Changes in Subjective Time and Self during Meditation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. General Procedure
2.4. Conditions
2.5. Physiological Recording
2.6. Questionnaires and Analogue Scales
2.6.1. Meditation Depth Questionnaire (MEDEQ)
2.6.2. Perceived Body Boundaries Scale
2.6.3. Inventory on Subjective Time, Self, and Space (STSS)
2.7. Metronome Task
2.8. Data Analysis
2.8.1. Breathing Rate
2.8.2. Heart Rate
3. Results
3.1. Between-Condition Differences in Physiological Variables
3.1.1. 2 × 2 Repeated-Measures ANOVA
3.1.2. T-Tests between Meditation and Story Conditions
3.1.3. T-Tests between Time Intervals
3.2. Temporal Integration of Metronome Beats
3.3. Subjective Time and Body-Self Boundaries
3.4. Meditation Condition: Correlations between Physiological Variables and Sense of Time and Body
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berkovich-Ohana, A.; Wittmann, M. A typology of altered states according to the consciousness state space (CSS) model: A special reference to subjective time. J. Conscious. Stud. 2017, 24, 37–61. [Google Scholar]
- Berkovich-Ohana, A.; Dor-Ziderman, Y.; Glicksohn, J.; Goldstein, A. Alterations in the sense of time, space, and body in the mindfulness-trained brain: A neurophenomenologically-guided MEG study. Front. Psychol. 2013, 4, 912. [Google Scholar] [CrossRef] [Green Version]
- Ben-Soussan, T.D.; Glicksohn, J.; De Fano, A.; Mauro, F.; Marson, F.; Modica, M.; Pesce, C. Embodied time: Time production in advanced Quadrato and Aikido practitioners. PsyCh J. 2019, 8, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittmann, M. Altered States of Consciousness: Experiences Out of Time and Self; MIT Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Droit-Volet, S.; Dambrun, M. Awareness of the passage of time and self-consciousness: What do meditators report? PsyCh J. 2019, 8, 51–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thönes, S.; Wittmann, M. Time perception in yogic mindfulness meditation—Effects on retrospective duration judgments and time passage. Psychol. Conscious. Theory Res. Pract. 2016, 3, 316–325. [Google Scholar] [CrossRef]
- Sedlmeier, P.; Winkler, I.; Lukina, A. How long did the time spent in meditation feel? “Attention. Attention. Attention”. Psychol. Conscious Theory Res. Pract. 2020. [Google Scholar] [CrossRef]
- Pfeifer, E.; Sarikaya, A.; Wittmann, M. Changes in states of consciousness during a period of silence after a session of depth relaxation music therapy (DRMT). Music Med. 2016, 8, 180–186. [Google Scholar] [CrossRef]
