Simple Summary
Acinetobacter baumannii (A. baumannii) is one of the ESKAPE organisms and has the competency to build biofilms. These biofilms account for the most nosocomial infections all over the world. This review reflects on the various physicochemical and environmental factors such as adhesion, pili expression, growth surfaces, drug-resistant genes, and virulence factors that profoundly affect its resistant forte. Emerging drug-resistant issues and limitations to newer drugs are other factors affecting the hospital environment. Here, we discuss newer and alternative methods that can significantly enhance the susceptibility to Acinetobacter spp. Many new antibiotics are under trials, such as GSK-3342830, The Cefiderocol (S-649266), Fimsbactin, and similar. On the other hand, we can also see the impact of traditional medicine and the secondary metabolites of these natural products’ application in searching for new treatments. The field of nanoparticles has demonstrated effective antimicrobial actions and has exhibited encouraging results in the field of nanomedicine. The use of various phages such as vWUPSU and phage ISTD as an alternative treatment for its specificity and effectiveness is being investigated. Cathelicidins obtained synthetically or from natural sources can effectively produce antimicrobial activity in the micromolar range. Radioimmunotherapy and photodynamic therapy have boundless prospects if explored as a therapeutic antimicrobial strategy.
Abstract
Acinetobacter species is one of the most prevailing nosocomial pathogens with a potent ability to develop antimicrobial resistance. It commonly causes infections where there is a prolonged utilization of medical devices such as CSF shunts, catheters, endotracheal tubes, and similar. There are several strains of Acinetobacter (A) species (spp), among which the majority are pathogenic to humans, but A. baumannii are entirely resistant to several clinically available antibiotics. The crucial mechanism that renders them a multidrug-resistant strain is their potent ability to synthesize biofilms. Biofilms provide ample opportunity for the microorganisms to withstand the harsh environment and further cause chronic infections. Several studies have enumerated multiple physiological and virulence factors responsible for the production and maintenance of biofilms. To further enhance our understanding of this pathogen, in this review, we discuss its taxonomy, pathogenesis, current treatment options, global resistance rates, mechanisms of its resistance against various groups of antimicrobials, and future therapeutics.
1. Introduction
The superior capability of A. baumannii strains to produce biofilms correspondingly facilitates its colonization on surfaces, including medically useful instruments, indwelling catheters, and endotracheal tubes [1]. Biofilms are defined as an accumulated mixture of the microbial cells enclosed by an autogenic polymeric exopolysaccharide matrix which are produced on complex biotic or abiotic surfaces. Structurally, it forms a conglomerate system that defends microbial communities and renders an enhanced protective mechanism against several antimicrobial agents and host immune defense as well as harsh physiological parameters. Biofilm-forming bacteria are responsible for causing 65–80% of infections in humans (mainly chronic infections) [2]. Compared to other species, the rate of biofilm formation in A. baumannii is nearly 80–91%, whereas it is approximately 5–24% in the other species [3]. Furthermore, the biofilm formation has led to an increased resistance mechanism of these strains of bacteria against antimicrobial stressors and antibiotics. Thus, biofilm is represented as one of the potent virulence factors [4].
2. Factors Involved in Biofilm Formation
The process of biofilm production is dynamic [3]. Several studies have enumerated multiple factors responsible for the production and maintenance of biofilms. This includes physicochemical and microbial determinants such as the aggregation of substances, adhesion of collagen, expression of pili, capsular polysaccharides, and resistance determinants. Secretion of macromolecules, cell communication, and surface-regulated attachment are other essential factors in producing the biofilm. However, there are mainly three mechanisms of interaction that prime the establishment of biofilms—first, the interaction between the microbial cells. Second, bacterial adherence to the surface of human tissues or objects, and, finally, through serine lactones acylation, exchange of information occurs in the surrounding medium (Figure 1) [5]. The study performed by Maria-Guadalupe Avila-Novoa et al., reported that MDR A. baumannii strains exhibiting 100% resistance to several antibiotics in antimicrobial susceptibility tests have the potential to form biofilms in the clinical environment [6].
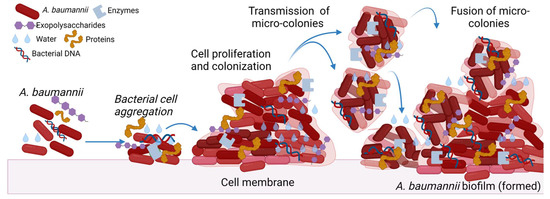
Figure 1.
A. baumannii biofilm formation. The figure was made with www.biorender.com (accessed on 25 July 2022).
Excessive production of the matrix formed by exo-polymeric substances, biological heterogeneity due to physicochemical changes and persisters, and variations in bacterial phenotypic and genotypic countenance due to aggressive reactions of microbial aggregation are the factors causing impaired drug diffusion and, eventually, amplifying drug resistance in the phenotype of biofilm. Consecutively, extracellular polysaccharides (EPS) form one and several microcolonies, which fuse with each other and with the liquid channels connected to create a mature biofilm eventually. The biofilm formation by A. baumannii isolates is usually associated with the upregulation of genes such as metal ions, plasmids, transposons, integrons, and outer membrane protein expression [7]. Adherence is the preliminary criteria for forming the biofilm, and Csu pili facilitate the formation of biofilms on any abiotic surface in A. baumannii [8].
2.1. Physiological Factors
Several environmental conditions such as temperature, availability of oxygen, pH, and surface hydrophobicity directly regulate the formation of biofilms. Any changes in such conditions facilitate the communication of cells with each other via a process of the cell-mediated density gradient, which in turn triggers the formation of biofilms with typical phenotypic and genotypic features.
Various studies have observed diverse environmental niches in promoting biofilm formation in A. baumannii strains. For example, a study conducted by Marti et al. reported that an Acinetobacter calcoaceticus—Acinetobacter baumannii complex (ACB complex) forms the biofilms at the (solid-liquid and air-liquid interphases) [9]. In their study, the other ACB complexes, A. baumaanii strains, produced the highest biofilm at the air-liquid interface. Similarly, Tomaras et al. showed that the pilus formation and usher chaperone mediate biofilm formation in A. baumannii cells on abiotic surfaces. Another study by Yassine et al. found that pellicle formation at the liquid-air interface leads to biofilm formation [10].
The temperature has also been widely reported to significantly alter the ability of the bacterial cells to mediate their attachment and generate biofilms on the surface. The study demonstrated by Marti et al. showed the elevated amount of biofilm formation by the Acinetobacter spp. at temperatures from 25 °C to 37 °C [9]. In another study, Pour N et al. showed that an optimum temperature of 30 °C with a constant pH of 7.0 supplemented with sodium chloride medium (at a concentration of 5 g/L−1) was the most favorable temperature for the formation of biofilm by A. baumannii isolates [10]. An increase in biofilm production was observed by Wei X et al. at an air-liquid interface which was mediated by isolates belonging to the ACB complex [11].
2.2. Type and Nutrient Availability
Nutrient availability and concentration have a profound effect on the accumulation of biofilms. A high concentration of nutrients negatively impacts biofilm formation due to the dissolution and minimum competition amongst the bacterial strains, which is otherwise necessary for the aggregation and forming biofilms. The frequency of biofilm formation is enhanced by the availability of low levels of nutrients. The growing proportion of biofilm is always doubled progressively in the availability of supplements, including sucrose, calcium, and phosphate. Excessive control of the nutrient supplement often results in the decreased production of exopolysaccharides and depletion in the level of biofilm production [11].
2.3. Growth Surface
The growth surface is one of the predisposing factors contributing to biofilm formation. The surface granularity and irregularities provide a shield to the bacteria by protecting it against the shear forces that allot turnaround time for the permanent attachment and hence promote biofilm production. The organic surfaces composed of different chemicals and nutrients facilitate the greater bacterial adherence. In electroactive microbes, biofilm production is influenced by factors including electrical changes on the surface and surface hydrophobicity [12].
2.4. Iron Concentration
The concentration of iron and sources of iron produces strain on the biofilm formation. Bap (biofilm-associated protein) is usually upregulated by limited iron availability. Increased iron concentration enables an increased hindrance to some selective antibiotics via signaling or by interacting with the antibiotics themselves. Most of the bacteria release potent iron-chelating substances known as sidephores to scavenge the surrounding iron available. Once iron is encountered by sidephores, it eventually forms an iron–sidephores complex. It binds to the specific outer membrane receptors, and this facilitates easy passage of molecules across the outer membrane. However, when the iron is available in higher concentrations, sidephores are insufficient to form complexes and cannot form passages in the outer membrane, thereby inhibiting the antibiotics from diffusing to the outer membrane. [13].
2.5. Expression of the Gene Involved in Biofilms
Some studies have demonstrated that the formation of biofilms is induced by biological signals and regulated by gene expression in a closed system. The genes reported to participate in the adherence and the generation of biofilm in A. baumannii strains are generally CsuC, CsuD, CsuE, OmpA, blaPER-1, abaI, epsA, bfin S&R, six genes of pilus synthetic system and gene sequence ST 25 and ST 78 identified by Multi-Locus Sequence typing [12]. The genes involved in biofilm development are summarized in Table 1. The expression of int I 1 mRNA is also increased markedly during the formation of biofilm by A. baumannii. Two genes of class I integron and 16S RNA methylase genes together are involved in gene movement and spread, which enhances the high expression of biofilm-related gene sequence [13]. One of the ribonuclease protein families T2 enables biofilm formation in Acinetobacter spp. as it promotes the adhesion and motility of A. baumannii.

Table 1.
Gene involvement in biofilm formations.
2.6. Virulence Factors Associated with Biofilms
There are multiple predisposing virulence factors that contribute to the formation of biofilms in the A. baumannii strain. It includes the outer membrane protein A (OmpA), biofilm-associated protein (Bap), chaperon-usher pilus assembly system of pili (Csu BABCDE), extracellular exopolysaccharide (EPS), two-component system (Bfm/S BfmR), poly-β-(1,6)-N-acetyl glucosamine (PNAG), PER-1 belonging to β-lactamase family, and the Quorum sensing system [21,22].
