Simple Summary
Extracellular vesicles are vesicles produced by cells and organisms, capable of developing different functions. In this sense, these vesicles have been involved in communication, in the production of damage during an infection and in the modulation of different cell pathways. These vesicles have also been proposed as key players for the diagnosis and treatment of several diseases. In this study, we report for the first time the production of extracellular vesicles by Naegleria fowleri, the free-living amoeba that causes primary amoebic meningoencephalitis, a severe infection of the central nervous system that is fatal in more than 95% of cases. The vesicles were isolated and size, surface charge and protein content were analyzed. The report of the production of extracellular vesicles by this amoeba is relevant for different reasons: i. these vesicles could be implicated during the processes of invasion and damage to the brain; ii. they could influence the environment to trigger specific responses by cells and, iii. specific vesicles secreted by the amoeba could be employed for diagnostic purposes. Several studies to reveal the role of extracellular vesicles of this pathogen are being performed in our laboratory.
Abstract
Extracellular vesicles (EVs) are small lipid vesicles released by both prokaryotic and eukaryotic cells, involved in intercellular communication, immunomodulation and pathogenesis. In this study, we performed a characterization of the EVs produced by trophozoites of a clinical isolate of the free-living amoeba Naegleria fowleri (N. fowleri). Size distribution, zeta potential, protein profile and protease activity were analyzed. Under our incubation conditions, EVs of different sizes were observed, with a predominant population ranging from 206 to 227 nm. SDS-PAGE revealed protein bands of 25 to 260 KDa. The presence of antigenic proteins was confirmed by Western blot, which evidenced strongest recognition by rat polyclonal antibodies raised against N. fowleri in the region close to 80 KDa and included peptidases, as revealed by zymography. Proteins in selected immunorecognized bands were further identified using nano-ESI-MS/MS. A preliminary proteomic profile of the EVs identified at least 184 proteins as part of the vesicles’ cargo. Protease activity assays, in combination with the use of inhibitors, revealed the predominance of serine proteases. The present characterization uncovers the complexity of EVs produced by N. fowleri, suggesting their potential relevance in the release of virulence factors involved in pathogenicity. Owing to their cargo’s diversity, further research on EVs could reveal new therapeutic targets or biomarkers for developing rapid and accurate diagnostic tools for lethal infections such as the one caused by this amoeba.
1. Introduction
Extracellular vesicles (EVs) are a heterogeneous group of nano to micro-sized vesicles, naturally released by both prokaryotic and eukaryotic cells. These vesicles are commonly surrounded by a phospholipid bilayer [1] and contain a bioactive cargo of proteins, metabolites, nucleic acids (DNA and RNA) and lipids. The three main subtypes of EVs include apoptotic bodies, microvesicles and exosomes, which are differentiated based upon their biogenesis, release pathways, size, content and function [2]. First considered as cell waste products or “platelet dust” [3], it has been confirmed the role of EVs in the regulation of physiological processes, emerging as key players in intercellular communication mainly through their ability to transfer biological content [4]. Furthermore, these vesicles have also been involved in pathogenesis, modulation and evasion of the immune response in pathophysiological processes. Diverse research groups have also evaluated the potential role of EVs for diagnostic (liquid biopsy) and therapeutic purposes [5,6,7].
Regarding parasites, results from different studies suggest that these organisms release different types of vesicles capable of inducing an efficient immune response or modulate the immune response by either triggering [8] or inhibiting the production of pro-inflammatory cytokines [9]. Besides this immunomodulatory activity, EVs could serve as carriers for virulence factors [10]. The study of EVs from parasites includes helminths such as Fasciola hepatica, [11], Heligmosomoides polygyrus [12], Schistosoma mansoni [13] and Echinostoma caproni [14], as well as protozoa such as Toxoplasma gondii [15], Trypanosoma cruzi [16,17,18,19,20], Leishmania [21], different Plasmodium species [22,23] and anaerobic protozoan parasites such as Entamoeba histolytica, Trichomonas vaginalis and Giardia intestinalis [24], among others. More recently, and in the case of free-living amoebae with pathogenic potential, the secretion of EVs has been described in Acanthamoeba, a genus related to the production of fatal granulomatous amoebic encephalitis, keratitis and skin lesions. In this specific case, biological and nanomechanical properties of extracellular vesicles produced by clinical and environmental isolates of Acanthamoeba have been recently reported [25,26,27,28].
To our knowledge, the secretion of EVs by trophozoites of Naegleria fowleri (N. fowleri) has not been described. In this work, we confirm the secretion of EVs by a clinical isolate of N. fowleri, a thermophilic free-living amoeba that produces primary amoebic meningoencephalitis (PAM), an infection of rapid progression of the central nervous system that is fatal in more than 95% of cases [29]. Since the description of the first clinical case and until 2019, more than 440 cases have been reported worldwide [29]. However, for example, an underestimation of the disease of up to 50% has been proposed for the United States, which makes it difficult to know the true incidence of the infection [30].
During the pathogenic process of primary amoebic encephalitis, certain events may be relevant, such as the trophozoites’ ability to attach to the nasal mucosa, the chemotactic response to components of the nerves and the increased locomotion rate of the amoebae [31,32]. Moreover, a significant immune response through activation of the innate immune system [31], the phagocytic activity by structures known as food cups, and the release of cytolytic molecules (phospholipases, acid hydrolases, neuraminidases, pore-forming polypeptides such as naegleriapores and phospholipolytic enzymes) play a role in host cell and nerve destruction. This combination of both, pathogenic mechanisms of N. fowleri and the intense immune response resulting from its presence, produce significant nerve damage and subsequent central nervous system tissue damage [33]. However, some specific factors involved in the pathogenesis of PAM are still unknown and need extensive research. As an important step towards gaining a deeper knowledge on the production of EVs by N. fowleri, here we address the characterization of several physical and biochemical parameters, including the proteomic profiling of their cargo and the immunogenicity of some of their proteins.
2. Materials and Methods
2.1. Axenic Culture of Naegleria fowleri Trophozoites
Trophozoites of a clinical isolate of Naegleria fowleri from Costa Rica (accession number: MT090627) [34] were cultured in 75 cm2 and 125 cm2 flasks (Nunc EasYFlask®, Thermo Fisher Scientific, Waltham, MA, USA) with 2% casein hydrolysate (Sigma Aldrich, St. Louis, MI, USA) culture medium, supplemented with 10% inactivated fetal bovine serum (Gibco, Grand Island, NY, USA) and antibiotics (penicillin/streptomycin). The flasks were incubated at 37 °C, with daily observation under an inverted microscope. The culture medium was replaced, at least, every two days.
The manipulation of N. fowleri and products derived from this organism was performed in biosafety level 2 facilities.
2.2. Isolation of Extracellular Vesicles (EVs)
EVs of trophozoites of N. fowleri were obtained as previously described by Retana Moreira et al. with some modifications [19,20], following the Minimal Information for the Study of Extracellular Vesicles (MISEV) guidelines [35]. Briefly, amoebae were washed 3 times using sterile PBS and then, 5 × 107 trophozoites were incubated for 5 h at 37 °C in 10 mL of 2% casein hydrolysate (Sigma Aldrich, St. Louis, MI, USA) culture medium without serum and antibiotics. After this incubation, the supernatants were collected and centrifuged at 2500× g for 15 min at 4 °C to discard possible remaining trophozoites. The resulting supernatants were collected for EV purification.
In order to eliminate larger vesicles and obtain exosome-enriched pellets, supernatants were centrifuged at 17,000× g for 30 min at 4 °C and then filtered through 0.22 µm-pore membranes (Sartorius, Göttingen, Germany), for subsequent ultracentrifugation at 120,000× g for 150 min in a Sorvall™ WX80 Ultracentrifuge (Thermo Fisher Scientific, Waltham, MA, USA). The resulting pellets were washed twice in sterile-filtered (0.22 µm pore) PBS at 120,000× g for 150 min and finally resuspended in 150 µL PBS. The samples were analyzed immediately or stored at 4 °C for a maximum period of 4 days.
The viability of trophozoites after the 5 h secretion period was evaluated using the trypan blue exclusion test, and the protein concentration of each sample was quantified using the Micro-BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA).
EV characterization techniques included transmission electron microscopy, atomic force microscopy, nanoparticle tracking analysis and dynamic light scattering, as previously reported [19,20] and briefly described below.