- Pfeifer, E.; Fiedler, H.; Wittmann, M. Enhanced relaxation in students after combined Depth Relaxation Music Therapy and silence in a natural setting. Arts Psychother. 2019, 63, 68–76. [Google Scholar] [CrossRef]
- Berkovich-Ohana, A.; Glicksohn, J.; Goldstein, A. Temporal cognition changes following mindfulness, but not transcendental meditation practice. Proc. Fechner Day 2011, 27, 245–250. [Google Scholar]
- Kramer, R.S.S.; Weger, U.W.; Sharma, D. The effect of mindfulness meditation on time perception. Conscious. Cogn. 2013, 22, 846–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Droit-Volet, S.; Fanget, M.; Dambrun, M. Mindfulness meditation and relaxation training increases time sensitivity. Conscious. Cogn. 2015, 31, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Kabat-Zinn, J. Coming to Our Senses; Hyperion: New York, NY, USA, 2005. [Google Scholar]
- Pöppel, E. A hierarchical model of temporal perception. Trends Cogn. Sci. 1997, 1, 56–61. [Google Scholar] [CrossRef]
- Kent, L. Duration perception versus perception duration: A proposed model for the consciously experienced moment. Timing Time Percept. 2019, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Singhal, I.; Srinivasan, N. Time and time again: A multi-scale hierarchical framework for time-consciousness and timing of cognition. Neurosci. Conscious. 2021, 2, niab020. [Google Scholar] [CrossRef] [PubMed]
- Linares Gutierrez, D.; Kübel, S.; Giersch, A.; Schmidt, S.; Meissner, K.; Wittmann, M. Meditation-induced states, vagal tone, and breathing activity are related to changes in auditory temporal integration. Behav. Sci. 2019, 9, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matko, K.; Ott, U.; Sedlmeier, P. What do meditators do when they meditate? Proposing a novel basis for future meditation research. Mindfulness 2021, 12, 1791–1811. [Google Scholar] [CrossRef]
- Matko, K.; Sedlmeier, P. What is meditation? Proposing an empirically derived classification system. Front. Psychol 2019, 10, 2276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szelag, E.; von Steinbüchel, N.; Reiser, M.; Gilles de Langen, E.; Pöppel, E. Temporal constraints in processing of nonverbal rhythmic patterns. Acta Neurobiol. Exp. 1996, 56, 215–225. [Google Scholar]
- Winter, U.; LeVan, P.; Borghardt, T.L.; Akin, B.; Wittmann, M.; Leyens, Y.; Schmidt, S. Content-free awareness: EEG-fcMRI correlates of consciousness as such in an expert meditator. Front. Psychol 2020, 10, 3064. [Google Scholar] [CrossRef] [Green Version]
