A.; Downward, J.; Szallasi, Z.; Tomlinson, I.P.; Howell, M.; et al. Chromosomal instability confers intrinsic multidrug resistance. Cancer Res. 2011, 71, 1858–1870. [Google Scholar] [CrossRef]
- Fidler, I.J. Commentary on tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 2016, 76, 3441–3442. [Google Scholar] [CrossRef]
- Vishwakarma, R.; McManus, K.J. Chromosome instability; implications in cancer development, progression, and clinical outcomes. Cancers 2020, 12, 824. [Google Scholar] [CrossRef]
- Holland, A.J.; Cleveland, D.W. Boveri revisited: Chromosome instability, aneuploidy and tumorigenesis. Nat. Rev. Mol. Cell Biol. 2009, 10, 478–487. [Google Scholar] [CrossRef]
- Storchova, Z.; Kuffer, C. The consequences of tetraploidy and aneuploidy. J. Cell Sci. 2008, 121, 3859–3866. [Google Scholar] [CrossRef]
- Pihan, G.A.; Wallace, J.; Zhou, Y.; Doxsey, S.J. Centrosome abnormalities and chromosome instability occur together in pre-invasive carcinomas. Cancer Res. 2003, 63, 1398–1404. [Google Scholar]
- Prime, S.S.; Cirillo, N.; Cheong, S.C.; Prime, M.S.; Parkinson, E.K. Targeting the genetic landscape of oral potentially malignant disorders has the potential as a preventative strategy in oral cancer. Cancer Lett. 2021, 518, 102–114. [Google Scholar] [CrossRef]
- Heng, J.; Heng, H.H. Genome chaos: Creating new genome information essential for cancer macroevolution. Semin. Cancer Biol. 2020, 81, 160–175. [Google Scholar] [CrossRef] [PubMed]
- Wood, H.M.; Daly, C.; Chalkley, R.; Senguven, B.; Ross, L.; Egan, P.; Chengot, P.; Graham, J.; Sethi, N.; Ong, T.K.; et al. The genomic road to invasion—Examining the similarities and differences in the genomes of associated oral pre-cancer and cancer samples. Genome Med. 2017, 9, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Gerstung, M.; Jolly, C.; Leshchiner, I.; Dentro, S.C.; Gonzales, S.; Rosebrock, D.; Mitchell, T.J.; Rubanova, Y.; Anur, P.; Yu, K.; et al. The evolutionary history of 2,658 cancers. Nature 2020, 578, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.R.; Chen, Z.; Faden, D.L.; Agrawal, N.; Hanna, G.J.; Iyer, N.G.; Boot, A.; Rozen, S.G.; Vettore, A.L.; Panda, B.; et al. The mutational landscape of early-onset oral tongue squamous cell carcinoma. Cancer 2020, 127, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Murti, P.R.; Warnakulasuriya, K.A.; Johnson, N.W.; Bhonsle, R.B.; Gupta, P.C.; Daftary, D.K.; Mehta, F.S. p53 expression in oral precancer as a marker for malignant potential. J. Oral Pathol. Med. 1998, 27, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Cruz, I.B.; Snijders, P.J.; Meijer, C.J.; Braakhuis, B.J.; Snow, G.B.; Walboomers, J.M.; van der Waal, I. P53 expression above the basal call layer in oral mucosa is an early event of malignant transformation and has predictive value for developing oral squamous cell carcinoma. J. Pathol. 1998, 184, 360–368. [Google Scholar] [CrossRef]
- Abbas, N.F.; El-Sharkawy, S.; Abbas, E.A.; Abdel Monem El-Shaer, M. Immunohistochemical study of p53 and angiogenesis in benign and preneoplastic oral lesions and oral squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, 385–390. [Google Scholar] [CrossRef]
- Shah, N.G.; Trivedi, T.I.; Tankshali, R.A.; Goswami, J.A.; Shah, J.S.; Jetly, D.H.; Kobawala, T.P.; Patel, K.C.; Shukla, S.N.; Shah, P.M.; et al. Molecular alterations in oral carcinogenesis: Significant risk predictors in malignant transformation and tumor progression. Int. J. Biol. Markers 2007, 22, 132–143. [Google Scholar] [CrossRef]
- Kumar, P.; Kane, S.; Rathod, G.P. Coexpression of p53 and Ki-67 and lack of c-erbB2 expression in oral leukoplakias in India. Braz. Oral Res. 2012, 26, 228–234. [Google Scholar] [CrossRef]
- Papadimitrakopoulou, V.; Izzo, J.; Lippman, S.M.; Lee, J.S.; Fan, Y.H.; Clayman, G.; Ro, J.Y.; Hittelman, W.N.; Lotan, R.; Hong, W.K.; et al. Frequent inactivation of p16INK4a in oral premalignant lesions. Oncogene 1997, 14, 1799–1803. [Google Scholar] [CrossRef]
- Kresty, L.A.; Mallery, S.R.; Knobloch, T.J.; Song, H.; Lloyd, M.; Casto, B.C.; Weghorst, C.M. Alterations of p16(INK4a) and p14(ARF) in patients with severe oral epithelial dysplasia. Cancer Res. 2002, 62, 5295–5300. [Google Scholar] [PubMed]
- Gologan, O.; Barnes, E.L.; Hunt, J.L. Potential diagnostic use of p16INK4A, a new marker that correlates with dysplasia in oral squamoproliferative lesions. Am. J. Surg. Pathol. 2005, 29, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Hall, G.L.; Shaw, R.J.; Field, E.A.; Rogers, S.N.; Sutton, D.N.; Woolgar, J.A.; Lowe, D.; Liloglou, T.; Field, J.K.; Risk, J.M. p16 promoter methylation is a potential predictor of malignant transformation in oral epithelial dysplasia. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2174–2179. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhou, J.; Gao, Y.; Gu, L.; Meng, H.; Liu, H.; Deng, D. Methylation of p16 CpG island associated with malignant progression of oral epithelial dysplasia: A prospective cohort study. Clin. Cancer Res. 2009, 15, 5178–5183. [Google Scholar] [CrossRef] [PubMed]
- Shridhar, K.; Walia, G.K.; Aggarwal, A.; Gulati, S.; Geetha, A.V.; Prabhakaran, D.; Dhillon, P.K.; Rajaraman, P. DNA methylation markers for oral pre-cancer progression: A critical review. Oral Oncol. 2016, 53, 1–9. [Google Scholar] [CrossRef]
