Commonly Prescribed Anticoagulants Exert Anticancer Effects in Oral Squamous Cell Carcinoma Cells In Vitro
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Culture Conditions
2.3. Anticoagulants and 5-FU
2.4. Proliferation Assays
2.5. Wound Healing Assays
2.6. Statistical Analysis
3. Results
3.1. Treatment with Anticoagulants Reduces OSCC Cell Proliferation In Vitro
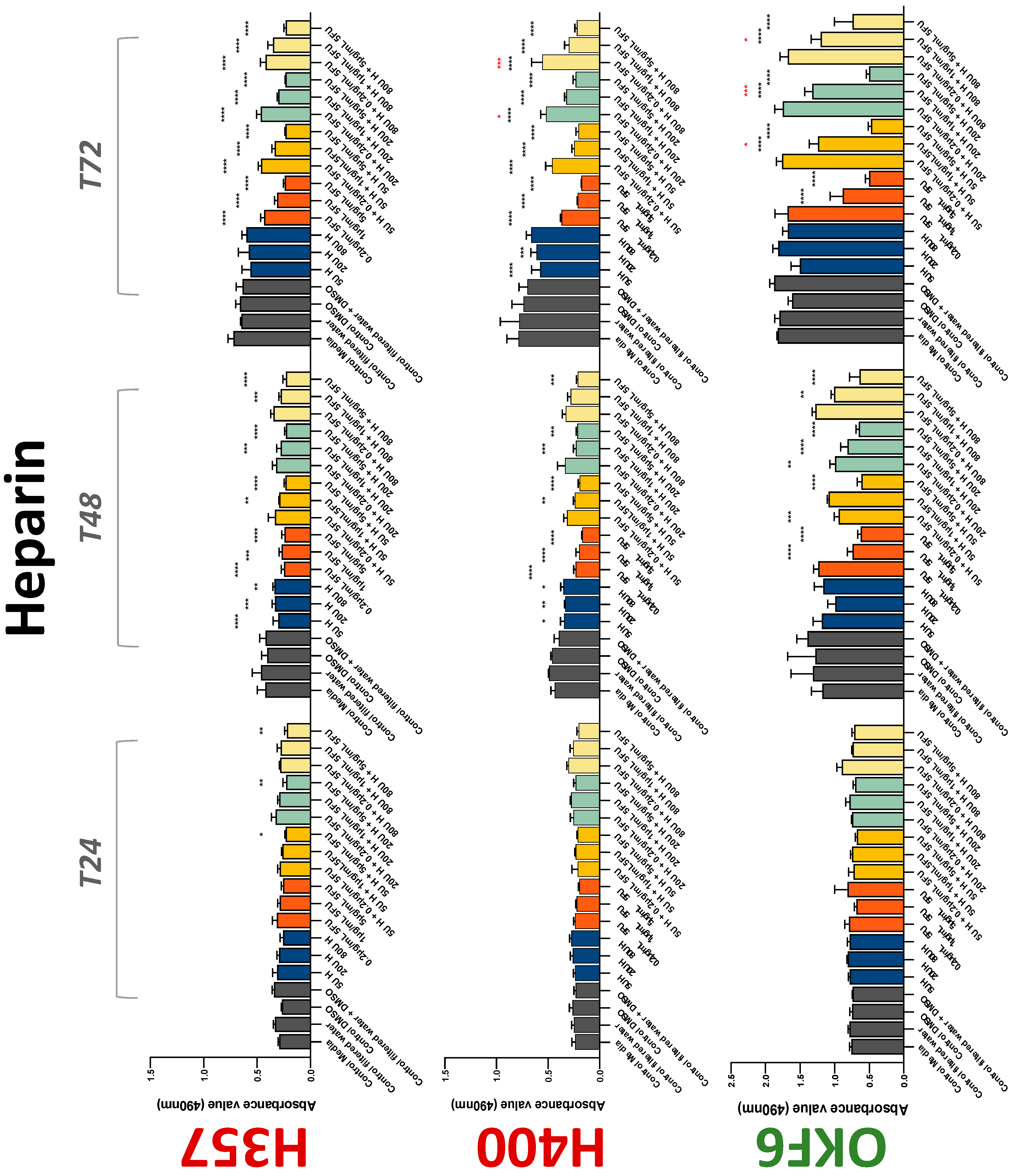
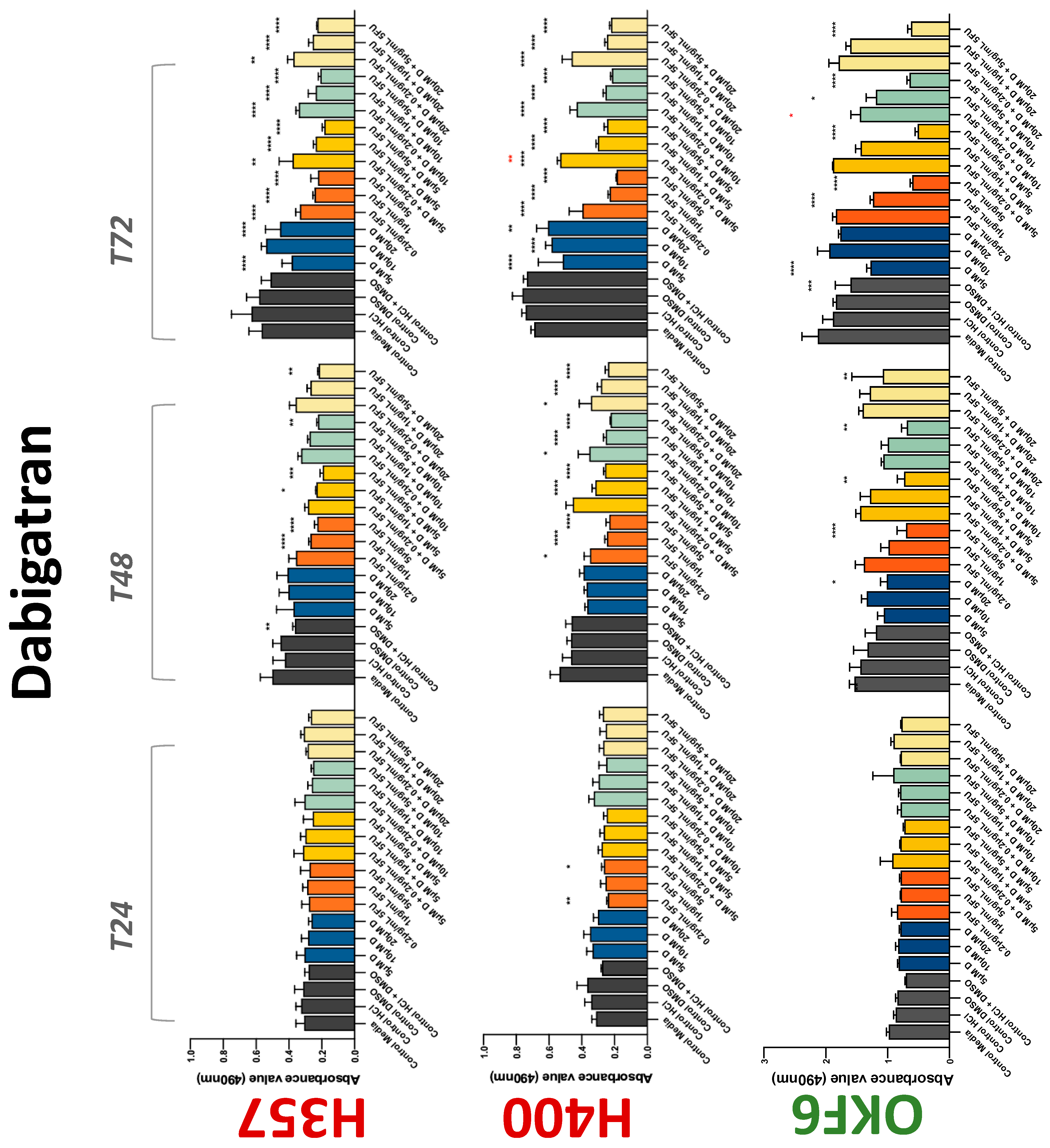
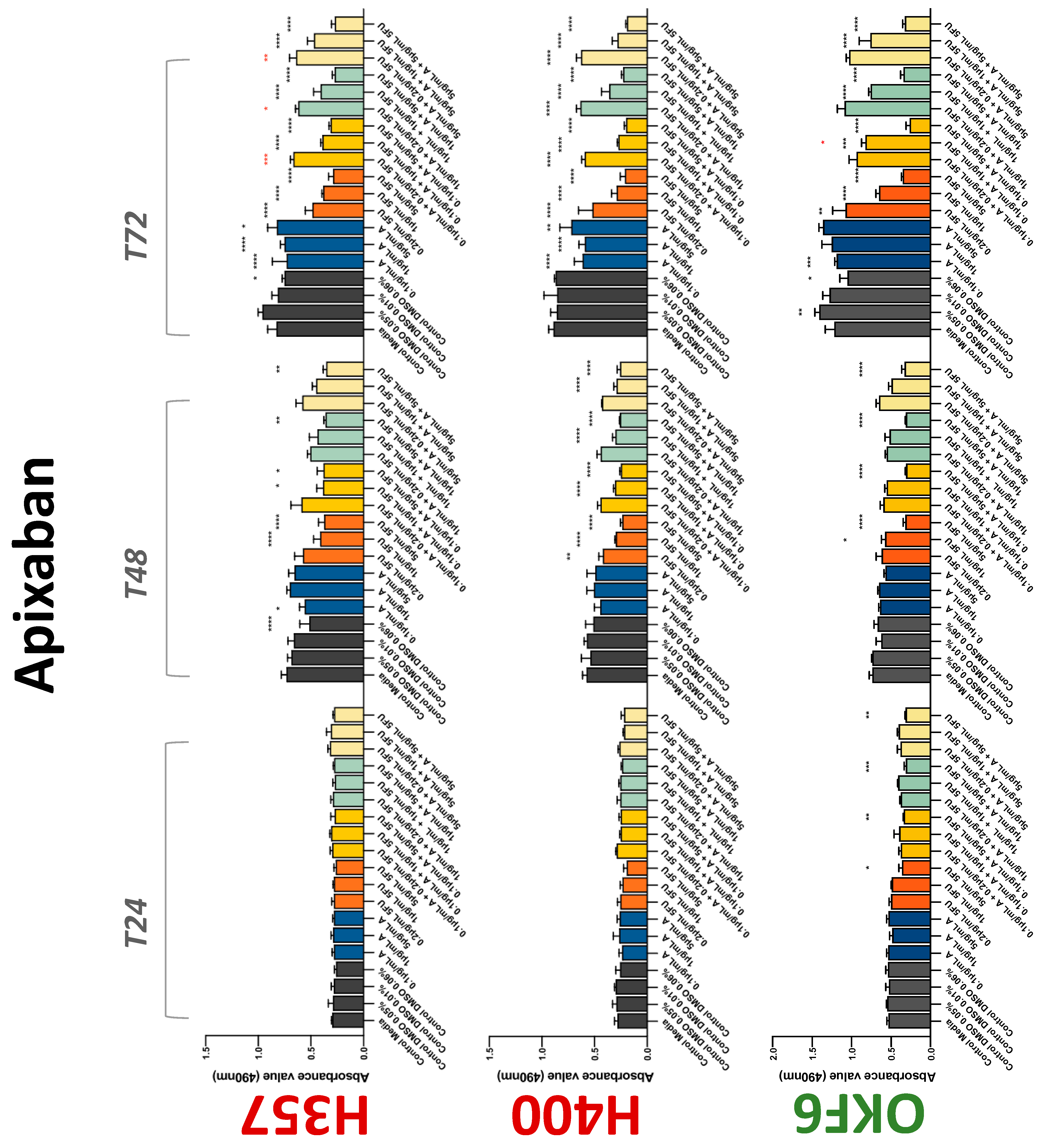
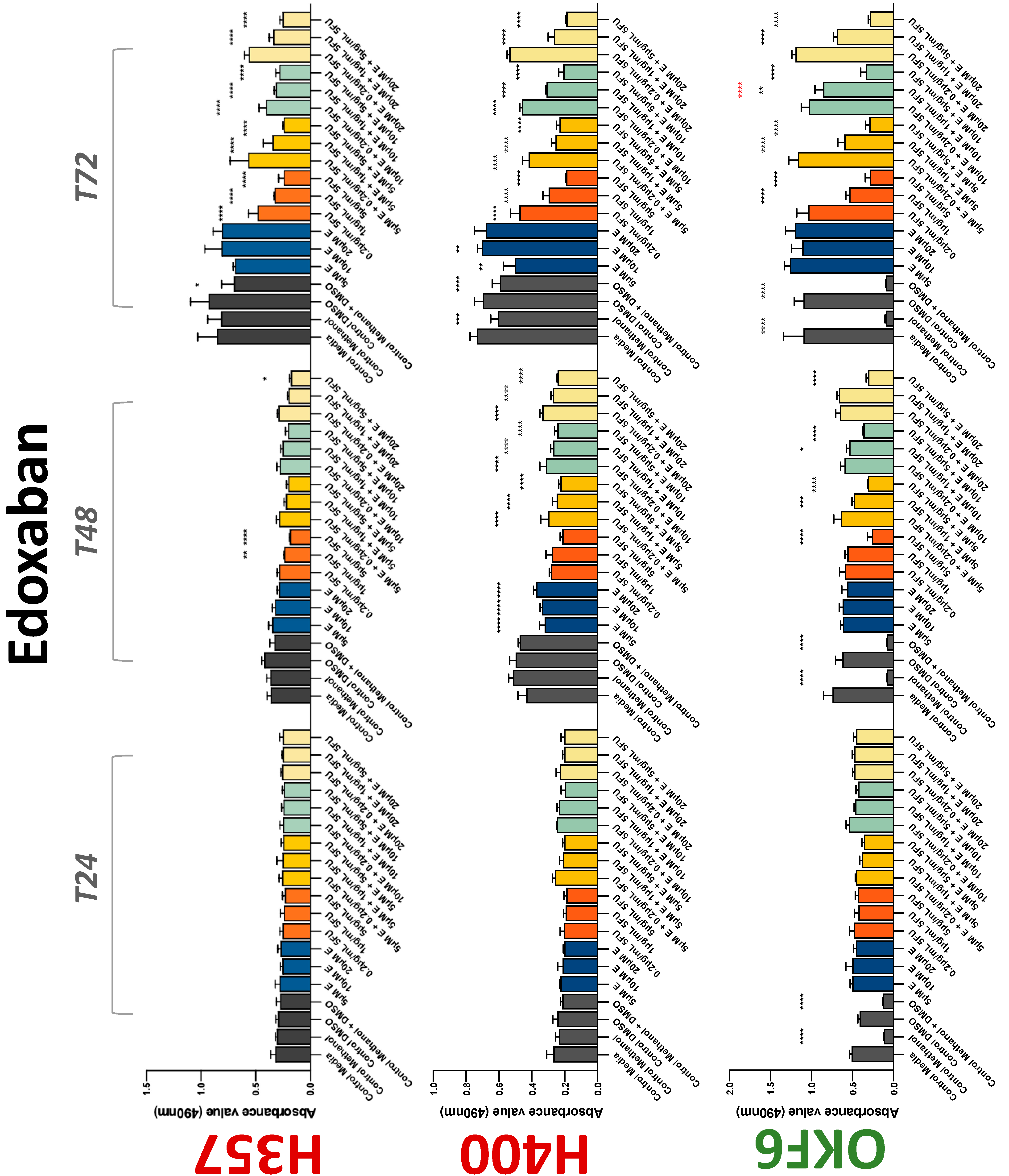
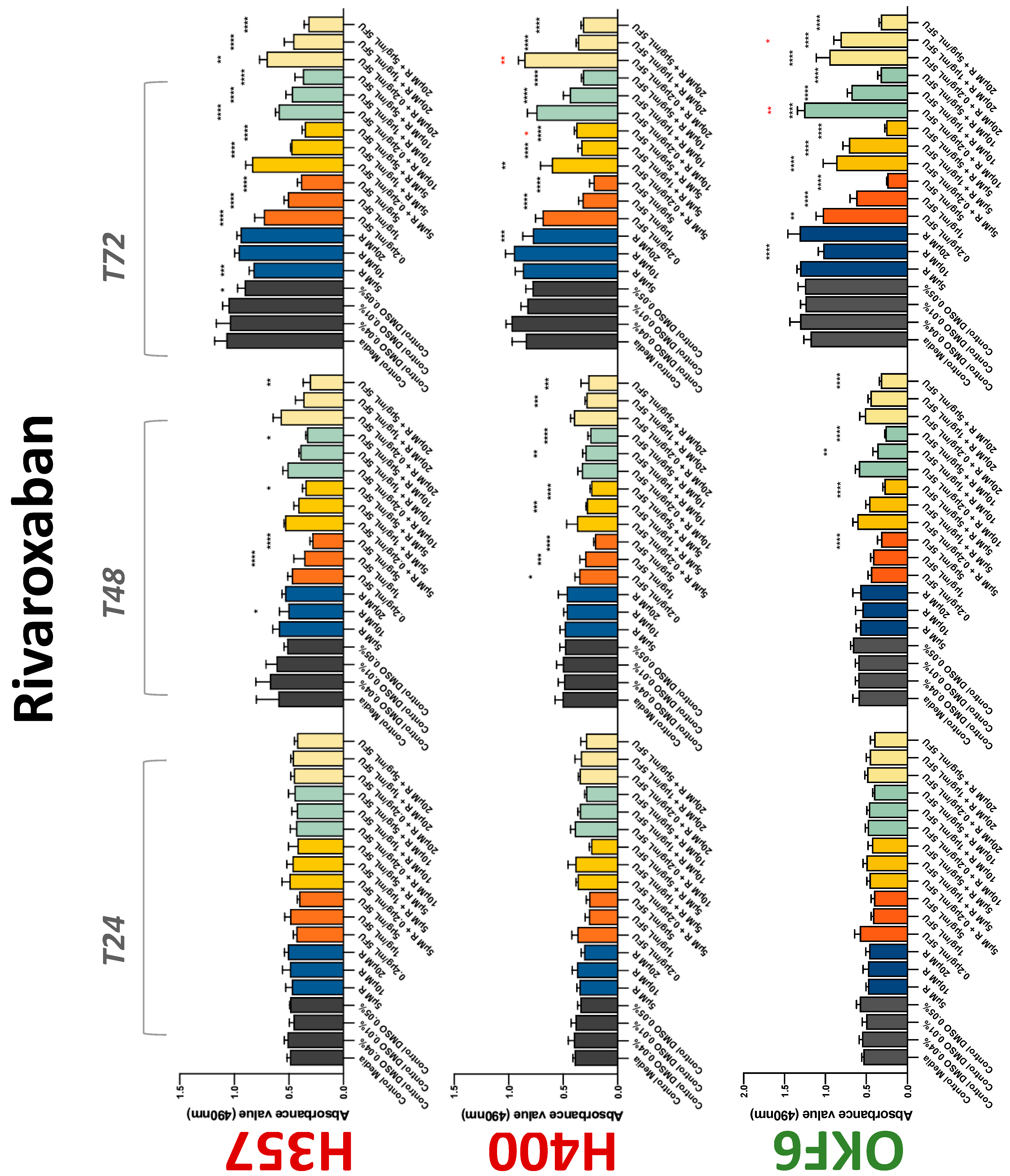
3.2. Anticoagulants Affect OSCC Cell Ability to Resist Chemotherapeutic Agent 5-FU
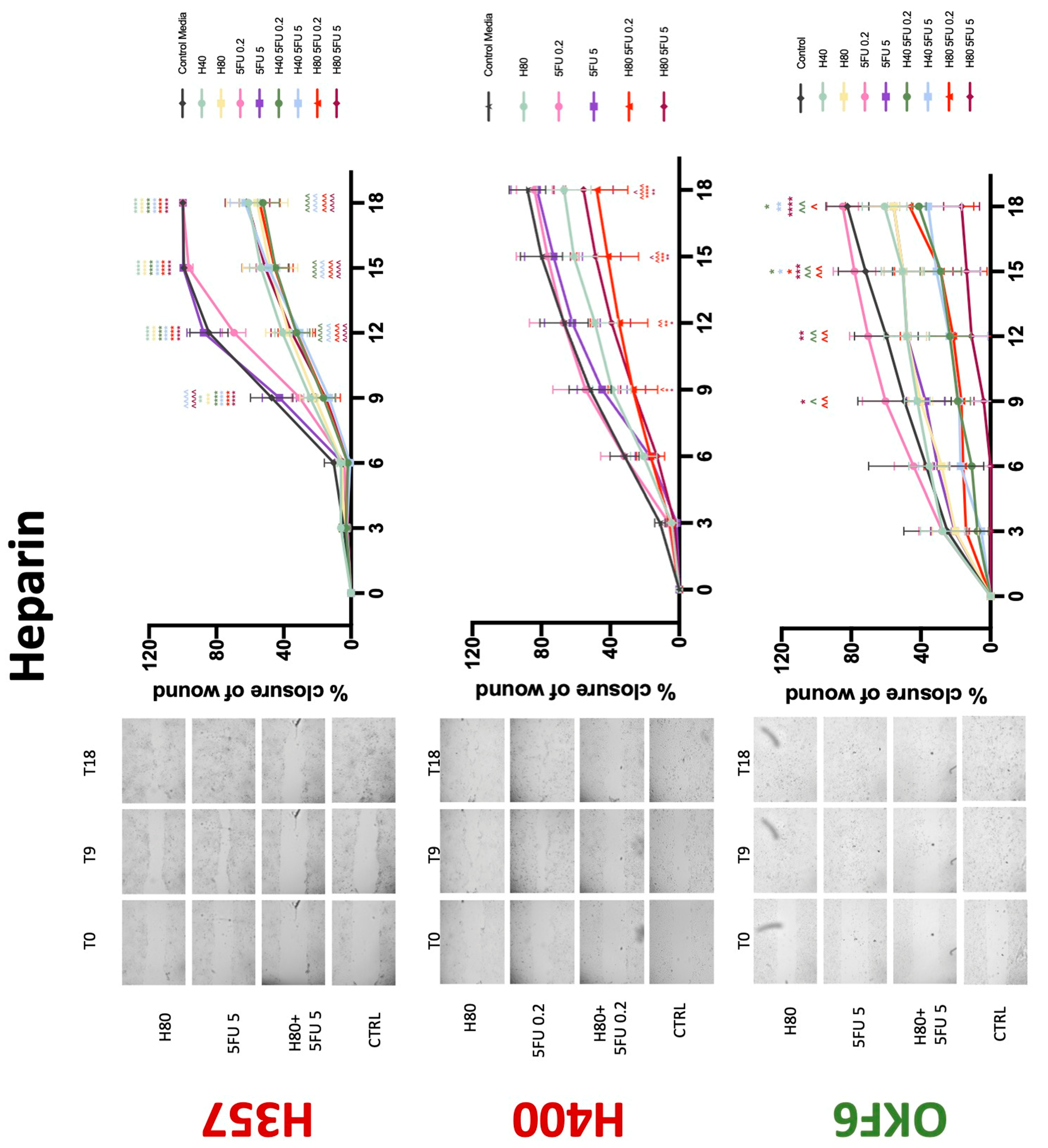
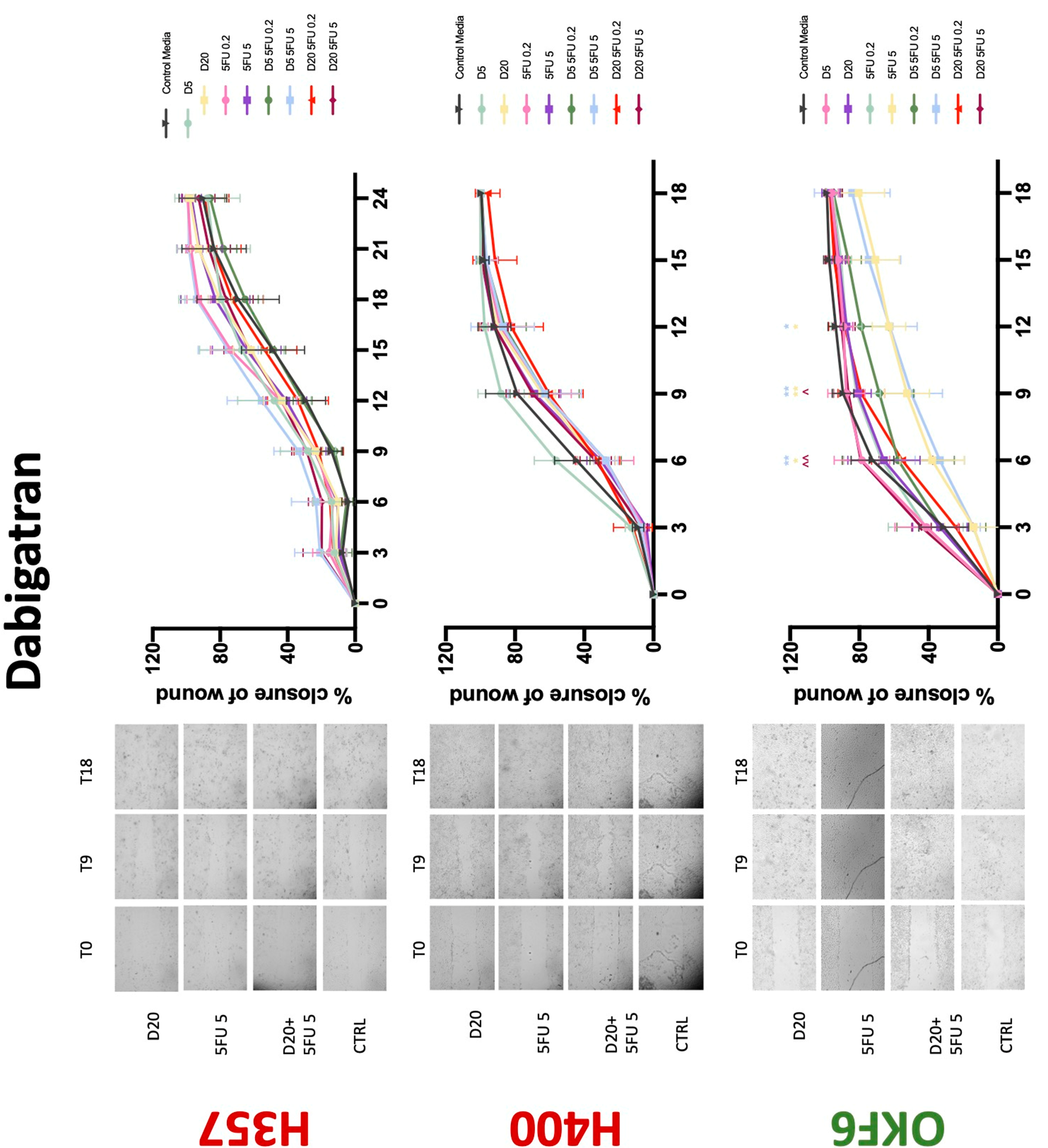
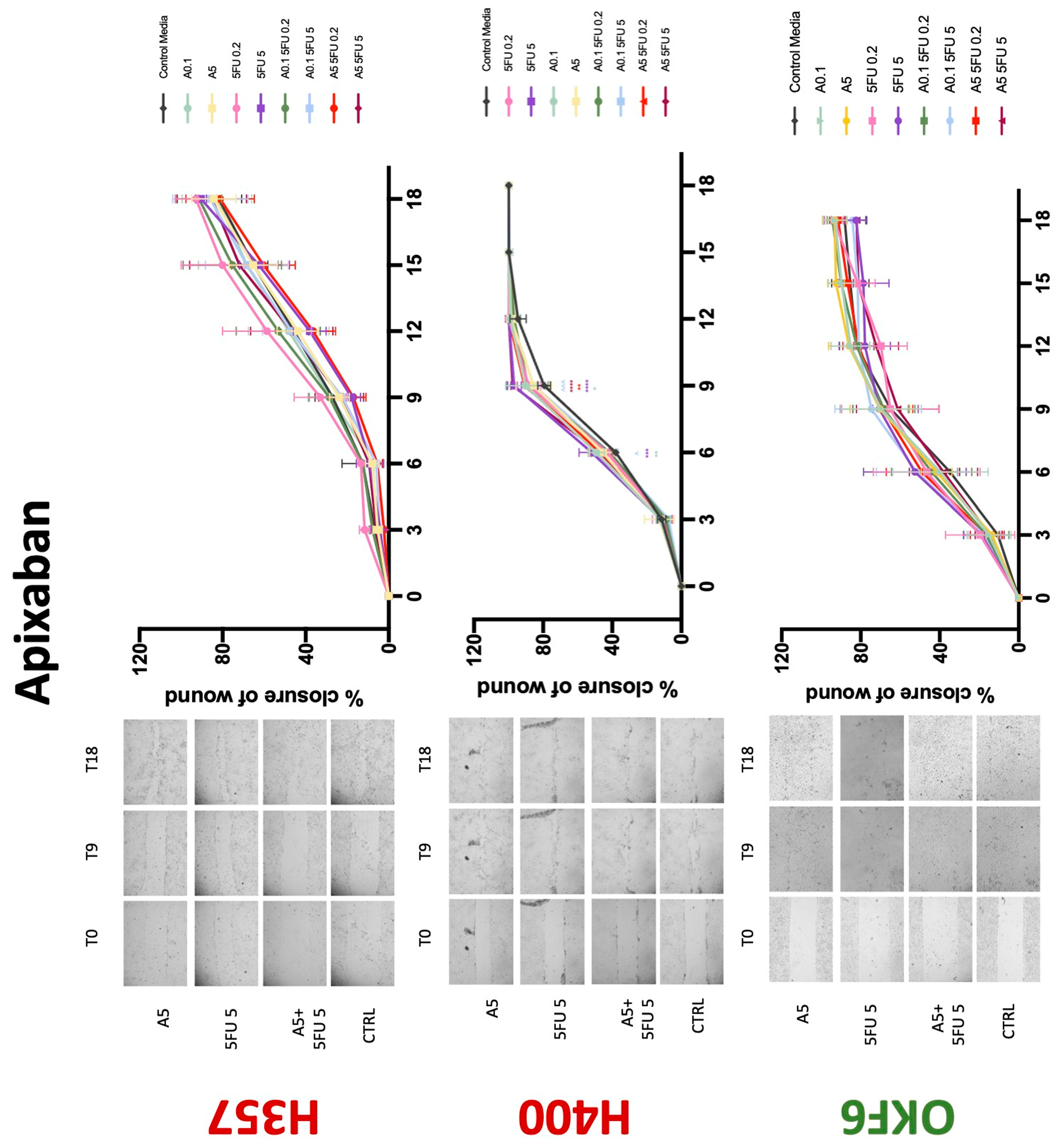
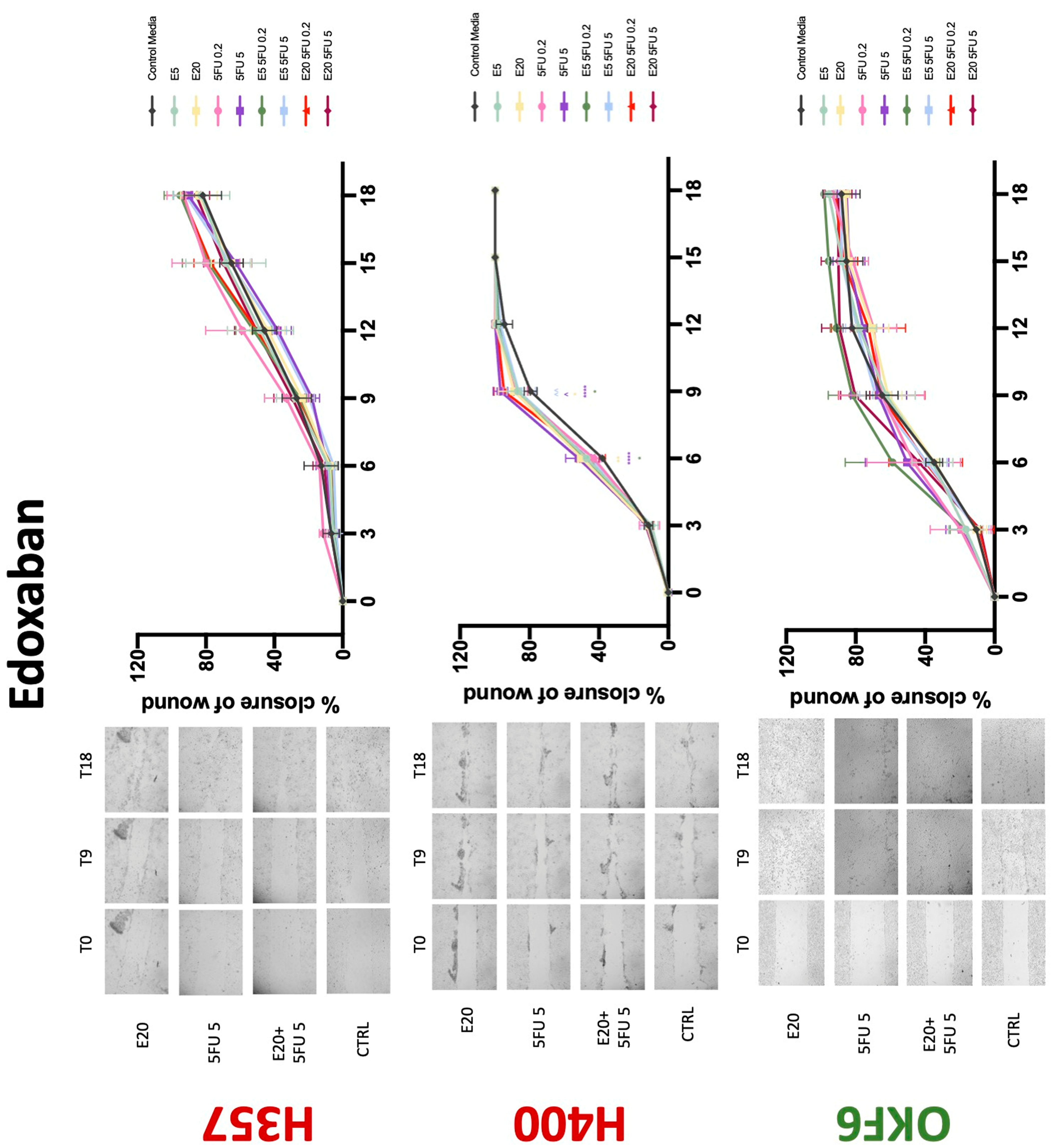
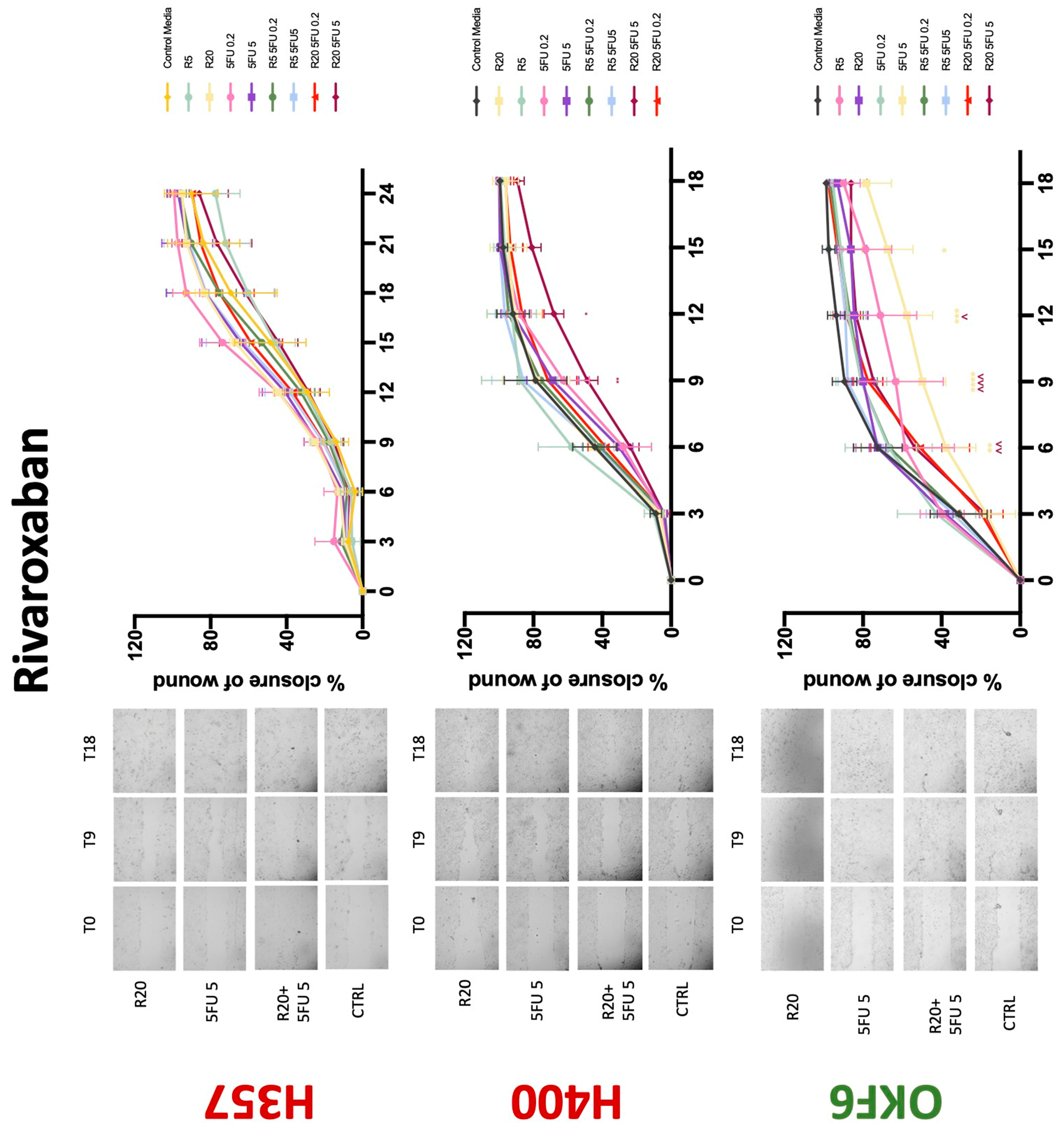
3.3. Treatment with Anticoagulants Induces a Reduction in OSCC Cell Migration
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar] [PubMed]
- Gaba, F.I.; Sheth, C.C.; Veses, V. Salivary biomarkers and their efficacies as diagnostic tools for Oral Squamous Cell Carcinoma: Systematic review and meta-analysis. J. Oral Pathol. Med. 2021, 50, 299–307. [Google Scholar] [CrossRef]
- Abdol Razak, N.B.; Jones, G.; Bhandari, M.; Berndt, M.C.; Metharom, P. Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers 2018, 10, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hejna, M.; Raderer, M.; Zielinski, C.C. Inhibition of metastases by anticoagulants. J. Natl. Cancer Inst. 1999, 91, 22–36. [Google Scholar] [CrossRef] [Green Version]
