Use of Lichens to Evaluate the Impact of Post-Earthquake Reconstruction Activities on Air Quality: A Case Study from the City of L’Aquila
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- LDVS > 50: very high naturalness
- 41 < LDVS ≤ 50: high naturalness
- 31 < LDVS ≤ 40: average naturalness
- 21 < LDVS ≤ 30: low naturalness
- 11 < LDVS ≤ 20: average alteration
- 1 ≤ LDVS ≤ 10: high alteration
- LDVS < 1: very high alteration
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hale, M.E. How to Know the Lichens, 1st ed.; W.C. Brown Company Publishers: Dubuque, IA, USA, 1969; pp. 1–226. [Google Scholar]
- Hale, M.E. The Biology of Lichens, 3rd ed.; Edward Arnold: London, UK, 1983; pp. 1–190. [Google Scholar]
- Ahmadjian, V. The Lichen Symbiosis, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1993; pp. 1–250. [Google Scholar]
- Conti, M.E. Lichens as Bioindicators of Air Pollution. In Biological Monitoring: Theory and Applications. Bioindicators and Biomarkers for Environmental Quality and Human Exposure Assessment; Conti, M.E., Ed.; WIT Press: Boston, MA, USA, 2008; Volume 17, pp. 111–162. [Google Scholar]
- Abas, A. A systematic review on biomonitoring using lichen as the biological indicator: A decade of practices, progress and challenges. Ecol. Indic. 2021, 121, 107197. [Google Scholar] [CrossRef]
- Sloof, J.E. Environment Lichenology: Biomonitoring Trace-Element Air Pollution. PhD Thesis, Delft University of Technology, Delft, The Netherlands, 1993; p. 202. [Google Scholar]
- Nash, T.H., III. Lichen Biology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 1–502. [Google Scholar]
- Szczepaniak, K.; Biziuk, M. Aspects of the biomonitoring studies using mosses and lichens as indicators of metal pollution. Environ. Res. 2003, 93, 221–230. [Google Scholar] [CrossRef]
- Amodio, M.; Catino, S.; Dambruoso, P.R.; De Gennaro, G.; Di Gilio, A.; Giungato, P.; Laiola, E.; Marzocca, A.; Mazzone, A.; Sardaro, A.; et al. Atmospheric Deposition: Sampling Procedures, Analytical Methods, and Main Recent Findings from the Scientific Literature. Adv. Meteorol. 2014, 2014, 161730. [Google Scholar] [CrossRef]
- Nimis, P.L. Linee-guida per la bioindicazione degli effetti dell’inquinamento tramite la biodiversità dei licheni epifiti. In Atti del Workshop Biomonitoraggio della Qualità dell’Aria sul territorio Nazionale; Piccini, C., Salvati, S., Eds.; Agenzia Nazionale per la Protezione dell’Ambiente: Roma, Italy, 1999; pp. 267–277. [Google Scholar]
- Liu, H.; Zhao, L.; Fang, S.; Liu, S.; Hu, J.; Wang, L.; Liu, X.; Wu, Q. Use of the lichen Xanthoria mandschurica in monitoring atmospheric elemental deposition in the Taihang Mountains, Hebei, China. Sci. Rep. 2016, 6, 23456. [Google Scholar] [CrossRef]
- Cansaran-Duman, D.; Atakol, O.; Aras, S. Assessment of air pollution genotoxicity by RAPD in Evernia prunastri (L.) Ach. from around iron-steel factory in Karabk, Turkey. J. Environ. Sci. 2011, 23, 1171–1178. [Google Scholar] [CrossRef]
- Hauck, M.; Bruyn, U.D.; Leuschner, C. Dramatic diversity losses in epiphytic lichens in temperate broad-leaved forests during the last 150 years. Biol. Cons. 2013, 157, 136–145. [Google Scholar] [CrossRef]
- El Rhzaoui, G.; Divakar, P.K.; Crespo, A.; Tahiri, H. Biomonitoring of air pollutants by using lichens (Evernia prunastri) in areas between Kenitra and Mohammedia cities in Morocco. Lazaroa 2015, 36, 21–30. [Google Scholar] [CrossRef]
- Loppi, S. Lichens as Bioindicators of Geothermal Air Pollution in Central Italy. Bryologist 1996, 99, 41–48. [Google Scholar] [CrossRef]