- Muntoni, A.; Fleming, J.; Gordon, K.E.; Hunter, K.; McGregor, F.; Parkinson, E.K.; Harrison, P.R. Senescing oral dysplasias are not immortalized by ectopic expression of hTERT alone without other molecular changes, such as loss of INK4A and/or retinoic acid receptor-beta: But p53 mutations are not necessarily required. Oncogene 2003, 22, 7804–7808. [Google Scholar] [CrossRef]
- De Boer, D.V.; Brink, A.; Buijze, M.; Stigter-van Walsum, M.; Hunter, K.D.; Yistra, B.; Bloemena, E.; Leemans, C.R.; Brakenhoff, R.H. Establishment and genetic landscape of precancer cell model systems from the head and neck mucosal lining. Mol. Cancer Res. 2019, 17, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, E.; Saeb, M.; Crum, C.P.; Woo, S.B.; McKee, P.H.; Rheinwald, J.G. Co-expression of p16(INK4A) and laminin gamma2 by microinvasive and superficial squamous cell carcinomas in vivo by migrating wound and senescent keratinocytes in culture. Am. J. Pathol. 2003, 163, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Gorgulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; Gil, J.; et al. Cellular senescence: Defining a path forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Mycielska, M.E.; James, E.N.; Parkinson, E.K. Metabolic alterations in cellular senescence: The role of citrate in ageing and age-related disease. Int. J. Mol. Sci. 2022, 23, 3652. [Google Scholar] [CrossRef]
- Yonish-Rouach, E.; Resnitsky, D.; Lotem, J.; Sachs, L.; Kimchi, A.; Oren, M. Wild-type p53 induces apoptosis of myeloid leukemic cells that is inhibited by interleukin-6. Nature 1991, 352, 345–347. [Google Scholar] [CrossRef]
- Meek, D.W. Regulation of the p53 response and its relationship to cancer. Biochem. J. 2015, 469, 325–346. [Google Scholar] [CrossRef] [PubMed]
- Meek, D.W.; Hupp, T.R. The regulation of MDM2 by multistage phosphorylation—Opportunities for molecular-based intervention to target tumors? Semin. Cancer Biol. 2010, 20, 19–28. [Google Scholar] [CrossRef]
- Xia, Z.; Kon, N.; Gu, A.P.; Tavana, O.; Gu, W. Deciphering the acetylation code of p53 in transcription regulation and tumor suppression. Oncogene 2022, 41, 3039–3050. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, L.R.; White, A.; Sprouse, J.; Livanos, E.; Jacks, T.; Tlsty, T.L. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell 1992, 70, 923–935. [Google Scholar] [CrossRef]
- Fujiwara, T.; Bandi, M.; Nitta, M.; Ivanova, E.V.; Bronson, R.T.; Pellman, D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 2005, 437, 1043–1047. [Google Scholar] [CrossRef]
- Serrano, M.; Hannon, G.J.; Beach, D. A new regulatory motif in cell cycle control causing specific inhibition of cyclinD/CDK4. Nature 1993, 366, 704–707. [Google Scholar] [CrossRef]
- Quelle, D.E.; Zindy, F.; Ashmun, R.A.; Sherr, C.J. Alternative reading frames of the INK4A tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell 1995, 83, 993–1000. [Google Scholar]
- Chandler, H.; Peters, G. Stressing the cell cycle in senescence and aging. Curr. Opin. Cell Biol. 2013, 25, 765–771. [Google Scholar] [CrossRef]
- Kamijo, T.; Zindy, F.; Roussel, M.F.; Quelle, D.E.; Downing, J.R.; Ashmun, R.A.; Grosveld, G.; Sherr, C.J. Tumor suppression at the mouse INK4A locus mediated by the alternate reading frame product p19ARF. Cell 1997, 91, 649–659. [Google Scholar] [CrossRef]
- Weber, H.O.; Samuel, T.; Rauch, P.; Funk, J.O. Human p14(ARF)-mediated cell cycle arrest strictly depends on intact p53 signaling pathways. Oncogene 2002, 21, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Eymin, B.; Leduc, C.; Coll, J.L.; Brambilla, E.; Gazzeri, S. p14ARF induces G2 arrest and apoptosis independently of p53 leading to regression of tumors established in nude mice. Oncogene 2003, 22, 1822–1835. [Google Scholar] [CrossRef] [PubMed]
- Michaloglou, C.; Vredeveld, L.C.W.; Soengas, M.S.; Denoyelle, C.; Kuilman, T.; van der Horst, C.M.A.M.; Majoorm, D., M.; Shay, J.W.; Mooi, W.J.; Peeper, D.S. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 2005, 436, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Bartkova, J.; Horejsi, Z.; Koed, K.; Kramer, A.; Tort, F.; Zieger, K.; Guldberg, P.; Sehested, M.; Nesland, J.M.; Lukas, C.; et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 2005, 434, 864–870. [Google Scholar] [CrossRef]
- Kang, T.-W.; Yevsa, T.; Woller, N.; Hoenicke, L.; Wuesterfeld, T.; Dauch, D.; Hohmeyer, A.; Gereke, M.; Rudalska, R.; Potapova, A.; et al. Senescence surveillance of premalignant hepatocytes limits liver cancer development. Nature 2011, 479, 547–551. [Google Scholar] [CrossRef]
- Krtolica, A.; Parrinello, S.; Lockett, S.; Desprez, P.Y.; Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: A link between cancer and aging. Proc. Natl. Acad. Sci. USA 2001, 98, 12072–12077. [Google Scholar] [CrossRef]
- Coppe, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Munoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.-Y.; Campisi, J. Senescent-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef]
- Ritschka, B.; Storer, M.; Mas, A.; Heinzman, F.; Ortells, M.C.; Morton, J.P.; Sansom, O.J.; Zender, L.; Keyes, W.M. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 2017, 31, 172–183. [Google Scholar] [CrossRef]
- Milanovic, M.; Fan, D.N.Y.; Belenki, D.; Dabritz, J.H.M.; Zhao, Z.; Yu, Y.; Dorr, J.R.; Dimitrova, L.; Lenze, D.; Barbosa, I.A.M.; et al. Senescence-associated reprogramming promotes cancer stemness. Nature 2018, 553, 96–100. [Google Scholar] [CrossRef]