2.3. Transmission Electron Microscopy
To confirm the production of EVs by trophozoites of N. fowleri, pellets of the samples obtained ultracentrifugation were fixed in 500 µL of Karnovsky’s fixative (2.5% glutaraldehyde and 2% formaldehyde in 0.1 M cacodylate buffer; 50 mg of CaCl2 in 100 mL) for 2 h at 37 °C. After this step, 25 µL of the samples was placed onto a parafilm piece and a zinc grid with a carbon film was disposed over the samples for 5 min. After this time, two washing steps were performed using ultrapure water and the samples were negative-stained and contrasted with 1% (v/v) uranyl acetate for 1 min. The grids were dried with filter paper and the final examination was achieved under a Carl Zeiss LIBRA 120 PLUS SMT electron microscope (Carl Zeiss, Oberkochen, Germany). Three samples from different days of isolation were analyzed.
2.4. Atomic Force Microscopy
Topographic imaging of EVs obtained after the ultracentrifugation step was performed using the non-contact AFM mode in an NX-10 instrument (Park Systems, Suwon, Korea) with TAP300 cantilevers (K = 40 N m−1 and f = 300 kHz). Briefly, each sample was diluted 1:10 in sterile-filtered PBS and 8 µL of the dilution were deposited onto freshly cleaved muscovite mica. After 10 min, the substrate with the sample was carefully rinsed three times with ultrapure water (MilliQ®, Millipore, Burlington, MA, USA) to remove salts and finally air-dried. Images were typically acquired as 256 × 256 pixels at a scan rate of 0.3–0.5 Hz and further processed and analyzed using XEI software (Park Systems, Suwon, Korea). Representative images of each sample were obtained by scanning at least 3 different locations on at least 3 different samples.
To confirm the secretion of EVs by N. fowleri, trophozoites were washed 3 times by centrifugation at 800× g or 10 min using sterile PBS. Then, 5 × 104 trophozoites were resuspended in 2% casein hydrolysate culture medium without serum and antibiotics and placed onto 18 mm round coverslips (Fisher Scientific, Portsmouth, NH, USA) that were previously rinsed and sterilized in an autoclave for 15 min at 121 °C. After 1 h of incubation at 37 °C, the coverslips with trophozoites were carefully washed in sterile filtered PBS and fixed in 4% paraformaldehyde for 30 min at room temperature. The coverslips were then washed three times in sterile filtered PBS (5 min each washing step) and air-dried, prior to atomic force microscopy analysis in a NX-10 instrument. For this purpose, the non-contact mode was also employed and images were acquired and analyzed as described.
2.5. Nanoparticle Tracking Analysis
Size, distribution and concentration of the EVs secreted by N. fowleri were determined by measuring the rate of Brownian motion according to the particle size in a Nanosight NS300 system (Malvern Panalytical, Worcestershire, UK). For the analysis, samples were diluted (1/100) in low-binding tubes (Eppendorf, Gamburg, Germany) using sterile-filtered PBS and measurements were performed at 25 °C. As previously reported, for data acquisition and information processing, the NTA software 3.2 Dev Build 3.2.16 was employed. The particle movement was analyzed by NTA software with the camera level at 16, slider shutter at 1200 and slider gain at 146, as previously reported [19]. The mean size distribution was calculated as a mean of three independent size distributions.
2.6. Dynamic Light Scattering
Dynamic light scattering analyses of isolated EVs were performed using a Zetasizer nano ZS90 Size Analyzer (Malvern Panalytical, Worcestershire, UK), following sample preparation and measurement conditions as described for nanoparticle tracking analysis. The Zetasizer Ver. 7.11 software was employed for data acquisition and information processing and the mean size distribution was calculated as a mean of five independent size distributions (10 runs per measurement).
2.7. Measurement of Zeta Potential
Zeta potential of EVs was measured in a Zetasizer NanoZeta ZS90 system (Malvern Panalytical, Worcestershire, United Kingdom), at 25 °C. Briefly, EV samples were resuspended in 1 mL PBS, pH 7.4, and the electrophoretic mobility (μe) was determined by Laser Doppler electrophoresis. Zeta potential was calculated by using the Smoluchowski equation and results were obtained from at least four independent measurements (20 runs each).
2.8. Protein Pattern and Recognition of Extracellular Vesicles by Rat Polyclonal Anti-Naegleria fowleri Antibodies
2.8.1. Preparation of Whole Protein Extracts of Naegleria fowleri Trophozoites
Whole protein extracts were obtained as follows: 5 × 107 trophozoites of N. fowleri were washed three times in sterile PBS, resuspended in 500 µL PBS and submitted to sonication in a 4710 series ultrasonic homogenizer (Cole-Parmer Instrument, Vernon Hills, IL, USA) by applying 3 cycles of 30 s, with pause of 60 s between cycles. The resulting extracts were used immediately or stored in aliquots at −80 °C until use.
Protein quantification of the whole protein extracts was performed using the Micro-BCA protein assay kit (Thermo Fischer Scientific, Waltham, MA, USA).
2.8.2. Electrophoretic Separation of Proteins Using SDS-PAGE
To obtain protein profiles, samples of EVs and whole protein extracts of N. fowleri were diluted 1:1 in reducing sample buffer [36], heated for 10 min at 98 °C and subsequently loaded onto 12% SDS-polyacrylamide gels (SDS-PAGE). Electrophoretic runs were performed for 90 min (120 V) and proteins were finally visualized by Coomassie blue R-250 (BioRad, Hercules, CA, USA) and silver stains, following previously described protocols [37].
2.8.3. Polyclonal Antibody Production
Two four-week old female Wistar rats were immunized with 40 μg of a whole protein extract of N. fowleri trophozoites (ATCC N. fowleri Carter 30808). The antigen was prepared by emulsification of the amoebae lysate (in sterile PSB) in complete Freund’s adjuvant (Sigma, Ronkonkoma, NY, USA), in a 1:1 ratio (final volume: 500 µL). This emulsion was administered intraperitoneally to the rats. For subsequent immunizations, the adjuvant was switched to incomplete Freund’s adjuvant (Sigma Aldrich, St. Louis, MI, USA). A total of 8 immunizations (1 per week) were performed. Finally, the animals were bled two weeks after the final immunization. The antibody response was evaluated using ELISA and Western blot, as described elsewhere [38].
2.8.4. Western Blot
The recognition of proteins in EVs and trophozoites by rat polyclonal anti-N. fowleri antibodies was analyzed by Western blot. Briefly, SDS-PAGE was performed as described and proteins were transferred to nitrocellulose membranes (60 min, 90 V) in an Enduro VE10 Vertical Gel System (Labnet International, Edison, NJ, USA). Then, the membranes were blocked overnight with 5% non-fat milk in PBS-0.1% Tween 20, washed four times in a solution of PBS-0.1% Tween 20 and incubated for 1 h at room temperature with the polyclonal anti-N. fowleri serum (1:10,000). After the incubation, the membranes were washed and incubated for 1 h at room temperature with peroxidase-conjugated goat anti-rat IgGs (1:10,000) (Thermo Fisher Scientific, Waltham, MA, USA). After four washing steps with PBS-0.1% Tween 20, the fluorogenic substrate reaction was visualized using the Clarity ECL Western substrate (BioRad, California, United States) in a ChemiDoc imaging system (BioRad, California, United States).
2.9. Evaluation of Protease Activity of Extracellular Vesicles
In order to evaluate protease activity of EVs, zymographic assays were performed as described by Herron et al. [39]. Briefly, protein extracts of EVs and whole protein extracts of N. fowleri trophozoites were subjected to electrophoresis on SDS-polyacrylamide gels containing gelatin (1 mg/mL) [40]. Then, SDS was removed by washing the gels twice in 1% Triton X-100 (w/v) for 30 min, for subsequent incubation overnight at 37 °C in a developing buffer (50 mM Tris-HCl, 10 mM CaCl2, pH 8.0). After the incubation, gels were rinsed and finally stained with Coomassie brilliant blue R-250 (BioRad, California, United States). Areas of gelatin digestion, which indicate protease activity, were seen as non-stained regions.
To characterize the general types of proteases present in EVs, the methodology described by Castro Artavia et al. [40] was employed. In this case, 1 mM phenylmethylsulfonyl fluoride (Sigma Aldrich, St. Louis, MI, USA) was employed as the serine proteases inhibitor, 1 mM 2-iodoacetamide (Merck, Hohebrunn, Germany) was selected as the cysteine proteases inhibitor and 10 mM EDTA (Sigma Aldrich, St. Louis, MI, USA) was employed as the metalloproteases inhibitor. To this end, EV samples were incubated for 30 min at 37 °C with each protease inhibitor and then submitted to SDS-PAGE in gels containing gelatin. Since EDTA is a reversible inhibitor, it was also included in the developing buffer.
