Hepatic Gene Expression Profiling of American Kestrels (Falco sparverius) Exposed In Ovo to Three Alternative Brominated Flame Retardants
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Exposures
2.2. Dosing Solutions and Chemical Analysis
2.3. Gene Expression Analysis
2.4. Statistical Analysis
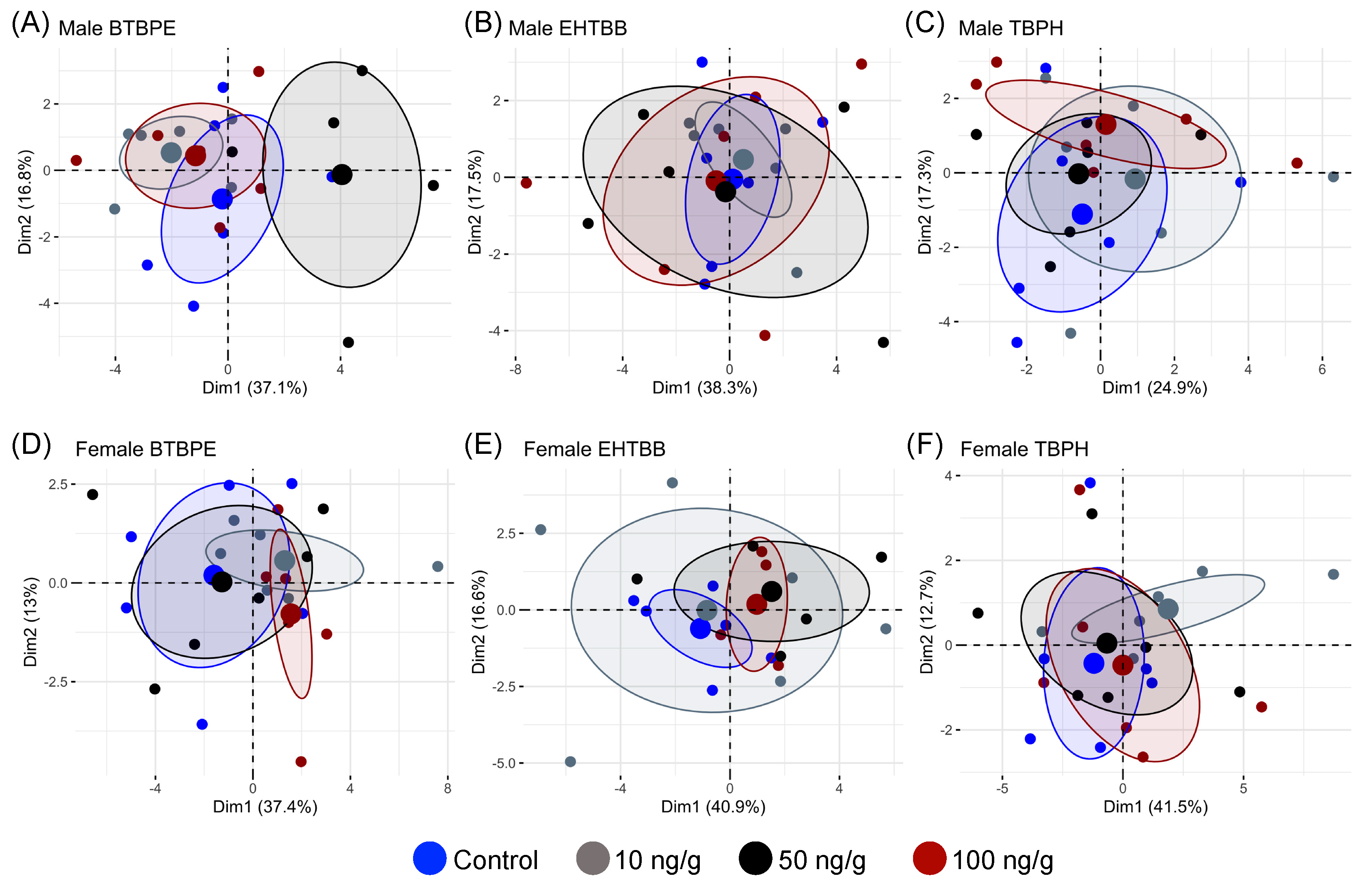
3. Results
3.1. Expression of Genes Related to Xenobiotic Metabolism and Oxidative Stress
3.2. Expression of Genes Related to Thyroid Metabolism and Transport
3.3. Expression of Genes Related to Lipid and Protein Metabolism
3.4. Expression of Immune System-Related Genes
4. Discussion
4.1. Effects on Genes Related to Thyroid Function
4.2. Effects on Genes Related to Xenobiotic Metabolism and Oxidative Stress
4.3. Effects on Genes Related to Lipid and Protein Metabolism
4.4. Effect on Genes Related to Immune Function
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Renner, R. In U.S., flame retardants will be voluntarily phased out. Environ. Sci. Technol. 2004, 38, 14a. [Google Scholar]
- Stapleton, H.M.; Allen, J.G.; Kelly, S.M.; Konstantinov, A.; Klosterhaus, S.; Watkins, D.; McClean, M.D.; Webster, T.F. Alternate and new brominated flame retardants detected in U.S. House Dust. Environ. Sci. Technol. 2008, 42, 6910–6916. [Google Scholar] [CrossRef]
- Tongue, A.D.W.; Fernie, K.J.; Harrad, S.; Drage, D.S.; McGill, R.A.R.; Reynolds, S.J. Interspecies comparisons of brominated flame retardants in relation to foraging ecology and behaviour of gulls frequenting a UK landfill. Sci. Total Environ. 2020, 764, 142890. [Google Scholar] [CrossRef] [PubMed]
- Tongue, A.D.W.; Reynolds, S.J.; Fernie, K.J.; Harrad, S. Flame retardant concentrations and profiles in wild birds associated with landfill: A critical review. Environ. Pollut. 2019, 248, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Möller, A.; Xie, Z.; Cai, M.; Zhong, G.; Huang, P.; Cai, M.; Sturm, R.; He, J.; Ebinghaus, R. Polybrominated Diphenyl Ethers vs. Alternate brominated flame retardants and dechloranes from east Asia to the arctic. Environ. Sci. Technol. 2011, 45, 6793–6799. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Venier, M.; Hites, R.A. 2-Ethylhexyl tetrabromobenzoate and Bis(2-ethylhexyl) tetrabromophthalate flame retardants in the great lakes atmosphere. Environ. Sci. Technol. 2012, 46, 204–208. [Google Scholar] [CrossRef]
- Newton, S.; Sellström, U.; de Wit, C.A. Emerging flame retardants, PBDEs, and HBCDDs in indoor and outdoor media in Stockholm, Sweden. Environ. Sci. Technol. 2015, 49, 2912–2920. [Google Scholar] [CrossRef]
- Sagerup, K.; Herzke, D.; Harju, M.; Evenset, A.; Christensen, G.N.; Routti, H.; Fuglei, E.; Aars, J.; Strøm, H.; Gabrielsen, G.W. New Brominated Flame Retardants in Arctic biota; Norwegian Climate and Pollution Agency: Oslo, Norway, 2010. [Google Scholar]
- Verreault, J.; Letcher, R.J.; Gentes, M.L.; Braune, B.M. Unusually high Deca-BDE concentrations and new flame retardants in a Canadian Arctic top predator, the glaucous gull. Sci. Total Environ. 2018, 219, 191–200. [Google Scholar] [CrossRef]
