Recovery of Ischemic Limb and Femoral Artery Endothelial Function Are Preserved in Mice with Dextran Sodium Sulfate-Induced Chronic Colitis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chronic DSS-Induced Colitis and Critical Limb Ischemia Mouse Model
2.3. Analysis of Colitis and Ischemic Limbs
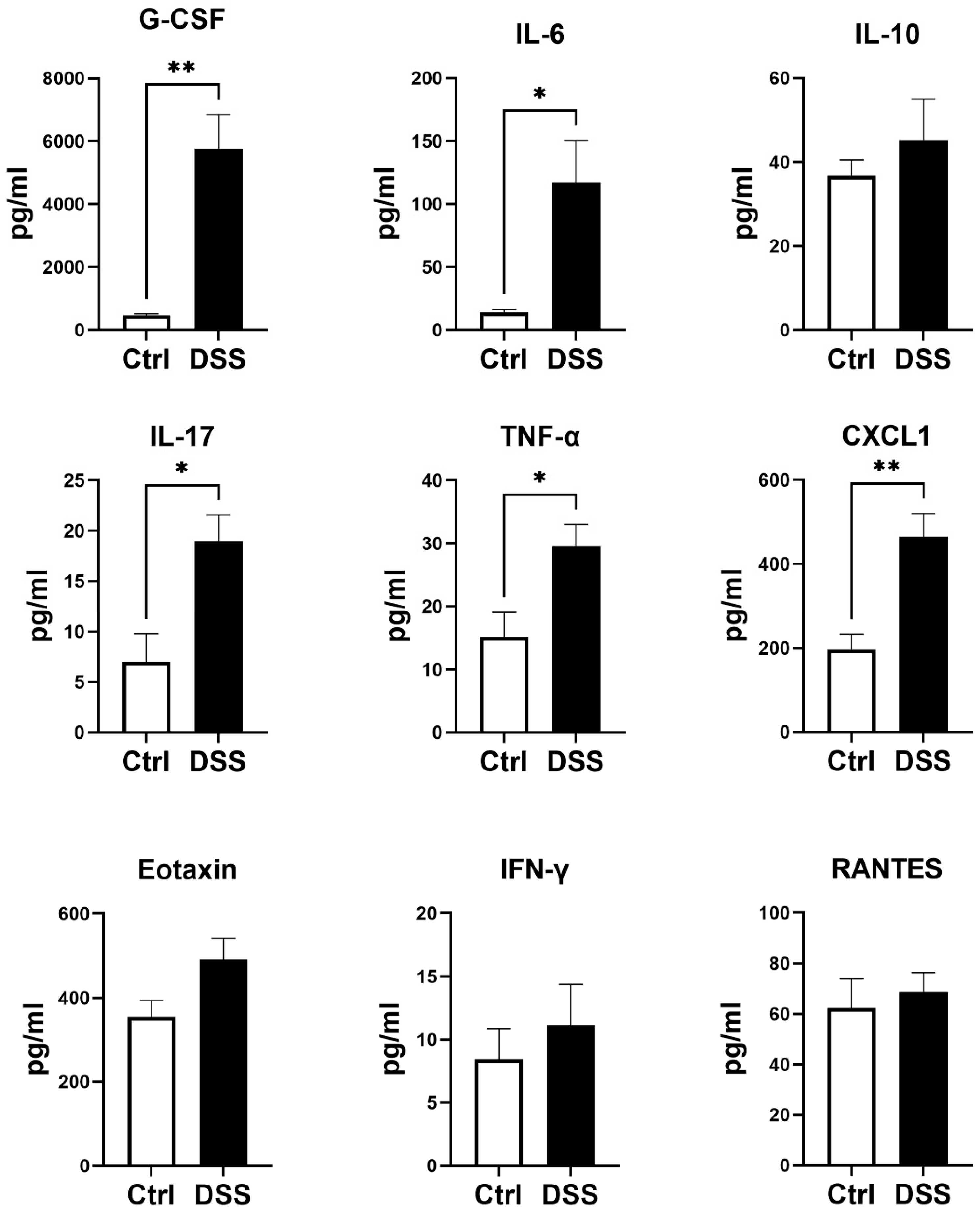
2.4. Analyses of Plasma Cytokines
2.5. Laser Doppler Perfusion Imaging and Function Assessment of Ischemic Limb
2.6. Analysis of Vascular Density and ROS Production
2.7. Evaluation of Femoral Artery Endothelial Function Ex Vivo
2.8. Statistical Analysis
3. Results
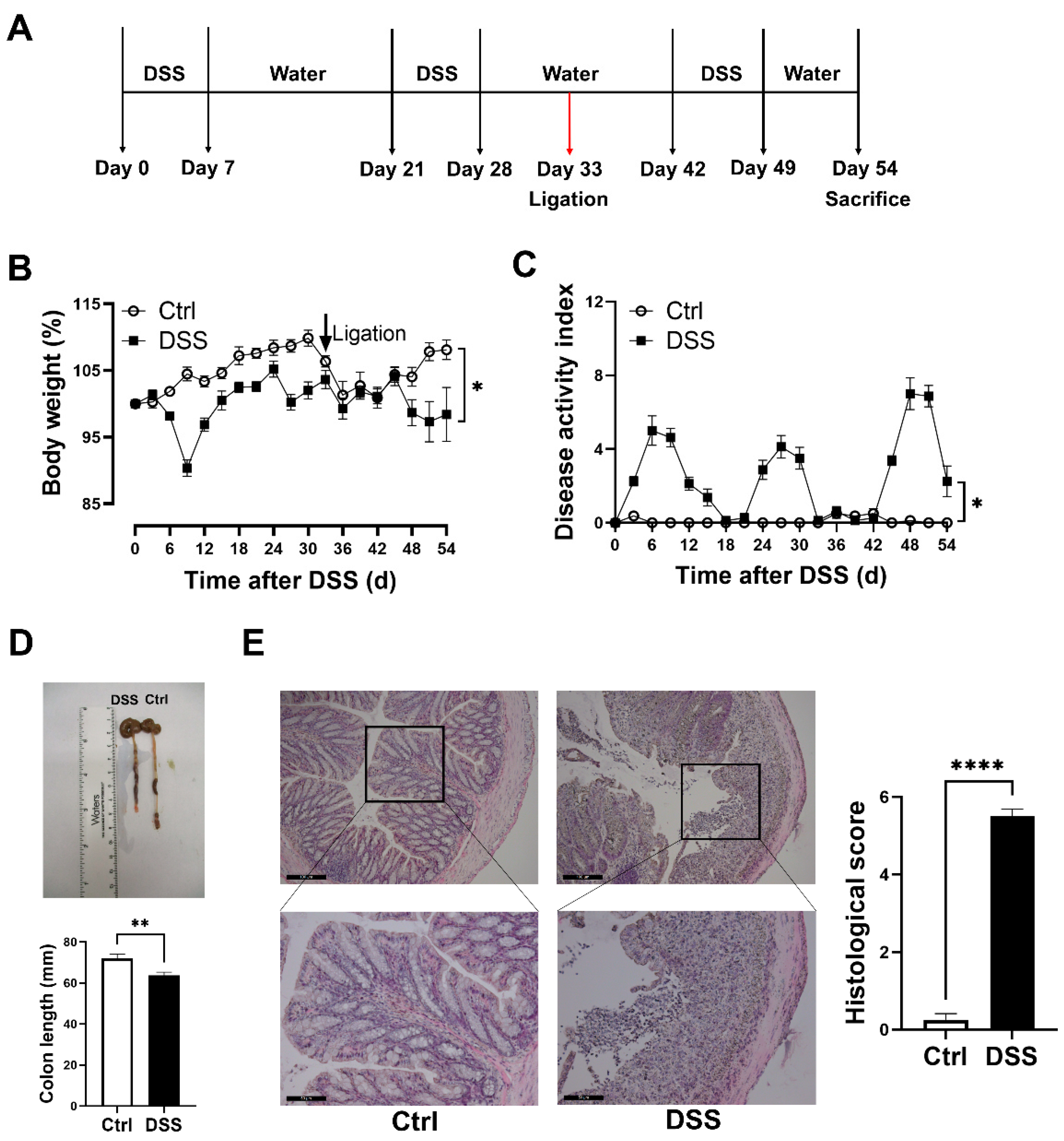
3.1. Evaluation of Chronic DSS-Induced Colitis Models
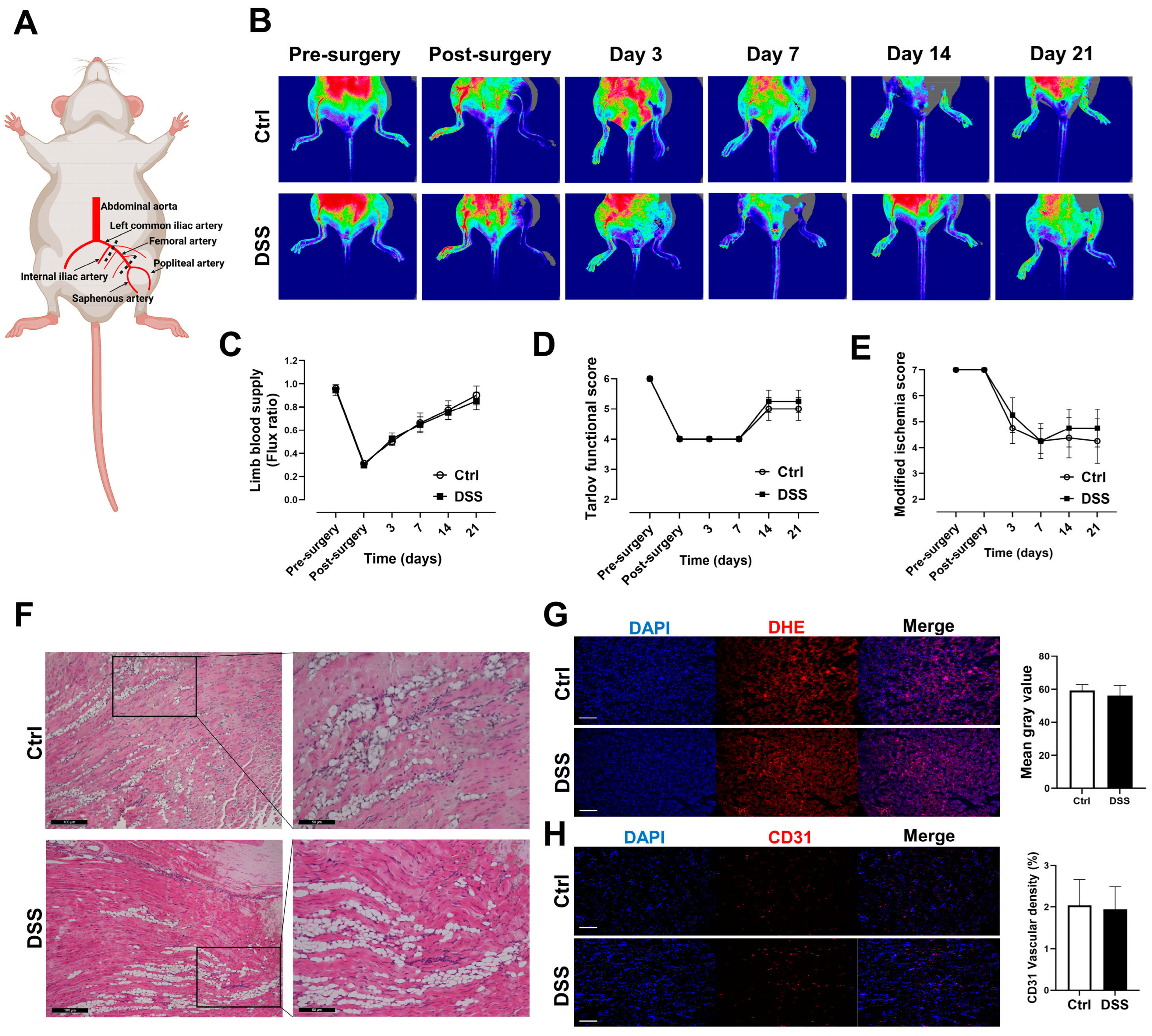
3.2. Blood Flow and Function Recoveries of the Ischemic Limb Were Preserved in Mice with Chronic Colitis
3.3. No Differences in Tissue ROS Level and Vascular Density in Mice with Chronic Colitis
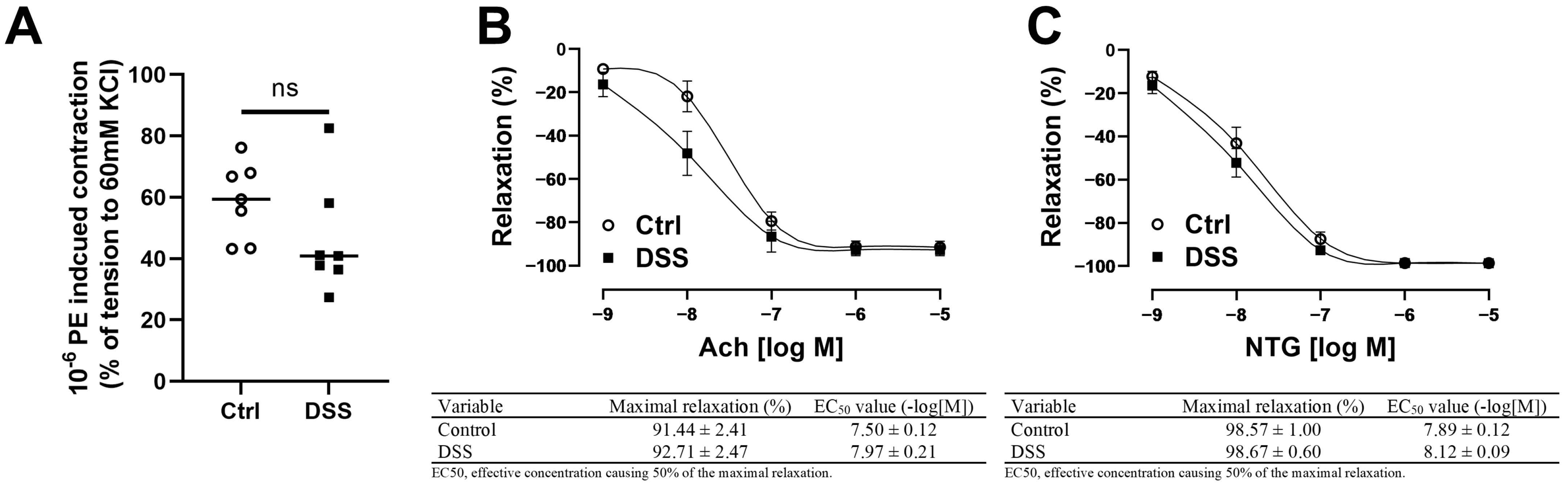
3.4. Femoral Artery Endothelium-Dependent and -Independent Vasodilation Were Preserved in Mice with Chronic Colitis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Wu, H.; Hu, T.; Hao, H.; Hill, M.A.; Xu, C.; Liu, Z. Inflammatory bowel disease and cardiovascular diseases: A concise review. Eur. Heart J. Open 2022, 2, oeab029. [Google Scholar] [CrossRef]
- Rungoe, C.; Basit, S.; Ranthe, M.F.; Wohlfahrt, J.; Langholz, E.; Jess, T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: A nationwide Danish cohort study. Gut 2013, 62, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Kirchgesner, J.; Beaugerie, L.; Carrat, F.; Andersen, N.N.; Jess, T.; Schwarzinger, M. Increased risk of acute