- Edington, K.G.; Loughran, O.P.; Berry, I.J.; Parkinson, E.K. Cellular immortality: A late event in the progression of human squamous cell carcinoma of the head and neck associated with p53 alteration and a high frequency of allele loss. Mol. Carcinogen. 1995, 13, 254–265. [Google Scholar] [CrossRef]
- Loughran, O.; Malliri, A.; Owens, D.; Gallimore, P.H.; Stanley, M.A.; Ozanne, B.; Frame, M.C.; Parkinson, E.K. Association of CDKN2A/p16INK4A with human head and neck keratinocyte replicative senescence: Relationship of dysfunction to immortalization and neoplasia. Oncogene 1996, 13, 561–568. [Google Scholar] [PubMed]
- Munro, J.; Stott, F.J.; Vousden, K.H.; Peters, G.; Parkinson, E.K. Role of the alternative INK4A proteins in human keratinocyte senescence: Evidence for the specific inactivation of p16INK4A upon immortalization. Cancer Res. 1999, 59, 2516–2521. [Google Scholar] [PubMed]
- McGregor, F.; Muntoni, A.; Fleming, J.; Brown, J.; Felix, D.H.; MacDonald, D.G.; Parkinson, E.K.; Harrison, P.R. Molecular changes associated with oral dysplasia progression and acquisition of immortality: Potential for its reversal by 5-azacytidine. Cancer Res. 2002, 62, 4757–4766. [Google Scholar]
- Hunter, K.D.; Thurlow, J.K.; Fleming, J.; Drake, P.J.; Vass, K.; Kalna, G.; Higham, D.J.; Herzyk, P.; Macdonald, D.G.; Parkinson, E.K.; et al. Divergent routes to oral cancer. Cancer Res. 2006, 66, 7405–7413. [Google Scholar] [CrossRef]
- Parkinson, E.K. Senescence as a modulator of oral squamous cell carcinoma development. Oral Oncol. 2010, 46, 840–853. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Hahn, W.C.; Ramsey, M.R.; Wu, J.Y.; Guo, Z.; Tsao, H.; De Luca, M.; Catricala, C.; O’Toole, K.M. A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol. Cell Biol. 2002, 22, 5157–5172. [Google Scholar] [CrossRef]
- Jacobs, J.J.L.; de Lange, T. Significant role for p16INK4a in p53-independent telomere-directed senescence. Curr. Biol. 2004, 14, 2302–2308. [Google Scholar] [CrossRef]
- Natarajan, E.; Omobono, J.D.; Guo, Z.; Hopkinson, S.; Lazar, A.J.; Brenn, T.; Jones, J.C.; Rheinwald, J.G. A keratinocyte hypermotility/growth-arrest response involving laminin 5 and p16INK4A activated in wound healing and senescence. Am. J. Pathol. 2006, 168, 1821–1837. [Google Scholar] [CrossRef]
- Artandi, S.E.; Chang, S.; Lee, S.L.; Alson, S.; Gottlieb, G.J.; Chin, L.; DePinho, R.A. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 2000, 406, 641–645. [Google Scholar] [CrossRef]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef]
- Chakravarty, D.; LaBella, K.Y.; DePinho, R.A. Telomeres: History, health and hallmarks of aging. Cell 2021, 184, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Aida, J.; Kobayashi, T.; Saku, T.; Yamaguchi, M.; Shimomura, N.; Nakamura, K.-I.; Ishikawa, N.; Maruyama, S.; Cheng, J.; Poon, S.S.; et al. Short telomeres in an oral precancerous lesion: Q-FISH analysis of leukoplakia. J. Oral Pathol. Med. 2012, 41, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Pal, J.; Raiput, Y.; Shrivastava, S.; Gahine, R.; Mungutwar, V.; Barardiya, T.; Chandraker, A.; Ramakrishna, P.P.; Mishra, S.S.; Banjara, H.; et al. A standalone approach to utilize telomere length measurement as a surveillance tool in oral leukoplakia. Mol. Oncol. 2021, 16, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, K.L.; Millard, M.; Bosenberg, M.W.; DePinho, R.A. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat. Genet. 2001, 28, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.; de Solorzano, C.O.; Knowles, D.; Jones, A.; Chou, W.; Rodriguez, E.G.; Kuo, W.L.; Ljung, B.M.; Chew, K.; Myambo, K.; et al. In situ analyses of genome instability in breast cancer. Nat. Genet. 2004, 36, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Meeker, A.K.; Hicks, J.L.; Iacobuzio-Donahue, C.A.; Montgomery, E.A.; Westra, W.H.; Chan, T.Y.; Ronnett, B.M.; DeMarzo, A.M. Telomere length abnormalities occur early in the initiation of epithelial carcinogenesis. Clin. Cancer Res. 2004, 10, 3317–3326. [Google Scholar] [CrossRef] [PubMed]
- Bau, D.-T.; Lippman, S.M.; Xu, E.; Gong, Y.; Lee, J.J.; Wu, X.; Gu, J. Short telomere lengths in peripheral blood leucocytes are associated with an increased risk of oral premalignant lesions and oral squamous cell carcinoma. Cancer 2013, 119, 4277–4283. [Google Scholar] [CrossRef]
- Harle-Bachor, C.; Boukamp, P. Telomerase activity in the regenerative basal layer of epidermis in human skin and in immortal and carcinoma-derived keratinocytes. Proc. Natl. Acad. Sci. USA 1996, 93, 6476–6481. [Google Scholar] [CrossRef]
- Ramirez, R.D.; Wright, W.E.; Shay, J.W.; Taylor, R.S. Telomerase activity concentrates in the mitotically active segments of human hair follicles. J. Investig. Dermatol. 1997, 108, 113–117. [Google Scholar] [CrossRef]
- Soder, A.I.; Hoare, S.F.; Muir, S.; Going, J.J.; Parkinson, E.K.; Keith, W.N. Amplification, increased dosage and in situ expression of the telomerase RNA gene in human cancer. Oncogene 1997, 14, 1013–1021. [Google Scholar] [CrossRef]
