Correlation between Carbonic Anhydrase Isozymes and the Evolution of Myocardial Infarction in Diabetic Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Aim of the Study
2.2. Materials
- Group 1—Included 30 diabetic patients with acute myocardial infarction
- Group 2—Included 30 non-diabetic patients with acute myocardial infarction
2.3. Methods
2.4. Statistical Analysis
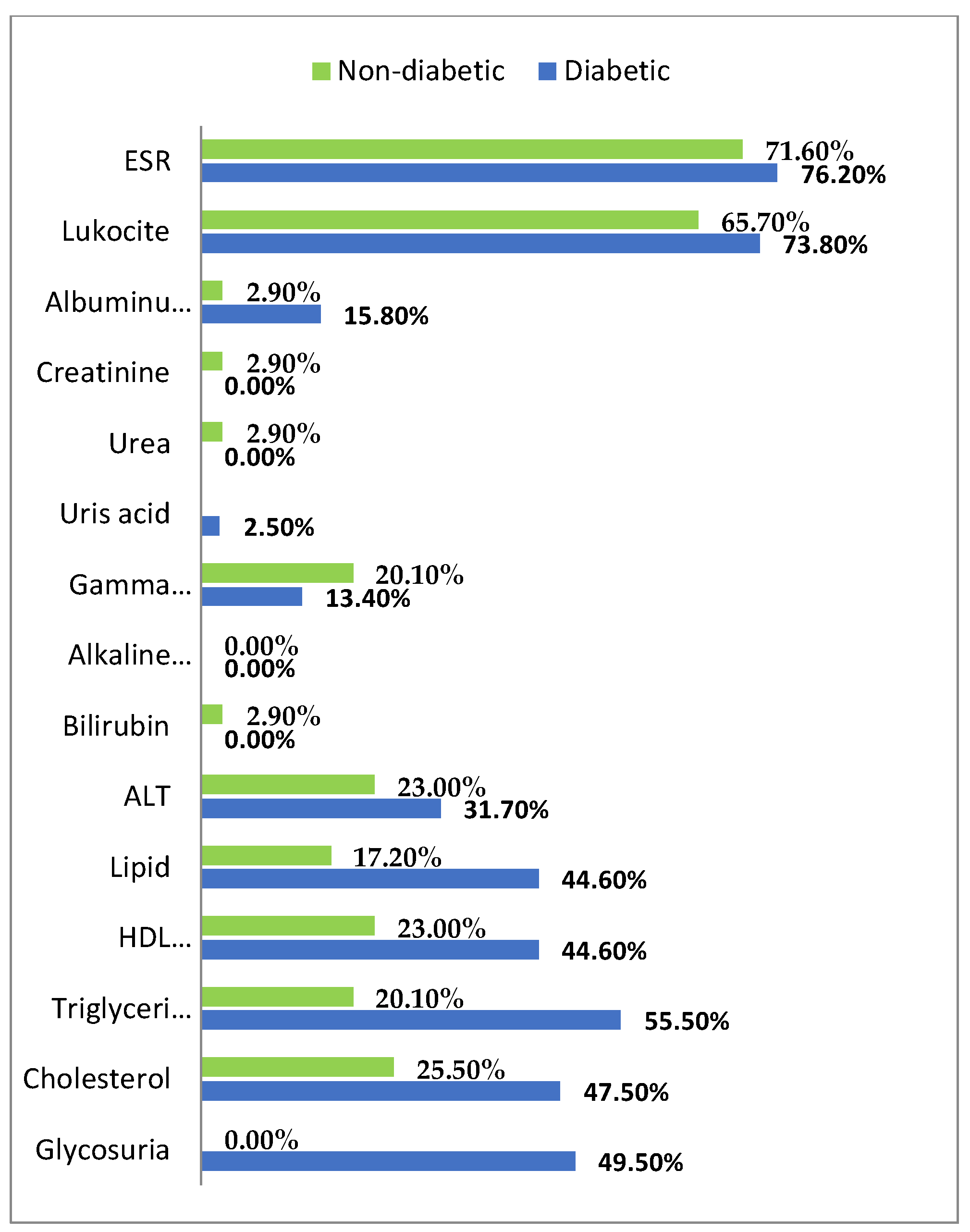
3. Results
