Simple Summary
The deciduous shrub Comptonia is a monotypic genus of Myricaceae, which currently is distributed only in eastern North America, with a smaller range than that in other periods of the Cenozoic. By analyzing the macroscopic and microscopic characteristics of the leaves, we describe a new species of Comptonia (i.e., Comptonia hirsuta) from the Hannuoba Formation in Zhuozi, Inner Mongolia, North China. The co-occurring fruits were also studied based on their morphological characteristics, which were assigned to Comptonia tymensis. Variation in the distribution range of Comptonia indicates the influence of global cooling on the expansion of this plant. Furthermore, the Bering Land Bridge played an important role in the migration from North America to East Asia. The Thulean route may have provided an opportunity for plant exchange between western Europe and eastern North America. Moreover, the reason for the disappearance of Comptonia from China according to the analysis of the changes in both the global climate and the distribution of Comptonia fossils is also discussed. It is suggested that the climatic changes after the late Miocene and the progenitive pattern of Comptonia together caused the disappearance of Comptonia in China.
Abstract
Comptonia (Myricaceae) is well known as a monotypic genus living only in eastern North America; however, fossils show that the genus occurred extensively in the Northern Hemisphere during the Cenozoic. We observed dozens of Comptonia leaf fossils from the early Miocene in Zhuozi, China. The leaf architecture characteristics and epidermal features of the fossil specimens are described in detail here for the first time, and they were assigned to a new species: Comptonia hirsuta. The fruit fossils collected simultaneously from the same layer were assigned to Comptonia tymensis. The global fossil records indicate that the spatial distribution range of Comptonia reached its peak in both the Eocene and Miocene as two warm periods and then gradually decreased in the Oligocene, as well as after the late Miocene, because of the cooling global climate. Furthermore, the Comptonia taxon in East Asia may have migrated from North America via the Bering route in the late Paleocene or Eocene. Plant exchange between western Europe and eastern North America possibly occurred during the Eocene via the Thulean route. Phytogeographic variation in the Comptonia fossils from China also indicates that the reason for the disappearance of Comptonia from China may not only be due to the prolonged cooling and drying after the late Miocene, but also due to its progenitive pattern.
1. Introduction
Comptonia L’Héritier de Brutelle ex Aiton is a monotypic genus of Myricaceae, which belongs to a kind of deciduous low shrub. It lives in the habitats with dry, sterile, sandy to rocky soils in pinelands or pine barren lands, clearings, or the edges of woodlots [1]. At present, only three taxa of Comptonia survive, including a single species, Comptonia peregrina (Linnaeus) Coulter, and two varieties, C. peregrina var. tomentosa A. Chevalier and C. peregrina var. aspleniifolia (L.) Fernald. They are presently limited to North America with a distribution range extending from Nova Scotia to North Carolina, western South Carolina, northern Georgia, and west to Saskatchewan, Minnesota, Illinois, and Tennessee [2,3,4].
Although the extant Comptonia populations live only in North America, the fossil records of this species indicate that it was once widely distributed in the Northern Hemisphere during the Paleogene and Neogene [3,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. Because of its distinctive leaf type, the earliest discovered fossils of Comptonia were incorrectly assigned to Aspleniopteris by Sternberg in 1825 [3,44]. Afterwards, Brongniart pointed out Sternberg’s error and then classified the fossils previously misidentified by Sternberg into Comptonia L’Héritier de Brutelle ex Aiton in 1828 [45]. Since then, Comptonia fossils have begun to attract more attention from researchers. Subsequently, an increasing number of fossils of this genus have been discovered and studied [3,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. Previous researches indicated that Comptonia appears to have originated in the Arctic during the Late Cretaceous and became widely distributed in the Northern Hemisphere during the Paleogene and Neogene with its dispersals [3], such as the late Eocene, Oligocene, Miocene, and late Pliocene in Russia [40]; the Paleogene and Neogene in North America [30,41]; the Miocene in Denmark [10,42]; the Oligocene to Miocene in Germany [43]; the Oligocene to Miocene in Bohemia and other parts of Europe [38]; and the late Eocene to Miocene in China [6,23,26,35,36,37].
In this paper, we describe a new species of Comptonia based on 93 recently collected leaves from the early Miocene Hannuoba Formation in Zhuozi County, Ulanqab City, Inner Mongolia Autonomous Region, North China. The new species is compared in detail with living equivalents and previously reported fossils on the gross morphology and cuticular microstructure of the leaves. The fruit fossils collected simultaneously from the same layer are identified according to the nutlet features. In addition, according to the global fossil records of Comptonia and global climate change during the Cenozoic, the reason for changes in the distribution of the Comptonia population during the Cenozoic is inferred. Finally, the reason for the disappearance of the Comptonia population from China is discussed.
2. Materials and Methods
2.1. Materials
Comptonia fossil leaves (ca. 93 pieces) and fruits (ca. 6 pieces) were collected from the Hannuoba Formation in Zhuozi County (40°45′01′′ N, 112°48′46′′ E), Ulanqab City, Inner Mongolia Autonomous Region, North China (Figure 1). This formation is mainly composed of olivine, pyroxene, and bubble basalts with fluvial and lacustrine sedimentary interlayers, which are known as Hannuoba basalts [46]. At this site, the fossiliferous layers include gray mudstones and black oil shales, yielding rich and well-preserved animal and plant fossils. In addition, the Hannuoba Formation was dated between 19.35 Ma and 22.84 Ma based on basalt K-Ar dating from Zuoyun County in Shanxi Province adjacent to Inner Mongolia [47]. Thus, the fossiliferous layer herein is considered to date as the early Miocene.
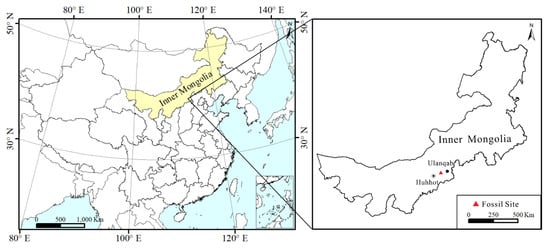
Figure 1.
Location of Comptonia fossils in Zhuozi County, Ulanqab City, Inner Mongolia Autonomous Region in North China.
To compare current fossils with living Comptonia peregrina, the extant leaf specimens were collected from Virginia, Maine, Massachusetts, and New Hampshire in the USA. All the materials are now preserved in the Geological Museum of Chang’an University.
