Modeling the Resistance Evolution to Insecticides Driven by Lepidopteran Species Competition in Cotton, Soybean, and Corn Crops
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioassays
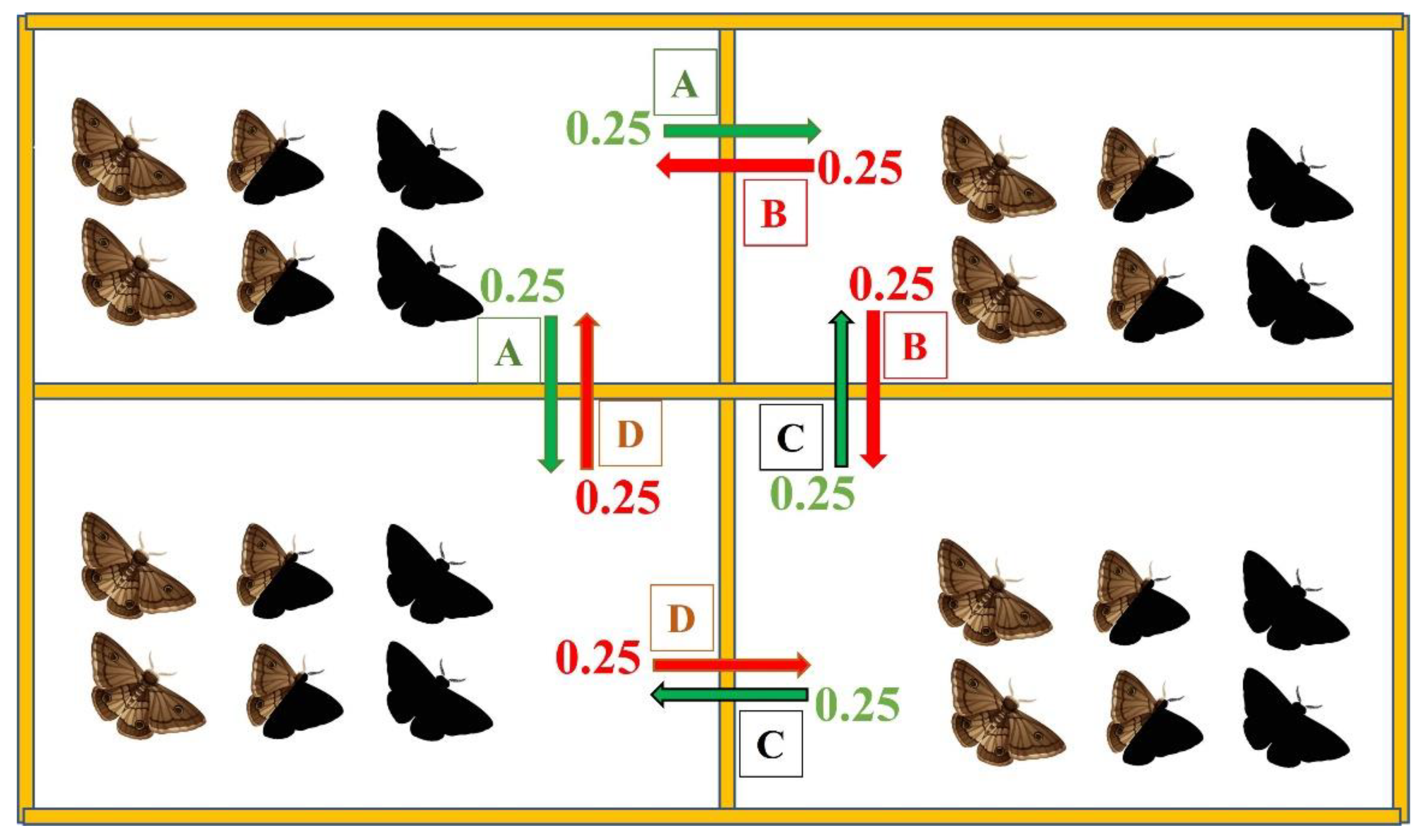
2.2. Computational Model
3. Results
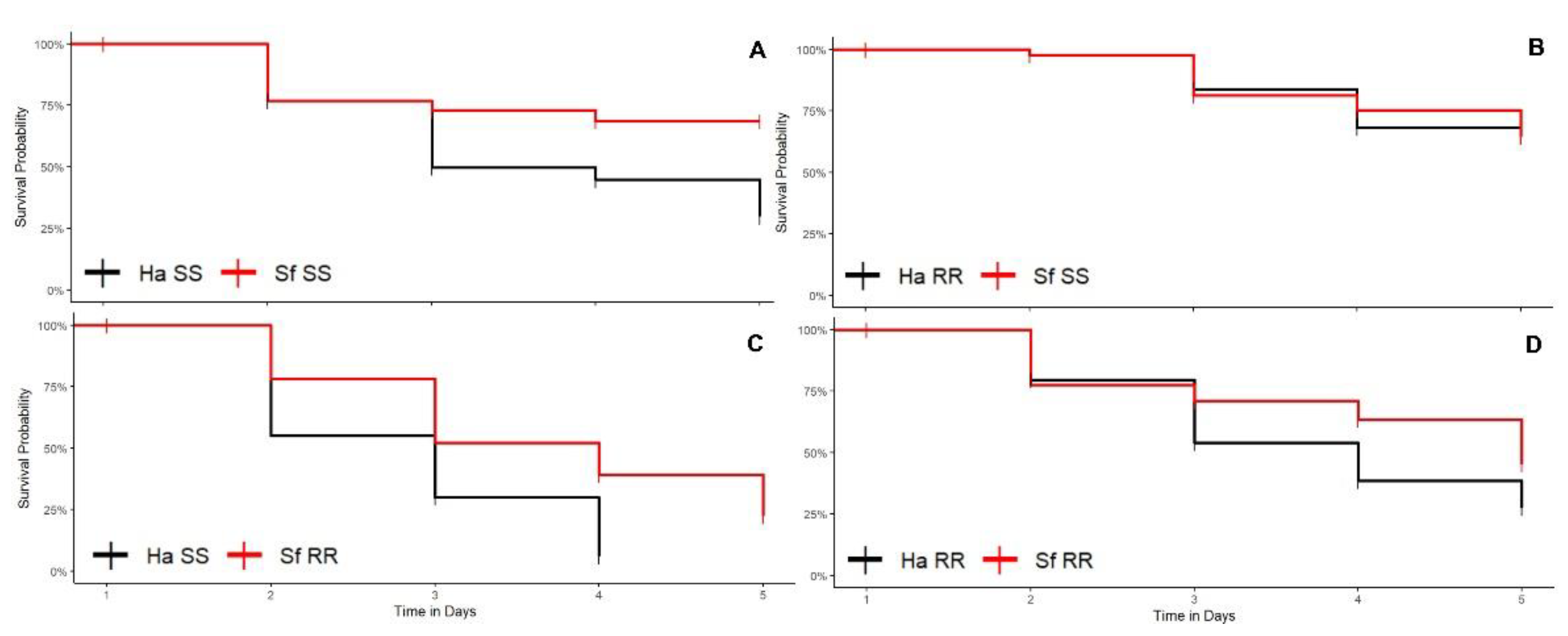
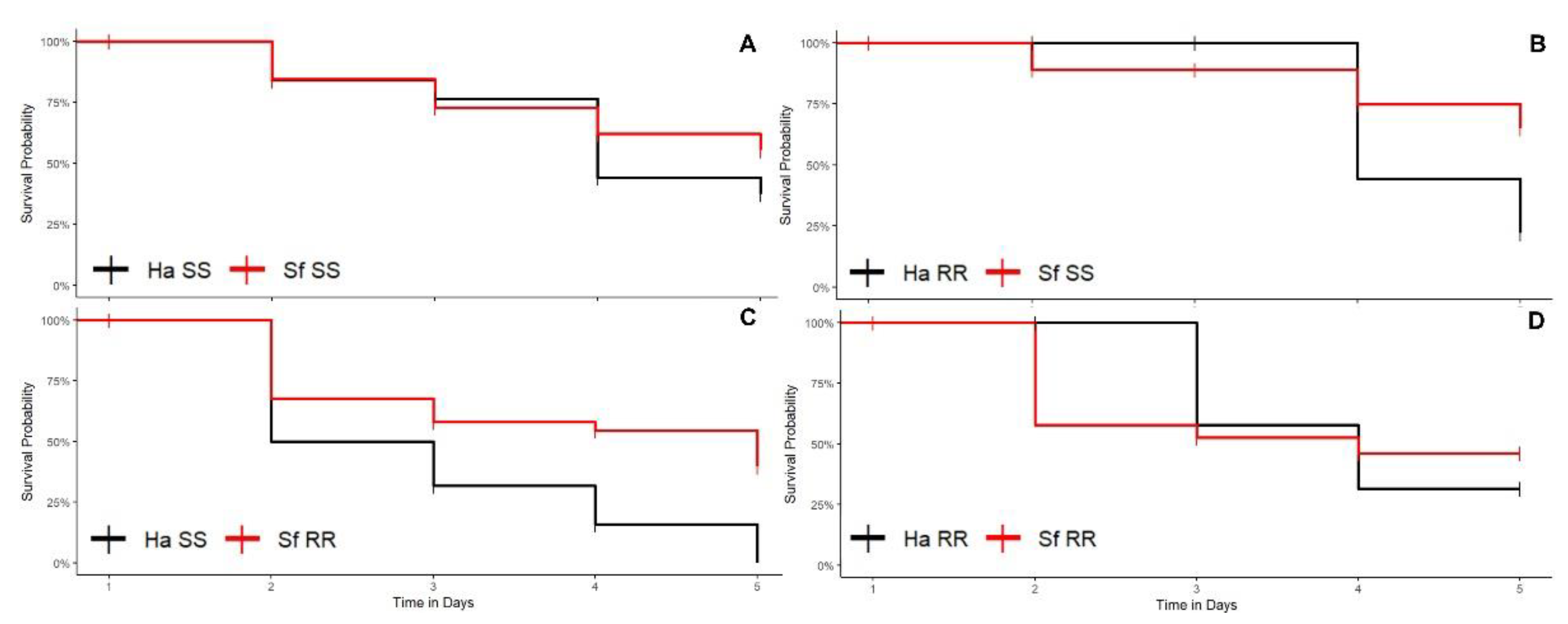
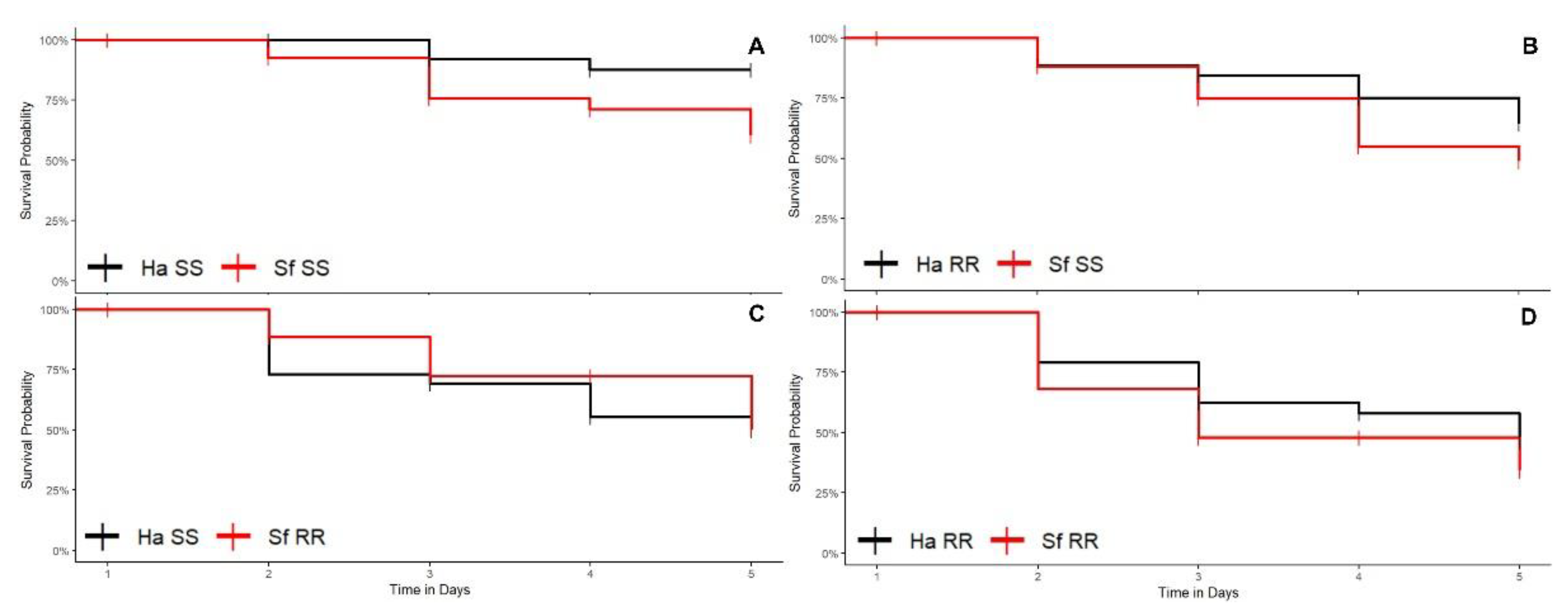
3.1. Bioassays
3.1.1. Cotton Plants
3.1.2. Corn Plants
3.1.3. Soybean Plants
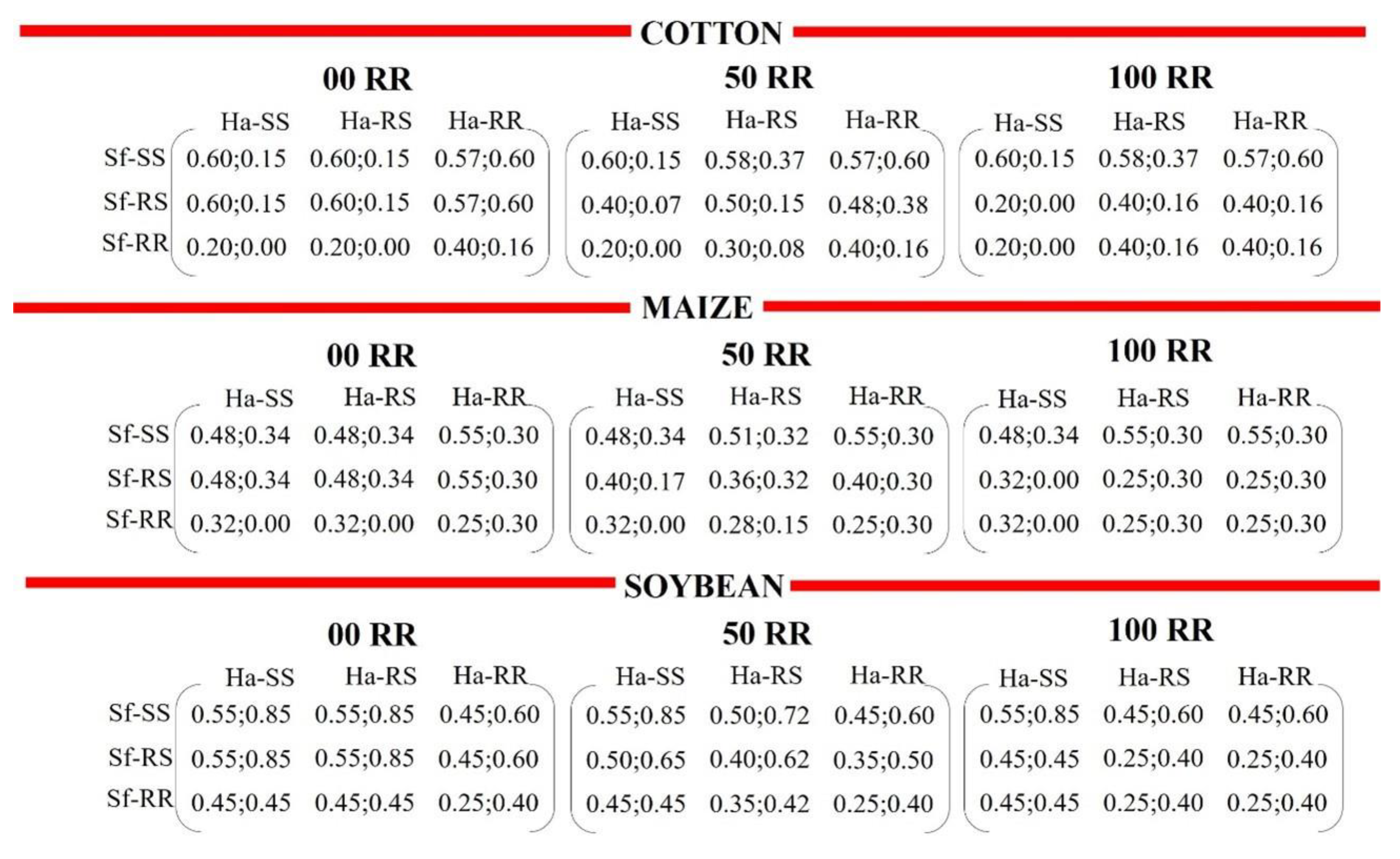
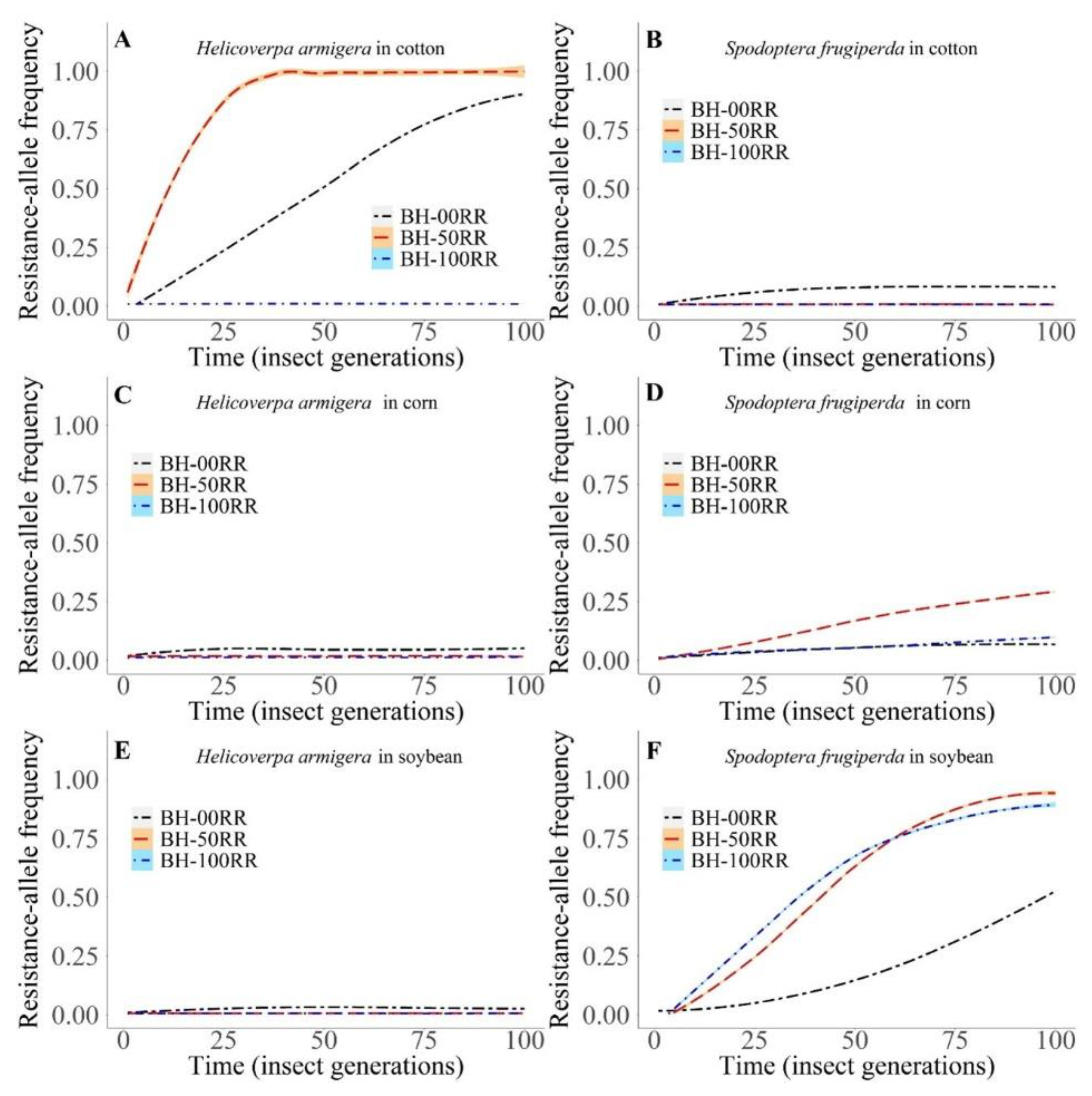
3.2. Computational Model
3.2.1. Intra- and Interspecific Interactions