- Khan, Y.; Zaidi, S.O.; Razak, B.S.; Zaki, M.; Malik, B.H. Use of New Oral Anticoagulants/Direct Oral Anticoagulants in Malignant Patients. Cureus 2020, 12, e7007. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Ling, L.R.; Ng, M.; Matlub, L.; Mehta, K.; Linus, R.A.; Looker, M.J.; Melia, Y.; Loong, J.; Paolini, R.; et al. The effect of anticoagulants on oral squamous cell carcinoma: A systematic review. J. Oral Pathol. Med. 2021, 50, 118–121. [Google Scholar] [CrossRef]
- Ueda, K.; Inoue, S.; Zhang, Y.; Kutsuna, T.; Inoue, S.; Noto, K.; Arai, N.; Noguchi, M. Heparin induces apoptosis through suppression of AKt in oral squamous cell carcinoma cells. Anticancer Res. 2009, 29, 1079–1088. [Google Scholar]
- Prime, S.S.; Nixon, S.V.; Crane, I.J.; Stone, A.; Matthews, J.B.; Maitland, N.J.; Remnant, L.; Powell, S.K.; Game, S.M.; Scully, C. The behaviour of human oral squamous cell carcinoma in cell culture. J. Pathol. 1990, 160, 259–269. [Google Scholar] [CrossRef]
- Celentano, A.; Yap, T.; Paolini, R.; Yiannis, C.; Mirams, M.; Koo, K.; McCullough, M.; Cirillo, N. Inhibition of matrix metalloproteinase-2 modulates malignant behaviour of oral squamous cell carcinoma cells. J. Oral Pathol. Med. 2021, 50, 323–332. [Google Scholar] [CrossRef]
- Celentano, A.; McCullough, M.; Cirillo, N. Glucocorticoids reduce chemotherapeutic effectiveness onOSCC cells via glucose-dependent mechanisms. J. Cell Physiol. 2018, 234, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.F.; Fan, L.L.; Li, B.W.; Zhao, R.R.; Jiang, L.G.; Zhang, B.C.; Lu, Y.S.; Shao, J.W. A study to evaluate herb-drug interaction underlying mechanisms: An investigation of ginsenosides attenuating the effect of warfarin on cardiovascular diseases. Eur. J. Pharm. Sci. 2020, 142, 105100. [Google Scholar] [CrossRef] [PubMed]