2.2. Experimental Methods
To obtain clean cuticles of the fossil leaves, we used a traditional method that was modified by Liang et al. [48]. The specimens were soaked in 20% HCl for 2 h to remove carbonate sediments and washed repeatedly with distilled water until the solution was neutral. Then, we soaked the leaves in 20% hydrofluoric acid for 12–24 h until the silicate deposits dissolved and washed them again until neutral. We moved the fossil leaves into 1% NaClO for 5–10 min and washed the solution to neutrality when the sample turned light yellow. Finally, the obtained leaf fossil cuticles were stained with 1% safranin stain and then mounted. Observations and photographs were performed using a Leica DM1000 light microscope (LM). Additionally, some specimens were selected and sprayed with gold for observation, and we took pictures with an FEI Quanta 650 FEG scan electron microscope (SEM).
The extant leaves were prepared with a 1:1 (v:v) solution of 60% glacial acetic acid and 30% H2O2, and then they were placed in a hot water bath at 60 °C for 8–12 h. Once the cuticles turned white and transparent, they could be easily separated. After completing the above steps, we stained the cuticles with safranine solution for 5–10 s and mounted them. For the LM and SEM preparations, the same procedures as those for the fossil leaves were then followed.
The fruit fossils were soaked in distilled water for 2 h. Then, we brushed off the mud and soaked them in 10% HCl for 12 h. We then washed them with distilled water until the solution was neutral. The specimens were soaked in 15% hydrofluoric acid for 12 h, and the solution was washed until neutral. Finally, we made observations and took photographs using a Leica M165 FC stereomicroscope.
3. Results
Order: Myricales.
Family: Myricaceae Richard ex Kunth (1817).
Genus: Comptonia L’Héritier ex Aiton (1789).
Species: Comptonia hirsuta Liang Xiao et De-Shuang Ji sp. nov.
Holotype: ELS-16-301AB (Figure 2A,C,G).

Figure 2.
Leaf compressions and impressions of Comptonia hirsuta from the early Miocene of Zhuozi, North China. (A) Holotype, No. ELS-16-301A. (B) No. ELS-16-464. (C) Holotype, No. ELS-16-301B. The counterpart of (A). (D) No. ELS-16-216. (E) No. ELS-16-435. (F) No. ELS-16-400. (G) Holotype, No. ELS-16-301A. Details of (A) show the semi-oval lobes in the middle of the leaf. (H) No. ELS-16-216. Details of (D) show the semi-oval lobes in the middle of the leaf. (I) No. ELS-16-410. Details of (K) showing the falcate lobes in the middle of the leaf. (J) No. ELS-16-215. (K) No. ELS-16-410. (L) No. ELS-16-400. The top of (F) shows triangular lobes. Scale bar: (A–F,J,K) = 1 cm; (G–I,L) = 2 mm.
Paratypes: ELS-16-216 (Figure 2D,H), ELS-16-464 (Figure 2B), ELS-16-435 (Figure 2E), ELS-16-400 (Figure 2F,L).
Etymology: The specific epithet is based on the evident epidermal features of the fossil leaves, meaning abundant trichomes.
Diagnosis: Laminas oblanceolate to mostly narrow elliptical; lobes pinnatisect; dense simple trichomes with unicellular base or two, four, six, eight unicellular fused base; and peltate glandular trichomes on both the adaxial cuticle and the abaxial cuticle.
Type locality: Yangjuanwan Village, Zhuozi County, Inner Mongolia, North China.
Stratum and age: the Hannuoba Formation, early Miocene.
Repository: Geological Museum of Chang’an University, Xi’an, China.
Description: The leaves are simple, 20–85 mm long, and 6–22 mm wide. Their petioles are short, 0.3–0.6 cm in length, expanding towards the base. The laminas are oblanceolate to mostly narrow, elliptical, pinnatisect, with alternate to opposite lobes (Figure 2). The lobes are entire-margined, broadly attached to the midrib, and separated by sinuses reaching up to the midrib. Individual lobes are triangular, falcate, semi-oval, and taper into the apex and base. The apex of the lobes is straight or acuminate. The upper side of the lobes is straight to slightly concave, and the bottom side is straight to convex. The basal pair of lobes is decurrent on the petiole. The apical lobe is elliptical without dissection. The venation is pinnate. The midrib is simple, 0.2–0.5 mm wide in the middle of the leaf length, and tapers towards the apex. Two to four secondary veins per lobe stretch from the midrib at an angle of 80°–90°. Two of them are thicker, craspedodromous, and converging to the apex of the lobe. The other two secondary veins are eucamptodromous. There are one to three intersecondary veins parallel to the secondaries. Their lengths are similar to or more than 50% of the subjacent secondary. The intersecondary distal course is basifixed, intersecting with adjacent secondary veins. The tertiary veins consist of two types, including intercostal and epimedial tertiaries. The intercostal tertiary veins cross between adjacent secondaries, which are divided into two forms. Some of them are mixed, including opposite and alternate percurrent courses. The others are transversely freely ramified. The epimedial tertiaries are ramified, and parallel to the intercostal tertiary veins. Some of the leaves show unobvious quaternary veins that are irregularly reticulate.
The epidermal anatomy of the above described leaves is well preserved. The adaxial cuticle is hairy, showing outlines of cells ca. 19.8–39 μm in length and ca. 15.6–21 μm in width, demarcated by almost straight to wavy anticlinal cell walls (Figure 3). The abaxial cuticle is hairy, showing outlines of cells ca. 14.6–21.4 μm in length and ca. 9.9–12.1 μm in width, and anomocytic, broadly elliptical stomata are 11–16 × 13–24 μm in size (Figure 4). Unicellular simple trichomes appear on both sides of the leaves, and their unicellular bases are surrounded by six to eight cells (sometimes two, four, six, or eight of the unicellular bases are fused; see Figure 3, Figure 4, Figure 5 and Figure 6). There are abundant trichomes on the abaxial cuticle. The glandular trichomes are peltate with the originally globular balloon-shaped heads on both the adaxial cuticle and the abaxial cuticle. The head cells of the peltate glandular trichomes are disc-shaped and compressed due to the process of fossilization, 50–110 μm in diameter (Figure 3A,E,G; Figure 4E,G; Figure 5I; Figure 6D,E,H,I). The simple stalks of the peltate glandular trichomes are 12–15 μm in diameter.
