Genistein Sensitizes Human Cholangiocarcinoma Cell Lines to Be Susceptible to Natural Killer Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
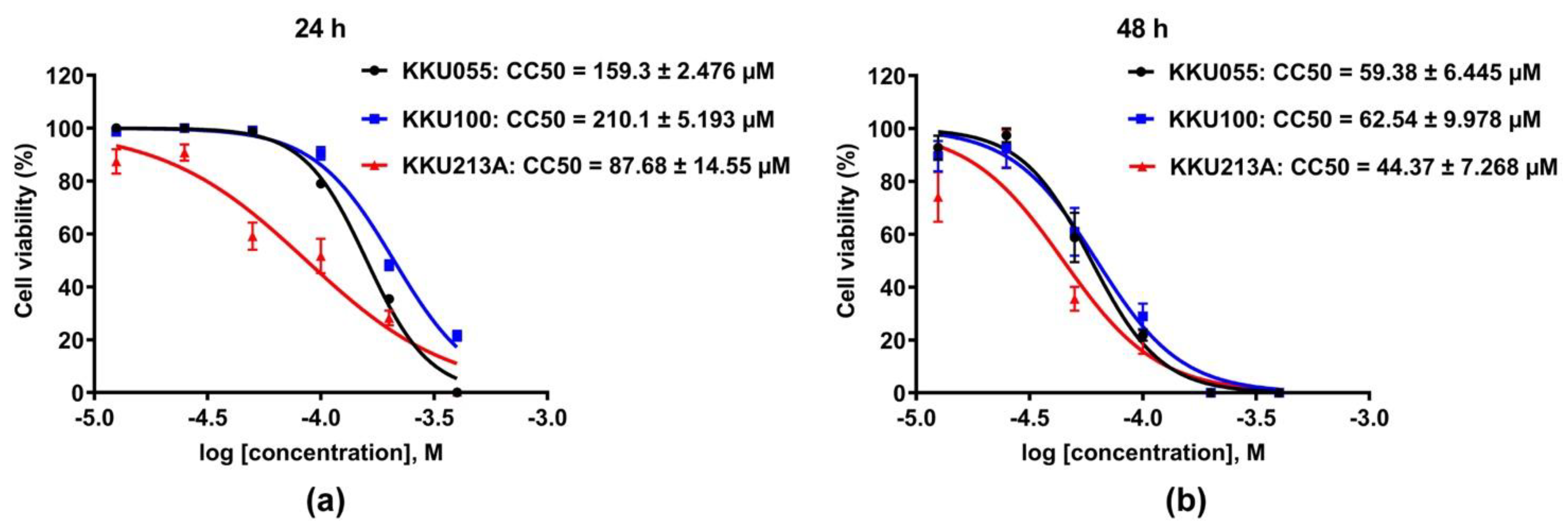
2.2. Cell Viability Assay
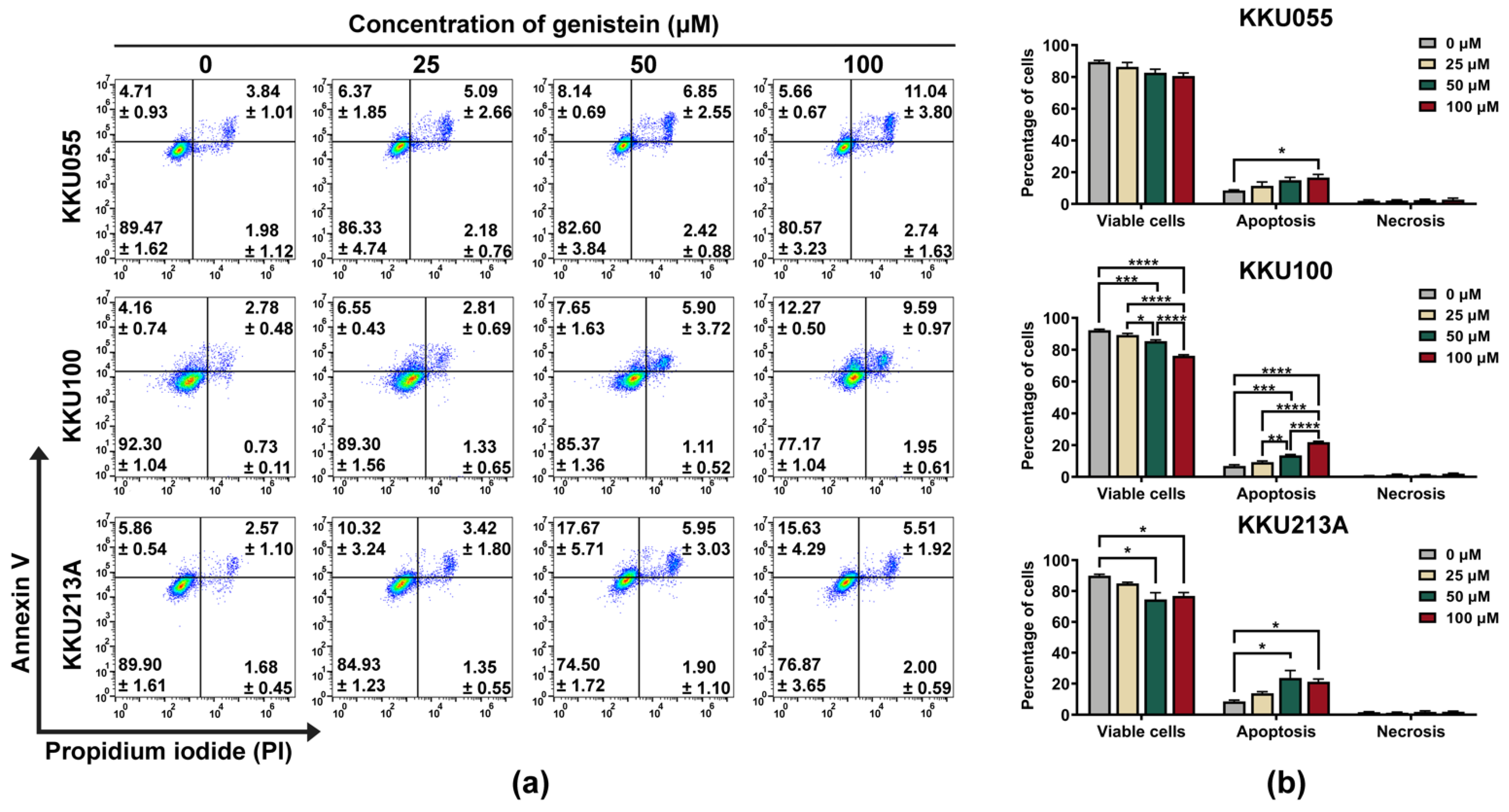
2.3. Flow Cytometry
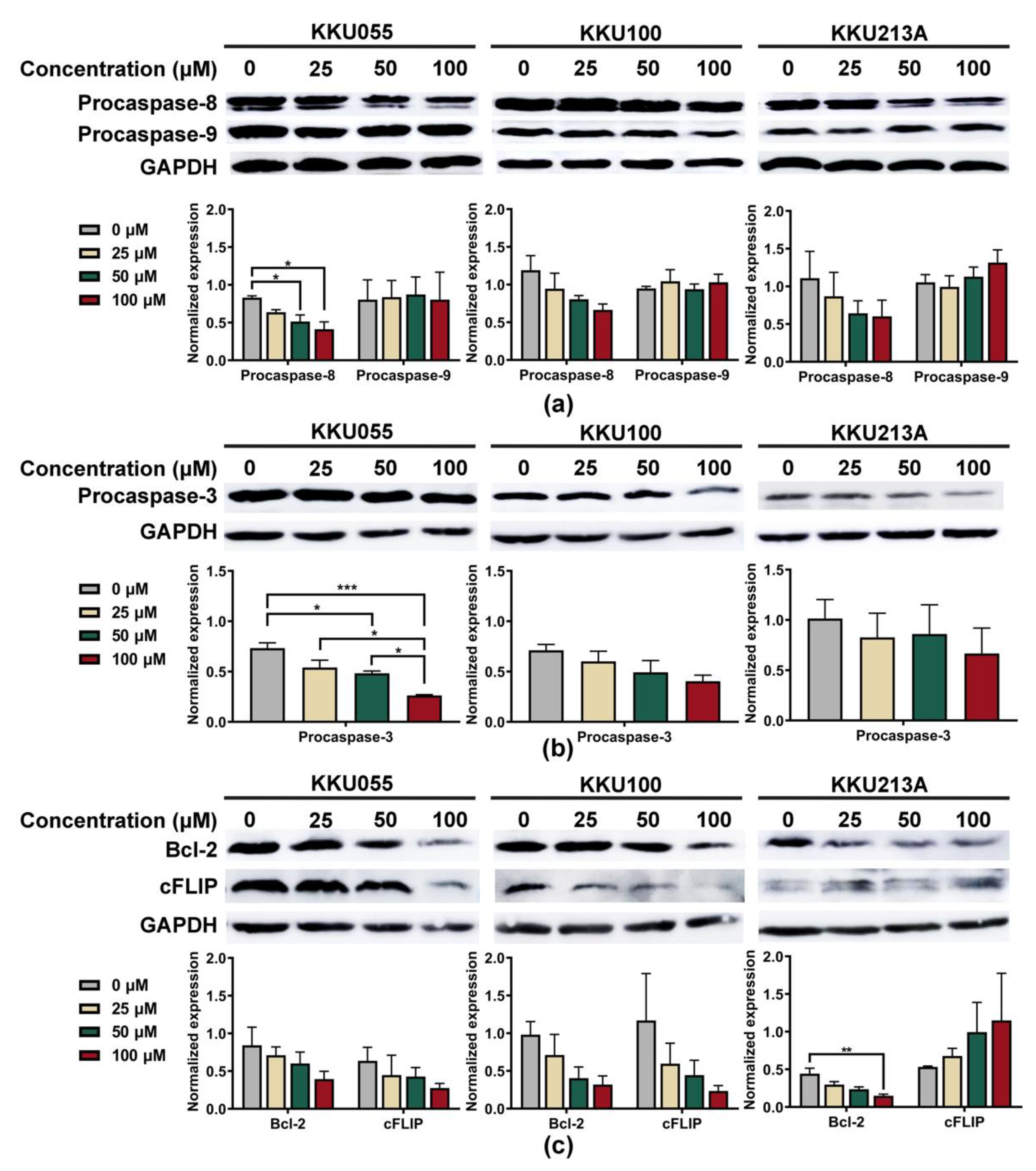
2.4. Immunoblotting Assay
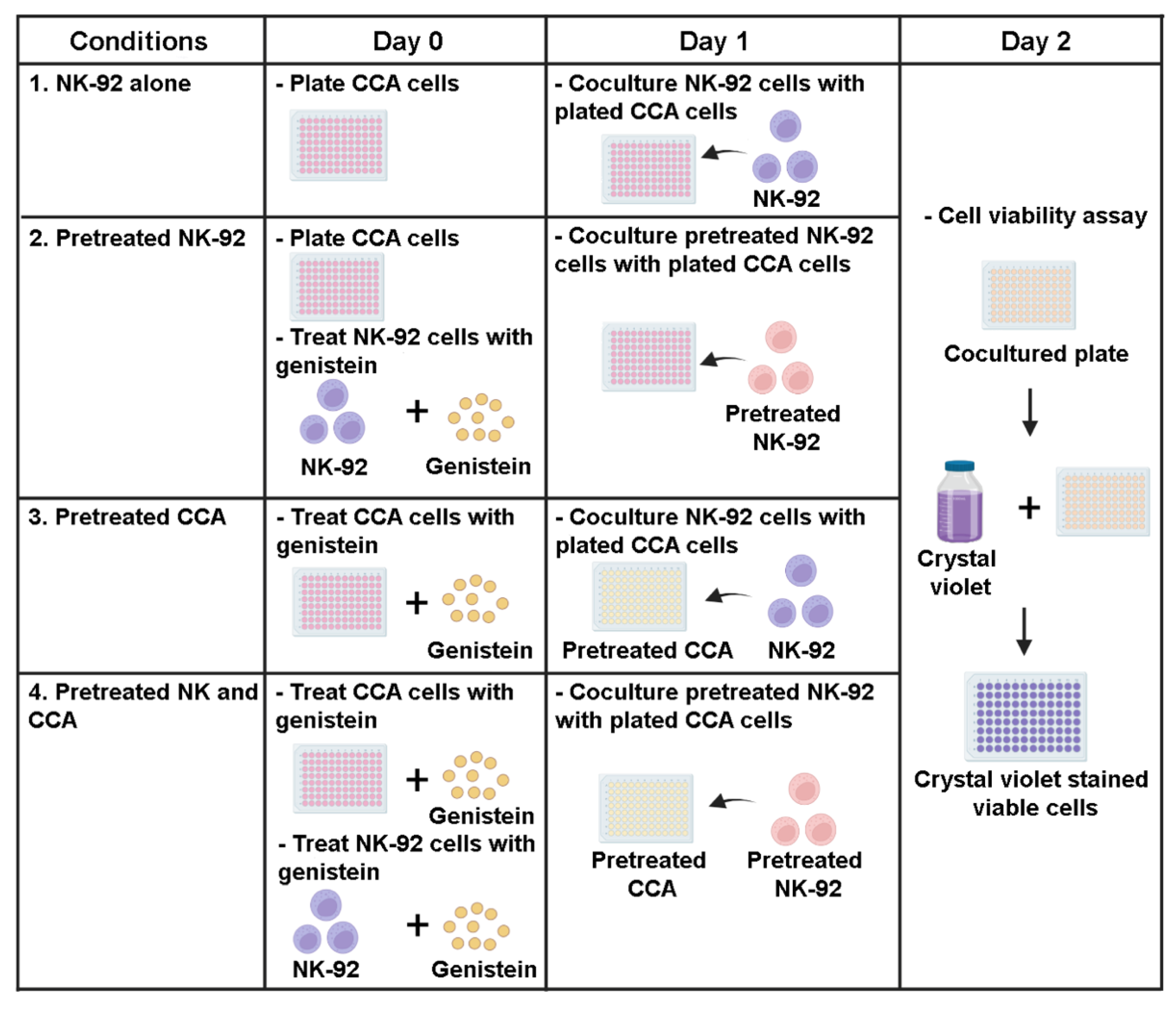
2.5. Killing Assay
2.6. Statistical Analysis
3. Results
3.1. Genistein Induced Cell Death in CCA Cell Lines
3.2. Genistein Activated Extrinsic Apoptotic Pathway in CCA Cell Lines
3.3. Genistein Enhanced Sensitivity of CCA Cell Lines to NK-92 Cell Killing
3.4. Genistein Increased Expression of Death Receptors on CCA Cell Lines
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Saha, S.K.; Zhu, A.X.; Fuchs, C.S.; Brooks, G.A. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016, 21, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Kamsa-ard, S.; Kamsa-ard, S.; Luvira, V.; Suwanrungruang, K.; Vatanasapt, P.; Wiangnon, S. Risk Factors for Cholangiocarcinoma in Thailand: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2018, 19, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Cardinale, V.; Bragazzi, M.C.; Carpino, G.; Torrice, A.; Fraveto, A.; Gentile, R.; Pasqualino, V.; Melandro, F.; Aliberti, C.; Bastianelli, C.; et al. Cholangiocarcinoma: Increasing burden of classifications. Hepatobiliary Surg. Nutr. 2013, 2, 272–280. [Google Scholar] [CrossRef]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.-Y.; Gong, W. Immunotherapy in cholangiocarcinoma: From concept to clinical trials. Surg. Pract. Sci. 2021, 5, 100028. [Google Scholar] [CrossRef]
- Piha-Paul, S.A.; Oh, D.Y.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int. J. Cancer 2020, 147, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.C.; Guo, Y.L.; Liu, Y.; Dai, H.R.; Wang, Y.; Lv, H.Y.; Huang, J.H.; Yang, Q.M.; Han, W.D. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J. Hematol. Oncol. 2017, 10, 4. [Google Scholar] [CrossRef]