2.10. Proteomic Analyses
2.10.1. Protein Identification from SDS-PAGE/Western Blot Immunogenic Bands
Immunogenic protein bands recognized by anti-N. fowleri antibodies in Western blots were matched to the corresponding bands on Coomassie blue stained gels. On the basis of immunoblotting results, 3 strongly recognized bands were manually excised from gels and submitted to tryptic digestion followed by nano-LC-MS/MS for protein identification, as described [41]. Proteins in gel plugs were reduced with 10 mM dithiothreitol, alkylated with 50 mM iodoacetamide and digested overnight in an automated workstation (Intavis, Tübingen, Germany) using sequencing-grade trypsin (Sigma Aldrich, St. Louis, MI, USA). The resulting peptides were dried, redissolved in water containing 0.1% formic acid and transferred to polypropylene nano-LC vials. Ten μL of each digest was loaded on a C18 trap column (75 μm × 2 cm, 3 μm particle; PepMap®, Thermo Fisher Scientific, Waltham, MA, USA), washed with 0.1% formic acid (solution A), and then separated at 200 nL/min with a 3 μm particle, 15 cm × 75 μm C18 Easy-spray® analytical column using a nano-Easy® 1200 chromatograph (Thermo Fisher Scientific, Waltham, MA, USA). A gradient from 0.1% formic acid (solution A) to 80% acetonitrile with 0.1% formic acid (solution B) was developed: 1–5% B in 1 min, 5–26% B in 25 min, 26–79% B in 4 min, 79–99% B in 1 min, and 99% B in 4 min, for a total time of 35 min. MS spectra were acquired in positive mode at 1.9 kV, with a capillary temperature of 200 °C, using 1 μscan at 400–1600 m/z, maximum injection time of 100 msec, AGC target of 3 × 106, and orbitrap resolution of 70,000. The top 10 ions with 2–5 positive charges were fragmented with AGC target of 1 × 105, maximum injection time of 110 msec, resolution 17,500, loop count 10, isolation window of 1.4 m/z, and a dynamic exclusion time of 5 s. The obtained MS/MS spectra were processed for peptide matching against protein sequences contained in the UniProt database for Naegleria, using Peaks X® (Bioinformatics Solutions, Waterloo, ON, Canada). Cysteine carbamidomethylation was set as a fixed modification, while deamidation of asparagine or glutamine, and methionine oxidation were set as variable modifications, allowing up to 2 missed cleavages by trypsin. Parameters for match acceptance were set to FDR < 1%, −10 lgP protein score ≥ 20, and ≥1 unique peptides.
2.10.2. Proteome Profiling of Whole EVs by Bottom-Up Shotgun Analysis
A preliminary proteome profiling of whole EV cargo was performed by a bottom-up shotgun strategy. To this end, EV proteins were concentrated by adding 4 volumes of cold acetone and incubating overnight at −20 °C [20]. The samples were submitted to centrifugation at 13,000× g for 10 min at 4 °C and two washing steps in 1 mL of cold acetone were performed. The acetone was then removed with a mixture of nitrogen and air stream and proteins were redissolved in one volume up to 12 µL of sterile PBS, mixed with electrophoresis sample buffer and loaded into wells of a 12% SDS-PAGE gel. The electrophoretic run was stopped as soon as the migration front entered 3 mm into the resolving gel, in order to concentrate the whole protein cargo as a single band in the stacking/resolving gel interface. This band was visualized by Coomassie stain, excised, destained in 50% acetonitrile, and finally submitted to tryptic digestion and nano-LC-MS/MS analysis as described above.
3. Results
3.1. Characterization of Extracellular Vesicles
3.1.1. Transmission Electron Microscopy and Atomic Force Microscopy
A standardized protocol that includes differential centrifugation, coupled to a filtration process through 0.22 µm pore membranes and ultracentrifugation was employed to evaluate the production of EVs by trophozoites of a clinical isolate of N. fowleri. After the isolation procedure, transmission electron microscopy and atomic force microscopy analyses were employed to identify the EVs secreted by this species.
In this work, 5 × 107 trophozoites were placed in cell culture flasks of 125 cm2 with 10 mL 2% casein hydrolysate culture medium and incubated for 5 h at 37 °C to further isolate the EVs. Under our incubation conditions, amoebae produced approximately 0.675 µg/μL protein in EVs. After the 5 h incubation, viability of trophozoites remained above 97.5% (mean percentage of viability of amoebae remaining in the supernatant after the first centrifugation step at 2500× g and amoebae in the surface of the culture flask).
Figure 1 shows a representative transmission electron microscopy image of the pellets collected after applying the EV isolation protocol. In this Figure, diameters of the vesicles ranged from 43.88 nm to 207.95 nm.
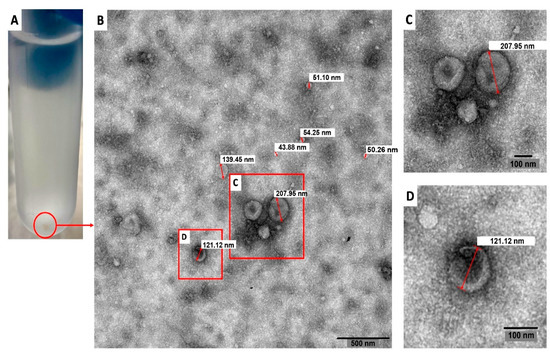
Figure 1.
Transmission electron microscopy image of extracellular vesicles secreted by trophozoites of Naegleria fowleri. A pellet of extracellular vesicles obtained after the isolation procedure employed is shown in (A). The content of these pellets was analyzed using transmission electron microscopy and vesicles of different diameters were obtained (B) (43.88 nm; 50.26 nm; 51.10 nm, 54.25 nm; 121.12 nm; 139.45 nm and 207.95 nm in this image). Magnified images of (C,D) are also shown. Representative image of three different samples analyzed.
The secretion of EVs by trophozoites of N. fowleri was also analyzed using atomic force microscopy (Figure 2, Figures S1 and S2). In this case, pellets of EVs obtained after ultracentrifugation and trophozoites incubated for 1 h following the conditions for EV production were analyzed. Figure 2 shows topography (A) and amplitude (B) images of a trophozoite of N. fowleri surrounded by EVs of different sizes. A topography image of the extracellular vesicles obtained after the isolation procedure is shown in C.
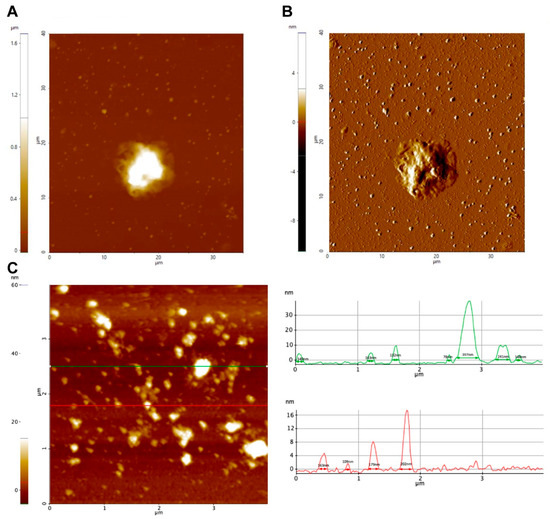
Figure 2.
Atomic force microscopy analysis of extracellular vesicles of Naegleria fowleri. (A) Trophozoite surrounded by secreted extracellular vesicles (1 h incubation): (A) Z-height image and (B) amplitude image. (C) Analysis of isolated pellets of EVs revealed the production of vesicles ranging from 30 nm to 400 nm in length and 4.5 nm to 40 nm in height. Representative images are shown.
3.1.2. Nanoparticle Tracking Analysis and Dynamic Light Scattering
Results of nanoparticle tracking analysis (NTA) and dynamic light scattering are summarized in Figure 3. For EVs secreted by trophozoites of N. fowleri, NTA revealed a mean size of 216 ± 83 nm and a mode of 206 nm (A-C). Using the same technique, it was also possible to determine that 5 × 107 trophozoites of N. fowleri secreted approximately 4.96 × 1010 particles.