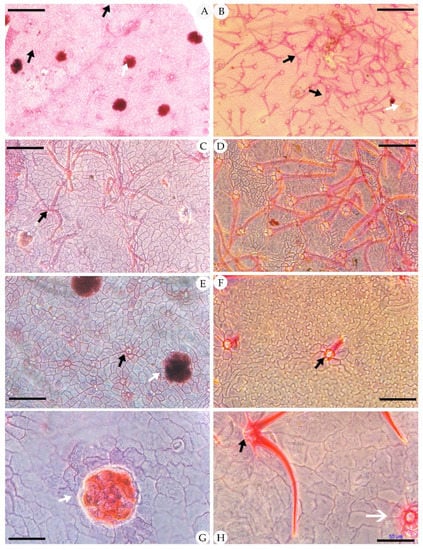
Figure 3.
Adaxial cuticle of fossil Comptonia hirsuta and extant C. peregrina. (A) The simple, paired trichome bases and peltate glandular trichomes of Comptonia hirsuta, holotype, ELS-16-301. (B) The adaxial cuticle of C. peregrina from Massachusetts, USA, showing the dense trichomes with simple, paired, and four-celled fused trichome bases. (C) The adaxial cuticle of Comptonia hirsuta, showing a four-celled fused trichome base, ELS-16-196. (D) The dense trichomes of C. peregrina from Massachusetts, USA. (E) The simple trichome bases and peltate glandular trichomes of Comptonia hirsuta, holotype, ELS-16-301. (F) Undulate epidermic cells and simple trichomes of C. peregrina from New Hampshire, USA. (G) Slightly undulate epidermic cells and peltate glandular trichomes of Comptonia hirsuta, ELS-16-217. (H) The paired trichome bases and peltate glandular trichomes of C. peregrina from Virginia, USA. Black arrows show the trichome bases, and white arrows show the peltate glandular trichomes. Scale bar: (A,B) = 200 μm; (C–F) = 100 μm; (G,H) = 50 μm.
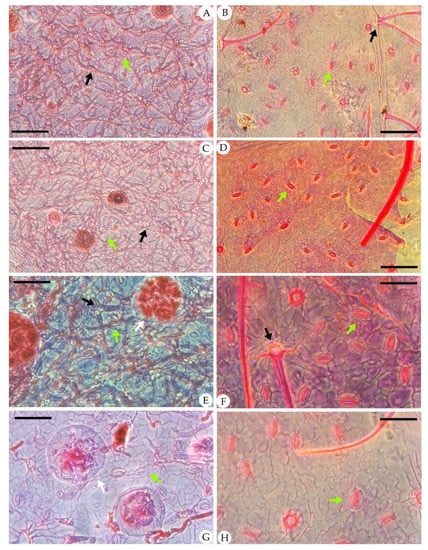
Figure 4.
Abaxial cuticle of fossil Comptonia hirsuta and extant C. peregrina. (A) The dense trichomes with simple, paired, and four-celled fused trichome bases; peltate glandular trichomes; and anomocytic broadly elliptical stomata of Comptonia hirsuta, ELS-16-270. (B) The abaxial cuticle of C. peregrina from Virginia, USA, showing the simple, paired trichome bases. (C) The abaxial cuticle of Comptonia hirsuta, showing dense trichomes, peltate glandular trichomes, and anomocytic stomata, ELS-16-216. (D) The anomocytic stomata and undulate epidermic cells of C. peregrina from Maine, USA. (E) The paired trichome bases, peltate glandular trichomes, and anomocytic stomata of Comptonia hirsuta, holotype, ELS-16-301. (F) Slightly undulate epidermic cells, simple trichomes, and anomocytic stomata of C. peregrina from New Hampshire, USA. (G) Anomocytic stomata and peltate glandular trichomes of Comptonia hirsuta, ELS-16-146. (H) The anomocytic stomata and slightly undulate epidermic cells of C. peregrina from Virginia, USA. The black arrows show the trichome bases; green arrows point to the stomata; and the white arrows show the peltate glandular trichomes. Scale bar: (A–D) = 100 μm; (E–H) = 50 μm.
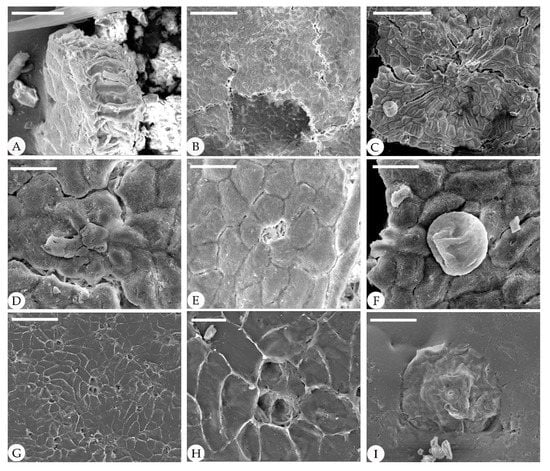
Figure 5.
Cuticle of fossil Comptonia hirsuta under SEM, ELS-16-216. (A) Cross-section of the fossil leaf showing the palisade tissue cells. (B–I) are the structures on the adaxial cuticle. (B) Straight epidermic cells with trichome bases. (C) Straight epidermic cells with a peltate glandular trichome. (D) Trichome base. (E) Details of (B), showing a trichome base surrounded by six cells. (F) Details of (C), showing a peltate glandular trichome. (G) Straight epidermic cells with trichome bases. (H) Details of (G), showing a three-cell fused trichome base. (I) A peltate glandular trichome. Scale bar: (A) = 30 μm; (B,C) = 100 μm; (D–F) = 20 μm; (G) = 50 μm; (H) = 10 μm; (I) = 40 μm.
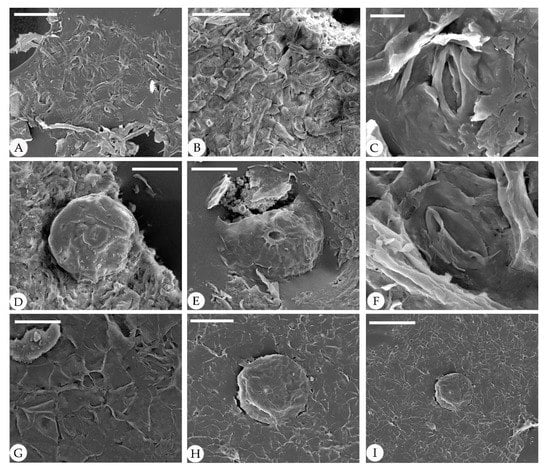
Figure 6.
Abaxial cuticle of fossil Comptonia hirsuta under SEM, ELS-16-216. (A) Dense trichomes. (B) Anomocytic stomata. (C) Details of anomocytic stomata. (D) A peltate glandular trichome with a one-celled stalk. (E) A broken peltate glandular trichome. (F) Anomocytic stomata. (G) Paired trichome base and anomocytic stomata. (H) Details of (I), showing a peltate glandular trichome with a one-celled stalk. (I) A peltate glandular trichome and trichome bases of simple trichomes. Scale bar: (A,C,F,H) = 50 μm; (B,D,E) = 40 μm; (G) = 20 μm; (I) = 100 μm.