- Nakano, K.; Watney, E.; McDougall, J.K. Telomerase activity and expression of telomerase RNA component and telomerase catalytic subunit gene in cervical cancer. Am. J. Pathol. 1998, 153, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, A.G.; Ouellette, M.; Frolkis, M.; Holt, S.E.; Chiu, C.P.; Morin, G.B.; Harley, C.B.; Shay, J.W.; Lichsteiner, S.; Wright, W.E. Extension of life-span by introduction of telomerase into normal human cells. Science 1998, 279, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Suram, A.; Kaplunov, J.; Patel, P.L.; Ruan, H.; Cerutti, A.; Boccardi, V.; Fumagalli, M.; Di Micco, R.; Mirani, N.; Gurung, R.L.; et al. Oncogene-induced telomere dysfunction enforces cellular senescence in human cancer precursor lesions. EMBO J. 2012, 31, 2839–2851. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.L.; Suram, A.; Mirani, N.; Bischof, O.; Herbig, U. Derepression of hTERT gene expression promotes escape from oncogene-induced cellular senescence. Proc. Natl. Acad. Sci. USA 2016, 113, E5024–E5033. [Google Scholar] [CrossRef]
- Veeramachaneni, R.; Walker, T.; Revil, T.; De Weck, A.; Badescu, D.; O’Sullivan, J.; Higgins, C.; Elliott, L.; Liloglou, T.; Risk, J.M.; et al. Analysis of head and neck cancer progression reveals novel and relevant stage-specific changes associated with immortalisation and malignancy. Sci. Rep. 2019, 9, 11912. [Google Scholar] [CrossRef]
- Meena, J.K.; Cerutti, A.; Beichler, C.; Morita, Y.; Bruhn, C.; Kumar, M.; Kraus, J.M.; Speicher, M.R.; Wang, Z.Q.; Kestler, H.A.; et al. Telomerase abrogates aneuploidy-induced telomere replication stress, senescence and cell depletion. EMBO J. 2015, 34, 1371–1384. [Google Scholar] [CrossRef]
- Kipling, D.; Faragher, R.G. Telomeres. Ageing hard or hardly ageing. Nature 1999, 398, 191–193. [Google Scholar] [CrossRef]
- Chang, K.P.; Wang, C.I.; Pickering, C.R.; Huang, Y.; Tsai, C.N.; Tsang, N.M.; Kao, H.K.; Cheng, M.H.; Myers, J.N. Prevalence of promoter mutations in the TERT gene in oral cavity squamous cell carcinoma. Head Neck 2017, 39, 1131–1137. [Google Scholar] [CrossRef]
- Annunziata, C.; Pezzuto, F.; Greggi, S.; Ionna, F.; Losito, S.; Botti, G.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Distinct profiles of TERT promoter mutations and telomerase expression in head and neck cancer and cervical carcinoma. Int. J. Cancer 2018, 143, 1153–1161. [Google Scholar] [CrossRef]
- Dokal, I. Dyskeratosis congenita. Haematol. Am. Soc. Hematol. Educ. Program 2011, 2011, 480–486. [Google Scholar] [CrossRef]
- Dorji, T.; Monti, V.; Fellagara, G.; Gabba, S.; Grazioli, V.; Repetti, E.; Marcialis, C.; Peluso, S.; Di Ruzza, D.; Neri, F.; et al. Gain of hTERC; a genetic marker of malignancy in oral potentially malignant lesions. Hum. Pathol. 2015, 46, 1275–1281. [Google Scholar] [CrossRef]
- Cristofari, G.; Lingner, J. Telomere length homeostasis requires that telomerase levels are limiting. EMBO J. 2006, 25, 565–574. [Google Scholar] [CrossRef]
- Wright, W.E.; Pereira-Smith, O.M.; Shay, J.W. Reversible cellular senescence: Implications for immortalization of normal human diploid fibroblasts. Mol. Cell Biol. 1989, 9, 3088–3092. [Google Scholar] [PubMed]
- Counter, C.M.; Avilion, A.A.; LeFeuvre, C.E.; Stewart, N.G.; Greider, C.W.; Harley, C.B.; Bacchetti, S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992, 11, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Gisselsson, D.; Jonson, T.; Petersen, A.; Strombeck, B.; Dal Cin, P.; Hoglund, M.; Mitelman, F.; Mertens, F.; Mandahl, N. Telomere dysfunction triggers extensive DNA fragmentation and evolution of complex chromosomal abnormalities in human malignant tumors. Proc. Natl. Acad. Sci. USA 2001, 98, 12683–12688. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Lorbeer, F.K.; Shain, A.H.; McSwiggen, D.T.; Schruf, E.; Oh, A.; Ryu, J.; Darzacq, X.; Bastian, B.C.; Hockemeyer, D. Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two-step mechanism. Science 2017, 357, 1416–1420. [Google Scholar] [CrossRef]
- Hannahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hastie, N.D.; Dempster, M.; Dunlop, M.G.; Thompson, A.M.; Green, D.K.; Allshire, R.C. Telomere reduction in human colorectal carcinoma and with ageing. Nature 1990, 346, 866–868. [Google Scholar] [CrossRef]
- Boscolo- Rizzo, P.; Giunco, S.; Rampazzo, E.; Brutti, M.; Spinato, G.; Menegaldo, A.; Stelin, M.; Mantovani, M.; Bandolin, L.; Rossi, M.; et al. TERT promoter hotspot mutations and their relationship with TERT levels and telomere erosion in patients with head and neck squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2020, 146, 381–389. [Google Scholar] [CrossRef]
- Donehower, L.A.; Godley, L.A.; Aldaz, C.M.; Pyle, R.; Shi, Y.P.; Pinkel, D.; Gray, J.; Bradley, A.; Medina, D.; Varmus, H.E. Deficiency of p53 accelerates mammary tumorigenesis in Wnt-1 transgenic mice and promotes chromosomal instability. Genes Dev. 1995, 9, 882–895. [Google Scholar] [CrossRef]
- Shao, C.; Deng, L.; Henegariu, O.; Liang, L.; Stambrook, P.J.; Tischfield, J.A. Chromosome instability contributes to loss of heterozygosity in mice lacking p53. Proc. Natl. Acad. Sci. USA 2000, 97, 7405–7410. [Google Scholar] [CrossRef] [PubMed]
- Borel, F.; Lohez, O.D.; Lacroix, F.B.; Margolis, R.L. Multiple centrosomes arise from tetraploidy checkpoint failure and mitotic centrosome clusters in p53 and RB pocket protein compromised cells. Proc. Natl. Acad. Sci. USA 2002, 99, 9819–9824. [Google Scholar] [CrossRef] [PubMed]