3.1. Characteristics of the Population (Age, Gender and Environment)
3.2. Electrocardiographic Evaluation
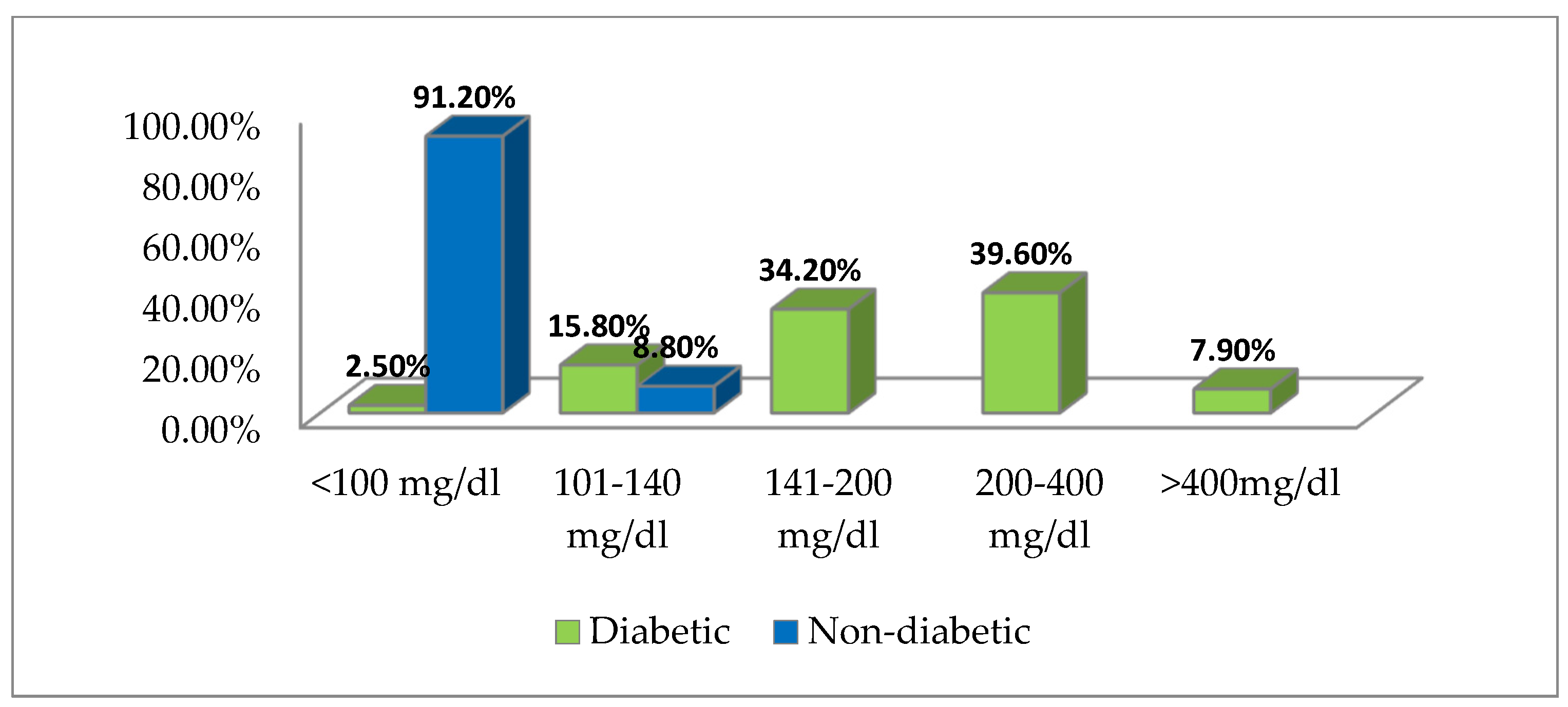
3.3. Blood Sugar Values Distribution
- creatine phosphokinase (CPK): VN = 25–90 U/I;
- creatine phosphokinase-MB (CPK-MB): VN < 5% of CK;
- lactic dehydrogenase (LDH): VN = 150–240 U/I;
- cardiac-specific troponins (cTnT and cTnI): VN < 0.1 ng/mL;
- oxaloacetic glutamic transaminase (GOT): VN < 35–40 IU.