arterial events in young patients and severely active IBD: A nationwide French cohort study. Gut 2018, 67, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Lerman, A.; Zeiher, A.M. Endothelial function: Cardiac events. Circulation 2005, 111, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Ozturk, K.; Guler, A.K.; Cakir, M.; Ozen, A.; Demirci, H.; Turker, T.; Demirbas, S.; Uygun, A.; Gulsen, M.; Bagci, S. Pulse Wave Velocity, Intima Media Thickness, and Flow-mediated Dilatation in Patients with Normotensive Normoglycemic Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 1314–1320. [Google Scholar] [CrossRef]
- Roifman, I.; Sun, Y.C.; Fedwick, J.P.; Panaccione, R.; Buret, A.G.; Liu, H.; Rostom, A.; Anderson, T.J.; Beck, P.L. Evidence of endothelial dysfunction in patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2009, 7, 175–182. [Google Scholar] [CrossRef]
- Wu, H.; Xu, M.; Hao, H.; Hill, M.A.; Xu, C.; Liu, Z. Endothelial Dysfunction and Arterial Stiffness in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 3179. [Google Scholar] [CrossRef]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef]
- Wu, H.; Hu, T.; Zhang, L.; Xia, X.; Liu, X.; Zhu, Q.; Wang, M.; Sun, Z.; Hao, H.; Cui, Y.; et al. Abdominal Aortic Endothelial Dysfunction Occurs in Female Mice With Dextran Sodium Sulfate-Induced Chronic Colitis Independently of Reactive Oxygen Species Formation. Front. Cardiovasc. Med. 2022, 9, 871335. [Google Scholar] [CrossRef] [PubMed]
- Niiyama, H.; Huang, N.F.; Rollins, M.D.; Cooke, J.P. Murine model of hindlimb ischemia. J. Vis. Exp. 2009, 23, e1035. [Google Scholar] [CrossRef]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar] [PubMed]