- Kobayashi, M.; Sakabe, T.; Abe, H.; Tanii, M.; Takahashi, H.; Chiba, A.; Yanagida, E.; Shibamoto, Y.; Ogasawara, M.; Tsujitani, S.; et al. Dendritic cell-based immunotherapy targeting synthesized peptides for advanced biliary tract cancer. J. Gastrointest. Surg. 2013, 17, 1609–1617. [Google Scholar] [CrossRef]
- Ricci, A.D.; Rizzo, A.; Brandi, G. Immunotherapy in biliary tract cancer: Worthy of a second look. Cancer Control. 2020, 27, 1073274820948047. [Google Scholar] [CrossRef]
- Rubnitz, J.E.; Inaba, H.; Ribeiro, R.C.; Pounds, S.; Rooney, B.; Bell, T.; Pui, C.H.; Leung, W. NKAML: A pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J. Clin. Oncol. 2010, 28, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.E.; Dowlati, E.P.; Geiger, A.E.; Chaudhry, K.; Tovar, M.A.; Bollard, C.M.; Cruz, C.R.Y. NK Cell Adoptive Immunotherapy of Cancer: Evaluating Recognition Strategies and Overcoming Limitations. Transplant. Cell Ther. 2021, 27, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Law, A.D.; Routy, B.; denHollander, N.; Gupta, V.; Wang, X.H.; Chaboureau, A.; Viswanathan, S.; Keating, A. A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget 2017, 8, 89256–89268. [Google Scholar] [CrossRef]
- Tonn, T.; Schwabe, D.; Klingemann, H.G.; Becker, S.; Esser, R.; Koehl, U.; Suttorp, M.; Seifried, E.; Ottmann, O.G.; Bug, G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 2013, 15, 1563–1570. [Google Scholar] [CrossRef]
- Lupo, K.B.; Matosevic, S. Natural Killer Cells as Allogeneic Effectors in Adoptive Cancer Immunotherapy. Cancers 2019, 11, 769. [Google Scholar] [CrossRef] [PubMed]
- Loeuillard, E.; Conboy, C.B.; Gores, G.J.; Rizvi, S. Immunobiology of cholangiocarcinoma. JHEP Rep. 2019, 1, 297–311. [Google Scholar] [CrossRef]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Payer, Á.R.; Gonzalez, S.; López-Soto, A. Mechanisms of Apoptosis Resistance to NK Cell-Mediated Cytotoxicity in Cancer. Int. J. Mol. Sci. 2020, 21, 3726. [Google Scholar] [CrossRef]