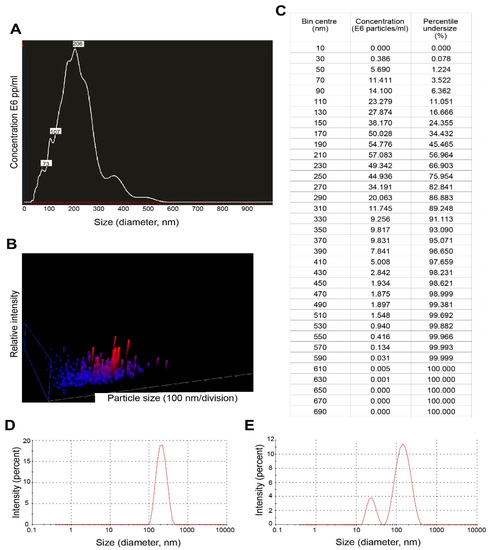
Figure 3.
Nanoparticle tracking analysis and dynamic light scattering results of extracellular vesicles secreted by trophozoites of Naegleria fowleri: (A) Graph showing sizes and relative concentration of the vesicles using NTA; (B) 3D plot of particle size/relative intensity using NTA and (C) table of concentration of particles of different sizes obtained during the NTA analysis. (D) Graph showing the size of EVs from the pellets using DLS and (E) graph showing size of EVs from the remaining supernatant (after the washing steps) using DLS.
For dynamic light scattering (DLS), measurements of the pellets of EVs and 1 mL of the remaining supernatant at the bottom of the tube (collected after the washing step by ultracentrifugation) were performed (Figure 3D,E). For the pellets, the mean size obtained for EVs was 227.13 ± 37.98 nm, while the remaining supernatant contained vesicles of 206.29 ± 37.08 nm. A second population of EVs of 24.24 ± 9.18 nm was obtained in one of the replicates of the supernatant.
Finally, to characterize the surface electrical charge of EVs of N. fowleri, zeta potential measurements were performed in different samples, resulting in a mean value of −12.228 ± 4.843 mV.
3.2. Protein Profile and Recognition of Extracellular Vesicles by Anti-Naegleria fowleri Polyclonal Antibodies
Figure 4 shows the electrophoretic protein profile of EVs produced by trophozoites of N. fowleri after Coomassie and silver stains (A-B). Protein bands ranging from approximately 25 KDa to 260 KDa were evidenced. However, low molecular weight proteins seem to be less abundant in both stains.
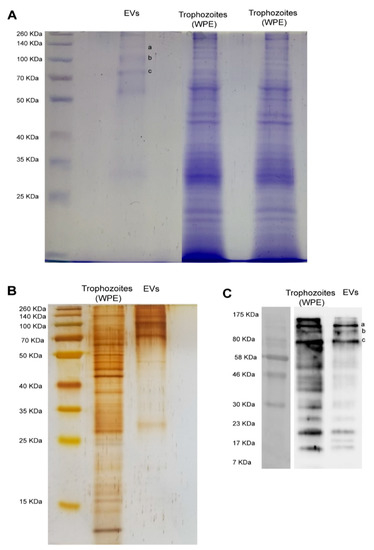
Figure 4.
Electrophoretic (SDS-PAGE) protein profile of extracellular vesicles and protein recognition by polyclonal anti-Naegleria fowleri antibodies using Western blot. (A) Coomassie and (B) silver stains showing similar band patterns in extracellular vesicles, which range from >25 KDa to 260 KDa; EVs: extracellular vesicles; WPE: whole protein extracts of trophozoites of N. fowleri. For Coomassie stain, approximately 30 µg of protein/sample were loaded onto the gel and for silver stain, approximately 9 µg of protein/sample were loaded onto the gel. (C) Western blot showing the recognition of 3 intense bands over 70–80 KDa ((C), a–c) and 3 less intense bands under 23 KDa, using the polyclonal anti-N. fowleri antibody (1:10,000) produced in rat. Bands over 70–80 KDa ((A), a–c) were excised from Coomassie stained gels and submitted to nano-LC-MS/MS. Protein identifications are listed in Table 1.
Western blot analysis using polyclonal anti-N. fowleri antibodies confirmed the immunorecognition of several EV proteins. Recognition was observed in bands from over 10 KDa to 175 KDa. The major recognition of proteins in EVs was observed close to 80 KDa, where 3 intense bands were identified. In addition, 3 less intense bands of <23 KDa were also observed (C).
Preliminary whole proteome analysis of EVs revealed a total of 184 proteins (Supplemental Table S1), of which 85 are still uncharacterized in the current Uniprot database. The list of proteins includes Rho GTPases, actin and actin related proteins, ubiquitin, dehydrogenases and fowlerpain, among others.
3.3. Protease Activity of Extracellular Vesicles
Results from protease activity of EVs of N. fowleri are presented in Figure 5. EVs and whole trophozoite extracts were submitted to zymography using gels with gelatin. From the Figure, protease activity in both type of samples is observed (A). EVs and trophozoite extracts showed similar protease activity, which resulted more evident in whole trophozoite extracts. In both types of samples, proteases appeared in the region of high molecular weight (from approximately 100 KDa to 260 KDa).
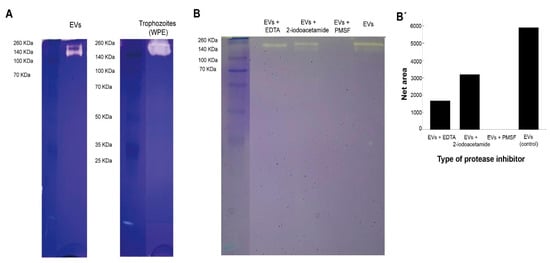
Figure 5.
Protease activity of extracellular vesicles and whole protein extracts of trophozoites of Naegleria fowleri showing clear areas of gelatin digestion. For this experiments, 2.5 µg of protein/sample were loaded onto the gel. (A) Protease activity of extracellular vesicles (EVs) and whole protein extracts (WPE) of trophozoites of N. fowleri. (B) Specific protease activity of EVs previously incubated with EDTA 10 mM (metalloproteases inhibitor), 2-iodoacetamide 1 mM (cysteine proteases inhibitor) and phenylmethylsulfonyl fluoride (PMSF) 1 mM (serine proteases inhibitor). Protease activity was fully inhibited by PMSF, which could indicate a predominance of serine proteases in the samples analyzed. Densitometry analysis of the gel was preformed using Image J software and band intensities are represented in (B′).
When the samples were treated with protease inhibitors, areas of gelatin digestion were not observed when incubating samples of EVs with phenylmethylsulfonyl fluoride, which could indicate the majority of proteases found in EVs of N. fowleri consist of serine proteases (B-B′). On the other hand, protease activity was partially inhibited when the samples were incubated with EDTA (metalloproteases inhibitor) and, apparently, less inhibition was found when incubating with 2-iodoacetamide (cysteine proteases inhibitor).
4. Discussion
EVs are small lipid vesicles released by prokaryotic and eukaryotic cells that contain nucleic acids, proteins, and small metabolites essential for cellular communication. Depending on the targeted cell, these vesicles can act either locally or in distant tissues in a paracrine, endocrine or juxtacrine cell signaling manner [42,43]. In EVs released by parasites, the analysis of the cargo and related functions suggest that these vesicles could be considered a mechanism of transferring biomolecules to host cells and tissues [44]. Recent reviews indicate that EVs released by protozoan parasites are capable of inducing an effective immune response, modulating the immune response and affecting the invasion process [45,46]. To our knowledge, this is the first study that evidences the production of EVs by N. fowleri; more specifically, a clinical isolate of this free-living amoeba that produces primary amoebic meningoencefalitis was employed.
In this study, trophozoites of N. fowleri incubated at 37 °C for 5 h in serum-free casein hydrolysate culture medium released EVs of different sizes. Transmission electron microscopy images showed vesicles ranging from ~44 nm to ~208 nm in diameter (Figure 1). Nanoparticle tracking analysis also revealed that ~76% of the vesicles were within <250 nm and only 2.3% of the population showed sizes of >390 nm (Figure 3), which could correspond to aggregates of vesicles, as ultracentrifugation could cause aggregation due to the high speed of centrifugation employed. The mean sizes of 206 nm or 227 nm, obtained by nanoparticle tracking analysis and dynamic light scattering, are slightly higher than those reported for EVs of Acanthamoeba, which range from 111.3 nm to 184.6 nm using DLS [27] and 166.7 nm using NTA [26]. For the latter genus, different size populations in the same sample of EVs were reported, especially when incubating trophozoites at 28 °C [27,46]. It is known that factors such as cell type, cell confluency or density, stimulation of cells with exogenous compounds, or abrupt change in culture conditions during the production of conditioned media might all potentially affect the yield outcome and the functional characteristics of the EVs secreted [47]. More studies are necessary to compare the EVs released under different culture conditions or by different isolates and/or species of Naegleria.