2.7. Outer Membrane Proteins
Acinetobacter species possess an outer membrane protein (OMP) that contributes to the pathogenicity and development of antibiotic resistance in an organism. The major factor that plays a key role in the bacterial pathogenesis of the bacteria is the presence of outer membrane proteins (OMPs) [23]. Porins act as an essential factor in microbial virulence. The bacteria can easily prevent the antimicrobial drugs from penetrating across the outer membrane channels with the help of porin. The most prominent porin in A. baumannii is OmpA, which acts by developing resistance to drugs, attaching to epithelial cells, and, thus, forming biofilms [24]. OmpA of A. baumannii (AbOmpA) is the most prominent surface protein with a molecular weight of 38 kDa that facilitates the transfer of small solutes [25]. It plays a significant role in displaying its function through attachment and attack in the epithelial cells with the help of fibronectin. These proteins also contribute to serum resistance, biofilm formation, persistence, induction of apoptosis, and development of antimicrobial resistance in A. baumannii [24]. OMPs enable cell membrane integrity and facilitate increased cell adhesion to the surfaces [26]. OMPs are essential for drug resistance and the production of biofilms as they modulate the formation of outer membrane vesicles [27]. OmpA causes apoptosis of eukaryotic cells. It progressively stimulates the dendrite cells, which eventually facilitates the differentiation of CD4T cells towards a Th1 phenotype, causing an immune escape [28].
2.8. Biofilm-Associated Protein
Bap (biofilm-associated protein) are proteins that are associated with the formation of biofilms. Bap is usually a higher molecular cell surface protein with a molecular weight of about (854-kDa) and 8620 amino acids residing on the surface of bacteria. The protein encoded by the Bap gene plays a significant role in the adhesion of intercellular cells, aggregation of bacterial cells, maturation, maintenance, and development of biofilm [29]. It also contains a core domain of tandem repeats, which flexibly provides bacteria with the tendency to produce a biofilm [30]. Loehfelm et al. demonstrated the presence of adhesion molecules of the bacterial Bap on the cellular surface of the Acinetobacter species for the first time in 2008. It was demonstrated as a highly conserved area, actively facilitating cellular adhesion and eventually leading to the maturation of biofilm in different substrata [31,32].
Acinetobacter spp. contains several different types of Bap proteins, all of which possess large central repetition regions. It is primarily composed of either 80–100 amino acids (aa) long repeat of Ig-like (alpha-type Bap or homopolymeric stretches of alternating amino acid (aa) residue (mainly the residues of serine and aspartic acid or serine and alanine) comprising nearly 500 dipeptides (β-types Bap). The alpha and beta-type Bap with repetitive region moves across the cell wall, mediating the exposure of the N-terminal region to the environment [33,34]. A study conducted by Brossard et al. revealed that the Bap is necessary for 3D tower structure and for aqueous channel formation on clinically important objects and areas, such as polypropylene, polystyrene, and titanium. The study also revealed the facilitation of enhanced hydrophobicity and adhesive property of the bacterial cellular surfaces by Bap protein [35]. Bap protein in A. baumannii is necessary for maintaining the stability of mature biofilms on glass surfaces, influencing both the biovolume and its thickness [36].
2.9. Chaperon-Usher Pilus Assembly System of Pili (Csu BABCDE)
Pili facilitate the adhesion and also generate the capacity to produce biofilm. The biofilm production on the abiotic surface by A. baumannii is mediated by Csu pili [37]. In A. baumannii, pilus-like bounded structures are formed from a clustered gene known as Csu operon. The Csu pilus is mainly composed of nearly four protein submits, Csu A/B, CsuA, CsuB, and CsuE, which function primarily through pathways known as archaic chaperone–usher (CU) pathways. The production of biofilms is based on the arrangement of the CsuA/B, CsuA, CsuB, CsuE, and CsuC-CsuD chaperone-usher secretion machinery, and the formation of pili is necessary for attachment to abiotic surfaces [38]. Among the entire CU system, archaic CU pili form a massive cluster of CU systems, and in combination with other substitutional CU families, it further forms a nonclassical branch of the CU superfamily [39]. Some relevant studies have demonstrated that the inactivation and deregulation of the CsuE gene abolish the formation of both pilus and biofilm. One of the prominent genes of the two-component system, namely, bfmSR, controls the expression of Csu operon. The bfmSR system mainly comprises the bfmS and bfmR gene [40]. BfmS gene is known as histidine sensor kinase that prominently identifies the environmental condition. BfmRs are the encoding genes that regulate the responses. A similar study conducted by Cerqueira GM related to a two-component system stated that the system termed GacSA displayed the regulated and controlled expression of Csu and indirectly impacted biofilm formation [41].
2.10. Extracellular Polysaccharides (EPS)
EPS play a leading role in the expansion and pathogenesis of biofilm. The main composition of EPS is alginate and antibiotics hydrolytic enzymes immobilized on biofilm, which functions by preventing the entry of antibacterial agents into the target and reducing the antibacterial activity [42]. EPS are mainly polysaccharides associated with a polypeptide chain that are negatively charged amino acid side chains that tend to attract the positively charged amino side chains. This force of attraction hinders the penetration of hydrophilic antibiotics into the cell bodies and considerably decreases the bactericidal ability. It is one of the most probable reasons why bacteria are not easily removed after biofilm formation. The multiresistant strain of A. baumannii has an O-glycosylation system and capsule synthesis with the major involvement of EPS [43].
2.11. Quorum Sensing (QS)
Quorum sensing (QS) is a series of events where microbes, mainly bacteria, exchange, sense, transport, and mediate active participation by releasing one or many chemical molecules of lesser molecular weight.
In A. baumannii, biofilm synthesis is controlled by the QS system caused by N-acyl-homoserine lactone (AHL) molecules which act as autoinducers. The information is mainly interchanged between bacteria through AHL molecules generated by a single bacterium which facilitates a huge accumulation of homologous bacteria. The massive number of intercellular adhesin (virulence factors) molecules in the polysaccharides are produced when the bacterial number reaches a critical level, and AHL becomes an effective sensing signal. When the population of bacteria reaches a certain level, biofilms are formed by embedding the microcolonies, and their production is facilitated by effective sensing of the signal by AHL [44]. However, Stacy et al. reported that with the help of the QS system, bacteria could possibly coordinate with each other as well as the different species to control their own behavior, and such interaction between different strains can lead to multiple infections [45]. The study by Liou et al. confirmed the involvement of another sensor in forming biofilms known as kinase BfmS. They demonstrated the significant reduction of biofilms in the absence of sensor kinase BfmS, which provided a new theory for further research on biofilm control [46]. The constrained iron supply enhances the expression of genes of the QS system that regulates the virulence factor based on the density of the bacterial species [47].
3. Current Antimicrobials for the Treatment of Acinetobacter Infections
The current treatment with antibiotics remains limited for Acinetobacter infections due to the increasing tendency to develop resistance to various antibiotics [48]. Generally, the non-baumannii species of Acinetobacter spp. are harmless to healthy individuals, but colonization led by A. baumannii can cause fatal infections in immunocompromised patients [49]. Therefore, the highly recommendable antibacterial agents for susceptible A. baumannii infections are mainly β-lactam antibiotics. Colistins, sulbactam coformulated with ampicillin, tigecycline, minocycline, carbapenems, piperacillin/tazobactam, cefepime, doxycycline, aminoglycosides, and quinolones are currently used as frontline antibiotics for the treatment of A. baumannii infections [50]. During Acinetobacter infections, the drugs generally used for the treatment are sulbactam, imipenem-cilastatin, meropenem, doripenem, amikacin tobramycin, colistin (colistimethate), polymyxin B, tigecycline, and minocycline. According to the CLSI guidelines 2022, the following lists of antimicrobial agents are used for Acinetobacter spp. infections (Figure 2) [51].
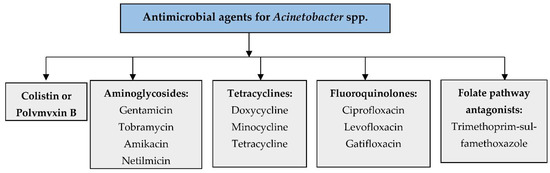
Figure 2.
Antimicrobial agents used for Acinetobacter spp. infections.
Combination Therapy
The formation of biofilms increases the resistance of Acinetobacter spp. to antibiotics. Thus, one of the possible and effective ways to treat biofilm-associated infections could be combination therapy. Due to the suboptimal pharmacokinetics of the drugs and rapid emergence of resistance, combination drug therapy is attracting frequent attention for the treatment of such infection. Recently, combination therapy and monotherapy (e.g., amikacin, minocycline or colistin, rifampicin) are also proving to be effective against A. baumannii infection. An excellent review study was made by Petrosillo et al. on colistin versus combination therapy. They demonstrated the relevance of only four clinical studies with respect to mortality and showed that only one study favored monotherapy, demonstrating statistical significance [52]. A prospective noncomparative study conducted by Bassetti et al. in severely sick patients with pneumonia and bacteremia demonstrated a colistin–rifampicin combination [53]. An in vitro antagonistic study was performed on biofilm-embedded A. baumannii strains to measure the strength and potency of antibiotics such as colistin, tigecycline, and levofloxacin solely or in amalgamation with clarithromycin and/or heparin as lock solutions. Biofilm-embedded A. baumannii strain showed bactericidal activity when treated with a combination of clarithromycin [54]. Another in vitro study to compare the efficacy of combined drugs, including colistin–levofloxacin, colistin–tigecycline, and tigecycline–levofloxacin-based catheter lock solutions, was performed [55]. The most potent antibacterial activity was displayed by colistin–levofloxacin, though the other drugs in combination also demonstrated bactericidal activity but at a lower level.
Song et al. evaluated the strength of imipenem and rifampicin solely and in combination against clinical isolates of A. baumannii in biofilm and planktonic culture. However, they observed that there was no significant reduction in the biofilm formation at the MIC of each of the antimicrobial agents of imipenem, colistin, and rifampicin when used individually. It was observed that imipenem, colistin, or rifampicin did not show any susceptibility against A. baumannii biofilms [56]. Compared to the positive control, tigecycline imipenem–rifampicin and colistin–rifampicin displayed a considerable reduction in biofilm synthesis after 48 h of incubation. They evaluated that combination therapy could be potent for controlling and reducing biofilm formed by A. baumannii strains by using antibiotics including tigecycline, imipenem–rifampicin, and colistin–rifampicin. For carbapenem-resistant and carbapenem-susceptible A. baumannii biofilms, the combination therapy of sulbactam–tigecycline was reported to be effective as an alternative treatment [56,57].