- Carnevale, G.; Carpino, G.; Cardinale, V.; Pisciotta, A.; Riccio, M.; Bertoni, L.; Gibellini, L.; De Biasi, S.; Nevi, L.; Costantini, D.; et al. Activation of Fas/FasL pathway and the role of c-FLIP in primary culture of human cholangiocarcinoma cells. Sci. Rep. 2017, 7, 14419. [Google Scholar] [CrossRef]
- George, A.; Sahin, I.; Carneiro, B.A.; Dizon, D.S.; Safran, H.P.; El-Deiry, W.S. Strategies to sensitize cancer cells to immunotherapy. Hum. Vaccines Immunother. 2021, 17, 2595–2601. [Google Scholar] [CrossRef]
- Wennerberg, E.; Sarhan, D.; Carlsten, M.; Kaminskyy, V.O.; D’Arcy, P.; Zhivotovsky, B.; Childs, R.; Lundqvist, A. Doxorubicin sensitizes human tumor cells to NK cell- and T-cell-mediated killing by augmented TRAIL receptor signaling. Int. J. Cancer 2013, 133, 1643–1652. [Google Scholar] [CrossRef]
- Sawasdee, N.; Wattanapanitch, M.; Thongsin, N.; Phanthaphol, N.; Chiawpanit, C.; Thuwajit, C.; Yenchitsomanus, P.T.; Panya, A. Doxorubicin sensitizes breast cancer cells to natural killer cells in connection with increased Fas receptors. Int. J. Mol. Med. 2022, 49, 40. [Google Scholar] [CrossRef] [PubMed]
- Panwong, S.; Wathikthinnakon, M.; Kaewkod, T.; Sawasdee, N.; Tragoolpua, Y.; Yenchitsomanus, P.T.; Panya, A. Cordycepin Sensitizes Cholangiocarcinoma Cells to Be Killed by Natural Killer-92 (NK-92) Cells. Molecules 2021, 26, 5973. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-R.; Chang, C.-H.; Hsu, C.-F.; Tsai, M.-J.; Cheng, H.; Leong, M.K.; Sung, P.-J.; Chen, J.-C.; Weng, C.-F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Tuzcu, M.; Basak, N.; Caglayan, B.; Kilic, U.; Sahin, F.; Kucuk, O. Sensitization of Cervical Cancer Cells to Cisplatin by Genistein: The Role of NFκB and Akt/mTOR Signaling Pathways. J. Oncol. 