Comparison: The above described leaf materials from the Zhuozi County, Inner Mongolia, North China, can be easily assigned to the genus Comptonia based on the typical pinnately lobed leaves with entire-margined lobes. The foliage of Comptonia hirsuta, as described above, has some common features with records from the Paleogene and Neogene in Czechia [38] and Weichang [36], including both gross morphology and epidermal features, such as the shape of the laminas, the features of veins, and the stomatal apparatus type. However, C. hirsuta has denser trichomes, more diverse forms of the lobes, and larger head cells of peltate glandular trichomes than those of C. naumannii. Moreover, the size of the foliage of C. difformis is larger than that of C. hirsuta in general, while the size of the head cells of the peltate glandular trichomes are smaller. In addition, the simple trichomes and peltate glandular trichomes are on both the adaxial cuticle and abaxial cuticle of C. hirsuta, while those of C. difformis were not observed on the adaxial cuticle because of the poorly preserved epidermal structure from Czechia [38]. The differences between C. hirsuta and late Eocene C. anderssonii Florin (1920) from Fushun, China, include the type of the stalks of the peltate glandular trichomes and the size of the laminas. C. hirsuta has only the unicellular stalks of the peltate glandular trichomes, while two-celled, three-celled, and four-celled stalks of the peltate glandular trichomes appear in C. anderssonii. Additionally, the size of the laminas in C. hirsuta is visibly larger than that of the laminas of C. anderssonii. Furthermore, C. hirsuta can be easily distinguished from C. columbiana [30,37,41] by the serrate leaf margin of the latter. Meanwhile, C. hirsuta has larger head cells of the peltate glandular trichomes than those of Eocene C. columbiana from Fushun and Yilan, China. Sadowski et al. [39] reported some amber fossil leaves assigned to Comptonia from Samland Peninsula near Kaliningrad (Russia) in the late Eocene (34–38 Ma) with head cells of the peltate glandular trichomes as large as the fossil leaves in this research. However, the former species has circular to elliptical trichome bases composed of up to 13 radially arranged cells, which are different from those of C. hirsuta. Although many fossil leaves have been described as Comptonia throughout geological history, none of the other fossil species confirmed the anticipated affinity to this genus according to the epidermal anatomical characteristics. Therefore, we prefer to identify the Comptonia fossil collected in Zhuozi County as a new species, namely, C. hirsuta, on account of the dense simple trichomes with a unicellular base or two, four, six, or eight unicellular fused base and the peltate glandular trichomes on both the adaxial cuticle and abaxial cuticle.
Species: Comptonia tymensis Dorofeev.
1994 Comptonia tymensis Dorofeev, Budantsev, p. 33, pl. 70, figs. 1–19.
2010 Comptonia tymensis Dorofeev, Liang et al., p. 55, pl. 5.
Material: ELS-18-116, ELS-18-205, ELS-18-154, ELS-18-246, ELS-18-232, ELS-16-432.
Description: Nutlets are elliptic to ovoid, 3.1–4.4 mm long and 1.6–2.9 mm wide. The length/width ratio is 1.42–1.87, the apex is acute or acuminate, and the base is widely cuneate to rounded with a knob-like blunt scar. The external surface has four to eight conspicuous longitudinal ribs extending from the basal scar and, rarely, to the apical part of the nutlets (Figure 7). The external surface of the endocarp is smooth with four to eight ribs corresponding to those of the nutlets (Figure 7B–D).

Figure 7.
Fruits of Comptonia hirsuta from early Miocene of Zhuozi. (A) No. ELS-18-116. (B) No. ELS-18-205A. (C) No. ELS-18-205B. (D) No. ELS-18-154. (E) No. ELS-18-246. (F) No. ELS-18-232. Scale bar: (A–F) = 1 mm.
Comparison: The fruits of this shrub can easily be classified as belonging to the genus Comptonia based on their typical shape. The morphological characteristics of the nutlets in Comptonia for identification at the species level include the size, shape, surface characteristics, and the number and characteristics of the ribs [38]. The above described fruits are similar to those of C. tymensis Dorofeev from the early Miocene of Weichang, except for their slightly smaller size and slightly larger number of ribs. The endocarps/nutlets of C. srodoniowae Friis are different from those of this research because of their shape and the style of the base. C. srodoniowae has a guttiform to fusiform shape and a long mucronate style base [10]. However, the fruits in this research have an elliptic to ovoid shape and a knob-like blunt scar at the base. In addition, the fruits of C. tymensis are also different from those of the extant C. peregrina because of their knob-like blunt scar.
Although the leaf specimens and co-occurring fruits were collected from the same layer of the same site in Zhuozi and seem to belong to the same species, they were not directly linked together. For the sake of caution, we did not assign the six fossil fruits and all the fossil leaves that co-occurred at the same time to the same species.
4. Discussion
4.1. Comparison between Comptonia hirsuta and C. peregrina
The fossils discovered from the Hannuoba Formation in Zhuozi, China, are similar to the living C. peregrina in leaf structure. They share many morphological features, such as the same pinnately lobed leaves, their leaf structure, and the similar shape of their leaves. On the other hand, they also have some common epidermal characteristics, including the anomocytic stomata on the abaxial cuticle, the dense unicellular simple trichomes, and peltate glandular trichomes on two sides of the epidermis. Teodoridis et al. [38] considered the simple and paired trichomes in C. peregrina to be more robust. However, the four-celled fused base also appeared in the adaxial cuticle of C. peregrina in our observation, and this feature can also be observed in C. hirsuta (Figure 3). Furthermore, there are abundant trichomes in the leaves of both extant C. peregrina and C. hirsuta. Nevertheless, there are some differences between these two species. Firstly, the size of the head cells of the glandular trichome is distinct. The size of the mature head cells of the glandular trichomes in extant C. peregrina is usually ca. 60 μm in diameter [49], while the head cells of the glandular trichomes on the cuticles of C. hirsuta can reach 50–110 μm in diameter. Additionally, the types of the stalks of the peltate glandular trichomes in extant C. peregrina are one-celled, two-celled, and four-celled [36], while the type of the stalks of the peltate glandular trichomes in C. hirsuta is only one-celled (Figure 4G, Figure 5I, Figure 6D,E,H,I).