- Calvelo, S.; Baccalá, N.; Liberatore, S. Environmental Bioindicators Lichens as Bioindicators of Air Quality in Distant Areas in Patagonia (Argentina). Environ. Bioindic. 2009, 4, 1–37. [Google Scholar] [CrossRef]
- Käffer, M.I.; Martins, S.M.; Alves, C.; Camejo-Pereira, V.; Fachel, J.; Ferrão-Vargas, V.M. Corticolous lichens as environmental indicators in urban areas in southern Brazil. Ecol. Indic. 2011, 11, 1319–1332. [Google Scholar] [CrossRef]
- Sigal, L.L. The Relationship of Lichen and Bryophytes Research to Regulatory Decisions in the United States. In Lichens, Bryophytes and Air Quality; Nash, T., Wirth, V., Eds.; Bibliotheca Lichenologica, Cramer: Berlin, Germany, 1988; Volume 30, pp. 269–287. [Google Scholar]
- McCune, B. Lichen communities as indicators of forest health. Bryologist 2000, 103, 353–356. [Google Scholar] [CrossRef]
- Nimis, P.L.; Skert, N.; Castello, M. Biomonitoraggio di metalli in traccia tramite licheni in aree a rischio nel Friuli-Venezia Giulia. Studia Geobot. 1999, 18, 3–49. [Google Scholar]
- Miani, N.; Skert, N.; Grahonja, R. Atlante dei licheni epifiti più comuni rinvenuti in studi di biomonitoraggio ambientale nella provincia di Trieste; ARPA FVG, Dipartimento di Trieste: Provincia di Trieste, Italy, 2006; pp. 1–54. [Google Scholar]
- LeBlanc, F.S.; De Sloover, J. Relation between industrialization and the distribution and growth of epiphytic lichens and mosses in Montreal. Can. J. Bot. 1970, 48, 1485–1496. [Google Scholar] [CrossRef]
- Brunialti, G.; Frati, L. Bioaccumulation with lichens: The Italian experience. Int. J. Environ. Stud. 2014, 71, 15–26. [Google Scholar] [CrossRef]
- Martinelli, A.; Cifani, G.; Petrucci, G.; Cialone, G.; Mancini, C.P.; Lemme, A. Sisma Abruzzo 2009—La situazione del centro storico della città dell’Aquila e l’avvio alla ricostruzione. L’edilizia 2010, 166, 116–125. [Google Scholar]
- Gizzi, S. The City of L’Aquila after the 2009 Earthquake: Review of Connections between Depopulation, Identity and Continuity. In Demographic Analysis—Selected Concepts, Tools, and Applications; Klimczuk, A., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Iannella, M.; Liberatore, L.; Biondi, M. The effects of a sudden urbanization on micromammal communities: A case study of post-earthquake L’Aquila (Abruzzi Region, Italy). Ital. J. Zool. 2016, 83, 255–262. [Google Scholar] [CrossRef][Green Version]
- Avveduto, A.; Ferella, F.; De Giovanni, M.; Innocenzi, V.; Pace, L.; Tripodi, P. L’Aquila smart clean air city: The Italian pilot project for healthy urban air. Environments 2017, 4, 78. [Google Scholar] [CrossRef]
- Curci, G.; Guijarro, J.A.; Di Antonio, L.; Di Bacco, M.; Di Lena, B.; Scorzini, A.R. Building a local climate reference dataset: Application to the Abruzzo region (Central Italy), 1930–2019. Int. J. Clim. 2021, 41, 4414–4436. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Giuliani, D.; Antenucci, F. Valori medi climatici dal 1951 al 2000 nella Regione Abruzzo, Servizio Presidi Tecnici di Supporto al Settore Agricolo—DPD023, Ufficio Coordinamento servizi vivaistici e agrimeteo. Scerni, Italy. 2017. Available online: https://www.regione.abruzzo.it/system/files/agricoltura/agrometereologia/VALORI_MEDI_CLIMATICI_NELLA_REGIONE_ABRUZZO.pdf (accessed on 1 March 2022).
- Biondi, E.; Baldoni, M. The climate and vegetation of peninsular Italy. Coll. Phytosoc. 1995, 23, 675–721. [Google Scholar]
- Ministero dell’Ambiente e della Tutela del Territorio e del Mare. Geoportale Nazionale. 2009. Available online: http://www.pcn.minambiente.it/mattm/ (accessed on 1 March 2022).