4. Future Therapies for the Treatment of Acinetobacter Infections
Numerous attempts have been made to develop alternative approaches with improved susceptibility to Acinetobacter spp. The future strategies which possibly could help to overcome the acquired resistance mechanism of antimicrobial agents are new antibiotics, natural products, nanoparticle technology, bacteriophage therapy, bactericidal gene transfer therapy, cathelicidins, radioimmunotherapy, and photodynamic therapy, as shown in Figure 3.
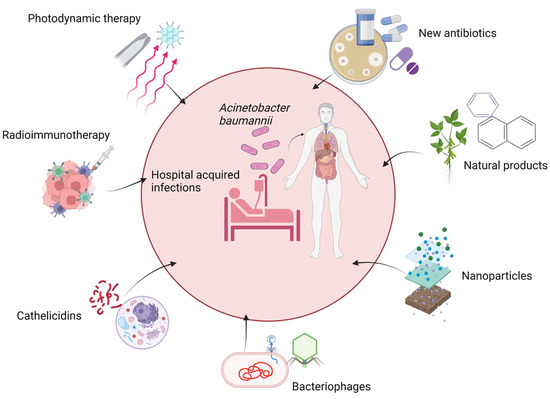
Figure 3.
Possible future therapies for A. baumannii infection. The figure was made with www.biorender.com (accessed on 25 July 2022).
4.1. New Antibiotics
Existing management choices for carbapenem-resistant A. baumannii (CRAB) are restricted and demonstrate many pharmacokinetic boundaries. Even with the last resort of drugs such as tigecycline or colistin, the drug-resistant A. baumannii progressively poses a severe hazard to public well-being worldwide. There is an urgent need for investments in new drugs.
Multiple companies are performing clinical trials to treat Acinetobacter spp effectively. Many of these are at the preclinical stage or at a very early stage of development. Under the siderophore cephalosporins, the GSK-3342830 is an injectable cephem antibacterial that targets Gram-negative bacteria developed by GlaxoSmithKline where considerable activity against CRAB was demonstrated [58]. The GT-1 action, contrary to CRAB in vitro and in animal study, has shown promising results as carried out by Geom Therapeutics [59]. Cefiderocol (S-649266), another novel cephalosporin conjugated with a catechol siderophore on its side chain, has revealed effective antibacterial activity against the carbapenem-resistant strains of A. baumannii with MIC50/90, 1–8 µg/mL [60]; however, it presented with less effective activity against the OXA-23 and OXA-40 [61]. Fimsbactin is a natural siderophore of A. baumannii; Ghosh M et al. [62] demonstrated in an in vivo study of mice that fimsbactin conjugate demonstrated good in vivo activity. Entasis Therapeutics has developed a drug that is a novel beta-lactamase inhibitor combined with sulbactam. It is a drug with a combination of Sulbactam and Durlobactam, which exhibited effective antimicrobial activity against clinical isolates of MDR strains of ABC complex [63].
Sulbactam-ETX2514, is a broad-spectrum diazabicyclooctanone (DBO) β-lactamase inhibitor. Hackel M et al. [64]’s study showed that it was active against 91% OXA carriers (MIC50/90, 1/4 µg/mL) and several colistin-resistant strains with an MIC of 2 µg/mL. Several trials are being conducted for investigating ETX2514 in combination with either sulbactam or imipenem-cilastatin, which have shown good results [65,66]. Another DBO β-lactamase inhibitor, the WCK 4234, was found to be effective against classes of carbapenemases as demonstrated in several studies [67,68]. The LN-1-255, was found to be active against class D-lactamases in vitro [69]. Under the new class of β-lactam antibiotics, FSI-1671 presented an efficient activity against CRAB [70]. Apramycin is an aminoglycoside. A study by Kang AD et al. [71] exhibited an MIC50/90 8/32 µg/mL; MIC range, 2 to 256 µg/mL against several A. baumannii isolates.
Spero Therapeutics has developed SPR741, which is a polymyxin-derived potential peptide that is combined with other antibiotics used against the other Gram-negative bacteria, including Acinetobacter. It is effective in reducing the infections caused by Enterobacteria, including Acinetobacter [66]. Melinta is developing a novel ribosomal protein synthesis inhibitor that specifically has an effective role in inhibiting Gram-negative activity against A. baumannii [72]. Tetraphase Pharmaceuticals is developing eravacycline, a novel fluorotetracycline that has expanded activity against Acinetobacter. Eravacycline has completed phase III clinical trials for complicated intra-abdominal infections (cIAI) and complicated urinary tract infections (cUTI); more trials will likely be required based on unexpectedly low cure rates for cUTI. This drug may provide an excellent treatment option for Acinetobacter infections [73]. A study by Seifert and colleagues [74] showed that eravacycline (TP-434), a flurocycline (tetracyclines), MICs were found to have a greater activity as compared to tigecycline MICs against CRAB. In several other studies, the isolates that produced carbapenemase genes demonstrated MIC50/90, 0.5/1 µg/mL [75,76,77]. Another novel drug TP-6076 has also displayed good results against clinical CRAB [78] by inhibiting bacterial protein synthesis. LpxC, another aminoglycoside, is a zinc-dependent deacetylase. The MIC50/90 values of a new LpxC inhibitor were 0.8/3.2 µg/mL (MIC range, 0.5 to 64 µg/mL) when tried on clinical A. baumannii isolates [79]. The RX-P873 also demonstrated effective activity against A. baumannii isolates with MIC50/90 values of 0.5/1 µg/mL (MIC range, 0.12 to 4 µg/mL) [80].
4.2. Natural Products
With the rise of drug resistance observed in the late 1970s, there was a dearth of drugs against various diseases caused by microorganisms. By the late 1990s, the effective and operative drug left to us was carbapenem, which also joined the drug resistance assemblage and made treatment challenging. Subsequently, having no innovative growth of drugs to counter the carbapenem-resistant strains, it has become necessary to emphasize the use of traditional medicine. The secondary metabolites mainly account for the antimicrobial activity of plants. There are several studies using plant extracts for evaluating the antimicrobial effect against drug-resistant pathogens. Some of the plants and their active compounds are listed in Table 2.

Table 2.
List of natural products used against A. baumannii.
Bulgecin A is a natural derivative of P. mesoacidophila. It acts as a lytic transglycosylase inhibitor and works synergistically with β-lactams. Bulgecin A could be used as an adjunctive compound to enhance the life of carbapenems against A. baumannii infections. It is accomplished by resisting the growth of carbapenem-resistant A. baumannii strains, thereby restoring the antimicrobial activity of meropenem. Likewise, farnesol is another natural product derived from Candida albicans applied for quorum-sensing, distorting the integrity of the cell membrane of A. baumannii, changing the cellular morphology, and, thereby, enhancing the susceptibility of MDR A. baumannii strains to colistin [75]. Several active compounds produced from herbs have strong antimicrobial activities against various bacteria, including carbapenem-resistant A. baumannii [90]. Similarly, oleanolic acid present in food, medicinal herbs, and several plants displays a potent antibacterial activity against many pathogenic bacteria as it contains a triterpenoid compound. In a study conducted by Shin et al., oleanolic acid enhanced aminoglycoside uptake by altering membrane permeability and energy metabolism in A. baumannii [91].
4.3. Nanoparticle Formulation
In nanotechnology, the nanoparticles of metals such as silver, gold, platinum, etc. have effective antimicrobial actions against microorganisms. These metals have demonstrated encouraging results in the field of nanomedicine and have exhibited both antibacterial and antifungal activities. This technology is novel, inexpensive, and easily approachable as it produces a topical class of antimicrobials. It has proved to be efficient for treating intricate cutaneous infections, including those caused by A. baumannii strains [92]. In another study, it was observed that attachment of silver nanoparticles to Acinetobacter was not only effective against several multidrug-resistant organisms but expressively lessened the biofilm activities of these drug-resistant organisms [93]. Nitric oxide (NO) has been demonstrated to display an effective antimicrobial activity as well as a potent immune modulator regulating wound healing. Friedman et al. [94] made a stable nitric oxide (NO)-releasing nanoparticle (NO-NPs) by using nanotechnology-based silane hydrogel. Mihu et al. [92] have demonstrated the efficacy of NO-NPs against A. baumannii using a murine wound and soft tissue model. In the study, NO-NP-treated mice showed considerable deductions in bacterial encumbrance in comparison to control animals, thereby increasing the rate of wound healing and reducing collagen degradation by bacterial collagenases. Helal et al. showed the action of silver nanoparticles (AgNPs) against A. baumannii isolates. They treated the bacteria with AgNPs, which substantially disrupted the bacterial growth and proliferation. The virulent and biofilm-related genes were downregulated by the AgNPs at the transcriptional level, which led to the interruption in the bacterial growth [95]. A previous study reported that the usage of silver nanoparticles inhibited the MDR A. baumannii and further prevented the colonization and formation of biofilm on the human lung epithelia with less toxicity [96]. Hemeg et al. published the concordant antibacterial effects of Ag, Au, and ZnO NPs against Gram-positive and Gram-negative bacteria. They deduced that NPs act by entering the bacterial cell membrane and interrupting the crucial molecular pathways, evading antimicrobial mechanisms. They also showed that NPs combined with several antibiotics, including polymyxin B, ciprofloxacin, ceftazidime, ampicillin, clindamycin, vancomycin, or erythromycin enhanced the antimicrobial effect and successfully worked against the MDR strains of bacteria including Acinetobacter [97]. Based on the study by Chen et al. [98], they determined that AgNPs can simultaneously induce apoptosis and inhibit new DNA synthesis in multidrug-resistant A. baumannii in a concentration-dependent manner using three different methods such as colony-forming unit (CFU) method, flow cytometry (FC), and a BrdU ELISA. (Biomedical). Another study by Banoub et al. [99] demonstrated the significant in vitro activities of chitosan nanoparticles either alone or in combination with various antibiotics against the MDR A. baumannii pathogens. Moreover, the use of chitosan is recommended as they are biodegradable polymers and are nontoxic. Wan et al. proved that upon taking AgNPs with antibiotics to treat carbapenem-resistant A. baumannii, there was complete inhibition of A. baumannii [100]. The AgNP treatment also presented synergistic effects with the antibiotics polymixin B, rifampicin, and tigecyline. Hence, based on the efficacy of the nanoparticles in various studies, we can include nanotechnology as an effective approach to combat the infections caused by biofilm-forming bacteria.