- Nesvold, A.; Fagerland, M.W.; Davanger, S.; Ellingsen, Ø.; Solberg, E.E.; Holen, A.; Sevre, K.; Atar, D. Increased heart rate variability during nondirective meditation. Eur. J. Prev. Cardiol. 2012, 19, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.; Muniyandi, M. Breath rate variability: A novel measure to study the meditation effects. Int. J. Yoga 2019, 12, 45–54. [Google Scholar] [PubMed]
- Peng, C.K.; Henry, I.C.; Mietus, J.E.; Hausdorff, J.M.; Khalsa, G.; Benson, H.; Goldberger, A.L. Heart rate dynamics during three forms of meditation. Int. J. Cardiol. 2004, 95, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Piron, H. The meditation depth index (MEDI) and the meditation depth questionnaire (MEDEQ). J. Medit. Medit. Res. 2001, 1, 69–92. [Google Scholar]
- Dambrun, M. When the dissolution of perceived body boundaries elicits happiness: The effect of selflessness induced by a body scan meditation. Conscious. Cogn. 2016, 46, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Bååth, R. Subjective rhythmization: A replication and an assessment of two theoretical explanations. Music. Percept. Interdiscip. J. 2015, 33, 244–254. [Google Scholar] [CrossRef]
- Nozaradan, S.; Peretz, I.; Missal, M.; Mouraux, A. Tagging the neuronal entrainment to beat and meter. J. Neurosci. 2011, 31, 10234–10240. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Rantaaho, P.O.; Karjalainen, P.A. An advanced detrending method with application to HRV analysis. IEEE Trans. Biomed. Eng. 2002, 49, 172–175. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart rate variability and cardiac vagal tone in psychophysiological research—Recommendations for experiment planning, data analysis, and data reporting. Front. Psychol 2017, 8, 213. [Google Scholar] [CrossRef] [Green Version]
- Friedman, B.H.; Allen, M.T.; Christie, I.C.; Santucci, A.K. Validity concerns of common heart-rate variability indices. IEEE Comput. Graph. Appl. 2002, 21, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T. Heart rate variability: Measurement and clinical utility. Ann. Noninvasive Electrocard 2005, 10, 88–101. [Google Scholar] [CrossRef]