- Gentes, M.L.; Letcher, R.J.; Caron-Beaudoin, É.; Verreault, J. Novel flame retardants in urban-feeding ring-billed gulls from the St. Lawrence River, Canada. Environ. Sci. Technol. 2012, 46, 9735–9744. [Google Scholar] [CrossRef]
- Zhang, X.L.; Luo, X.J.; Liu, H.Y.; Yu, L.H.; Chen, S.J.; Mai, B.X. Bioaccumulation of several brominated flame retardants and dechlorane plus in waterbirds from an e-waste recycling region in south China: Associated with trophic level and diet sources. Environ. Sci. Technol. 2011, 45, 400–405. [Google Scholar] [CrossRef]
- Jin, X.; Lee, S.; Jeong, Y.; Yu, J.P.; Baek, W.K.; Shin, K.H.; Kannan, K.; Moon, H.B. Species-specific accumulation of polybrominated diphenyl ethers (PBDEs) and other emerging flame retardants in several species of birds from Korea. Environ. Pollut. 2016, 219, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Sagerup, K.; Leonards, P.; Routti, H.; Fuglei, E.; Aars, J.; Strøm, H.; Kovacs, K.; Lydersen, C.; Gabrielsen, G. Organophosphorus Flame Retardants in Arctic biota; Norwegian Climate and Pollution Agency: Oslo, Norway, 2011. [Google Scholar]
- Gauthier, L.T.; Hebert, C.E.; Weseloh, D.V.C.; Letcher, R.J. Current-use flame retardants in the eggs of herring gulls (Larus argentatus) from the Laurentian Great Lakes. Environ. Sci. Technol. 2007, 41, 4561–4567. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.T.; Hebert, C.E.; Weseloh, D.V.C.; Letcher, R.J. Dramatic changes in the temporal trends of polybrominated diphenyl ethers (PBDEs) in herring gull eggs from the Laurentian Great Lakes: 1982–2006. Environ. Sci. Technol. 2008, 42, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Ericson, I.; van Bavel, B.; Jensen, J.K.; Dam, M. Levels of brominated flame retardants in Northern Fulmar (Fulmarus glacialis) eggs from the Faroe Islands. Sci. Total Environ. 2006, 367, 840–846. [Google Scholar] [CrossRef]
- Vorkamp, K.; Falk, K.; Møller, S.; Rigét, F.F.; Sørensen, P.B. Regulated and unregulated halogenated flame retardants in peregrine falcon eggs from Greenland. Environ. Sci. Technol. 2018, 52, 474–483. [Google Scholar] [CrossRef]
- Guerra, P.; Alaee, M.; Jiménez, B.; Pacepavicius, G.; Marvin, C.; MacInnis, G.; Eljarrat, E.; Barceló, D.; Champoux, L.; Fernie, K. Emerging and historical brominated flame retardants in peregrine falcon (Falco peregrinus) eggs from Canada and Spain. Environ. Int. 2012, 40, 179–186. [Google Scholar] [CrossRef]
- Fernie, K.J.; Chabot, D.; Champoux, L.; Brimble, S.; Alaee, M.; Marteinson, S.; Chen, D.; Palace, V.; Bird, D.M.; Letcher, R.J. Spatiotemporal patterns and relationships among the diet, biochemistry, and exposure to flame retardants in an apex avian predator, the peregrine falcon. Environ. Res. 2017, 158, 43–53. [Google Scholar] [CrossRef]
- Guo, J.; Simon, K.; Romanak, K.; Bowerman, W.; Venier, M. Accumulation of flame retardants in paired eggs and plasma of bald eagles. Environ. Pollut. 2018, 237, 499–507. [Google Scholar] [CrossRef]