- ANPA—Agenzia Nazionale Protezione dell’Ambiente. I.B.L.: Indice di Biodiversità Lichenica; Manuale ANPA, Serie manuali e linee guida 2/2001; ANPA: Rome, Italy, 2001; pp. 1–90. [Google Scholar]
- Purvis, O.W.; Coppins, B.J.; Hawksworth, D.L.; James, P.W.; Moore, D.M. The Lichen Flora of Great Britain and Ireland; Natural History Museum Publications: London, UK, 1992; pp. 1–710. [Google Scholar]
- Nimis, P.L. I macrolicheni d’Italia. Chiavi analitiche per la determinazione. Gortania 1987, 8, 101–220. [Google Scholar]
- Nimis, P.L.; Martellos, S. Materiali per una guida ai licheni epifiti d’Italia. Available online: http://dbiodbs.units.it/carso/chiavi_pub21?sc=120 (accessed on 29 June 2022).
- Nimis, P.L.; Martellos, S. ITALIC—The Information System on Italian Lichens. Version 7.0, 2022. University of Trieste, Dept. of Biology. Available online: https://italic.units.it/index.php?procedure=idkeys (accessed on 29 June 2022).
- Asta, J.; Erhardt, W.; Ferretti, M.; Fornasier, F.; Kirschbaum, U.; Nimis, P.L.; Wirth, V. Mapping lichen diversity as an indicator of environmental quality. In Monitoring with Lichens—Monitoring Lichens; NATO Science Series. Series IV: Earth and Environmental Sciences; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; Springer: Dordrecht, The Netherlands, 2002; Volume 7, pp. 273–279. [Google Scholar]
- Brunialti, G.; Frati, L.; Malegori, C.; Giordani, P.; Malaspina, P. Do Different Teams Produce Different Results in Long-Term Lichen Biomonitoring? Diversity 2019, 11, 43. [Google Scholar] [CrossRef]
- Llewellyn, T.; Gaya, E.; Murrell, D.J. Are Urban Communities in Successional Stasis? A Case Study on Epiphytic Lichen Communities. Diversity 2020, 12, 330. [Google Scholar] [CrossRef]
- Cook, R.R.; Angermeier, P.L.; Finn, D.S.; Poff, N.L.; Krueger, K.L. Geographic variation in patterns of nestedness among local stream fish assemblages in Virginia. Oecologia 2004, 140, 639–649. [Google Scholar] [CrossRef]
- Staniczenko, P.P.A.; Kopp, J.C.; Allesina, S. The ghost of nestedness in ecological networks. Nat. Commun. 2013, 4, 1391. [Google Scholar] [CrossRef]
- Strona, G.; Fattorini, S. On the Methods to Assess Significance in Nestedness Analyses. Theory Biosci. 2014, 133, 179–186. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing. 2022. Available online: http://www.r-project.org/ (accessed on 15 March 2022).
- Strona, G.; Galli, P.; Seveso, D.; Montano, S.; Fattorini, S. Nestedness for Dummies (NeD): A user friendly web interface for exploratory nestedness analysis. J. Stat. Softw. 2014, 59, 1–9. [Google Scholar] [CrossRef]
- Strona, G.; Fattorini, F. NeD—Nestedness for Dummies. 2014. Available online: https://ecosoft.alwaysdata.net/ (accessed on 1 March 2022).
- Nash, T.H.; Gries, C. Lichens as Indicators of Air Pollution. In The Handbook of Environmental Chemistry. Air Pollution; Hutzinger, O., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; Volume 4, pp. 1–29. [Google Scholar]
- Nimis, P.L. ITALIC—The Information System on Italian Lichens. Version 7.0. University of Trieste, Dept of Biology. Available online: https://italic.units.it/index.php?procedure=qtaxon (accessed on 29 June 2022).