4.4. Bacteriophage and Bactericidal Gene Transfer Therapy
Currently, the research area revolving around antibacterial phage therapy has gained a considerable interest. To counteract the phenomenon of antibiotic resistance, bacteriophage therapy is being re-evaluated as a substitutional treatment because of its high specificity and efficient role. Bacteriophages are viruses that invade and kill target bacteria by lysis. These phages are specific for different bacteria. They are known to bind to the specific receptors present on the cell walls of the bacteria [101]. They introduce the deoxyribonucleic acid into the cell and, in that process, lyse the cell. Various studies have been conducted with lytic bacteriophage therapy to combat drug resistance issues [102,103]. Schooley et al. demonstrated that phages were used on MDR A. baumannii pancreatic pseudocyst infection, resulting in a cure of the infection and complete clinical recovery [104]. In fact, recently, a study conducted by Yang et al. showed the efficacy of virulent AB1 bacteriophage against A. baumannii by isolating and characterizing virulent AB1 bacteriophage representing it as a unique therapeutic with some potent efficacy [105]. The amalgamation of phage-vWUPSU, family Myoviridae and sacha inchi oil as antimicrobial agents suggestively repressed and detached biofilms, compared with the effects of either single treatment [106]. In recent years, the antibacterial and anti-biofilm activities of several phages targeting MDR A. baumannii have been characterized [107]. Some of the phages are listed as: FAB1- and phage Abp2-specific MDR A. baumannii [108], Phage ISTD [109], Phage IsfAB78 [110], and Phage B-R2096 [111]. Another possible future therapy includes-bactericidal gene transfer therapy. Bactericidal gene transfer therapy is the process of representation and incorporation of vectors possessing the genes of bacteria into pathogenic recipient organisms by the process of conjugation using attenuated donor cells. Despite the limitation in the therapeutic potential, the necessity of incorporation of donor cells to the pathogen (to facilitate vector transfer) resulted in murine burn infection models, which showed positive effects of bactericidal gene transfer. A similar approach was taken by Ebrahimi et al., who demonstrated the efficacy of bactericidal genes in mice treated with a single dose of 1010 CFU of donor cells. They reported lower levels of A. baumannii in burn wounds than in untreated mice [112].
4.5. Other Products
Gallium is used as one of the treatment strategies to combat the biofilms formation in Acinetobacter species. Gallium is a group 13 semimetallic element. It is the element that participates in iron-binding sites of the chelators and proteins. Gallium binds to biological complexes containing Fe3+ and significantly destroys a vital redox-driven biological process [113]. Gallium is generally used either in complex form with inorganic compounds or as a simple inorganic or organic salt. Studies have reported that gallium nitrate or gallium protoporphyrin IX can probably be a potent therapeutic choice for treating MDR A. baumannii [114,115]. The D-amino acids have verified that D-His and D-Cys interrupt the biofilm formation, adherence, and advance proliferation of eukaryotic cells in A. baumannii [116].
Probiotics can be utilized to protect the host from MDR A. baumannii pathogens. Probiotics are “protective live microorganisms which when administered sufficiently can provide a potent health benefit to the host” [117]. Asahara et al. demonstrated the potential of probiotic (Bifidobacterium breve) to provide protection against MDR A. baumannii infections in the intestine [118]. The use of probiotics and immunomodulators, such as lysophosphatidylcholine [119], can decrease the severity of infection caused by A. baumannii. In addition, macrolide antibiotics such as clarithromycin can be used in combination with other antibiotics such as colistin, tigecycline, or imipenem, which could potentially reduce infection [119].
4.6. Cathelicidins
Antimicrobial peptides (AMPs) are an evolutionarily conserved, heterogeneous group of short oligopeptides produced by the innate immune system and shown to have broad-spectrum bactericidal activity against pathogens, including viruses, bacteria, and parasites, serving as an integral part of the immune system’s first line of defense [120]. Multiple AMPs have been isolated from natural sources, and many others have been synthetically produced [121,122]. They demonstrate antimicrobial activity in the micromolar range, and, compared with traditional antibiotics, kill bacteria very rapidly [120,122]. Cathelicidins are the antimicrobial polypeptides (AMPs) identified from prokaryotic to eukaryotic kingdoms, including bacteria, fungi, plantae, and animalia composed of an N-terminal signal peptide (about 30 amino acids), a highly conserved cathelicidin domain (99–114 residues) between signal peptide and mature peptide and a C-terminal mature peptide (12–100 residues) with diverse structures (sequence and length) and functions [121,122]. In humans, the only cathelicidin studied is human LL-37; it displays both antitumor and anti-HIV activity. The cathelicidin providing effective results against Acinetobacter species is Tammar Wallaby cathelicidinWAM1, and the action of WAM1 against bacterial pathogens is 3–80 times more potent than LL-37. WAM1 can be used parenterally in humans with enhanced potential as it is nonhemolytic against human red blood cells. Indeed, for future in vivo studies, WAM1 can be considered one of the potential candidates due to its ability to tolerate high salt concentrations and antimicrobial activity [123].
As they have strong bactericidal properties without significant toxicity, several cathelicidin-derived peptide antibiotics have been tested in clinical trials. Some of the cathelicidins used for the treatment are given in Table 3:

Table 3.
Cathelicidins used for the treatment of A. baumannii.
4.7. Radioimmunotherapy
Clinically, though this method has not been explored as a therapeutic antimicrobial strategy, such as in cancer cells, radioimmunotherapy has much potential and can target microorganisms rapidly and powerfully [131]. The principle of the technique is based on the specificity of antigen–antibody interactions. Radionuclide are delivered and release a lethal dose of cytotoxic radiation directly to the target cell leading to the lysis of the targeted cell. Radioimmunotherapy has effectively opted for the treatment of bacterial, fungal, and viral infections. As in in vitro studies, it has produced only temporary hematological toxicity in experimental animals. Based on the production of antibodies against A. baumannii, radioimmunotherapy can be opted as a novel therapeutic strategy that could possibly be used to treat infections caused by A. baumannii [132].
4.8. Photodynamic Therapy
Photodynamic therapy is a process that successfully combines nontoxic photosensitizers (PSs) with oxygen. These PSs, in combination with oxygen, visibly generate a reactive oxygen species (ROS), which oxidizes biomolecules, thereby lysing the infected cells. The application of photodynamic therapy (PDT) involves the treatment of localized bacterial infections by its topical application into the infected tissue, followed by illumination with red (or near-infrared) light with the potential of penetrating the infected tissue [133]. The experimental study conducted on the murine burn wound model demonstrated the efficacy of this technique against A. baumannii, displaying no harmful effects on wound healing. Recently, Tsai et al. conducted a study to investigate the increasing efficacy of PDT against many resistant pathogens such as Acinetobacter by making use of polycationic biopolymer chitosan. The study displayed a bactericidal effect on a 2–4 log scale, the complete eradication of bacteria within 30 min in the subsequent chitosan treatment (0.025%) combined with hematoporphyrin-PDT (initial inoculation of 108 CFU/mL). The considerable antimicrobial activity was not displayed by chitosan alone without prior PDT; rather, the effective response of chitosan was caused due to induction by PDT [134].
5. Conclusions
A. baumannii, being an opportunistic nosocomial pathogen, gained advert importance due to its emerging multidrug-resistant characteristics caused by several virulence factors, including biofilm formation. It has been estimated that the formation of biofilm renders bacteria more resistant to antibiotics compared to other free-living cells [135,136,137,138]. The factors that initiate the formation of biofilm have been well-understood by the main interaction between the ambient environmental factors and the bacterial cells [138,139,140]. In hospital surroundings, mainly in ICUs, A. baumannii currently exists as a potent drug-resistant bacterium. Despite several comprehensive studies, the pathogenesis and toxicity of A. baumannii strains still remain vague. The extensive studies on antibiotics and combination therapy direct the necessity for profuse studies that ascertain the pharmacodynamics of antibiotics in monotherapy and combination therapy [141]. The multi-drug resistance capability of A. baumannii needs comprehensive research to understand its overall resistance mechanism. Newly developed antibiotics can be useful to manage multidrug-resistant A. baumannii [142]. The study primarily focuses on the emergence of biofilms, their process of formation, biomarkers, and specific determinants of their EPS matrix. Study is also required so that effective and potent antibiofilm drugs can be produced. Thus, more insightful research should be performed soon to anticipate the pathogenesis of A. baumannii and perform a thorough study on the formation of biofilm and its virulence determinants so that it can lay the cornerstone for the development of potent antibiotics.
Author Contributions
Conceptualization, K.G.D., V.N. and P.W.; methodology, K.G.D., R.K., A.K.P., B.K., C.G. and D.G.; validation, K.G.D., M.R., M.d.L.P., P.W., C.W., R.K.G. and V.N.; investigation, K.G.D., B.K., C.G., D.G., M.G., C.W. and V.N.; resources, K.G.D., P.W. and V.N.; data curation, K.G.D.; writing—original draft preparation, K.G.D., R.K., A.K.P., M.d.L.P., P.W., B.K., C.G. and D.G.; writing—review and editing, K.G.D., A.K.P., M.R., M.G., R.K.G., C.W. and V.N.; visualization, A.K.P. and R.K.; supervision, K.G.D., V.N. and P.W.; project administration, K.G.D.; funding acquisition, K.G.D. and P.W. All authors have read and agreed to the published version of the manuscript.
Funding
K.G.D. acknowledges the TMA Pai Major Grant 2018-19 of Sikkim Manipal Institute of Medical Sciences, Sikkim Manipal University, Sikkim, 737102, India. Thanks to Project CICECO-Aveiro Institute of Materials, UIDB/50011/2020, UIDP/50011/2020 and LA/P/0006/2020, financed by national funds through the FCT/MEC (PIDDAC).