- Bardo, L.; Bird, D.M. The use of captive American kestrels (Falco sparverius) as wildlife models: A review. J. Raptor Res. 2009, 43, 345–364. [Google Scholar] [CrossRef]
- Eng, M.L.; Karouna-Renier, N.K.; Henry, P.F.P.; Letcher, R.J.; Schultz, S.L.; Bean, T.G.; Peters, L.E.; Palace, V.P.; Williams, T.D.; Elliott, J.E.; et al. In ovo exposure to brominated flame retardants Part II: Assessment of effects of TBBPA-BDBPE and BTBPE on hatching success, morphometric and physiological endpoints in American kestrels. Ecotoxicol. Environ. Saf. 2019, 179, 151–159. [Google Scholar] [CrossRef]
- Goodchild, C.; Karouna-Renier, N.K.; Henry, P.F.P.; Letcher, R.J.; Schultz, S.L.; Maddox, C.M.; Bean, T.G.; Peters, L.E.; Palace, V.; Fernie, K.J. Thyroid disruption and oxidative stress in American kestrels following embryonic exposure to the alternative flame retardants, EHTBB and TBPH. Environ. Int. 2021, 157, 106826. [Google Scholar] [CrossRef] [PubMed]
- Mingming, T.; Pu, X.; Xiaowei, Z. Applications of functional genomics in uncovering the toxicity mechanisms of environmental chemicals. Asian J. Ecotoxicol. 2022, 17, 1–17. [Google Scholar]
- Crump, D.; Williams, K.L.; Chiu, S.; Letcher, R.J.; Periard, L.; Kennedy, S.W. Biochemical and transcriptomic effects of herring gull egg extracts from variably contaminated colonies of the Laurentian Great Lakes in chicken hepatocytes. Environ. Sci. Technol. 2015, 49, 10190–10198. [Google Scholar] [CrossRef] [PubMed]
- Klimstra, J.D.; Stebbins, K.R.; Heinz, G.H.; Hoffman, D.J.; Kondrad, S.R. Factors related to the artificial incubation of wild bird eggs. Avian Biol. Res. 2009, 2, 121–131. [Google Scholar] [CrossRef]
- Rattner, B.A.; Lazarus, R.S.; Heinz, G.H.; Karouna-Renier, N.K.; Schultz, S.L.; Hale, R.C. Comparative embryotoxicity of a pentabrominated diphenyl etheer mixture to common terns (Sterna hirundo) and American kestrels (Falco sparverius). Chemosphere 2013, 93, 441–447. [Google Scholar] [CrossRef][Green Version]
- Guigueno, M.F.; Karouna-Renier, N.K.; Henry, P.F.P.; Peters, L.E.; Palace, V.P.; Letcher, R.J.; Fernie, K.J. Sex-specific responses in neuroanatomy of hatchling American kestrels in response to embryonic exposure to the flame retardants bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate and 2-ethylhexyl-2,3,4,5-tetrabromobenzoate. Environ. Toxicol. Chem. 2018, 37, 3032–3040. [Google Scholar] [CrossRef]
- Wang, H.; Zhai, T.; Wang, C. NanoStringDiff: Differential expression analysis of NanoString nCounter data. R Package Version 2020, 1–17. [Google Scholar]
- Smythe, T.A.; Butt, C.M.; Stapleton, H.M.; Pleskach, K.; Ratnayake, G.; Song, C.Y.; Riddell, N.; Konstantinov, A.; Tomy, G.T. Impacts of unregulated novel brominated flame retardants on human liver thyroid deiodination and sulfotransferation. Environ. Sci. Technol. 2017, 51, 7245–7253. [Google Scholar] [CrossRef]