- Llop, E.; Pinho, P.; Matos, P.; Pereira, M.J.; Branquinho, C. The use of lichen functional groups as indicators of air quality in a Mediterranean urban environment. Ecol. Indic. 2012, 13, 215–221. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Rose, F. Qualitative scale for estimating sulphur dioxide air pollution in England and Wales using epiphytic lichens. Nature 1970, 227, 145–148. [Google Scholar] [CrossRef]
- Nimis, P.L.; Castello, M.; Perotti, M. Lichens as biomonitors of sulphur dioxide pollution in La Spezia (Northern Italy). Lichenologist 1990, 22, 333–344. [Google Scholar] [CrossRef]
- Nimis, P.L.; Lazzarin, A.; Lazzarin, G.; Gasparo, D. Lichens as bioindicators of air pollution by S02 in the Veneto region (NE Italy). Studia Geobot. 1991, 11, 3–76. [Google Scholar]
- van Dobben, H.F.; de Bakker, A.J. Re-mapping epiphytic lichen biodiversity in The Netherlands: Effects of decreasing SO2 and increasing NH3. Acta Bot. Neerl. 1996, 45, 55–71. [Google Scholar] [CrossRef]
- Kricke, R.; Loppi, S. Bioindication: The I.A.P. Approach. In Monitoring with Lichens—Monitoring Lichens; NATO Science Series; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; Springer: Dordrecht, The Netherlands, 2002; Volume 7, pp. 21–37. [Google Scholar]
- Davies, L.; Bates, J.; Bell, J.; James, P.; Purvis, O. Diversity and sensitivity of epiphytes to oxides of nitrogen in London. Environ. Pollut. 2007, 146, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Grindon, L.H. The Manchester Flora; William White: London, UK, 1859; p. 565. [Google Scholar]
- Nylander, W. Les Lichen du Jardin du Luxembourg. Bull. Soc. Bot. 1866, 13, 364–372. [Google Scholar] [CrossRef]
- Forman, R.T.T. Urban Ecology. Science of Cities; Cambridge University Press: Cambridge, UK, 2014; pp. 1–462. [Google Scholar]
- Karagulian, F.; Belis, C.A.; Dora, C.F.C.; Prüss-Ustün, A.M.; Bonjour, S.; Adair-Rohani, H.; Amann, M. Contributions to cities’ ambient particulate matter (PM): A systematic review of local source contributions at global level. Atmos. Environ. 2015, 120, 475–483. [Google Scholar] [CrossRef]
- Fattorini, S. Ecologia Urbana; Ediesse: Roma, Italy, 2019; pp. 1–300. [Google Scholar]
- Thunis, P.; Clappier, A.; de Meij, A.; Pisoni, E.; Bessagnet, B.; Tarrason, L. Why is the city’s responsibility for its air pollution often underestimated? A focus on PM2.5. Atmos. Chem. Phys. 2021, 21, 18195–18212. [Google Scholar] [CrossRef]
- Forman, R.T.T. Towns, Ecology, and the Land; Cambridge University Press: Cambridge, UK, 2019; pp. 1–586. [Google Scholar]
- Hölscher, K.; Frantzeskaki, N. Perspectives on urban transformation research: Transformations in, of, and by cities. Urban Transform. 2021, 3, 2. [Google Scholar] [CrossRef]
- Beck, C.M.; Geyh, A.; Srinivasan, A.; Breysse, P.N.; Eggleston, P.A.; Buckley, T.J. The Impact of a Building Implosion on Airborne Particulate Matter in an Urban Community. J. Air Waste Manag. Assoc. 2003, 53, 1256–1264. [Google Scholar] [CrossRef][Green Version]
- Dorevitch, S.; Demirtas, H.; Perksy, V.W.; Erdal, S.; Conroy, L.; Schoonover, T.; Scheff, P.A. Demolition of high-rise public housing increases particulate matter air pollution in communities of high-risk asthmatics. J. Air Waste Manag. Assoc. 2006, 56, 1022–1032. [Google Scholar] [CrossRef]
- Brown, A.; Barrett, J.E.; Robinson, H.; Potgieter-Vermaak, S. Risk assessment of exposure to particulate output of a demolition site. Environ. Geochem. Health 2015, 37, 675–687. [Google Scholar] [CrossRef]
- Institute of Air Quality Management. Guidance on Monitoring in the Vicinity of Demolition and Construction Sites (Version 1.1); Institute of Air Quality Management: London, UK, 2018; p. 31. Available online: https://www.the-ies.org/sites/default/files/reports/Monitoring%20Construction%20Sites.pdf (accessed on 1 March 2022).