Acknowledgments
K.G.D. would like to thank M.D. Venkatesh and Karma S. Sherpa, Sikkim Manipal University for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Duarte, A.; Ferreira, S.; Almeida, S.; Domingues, F.C. Clinical isolates of Acinetobacter baumannii from a Portuguese hospital: PFGE characterization, antibiotic susceptibility and biofilm-forming ability. Comp. Immunol. Microbiol. Infect. Dis. 2016, 45, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, J.A.; Tomaras, A.P.; Actis, L.A. Acinetobacter baumannii outer membrane protein A is involved in attachment to biotic and abiotic surfaces and killing of Candida albicans filaments. In Proceedings of the 108th American Society for Microbiology General Meeting, Boston, MA, USA, 1–5 June 2008. [Google Scholar]
- Abdi-Ali, A.; Hendiani, S.; Mohammadi, P.; Gharavi, S. Assessment of biofilm formation and resistance to imipenem and ciprofloxacin among clinical isolates of Acinetobacter baumannii in Tehran. Jundishapur J. Microbiol. 2014, 7, 8606. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, N.; Sharma, P.; Capalash, N. N-acyl homoserine lactone mediated interspecies interactions between A. baumannii and P. aeruginosa. Biofouling 2012, 28, 813–822. [Google Scholar] [CrossRef]
- Rumbo-Feal, S.; Gómez, M.J.; Gayoso, C.; Álvarez-Fraga, L.; Cabral, M.P.; Aransay, A.M.; Rodríguez-Ezpeleta, N.; Fullaondo, A.; Valle, J.; Tomás, M.; et al. Whole transcriptome analysis of Acinetobacter baumannii assessed by RNA-sequencing reveals different mRNA expression profiles in biofilm compared to planktonic cells. PLoS ONE 2013, 8, e72968. [Google Scholar] [CrossRef]
- Avila-Novoa, M.G.; Solís-Velázquez, O.A.; Rangel-López, D.E.; González-Gómez, J.P.; Guerrero-Medina, P.J.; Gutiérrez-Lomelí, M. Biofilm formation and detection of fluoroquinolone- and carbapenem-resistant genes in multidrug-resistan Acinetobacter baumannii. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 3454907. [Google Scholar] [CrossRef]
- Monem, S.; Furmanek-Blaszk, B.; Lupkowska, A.; Kuczynska-Wisnik, D.; Stojowska-Swedrzynska, K.; Laskowska, E. Mechanisms protecting Acinetobacter baumannii against multiple stresses triggered by the host immune response, antibiotics and outside-host environment. Int. J. Mol. Sci. 2020, 21, 5498. [Google Scholar] [CrossRef]
- Assaidi, A.; Ellouali, M.; Latrache, H.; Mabrouki, M.; Hamadi, F.; Timinouni, M.; Zahir, H.; El Mdaghri, N.; Barguigua, A.; Mliji, E.M. Effect of temperature and plumbing materials on biofilm formation by Legionella pneumophila serogroup 1 and 2–15. J. Adhes Sci. Technol. 2018, 13, 1–14. [Google Scholar]
- Martí, S.; Rodríguez-Baño, J.; Catel-Ferreira, M.; Jouenne, T.; Vila, J.; Seifert, H.; Dé, E. Biofilm formation at the solid-liquid and air-liquid interfaces by Acinetobacter species. BMC Res. Notes 2011, 4, 5. [Google Scholar] [CrossRef]
- Pour, N.K.; Dusane, D.H.; Dhakephalkar, P.K.; Zamin, F.R.; Zinjarde, S.S.; Chopade, B.A. Biofilm formation by Acinetobacter baumannii strains isolated from urinary tract infection and urinary catheters. FEMS Immunol. Med. Microbiol. 2011, 62, 328–338. [Google Scholar] [CrossRef]
- Wei, X.; Shen, D.; Luo, Y. Molecular mechanism of biofilm formation in Acinetobacter baumannii. Chin. J. Nosocomiol. 2010, 18, 2735–2738. [Google Scholar]
- Dong, R.; Guan, C.; Hu, D.; Xin, T.T.; Qu, Y. The correlation study on antimicrobial resistance and biofilm-related genes in clinical isolates of Acinetobacter baumannii. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2013, 25, 493–494. [Google Scholar] [PubMed]
- Jacobs, A.C.; Blanchard, C.E.; Catherman, S. Anribonuclease T2 family protein modulates Acinetobacter baumannii. Abiotic Surf. Colonization. 2014, 9, e85729. [Google Scholar]
- Wright, M.S.; Jacobs, M.R.; Bonomo, R.A.; Adams, M.D. Transcriptome remodeling of Acinetobacter baumannii during infection and treatment. MBio 2017, 8, e02193-16. [Google Scholar] [CrossRef] [PubMed]
- Rumbo, C.; Gato, E.; López, M.; Ruiz de Alegría, C.; Fernández-Cuenca, F.; Martínez-Martínez, L.; Vila, J.; Pachón, J.; Cisneros, J.M.; Rodríguez-Baño, J.; et al. The contribution of efflux pumps, porins and β-lactamases to multidrugresistance in clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother 2013, 57, 5247–5257. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.H.; Yarhui, N.B.; Nastro, M.; Quezada, T.N.; Cañarte, G.C.; Ventura, R.M.; Cuba, T.U.; Valenzuela, N.; Roach, F.; Mota, M.I.; et al. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii in South America. J. Med. Microbiol. 2016, 65, 1088–1091. [Google Scholar] [CrossRef]
- Badave, G.K. Biofilm producing multidrug resistant Acinetobacter baumannii: An emerging challenge. J. Clin. Diagn. Res. 2015, 9, DC08-10. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, Y.; Chen, F.; Xia, Y.; Lou, J.; Zhang, X.; Yang, N.; Sun, X.; Zhang, Q.; Zhuo, C.; et al. A novel signal transduction pathway that modulates rhl quorum sensing and bacterial virulence in Pseudomonas aeruginosa. PLOS Pathog. 2014, 10, e1004340. [Google Scholar] [CrossRef]
- Russo, T.A.; MacDonald, U.; Beanan, J.M.; Olson, R.; MacDonald, I.J.; Sauberan, S.L.; Luke, N.R.; Wayne Schultz, L.; Umland, T.C. Penicillin-binding protein 7/8 contributes to the survival of Acinetobacter baumanniiin in vitro and in vivo. J. Infect Immun. 2009, 199, 513–521. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, M.S.; Kim, S.; Son, J.H.; Kim, S.; Lee, Y.C.; Shin, M.; Oh, M.H.; Lee, J.C. The sensor kinase BfmS controls production of outer membrane vesicles in Acinetobacter baumannii. BMC Microbiol. 2019, 19, 301. [Google Scholar] [CrossRef]
- Aly, M.M.; Abu Alsoud, N.M.; Elrobh, M.S.; Al Johani, S.M.; Balkhy., H.H. High prevalence of the PER-1 gene among carbapenem-resistant Acinetobacter baumannii in Riyadh, Saudi Arabia. Eur. J. Clin. Microbiol. Infect Dis. 2016, 35, 1759–1766. [Google Scholar] [CrossRef]
- Ryu, S.Y.; Baek, W.K.; Kim, H.A. Association of biofilm production with colonization among clinical isolates of Acinetobater baumannii. Korean J. Intern. Med. 2017, 32, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Dahdouh, E.; Gómez-Gil, R.; Pacho, S.; Mingorance, J.; Daoud, Z.; Suárez, M. Clonality, virulence determinants, and profiles of resistance of clinical Acinetobacter baumannii isolates obtained from a Spanish hospital. PLoS ONE 2017, 12, e0176824. [Google Scholar] [CrossRef] [PubMed]
- Chapartegui-González, I.; lázaro-Díez, M.; Bravo, Z.; Navas, J.; Icardo, J.M.; Ramos-Vivas, J. Acinetobacter baumannii maintains its virulence after long-time starvation. PLoS ONE 2018, 13, e0201961. [Google Scholar] [CrossRef]
- Choi, C.H.; Lee, J.S.; Lee, Y.C.; Park, T.I.; Lee, J.C. Acinetobacter baumannii invades epithelial cells and outer membrane protein A mediates interactions with epithelial cells. BMC Microbiol. 2008, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Smani, Y.; Fàbrega, A.; Roca, I.; Sánchez-Encinales, V.; Vila, J.; Pachón, J. Role of OmpA in the multidrug resistance phenotype of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2014, 58, 1806–1808. [Google Scholar] [CrossRef]
- Gaddy, J.A.; Actis, L.A. Regulation of Acinetobacter baumannii biofilm formation. Futur. Microbiol. 2009, 4, 273–278. [Google Scholar] [CrossRef]
- Fattahian, Y.; Rasooli, I.; Gargari, S.L.M.; Rahbar, M.R.; Astaneh, S.D.A.; Amani, J. Protection against Acinetobacter baumannii infection via its functional deprivation of biofilm-associated protein (Bap). Microb. Pathog. 2011, 51, 402–406. [Google Scholar] [CrossRef]
- Ghasemi, E.; Ghalavand, Z.; Goudarzi, H.; Yeganeh, F.; Hashemi, A.; Dabiri, H.; Mirsamadi, E.S.; Foroumand, M. Phenotypic and genotypic investigation of biofilm formation in clinical and environmental isolates of Acinetobacter baumannii. Arch. Clin. Infect. Dis. 2018, 13, 12914. [Google Scholar] [CrossRef]
- Loehfelm, T.W.; Luke, N.R.; Campagnari, A.A. Identification and characterization of an Acinetobacter baumannii biofilm associated protein. J. Bacteriol. 2008, 190, 1036–1044. [Google Scholar] [CrossRef]
- Bodelon, G.; Palomino, C.; Fernandez, L.A. Immunoglobulin domains in Escherichia coli and other enterobacteria: From pathogenesis to applications in antibody technologies. FEMS Microbiol. Rev. 2013, 37, 204–250. [Google Scholar] [CrossRef]
- Hazenbos, W.L.W.; Kajihara, K.K.; Vandlen, R.; Morisaki, J.H.; Lehar, S.M.; Kwakkenbos, M.J.; Beaumont, T.; Bakker, A.Q.; Phung, Q.; Swem, L.R.; et al. Novel staphylococcal glyco-syltransferases SdgA and SdgB mediate immunogenicity and protection of virulence-associated cell wall proteins. PLoS Pathog. 2013, 9, e1003653. [Google Scholar] [CrossRef] [PubMed]