2012, 2012, 461562. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances. Front. Pharmacol. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Dhandayuthapani, S.; Marimuthu, P.; Hörmann, V.; Kumi-Diaka, J.; Rathinavelu, A. Induction of apoptosis in HeLa cells via caspase activation by resveratrol and genistein. J. Med. Food 2013, 16, 139–146. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, J.; Mi, M.; Chen, W.; Pan, Q.; Wei, M. Anti-angiogenic genistein inhibits VEGF-induced endothelial cell activation by decreasing PTK activity and MAPK activation. Med. Oncol. 2012, 29, 349–357. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.Z.; Du, G.J.; Qi, L.W.; Calway, T.; He, T.C.; Du, W.; Yuan, C.S. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int. J. Oncol. 2013, 43, 289–296. [Google Scholar] [CrossRef]
- Lee, S.R.; Kwon, S.W.; Lee, Y.H.; Kaya, P.; Kim, J.M.; Ahn, C.; Jung, E.M.; Lee, G.S.; An, B.S.; Jeung, E.B.; et al. Dietary intake of genistein suppresses hepatocellular carcinoma through AMPK-mediated apoptosis and anti-inflammation. BMC Cancer 2019, 19, 6. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Steven, A.; Seliger, B. The Role of Immune Escape and Immune Cell Infiltration in Breast Cancer. Breast Care 2018, 13, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Doherty, B.; Nambudiri, V.E.; Palmer, W.C. Update on the Diagnosis and Treatment of Cholangiocarcinoma. Curr. Gastroenterol. Rep. 2017, 19, 2. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, P.; Xie, X.; Yu, H.; Wang, Y.; Chen, G. The role of tumour microenvironment: A new vision for cholangiocarcinoma. J. Cell Mol. Med. 2019, 23, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Hunsawong, T.; Singsuksawat, E.; In-chon, N.; Chawengrattanachot, W.; Thuwajit, C.; Sripa, B.; Paupairoj, A.; Chau-in, S.; Thuwajit, P. Estrogen is increased in male cholangiocarcinoma patients’ serum and stimulates invasion in cholangiocarcinoma cell lines in vitro. J. Cancer Res. Clin. Oncol. 2012, 138, 1311–1320. [Google Scholar] [CrossRef]