3.4. Correlation of Lactic Acid Values with ST-Segment Elevation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thielmann, M.; Massoudy, P.; Neuhäuser, M.; Tsagakis, K.; Marggraf, G.; Kamler, M.; Mann, K.; Erbel, R.; Jakob, H. Prognostic value of preoperative cardiac troponin I in patients undergoing emergency coronary artery bypass surgery with non-ST-elevation or ST-elevation acute coronary syndromes. Circulation 2006, 114 (Suppl. S1), I-448–I-453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Than, M.P.; Aldous, S.J.; Troughton, R.W.; Pemberton, C.J.; Richards, A.M.; Frampton, C.M.; Florkowski, C.M.; George, P.M.; Bailey, S.; Young, J.M.; et al. Detectable high-sensitivity cardiac troponin within the population reference interval conveys high 5-year cardiovascular risk: An observational study. Clin. Chem. 2018, 64, 1044–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freisinger, E.; Fuerstenberg, T.; Malyar, N.M.; Wellmann, J.; Keil, U.; Breithardt, G.; Reinecke, H. German nationwide data on current trends and management of acute myocardial infarction: Discrepancies between trials and real-life. Eur. Heart J. 2014, 35, 979–988. [Google Scholar] [CrossRef] [Green Version]
- De Waha, S.; Patel, M.R.; Granger, C.B.; Ohman, E.M.; Maehara, A.; Eitel, I.; Ben-Yehuda, O.; Jenkins, P.; Thiele, H.; Stone, G.W. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: An individual patient data pooled analysis from seven randomized trials. Eur. Heart J. 2017, 38, 3502–3510. [Google Scholar] [CrossRef]
- Tasar, O.; Karabay, A.K.; Oduncu, V.; Kirma, C. Predictors and outcomes of no-reflow phenomenon in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Coron. Artery Dis. 2019, 30, 270–276. [Google Scholar] [CrossRef]
- Califf, R.M.; Abdelimeguid, A.E.; Kuntz, R.E.; Popma, J.J.; Davidson, C.J.; Cohen, E.A.; Kleiman, N.S.; Mahaffey, K.W.; Topol, E.J.; Pepine, C.J.; et al. Myonecrosis after revascularization procedures. J. Am. Coll. Cardiol. 1998, 31, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Nam, K.; Jeon, Y.; Kim, W.H.; Jung, D.E.; Kwon, S.M.; Kang, P.; Cho, Y.J.; Kim, T.K. Intraoperative glucose variability, but not average glucose concentration, may be a risk factor for acute kidney injury after cardiac surgery: A retrospective study. Can. J. Anaesth. 2019, 66, 921–933. [Google Scholar] [CrossRef] [Green Version]
- Stalikas, N.; Papazoglou, A.S.; Karagiannidis, E.; Panteris, E.; Moysidis, D.; Daios, S.; Anastasiou, V.; Patsiou, V.; Koletsa, T.; Sofidis, G.; et al. Association of stress induced hyperglycemia with angiographic findings and clinical outcomes in patients with ST-elevation myocardial infarction. Cardiovasc. Diabetol. 2022, 21, 140. [Google Scholar] [CrossRef]
- Mone, P.; Gambardella, J.; Minicucci, F.; Lombardi, A.; Mauro, C.; Santulli, G. Hyperglicemia Drives Stent Restenosis in STEMI Patients. Diabetes Care 2021, 44, e192–e193. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Y.-M.; Zhu, J.; Wu, S.; Wang, J.; Zhang, H.; Shao, X.-H. Predictive value of the stress hyperglycemia ratio in patients with acute ST-segment elevation myocardial infarction: Insights from a multi-center observational study. Cardiovasc. Diabetol. 2022, 21, 48. [Google Scholar] [CrossRef]