- Ogrodnik, M.; Zhu, Y.; Langhi, L.G.P.; Tchkonia, T.; Krüger, P.; Fielder, E.; Victorelli, S.; Ruswhandi, R.A.; Giorgadze, N.; Pirtskhalava, T.; et al. Obesity-Induced Cellular Senescence Drives Anxiety and Impairs Neurogenesis. Cell Metab. 2019, 29, 1061–1077.e8. [Google Scholar] [CrossRef]
- Wang, H.; Agarwal, P.; Xiao, Y.; Peng, H.; Zhao, S.; Liu, X.; Zhou, S.; Li, J.; Liu, Z.; He, X. A Nano-In-Micro System for Enhanced Stem Cell Therapy of Ischemic Diseases. ACS Cent. Sci. 2017, 3, 875–885. [Google Scholar] [CrossRef]
- Tarlov, I.M. Spinal cord compression studies. III. Time limits for recovery after gradual compression in dogs. AMA Arch. Neurol. Psychiatry 1954, 71, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Westvik, T.S.; Fitzgerald, T.N.; Muto, A.; Maloney, S.P.; Pimiento, J.M.; Fancher, T.T.; Magri, D.; Westvik, H.H.; Nishibe, T.; Velazquez, O.C.; et al. Limb ischemia after iliac ligation in aged mice stimulates angiogenesis without arteriogenesis. J. Vasc. Surg. 2009, 49, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Rabiolo, A.; Bignami, F.; Rama, P.; Ferrari, G. VesselJ: A New Tool for Semiautomatic Measurement of Corneal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8199–8206. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zou, M.H. Measurement of Reactive Oxygen Species (ROS) and Mitochondrial ROS in AMPK Knockout Mice Blood Vessels. Methods Mol. Biol. 2018, 1732, 507–517. [Google Scholar] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Mason, J.C.; Libby, P. Cardiovascular disease in patients with chronic inflammation: Mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur. Heart J. 2015, 36, 482–489c. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, D.H.; Shin, D.W.; Han, K.D.; Yoon, H.; Shin, C.M.; Park, Y.S.; Kim, N. Patients with inflammatory bowel disease have an increased risk of myocardial infarction: A nationwide study. Aliment. Pharmacol. Ther. 2019, 50, 769–779. [Google Scholar] [CrossRef]
- Criqui, M.H.; Matsushita, K.; Aboyans, V.; Hess, C.N.; Hicks, C.W.; Kwan, T.W.; McDermott, M.M.; Misra, S.; Ujueta, F.; on behalf of the American Heart Association Council on Epidemiology and Prevention; et al. Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e171–e191. [Google Scholar] [CrossRef]
- Lin, T.Y.; Chen, Y.G.; Lin, C.L.; Huang, W.S.; Kao, C.H. Inflammatory Bowel Disease Increases the Risk of Peripheral Arterial Disease: A Nationwide Cohort Study. Medicine 2015, 94, e2381. [Google Scholar] [CrossRef]
- Pande, R.L.; Brown, J.; Buck, S.; Redline, W.; Doyle, J.; Plutzky, J.; Creager, M.A. Association of monocyte tumor necrosis factor α expression and serum inflammatory biomarkers with walking impairment in peripheral artery disease. J. Vasc. Surg. 2015, 61, 155–161. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, F.; Pan, H.; Zhao, Y.; Wang, S.; Sun, S.; Li, J.; Hu, X.; Wang, L. Correlation of peripheral Th17 cells and Th17-associated cytokines to the severity of carotid artery plaque and its clinical implication. Atherosclerosis 2012, 221, 232–241. [Google Scholar] [CrossRef]
- Danese, S.; Sans, M.; de la Motte, C.; Graziani, C.; West, G.; Phillips, M.H.; Pola, R.; Rutella, S.; Willis, J.; Gasbarrini, A.; et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology 2006, 130, 2060–2073. [Google Scholar] [CrossRef]