- Augenti, N.; Parisi, R. Learning from Construction Failures due to the 2009 L’Aquila, Italy, Earthquake. J. Perform. Constr. Facil. 2010, 24, 536–555. [Google Scholar] [CrossRef]
- Opdyke, M.R.; Dolney, B.E.; Frost, L.L.; Roy, J.D. A Study of Epiphytic Lichen Communities in Urban and Rural Environments in Southwestern Pennsylvania. J. Pa. Acad. Sci. 2011, 85, 151–158. [Google Scholar] [CrossRef]
- Lättman, H.; Bergman, K.O.; Malin, R.; Malin, T.; Lars, W.; Per, M. Decline in lichen biodiversity on oak trunks due to urbanization. Nord. J. Bot. 2014, 32, 518–528. [Google Scholar] [CrossRef]
- Mcmullin, R.; Bennett, L.; Bjorgan, O.; Bourque, D.; Burke, C.; Clarke, M.; Gutgesell, M.K.; Krawiec, P.L.; Malyon, R.; Mantione, A.; et al. Relationships between air pollution, population density, and lichen biodiversity in the Niagara Escarpment World Biosphere Reserve. Lichenologist 2016, 48, 593–605. [Google Scholar] [CrossRef]
- Iannarelli, A. Biomonitoraggio della qualità dell’aria nella Provincia dell’Aquila tramite licheni epifiti. Rapporto tecnico. Arta Abruzzo. 2018. Available online: https://www.artaabruzzo.it/download/pubblicazioni/20211005_rapporto_tecnico_licheni_2018.pdf (accessed on 2 August 2022).
- Nimis, P.L. The Lichens of Italy. A Second Annotated Catalogue; EUT Edizioni Università di Trieste: Trieste, Italy, 2016; pp. 1–740. [Google Scholar]
- Loppi, S.; Pirintsos, S.A.; De Dominicis, V. Soil contribution to the elemental composition of epiphytic lichens (Tuscany, central Italy). Environ. Monit. Assess. 1999, 58, 121–131. [Google Scholar] [CrossRef]
- Mikhaylov, A. Lichens as indicators of atmospheric pollution in urban ecosystems. Isr. J. Ecol. Evol. 2020, 67, 60–68. [Google Scholar] [CrossRef]