- Ciriello, G.; Miller, M.L.; Aksoy, B.A.; Senbabaoglu, Y.; Schultz, N.; Sander, C. Emerging landscape of oncogenic signatures across human cancers. Nat. Genet. 2013, 45, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Shih, J.; Ha, G.; Gao, G.F.; Zhang, X.; Berger, A.C.; Schummacher, S.E.; Wang, C.; Hu, H.; Liu, J.; et al. Genomic and functional approaches to understanding cancer aneuploidy. Cancer Cell 2018, 33, 676–689. [Google Scholar] [CrossRef]
- Rausch, T.; Jones, D.T.; Zapatka, M.; Stutz, A.M.; Zichner, T.; Weischenfeldt, J.; Jager, N.; Remke, M.; Shih, D.; Northcott, P.A.; et al. Genomic sequencing of pediatric medulloblastoma links catastrophic DNA rearrangements with TP53 mutations. Cell 2012, 148, 59–71. [Google Scholar] [CrossRef]
- Bischoff, F.Z.; Yim, S.O.; Parthak, S.; Grant, G.; Siciliano, M.J.; Giovanella, B.C.; Strong, L.C.; Tainsky, M. Spontaneous abnormalities in normal fibroblasts from patients with Li-Fraumeni cancer syndrome: Aneuploidy and immortalization. Cancer Res. 1990, 50, 7979–7984. [Google Scholar]
- Boyl, J.M.; Mitchell, E.L.; Greaves, M.J.; Roberts, S.A.; Tricker, K.; Burt, E.; Varley, J.M.; Birch, J.M.; Scott, D. Chromosome instability is a predominant trait of fibroblasts from Li Fraumeni families. Br. J. Cancer 1998, 77, 2181–2192. [Google Scholar] [CrossRef]
- Liu, P.K.; Kraus, E.; Wu, T.A.; Strong, L.C.; Tainsky, M.A. Analysis of genomic instability in Li-Fraumeni fibroblasts with germline p53 mutations. Oncogene 1996, 12, 2267–2278. [Google Scholar]
- Gualberto, A.; Aldape, K.; Kozakiewicz, K.; Tlsty, T.D. An oncogenic form of p53 confers a dominant, gain of function phenotype that disrupts spindle checkpoint control. Genetics 1998, 95, 5166–5171. [Google Scholar] [CrossRef]
- Talos, F.; Nemajerova, A.; Flores, E.R.; Petrenko, O.; Moll, U.M. p73 suppresses polyploidy and aneuploidy in the absence of functional p53. Mol. Cell 2007, 27, 647–659. [Google Scholar] [CrossRef]
- Shlien, A.; Tabori, U.; Marshall, C.R.; Pienkowska, M.; Fenk, L.; Novokmet, A.; Nanda, S.; Druker, H.; Scherer, S.W.; Malkin, D. Excessive DNA copy number variation in the Li-Fraumeni cancer predisposition syndrome. Proc. Natl. Acad. Sci. USA 2008, 105, 11264–11269. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.L.; Compton, D.A. Proliferation of aneuploidy cells is limited by a p53-dependent mechanism. J. Cell Biol. 2010, 188, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kschischo, M. Distinct and common features of numerical and structural chromosome instability across different cancer types. Cancers 2022, 14, 1424. [Google Scholar] [CrossRef]
- Elmore, L.W.; Turner, K.C.; Gollahon, L.S.; Landon, M.R.; Jackson-Cook, C.K.; Holt, S.E. Telomerase protects cancer-prone human cells from chromosomal instability and spontaneous immortalization. Cancer Biol. Ther. 2002, 1, 391–397. [Google Scholar] [CrossRef]
- O’Hagan, R.C.; Chang, S.; Maser, R.S.; Mohan, R.; Artandi, S.E.; Chin, L.; DePinho, R.A. Telomere dysfunction promotes regional amplification and deletion in cancer genomes. Cancer Cell 2002, 2, 149–155. [Google Scholar] [CrossRef]
- Redman-Rivera, L.N.; Shaver, T.M.; Jin, H.; Marshall, C.B.; Schafer, J.M.; Sheng, Q.; Hongo, R.A.; Beckermann, K.E.; Wheeler, F.C.; Lehmann, B.D.; et al. Acquisition of aneuploidy drives mutant p53-associated gain of function phenotypes. Nat. Commun. 2021, 12, 5184. [Google Scholar] [CrossRef]
- Helias-Rodzewicz, Z.; Lourenco, N.; Bakari, M.; Capron, C.; Emile, J.F. CDKN2A depletion causes aneuploidy and enhances cell proliferaton in non-immortalised normal human cells. Cancer Investig. 2018, 36, 338–348. [Google Scholar] [CrossRef]
- Patel, S.; Wilkinson, C.J.; Sviderskaya, E.V. Loss of both CDKN2A and CDKN2B allows for centrosome overduplication in melanoma. J. Investig. Dermatol. 2020, 140, 1837–1846.e1. [Google Scholar] [CrossRef]
- Lee, J.K.; Choi, Y.L.; Kwon, M.; Park, P.J. Mechanisms and consequences of cancer genome instability: Lessons from genome sequencing studies. Annu. Rev. Pathol. 2016, 11, 283–312. [Google Scholar] [CrossRef]
- Cui, H.; Kong, Y.; Xu, M.; Zhang, H. Notch 3 functions as a tumor suppressor by controlling cellular senescence. Cancer Res. 2013, 73, 3451–3459. [Google Scholar] [CrossRef]
- Kagawa, S.; Natsuizaka, M.; Wheelan, K.A.; Facompre, N.; Naganuma, S.; Ohashi, S.; Kinugasa, H.; Egloff, A.M.; Basu, D.; Gimotty, P.A.; et al. Cellular senescence checkpoint function determines differential Notch1-dependent oncogenic and tumor-suppressor activities. Oncogene 2015, 34, 2347–2359. [Google Scholar] [CrossRef] [PubMed]
- Hoare, M.; Ito, Y.; Kang, T.-W.; Weekes, M.P.; Matheson, N.J.; Patten, D.A.; Shetty, S.; Parry, A.J.; Menon, S.; Salama, R.; et al. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat. Cell Biol. 2016, 18, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Demehri, S.; Turkoz, A.; Kopan, R. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal environment. Cancer Cell 2009, 16, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, M.; Wolfer, A.; Raj, K.; Kummer, J.A.; Mill, P.; van Noort, M.; Hui, C.; Clevers, H.; Dotto, G.P.; Radtke, F. Notch1 functions as a tumor suppressor in mouse skin. Nat. Genet. 2003, 33, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Martincorena, I.; Fowler, J.C.; Wabik, A.; Lawson, A.R.J.; Abascal, F.; Hall, M.W.J.; Cagan, A.; Murai, K.; Mahbubani, K.; Stratton, M.R.; et al. Somatic mutant clones colonize the human esophagus with age. Science 2018, 362, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Yang, S.-A.; Gong, S.; Chang, C.-H.; Portilla, J.M.; Chatterjee, D.; Irianto, J.; Bao, H.; Huang, Y.-C.; Dengm, W.-M. Polyploid mitosis and depolyploidization promote chromosomal instability and tumor progression in a Notch-induced tumor model. Dev. Cell 2021, 56, 1976–1988. [Google Scholar] [CrossRef]