In addition to the differences presented above, the density of trichomes in C. naumannii is lower than that in the two above mentioned species. Normally, plants living in arid environments have more trichomes than those living in humid environments. This may indicate that the early Miocene C. hirsuta of Zhuozi lived in a drier environment than the C. naumannii in Weichang at the same time. It is noteworthy that the similarity of the leaves of the Comptonia fossils from the Neogene and Quaternary (e.g., C. naumannii, C. hirsuta, and C. difformis) and those of the extant C. peregrina is very high, which may indicate that the morphological evolution of the genus, at least since the early Miocene, was relatively slow. Comptonia was widely distributed in the Northern Hemisphere during the Paleogene and Neogene periods. However, after the Quaternary ice age, it became a monotypic genus and its distribution was limited to North America, indicating that Comptonia is a relict and can also be regarded as a “living fossil”.
4.2. Phytogeographic History of Comptonia
Comptonia is a deciduous tree that does not tolerate shade and naturally grows in well-drained, dry, acidic, sandy, or gravelly clearings in coniferous forests. It is presently living in eastern North America as one of the refugia. However, the Comptonia population was once widely distributed in the Northern Hemisphere during the Paleogene and Neogene [3,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43].
Fossil records of Comptonia have been found in North America (e.g., New Jersey), Greenland, and Europe (e.g., Transylvania, Central Europe, and Russia), from the Late Cretaceous [3,40]. It seems to have originated in the Northern Hemisphere at high latitudes in at least the Late Cretaceous. However, these fossils are still questionable regarding their age and their systematic identity; thus, they need to be researched in more detail [36]. In the Paleocene, reliably identified Comptonia only appeared in high latitudes, such as Axel Herberg Island and Alaska [21,29], which also indicates that the Comptonia taxon lived mainly at high latitudes in the Northern Hemisphere. It was widely distributed in the Northern Hemisphere during the Eocene, including western North America, Europe, and East Asia [5,6,8,18,23,26,27,30,33,39]. As is known, the Turgai Strait, which was the barrier between Europe and East Asia from the mid-Mesozoic to the late Eocene, limited migration between Europe and Asia [50]. Therefore, the Comptonia taxon in East Asia is more likely to have migrated from Alaska, North America, via the Bering route in the late Paleocene or Eocene (Figure 8) [50]. On the other hand, the fossil records in western Europe and eastern North America during the Eocene may be due to numerous biological exchanges in the Eocene by the Thulean route, as suggested by Brikiatis (2014) [51]. In the Oligocene, Comptonia was mainly distributed in western North America, Europe, and Asia (Figure 8). Compared to that in the Eocene, the distribution range in the Oligocene was narrowed. Moreover, there are no fossil records from high-latitude regions during the Oligocene. The change in the distribution from the Eocene to the Oligocene is possibly due to the climatic cooling that happened in the earliest Oligocene (i.e., Oi-1 Glaciation) [52]. Moreover, the distribution of Comptonia spread into Kazakhstan in central Asia, belonging to the Turgai region, which may be related to the closure of the Turgai Strait in the early Oligocene. In the Miocene, Comptonia taxa achieved another peak in their spatial distribution, which came to include North America, western Europe, East Asia, and Iceland [7,10,16,18,22,23,24,36,38,42], corresponding exactly to the warm Miocene, especially the mid-Miocene climatic optimum (i.e., MMCO) [52]. After the Miocene, the distribution range of this genus was reduced obviously, and it disappeared from East Asia. In the Pliocene, the Comptonia taxon only occurred in Alaska, Yukon, and Russia [19,40]. The decrease in the distribution range of Comptonia may be the result of the cooling global climate after the late Miocene [52]. Because of this, the Comptonia taxon is only currently found in eastern North America.
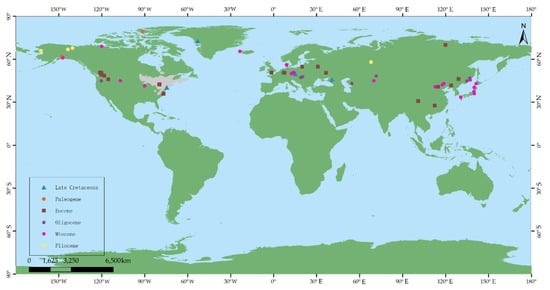
Figure 8.
The fossil records and the present distribution range (represented in grey) of Comptonia in the world. Base maps were constructed with ArcGIS 10.2 based on data from the Resource and Environment Data Cloud Platform [53].
In summary, Comptonia may have originated from the Northern Hemisphere at high latitudes before the early Paleocene, and then migrated to East Asia in the late Paleocene or Eocene via the Bering route. Plant exchange between western Europe and eastern North America possibly occurred during the Eocene via the Thulean route (Figure 8). Afterwards, this genus reached its peak spatial distribution range in the warm periods of both the Eocene and Miocene and gradually decreased in the Oligocene as well as after the late Miocene. The distribution of Comptonia was eventually limited to eastern North America, where it occurs at present.
4.3. Phytogeographic Evolution of Comptonia in China
The global paleogeographic distribution of Comptonia has been researched in a general manner. However, the paleogeographic changes in China have not been studied in detail. In this research, we noticed that the genus Comptonia in particular has a conspicuous variation in its distribution in China (Figure 9).
In China, Comptonia fossils were found to occur in more widespread areas during the Eocene, which were in or near the humid zone of China (Figure 9A), such as Yilan, Heilongjiang [37]; Fushun, Liaoning [26,35,37]; Zhangjiakou, Hebei [26]; Litang, Sichuan [6]; and Xiangxiang, Hunan [26]. The warm climate of the Eocene, as well as the humid environment, provided good conditions for the survival of Comptonia. However, Comptonia fossils were not found in China during the Oligocene, possibly due to the poorer preservation of the Oligocene terrestrial strata relative to the Eocene strata and the Miocene strata, which were influenced by the uplift of some basins in the mainland of China [54]. Moreover, the global cooling event from the late Eocene to the Oligocene may also have influenced the distribution of Comptonia. In the Miocene, the distribution range of Comptonia is significantly reduced relative to that in the Eocene. During the Miocene, Comptonia remains were only found in the northern regions of China, such as Chifeng, Zhuozi of the Inner Mongolia Autonomous Region, and Weichang of Hebei (Figure 9C). This may be due to the apparent cooling event from the late Oligocene to the early Miocene [52] that had some impact on the survival of the genus. On the other hand, the geological age of the fossil site in Liangcheng, where Comptonia leaf fossils were reported by Zhang [55], is actually of the Miocene rather than the Pliocene, according to the investigation of stratigraphic correlations. In fact, after the Miocene, Comptonia fossils have hitherto not been found in China. However, the Pliocene strata is extensively exposed in China [54]. Thus, the preservation bias of Comptonia fossils should be excluded. An interesting question concerns the reason for the disappearance of Comptonia in China after the Miocene.