- Sripa, B.; Leungwattanawanit, S.; Nitta, T.; Wongkham, C.; Bhudhisawasdi, V.; Puapairoj, A.; Sripa, C.; Miwa, M. Establishment and characterization of an opisthorchiasis-associated cholangiocarcinoma cell line (KKU-100). World J. Gastroenterol. 2005, 11, 3392–3397. [Google Scholar] [CrossRef]
- Dokduang, H.; Namwat, N.; Jusakul, A.; Bhudhisawasdi, V.; Loilome, W.; Yongvanit, P.; Sripa, B.; Tassaneeyakul, W. Determination of Growth Inhibitory Effect of Gemcitabine on Human Intrahepatic Cholangiocarcinoma Cell lines and Comparison of its Inhibition Between the Generic and Reference Formulation. Srinagarind Med. J. 2010, 25, 1–5. [Google Scholar]
- Zaal, E.A.; Berkers, C.R. The Influence of Metabolism on Drug Response in Cancer. Front. Oncol. 2018, 8, 500. [Google Scholar] [CrossRef]
- Tepsiri, N.; Chaturat, L.; Sripa, B.; Namwat, W.; Wongkham, S.; Bhudhisawasdi, V.; Tassaneeyakul, W. Drug sensitivity and drug resistance profiles of human intrahepatic cholangiocarcinoma cell lines. World J. Gastroenterol. 2005, 11, 2748–2753. [Google Scholar] [CrossRef] [PubMed]
- Wathikthinnakon, M.; Luangwattananun, P.; Sawasdee, N.; Chiawpanit, C.; Lee, V.S.; Nimmanpipug, P.; Tragoolpua, Y.; Rotarayanont, S.; Sangsuwannukul, T.; Phanthaphol, N.; et al. Combination gemcitabine and PD-L1xCD3 bispecific T cell engager (BiTE) enhances T lymphocyte cytotoxicity against cholangiocarcinoma cells. Sci. Rep. 2022, 12, 6154. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, G.; Saidijam, M.; Tavilani, H.; Ghasemkhani, N.; Khodadadi, I. Genistein Induces Apoptosis and Inhibits Proliferation of HT29 Colon Cancer Cells. Int. J. Mol. Cell. Med. 2016, 5, 178–191. [Google Scholar]