- Luft, C.D.B.; Takase, E.; Darby, D. Heart rate variability and cognitive function: Effects of physical effort. Biol. Psychol. 2009, 82, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Khandoker, A.H.; Karmakar, C.; Brennan, M.; Palaniswami, M.; Voss, A. Poincaré Plot Methods for Heart Rate Variability Analysis; Springer: New York, NY, USA, 2013. [Google Scholar]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, L.K.; Siebenbrock, A. Are all measures created equal? Heart rate variability and respiration. Biomed. Sci. Instrum. 2009, 45, 71–76. [Google Scholar]
- Penttilä, J.; Helminen, A.; Jartti, T.; Kuusela, T.; Huikuri, H.V.; Tulppo, M.P.; Coffeng, R.; Scheinin, H. Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: Effects of various respiratory patterns. Clin. Physiol. 2001, 21, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef] [Green Version]
- Berntson, G.G.; Bigger, J.T.; Eckberg, D.L.; Grossman, P.; Kaufmann, P.G.; Malik, M.; Nagaraja, H.N.; Porges, S.W.; Saul, J.P.; Stone, P.H.; et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiol 1997, 34, 623–648. [Google Scholar] [CrossRef]
- Pincus, S.M. Approximate entropy as a measure of system complexity. Proc. Natl. Acad. Sci. USA 1991, 88, 2297–2301. [Google Scholar] [CrossRef] [Green Version]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, 2039–2049. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.M.; Kim, J.K. Approximate entropy for heart rate variability: Effect of tolerance and data length. Int. J. Eng. Res. Technol. 2019, 12, 1992–1999. [Google Scholar]
- Carhart-Harris, R.L. The entropic brain-revisited. Neuropharmacol 2018, 142, 167–178. [Google Scholar] [CrossRef]
- Millière, R.; Carhart-Harris, R.L.; Roseman, L.; Trautwein, F.M.; Berkovich-Ohana, A. Psychedelics, meditation, and self-consciousness. Front. Psychol. 2018, 9, 1475. [Google Scholar] [CrossRef] [PubMed]
- Linares Gutierrez, D. Effects of meditation-induced mental states and individual differences on subjective time. Ph.D. Thesis, University Freiburg, Baden, Germany, 6 May 2021. [Google Scholar]
- Brochard, R.; Abecasis, D.; Potter, D.; Ragot, R.; Drake, C. The “ticktock” of our internal clock: Direct brain evidence of subjective accents in isochronous sequences. Psychol. Sci. 2003, 14, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Kornmeier, J.; Bach, M. Bistable perception—along the processing chain from ambiguous visual input to a stable percept. Int. J. Psychophysiol. 2016, 62, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Sauer, S.; Lemke, J.; Wittmann, M.; Kohls, N.; Mochty, U.; Walach, H. How long is now for mindfulness meditators? Personal. Individ. Differ. 2012, 52, 750–754. [Google Scholar] [CrossRef]