- Brossard, K.A.; Campagnari, A.A. The Acinetobacter baumannii biofilm-associated protein plays a role in adherence to human epithelial cells. Infect. Immun. 2012, 80, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Lasa, I.; Penadés, J.R. Bap: A family of surface proteins involved in biofilm formation. Res. Microbiol. 2006, 157, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Tomaras, A.P.; Dorsey, C.W.; Edelmann, R.E.; Actis, L.A. Attachment to and biofilm formation on abiotic surfaces by Acineto-bacter baumannii: Involvement of a novel chaperone-usher pili assembly system. Microbiology 2003, 149, 3473–3484. [Google Scholar] [CrossRef]
- Pakharukova, N.; Tuittila, M.; Paavilainen, S.; Malmi, H.; Parilova, O.; Teneberg, S.; Knight, S.D.; Zavialov, A.V. Structural basis for Acinetobacter baumannii biofilm formation. Proc. Natl. Acad. Sci. USA 2018, 115, 5558–5563. [Google Scholar] [CrossRef]
- Pakharukova, N.; Garnett, J.A.; Tuittila, M.; Paavilainen, S.; Diallo, M.; Xu, Y.; Matthews, S.J.; Zavialov, A.V. Structural insight into archaic and alternative chaperone usher pathways reveals a novel mechanism of pilus biogenesis. PLoS Pathog. 2015, 11, e1005269.8. [Google Scholar] [CrossRef]
- Nuccio, S.-P.; Baumler, A.J. Evolution of the chaperone/usher assembly pathway: Fimbrial classification goes greek. Microbiol. Mol. Biol. Rev. 2007, 71, 551–575. [Google Scholar] [CrossRef]
- Choudhury, D.; Thompson, A.; Stojanoff, V.; Langermann, S.; Pinkner, J.; Hultgren, S.J.; Knight, S.D. X-ray structure of the FimC-FimH chaperone-adhesin complex from uropathogenic Escherichia coli. Science 1999, 285, 1061–1066. [Google Scholar] [CrossRef]
- Morgan, D.J.; Liang, S.Y.; Smith, C.L.; Kristie Johnson, J.; Harris, A.D.; Furuno, J.P.; Thom, K.A.; Snyder, G.M.; Day, H.R.; Perencevich, E.N. Frequent multidrug-resistant Acinetobacter baumannii contamination of gloves, gowns, and hands of healthcare workers. Infect. Control. Hosp. Epidemiol. 2010, 31, 716–721. [Google Scholar] [CrossRef]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Genet. 2017, 16, 91–102. [Google Scholar] [CrossRef]
- Lewis, K. Multidrug tolerance of biofilms and persister cells. In Current Topics in Microbiology and Immunology; Romeo, T., Ed.; Springer: Berlin, Heidelberg, 2008; Volume 322, pp. 107–131. [Google Scholar] [CrossRef]
- Cuccui, J.; Wren, B.W. Bacteria like sharing their sweets. Mol. Microbiol. 2013, 89, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Bitrian, M.; Solari, C.M.; González, R.H.; Nudel, C.B. Identification of virulence markers in clinically relevant strains of Acineto-bacter genospecies. Int. Microbiol. 2012, 15, 79–88. [Google Scholar] [PubMed]
- Stacy, D.M.; Welsh, M.A.; Rather, P.N.; Blackwell, H.E. Attenuation of quorum sensing in the pathogen Acinetobacter baumannii using non-native N-Acyl homoserine lactones. ACS Chem. Biol. 2012, 7, 1719–1728. [Google Scholar] [CrossRef]
- Liou, M.L.; Soo, P.C.; Ling, S.R.; Kuo, H.Y.; Tang, C.Y.; Chang, K.C. The sensor kinase BfmS mediates virulence in Acinetobacter baumannii. J. Microbiol. Immunol. Infect. 2014, 47, 275–281. [Google Scholar] [CrossRef]
- Kim, I.H.; Wen, Y.; Son, J.S.; Lee, K.H.; Kim, K.S. The fur-iron complex modulates expression of the quorum-sensing master regulator, SmcR, to control expression of virulence factors in Vibrio vulnificus. Infect. Immun. 2013, 81, 2888–2898. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Maragakis, L.L.; Perl, T.M. Acinetobacter baumannii: Epidemiology, antimicrobial resistance, and treatment options. Clin. Infect. Dis. 2008, 46, 1254–1263. [Google Scholar] [CrossRef]
- Emmanuel, C.E.; Hafizah, Y.C.; Mohamed, E.Z. Acinetobacter baumannii biofilms: Effects of physico-chemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infect. Drug Resist. 2018, 11, 2277–2299. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI supplement M100 (ISBN 978-1-68440-134-5 [Print]; ISBN 978-1-68440-135-2 [Electronic]); Clinical and Laboratory Standards Institute: Wayne, PN, USA, 2022. [Google Scholar]
- Petrosillo, N.; Ioannidou, E.; Falagas, M. Colistin monotherapy vs. combination therapy: Evidence from microbiological, animal and clinical studies. Clin. Microbiol. Infect. 2008, 14, 816–827. [Google Scholar] [CrossRef]
- Bassetti, M.; Poulakou, G.; Giamarellou, H. Is there a future for tigecycline? Intensiv. Care Med. 2014, 40, 1039–1045. [Google Scholar] [CrossRef]
- Sader, H.S.; Castanheira, M.; Flamm, R.K.; Mendes, R.E.; Farrell, D.J.; Jones, R.N. Ceftazidime/avibactam tested against Gram-negative bacteria from intensive care unit (ICU) and non-ICU patients, including those with ventilator-associated pneumonia. Int. J. Antimicrob. Agents 2015, 46, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Dijkshoorn, L.; Aucken, H.; Gerner-Smidt, P.; Janssen, P.; Kaufmann, M.E.; Garaizar, J.; Ursing, J.; Pitt, T.L. Comparison of outbreak and non-outbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J. Clin. Microbiol. 1996, 34, 1519–1525. [Google Scholar] [CrossRef]
- Song, J.Y.; Cheong, H.J.; Noh, J.Y.; Kim, W.J. In vitro comparison of antibiofilm effects against carbapenem-resistant Acineto-bacter baumannii: Imipenem, colistin, tigecycline, rifampicin and combinations. Infect. Chemother. 2015, 47, 27–32. [Google Scholar] [CrossRef] [PubMed]
- McLeod, S.M.; Moussa, S.H.; Hackel, M.A.; Miller, A.A. In vitro activity of sulbactam-durlobactam against Acinetobacter baumannii- calcoaceticus complex isolates collected globally in 2016 and 2017. Antimicrob. Agents Chemother. 2020, 64, e02534-19. [Google Scholar] [CrossRef] [PubMed]
- Rhomberg, P.R.; Shortridge, D.; Huband, M.D.; Butler, D.; West, J.; Flamm, R.K. Multilaboratory broth microdilution MIC reproducibility study for GSK3342830, a novel catechol-cephem, abstr SATURDAY287. In Proceedings of the ASM Microbe, New Orleans, LA, USA, 1–5 June 2017; GlaxoSmithKline: Brentford, UK, 2017. [Google Scholar]
- Geom Therapeutics. Geom Therapeutics Announces Agreement with National Institute of Allergy and Infectious Diseases (NIAID) for Clinical Advancement of GT-1; Geom Therapeutics: San Francisco, CA, USA, 2017. [Google Scholar]
- Ito, A.; Sato, T.; Ota, M.; Takemura, M.; Nishikawa, T.; Toba, S.; Kohira, N.; Miyagawa, S.; Ishibashi, N.; Matsumoto, S.; et al. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against Gram-negative bacteria. Antimicrob. Agents Chemother. 2017, 23, e01454-17. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Kazmierczak, K.M.; Hackel, M.; Echols, R.; Yamano, Y.; Sahm, D.F. Cefiderocol (S-649266) susceptibility against globally isolated meropenem non-susceptible Gram-negative bacteria containing serine- and metal-lo-carbapenemase genes. abstr SUNDAY-25. In Proceedings of the ASM Microbe, New Orleans, LA, USA, 1–5 June 2017. [Google Scholar]
- Ghosh, M.; Miller, P.A.; Möllmann, U.; Claypool, W.D.; Schroeder, V.A.; Wolter, W.R.; Suckow, M.; Yu, H.; Li, S.; Huang, W.; et al. Targeted antibiotic delivery: Selective siderophore conjugation with daptomycin confers potent activity against multidrug resistant Acinetobacter baumannii both in Vitro and in vivo. J. Med. Chem. 2017, 60, 4577–4583. [Google Scholar] [CrossRef]
- Entasis Therapeutics. Evaluation of Safety and Efficacy of Intravenous Sulbactam-ETX2514 in the Treatment of Hospitalized Adults with Complicated Urinary Tract Infections. Accession no. NCT03445195. ClinicalTrials.gov, NIH. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03445195?termNCT03445195&rank1.LA (accessed on 17 August 2022).
- Hackel, M.; Bouchillon, S.; deJonge, B.; Lawrence, K.; Mueller, J.; Tommasi, R.; Miller, A. Global Surveillance of the Activity of Sulbactam Combined with the Novel-Lactamase Inhibitor ETX2514 against Clinical Isolates of Acinetobacter baumannii from 2014; Entasis Therapeutics: Waltham, MA, USA, 2017. [Google Scholar]
- Evaluation of the Pharmacokinetics, Safety, and Tolerability of Intravenous ETX2514 and Sulbactam Administered Concurrently to Subjects with Various Degrees of Renal Impairment and Healthy Matched Control Subjects. Accession no. NCT03310463. ClinicalTrials.gov, NIH. Available online: https://clinicaltrials.gov/ct2/show/NCT03310463term-NCT03310463&rank1 (accessed on 17 August 2022).
- Spero Therapeutics Unveils Data on Lead Potentiator Candidate for the Treatment of Multidrug-Resistant Gram-Negative Infections at ASM Microbe 2016; Spero Therapeutics: Cambridge, MA, USA, 2016.