- Prietsch, R.F.; Monte, L.G.; da Silva, F.A.; Beira, F.T.; Del Pino, F.A.; Campos, V.F.; Collares, T.; Pinto, L.S.; Spanevello, R.M.; Gamaro, G.D.; et al. Genistein induces apoptosis and autophagy in human breast MCF-7 cells by modulating the expression of proapoptotic factors and oxidative stress enzymes. Mol. Cell Biochem. 2014, 390, 235–242. [Google Scholar] [CrossRef]
- Matsukawa, Y.; Marui, N.; Sakai, T.; Satomi, Y.; Yoshida, M.; Matsumoto, K.; Nishino, H.; Aoike, A. Genistein arrests cell cycle progression at G2-M. Cancer Res. 1993, 53, 1328–1331. [Google Scholar] [PubMed]
- Li, Z.; Li, J.; Mo, B.; Hu, C.; Liu, H.; Qi, H.; Wang, X.; Xu, J. Genistein induces G2/M cell cycle arrest via stable activation of ERK1/2 pathway in MDA-MB-231 breast cancer cells. Cell Biol. Toxicol. 2008, 24, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Tanjak, P.; Thiantanawat, A.; Watcharasit, P.; Satayavivad, J. Genistein reduces the activation of AKT and EGFR, and the production of IL6 in cholangiocarcinoma cells involving estrogen and estrogen receptors. Int. J. Oncol. 2018, 53, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Sydor, S.; Jafoui, S.; Wingerter, L.; Swoboda, S.; Mertens, J.C.; Gerken, G.; Canbay, A.; Paul, A.; Fingas, C.D. Bcl-2 degradation is an additional pro-apoptotic effect of polo-like kinase inhibition in cholangiocarcinoma cells. World J. Gastroenterol. 2017, 23, 4007–4015. [Google Scholar] [CrossRef]
- Liu, D.; Fan, Y.; Li, J.; Cheng, B.; Lin, W.; Li, X.; Du, J.; Ling, C. Inhibition of cFLIP overcomes acquired resistance to sorafenib via reducing ER stress-related autophagy in hepatocellular carcinoma. Oncol. Rep. 2018, 40, 2206–2214. [Google Scholar] [CrossRef]
- Mora-Molina, R.; Stöhr, D.; Rehm, M.; López-Rivas, A. cFLIP downregulation is an early event required for endoplasmic reticulum stress-induced apoptosis in tumor cells. Cell Death Dis. 2022, 13, 111. [Google Scholar] [CrossRef]