- Kornmeier, J.; Friedel, E.; Wittmann, M.; Atmanspacher, H. EEG correlates of cognitive time scales in the Necker-Zeno model for bistable perception. Conscious. Cogn. 2017, 53, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, N.; Tripathi, S.; Singhal, I. Meditators exercise better endogenous and exogenous control of visual awareness. Mindfulness 2020, 11, 2705–2714. [Google Scholar] [CrossRef]
- Al Zoubi, O.; Misaki, M.; Bodurka, J.; Kuplicki, R.; Wohlrab, C.; Schoenhals, W.A.; Refai, H.H.; Khalsa, S.S.; Stein, M.B.; Paulus, M.P.; et al. Taking the body off the mind: Decreased functional connectivity between somatomotor and default-mode networks following floatation-REST. Hum. Brain Mapp. 2021, 42, 3216–3227. [Google Scholar] [CrossRef]
- Preller, K.H.; Razi, A.; Zeidman, P.; Stämpfli, P.; Friston, K.J.; Vollenweider, F.X. Effective connectivity changes in LSD-induced altered states of consciousness in humans. Proc. Natl. Acad. Sci. 2019, 116, 2743–2748. [Google Scholar] [CrossRef] [Green Version]
- Shanon, B. Altered temporality. J. Conscious. Stud. 2001, 8, 35–58. [Google Scholar]
- Fingelkurts, A.A.; Fingelkurts, A.A.; Kallio-Tamminen, T. Self, Me and I in the repertoire of spontaneously occurring altered states of Selfhood: Eight neurophenomenological case study reports. Cogn. Neurodynamics 2022, 16, 255–282. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Leshem, R.; Pellegrino, M.; Ben-Soussan, T.D. Tackling the electro-topography of the selves through the sphere model of consciousness. Front. Psychol. 2020, 13, 836290. [Google Scholar] [CrossRef] [PubMed]
- Lindström, L.; Kajonius, P.; Cardeña, E. Dissolution of what? The self lost in self-transcendent experiences. J. Conscious. Stud. 2022, 29, 75–101. [Google Scholar] [CrossRef]
- Nave, O.; Trautwein, F.M.; Ataria, Y.; Dor-Ziderman, Y.; Schweitzer, Y.; Fulder, S.; Berkovich-Ohana, A. Self-boundary dissolution in meditation: A phenomenological investigation. Brain Sci. 2021, 11, 819. [Google Scholar] [CrossRef] [PubMed]
| Variable | Value |
|---|---|
| Age (mean ± SD) | 47.2 ± 12.45 |
| Gender: female (%) | 12 (55) |
| Educational level: | |
| high-school degree n (%) | 7 (31.8) |
| university degree n (%) | 15 (68.2) |
| Meditation experience: | |
| lifetime in years (mean ± SD) | 19.2 ± 14.83 |
| lifetime in hours (mean ± SD) | 2994.2 ± 3213.44 |
| hours per week (mean ± SD) | 4.0 ± 2.08 |
| Physiological Variable 5 Minute Baseline (BL) | Meditation Condition (n = 22) | Story Condition (n = 22) | Δ Score: Meditation-Story | t = | p = |