Atomic force microscopy also confirmed the secretion of EVs by N. fowleri (Figure 2, Figures S1 and S2). In this case, samples of trophozoites and EVs collected after the isolation procedure employed were analyzed. For EVs, heights of 4.5 nm to 40 nm and diameters of 30 nm to 400 nm were obtained; diameters >220 nm could consist of EV aggregates. EVs of more heterogeneous sizes were observed when analyzing samples of trophozoites incubated at 37 °C for 1 h in serum-free casein hydrolysate culture medium.
Besides imaging, particle number and size, determination of zeta potential was also included in the EV characterization analyses, which resulted in a value of −12.228 ± 4.843 mV. The zeta potential in a dispersed system is a measure of charge stability and affects particle-particle interaction [48]. This is considered a popular method to determine the surface potential of EVs, while used as an indicator of surface charge and colloidal stability, influenced by surface chemistry, bioconjugation and the theoretical model applied [49]. As the electrical charge of the surface of EVs is reflected in the zeta potential, it could be also considered as a characteristic of the vesicle´s population [19]. Determination of zeta potential is also considered one of the most useful tools to investigate the collective behavior of nanoparticles, such as EVs in dispersed systems, and thus, holds promise as a method for studying the activity of EVs in biological processes [49]. In this sense, the surface charge is known to influence different biological processes related to vesicles, such as cellular uptake and cytotoxicity. For example, charge-dependent differences in the mode of cytotoxic action have been reported using synthetic nanoparticles (NPs); it appears that positively charged NPs cause membrane damage, whereas anionic particles cause intracellular damage [50]. Studies on the effect of charge density and the kind of charge (positive, negative) in nonphagocytic cells have also shown that charged NPs (polystyrene and iron oxide) are taken up better than their uncharged counterparts [50]; moreover, anionic NPs seem to be better ingested in phagocytic cells [50]. These observations could suggest that, due to the mean zeta potential obtained in this study, EVs of N. fowleri could easily be captured by host cells and release their contents within the cells, including virulence factors, as will be discussed below. Uptake experiments to evaluate the entrance of EVs of N. fowleri in different cell lines are being currently performed by our research group.
Literature reports that include zeta potential values of EVs of parasitic origin are very scarce. Mean values of zeta potential of −16 ± 4 mV and −18 ± 8 mV have been reported for trypomastigotes and epimastigotes of Trypanosoma cruzi [19]. In this study, the mean zeta potential value obtained in EVs secreted by trophozoites of N. fowleri is considered within the range previously reported by different EV research groups. It is important to mention that, according to several authors, there are still limitations when interpreting the results of zeta potential of EVs [51] and the isolation methodology should be taken into account when performing the interpretations.
To evaluate the presence of immunogenic cargo in EVs released by our clinical isolate of N. fowleri, Western blots were performed using polyclonal antibodies raised in rats against a whole protein extract of trophozoites of N. fowleri (ATCC N. fowleri Carter 30,808). Results showed a similar recognition pattern in EVs and protein extracts of trophozoites; however, in EVs, the stronger recognition was observed in a group of 3 bands above 70–80 KDa and 3 bands below 23 KDa (Figure 4). Similarities found in the recognition patterns of whole protein extracts and EVs suggest that antigenic components—either cytosolic or from the plasma membrane of trophozoites—could be packed within the EVs secreted by the amoeba, components that may possibly stimulate the immune response during the invasion process of the amoeba to the host. In recent years, the ability of EVs to elicit an effective immune response has been the subject of extensive research related to diagnosis and immunotherapy in parasitic diseases produced by helminths and protozoan microorganisms [8]. For example, in the case of malaria, evidence suggests that EVs are a source of circulating virulence factors that appear to influence disease severity [52]. Moreover, the release of EVs by Plasmodium berghei elicits a strong proinflammatory reaction thtrough macrophage activation [53], while the immunization with EVs of P. yoelii seems to induce a protective response in a murine model, triggering the production of IgG and stimulating reticulocitosys [22]. These findings suggest that EVs could be employed as antigen carriers for novel vaccination strategies. In this sense, it has been shown that EVs secreted by tachyzoites of Toxoplasma gondii are highly immunogenic, capable of inducing both protective humoral and cellular immune responses [54,55] and triggering the production of IL-10, TNFα and iNOS in an in vitro model using murine macrophages [56]. However, in another protozoan parasite, Leishmania, exosomes have shown an immunosuppresive role, inhibiting the production of IFN-γ and IL-10 by monocyte-derived dendritic cells [57]. Besides this immunomodulatory activity, EVs could act as vehicles for virulence factors like the major surface protease (MSP), also known as GP63 [58,59]. Regarding free-living amoebae, Costa et al. have demonstrated that EVs of clinical and environmental isolates of Acanthamoeba are capable of inducing the production of nitric oxide via TLR4 in an in vitro model using murine macrophages [28]. These authors concluded that EVs carry different types of PAMPs that could trigger the innate immune response during the infection process.
In order to identify the predominant proteins in EVs recognized by the polyclonal anti-N. fowleri antibodies, the 3 more intense bands over 70–80 KDa were submitted to proteomic analysis. In this case, 36, 28 and 35 proteins were identified in bands a, b, and c, respectively. From these proteins, actin (B5M6J9), Hsp70 (Q6B3P1) and glyceraldehyde-3-phosphate dehydrogenase (D2W142) are proteins that had been previously reported by Gutiérrez Sanchez et al. in 2020, showing differential expression between N. fowleri and N. lovaniensis [60]. Another protein of interest is adenosyl homocysteinase (A0A6A5BYK8) (Table 1), a protein that has been considered as potential target in the design of new drugs for the treatment of primary amoebic meningoencephalitis. This protein participates in methylation reactions required for growth and gene regulation; for this reason, it has been considered an ideal target for antimicrobial drugs, especially for eukaryotic parasites [61]. Regarding peptidases, fowlerpain (a cysteine protease) and leucine aminopeptidase were also identified in EVs of N. fowleri (Table 1 and Table S1). In general terms, peptidases are considered virulence factors in different parasitic infections, being implicated in immune evasion and in host invasion. In N. fowleri infections, cysteine proteases present in trophozoites and conditioned media have been considered essential during the invasion process of the amoeba to the central nervous system and its survival in brain tissue [62,63]. It is important to highlight the presence of the elongation factor 1-alpha (eeEF1 α), identified in one of the bands (Table 1 and Table S1). This protein has been found in exosomes of Leishmania in early infections and has been identified as an important factor for immunosuppression and priming of host cells for Leishmania invasion [64,65].

Table 1.
Proteins identified by nano-LC-MS/MS in trypsin-digested immunorecognized bands (a–c) of extracellular vesicles of Naegleria fowleri after SDS-PAGE/Western blot analysis using rat anti-N. fowleri antibodies *.
Besides the identification of immunogenic proteins, a preliminary whole proteome analysis of N. fowleri EVs was also obtained by a shotgun approach, revealing the presence of 184 proteins in vesicles obtained under our experimental conditions (Table S1). The presence of Rho GTPases, actin and actin related proteins, ubiquitin, dehydrogenases and fowlerpain was confirmed in EVs (Table S1). However, 85 of the detected proteins are still uncharacterized in databases. Previous studies have identified the presence of 110, 148 and 130 proteins in EVs of another free-living amoeba, Acanthamoeba, grown under different experimental conditions [25,46]. Results obtained after quantitative analysis showed that the largest protein families in EVs of Acanthamoeba were categorized into the classes of hydrolases and oxidoreductases.