- Papp-Wallace, K.M.; Nguyen, N.Q.; Jacobs, M.R.; Bethel, C.R.; Barnes, M.D.; Kumar, V.; Bajaksouzian, S.; Rudin, S.D.; Rather, P.N.; Bhavsar, S.; et al. Strategic approaches to overcome resistance against gram-negative pathogens using β-lactamase inhibitors and β-lactam enhancers: Activity of three novel diazabicyclooctanes WCK 5153, Zidebactam (WCK 5107), and WCK 4234. J. Med. Chem. 2018, 61, 4067–4086. [Google Scholar] [CrossRef]
- Castanheira, M.; Rhomberg, P.R.; Lindley, J.M.; Jones, R.N.; Sader, H.S. Activity of the new carbapenem/-lactamase inhibitor combination WCK 5999 against Gram-negative isolates producing oxacillinases (OXAs), abstr MON-DAY-422. In Proceedings of the ASM Microbe, Boston, MA, USA, 16–20 June 2016. [Google Scholar]
- Vázquez-Ucha, J.C.; Maneiro, M.; Martínez-Guitián, M.; Buynak, J.; Bethel, C.R.; Bonomo, R.A.; Bou, G.; Poza, M.; González-Bello, C.; Beceiro, A. Activity of the β-lactamase inhibitor LN-1-255 against carbapenem-hydrolyzing class D β-lactamases from Acinetobacter baumannii. Antimicrob. Agents Chemother. 2017, 61, e01172-17. [Google Scholar] [CrossRef]
- Joo, H.Y.; Kim, D.I.; Kowalik, E.; Li, Y.; Mao, S.; Liu, S.; Hager, M.W.; Choi, W.B. FSI-1671, a novel anti-Acinetobacter carbapenem; in vitro activities of FSI-1671 and FSI-1671/sulbactam against MDR-A. baumannii, abstr F-1202. In Proceedings of the 53rd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Denver, CO, USA, 10–13 September 2013. [Google Scholar]
- Kang, A.D.; Smith, K.P.; Eliopoulos, G.M.; Berg, A.H.; McCoy, C.; Kirby, J.E. In vitro Apramycin Activity against multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Diagn. Microbiol. Infect. Dis. 2017, 88, 188–191. [Google Scholar] [CrossRef]
- Vickers, A.; Mushtaq, S.; Woodford, N.; Doumith, M.; David, M. Activity of RX-04 Pyrrolocytosine protein synthesis inhibitors against multidrug-resistant Gram-negative bacteria. Antimicrob. Agents Chemother. 2018, 62, e00689-18. [Google Scholar] [CrossRef]
- Tetra Phase Pharmaceuticals. Tetra Phase Pharmaceuticals Provides Update on Eravacycline Regulatory and Development Status; Tetraphase Pharmaceuticals: Watertown, MA, USA, 2016. [Google Scholar]
- Seifert, H.; Stefanik, D.; Sutcliffe, J.A.; Higgins, P.G. In-vitro activity of the novel fluorocycline eravacycline against carbapenem non-susceptible Acinetobacter baumannii. Int. J. Antimicrob. Agents 2018, 51, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Skalweit, M.J.; Li, M. Bulgecin A as a β-lactam enhancer for carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Acinetobacter baumannii clinical isolates containing various resistance mechanisms. Drug Des. Dev. Ther. 2016, 10, 3013–3020. [Google Scholar] [CrossRef]
- Livermore, D.M.; Mushtaq, S.; Warner, M.; Woodford, N. In-vitro activity of eravacycline against carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 4, 3840–3844. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Olafisoye, O.; Cortes, C.; Urban, C.; Landman, D.; Quale, J. Activity of eravacycline against Enterobacteriaceae and Acinetobacter baumannii, including multidrug-resistant isolates, from New York City. Antimicrob. Agents Chemother. 2015, 59, 1802–1805. [Google Scholar] [CrossRef] [PubMed]
- Seifert, H.; Stefanik, D.; Sutcliffe, J.; Higgins, P.G. In-vitro activity of the novel fluorocycline TP-6076 against car-bapenem non-susceptible Acinetobacter baumannii, abstr P-1364. In Proceedings of the 27th Europe Cong Clinical Microbiology Infection Disease, Vienna, Austria, 22–25 April 2017. [Google Scholar]
- Lemaitre, N.; Liang, X.; Najeeb, J.; Lee, C.J.; Titecat, M.; Leteurtre, E.; Simonet, M.; Toone, E.J.; Zhou, P.; Sebbane, F. Curative treatment of severe Gram-negative bacterial infections by a new class of antibiotics targeting LpxC. MBio 2017, 8, e00674-17. [Google Scholar] [CrossRef] [PubMed]
- Flamm, R.K.; Rhomberg, P.R.; Jones, R.N.; Farrell, D.J. In vitro activity of RX-P873 against Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter baumannii. Antimicrob. Agents Chemother. 2015, 59, 2280–2285. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guclu, E.; Genc, H.; Zengin, M.; Karabay, O. Antibacterial activity of Lythrum salicaria against multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Annu. Res. Rev. Biol. 2014, 4, 1099–1105. [Google Scholar] [CrossRef]
- Miyasaki, Y.; Rabenstein, J.D.; Rhea, J.; Crouch, M.-L.; Mocek, U.M.; Kittell, P.E.; Morgan, M.A.; Nichols, W.S.; Van Benschoten, M.M.; Hardy, W.D.; et al. Isolation and characterization of antimicrobial compounds in plant extracts against multidrug-resistant Acinetobacter baumannii. PLoS ONE 2013, 8, e61594. [Google Scholar] [CrossRef]
- Montagu, A.; Saulnier, P.; Cassisa, V.; Rossines, E.; Eveillard, M.; Joly-Guillou, M.L. Aromatic and terpenic compounds loaded in lipidic nanocapsules: Activity against multi-drug resistant Acinetobacter baumannii assessed in vitro and in a murine model of sepsis. J. Nanomed. Nanotechnol. 2014, 5, 1000206. [Google Scholar] [CrossRef]
- Pelletier, R.P. Effect of Plant-Derived Molecules on Acinetobacter baumannii Biofilm on Abiotic Surfaces. Ph.D. Thesis, University of Connecticut, Storrs, CT, USA, 2012. [Google Scholar]
- Betts, J.W.; Wareham, D.W. In vitro activity of curcuminin combination with Epigallocatechingallate (EGCG) versus multidrug-resistant Acinetobacter baumannii. BMC Microbiol. 2014, 14, 172. [Google Scholar] [CrossRef]
- Betts, J.W.; Kelly, S.M.; Haswell, S.J. Antibacterial effects of theaflavin and synergy with epicatechin against clinical isolates of Acinetobacter baumannii and Stenotrophomonas maltophilia. Int. J. Antimicrob. Agents. 2011, 38, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.C.L.; Lau, C.B.S.; Jolivalt, C.; Lui, S.L.; Ganem-Elbaz, C.; Paris, J.M. Chinese medicinal herbs against an-tibiotic-resistant bacterial pathogens. In Science against Microbial Pathogens: Communicating Current Research Technology Advance; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; Volume 2, pp. 73–781. [Google Scholar]
- Osterburg, J.A.; Gardner, S.; Hyon, H.; Neely, A.; Babcock, G. Highly antibiotic-resistant Acinetobacter baumannii clinical isolates are killed by the green tea polyphenol (–)-epigallocatechin-3-gallate (EGCG). Clin. Microbiol. Infect. 2009, 15, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.N.; Stavrinides, J. Pantoea natural product 3 is encoded by an eight-gene biosynthetic gene cluster and exhibits antimicrobial activity against multi-drug resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Microbiol. Res. 2020, 234, 126412. [Google Scholar] [CrossRef] [PubMed]
- Kostoulias, X.; Murray, G.L.; Cerqueira, G.M.; Kong, J.B.; Bantun, F.; Mylonakis, E.; Khoo, C.A.; Peleg, A.Y. Impact of a Cross-Kingdom Signaling Molecule of Candida albicans on Acinetobacter baumannii Physiology. Antimicrob. Agents Chemother. 2016, 60, 161–167. [Google Scholar] [CrossRef]
- Shin, B.; Park, W. Synergistic effect of oleanolic acid on aminoglycoside antibiotics against Acinetobacter baumannii. PLoS ONE 2015, 10, e0137751. [Google Scholar] [CrossRef]
- Mihu, M.R.; Sandkovsky, U.; Han, G.; Friedman, J.M.; Nosanchuk, J.D.; Martinez, L.R. The use of nitric oxide releasing nanoparticles as a treatment against Acinetobacter baumannii in wound infections. Virulence 2010, 1, 62–67. [Google Scholar] [CrossRef]
- Shaker, M.A.; Shaaban, M.I. Synthesis of silver nanoparticles with antimicrobial and anti-adherence activities against multidrug-resistant isolates from Acinetobacter baumannii. J. Taibah Univ. Med. Sci. 2017, 12, 291–297. [Google Scholar] [CrossRef]
- Friedman, A.; Friedman, J. New biomaterials for the sustained release of nitric oxide: Past, present and future. Expert Opin. Drug Deliv. 2009, 6, 1113–1122. [Google Scholar] [CrossRef]
- Hetta, H.F.; Al-Kadmy, M.S.I.; Khazaal, S.S.; Abbas, S.; Suhail, A.; El-Mokhtar, M.A.; Abd Ellah, N.H.; Ahmed, E.A. Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2021, 11, 10751. [Google Scholar] [CrossRef]
- Wintachai, P.; Paosen, S.; Yupanqui, C.T.; Voravuthikunchai, S.P. Silver nanoparticles synthesized with Eucalyptus critriodora ethanol leaf extract stimulate antibacterial activity against clinically multidrug-resistant Acinetobacter baumannii isolated from pneumonia patients. Microb. Pathog. 2018, 126, 245–257. [Google Scholar] [CrossRef]
- Hemeg, H.A. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yu, X.; Huo, Q.; Yuan, X.Q.; Li, X.; Xu, C.; and Bao, H. Biomedical potentialities of silver nanoparticles for clinical multiple drug-resistant Acinetobacter baumannii. J. Nanomater. 2019, 2019, 3754018. [Google Scholar] [CrossRef]
- Banoub, N.G.; E Saleh, S.; Helal, H.S.; Aboshanab, K.M. Antibiotics combinations and chitosan nanoparticles for combating multidrug resistance Acinetobacter baumannii. Infect. Drug Resist. 2021, 14, 3327–3339. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Ruan, L.; Yin, Y.; Yang, T.; Ge, M.; Cheng, X. Effects of silver nanoparticles in combination with antibiotics on the resistant bacteria Acinetobacter baumannii. Int. J. Nanomed. 2016, 11, 3789–3800. [Google Scholar] [CrossRef]
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef]
- Rhoads, D.D.; Wolcott, R.D.; Kuskowski, M.A.; Wolcott, B.M.; Ward, L.S.; Sulakvelidze, A. Bacteriophage therapy of venous leg ulcers in humans: Results of a phase I safety trial. J. Wound Care 2009, 18, 237–243. [Google Scholar] [CrossRef]