|---|---|---|---|---|---|
| BL BR: breathing period [s] (mean ± SD) | 4.22 ± 0.64 | 4.23 ± 0.70 | −0.01 | −0.034 | 0.974 |
| BL RMSSD (mean ± SD) | 29.36 ± 17.74 | 27.35 ± 13.82 | 2.01 | 0.382 | 0.415 |
| BL HF (mean ± SD) | 39.04 ± 19.90 | 36.94 ± 18.92 | 2.10 | 0.406 | 0.689 |
| BL α 1 (mean ± SD) | 1.16 ± 0.26 | 1.18 ± 0.29 | −0.02 | −0.380 | 0.707 |
| BL α 2 (mean ± SD) | 0.29 ± 0.14 | 0.33 ± 0.19 | −0.04 | −0.915 | 0.370 |
| BL ApEn (mean ± SD) | 1.13 ± 0.24 | 1.08 ± 0.17 | 0.05 | 0.873 | 0.392 |
| BL SampEn (mean ± SD) | 1.47 ± 0.28 | 1.49 ± 0.33 | −0.02 | −0.285 | 0.779 |
| 20 min experimental sessions | |||||
| Session BR: breathing period [s] (mean ± SD) | 4.88 ± 0.84 | 4.18 ± 0.69 | 0.70 | 2.87 | 0.012 * FDR, ANOVA |
| Session RMSSD (mean ± SD) | 27.84 ± 11.88 | 28.23 ± 14.19 | −0.39 | −0.15 | 0.881 |
| Session HF (mean ± SD) | 33.11 ± 17.49 | 38.10 ± 24.54 | −4.99 | −1.12 | 0.281 |
| Session α 1 (mean ± SD) | 1.35 ± 0.23 | 1.17 ± 0.35 | 0.18 | 2.74 | 0.012 * FDR, ANOVA |
| Session α 2 (mean ± SD) | 0.27 ± 0.10 | 0.35 ± 0.12 | −0.08 | −2.78 | 0.011 * FDR, b |
| Session ApEn (mean ± SD) | 1.23 ± 0.20 | 1.38 ± 0.17 | −0.15 | −3.27 | 0.004 ** FDR, ANOVA |
| Session SampEn (mean ± SD) | 1.32 ± 0.27 | 1.55 ± 0.29 | −0.23 | 3.50 | 0.002 ** FDR, ANOVA |
| Physiological Variable | Session | 5 Min Baseline (BL) | 20 Min Sessions | Δ Score: 20 Min. Session–5 Min. BL | t = | p = |
|---|---|---|---|---|---|---|
| BR: breathing period [s] (mean ± SD) | Meditation | 4.22 ± 0.64 | 4.88 ± 0.84 | 0.66 | 4.277 | 0.001 *** ANOVA |
| Story | 4.23 ± 0.70 | 4.18 ± 0.69 | −0.05 | −0.763 | 0.458 | |
| RMSSD (mean ± SD) | Meditation | 29.36 ± 17.74 | 27.84 ± 11.88 | −1.52 | −0.607 | 0.550 |
| Story | 27.35 ± 13.82 | 28.23 ± 14.19 | 0.88 | 0.625 | 0.539 | |
| HF (mean ± SD) | Meditation | 39.04 ± 19.90 | 33.11 ± 17.49 | −5.93 | −1.342 | 0.200 |
| Story | 36.94 ± 18.92 | 38.10 ± 24.54 | 1.16 | 0.646 | 0.528 | |
| α 1 (mean ± SD) | Meditation | 1.16 ± 0.26 | 1.35 ± 0.23 | 0.19 | 3.741 | 0.001 *** ANOVA |
| Story | 1.18 ± 0.29 | 1.19 ± 0.35 | 0.01 | −0.307 | 0.762 | |
| α 2 (mean ± SD) | Meditation | 0.29 ± 0.14 | 0.27 ± 0.10 | −0.02 | −0.643 | 0.527 |
| Story | 0.33 ± 0.19 | 0.35 ± 0.12 | 0.02 | 0.711 | 0.485 | |
| ApEn (mean ± SD) | Meditation | 1.13 ± 0.24 | 1.23 ± 0.20 | 0.10 | 2.004 | 0.058 |
| Story | 1.08 ± 0.17 | 1.38 ± 0.17 | 0.30 | 8.405 | 0.001 *** ANOVA | |
| SampEn (mean ± SD) | Meditation | 1.47 ± 0.28 | 1.32 ± 0.27 | −0.15 | −4.049 | 0.001 *** ANOVA |