- Mone, P.; Pansini, A.; Rizzo, M.; Minicucci, F.; Mauro, C. ST-Elevation Myocardial Infarction Patients with Hyperglycemia: Effects of Intravenous Adenosine. Am. J. Med. Sci. 2022, 363, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Bellou, V.; Belbasis, L.; Tzoulaki, I.; Evangelou, E. Risk factors for type 2 diabetes mellitus: An exposure-wide umbrella review of meta-analyses. PLoS ONE 2018, 13, e0194127. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Fujihara, K.; Horikawa, C.; Sato, T.; Iwanaga, M.; Yamada, T.; Kato, K.; Watanabe, K.; Shimano, H.; Izumi, T.; et al. Diabetes mellitus and risk of new-onset and recurrent heart failure: A systematic review and meta-analysis. ESC Heart Fail. 2020, 7, 2146–2174. [Google Scholar] [CrossRef]
- Bouthoorn, S.; Valstar, G.B.; Gohar, A.; den Ruijter, H.M.; Reitsma, H.B.; Hoes, A.W.; Rutten, F.H. The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: A systematic review and meta-analysis. Diab. Vasc. Dis. Res. 2018, 15, 477–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alterio, V.; Monti, S.M.; de Simone, G. Thermal-stable carbonic anhydrases: A structural overview. In Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications, 1st ed.; Frost, S.C., McKenna, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 387–404. [Google Scholar]
- Maren, T.H. CARBONI Canhydrase: Chemistry, physiology and inhibition. Physiol. Rev. 1967, 47, 595–781. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Emerging role of carbonic anhydrase inhibitors. Clin. Sci. 2021, 135, 1233–1249. [Google Scholar] [CrossRef]
- Parkkila, S.; Kivela, A.J.; Kaunisto, K.; Parkkila, A.K.; Hakkola, J.; Rajaniemi, H.; Waheed, A.; Sly, W.S. The plasma membrane CARBONIC anhydrase in murine hepatocytes identified as isozyme XIV. BMC Gastroenterol. 2002, 2, 13–16. [Google Scholar] [CrossRef] [Green Version]
- Pastoreková, S.; Parkkila, S.; Parkkila, A.K.; Opavský, R.; Zelník, V.; Saarnio, J.; Pastorek, J. Carbonic anhydrase IX, MN/CA IX: Analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology 1997, 112, 398–408. [Google Scholar] [CrossRef]
- Parkkila, S. An overview of the distribution and function of CARBONIC anhydrase in mammals. In The Carbonic Anhydrases; Chegwidden, W.R., Carter, N.D., Edwards, Y.H., Eds.; Birkhäuser: Basel, Switzerland, 2000; pp. 79–93. [Google Scholar]
- Puscas, I.; Coltau, M.; Baican, M.; Pasca, R.; Domuta, G.; Hecht, A. Vasoconstrictive drugs increase CARBONICic anhydrase I in vascular smooth muscle while vasodilating drugs reduce the activity of this isozyme by a direct mechanism of action. Drugs Exp. Clin. Res. 2001, 27, 231–238. [Google Scholar]
- Benkovic, S.; Hammes-Schiffer, S. A perspective on enzyme catalysis. Science 2003, 301, 1196–1202. [Google Scholar] [CrossRef] [Green Version]