- Taghizadeh, F.; Jafari, A.J.; Gholami, M.; Kermani, M.; Arfaeinia, H.; Mohammadi, S.; Dowlati, M.; Shahsavani, A. Monitoring of airborne asbestos fibers in an urban ambient air of Shahryar City, Iran: Levels, spatial distribution, seasonal variations, and health risk assessment. Environ. Sci. Pollut. Res. Int. 2019, 26, 6450–6459. [Google Scholar] [CrossRef] [PubMed]
- Kakooei, H.; Yunesian, M.; Marioryad, H.; Azam, K. Assessment of airborne asbestos fiber concentrations in urban area of Tehran, Iran. Air Qual. Atmos. Health 2009, 2, 39–45. [Google Scholar] [CrossRef]
- Jung, H.S.; Jang, J.; Cho, Y.; Lee, J.C.; Kim, H. Asbestos in the ambient air from rural, urban, residential, baseball and mining areas in South Korea. Environ. Chem. Lett. 2021, 19, 3487–3495. [Google Scholar] [CrossRef]
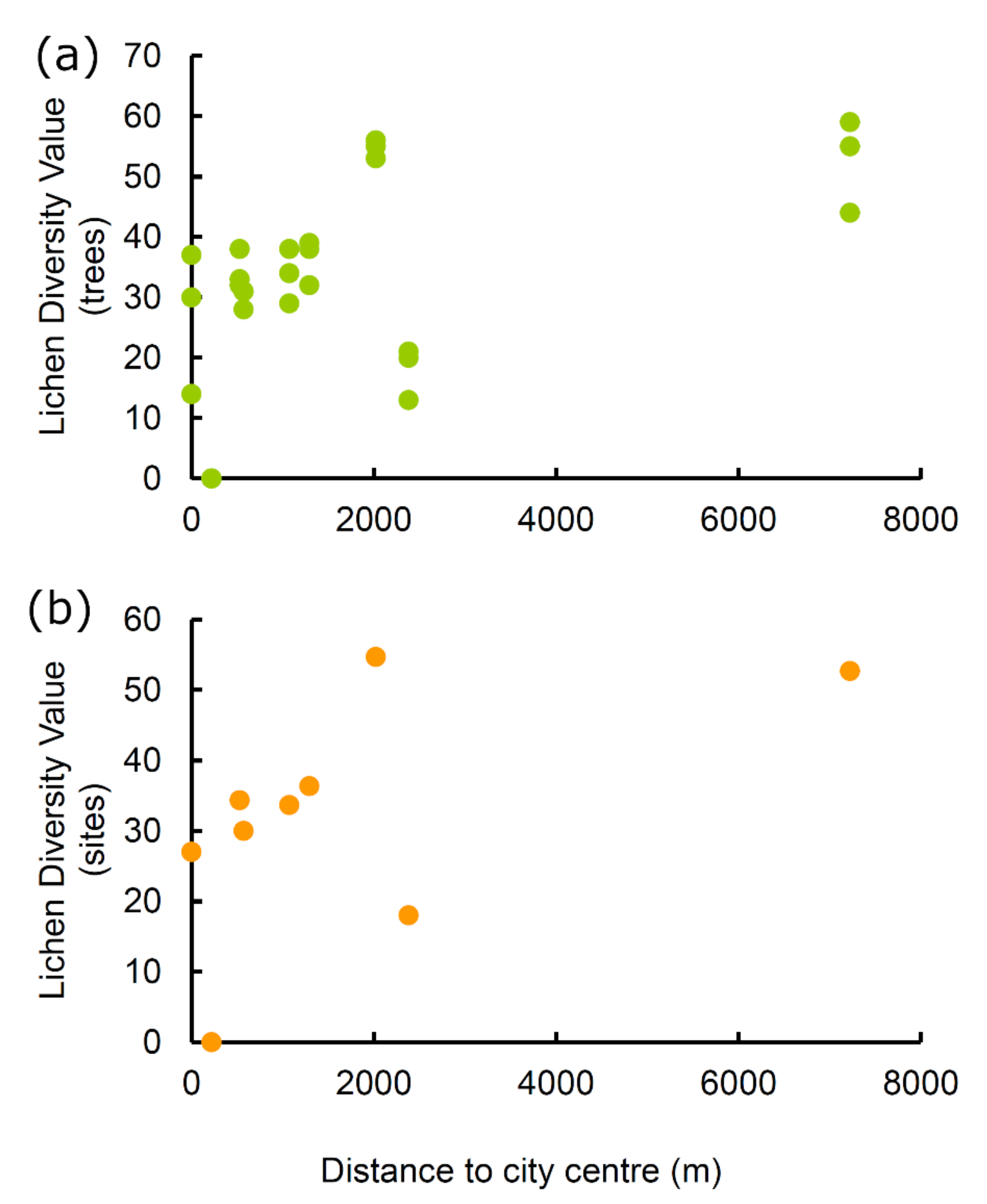

| Site | Geographic Location | Distance from City Centre (Piazza Palazzo) (m) | Lichen Diversity Value (LDVS) | Environmental Quality | Sampled Tree |
|---|---|---|---|---|---|
| 1 Piazza Palazzo | 42.351185 N 13.398683 E | 0 | 27.000 | Low naturalness | Tilia platyphyllos Scop. |
| 2 Piazza dei Nove Martiri | 42.349842 N 13.400554 E | 218 | 0.000 | Very high alteration | Quercus ilex L. |
| 3 Giovanni XXIII | 42.351226 N 13.392293 E | 527 | 34.333 | Average naturalness | Tilia platyphyllos Scop. |
| 4 Via dei Giardini | 42.346035 N 13.398791 E | 572 | 30.000 | Low naturalness | Cercis siliquastrum L. |
| 5 Via XXIV Maggio | 42.341733 N 13.395884 E | 1072 | 33.667 | Average naturalness | Tilia platyphyllos Scop. |
| 6 Via Colagrande | 42.360944 N 13.406858 E | 1293 | 36.333 | Average naturalness | Aesculus hippocastanum L. |