3.2.2. Intraspecific Interactions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Townsend, C.R.; Begon, M.; Harper, J.L. Fundamentos em Ecologia; Artmed: Bucuresti, Romania, 2010; 576p. [Google Scholar]
- McNutt, D.W.; Underwood, N. Variation in plant-mediated intra-and interspecific interactions among insect herbivores: Effects of host genotype. Ecosphere 2016, 7, e01520. [Google Scholar] [CrossRef]
- Barros, E.M.; Torres, J.B.; Ruberson, J.R.; Oliveira, M.D. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomol. Exp. Appl. 2010, 137, 237–245. [Google Scholar] [CrossRef]
- Reigada, C.; Guimarães, K.F.; Parra, J.R.P. Relative fitness of Helicoverpa armigera (Lepidoptera: Noctuidae) on seven host plants: A perspective for IPM in Brazil. J. Ins. Sci. 2016, 16, 1–5. [Google Scholar] [CrossRef]
- Reigada, C.; Andrade Moral, R.; Demétrio, C.G.B.; Parra, J.R.P. Cross-crop effects on larval growth, survivorship and fecundity of Helicoverpa armigera. J. Pest. Sci. 2018, 91, 121–131. [Google Scholar] [CrossRef]
- Bolzan, A.; Padovez, F.E.O.; Nascimento, A.R.B.; Kaiser, I.S.; Lira, E.C.; Amaral, F.S.A.; Kanno, R.H.; Malaquias, J.B.; Omoto, C. Selection and characterization of the inheritance of resistance of Spodoptera frugiperda (Lepidoptera: Noctuidae) to chlorantraniliprole and cross-resistance to other diamide insecticides. Pest Manag. Sci. 2019, 75, 2682–2689. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.M.; Abbade-Neto, D.; Amado, D.; Durigan, M.R.; Franciscatti, R.A.; Mocheti, M.; Omoto, C. Baseline