- Sainson, R.C.; Johnston, D.A.; Chu, H.C.; Holderfield, M.T.; Nakatsu, M.N.; Crampton, S.P.; Davis, J.; Conn, E.; Hughes, C.C.W. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood 2008, 111, 4997–5007. [Google Scholar] [CrossRef]
- Luo, D.; Luo, Y.; He, Y.; Zhang, H.; Zhang, R.; Li, X.; Dobrucki, W.; Sinusas, A.J.; Sessa, W.; Min, W. Differential functions of tumor necrosis factor receptor 1 and 2 signaling in ischemia-mediated arteriogenesis and angiogenesis. Am. J. Pathol. 2006, 169, 1886–1898. [Google Scholar] [CrossRef]
- Ryan, E.; McNicholas, D.; Creavin, B.; Kelly, M.E.; Walsh, T.; Beddy, D. Sarcopenia and Inflammatory Bowel Disease: A Systematic Review. Inflamm. Bowel Dis. 2019, 25, 67–73. [Google Scholar] [CrossRef]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Wassmann, S.; Stumpf, M.; Strehlow, K.; Schmid, A.; Schieffer, B.; Böhm, M.; Nickenig, G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ. Res. 2004, 94, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Belmadani, S.; Picchi, A.; Xu, X.; Potter, B.J.; Tewari-Singh, N.; Capobianco, S.; Chilian, W.M.; Zhang, C. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation 2007, 115, 245–254. [Google Scholar] [CrossRef]
- Nguyen, H.; Chiasson, V.L.; Chatterjee, P.; Kopriva, S.E.; Young, K.J.; Mitchell, B.M. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc. Res. 2013, 97, 696–704. [Google Scholar] [CrossRef]
- Watts, M.N.; Leskova, W.; Carter, P.R.; Zhang, S.; Kosloski-Davidson, M.; Grisham, M.B.; Harris, N.R. Ocular dysfunction in a mouse model of chronic gut inflammation. Inflamm. Bowel Dis. 2013, 19, 2091–2097. [Google Scholar] [CrossRef]