- Kirane, A.; Ludwig, K.F.; Sorrelle, N.; Haaland, G.; Sandal, T.; Ranaweera, R.; Toombs, J.E.; Wang, M.; Dineen, S.P.; Micklem, D.; et al. Warfarin blocks Gas6-mediated Axl activation required for pancreatic cancer epithelial plasticity and metastasis. Cancer Res. 2015, 75, 3699–3705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, G.; Zhao, D.; Ran, X.; Zhang, L.; Zhao, D. Novel hybrids of podophyllotoxin and coumarin inhibit the growth and migration of human oral squamous carcinoma cells. Front. Chem. 2021, 8, 1214. [Google Scholar] [CrossRef]
- McCulloch, P.; George, W.D. Warfarin inhibits metastasis of Mtln3 rat mammary carcinoma without affecting primary tumour growth. Br. J. Cancer 1989, 59, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Ryan, J.J.; Ketcham, A.S.; Wexler, H. Warfarin treatment of mice bearing autochthonous tumors: Effect on spontaneous metastases. Science 1968, 162, 1493–1494. [Google Scholar] [CrossRef]
- Haaland, G.S.; Falk, R.S.; Straume, O.; Lorens, J.B. Association of warfarin use with lower overall cancer incidence among patients older than 50 years. JAMA Intern. Med. 2017, 177, 1774–1780. [Google Scholar] [CrossRef]
- Tagalakis, V.; Tamim, H.; Blostein, M.; Collet, J.P.; Hanley, J.A.; Kahn, S.R. Use of warfarin and risk of urogenital cancer: A population-based, nested case-control study. Lancet Oncol. 2007, 8, 395–402. [Google Scholar] [CrossRef]
- Tsou, W.I.; Nguyen, K.Q.; Calarese, D.A.; Garforth, S.J.; Antes, A.L.; Smirnov, S.V.; Almo, S.C.; Birge, R.B.; Kotenko, S.V. Receptor tyrosine kinases, TYRO3, AXL, and MER, demonstrate distinct patterns and complex regulation of ligand-induced activation. J. Biol. Chem. 2014, 289, 25750–25763. [Google Scholar] [CrossRef] [Green Version]