Protease activity of EVs of N. fowleri was analyzed using zymography (Figure 5). In this case, protease activity between 140 KDa and 260 KDa, at 37 °C and pH 8, was identified. A complete and partial inhibition of protease activity was achieved when incubating EVs with phenylmethylsulfonyl fluoride and EDTA, respectively. These results suggest that the predominant enzymatic activity in EVs is related to serine proteases, followed by a less intense metalloprotease activity (Figure 4), similar to that described for clinical and environmental isolates of Acanthamoeba [25,26,28,46]. In contrast, published reports of protease activity of whole protein extracts of N. fowleri trophozoites suggest a predominance of cysteine proteases [63,66]. The release of serine proteases via EVs could contribute to the degradation of the extracellular matrix during the initial stages of the infection with this amoeba, thus facilitating tissue invasion as reported in in vitro infections with Acanthamaoeba genotype T4 [67,68,69]. Moreover, proteases could exert an effect over the immune response of the host, as reported for several parasites. For example, it has been demonstrated that serine proteases secreted by Leishmania donovani downregulate macrophages microbicidal activity [70], while in infections with Acanthamoeba, these enzymes are capable of inducing the production of IL-12 and mostly IL-6 by these cells [71].
In general terms, protease activity in protozoan related infections, including those produced by free-living amoebae, has been demonstrated and extensively discussed as a virulence factor. Proteases participate in the invasion and egress process of intracellular parasites like Plasmodium falciparum [72,73,74,75] and Toxoplasma gondii [76]; in the modulation of the immune response of macrophages during an infection with Leishmania Mexicana and L. donovani [77,78]; in the pathogenesis of the intestinal infection with Entamoeba histolytica [79,80], among others. Regarding free-living amoebae, the secretion and release of proteases has been related to the increase in cell permeability and in permeability of mitochondrial membranes, in extracellular matrix degradation, and in apoptosis induction and cell death [81,82]. More recently, the release in Leishmania infantum of the major surface protease, a metalloprotease responsible of the suppression of the immune response, was confirmed in EVs [59]. Ongoing studies to evaluate the significance of this secretion pathway of proteases in infections by free-living amoeba such as N. fowleri are being carried out in our laboratory.
5. Conclusions
Since 2012, scientific literature regarding EVs has increased at an exponential rate [83]. In parasitology, evidence suggests that EVs could be considered as key players in a pathway for the release of virulence factors. Research on EVs secreted by emerging pathogens, including free-living amoebae, could help to better understand pathogenic-related aspects in infections. The present study provides an extensive characterization of general properties and protein cargo for the EVs of a clinical isolate of N. fowleri, representing a valuable platform of information to pursue further investigations on this protozoon. Research in this field focusing on EVs could open new possibilities for the discovery of therapeutic targets and possible diagnostic biomarkers for infections such as the highly lethal primary acute meningoencephalitis caused by N. fowleri.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology11070983/s1. Figure S1: Atomic force microscopy images of a trophozoite of Naegleria fowleri surrounded by secreted extracellular vesicles: (A) Z-height image and (B) amplitude image; Figure S2: Atomic force microscopy images of extracellular vesicles secreted by trophozoites of Naegleria fowleri: A. and B. Z-height images, C. and D. amplitude images; Figure S3: Electrophoretic protein profile of extracellular vesicles and trophozoites of Naegleria fowleri after Coomassie stain; Figure S4: Electrophoretic protein profile of extracellular vesicles and trophozoites of Naegleria fowleri after silver stain; Figure S5: Molecular recognition of proteins of extracellular vesicles of Naegleria fowleri by polyclonal anti-Naegleria fowleri antibodies, using Western blot: A. molecular weight marker; B. samples of extracellular vesicles and trophozoites of Naegleria fowleri; Figure S6: Protease activity of extracellular vesicles (A) and trophozoites (B) of Naegleria fowleri; Figure S7: Determination of the protease types in extracellular vesicles of Naegleria fowleri using zymography. Table S1: Proteins identified in extracellular vesicles of Naegleria fowleri (total proteome).
Author Contributions
Conceptualization, L.R.M. and E.A.S.; methodology, L.R.M., E.A.S., G.S.-A. and B.L.; validation, E.A.S., L.R.M., B.L., G.S.-A. and A.O.; formal analysis, L.R.M., E.A.S., G.S.-A. and B.L.; investigation, L.R.M., E.A.S., M.F.S.E., A.C.-G., N.C.C., G.S.-A. and B.L.; resources, E.A.S., L.R.M., G.S.-A., B.L. and A.O.; data curation, L.R.M., B.L., G.S.-A. and E.A.S.; writing—original draft preparation, L.R.M., E.A.S., B.L. and G.S.-A.; writing—review and editing, E.A.S., L.R.M., B.L., G.S.-A. and A.O.; funding acquisition, E.A.S. and L.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Vicerrectoría de Investigación” of the “Universidad de Costa Rica”, by supporting the research projects C-1061: “Caracterización de antígenos de excreción/secreción y antígenos somáticos en amebas de vida libre mediante empleo de anticuerpos policlonales producidos en roedores” and C-2600: “Secreción de vesículas extracelulares por Naegleria fowleri y evaluación de su potencial rol inmunomodulador en un modelo in vitro”.
Institutional Review Board Statement
The use of animals was performed following the institutional guidelines (Spanish government regulations (Real Decreto RD1201/05)) and the guidelines of the European Union (European Directive 2010/63/EU). These experiments were approved by the Ethical Committee of the University of Granada (235-CEEA-OH-2018) and by the authorities of the Regional Government of Andalucía (JJAA) (number 12/11/2017/162).
Informed Consent Statement
Not applicable.
Data Availability Statement
Mass spectrometry raw files of the data presented in this work are available from the authors upon reasonable request.
Acknowledgments
Transmission electron microscopy was performed at the “Centro de Instrumentación Científica” of the “Universidad de Granada”. Dynamic light scattering was performed at the “Laboratorio de Polímeros” of the School of Chemistry, “Universidad Nacional” and atomic force microscopy imaging was performed at the Physics Department of the same university. Proteomic analyses were performed at the “Instituto Clodomiro Picado”, of the “Universidad de Costa Rica”. Special thanks to Oscar Rojas Carrillo for allowing the access to the equipment and for his methodological support during the dynamic light scattering analyses and Desire Arrieta Murillo and Jocelyn Cortés Espínola for the technical support during the atomic force microscopy analyses. G.S.-A. wants to thank “Plan de Mejoramiento Institucional UNA BM-04”, Consejo Nacional de Rectores (FEES-CONARE) and the “Vicerrectoría de Investigación” of Universidad Nacional de Costa Rica for funding for equipment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- van der Pol, E.; Boing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, M.L.; Wang, M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [Green Version]
- Hargett, L.A.; Bauer, N.N. On the origin of microparticles: From “platelet dust” to mediators of intercellular communication. Pulm. Circ. 2013, 3, 329–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular vesicle heterogeneity: Subpopulations, isolation techniques, and diverse functions in cancer progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef] [Green Version]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular vesicles as biomarkers and therapeutic tools: From pre-clinical to clinical applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Kodam, S.P.; Ullah, M. Diagnostic and therapeutic potential of extracellular vesicles. Technol. Cancer Res. Treat. 2021, 20, 15330338211041203. [Google Scholar] [CrossRef]