- Hill, K.L.; Mortensen, Å.-K.; Teclechiel, D.; Willmore, W.G.; Sylte, I.; Jenssen, B.M.; Letcher, R.J. In Vitro and in silico competitive binding of brominated polyphenyl ether contaminants with human and gull thyroid hormone transport proteins. Environ. Sci. Technol. 2018, 52, 1533–1541. [Google Scholar] [CrossRef]
- Mortensen, Å.-K.; Mæhre, S.; Kristiansen, K.; Heimstad, E.S.; Gabrielsen, G.W.; Jenssen, B.M.; Sylte, I. Homology modeling to screen for potential binding of contaminants to thyroid hormone receptor and transthyretin in glaucous gull (Larus hyperboreus) and herring gull (Larus argentatus). Comput. Toxicol. 2020, 13, 100120. [Google Scholar] [CrossRef]
- Egloff, C.; Crump, D.; Chiu, S.; Manning, G.; McLaren, K.K.; Cassone, C.G.; Letcher, R.J.; Gauthier, L.T.; Kennedy, S.W. In vitro and in ovo effects of four brominated flame retardants on toxicity and hepatic mRNA expression in chicken embryos. Toxicol. Lett. 2011, 207, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ourlin, J.-C.; Baader, M.; Fraser, D.; Halpert, J.R.; Meyer, U.A. Cloning and functional expression of a first inducible avian cytochrome P450 of the CYP3A subfamily (CYP3A37). Arch. Biochem. Biophys. 2000, 373, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.P.; Kawai, Y.K.; Ikenaka, Y.; Kawata, M.; Ikushiro, S.I.; Sakaki, T.; Ishizuka, M. Avian cytochrome P450 (CYP) 1-3 family genes: Isoforms, evolutionary relationships, and mRNA expression in chicken liver. PLoS ONE 2013, 8, e75689. [Google Scholar] [CrossRef] [PubMed]
- Barouki, R.; Morel, Y. Repression of cytochrome P450 1A1 gene expression by oxidative stress: Mechanisms and biological implications. Biochem. Pharmacol. 2001, 61, 511–516. [Google Scholar] [CrossRef]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Morel, Y.; Barouki, R. Down-regulation of Cytochrome P450 1A1 Gene Promoter by Oxidative Stress: Critical Contribution of Nuclear Factor 1 *. J. Biol. Chem. 1998, 273, 26969–26976. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Pircalabioru, G.G.; Bezirtzoglou, E. The Role of Cytochromes P450 in Infection. Front. Immunol. 2018, 9, 89. [Google Scholar] [CrossRef]
- Porter, E.; Crump, D.; Egloff, C.; Chiu, S.; Kennedy, S.W. Use of an avian hepatocyte assay and the avian toxchip polymerse chain reaction array for testing prioritization of 16 organic flame retardants. Environ. Toxicol. Chem. 2014, 33, 573–582. [Google Scholar] [CrossRef]
- Farhat, A.; Crump, D.; Chiu, S.; Williams, K.L.; Letcher, R.J.; Gauthier, L.T.; Kennedy, S.W. In ovo effects of two organophosphate flame retardants-TCPP and TDCPP-on pipping success, development, mRNA expression, and thyroid hormone levels in chicken embryos. Toxicol. Sci. 2013, 134, 92–102. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Monaghan, P.; Metcalfe, N.B.; Torres, R. Oxidative stress as a mediator of life history trade-offs: Mechanisms, measurements and interpretation. Ecol. Lett. 2009, 12, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Deeming, D.C.; Pike, T.W. Embryonic growth and antioxidant provision in avian eggs. Biol. Lett. 2013, 9, 20130757. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Up to date on cholesterol 7 alpha-hydroxylase (CYP7A1) in bile acid synthesis. Liver Res. 2020, 4, 47–63. [Google Scholar] [CrossRef]