- Baia, G.S.; Stifani, S.; Kimura, E.T.; McDermott, M.W.; Pieper, R.O.; Lal, A. Notch activation is associated with tetraploidy and enhanced chromosomal instability in meningiomas. Neoplasia 2008, 10, 604–612. [Google Scholar] [CrossRef]
- Agarwal, V.; Subash, A.; Nayar, R.C.; Rao, V. Is EGFR really a therapeutic target in head and neck cancer? J. Surg. Oncol. 2019, 119, 685–686. [Google Scholar] [CrossRef]
- Ries, J.; Vairaktaris, E.; Agaimy, A.; Bechtold, M.; Gorecki, P.; Neukam, F.W.; Nkenke, E. The relevance of EGFR overexpression for the prediction of the malignant transformation of oral leukoplakia. Oncol. Rep. 2013, 30, 1149–1156. [Google Scholar] [CrossRef]
- Tsiambas, E.; Mastronikolis, N.S.; Lefas, A.Y.; Georgiannos, S.N.; Ragos, V.; Foliades, P.P.; Tsoukalas, N.; Kavantzas, N.; Karameris, A.; Peschos, D.; et al. Chromosome 7 multiplication in EGFR-positive lung carcinomas based on tissue microarray analysis. In Vivo 2017, 31, 641–648. [Google Scholar]
- Crowell, R.E.; Gilliland, F.D.; Temes, R.T.; Harms, H.J.; Neft, R.E.; Heaphy, E.; Auckley, D.H.; Crooks, L.A.; Jordan, S.W.; Samet, J.M.; et al. Detection of trisomy 7 in nonmalignant bronchial epithelium from lung cancer patients and individuals at risk for lung cancer. Cancer Epidemiol. Biomark. Prev. 1996, 5, 631–637. [Google Scholar]
- Nimeus, E.; Baldetorp, B.; Bendahl, P.-O.; Rennstam, K.; Wennerberg, J.; Akervall, J.; Ferno, M. Amplification of the cyclin D1 gene is associated with tumor subsite, DNA non-diploidy and high S-phase fraction in squamous cell carcinoma of the head and neck. Oral Oncol. 2004, 40, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Watkins, T.B.K.; Lim, E.L.; Petkovic, M.; Elizalde, S.; Birbak, N.J.; Wilson, G.A.; Moore, D.A.; Gronroos, E.; Rowan, A.; Dewhurst, S.M.; et al. Pervasive chromosome instability and karyotype order in tumour evolution. Nature 2020, 587, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-T.; Cheng, S.-D.; Liao, C.-T.; Wang, H.-M.; Huang, S.-F. Amplification of the EGFR and CCND1 are coordinated and play important roles in the progression of oral squamous cell carcinomas. Cancers 2019, 11, 760. [Google Scholar] [CrossRef]
- Murugan, A.K.; Munirajan, A.K.; Tsuchida, N. Genetic deregulation of the PIK3CA oncogene in oral cancer. Cancer Lett. 2013, 338, 193–203. [Google Scholar] [CrossRef]
- Vander Broek, R.; Mohan, S.; Eytan, D.F.; Chen, Z.; Van Waes, C. The PI3K/Akt/mTOR axis in head and neck cancer: Functions, aberrations, cross-talk, and therapies. Oral Dis. 2015, 21, 815–825. [Google Scholar] [CrossRef]
- Tsui, I.F.L.; Poh, C.F.; Garnis, C.; Rosin, M.P.; Zhang, L.; Lam, W.L. Multiple pathways in the FGF signaling network are frequently deregulated by gene amplification in oral dysplasias. Int. J. Cancer 2009, 125, 2219–2228. [Google Scholar] [CrossRef]
- Squarize, C.H.; Castilho, R.M.; Abraham, A.C.; Molinolo, A.; Lingen, M.W.; Gutkind, J.S. PTEN deficiency contributes to the development and progression of head and neck cancer. Neoplasia 2013, 15, 461–471. [Google Scholar] [CrossRef]
- Miyahara, L.A.N.; Pontes, F.S.C.; Burbano, R.M.R.; Conte Neto, N.; Guimaraes, D.M.; Fonseca, F.P.; Pontes, H.A.R. PTEN allelic loss is an important mechanism in the late stage of development of oral leukoplakia into oral squamous cell carcinoma. Histopathology 2018, 72, 330–338. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Bilanges, B.; Madsenm, R.R.; Dalem, K.L.; Laum, E.; Vladimiroumm, E. Perspective: Potential impact and therapeutic implications of oncogenic PI3K activation on chromosomal instability. Biomolecules 2019, 9, 331. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, C.; He, J.; Lamb, K.L.; Kang, X.; Gu, T.; Shen, W.H.; Yin, Y. PTEN C-terminal deletion causes genomic instability and tumor development. Cell Rep. 2014, 6, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Vidotto, T.; Guimaraes-Tiezz, D.; Squire, J.A. Distinct subtypes of genomic PTEN deletion size influence the landscape of aneuploidy and outcome in prostate cancer. Mol. Cytogenet. 2018, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.Q.; Ouyang, M.; Brandmaier, A.; Hao, H.; Shen, W.H. Pten in the maintenance of genomic integrity: From DNA replication to chromosome segregation. Bioessays 2017, 39, 82. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.; Silva, P.; Delgado, L.; Amaral, B.; Garces, F.; Salazar, F.; Pacheco, J.-J.; Lopes, C.; Bousbaa, H.; Warnakulasuriya, S. Expression of spindle assembly checkpoint proteins BubR1 and Mad2 expression as potential biomarkers of malignant transformation of oral leukoplakia: An observational cohort study. J. Oral Med. Oral Patol. Oral Cir. Bucal. 2021, 26, e719–e728. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Okami, K.; Hibi, K.; Wehage, S.L.; Jen, J.; Sidransky, D. Mutation analysis of hBUB1 in aneuploidy HNSCC and lung cancer cell lines. Cancer Lett. 1999, 139, 183–187. [Google Scholar] [CrossRef]