susceptibility and frequency of resistance to diamide insecticides in Helicoverpa armigera (Lepidoptera: Noctuidae) populations in Brazil. Crop Prot. 2020, 137, 105266. [Google Scholar] [CrossRef]
- Malaquias, J.B.; Santana, D.R.S.; Degrande, P.E.; Ferreira, C.P.; Melo, E.P.; Godoy, W.A.C.; Pachú, J.K.S.; Ramalho, F.S.; Omoto, C.; Pereira, A.I.A.; et al. Shifts in ecological dominance between two lepidopteran species in refuge areas of bt cotton. Insects 2021, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Malaquias, J.B.; Godoy, W.A.; Garcia, A.G.; Ramalho, F.D.S.; Omoto, C. Larval dispersal of Spodoptera frugiperda strains on Bt cotton: A model for understanding resistance evolution and consequences for its management. Sci. Rep. 2017, 7, 16109. [Google Scholar] [CrossRef]
- Malaquias, J.B.; Caprio, M.A.; Godoy, W.A.; Omoto, C.; Ramalho, F.S.; Pachú, J.K. Experimental and theoretical landscape influences on Spodoptera frugiperda movement and resistance evolution in contaminated refuge areas of Bt cotton. J. Pest Sci. 2020, 93, 329–340. [Google Scholar] [CrossRef]
- Malaquias, J.B.; Godoy, W.A.C.; Caprio, M.A.; Pachu, J.K.D.S.; Ramalho, F.S.; Omoto, C.; Ferreira, C.P. Evolutionary process modeling with Bayesian inference of Spodoptera frugiperda ballooning and walking dispersal in Bt and non-Bt cotton plant mixtures. Entomol. Exp. Appl. 2021, 169, 721–731. [Google Scholar] [CrossRef]
- Hackett, S.C.; Bonsall, M.B. Type of fitness cost influences the rate of evolution of resistance to transgenic Bt crops. J. Appl. Ecol. 2016, 53, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, K. Game Theory. In Encyclopedia of Animal Behavior; Choe, J.C., Ed.; Academic Press: New York, NY, USA, 2019; pp. 45–50. [Google Scholar]
- Turchin, P. Quantitative Analysis of Movement Measuring and Modeling Population Redistribution of Plants and Animals; Sinauer Associates: Sunderland, MA, USA, 1998; p. 390. [Google Scholar]
- Kassambara, A.; Kosinski, M.; Biecek, P. Survminer: Drawing Survival Curves Using ‘ggplot2’. R Package Version 0.4.9. 2021. Available online: https://CRAN.R-project.org/package=survminer (accessed on 2 October 2021).