- Aamand, R.; Dalsgaard, T.; Jensen, F.B.; Simonsen, U.; Roepstorff, A.; Fago, A. Generation of nitric oxide from nitrite by carbonic anhydrase: A possible link between metabolic activity and vasodilation. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H2068–H2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demandt, J.A.F.; Dubois, L.J.; van Kuijk, K.; Zat’ovičová, M.; Jin, H.; Parkkila, S.; van der Laan, S.W.; Jelenska, L.; Mees, B.M.E.; Reutelingsperger, C.P.M.; et al. The hypoxia-sensor carbonic anhydrase IX affects macrophage metabolism, but is not a suitable biomarker for human cardiovascular disease. Sci. Rep. 2021, 11, 425. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Małek, L.A.; Maciejewski, P.; Wąsek, W.; Niewada, M.; Kamiński, B.; Wiecki, J.D.; Kośmider, M.; Kubica, J.; Rużyłło, W.; et al. Impact of diabetes on survival in patients with ST-segment elevation myocardial infarction treated by primary angioplasty: Insights from the POLISH STEMI registry. Atherosclerosis 2010, 210, 516–520. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Qiu, C.; Zhu, H.; Pan, S.; Jia, H.; Kang, H.; Guan, G.; Hui, R.; Zhu, L.; et al. Diabetes mellitus exacerbates post-myocardial infarction heart failure by reducing sarcolipin promoter methylation. ESC Heart Fail. 2020, 7, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Khalifah, R.G. The CARBONIC dioxide hidration activity of CARBONIC anhydrase: Stop-flow kinetic studies on the native human isozymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrases—An overview. Curr. Pharm. Des. 2008, 14, 603–614. [Google Scholar] [CrossRef]
- Kosiborod, M.; Gomes, M.B.; Nicolucci, A.; Pocock, S.; Rathmann, W.; Shestakova, M.V.; Watada, H.; Shimomura, I.; Chen, H.; Cid-Ruzafa, J.; et al. Vascular Complications in Patients with Type 2 Diabetes: Prevalence and Associated Factors in 38 Countries (the DISCOVER Study Program). Cardiovasc. Diabetol. 2018, 17, 150. [Google Scholar] [CrossRef] [Green Version]
- El-Lebedy, D.; Raslan, H.M.; Mohammed, A.M. Apolipoprotein E Gene Polymorphism and Risk of Type 2 Diabetes and Cardiovascular Disease. Cardiovasc. Diabetol. 2016, 15, 12. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Xu, C.; Tang, X.; Liu, Z.; Lin, X.; Meng, H.; Shi, C.; Ma, K.; Xiao, B.; Li, L. Endoplasmic reticulum stress -related secretory proteins as biomarkers of early myocardial ischemia-induced sudden cardiac deaths. Int. J. Legal Med. 2022, 136, 159–169. [Google Scholar] [CrossRef]
- Xu, C.; Yu, B.; Zhao, X.; Lin, X.; Tang, X.; Liu, Z.; Gao, P.; Ge, J.; Wang, S.; Li, L. Valosin Containing Protein as a Specific Biomarker for Predicting the Development of Acute Coronary Syndrome and Its Complication. Front. Cardiovasc. Med. 2022, 9, 803532. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, X.; Chen, R.; Li, J.; Zhou, J.; Liu, C.; Zhou, P.; Wang, Y.; Chen, Y.; Zhao, H.; et al. Association of PCSK9 with inflammation and platelet activation markers and recurrent cardiovascular risks in STEMI patients undergoing primary PCI with or without diabetes. Cardiovasc. Diabetol. 2022, 21, 80. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics of the Diabetic Group and Non-Diabetic Group | Diabetic MD ± DS | Non-Diabetic MD ± DS | ||
|---|---|---|---|---|
| Age (years) | 56.7 ± 7.3 | 64.6 ± 6.9 | ||
| Gender Women | 19 | Percentage% 63.33 | 17 | Percentage% 56.66 |
| Men | 11 | 36.66 | 13 | 43.33 |
| Environment Urban | 28 | 93.33 | 25 | 83.33 |
| Rural | 2 | 6.66 | 5 | 16.66 |
| Electrocardiographic Evaluation | Diabetic | Non-Diabetic |
|---|---|---|
| % | % | |
| Pathological Q wave | 18.3 | 8.8 |