- Chan, K.L.; Palmai-Pallag, T.; Ying, S.; Hickson, I.D. Replication stress induces sister-chromatid bridging at fragile site loci in mitosis. Nat. Cell Biol. 2009, 11, 753–760. [Google Scholar] [CrossRef]
- Doksani, Y. The response to DNA damage at telomeric repeats and its consequence for telomere function. Genes 2019, 10, 318. [Google Scholar] [CrossRef]
- Umbreit, N.T.; Zhang, C.-Z.; Lynch, L.D.; Blaine, L.J.; Cheng, A.M.; Tourdot, R.; Sun, L.; Almubarak, H.F.; Judge, K.; Mitchell, T.J.; et al. Mechanisms generating cancer genome complexity from a single cell division error. Science 2020, 368, eaba0712. [Google Scholar] [CrossRef]
- Bryan, T.M.; Englezou, A.; Dunham, M.A.; Reddel, R.R. Telomere length dynamics in telomerase-positive immortal human cell populations. Exp. Cell Res. 1998, 239, 370–378. [Google Scholar] [CrossRef]
- Counter, C.M.; Hahn, W.C.; Wei, W.; Caddle, S.D.; Beijersbergen, R.L.; Lansdorp, P.M.; Sedivy, J.M.; Wenberg, R.A. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc. Natl. Acad. Sci. USA 1998, 95, 14723–14728. [Google Scholar] [CrossRef]
- Gordon, K.E.; Ireland, H.; Roberts, M.; Steeghs, K.; McCaul, J.A.; MacDonald, D.G.; Parkinson, E.K. High levels of telomere dysfunction bestow a selective disadvantage during the progression of human oral squamous cell carcinoma. Cancer Res. 2003, 63, 458–467. [Google Scholar] [PubMed]
- Davoli, T.; Xu, A.W.; Mengwasser, K.E.; Sack, L.M.; Yoon, J.C.; Park, P.J.; Elledge, S.J. Cumulative haploinsufficiency and triplosensitivity drive aneuploidy patterns and shape the cancer genome. Cell 2013, 155, 948–962. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tsai, H.J.; Gordon, M.R.; Li, R. Cellular stress associated with aneuploidy. Dev. Cell 2018, 44, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Chunduri, N.K.; Storchova, Z. The diverse consequences of aneuploidy. Nat. Cell Biol. 2019, 21, 54–62. [Google Scholar] [CrossRef]
- Ben-David, U.; Amon, A. Context is everything: Aneuploidy in cancer. Nat. Rev. Genet. 2020, 21, 44–62. [Google Scholar] [CrossRef]
- Williams, B.R.; Prabhu, V.R.; Hunter, K.E.; Glazier, C.M.; Whittaker, C.A.; Housman, D.E.; Amon, A. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science 2008, 322, 703–709. [Google Scholar] [CrossRef]
- Stingele, S.; Stoehr, G.; Peplowska, K.; Cox, J.; Mann, M.; Storchova, Z. Global analysis of genome, transcriptome and proteome reveals the response to aneuploidy in human cells. Mol. Syst. Biol. 2012, 8, 608. [Google Scholar] [CrossRef]
- Kim, C.; Gao, R.; Sei, E.; Brandt, R.; Hartman, J.; Hatschek, T.; Crosetto, N.; Foukakis, T.; Navin, N.E. Chemoresistance evolution in triple-negative breast cancer delineated by single-cell sequencing. Cell 2018, 173, 879–893. [Google Scholar] [CrossRef]
- Davoli, T.; Uno, H.; Wooten, E.C.; Elledge, S.J. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science 2017, 355, eaaf8399. [Google Scholar] [CrossRef]
- Vasudevan, A.; Schukken, K.M.; Sausville, E.L.; Girish, V.; Adebambo, O.A.; Sheltzer, J.M. Aneuploidy as a promoter and suppressor of malignant growth. Nat. Rev. Cancer 2021, 21, 89–103. [Google Scholar] [CrossRef]
- Bakhoum, S.F.; Ngo, B.; Laughney, A.M.; Cavallo, J.A.; Murphy, C.J.; Ly, P.; Shah, P.; Sriram, R.K.; Watkins, T.B.K.; Taunk, N.K.; et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 2018, 553, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, G.; Bruno, S.; Padella, A.; Tenti, E.; Martinelli, G. Aneuploidy: Cancer strength or vulnerability. Int. J. Cancer 2019, 144, 8–25. [Google Scholar] [CrossRef]
- Sheltzer, J.M.; Ko, J.H.; Replogle, J.M.; Habibe Burgos, N.C.; Chung, E.S.; Meehl, C.M.; Sayles, N.M.; Passerini, V.; Storchova, Z.; Amon, A. Single chromosome gains commonly function as tumor suppressors. Cancer Cell 2017, 31, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Sheltzer, J.M.; Amon, A. The aneuploidy paradox: Costs and benefits of an incorrect karyotype. Trends Genet. 2011, 27, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Foijer, F.; DiTommaso, T.; Hautaviita, K.; Xie, S.Z.; Heath, E.; Smyth, I.; Watt, F.M.; Sorger, P.K.; Bradley, A. Spindle checkpoint deficiency is tolerated by murine epidermal cells but not by hair follicle stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 2928–2933. [Google Scholar] [CrossRef]
- Jilderda, L.J.; Zhou, L.; Fijer, F. Understanding how genetic mutations collaborate with genomic instability in cancer. Cells 2021, 10, 342. [Google Scholar] [CrossRef]
- Garcia-Cao, I.; Garcia-Cao, M.; Martin-Caballero, J.; Criado, L.M.; Klatt, P.; Flores, J.M.; Blasco, M.A.; Serrano, M. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 2002, 21, 6225–6235. [Google Scholar] [CrossRef]
- Hoevenaar, W.H.M.; Janssen, A.; Quirindongo, A.I.; Ma, H.; Klaasen, S.; Teixeira, A.; van Gerwen, B.; Lansu, N.; Morsink, F.H.M.; Offerhaus, G.J.A.; et al. Degree and site of chromosomal instability define its oncogenic potential. Nat. Commun. 2020, 11, 1501. [Google Scholar] [CrossRef]
- Shoshani, O.; Bakker, B.; de Haan, L.; Tijhuis, A.E.; Wang, Y.; Kim, D.H.; Maldonado, M.; Demarest, M.A.; Artates, J.; Zhengyu, O.; et al. Transient genomic instability drives tumorigenesis through accelerated clonal evolution. Genes Dev. 2021, 35, 1093–1108. [Google Scholar] [CrossRef]