- Burg, J.S.; Espenshade, P.J. Regulation of HMG-CoA reductase in mammals and yeast. Prog. Lipid Res. 2011, 50, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Crump, D.; Porter, E.; Egloff, C.; Williams, K.L.; Letcher, R.J.; Gauthier, L.T.; Kennedy, S.W. 1,2-Dibromo-4-(1,2-dibromoethyl)-cyclohexane and tris(methylphenyl) phosphate cause significant effects on development, mRNA expression, and circulating bile acid concentrations in chicken embryos. Toxicol. Appl. Pharmacol. 2014, 277, 279–287. [Google Scholar] [CrossRef]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as metabolic regulators in the liver: Lessons from liver-specific PPAR-null mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef]
- Ali, S.A.-F.; Ismail, A.A.; Abdel-Hafez, S.A.; El-Genaidy, H.M.A. Influence of thermally oxidized palm oil on growth performance and PPAR-α gene expression in broiler chickens. Egypt. Acad. J. Biol. Sciences. C Physiol. Mol. Biol. 2020, 12, 23–37. [Google Scholar] [CrossRef]
- Parada, R.; Malewski, T.; Jaszczak, K.; Kawka, M. Alternative transcription of peroxisome proliferator-activated receptor gamma in the liver is associated with fatness of chickens. Braz. J. Poult. Sci. 2018, 20, 447–454. [Google Scholar] [CrossRef]
- Guo, W.; Lei, L.; Shi, X.; Li, R.; Wang, Q.; Han, J.; Yang, L.; Chen, L.; Zhou, B. Nonalcoholic fatty liver disease development in zebrafish upon exposure to bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate, a novel brominated flame retardant. Environ. Sci. Technol. 2021, 55, 6926–6935. [Google Scholar] [CrossRef]
- Crump, D.; Egloff, C.; Chiu, S.; Letcher, R.J.; Chu, S.; Kennedy, S.W. Pipping success, isomer-specific accumulation, and hepatic mRNA expression in chicken embryos exposed to HBCD. Toxicol. Sci. 2010, 115, 492–500. [Google Scholar] [CrossRef]
- Giraudo, M.; Douville, M.; Letcher, R.J.; Houde, M. Effects of food-borne exposure of juvenile rainbow trout (Oncorhynchus mykiss) to emerging brominated flame retardants 1,2-bis(2,4,6-tribromophenoxy)ethane and 2-ethylhexyl-2,3,4,5-tetrabromobenzoate. Aquat. Toxicol. 2017, 186, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Karouna-Renier, N.K.; Braham, R.P. Hepatic gene expression changes in American kestrel hatchlings. USGS Data Release 2022. [Google Scholar] [CrossRef]



| Symbol | Gene Name | Function |
|---|---|---|
| EEF1A | Eukaryotic translation elongation factor 1 alpha 1 | Housekeeping gene |
| PPIA | Peptidylprolyl isomerase A | Housekeeping gene |
| TBP | TATA box-binding protein | Housekeeping gene |
| IFNB | Interferon beta | Immune function |
| IFNG | Interferon gamma | Immune function |
| IL18 | Interleukin 18 | Immune function |
| IL6 | Interleukin 6 | Immune function |
| IRF7 | IFN regulatory factor 7 | Immune function |
| RACK1 | Receptor for activated C kinase 1 | Immune function |