- Wright, A.; Hawkins, C.H.; Änggård, E.E.; Harper, D.R. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol. Allied Sci. 2009, 34, 349–357. [Google Scholar] [CrossRef]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2017, 61, e00954-17. [Google Scholar] [CrossRef]
- Yang, H.; Liang, L.; Lin, S.; Jia, S. Isolation and characterization of a virulent bacteriophage AB1 of Acinetobacter baumannii. BMC Microbiol. 2010, 10, 131. [Google Scholar] [CrossRef]
- Wintachai, P.; Voravuthikunchai, S.P. Characterization of novel lytic Myoviridae phage infecting multidrug-resistant Acinetobacter baumannii and synergistic antimicrobial efficacy between phage and Sacha Inchi oil. Pharmaceuticals 2022, 15, 291. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Babakhani, S.; Moradi, L.; Karami, S.; Shahbandeh, M.; Mirshekar, M.; Mohebi, S.; Moghadam, M.T. Bacteriophage as a novel therapeutic weapon for killing colistin-resistant multi-drug-resistant and extensively drug-resistant gram-negative bacteria. Curr. Microbiol. 2021, 78, 4023–4036. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, X.; Shi, Y.; Yin, S.; Shen, W.; Chen, J.; Chen, Y.; Chen, Y.; You, B.; Gong, Y.; et al. Characterization and genome annotation of a newly detected bacteriophage infecting multidrug-resistant Acinetobacter baumannii. Arch. Virol. 2019, 164, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Lin, N.T.; Hu, A.; Lin, Y.S.; Chen, L.K.; Lai, M.J. Genomic analysis of bacteriophage varphiAB1, a varphiKMV-like virus infecting multidrug-resistant Acinetobacter baumannii. Genomics 2011, 97, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Vukotic, G.; Obradovic, M.; Novovic, K.; Di Luca, M.; Jovcic, B.; Fira, D.; Neve, H.; Kojic, M.; McAuliffe, O. Characterization, antibiofilm, and depolymerizing activity of two phages active on carbapenem-resistant Acinetobacter baumannii. Front. Med. 2020, 7, 426. [Google Scholar] [CrossRef]
- Jeon, J.; Park, J.H.; Yong, D. Efficacy of bacteriophage treatment against carbapenem-resistant Acinetobacter baumannii in Galleria mellonella larvae and a mouse model of acute pneumonia. BMC Microbiol. 2019, 19, 70. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Sisakhtpour, B.; Mirzaei, A.; Karbasizadeh, V.; Moghim, S. Efficacy of isolated bacteriophage against biofilm embedded colistin-resistant Acinetobacter baumannii. Gene Rep. 2020, 22, 100984. [Google Scholar] [CrossRef]
- Bernstein, L.R. Mechanisms of therapeutic activity for gallium. Pharmacol. Rev. 1998, 50, 665–682. [Google Scholar]
- Antunes, L.C.S.; Imperi, F.; Minandri, F.; Visca, P. In vitro and in vivo antimicrobial activities of gallium nitrate against multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2012, 56, 5961–5970. [Google Scholar] [CrossRef]
- Arivett, B.A.; Fiester, S.E.; Ohneck, E.J.; Penwell, W.F.; Kaufman, C.M.; Relich, R.F.; Actis, L.A. Antimicrobial activity of gallium protoporphyrin IX AGAINST Acinetobacter baumannii strains displaying different antibiotic resistance phenotypes. Antimicrob. Agents Chemother. 2015, 59, 7657–7665. [Google Scholar] [CrossRef]
- Rumbo, C.; Fernández-Moreira, E.; Merino, M.; Poza, M.; Mendez, J.A.; Soares, N.C.; Mosquera, A.; Chaves, F.; Bou, G. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: A new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2011, 55, 3084–3090. [Google Scholar] [CrossRef]
- Reid, M. The importance of guidelines in the development and application of probiotics. Curr. Pharm. Des. 2005, 11, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Takahashi, A.; Yuki, N.; Kaji, R.; Takahashi, T.; Nomoto, K. Protective effect of a synbiotic against multidrug-resistant Acinetobacter baumannii in a murine infection model. Antimicrob. Agents Chemother. 2016, 60, 3041–3050. [Google Scholar] [CrossRef] [PubMed]
- Parra Millán, R.; Jiménez Mejías, M.E.; Sánchez Encinales, V.; Ayerbe Algaba, R.; Gutiérrez Valencia, A.; Pachón Ibáñez, M.E.; Díaz, C.; Pérez del Palacio, J.; López Cortés, L.F.; Pachón, J.; et al. Efficacy of lysophosphatidylcholine in combination with antimicrobial agents against Acinetobacter baumannii in experimental murine peritoneal sepsis and pneumonia models. Antimicrob. Agents Chemother. 2016, 60, 4464–4470. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Das, S. Mammalian antimicrobial peptides: Promising therapeutic targets against infection and chronic inflammation. Curr. Top. Med. Chem. 2015, 16, 99–129. [Google Scholar] [CrossRef]
- Bahar, A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Berisio, R.; Grieco, P.; Morelli, G.; Galdiero, M. Antimicrobial peptides as an opportunity against bacterial diseases. Curr. Med. Chem. 2015, 22, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Jang, A.R.; Park, J.Y.; Ahn, J.H.; Lee, T.S.; Kim, D.Y.; Jung, D.H.; Song, E.J.; Hong, J.J.; Park, J.H. Cathelicidin-related antimicrobial peptide contributes to host immune responses against pulmonary infection with Acinetobacter baumannii in mice. Immune Netw. 2020, 20, e25. [Google Scholar] [CrossRef]
- Esfandiyari, R.; Halabian, R.; Behzadi, E.; Sedighian, H.; Jafari, R.; Fooladi, A.A.I. Performance evaluation of antimicrobial peptide ll-37 and hepcidin and β-defensin-2 secreted by mesenchymal stem cells. Heliyon 2019, 5, e02652. [Google Scholar] [CrossRef]
- Neshani, A.; Zare, H.; Eidgahi, M.R.A.; Kakhki, R.K.; Safdari, H.; Khaledi, A.; Ghazvini, K. LL-37: Review of antimicrobial profile against sensitive and antibiotic-resistant human bacterial pathogens. Gene Rep. 2019, 17, 100519. [Google Scholar] [CrossRef]
- de Breij, A.; Riool, M.; Cordfunke, R.A.; Malanovic, N.; de Boer, L.; Koning, R.I.; Ravensbergen, E.; Franken, M.; van der Heijde, T.; Boekema, B.K.; et al. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci. Transl. Med. 2018, 10, 4044. [Google Scholar] [CrossRef]
- Mwangi, J.; Yin, Y.; Wang, G.; Yang, M.; Li, Y.; Zhang, Z.; Lai, R. The antimicrobial peptide ZY4 combats multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii infection. Proc. Natl. Acad. Sci. USA 2019, 116, 26516–26522. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Lan, X.Q.; Du, Y.; Chen, P.Y.; Zhao, J.; Zhao, F.; Lee, W.-H.; Zhang, Y. King cobra peptide OH-CATH30 as a potential candidate drug through clinic drug-resistant isolates. Zool Res. 2018, 39, 87–96. [Google Scholar] [PubMed]
- Barksdale, S.M.; Hrifko, E.J.; van Hoek, M.L. Cathelicidin antimicrobial peptide from Alligator mississippiensis has antibacterial activity against multi-drug resistant Acinetobacter baumanii and Klebsiella pneumoniae. Dev. Comp. Immunol. 2017, 70, 135–144. [Google Scholar] [CrossRef]
- Xie, F.; Zan, Y.; Zhang, X.; Zhang, H.; Jin, M.; Zhang, W.; Zhang, Y.; Liu, S. Differential abilities of mammalian cathelicidins to inhibit bacterial biofilm formation and promote multifaceted immune functions of neutrophils. Int. J. Mol. Sci. 2020, 21, 1871. [Google Scholar] [CrossRef] [PubMed]
- Milenic, D.E.; Brady, E.D.; Brechbiel, M.W. Antibody-targeted radiation cancer therapy. Nat. Rev. Drug Discov. 2004, 3, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Dadachova, E.; Casadevall, A. Antibodies as delivery vehicles for radioimmunotherapy of infectious diseases. Expert Opin. Drug Deliv. 2005, 2, 1075–1084. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Wu, M.X.; Hamblin, M.R. Photodynamic therapy plus low-dose cyclophosphamide generates antitumor immunity in a mouse model. Proc. Natl. Acad. Sci. USA 2008, 105, 5495–5500. [Google Scholar] [CrossRef]
- Dai, T.; Tegos, G.P.; Lu, Z.; Huang, L.; Zhiyentayev, T.; Franklin, M.J.; Baer, D.G.; Hamblin, M.R. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob. Agents Chemother. 2009, 53, 3929–3934. [Google Scholar] [CrossRef]
- Li, Z.; Ding, Z.; Liu, Y.; Jin, X.; Xie, J.; Li, T.; Zeng, Z.; Wang, Z.; Liu, J. Phenotypic and genotypic characteristics of biofilm formation in clinical isolates of Acinetobacter baumannii. Infect. Drug Resist. 2021, 14, 2613–2624. [Google Scholar] [CrossRef]
- Donadu, M.G.; Mazzarello, V.; Cappuccinelli, P.; Zanetti, S.; Madléna, M.; Nagy, Á.L.; Stájer, A.; Burián, K.; Gajdács, M. Relationship between the biofilm-forming capacity and antimicrobial resistance in clinical Acinetobacter baumannii isolates: Results from a laboratory-based in vitro study. Microorganisms 2021, 9, 2384. [Google Scholar] [CrossRef]
- Ababneh, Q.; Abulaila, S.; Jaradat, Z. Isolation of extensively drug resistant Acinetobacter baumannii from environmental surfaces inside intensive care units. Am. J. Infect. Control 2021, 50, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Ababneh, Q.; Al-Rousan, E.; Jaradat, Z. Fresh produce as a potential vehicle for transmission of Acinetobacter baumannii. Int. J. Food Contam. 2022, 9, 1–9. [Google Scholar] [CrossRef]
- Greene, C.; Vadlamudi, G.; Newton, D.; Foxman, B.; Xi, C. The influence of biofilm formation and multidrug resistance on environmental survival of clinical and environmental isolates of Acinetobacter baumannii. Am. J. Infect. Control 2016, 44, e65–e71. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.D.; Zhu, Y.; Azad, M.A.K.; Han, M.L.; Wang, J.; Wang, L.; Yu, H.H.; Horne, A.S.; Pinson, J.A.; Rudd, D.; et al. A synthetic lipopeptide targeting top-priority multidrug-resistant Gram-negative pathogens. Nat. Commun. 2022, 13, 1625. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).