- Brand, T.M.; Iida, M.; Stein, A.P.; Corrigan, K.L.; Braverman, C.M.; Coan, J.P.; Pearson, H.E.; Bahrar, H.; Fowler, T.L.; Bednarz, B.P.; et al. AXL is a logical molecular target in head and neck squamous cell carcinoma. Clin. Cancer Res. 2015, 21, 2601–2612. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Yen, C.Y.; Liu, S.Y.; Chen, C.K.; Chiang, C.F.; Shiah, S.G.; Chen, P.H.; Shieh, Y.S. Axl is a prognostic marker in oral squamous cell carcinoma. Ann. Surg. Oncol. 2012, 19, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Paolino, M.; Choidas, A.; Wallner, S.; Pranjic, B.; Uribesalgo, I.; Loeser, S.; Jamieson, A.M.; Langdon, W.Y.; Ikeda, F.; Fededa, J.P.; et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature 2014, 507, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Alturkistani, A.; Ghonem, N.; Power-Charnitsky, V.A.; Pino-Figueroa, A.; Migliore, M.M. Inhibition of PAR-1 receptor signaling by enoxaparin reduces cell proliferation and migration in A549 cells. Anticancer Res. 2019, 39, 5297–5310. [Google Scholar] [CrossRef] [Green Version]
- Schlesinger, M.; Roblek, M.; Ortmann, K.; Naggi, A.; Torri, G.; Borsig, L.; Bendas, G. The role of VLA-4 binding for experimental melanoma metastasis and its inhibition by heparin. Thromb. Res. 2014, 133, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Featherby, S.; Xiao, Y.P.; Ettelaie, C.; Nikitenko, L.L.; Greenman, J.; Maraveyas, A. Low molecular weight heparin and direct oral anticoagulants influence tumour formation, growth, invasion and vascularisation by separate mechanisms. Sci. Rep. 2019, 9, 6272. [Google Scholar] [CrossRef] [PubMed]
- Mantziou, S.; Markopoulos, G.; Thrasyvoulou, S.; Noutsopoulos, D.; Gkartziou, F.; Artholomatos, G.; Tzavaras, T. Tinzaparin inhibits VL30 retrotransposition induced by oxidative stress and/or VEGF in HC11 mouse progenitor mammary cells: Association between inhibition of cancer stem cell proliferation and mammosphere disaggregation. Oncol. Rep. 2021, 46, 1–2. [Google Scholar] [CrossRef]