- Watts, M.N.; Eshaq, R.S.; Carter, P.R.; Harris, N.R. Decreased retinal blood flow in experimental colitis; improvement by eye drop administration of losartan. Exp. Eye Res. 2013, 115, 22–26. [Google Scholar] [CrossRef][Green Version]
- Norton, C.E.; Grunz-Borgmann, E.A.; Hart, M.L.; Jones, B.W.; Franklin, C.L.; Boerman, E.M. Role of perivascular nerve and sensory neurotransmitter dysfunction in inflammatory bowel disease. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1887–H1902. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Zhu, Q.; Liu, X.; Hao, H.; Sun, Z.; Wang, M.; Hill, M.A.; Xu, C.; Liu, Z. Recovery of Ischemic Limb and Femoral Artery Endothelial Function Are Preserved in Mice with Dextran Sodium Sulfate-Induced Chronic Colitis. Biology 2022, 11, 1169. https://doi.org/10.3390/biology11081169
Wu H, Zhu Q, Liu X, Hao H, Sun Z, Wang M, Hill MA, Xu C, Liu Z. Recovery of Ischemic Limb and Femoral Artery Endothelial Function Are Preserved in Mice with Dextran Sodium Sulfate-Induced Chronic Colitis. Biology. 2022; 11(8):1169. https://doi.org/10.3390/biology11081169
Chicago/Turabian StyleWu, Hao, Qiang Zhu, Xuanyou Liu, Hong Hao, Zhe Sun, Meifang Wang, Michael A. Hill, Canxia Xu, and Zhenguo Liu. 2022. "Recovery of Ischemic Limb and Femoral Artery Endothelial Function Are Preserved in Mice with Dextran Sodium Sulfate-Induced Chronic Colitis" Biology 11, no. 8: 1169. https://doi.org/10.3390/biology11081169
APA StyleWu, H., Zhu, Q., Liu, X., Hao, H., Sun, Z., Wang, M., Hill, M. A., Xu, C., & Liu, Z. (2022). Recovery of Ischemic Limb and Femoral Artery Endothelial Function Are Preserved in Mice with Dextran Sodium Sulfate-Induced Chronic Colitis. Biology, 11(8), 1169. https://doi.org/10.3390/biology11081169