- Barnie, P.A.; Afrifa, J.; Gyamerah, E.O.; Amoani, B. Extracellular vesicles as biomarkers and therapeutic targets in cancers. In Extracellular Vesicles—Role in Diseases Pathogenesis and Therapy; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Khosravi, M.; Mirsamadi, E.S.; Mirjalali, H.; Zali, M.R. Isolation and functions of extracellular vesicles derived from parasites: The promise of a new era in immunotherapy, vaccination, and diagnosis. Int. J. Nanomed. 2020, 15, 2957–2969. [Google Scholar] [CrossRef]
- Silverman, J.M.; Clos, J.; de’Oliveira, C.C.; Shirvani, O.; Fang, Y.; Wang, C.; Foster, L.J.; Reiner, N.E. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J. Cell Sci. 2010, 123, 842–852. [Google Scholar] [CrossRef] [Green Version]
- Torrecilhas, A.C.; Schumacher, R.I.; Alves, M.J.; Colli, W. Vesicles as carriers of virulence factors in parasitic protozoan diseases. Microbes Infect. 2012, 15, 1465–1474. [Google Scholar] [CrossRef]
- Cwiklinski, C.; de la Torre-Escudero, E.; Trelis, M.; Bernal, D.; Dufresne, P.J.; Brennan, G.P.; O’Neill, S.; Tort, J.F.; Paterson, S.; Marcilla, A.; et al. The extracellular vesicles of the helminth pathogen, Fasciola hepatica: Biogenesis pathways and cargo molecules involved in parasite pathogenesis. Mol. Cell. Proteom. 2015, 14, 3258–3273. [Google Scholar] [CrossRef] [Green Version]
- Buck, A.H.; Coakley, G.; Simbari, F.; McSorley, H.J.; Quintana, J.F.; Le Bihan, T.; Kumar, S.; Abreu-Goodger, C.; Lear, M.; Harcus, Y.; et al. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gasan, T.A.; Kuipers, M.E.; Roberts, G.H.; Padalino, G.; Forde-Thomas, J.E.; Wilson, S.; Wawrzyniak, J.; Tukahebwa, E.M.; Hoffmann, K.F.; Chalmers, I.W. Schistosoma mansoni larval extracellular vesicle protein 1 (SmLEV1) is an immunogenic antigen found in EVs released from pre-acetabular glands of invading cercariae. PLoS Negl. Trop. Dis. 2021, 15, e0009981. [Google Scholar] [CrossRef] [PubMed]
- Marcilla, A.; Trelis, M.; Cortés, A.; Sotillo, J.; Cantalapiedra, F.; Minguez, M.T.; Valero, M.L.; del Pino, M.S.; Muñoz-Antoli, C.; Toledo, R.; et al. Extracellular vesicles from parasitic helminths contain specific excretory/secretory proteins and are internalized in intestinal host cells. PLoS ONE 2012, 7, e45974. [Google Scholar] [CrossRef]
- Aline, F.; Bout, D.; Amigorena, S.; Roingeard, P.; Dimier Poisson, I. Toxoplasma gondii antigen-pulsed dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect. Immun. 2004, 72, 4127–4137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrecilhas, A.C.; Tonelli, R.R.; Pavanelli, W.R.; da Silva, J.S.; Schumacher, R.I.; de Souza, W.; de Silva, N.C.; Abrahamsohn, I.A.; Colli, W.; Manso Alves, M.J. Trypanosoma cruzi: Parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes. Infect. 2009, 11, 29–39. [Google Scholar] [CrossRef]
- Cestari, I.; Ansa Addo, E.; Deolindo, P.; Inal, J.M.; Ramirez, M.I. Trypanosoma cruzi immune evasion mediated by host cell derived microvesicles. J. Immunol. 2012, 188, 1942–1952. [Google Scholar] [CrossRef] [Green Version]
- Díaz Lozano, I.M.; de Pablos, L.M.; Longhi, S.A.; Zago, M.P.; Schijman, A.G.; Osuna, A. Immune complexes in chronic Chagas disease patients are formed by exovesicles from Trypanosoma cruzi carrying the conserved MASPs N-terminal region. Sci. Rep. 2017, 7, 44451. [Google Scholar] [CrossRef] [Green Version]
- Retana Moreira, L.; Rodríguez Serrano, F.; Osuna, A. Extracellular vesicles of Trypanosoma cruzi tissue-culture cell-derived trypomastigotes: Induction of physiological changes in non-parasitized culture cells. PLoS Negl. Trop. Dis. 2019, 13, e0007163. [Google Scholar] [CrossRef] [Green Version]
- Retana Moreira, L.; Prescilla-Ledezma, A.; Cornet-Gomez, A.; Linares, F.; Jódar-Reyes, A.B.; Fernandez, J.; Ibarrola Vannucci, A.K.; De Pablos, L.M.; Osuna, A. Biophysical and biochemical comparison of extracellular vesicles produced by infective and non-infective stages of Trypanosoma cruzi. Int. J. Mol. Sci. 2021, 22, 5183. [Google Scholar] [CrossRef]
- Zauli, R.C.; Vidal, A.S.; Dupin, T.V.; de Morais, A.C.C.; Batista, W.L.; Xander, P. Extracellular vesicles released by Leishmania: Impact on disease development and immune system cells. In Leishmaniasis—General Aspects of a Stigmatized Disease; de Azevedo Calderonon, L., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Martin Jaular, L.; Nakayasu, E.S.; Ferrer, M.; Almeida, I.C.; del Portillo, H.A. Exosomes from Plasmodium yoelii-infected reticulocytes protect mice from lethal infections. PLoS ONE 2011, 6, e26588. [Google Scholar] [CrossRef] [Green Version]
- Schorey, J.S.; Cheng, Y.; Singh, P.P.; Smith, V.L. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015, 16, 24–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nievas, Y.R.; Lizarraga, A.; Salas, N.; Cóceres, V.M.; de Miguel, N. Extracellular vesicles released by anaerobic protozoan parasites: Current situation. Cell Microbiol. 2020, 22, e13257. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.; da Silva Ferreira, M.; Guimarães, A.J. Extracellular vesicles from the protozoa Acanthamoeba castellanii: Their role in pathogenesis, environmental adaptation and potential applications. Bioengineering 2019, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.C.; Tsai, C.Y.; Huang, J.M.; Wu, S.R.; Chu, L.J.; Huang, K.Y. Quantitative proteomic analysis and functional characterization of Acanthamoeba castellanii exosome-like vesicles. Parasit. Vectors 2019, 12, 467. [Google Scholar] [CrossRef] [PubMed]
- Retana Moreira, L.; Vargas Ramírez, D.; Linares, F.; Prescilla Ledezma, A.; Vaglio Garro, A.; Osuna, A.; Lorenzo Morales, J.; Abrahams Sandí, E. Isolation of Acanthamoeba T5 from water: Characterization of its pathogenic potential, including the production of extracellular vesicles. Pathogens 2020, 9, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, A.O.; Chagas, I.A.R.; de Menezes-Neto, A.; Rêgo, F.D.; Nogueira, P.M.; Torrecilhas, A.C.; Furst, C.; Fux, B.; Soares, R.P. Distinct immunomodulatory properties of extracellular vesicles released by different strains of Acanthamoeba. Cell Biol. Int. 2021, 45, 1060–1071. [Google Scholar] [CrossRef]
- Jahangeer, M.; Mahmood, Z.; Munir, N.; Waraich, U.; Mahmood Tahir, I.; Muhammad, A.; Muhammad Ali Shah, S.; Zulfqar, A.; Zainab, R. Naegleria fowleri: Sources of infection, pathophysiology, diagnosis and management; a review. Clin. Exp. Pharmacol. Physiol. 2019, 47, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Matanock, A.; Mehal, J.M.; Liu, L.; Blau, D.M.; Cope, J.R. Estimation of undiagnosed Naegleria fowleri primary amebic meningoencephalitis, United States. Emerg. Inf. Dis. 2018, 24, 162–164. [Google Scholar] [CrossRef] [Green Version]
- Marciano-Cabral, F.; Cabral, G.A. The immune response to Naegleria fowleri amebae and pathogenesis of infection. FEMS Immunol. Med. Microbiol. 2007, 51, 243–259. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, A.A.; Yazdani, N.; Ahmad, R.; Zehra, F.; Ahmad, N. Epidemiology of primary amoebic meningoencephalitis-related deaths due to Naegleria fowleri infections from freshwater in Pakistan: An analysis of 8-year dataset. Arch. Pharm. Pract. 2016, 7, 11–19. [Google Scholar] [CrossRef]
- Grace, E.; Asbill, S.; Virga, K. Naegleria fowleri: Pathogenesis, diagnosis, and treatment options. Antimicrob. Agents Chemother. 2015, 59, 6677–6681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Retana Moreira, L.; Zamora Rojas, L.; Grijalba Murillo, M.; Molina Castro, S.E.; Abrahams Sandí, E. Primary amebic meningoencephalitis related to groundwater in Costa Rica: Diagnostic confirmation of three cases and environmental investigation. Pathogens 2020, 9, 629. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.; Aikawa, E.; Alacaraz, M.J.; Anderson, J.D.; Andriantsitohaina, A.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV): A position statement of the International Society for Extracellular Vesicles and update of the MISEV guidelines. J. Extracel. Ves. 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Heukeshoven, J.; Dernick, R. Improved silver staining procedure for fast staining in PhastSystem Development Unit. I. Staining of sodium dodecyl sulfate gels. Electrophoresis 1988, 9, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [Green Version]