- Chang, D.W.; Xing, Z.; Pan, Y.; Algeciras-Schimnich, A.; Barnhart, B.C.; Yaish-Ohad, S.; Peter, M.E.; Yang, X. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002, 21, 3704–3714. [Google Scholar] [CrossRef] [PubMed]
- Safa, A.R.; Kamocki, K.; Saadatzadeh, M.R.; Bijangi-Vishehsaraei, K. c-FLIP, a Novel Biomarker for Cancer Prognosis, Immunosuppression, Alzheimer’s Disease, Chronic Obstructive Pulmonary Disease (COPD), and a Rationale Therapeutic Target. Biomark J. 2019, 5, 4. [Google Scholar] [CrossRef]
- Oyarzo, M.P.; Medeiros, L.J.; Atwell, C.; Feretzaki, M.; Leventaki, V.; Drakos, E.; Amin, H.M.; Rassidakis, G.Z. c-FLIP confers resistance to FAS-mediated apoptosis in anaplastic large-cell lymphoma. Blood 2006, 107, 2544–2547. [Google Scholar] [CrossRef] [PubMed]
- Prager, I.; Watzl, C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Prager, I.; Liesche, C.; van Ooijen, H.; Urlaub, D.; Verron, Q.; Sandström, N.; Fasbender, F.; Claus, M.; Eils, R.; Beaudouin, J.; et al. NK cells switch from granzyme B to death receptor–mediated cytotoxicity during serial killing. J. Exp. Med. 2019, 216, 2113–2127. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Galat, V.; Galat, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK cell-based cancer immunotherapy: From basic biology to clinical development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef]
- Ames, E.; Hallett, W.H.D.; Murphy, W.J. Sensitization of human breast cancer cells to natural killer cell-mediated cytotoxicity by proteasome inhibition. Clin. Exp. Immunol. 2009, 155, 504–513. [Google Scholar] [CrossRef]
- Hallett, W.H.; Ames, E.; Motarjemi, M.; Barao, I.; Shanker, A.; Tamang, D.L.; Sayers, T.J.; Hudig, D.; Murphy, W.J. Sensitization of tumor cells to NK cell-mediated killing by proteasome inhibition. J. Immunol. 2008, 180, 163–170. [Google Scholar] [CrossRef]