The vegetation types in Zhuozi, Chifeng, and Weichang during the Miocene period belong to mixed northern hardwood forests [54]. The climate of central Inner Mongolia during this period was warm and humid, similar to that of present-day eastern North America, and suitable for the growth of the Comptonia population. After the late Miocene, the global climate gradually cooled, leading to the global Great Ice Age of the Quaternary. Such drastic climatic change caused the vegetation type of the Inner Mongolia region to change from mixed northern hardwood forests to temperate grasslands. Moreover, central Inner Mongolia changed from being a humid area to a dry or semi-dry zone because of the aridification of the Chinese inland area. Tredici and Torrey [56] found that germination of Comptonia seeds is very difficult, meaning that Comptonia cannot reproduce annually by seed germination. In this case, root tillers became another important propagational pattern of Comptonia (i.e., new plantlets are formed by growing adventitious shoots on the roots that stick out of the ground), which would limit its dispersal scope. Moreover, the prolonged cooling and drying climates covering in the Inner Mongolia region after the late Miocene were not suitable for the survival of Comptonia. As a result, both climatic and progenitive factors may together have resulted in the disappearance of Comptonia in China.
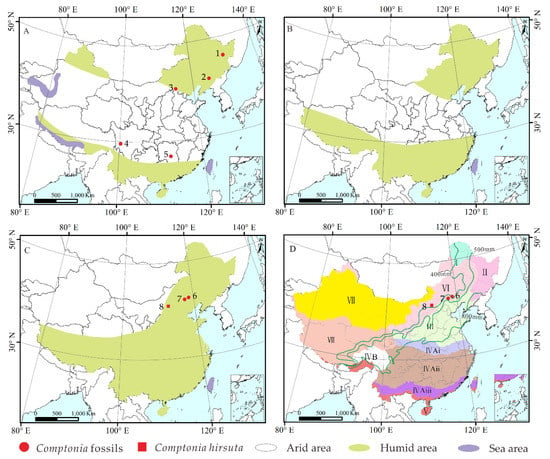
Figure 9.
Terrestrial climate changes from the Eocene to Miocene with the megafossil records of Comptonia in China. (A) Eocene, (B) Oligocene, and (C) Miocene (redrawn with permission from Ref. [57]. Copyright 2005 Palaeogeography, Palaeoclimatology, Palaeoecology and Ref. [58]. Copyright 2021 Taylor & Fancis). (D) Current vegetation distribution with the respect to annual precipitation in China (redrawn from EBVMC [59]). Base maps were constructed with ArcGIS 10.2 based on data from the Resource and Environment Data Cloud Platform [53]. The Arabic numbers in (A,C) represent the sites where Comptonia megafossils were collected in China, respectively: 1, Eocene of Yilan, Heilongjiang; 2, Eocene of Fushun, Liaoning; 3, Eocene of Zhangjiakou, Hebei; 4, Litang, Sichuan; 5, Xiangxiang, Hunan; 6, Chifeng, Inner Mongolia; 7, Weichang, Hebei; 8, early Miocene of Zhuozi, Inner Mongolia. The green lines represent the boundary of the precipitation. The Roman numerals in (D) designate different climate and vegetation types: I, cold-temperate deciduous coniferous forest; II, temperate mixed coniferous and deciduous broadleaved forest; III, warm-temperate deciduous broadleaved forest; IVAi, northern subtropical evergreen and deciduous broadleaved forest; IVAii, middle subtropical evergreen broadleaved forest; IVAiii, southern subtropical evergreen broadleaved forest; IVB, subtropical alpine coniferous forest; V, tropical monsoon rainforest and rainforest; VI, temperate steppe; VII, temperate desert; VIII, Tibetan Plateau alpine vegetation.
Author Contributions
Conceptualization, D.J. and L.X.; Field sampling, data processing, and measurement, D.J., L.X., L.G., X.L., J.L., Z.W., M.W., X.X., N.S. and C.F.; writing—original draft preparation, D.J. and L.X.; writing—review and editing, L.X. and L.G. All authors contributed suggestions and revised the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (No. 41872017); the Fundamental Research Funds for the Central Universities, CHD (Nos. 300102272206, 300102262903, 300102272901); the Foundation of State Key Laboratory of Palaeobiology and Stratigraphy (Nanjing Institute of Geology and Palaeontology, CAS) (Nos. 183125, 193113); Chang’an University Students’ Innovation and Entrepreneurship Training Program (No. G202010710054).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the editor and anonymous reviewers for their constructive comments and helpful suggestion; Qin Leng (Bryant University, USA) and Li Wang (Xishuangbanna Tropical Botanical Garden, China) for the collection of living plant specimens. We are also grateful to Xing Wang, Hongyu Wang, Jian Wang, and Man Yuan for collecting the fossils. And we would like to thank Chuntao Yin for drawing the diagrams.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Flora of North America. Available online: http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=233500420 (accessed on 29 July 2022).