- Mousa, S.A.; Linhardt, R.; Francis, J.L.; Amirkhosravi, A. Anti-metastatic effect of a non-anticoagulant low-molecular-weight heparin versus the standard low-molecular-weight heparin, enoxaparin. Thromb. Haemost. 2006, 96, 816–821. [Google Scholar] [PubMed] [Green Version]
- Rousseau, A.; Van Dreden, P.; Mbemba, E.; Elalamy, I.; Larsen, A.; Gerotziafas, G.T. Cancer cells BXPC3 and MCF7 differentially reverse the inhibition of thrombin generation by apixaban, fondaparinux and enoxaparin. Thromb. Res. 2015, 136, 1273–1279. [Google Scholar] [CrossRef]
- Gerotziafas, G.T.; Galea, V.; Mbemba, E.; Sassi, M.; Roman, M.P.; Khaterchi, A.; van Dreden, P.; Japcowitz, M.; Lotz, J.P.; Bernaudin, J.F.; et al. Effect of low molecular weight heparins and fondaparinux upon thrombin generation triggered by human pancreatic cells BXPC3. Curr. Vasc. Pharmacol. 2014, 12, 893–902. [Google Scholar] [CrossRef]
- Abu Arab, W.; Kotb, R.; Sirois, M.; Rousseau, E. Concentration and time dependent effects of enoxaparin on human adenocarcinomicapithelial cell line A549 proliferation in vitro. Can. J. Physiol. Pharmacol. 2011, 89, 705–711. [Google Scholar] [CrossRef]
- Seeholzer, N.; Thürlimann, B.; Köberle, D.; Dagmar, H.; Korte, W. Combining chemotherapy and low-molecular-weight heparin for the treatment of advanced breast cancer: Results on clinical response, transforming growth factor-beta 1 and fibrin monomer in a phase II study. Blood Coagul. Fibrinolysis 2007, 18, 415–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balzarotti, M.; Fontana, F.; Marras, C.; Boiardi, A.; Croci, D.; Ciusani, E.; Salmaggi, A. In vitro of low molecular weight heparin effect on cell growth and cell invasion in