- Vasudevan, A.; Baruah, P.S.; Smith, J.C.; Wang, Z.; Sayles, N.M.; Andrews, P.; Kendall, J.; Leu, J.; Chunduri, N.K.; Levy, D.; et al. Single-chromosome gains can function as metastasis suppressors and promoters in colon cancer. Dev. Cell 2020, 52, 413–428. [Google Scholar] [CrossRef]
- Bach, D.H.; Zhang, W.; Sood, A.K. Chromosomal instability in tumor initiation and development. Cancer Res. 2019, 79, 3995–4002. [Google Scholar] [CrossRef] [PubMed]
- Snape, K.; Hanks, S.; Ruark, E.; Barros-Nunez, P.; Elliot, A.; Murray, A.; Lane, A.H.; Shannon, N.; Callier, P.; Chitayat, D.; et al. Mutations in CEP57 cause mosaic variegated aneuploidy syndrome. Nat. Genet. 2011, 43, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.A.; Komarova, N.L.; Sengupta, A.; Jallepalli, P.V.; Shih, M.; Vogelstein, B.; Lengauer, C. The role of chromosomal instability in tumor initiation. Proc. Natl. Acad. Sci. USA 2002, 99, 16226–16231. [Google Scholar] [CrossRef]
- Giam, M.; Rancati, G. Aneuploidy and chromosome instability in cancer: A jackpot to chaos. Cell Div. 2015, 10, 3. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Beckett, M.A. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981, 41, 1657–1663. [Google Scholar] [PubMed]
- Branch, P.; Hampson, R.; Karran, P. DNA mismatch binding defects, DNA damage tolerance and mutator phenotypes in human colorectal carcinoma cell lines. Cancer Res. 1995, 55, 2304–2309. [Google Scholar] [PubMed]
- Vendramin, R.; Litchfieldm, K.; Swanton, C. Cancer evolution: Darwin and beyond. EMBO J. 2021, 40, e108389. [Google Scholar] [CrossRef] [PubMed]
- Trakala, M.; Aggarwal, M.; Sniffen, C.; Zasadil, L.; Carroll, A.; Ma, D.; Su, X.A.; Wangsa, D.; Meyer, A.; Sieben, C.J.; et al. Clonal selection of stable aneuploidies in progenitor cells drives high-prevalence tumorigenesis. Genes Dev. 2021, 35, 1079–1092. [Google Scholar] [CrossRef]
- Kogan-Sakin, I.; Tabach, Y.; Buganim, Y.; Molchadsky, A.; Solomon, H.; Madar, S.; Kamer, I.; Stambolsky, P.; Shelley, A.; Goldfinger, N.; et al. Mutant p53 (R175H) upregulates Twist1 expression and promotes epithelial-mesenchymal transition in immortalized prostate cells. Cell Death Differ. 2011, 18, 271–281. [Google Scholar] [CrossRef]
- Dong, P.; Karaayvaz, M.; Jia, N.; Kaneuchi, M.; Hamada, J.; Watari, H.; Sudo, S.; Ju, J.; Sakuragi, N. Mutant p53 gain-of-function induces epithelial-mesenchymal transition through modulation of the miR-130b-ZEB1 axis. Oncogene 2013, 32, 3286–3295. [Google Scholar] [CrossRef]
- Hassona, Y.; Cirillo, N.; Lim, K.P.; Herman, A.; Mellone, M.; Thomas, G.J.; Pitiyage, G.N.; Parkinson, E.K.; Prime, S.S. Progression of genotype-specific oral cancer leads to senescence of cancer-associated fibroblasts and is mediated by oxidative stress and TGF-β. Carcinogenesis 2013, 34, 1286–1295. [Google Scholar] [CrossRef]
- Cirillo, N.; Hassona, Y.; Celentano, A.; Lim, K.P.; Manchella, S.; Parkinson, E.K.; Prime, S.S. Cancer-associated fibroblasts regulate keratinocyte cell-cell adhesion via TGF-β-dependent pathways in genotype-specific oral cancer. Carcinogenesis 2017, 38, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Odell, E.W. Aneuploidy and loss of heterozygosity as risk markers for malignant transformation in oral mucosa. Oral Dis. 2021, 27, 1993–2007. [Google Scholar] [CrossRef] [PubMed]
- Douville, C.; Cohen, J.D.; Ptak, J.; Popoli, M.; Schaefer, J.; Silliman, N.; Dobbyn, L.; Schoen, R.E.; Tie, J.; Gibbs, P.; et al. Assessing aneuploidy with repetitive element sequencing. Proc. Natl. Acad. Sci. USA 2020, 117, 4858–4863. [Google Scholar] [CrossRef] [PubMed]
- Mattox, A.K.; Douville, C.; Silliman, N.; Ptak, J.; Dobbyn, L.; Schaefer, J.; Popoli, M.; Blair, C.; Judge, K.; Pollard, K.; et al. Detection of malignant nerve sheath tumors in patients with neurofibromatosis using aneuploidy and mutation identification in plasma. eLife 2022, 11, e74238. [Google Scholar] [CrossRef]
- Thompson, L.L.; Jeusset, L.M.-P.; Lepage, C.C.; McManus, K.J. Evolving therapeutic strategies to exploit chromosome instability in cancer. Cancers 2017, 9, 151. [Google Scholar] [CrossRef]
- Bakhoum, S.F.; Cantley, L.C. The multifaceted role of chromosomal instability in cancer and its microenvironment. Cell 2018, 174, 1347–1360. [Google Scholar] [CrossRef]
- Cohen-Sharir, Y.; McFarland, J.M.; Abdusamad, M.; Marquis, C.; Bernhard, S.V.; Kazachkova, M.; Tang, H.; Ippolito, M.R.; Laue, K.; Zerbib, J.; et al. Aneuploidy renders cancer cells vulnerable to mitotic checkpoint inhibition. Nature 2021, 590, 486–491. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prime, S.S.; Cirillo, N.; Parkinson, E.K. Escape from Cellular Senescence Is Associated with Chromosomal Instability in Oral Pre-Malignancy. Biology 2023, 12, 103. https://doi.org/10.3390/biology12010103
Prime SS, Cirillo N, Parkinson EK. Escape from Cellular Senescence Is Associated with Chromosomal Instability in Oral Pre-Malignancy. Biology. 2023; 12(1):103. https://doi.org/10.3390/biology12010103
Chicago/Turabian StylePrime, Stephen S., Nicola Cirillo, and E. Kenneth Parkinson. 2023. "Escape from Cellular Senescence Is Associated with Chromosomal Instability in Oral Pre-Malignancy" Biology 12, no. 1: 103. https://doi.org/10.3390/biology12010103
APA StylePrime, S. S., Cirillo, N., & Parkinson, E. K. (2023). Escape from Cellular Senescence Is Associated with Chromosomal Instability in Oral Pre-Malignancy. Biology, 12(1), 103. https://doi.org/10.3390/biology12010103