- Dow, M.A.; Schwintzer, C.R. Seed germination, seedling emergence, and seed bank ecology of sweet fern (Comptonia peregrina (L.) Coult.). Can. J. Bot. 1999, 77, 1378–1386. [Google Scholar] [CrossRef]
- Berry, E.W. Living and Fossil Species of Comptonia. Am. Nat. 1906, 40, 485–524. [Google Scholar] [CrossRef]
- Elias, T.S. The genera of Myricaceae in the southeastern United States. J. Arnold Arbor. 1971, 52, 305–318. [Google Scholar] [CrossRef]
- Baghai, N.L.; Jorstad, R.B. Paleontology, paleoclimatology and paleoecology of the late middle Miocene musselshell creek flora, clearwater county Idaho. A preliminary study of a new fossil flora. Palaios 1995, 10, 424–436. [Google Scholar] [CrossRef]
- Chen, M.H.; Kong, Z.C.; Chen, Y. On the discovery of Palaeogene Flora from the Western Sichuan Pupperau and its significance in phytogeography. Acta Bot. Sinica. 1983, 25, 482–491. (In Chinese) [Google Scholar]
- Denk, T.; Grímsson, F.G.; Kvaček, Z. The Miocene floras of Iceland and their significance for late Cainozoic North Atlantic biogeography. Bot. J. Linn. Soc. 2005, 149, 369–417. [Google Scholar] [CrossRef]
- Dillhoff, R.M.; Leopold, E.B.; Manchester, S.R. The McAbee flora of British Columbia and its relation to the Early Middle Eocene Okanagan Highlands flora of the Pacific Northwest. Can. J. Earth Sci. 2005, 42, 151–166. [Google Scholar] [CrossRef]
- Ferguson, D.K. The contribution of micromorphology to the taxonomy and fossil record of the Myricaceae. Taxon 1998, 47, 333–335. [Google Scholar] [CrossRef]
- Friis, E.M. The Damgaard flora: A new middle Miocene flora from Denmark. Bull. Geol. Soc. Den. 1979, 27, 117–142. [Google Scholar] [CrossRef]
- Greenwood, D.R.; Archibald, S.B.; Mathewes, R.W.; Moss, P.T. Fossil biotas from the Okanagan Highlands, southern British Columbia and northeastern Washington State: Climates and ecosystems across an Eocene landscape. Can. J. Earth Sci. 2005, 42, 167–185. [Google Scholar] [CrossRef]
- Grímsson, F.; Denk, T. Floristic turnover in Iceland from 15 to 6 Ma extracting biogeographical signals from fossil floral assemblages. J. Biogeogr. 2007, 34, 1490–1504. [Google Scholar] [CrossRef]
- Hably, L.; Fernandez, M.T. A comparison of the Oligocene floras of the Tethyan and Central-Paratethyan areas on the basis of Spanish and Hungarian macroflora. Tert. Res. 1998, 18, 67–76. [Google Scholar]
- Iljinskaja, I.A.; Pneva, G.P.; Schvareva, N.Y. The Mamontova Gora Flora through Leaf Impressions. In The Miocene of Mamontova Gora (Stratigraphy and Paleoflora); Baranova, J.P., Il’jinskaja, I.A., Nikitin, V.P., Pneva, G.P., Fradkina, A.F., Schvareva, N.Y.A., Eds.; Nauka: Moscow, Russia, 1976; pp. 66–130. [Google Scholar]
- Ina, H. Plants from the middle Miocene Shukunohora sandstone facies of the Mizunama group, Mizunama city, Gifu prefecture, Central Japan. Bull. Mizunami Foss. Mus. 2004, 31, 73–76. (In Japanese) [Google Scholar]
- Kvaček, Z. Bílina: A window on early Miocene marshland environments. Rev. Palaeobot. Palyno. 1998, 101, 111–123. [Google Scholar] [CrossRef]
- Kvaček, Z.; Walther, H. Oligocene flora of Bechlejovice at Decin from the neovolcanic area of the Ceske stredohori Mountains, Czech Republic. Acta Musei. Nat. Pragae. 2004, 60, 9–60. [Google Scholar] [CrossRef]
- Manchester, S.R. Biogeographical relationships of North American Tertiary floras. Ann. Mo. Bot. Grad. 1999, 86, 472–522. [Google Scholar] [CrossRef]
- Matthews, J.V., Jr.; Ovenden, L.E. Upper Tertiary plant macrofossils from localities in Arctic/Subarctic North America: A review of the data. Arctic 1990, 43, 364–392. [Google Scholar] [CrossRef]
- McClaughry, J.D.; Gaylord, D.R. Middle Eocene sedimentary and volcanic infilling of an evolving supradetachment basin: White Lake Basin, south-central British Columbia. Cab. J. Earth Sci. 2005, 42, 49–66. [Google Scholar] [CrossRef]
- McIver, E.E.; Basinger, J.F. Early Tertiary floral evolution in the Arctic. Ann. Mo. Bot. Gard. 1999, 86, 523–545. [Google Scholar] [CrossRef]
- Momohara, A. Paleoecology and History of Metasequoia in Japan, with Reference to its Extinction and Survival in East Asia. In The Geobiology and Ecology of Metasequoia; LePage, B.A., Williams, C.J., Yang, H., Eds.; Springer: Berlin, Germany, 2005; pp. 115–136. [Google Scholar]
- Tao, J.R. The Evolution of the Upper Cretaceous Cenozoic Floras in China; Science Press: Beijing, China, 2000; pp. 1–282. (In Chinese) [Google Scholar]
- Teodoridis, V. Tertiary flora and vegetation of the locality Záhořínear Žatec (Most Basin, Czech Republic). Bull. Geosci. 2003, 78, 261–276. [Google Scholar]
- Teodoridis, V.; Kvaček, Z. Palaeobotanical research of the early Miocene deposits overlying the main coal seam (Libkovice and Lom Members) in the Most Basin (Czech Republic). Bull. Geosci. 2006, 81, 93–113. [Google Scholar] [CrossRef]
- Writing Group of Cenozoic Plants of China (WGCPC) (Ed.) Cenozoic Plants from China. In Fossil Plants of China; Science Press: Beijing, China, 1978; pp. 1–232. (In Chinese) [Google Scholar]
- Walther, H. Die Tertiärflora von Kleinsaubernitz bei Bautzen. Palaeontogr. Abt. B 1999, 249, 63–174. (In German) [Google Scholar] [CrossRef]
- Wilde, V.; Frankenhäuser, H. Comptonia-like leaves from the German Middle Eocene. Acta Palaeobot. Sup. 1999, 2, 447–463. [Google Scholar]
- Wolfe, J.A. Tertiary Plants from the Cook Inlet Region, Alaska; United States Geological Survey Professional Paper; United States Government Printing Office: Washington, DC, USA, 1966; Volume 398-B, pp. 1–32. [Google Scholar]
- Wolfe, J.A.; Wehr, W. Middle Eocene dicotyledonous plants from Republic, northeastern Washington. U. S. Geo. Surv. Bull. 1987, 1597, 1–26. [Google Scholar]