| 7 Via Mariana di Poggio di Roio | 42.336272 N 13.384677 E | 2022 | 54.667 | Very high naturalness | Quercus pubescens Will. |
| 8 Via Amiternum | 42.366085 N 13.377949 E | 2382 | 18.000 | Average alteration | Juglans regia L. |
| 9 Doline Monticchio/Ocre | 42.312348 N 13.469104 E | 7225 | 52.667 | Very high naturalness | Ostrya carpinifolia Scop. |
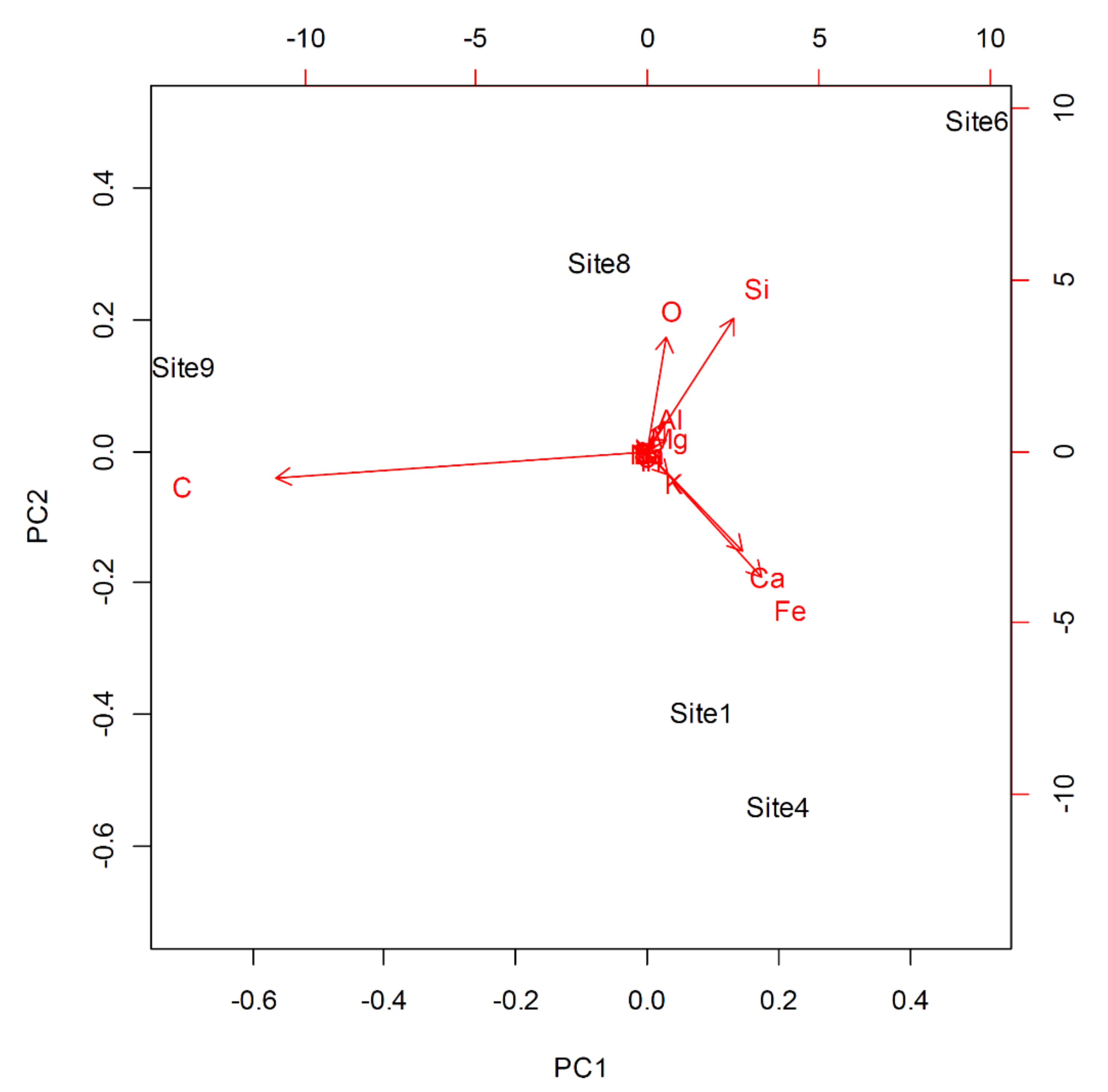
| PC | Location Eigenvalue | Percentage of Explained Variance | Percentage of Cumulative Variance |
|---|---|---|---|
| 1 | 45.091 | 69.448 | 69.448 |
| 2 | 15.552 | 23.953 | 93.401 |
| 3 | 2.85773 | 4.401 | 97.802 |
| 4 | 1.42676 | 2.198 | 100.000 |
| 5 | 4.30 × 10−31 | 6.62 × 10−33 | 100.000 |
| Element | PC1 | PC2 | PC3 | PC4 | PC5 |
|---|---|---|---|---|---|
| C | −0.903 | −0.109 | 0.044 | −0.028 | −0.356 |
| O | 0.048 | 0.474 | −0.675 | 0.441 | −0.341 |
| K | 0.049 | −0.100 | 0.082 | 0.046 | −0.353 |
| S | −0.012 | 0.003 | 0.044 | −0.076 | −0.275 |
| Si | 0.212 | 0.547 | 0.466 | −0.295 | −0.470 |
| Mg | 0.045 | 0.045 | 0.147 | 0.016 | −0.216 |
| Al | 0.043 | 0.116 | 0.079 | −0.034 | 0.113 |
| P | 0.004 | −0.021 | 0.010 | −0.002 | −0.047 |
| Fe | 0.277 | −0.519 | 0.251 | 0.568 | −0.374 |
| Cl | 0.002 | −0.012 | −0.023 | −0.074 | −0.047 |
| Ca | 0.233 | −0.410 | −0.471 | −0.615 | −0.353 |
| Na | 0.000 | 0.000 | 0.001 | −0.013 | −0.013 |
| Ti | 0.006 | −0.020 | 0.060 | 0.045 | −0.082 |
| Br | 0.000 | −0.002 | −0.005 | −0.006 | −5.605 × 10−5 |
| Element | PC1 | PC2 | PC3 | PC4 | PC5 |
|---|---|---|---|---|---|
| C | −0.997 | −0.071 | 0.012 | −0.005 | 0.103 |
| O | 0.142 | 0.821 | −0.501 | 0.232 | 0.267 |
| K | 0.614 | −0.739 | 0.258 | 0.103 | −0.406 |
| S | −0.559 | 0.095 | 0.524 | −0.635 | 0.147 |