| Story | 1.49 ± 0.33 | 1.55 ± 0.29 | 0.06 | 1.381 | 0.182 |
| Variable | Time | Meditation Condition | Story Condition |
|---|---|---|---|
| TI at 2 s. ISI (mean ± SD) | Pre- | 3.26 ± 1.66 | 3.15 ± 1.38 |
| Post- | 3.57 ± 1.95 | 3.05 ± 1.68 | |
| TI at 1.33 s. ISI (mean ± SD) | Pre- | 2.87 ± 1.19 | 2.94 ± 1.37 |
| Post- | 2.48 ± 1.08 | 2.80 ± 1.16 | |
| TI at 1 s. ISI (mean ± SD) | Pre- | 2.05 ± 0.62 | 2.57 ± 1.12 |
| Post- | 2.26 ± 1.19 | 2.15 ± 0.83 | |
| TI at 0.5 s. ISI (mean ± SD) | Pre- | 1.63 ± 0.92 | 1.57 ± 0.93 |
| Post- | 1.44 ± 0.83 | 1.63 ± 0.99 | |
| TI at 0.33 s. ISI (mean ± SD) | Pre- | 1.26 ± 0.83 | 1.26 ± 0.85 |
| Post- | 1.04 ± 0.63 | 0.88 ± 0.45 |
| Variable | Meditation Condition | Story Condition | p-Values a |
|---|---|---|---|
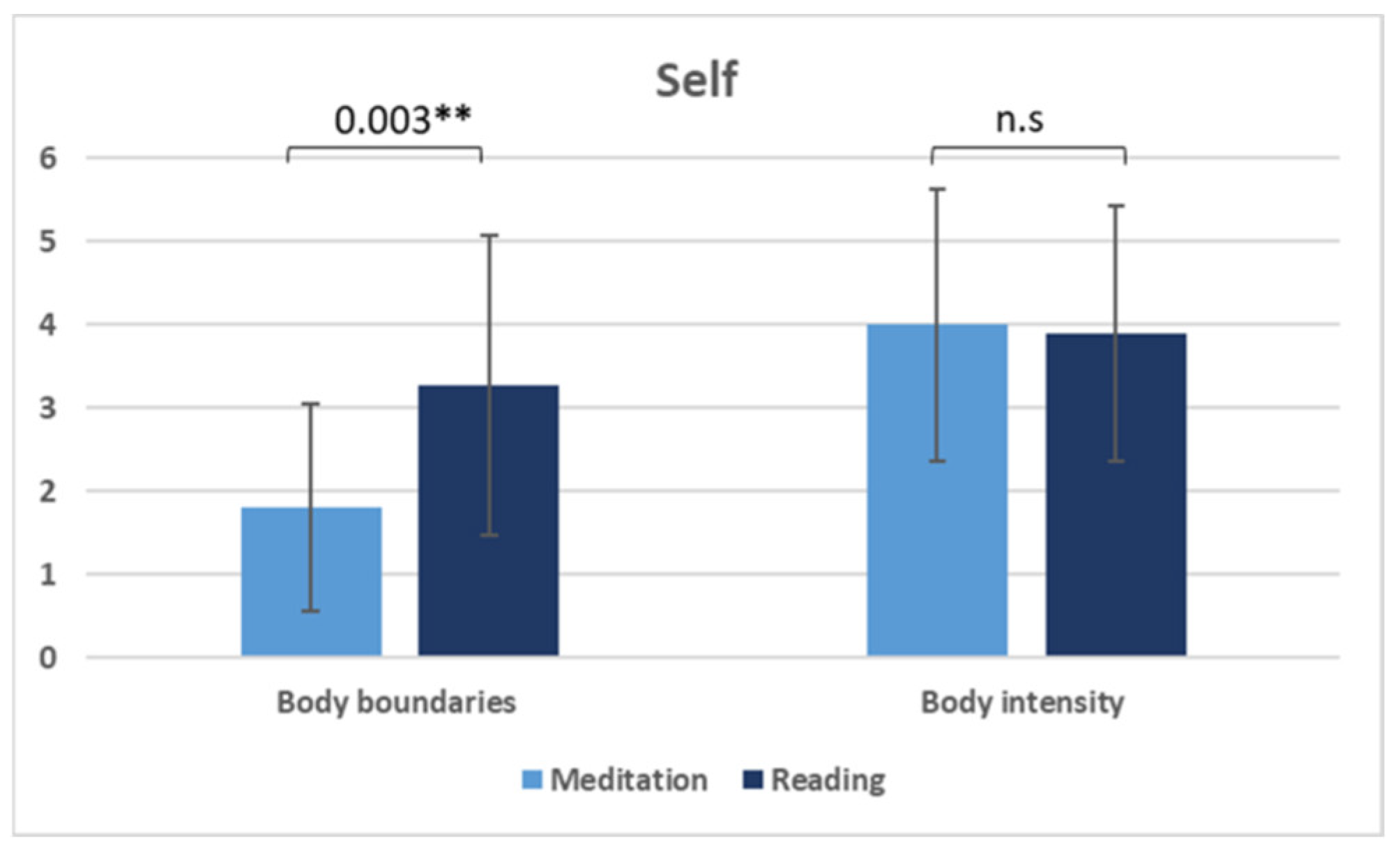
| Body boundaries (mean ± SD) | 1.81 ± 1.25 | 3.27 ± 1.80 | 0.003 ** b; FDR |
| Body intensity (mean ± SD) | 4.00 ± 1.63 | 3.90 ± 1.54 | 0.883 |
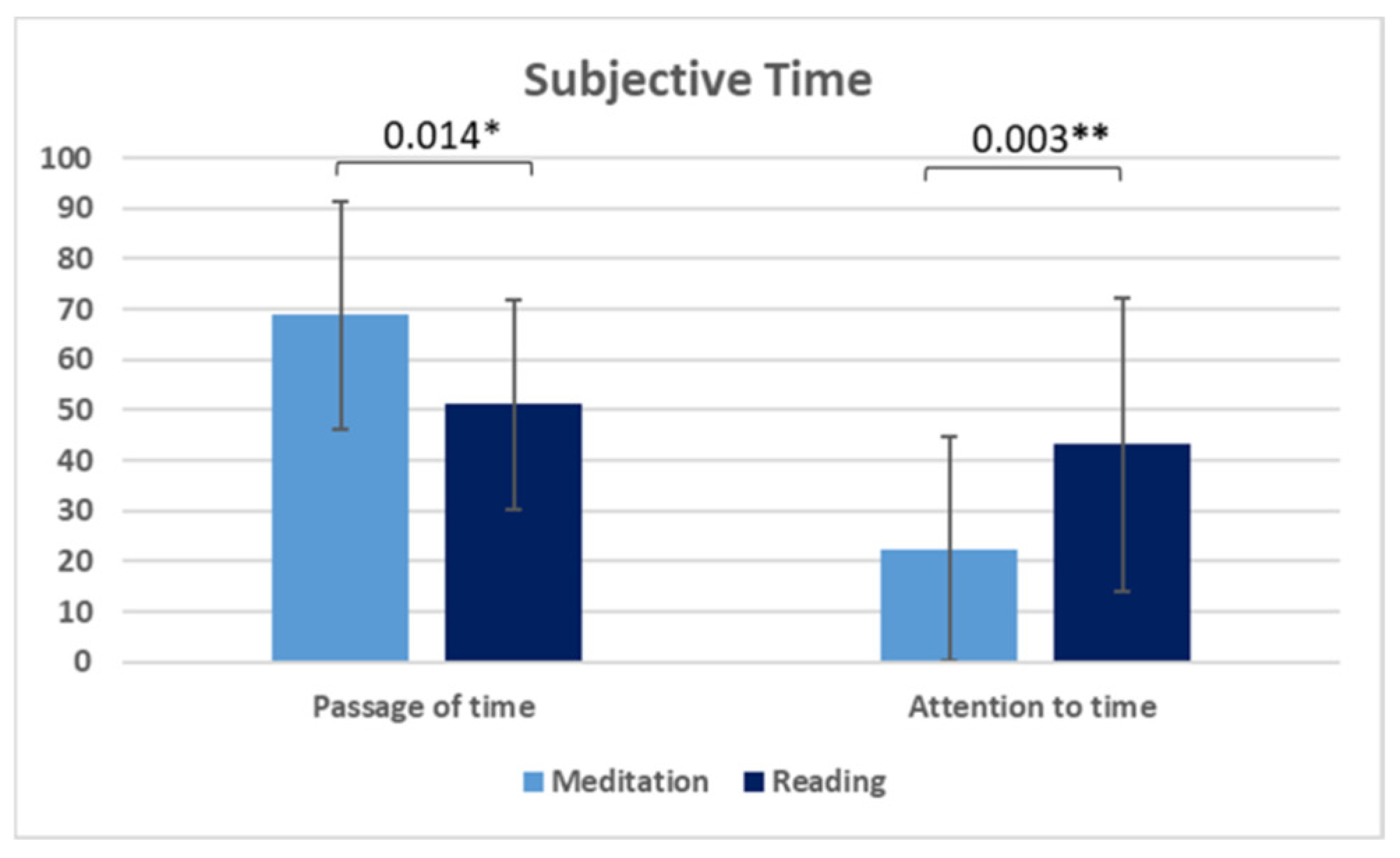
| Passage of time (mean ± SD) | 68.76 ± 22.56 | 51.14 ± 20.85 | 0.014 * b; FDR |
| Attention to time (mean ± SD) | 22.38 ± 22.15 | 43.14 ± 29.04 | 0.003 ** b; FDR |
| Variable | BR | α 1 | α 2 | ApEn | SampEn |
|---|---|---|---|---|---|
| Body boundaries | −0.297 | −0.041 | −0.222 | 0.095 | 0.080 |
| Body intensity | 0.009 | 0.148 | 0.105 | 0.019 | 0.018 |
| Attention to time | 0.154 | 0.312 | −0.402 | 0.112 | 0.042 |
| Passage of time | 0.297 | 0.273 | −0.029 | −0.497 * b | −0.507 * b |
| Variable | MEDEQ |
|---|---|
| Body boundaries | −0.384 |
| Body intensity | −0.332 |
| Attention to time | −0.338 |
| Passage of time | 0.104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linares Gutiérrez, D.; Schmidt, S.; Meissner, K.; Wittmann, M. Changes in Subjective Time and Self during Meditation. Biology 2022, 11, 1116. https://doi.org/10.3390/biology11081116
Linares Gutiérrez D, Schmidt S, Meissner K, Wittmann M. Changes in Subjective Time and Self during Meditation. Biology. 2022; 11(8):1116. https://doi.org/10.3390/biology11081116
Chicago/Turabian StyleLinares Gutiérrez, Damisela, Stefan Schmidt, Karin Meissner, and Marc Wittmann. 2022. "Changes in Subjective Time and Self during Meditation" Biology 11, no. 8: 1116. https://doi.org/10.3390/biology11081116
APA StyleLinares Gutiérrez, D., Schmidt, S., Meissner, K., & Wittmann, M. (2022). Changes in Subjective Time and Self during Meditation. Biology, 11(8), 1116. https://doi.org/10.3390/biology11081116