- Yasuno, T. Early Miocene pharyngeal teeth (Cyprinidae) fossils from northwestern Shan-in and Kyushu, Japan (I). Bull. Fukui City Mus. Nat. Hist. 2003, 50, 1–8. [Google Scholar]
- Yasuno, T. Early Miocene fossil assemblages discovered from Takeno Coast, Tyooka City, Hyogo Prefecture, central Japan. Bull. Fukui City Mus. Nat. Hist. 2005, 52, 43–65. [Google Scholar]
- Zhilin, S.G. History of the development of the temperate forest flora in Kazakhstan, U.S.S.R. from the Oligocene to the lower Miocene. Bot. Rev. 1989, 55, 205–330. [Google Scholar] [CrossRef]
- Zhilin, S.G.; Vikulin, S.V. Comptonia dryandrifolia (Myricaceae) from the Eocene of the south of Middle-Russian upland. Bot. J. 1986, 71, 148–153. [Google Scholar]
- Florin, R. Einige chinesische Tertiärpflanzen. Sven. Bot. Tidskr. 1920, 14, 239–243. (In German) [Google Scholar]
- Liang, X.Q.; Wilde, V.; Ferguson, D.K.; Kvaček, Z.; Ablaev, A.G.; Wang, Y.F.; Li, C.S. Comptonia naumannii (Myricaceae) from the early Miocene of Weichang, China, and the palaeobiogeographical implication of the genus. Rev. Palaeobot. Palyno. 2010, 163, 52–63. [Google Scholar] [CrossRef]
- Lu, P.; Liang, X.Q.; Li, Y.; Li, C.S. Leaves of Comptonia Columbiana from the Eocene of Northeast China and Its Paleobiogeographic Implications. J. Yuxi No. Univ. 2016, 8, 41–46. (In Chinese) [Google Scholar]
- Teodoridis, V.Z.; Kvaček, K.; Mach, J.; Sakala, J.; Daškova, P.R. Fossil Comptonia difformis (Sternberg) Berry (Myricaceae) from the type area in North Bohemia with comments on foliage anatomy and associated fruits. Bull. Geosci. 2017, 92, 185–210. [Google Scholar] [CrossRef][Green Version]
- Sadowski, E.M.; Seyfullah, L.J.; Regalado, L.; Skadell, L.E.; Gehler, A.; Gröhn, C.; Hoffeins, C.; Hoffeins, H.W.; Neumann, C.; Schneider, H.; et al. How diverse were ferns in the Baltic amber forest? J. Syst. Evol. 2019, 57, 305–328. [Google Scholar] [CrossRef]
- Budantsev, L. Magnoliophyta fossilia Rossiae et Civitatum Finitimarum, 3, Leitneriaceae Juglandaceae; Institutum Botanicum Komarovianum Academiae Scientiarum Rossicum: St. Petersburg, Russia, 1994; pp. 1–118. (In Russian) [Google Scholar]
- Meyer, W.; Manchester, S.R. The Oligocene Bridge Creek flora of the John Day Formation, Oregon. Univ. Calif. Publ. Geol. Sci. 1997, 141, 1–195. [Google Scholar]
- Christensen, E.F. The Søby Flora: Fossil plants from middle Miocene delta deposits of the Søby-Fastenholt area, Central Jutland, Denmark. Part I. Geol. Surv. Den. Greenl. Bull. 1975, 103, 1–41. [Google Scholar] [CrossRef]
- Mai, D.H.; Walther, H. Die Floren der Haselbacher Serie im Weißelster-Becken (Bezirk Leipzig, DDR). Abha. Staat. Muse. Mineral. Geol. Dres. 1978, 28, 1–200. (In German) [Google Scholar]
- Kvaček, J. Comments on the nomenclature of a fossil sweet-fern Comptonia difformis (Sternb.) Berry (Myricaceae). Taxon 2004, 53, 548–550. [Google Scholar] [CrossRef]
- Brongniart, A. Prodrome d’une Histoire des Végétaux Fossils; F. G. Levrault: Paris, France, 1828; p. 223. [Google Scholar]
- Wang, H.F.; Dai, T.M.; Fan, S.K.; Yang, X.C. K-Ar age timing of Hanuoba basalt in Zhangjiakou. Geochemistry 1985, 3, 206–215. (In Chinese) [Google Scholar]
- Li, D.M.; Li, Q. Systematic K-r dating of volcanic rock profile in Zuoyun, Shanxi. J. Earth 2003, 6, 559–562. (In Chinese) [Google Scholar]
- Liang, J.Q.; Leng, Q.; Höfig, D.F.; Niu, G.; Wang, L.; Royer, D.L.; Royer, D.L.; Burke, K.; Xiao, L.; Zhang, Y.G.; et al. Constraining conifer physiological parameters in leaf gas-exchange models for ancient CO2 reconstruction. Glob. Planet Change 2022, 209, 103737. [Google Scholar] [CrossRef]
- Bell, J.M.; Curtis, J.D. Development and ultrastructure of foliar glands of Comptonia peregrina (Myricaceae). Bot. Gazet. 1985, 146, 288–292. [Google Scholar] [CrossRef]
- Tiffney, B.H.; Manchester, S.R. The Use of Geological and Paleontological Evidence in Evaluating Plant Phylogeographic Hypotheses in the Northern Hemisphere Tertiary. Int. J. Plant Sci. 2001, 162, S3–S17. [Google Scholar] [CrossRef]
- Brikiatis, L. The De Geer, Thulean and Beringia routes: Key concepts for understanding early Cenozoic biogeography. J. Biogeogr. 2014, 41, 1036–1054. [Google Scholar] [CrossRef]
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef]
- Resource and Environment Data Cloud Platform. Available online: http://www.resdc.cn/Default.aspx (accessed on 19 May 2022).
- Liu, Y.S.; Zheng, Y.H. Flora of the Late Tertiary period. In The Flora of China during Geological Time; Li, X.X., Zhou, Z.Y., Cai, C.Y., Sun, G., Ouyang, S., Deng, L.H., Eds.; Science and Technology of Guangdong Press: Guangzhou, China, 1995; pp. 383–416. ISBN 7-5359-1536-1. (In Chinese) [Google Scholar]
- Zhang, Z.C. Angiosperm. In Atlas of Paleontology in North of China, Series 2nd; Writing Group of Atlas of Paleontology in North of China, Ed.; Geology Press: Beijing, China, 1976; pp. 1–261. (In Chinese) [Google Scholar]
- Del Tredici, P.; Torrey, J.G. On the germination of seeds of Comptonia peregrina, the sweet fern. Bot. Gazet. 1976, 137, 262–268. [Google Scholar] [CrossRef]
- Sun, X.; Wang, P. How old is the Asian monsoon system?—Palaeobotanical records from China. Palaeogeogr. Palaeocl. 2005, 222, 181–222. [Google Scholar] [CrossRef]
- Li, X.C.; Manchester, S.R.; Xiao, L.; Wang, Q.; Hu, Y.; Sun, B.N. Ormosia (Fabaceae: Faboideae) from the Miocene of southeastern China support historical expansion of the tropical genus in East Asia. Hist. Biol. 2021, 33, 3561–3578. [Google Scholar] [CrossRef]
- EBVMC (Editor Board of Vegetation Map of China, Chinese Academy of Sciences). Vegetation Map of the People s Republic of China (1:1,000,000); Geology Publishing House Press: Beijing, China, 2007; (In Chinese with English abstract). [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).