| ST segment change | 81.2 | 91.6 |
| ST Elevation | 78.7 | 82.8 |
| ST Subleveling | 2.5 | 8.8 |
| T wave change | 31.7 | 25.5 |
| Location of the Infarction | Diabetic | Non-Diabetic |
|---|---|---|
| % | % | |
| Lower | 31.7 | 28.9 |
| Previous | 7.9 | 20.1 |
| Anteroseptal | 21.3 | 14.2 |
| Previously stretched | 18.3 | 5.9 |
| Side | 20.8 | 30.9 |
| Normal Values | Increased Values | p Value | |||
|---|---|---|---|---|---|
| Parameter | % | Mp ± DS | % | Mp ± DS | |
| Diabetic | |||||
| CPK | 23.8 | 68.1 ± 8.5 | 76.2 | 458.5 ± 40.7 | p < 0.001 |
| CPK-MB | 21.3 | 5.2 ± 1.3 | 78.7 | 68.2 ± 7.9 | p < 0.01 |
| LDH | 2.5 | 159.7 ± 21.6 | 97.5 | 625.0 ± 64.3 | p < 0.001 |
| cTnT- cTnI | 0 | - | 100 | 2.2 ± 0.3 | - |
| GOT | 26.2 | 27.5 ± 3.9 | 73.8 | 116.6 ± 12.3 | p < 0.001 |
| Non-diabetic | |||||
| CPK | 11.3 | 56.3 ± 6.6 | 88.7 | 399.4 ± 41.3 | p < 0.001 |
| CPK-MB | 8.8 | 5.0 ± 1.2 | 91.2 | 43.4 ± 5.7 | p < 0.001 |
| LDH | 0 | - | 100 | 575.3 ± 61.8 | - |
| cTnT- cTnI | 0 | - | 100 | 2.1 ± 0.2 | - |
| GOT | 40.2 | 25.7 ± 3.2 | 59.8 | 107.2 ± 11.8 | p < 0.01 |
| Lactic Acid | Diabetic | Non-Diabetic | p Value |
|---|---|---|---|
| % | % | ||
| <25 mg/dL | 27.0 | 30.0 | p > 0.05 |
| 25–35 mg/dL | 29.7 | 33.3 | p < 0.01 |
| >35 mg/dL | 43.2 | 36.7 | p < 0.001 |
| Lactic Acid | Diabetic | Non-Diabetic | ||
|---|---|---|---|---|
| ST-Segment Elevation | <30% | >30% | <30% | >30% |
| <25 mg/dL p > 0.05 | 20.0 | 80.0 | - | 100.0 |
| 25–35 mg/dL | 36.4 | 63.6 | 20.0 | 80.0 |
| >35 mg/dL p < 0.001 | 75.0 | 25.0 | 55.6 | 44.4 |
| Red Blood Cells (UE/mL) | Normal Values | Diabetic | Non-Diabetic | p Value |
|---|---|---|---|---|
| CA I red blood cells (UE/mL) | 0.262 ± 0.011 | 0.582 ± 0.021 * | 0.574 ± 0.018 * | p < 0.01 |
| CA II red blood cells (UE/mL) | 1.015 ± 0.083 | 1.701 ± 0.118 * | 1.042 ± 0.105 | p < 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magheru, S.; Magheru, C.; Maghiar, F.; Sachelarie, L.; Marc, F.; Moldovan, C.M.; Romila, L.; Hoza, A.; Farcas, D.M.; Gradinaru, I.; et al. Correlation between Carbonic Anhydrase Isozymes and the Evolution of Myocardial Infarction in Diabetic Patients. Biology 2022, 11, 1189. https://doi.org/10.3390/biology11081189
Magheru S, Magheru C, Maghiar F, Sachelarie L, Marc F, Moldovan CM, Romila L, Hoza A, Farcas DM, Gradinaru I, et al. Correlation between Carbonic Anhydrase Isozymes and the Evolution of Myocardial Infarction in Diabetic Patients. Biology. 2022; 11(8):1189. https://doi.org/10.3390/biology11081189
Chicago/Turabian StyleMagheru, Sorina, Calin Magheru, Florin Maghiar, Liliana Sachelarie, Felicia Marc, Corina Maria Moldovan, Laura Romila, Anica Hoza, Dorina Maria Farcas, Irina Gradinaru, and et al. 2022. "Correlation between Carbonic Anhydrase Isozymes and the Evolution of Myocardial Infarction in Diabetic Patients" Biology 11, no. 8: 1189. https://doi.org/10.3390/biology11081189
APA StyleMagheru, S., Magheru, C., Maghiar, F., Sachelarie, L., Marc, F., Moldovan, C. M., Romila, L., Hoza, A., Farcas, D. M., Gradinaru, I., & Hurjui, L. L. (2022). Correlation between Carbonic Anhydrase Isozymes and the Evolution of Myocardial Infarction in Diabetic Patients. Biology, 11(8), 1189. https://doi.org/10.3390/biology11081189