primary cell cultures of high-grade gliomas. Oncol. Res. 2006, 16, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Alonso, F.; Gómez-Albentosa, T.; Oñate-Sánchez, R.E.; Tudela-Mulero, M.R.; Sánchez-Siles, M.; Gómez-García, F.J.; Guerrero-Sánchez, Y. In Vitro Study of Synergic Effect of Cisplatin and Low Molecular Weight Heparin on Oral Squamous Cell Carcinoma. Front. Oncol. 2020, 10, 5494122588. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Younes, M.; Swan, E.A.; Jasser, S.A.; Doan, D.; Yigitbasi, O.; McMurphey, A.; Ludwick, J.; El-Naggar, A.K.; Bucana, C.; et al. The Akt inhibitor KP372-1 inhibits proliferation and induces apoptosis and anoikis in squamous cell carcinoma of the head and neck. Oral Oncol. 2006, 42, 430–439. [Google Scholar] [CrossRef] [Green Version]
- Amornphimoltham, P.; Sriuranpong, V.; Patel, V.; Benavides, F.; Conti, C.J.; Sauk, J.; Sausville, E.A.; Molinolo, A.A.; Gutkind, J.S. Persistent activation of the Akt pathway in head and neck squamous cell carcinoma: A potential target for UCN-01. Clin. Cancer Res. 2004, 10, 4029–4037. [Google Scholar] [CrossRef] [Green Version]
- Buijs, J.T.; Laghmani, E.H.; van den Akker, R.F.; Tieken, C.; Vletter, E.M.; van der Molen, K.M.; Crooijmans, J.J.; Kroone, C.; Le Dévédec, S.E.; van der Pluijm, G.; et al. The direct oral anticoagulants rivaroxaban and dabigatran do not inhibit orthotopic growth and metastasis of human breast cancer in mice. J. Thromb. Haemost. 2019, 17, 951–963. [Google Scholar] [CrossRef] [Green Version]
- Vianello, F.; Sambado, L.; Goss, A.; Fabris, F.; Prandoni, P. Dabigatran antagonizes growth, cell-cycle progression, migration, and endothelial tube formation induced by thrombin in breast and glioblastoma cell lines. Cancer Med. 2016, 5, 2886–2898. [Google Scholar] [CrossRef]