| Si | 0.523 | 0.792 | 0.289 | −0.129 | −0.086 |
| Mg | 0.703 | 0.411 | 0.579 | 0.044 | −0.473 |
| Al | 0.519 | 0.817 | 0.238 | −0.072 | 0.087 |
| P | 0.299 | −0.935 | 0.191 | −0.020 | −0.260 |
| Fe | 0.646 | −0.711 | 0.147 | 0.236 | −0.421 |
| Cl | 0.096 | −0.446 | −0.358 | −0.815 | 0.755 |
| Ca | 0.627 | −0.647 | −0.319 | −0.294 | 0.282 |
| Na | 0.001 | −0.073 | 0.105 | −0.992 | 0.605 |
| Ti | 0.275 | −0.551 | 0.695 | 0.371 | −0.839 |
| Br | 0.102 | −0.492 | −0.625 | −0.597 | 0.787 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Biase, L.; Di Lisio, P.; Pace, L.; Arrizza, L.; Fattorini, S. Use of Lichens to Evaluate the Impact of Post-Earthquake Reconstruction Activities on Air Quality: A Case Study from the City of L’Aquila. Biology 2022, 11, 1199. https://doi.org/10.3390/biology11081199
Di Biase L, Di Lisio P, Pace L, Arrizza L, Fattorini S. Use of Lichens to Evaluate the Impact of Post-Earthquake Reconstruction Activities on Air Quality: A Case Study from the City of L’Aquila. Biology. 2022; 11(8):1199. https://doi.org/10.3390/biology11081199
Chicago/Turabian StyleDi Biase, Letizia, Paolo Di Lisio, Loretta Pace, Lorenzo Arrizza, and Simone Fattorini. 2022. "Use of Lichens to Evaluate the Impact of Post-Earthquake Reconstruction Activities on Air Quality: A Case Study from the City of L’Aquila" Biology 11, no. 8: 1199. https://doi.org/10.3390/biology11081199
APA StyleDi Biase, L., Di Lisio, P., Pace, L., Arrizza, L., & Fattorini, S. (2022). Use of Lichens to Evaluate the Impact of Post-Earthquake Reconstruction Activities on Air Quality: A Case Study from the City of L’Aquila. Biology, 11(8), 1199. https://doi.org/10.3390/biology11081199