- Sarhan, D.; Wennerberg, E.; D’Arcy, P.; Gurajada, D.; Linder, S.; Lundqvist, A. A novel inhibitor of proteasome deubiquitinating activity renders tumor cells sensitive to TRAIL-mediated apoptosis by natural killer cells and T cells. Cancer Immunol. Immunother. 2013, 62, 1359–1368. [Google Scholar] [CrossRef]
- Hu, W.; Zheng, R.R.; Cui, H.X.; Yue, D.; Wang, Y.; Jiang, Y.H. Effects of bortezomib in sensitizing human prostate cancer cell lines to NK-mediated cytotoxicity. Asian J. Androl. 2012, 14, 695–702. [Google Scholar] [CrossRef]
- Ullmann, U.; Metzner, J.; Frank, T.; Cohn, W.; Riegger, C. Safety, tolerability, and pharmacokinetics of single ascending doses of synthetic genistein (Bonistein™) in healthy volunteers. Adv. Ther. 2005, 22, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D. Assessing risks and benefits of genistein and soy. Environ. Health Perspect. 2006, 114, A332–A333. [Google Scholar] [CrossRef] [PubMed][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiawpanit, C.; Panwong, S.; Sawasdee, N.; Yenchitsomanus, P.-t.; Panya, A. Genistein Sensitizes Human Cholangiocarcinoma Cell Lines to Be Susceptible to Natural Killer Cells. Biology 2022, 11, 1098. https://doi.org/10.3390/biology11081098
Chiawpanit C, Panwong S, Sawasdee N, Yenchitsomanus P-t, Panya A. Genistein Sensitizes Human Cholangiocarcinoma Cell Lines to Be Susceptible to Natural Killer Cells. Biology. 2022; 11(8):1098. https://doi.org/10.3390/biology11081098
Chicago/Turabian StyleChiawpanit, Chutipa, Suthida Panwong, Nunghathai Sawasdee, Pa-thai Yenchitsomanus, and Aussara Panya. 2022. "Genistein Sensitizes Human Cholangiocarcinoma Cell Lines to Be Susceptible to Natural Killer Cells" Biology 11, no. 8: 1098. https://doi.org/10.3390/biology11081098
APA StyleChiawpanit, C., Panwong, S., Sawasdee, N., Yenchitsomanus, P.-t., & Panya, A. (2022). Genistein Sensitizes Human Cholangiocarcinoma Cell Lines to Be Susceptible to Natural Killer Cells. Biology, 11(8), 1098. https://doi.org/10.3390/biology11081098