- DeFeo, K.; Hayes, C.; Chernick, M.; Van Ryn, J.; Gilmour, S.K. Use of dabigatran etexilate to reduce breast cancer progression. Cancer Biol. Ther. 2010, 10, 1001–1008. [Google Scholar] [CrossRef] [Green Version]
- Alexander, E.T.; Minton, A.R.; Hayes, C.S.; Goss, A.; Van Ryn, J.; Gilmour, S.K. Thrombin inhibition and cyclophosphamide synergistically block tumor progression and metastasis. Cancer Biol. Ther. 2015, 16, 1802–1811. [Google Scholar] [CrossRef] [Green Version]
- Shi, K.; Damhofer, H.; Daalhuisen, J.; Ten Brink, M.; Richel, D.J.; Spek, C.A. Dabigatran potentiates gemcitabine-induced growth inhibition of pancreatic cancer in mice. Mol. Med. 2017, 23, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Maqsood, A.; Hisada, Y.; Garratt, K.B.; Homeister, J.; Mackman, N. Rivaroxaban does not affect growth of human pancreatic tumors in mice. J. Thromb. Haemost. 2019, 17, 2169–2173. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Wilgenbus, P.; Pagel, S.; Pott, J.; Marini, F.; Reyda, S.; Kitano, M.; Macher-Göppinger, S.; Weiler, H.; Ruf, W. Myeloid cell-synthesized coagulation factor X dampens antitumor immunity. Sci. Immunol. 2019, 4, eaaw8405. [Google Scholar] [CrossRef] [PubMed]
- Guasti, L.; Squizzato, A.; Moretto, P.; Vigetti, D.; Ageno, W.; Dentali, F.; Maresca, A.M.; Campiotti, L.; Grandi, A.M.; Passi, A. In vitro effects of Apixaban on 5 different cancer cell lines. PLoS ONE 2017, 12, e0185035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Versteeg, H.H.; Heemskerk, J.W.; Levi, M.; Reitsma, P.H. New fundamentals in hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nierodzik, M.L.; Karpatkin, S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell 2006, 10, 355–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Proliferation | Migration | 5-FU Effectiveness | |||||||