| RIGI | Retinoic acid inducible gene I | Immune function |
| STAT3 | Signal transducer and activator of transcription 3 | Immune function |
| TLR3 | Toll-like receptor 3 | Immune function |
| CEBPB | CCAAT/enhancer-binding protein beta | Immune function |
| CYP7B1 | Cytochrome P450, family 7, subfamily B, polypeptide 1 | Lipid homeostasis |
| FABP1 | Fatty acid-binding protein 1, liver | Lipid homeostasis |
| FABP4 | Fatty acid-binding protein 4 | Lipid homeostasis |
| HMGCR | 3-Hydroxy-3-methylglutaryl-coenzyme A reductase | Lipid homeostasis |
| LPL | Lipoprotein lipase | Lipid homeostasis |
| GPX1 | Glutathione peroxidase 1 | Oxidative stress |
| GSTA | Glutathione S-transferase class-alpha | Oxidative stress |
| SOD | Superoxide dismutase | Oxidative stress |
| PPARD | Peroxisome proliferator-activated receptor delta | PPAR signaling pathway |
| PPARA | Peroxisome proliferator-activated receptor alpha | PPAR signaling pathway |
| PPARG | Peroxisome proliferator-activated receptor gamma | PPARsignaling pathway |
| DIO1 | Iodothyronine deiodinase 1 | Thyroid hormone pathway |
| DIO2 | Iodothyronine deiodinase 2 | Thyroid hormone pathway |
| THRA | Thyroid hormone receptor alpha | Thyroid hormone pathway |
| THRB | Thyroid hormone receptor beta | Thyroid hormone pathway |
| TTR | Transthyretin | Thyroid hormone pathway |
| AHR | Aryl hydrocarbon receptor | Xenobiotic metabolism |
| CYP1A4 | Cytochrome P450 1A4 | Xenobiotic metabolism |
| CYP2H1 | cytochrome P450 2H1 | Xenobiotic metabolism |
| CYP3A37 | Cytochrome P450 A 37 | Xenobiotic metabolism |
| SULT1B1 | Sulfotransferase family, cytosolic, 1B, member 1 | Xenobiotic metabolism |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goodchild, C.G.; Karouna-Renier, N.K.; Braham, R.P.; Henry, P.F.P.; Letcher, R.J.; Fernie, K.J. Hepatic Gene Expression Profiling of American Kestrels (Falco sparverius) Exposed In Ovo to Three Alternative Brominated Flame Retardants. Biology 2022, 11, 1341. https://doi.org/10.3390/biology11091341
Goodchild CG, Karouna-Renier NK, Braham RP, Henry PFP, Letcher RJ, Fernie KJ. Hepatic Gene Expression Profiling of American Kestrels (Falco sparverius) Exposed In Ovo to Three Alternative Brominated Flame Retardants. Biology. 2022; 11(9):1341. https://doi.org/10.3390/biology11091341
Chicago/Turabian StyleGoodchild, Christopher G., Natalie K. Karouna-Renier, Ryan P. Braham, Paula F. P. Henry, Robert J. Letcher, and Kim J. Fernie. 2022. "Hepatic Gene Expression Profiling of American Kestrels (Falco sparverius) Exposed In Ovo to Three Alternative Brominated Flame Retardants" Biology 11, no. 9: 1341. https://doi.org/10.3390/biology11091341
APA StyleGoodchild, C. G., Karouna-Renier, N. K., Braham, R. P., Henry, P. F. P., Letcher, R. J., & Fernie, K. J. (2022). Hepatic Gene Expression Profiling of American Kestrels (Falco sparverius) Exposed In Ovo to Three Alternative Brominated Flame Retardants. Biology, 11(9), 1341. https://doi.org/10.3390/biology11091341