- Thermae, T. A Package for Survival Analysis in R. R Package Version 3.2-13. 2021. Available online: https://CRAN.R-project.org/package=survival (accessed on 3 December 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 3 October 2021).
- Gomes, E.S.; Santos, V.; Ávila, C.J. Biology and fertility life table of Helicoverpa armigera (Lepidoptera: Noctuidae) in different hosts. Entomol. Sci. 2017, 20, 419–426. [Google Scholar] [CrossRef]
- Monteiro, G.F.; Macedo-Reis, L.E.; Dáttilo, W.; Fernandes, G.W.; Siqueira de Castro, F.; Neves, F.S. Ecological interactions among insect herbivores, ants and the host plant Baccharis dracunculifolia in a Brazilian mountain ecosystem. Austral. Ecol. 2020, 45, 158–167. [Google Scholar] [CrossRef]
- Dejean, A.; Grangier, J.; Leroy, C.; Orivel, J. Predation and aggressiveness in host plant protection: A generalization using ants from the genus Azteca. Naturwissenschaften 2009, 96, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Abbade Neto, D.O. Dinâmica da resistência an inseticidas diamidas em populações de Helicoverpa armigera (Lepidoptera: Noctuidae) do Brasil. Ph.D. Thesis, University of Sao Paulo, Sao Paulo, Brazil, 2021. [Google Scholar] [CrossRef]
- da Silva, D.M.; Bueno, A.F.; Andrade, K.; Stecca, C.S.; Neves, P.M.O.J.; Oliveira, M.C.N. Biology and nutrition of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on different food sources. Sci. Agric. 2017, 74, 18–31. [Google Scholar] [CrossRef]
- Vergara, F.; Shino, A.; Kikuchi, J. Cannibalism affects core metabolic processes in Helicoverpa armigera larvae—A 2D NMR metabolomics study. Int. J. Mol. Sci. 2016, 17, 1470. [Google Scholar] [CrossRef] [PubMed]
- Raffa, K.F. Effect of host plant on cannibalism rates by fall armyworm (Lepidoptera: Noctuidae). Environ. Entomol. 1987, 16, 672–675. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malaquias, J.B.; Ferreira, C.P.; Ramalho, F.d.S.; Godoy, W.A.C.; Pachú, J.K.S.; Omoto, C.; Neto, D.d.O.A.; Padovez, F.E.O.; Silva, L.B. Modeling the Resistance Evolution to Insecticides Driven by Lepidopteran Species Competition in Cotton, Soybean, and Corn Crops. Biology 2022, 11, 1354. https://doi.org/10.3390/biology11091354
Malaquias JB, Ferreira CP, Ramalho FdS, Godoy WAC, Pachú JKS, Omoto C, Neto DdOA, Padovez FEO, Silva LB. Modeling the Resistance Evolution to Insecticides Driven by Lepidopteran Species Competition in Cotton, Soybean, and Corn Crops. Biology. 2022; 11(9):1354. https://doi.org/10.3390/biology11091354
Chicago/Turabian StyleMalaquias, José B., Cláudia P. Ferreira, Francisco de S. Ramalho, Wesley A. C. Godoy, Jéssica K. S. Pachú, Celso Omoto, Dyrson de O. A. Neto, Fernando E. O. Padovez, and Luciana Barboza Silva. 2022. "Modeling the Resistance Evolution to Insecticides Driven by Lepidopteran Species Competition in Cotton, Soybean, and Corn Crops" Biology 11, no. 9: 1354. https://doi.org/10.3390/biology11091354
APA StyleMalaquias, J. B., Ferreira, C. P., Ramalho, F. d. S., Godoy, W. A. C., Pachú, J. K. S., Omoto, C., Neto, D. d. O. A., Padovez, F. E. O., & Silva, L. B. (2022). Modeling the Resistance Evolution to Insecticides Driven by Lepidopteran Species Competition in Cotton, Soybean, and Corn Crops. Biology, 11(9), 1354. https://doi.org/10.3390/biology11091354