|---|---|---|---|---|---|---|---|---|---|
| H357 | H400 | OKF6 | H357 | H400 | OKF6 | H357 | H400 | OKF6 | |
| Warfarin | −(72) | +(48) | −(48) | 0 | − | 0/− | −(24) | 0 | −(72) |
| Heparin | −(48) | −(72) | 0 | − | 0/− | 0/− | 0 | −(72) | −(72) |
| Dabigatran | −(72) | −(72) | −(48) | 0 | 0 | + | 0 | −(72) | +(72) |
| Apixaban | −(72) | −(72) | −(72) | 0 | + | 0 | −(72) | 0 | −(72) |
| Edoxaban | 0 | +/−(72) | 0 | 0 | + | 0 | −(72) | −(48) | −(72) |
| Rivaroxaban | −(48) | −(72) | −(72) | 0 | 0/− | 0 | 0 | 0 | −(72) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, L.-Q.R.; Lin, Z.; Paolini, R.; Farah, C.S.; McCullough, M.; Lim, M.A.W.T.; Celentano, A. Commonly Prescribed Anticoagulants Exert Anticancer Effects in Oral Squamous Cell Carcinoma Cells In Vitro. Biology 2022, 11, 596. https://doi.org/10.3390/biology11040596
Ling L-QR, Lin Z, Paolini R, Farah CS, McCullough M, Lim MAWT, Celentano A. Commonly Prescribed Anticoagulants Exert Anticancer Effects in Oral Squamous Cell Carcinoma Cells In Vitro. Biology. 2022; 11(4):596. https://doi.org/10.3390/biology11040596
Chicago/Turabian StyleLing, Li-Qiao R., Zichen Lin, Rita Paolini, Camile S. Farah, Michael McCullough, Mathew A. W. T. Lim, and Antonio Celentano. 2022. "Commonly Prescribed Anticoagulants Exert Anticancer Effects in Oral Squamous Cell Carcinoma Cells In Vitro" Biology 11, no. 4: 596. https://doi.org/10.3390/biology11040596
APA StyleLing, L.-Q. R., Lin, Z., Paolini, R., Farah, C. S., McCullough, M., Lim, M. A. W. T., & Celentano, A. (2022). Commonly Prescribed Anticoagulants Exert Anticancer Effects in Oral Squamous Cell Carcinoma Cells In Vitro. Biology, 11(4), 596. https://doi.org/10.3390/biology11040596