- Herron, G.S.; Banda, M.J.; Clark, E.J.; Gavrilovic, J.; Werb, Z. Secretion of metalloproteinases by stimulated capillary endothelial cells. II. Expression of collagenase and stromelysin activities is regulated by endogenous inhibitors. J. Biol. Chem. 1986, 261, 2814–2818. [Google Scholar] [CrossRef]
- Castro Artavia, E.; Retana Moreira, L.; Lorenzo-Morales, J.; Abrahams Sandí, E. Potentially pathogenic Acanthamoeba genotype T4 isolated from dental units and emergency combination showers. Mem. Inst. Oswaldo Cruz 2017, 112, 817–821. [Google Scholar] [CrossRef] [Green Version]
- Lomonte, B.; Fernández, J. Solving the microheterogeneity of Bothrops asper myotoxin-II by high-resolution mass spectrometry: Insights into C-terminal region variability in Lys49-phospholipase A2 homologs. Toxicon 2022, 210, 123–131. [Google Scholar] [CrossRef]
- De Pablos Torró, L.M.; Retana Moreira, L.; Osuna, A. Extracellular vesicles in Chagas disease: A new passenger for an old disease. Front. Microbiol. 2018, 9, 1190. [Google Scholar] [CrossRef] [Green Version]
- Nederveen, J.P.; Warnier, G.; Di Carlo, A.; Nilsson, M.I.; Tarnopolsky, M.A. Extracellular vesicles and exosomes: Insights from exercise science. Front. Physiol. 2021, 11, 604274. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.; Cwiklinski, K.; Lalor, R.; O’Connell, B.; Robinson, M.W.; Gerlach, J.; Joshi, L.; Kilcoyne, M.; Dalton, J.P.; O’Neill, S.M. Fasciola hepatica extracellular vesicles isolated from excretory-secretory products using a gravity flow method modulate dendritic cell phenotype and activity. PLoS Negl. Trop. Dis. 2020, 14, e0008626. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, L.; Li, J.; Wang, L.; Wu, Z.; Sun, X. Extracellular vesicle-mediated communication within host-parasite interactions. Front. Immunol. 2019, 15, 3066. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.S.; Ferreira, M.D.S.; Liedke, S.C.; Gomes, K.X.; de Oliveira, G.A.; Leão, P.E.L.; Cesar, G.V.; Seabra, S.H.; Cortines, J.R.; Casadevall, A.; et al. Extracellular vesicles and vesicle-free secretome of the protozoa Acanthamoeba castellanii under homeostasis and nutritional stress and their damaging potential to host cells. Virulence 2018, 9, 818–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudbergsson, J.M.; Johnsen, K.B.; Skov, M.N.; Duroux, M. Systematic review of factors influencing extracellular vesicle yield from cell cultures. Cytotechnology 2016, 68, 579–592. [Google Scholar] [CrossRef] [Green Version]
- Beit Yannai, E.; Tabak, S.; Stamer, W.D. Physical exosome:exosome interactions. J. Cell. Mol. Med. 2018, 22, 2001–2006. [Google Scholar] [CrossRef] [Green Version]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta potential of extracellular vesicles: Toward understanding the attributes that determine colloidal stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [Green Version]
- Soares Martins, T.; Catita, J.; Martins Rosa, I.; da Cruz, A.B.E.; Silva, O.; Henriques, A.G. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE 2018, 13, e0198820. [Google Scholar] [CrossRef] [Green Version]
- Sampaio, N.G.; Cheng, L.; Eriksson, E.M. The role of extracellular vesicles in malaria biology and pathogenesis. Malar J. 2017, 16, 245. [Google Scholar] [CrossRef] [Green Version]
- Couper, K.N.; Barnes, T.; Hafalla, J.C.; Combes, V.; Ryffel, B.; Secher, T.; Grau, G.; Riley, E.M.; De Souza, J.B. Parasite-derived plasma microparticles contribute significantly to malaria infection-induced inflammation through potent macrophage stimulation. PLoS Pathog. 2010, 6, e1000744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prigione, I.; Facchetti, P.; Lecordier, L.; Deslée, D.; Chiesa, S.; Cesbron-Delauw, M.F.; Pistoia, V. T cell clones raised from chronically infected healthy humans by stimulation with Toxoplasma gondii excretory-secretory antigens cross-react with live tachyzoites: Characterization of the fine antigenic specificity of the clones and implications for vaccine development. J. Immunol. 2000, 164, 3741–3748. [Google Scholar] [CrossRef] [Green Version]
- Costa-Silva, T.A.; Borges, M.M.; Galhardo, C.S.; Pereira-Chioccola, V.L. Immunization with excreted/secreted proteins in AS/n mice activating cellular and humoral response against Toxoplasma gondii infection. Acta Trop. 2012, 124, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.O.; Maia, M.M.; Torrecilhas, A.C.; Taniwaki, N.N.; Namiyama, G.M.; Oliveira, K.C.; Ribeiro, K.S.; Toledo, M.D.S.; Xander, P.; Pereira-Chioccola, V.L. Extracellular vesicles isolated from Toxoplasma gondii induce host immune response. Parasite Immunol. 2018, 40, e12571. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.M.; Clos, J.; Horakova, E.; Wang, A.Y.; Wiesgigl, M.; Kelly, I.; Lynn, M.A.; McMaster, W.R.; Foster, L.J.; Levings, M.; et al. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J. Immunol. 2010, 185, 5011–5022. [Google Scholar] [CrossRef] [Green Version]
- Hassani, K.; Antoniak, E.; Jardim, A.; Olivier, M. Temperature-induced protein secretion by Leishmania mexicana modulates macrophage signalling and function. PLoS ONE 2011, 6, e18724. [Google Scholar] [CrossRef] [Green Version]
- Marshall, S.; Kelly, P.H.; Singh, B.K.; Pope, R.M.; Kim, P.; Zhanbolat, B.; Wilson, M.E.; Yao, C. Extracellular release of virulence factor major surface protease via exosomes in Leishmania infantum promastigotes. Parasit. Vectors 2018, 11, 355. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, M.; Carrasco-Yepez, M.M.; Herrera-Díaz, J.; Rojas-Hernández, S. Identification of differential protein recognition pattern between Naegleria fowleri and Naegleria lovaniensis. Parasite Immunol. 2020, 42, e12715. [Google Scholar] [CrossRef]
- Tillery, L.; Barrett, K.; Goldstein, J.; Lassner, J.W.; Osterhout, B.; Tran, N.L.; Xu, L.; Young, R.M.; Craig, J.; Chun, I.; et al. Naegleria fowleri: Protein structures to facilitate drug discovery for the deadly, pathogenic free-living amoeba. PLoS ONE 2021, 16, e0241738. [Google Scholar] [CrossRef]
- Martínez-Castillo, M.; Ramírez-Rico, G.; Serrano-Luna, J.; Shibayama, M. Iron-binding protein degradation by cysteine proteases of Naegleria fowleri. BioMed Res. Int. 2015, 2015, 416712. [Google Scholar] [CrossRef] [Green Version]
- Vyas, I.K.; Jamerson, M.; Cabral, G.A.; Marciano-Cabral, F. Identification of peptidases in highly pathogenic vs. weakly pathogenic Naegleria fowleri amebae. J. Eukaryot. Microbiol. 2015, 62, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.M.; Reiner, N.E. Leishmania exosomes deliver preemptive strikes to create an environment permissive for early infection. Front. Cell. Infect. Microbiol. 2012, 1, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timm, T.; Annoscia, G.; Klein, J.; Lochnit, G. The eukaryotic elongation factor 1 alpha (eEF1α) from the parasite Leishmania infantum is modified with the immunomodulatory substituent phosphorylcholine (PC). Molecules 2017, 22, 2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano-Luna, J.; Cervantes-Sandoval, I.; Tsutsumi, V.; Shibayama, M. A biochemical comparison of proteases from pathogenic Naegleria fowleri and non-pathogenic Naegleria gruberi. J. Eukaryot. Microbiol. 2007, 54, 411–417. [Google Scholar] [CrossRef]
- Khan, N.A.; Jarroll, E.L.; Panjwani, N.; Cao, Z.; Paget, T.A. Proteases as markers for differentiation of pathogenic and nonpatogenic species of Acanthamoeba. J. Clin. Microbiol. 2000, 38, 2858–2861. [Google Scholar] [CrossRef] [Green Version]
- Alsam, S.; Sissons, J.; Jayasekera, S.; Khan, N.A. Extracellular proteases of Acanthamoeba castellanii (encephalitis isolate belonging to T1 genotype) contribute to increased permeability in an in vitro model of the human blood-brain barrier. J. Infect. 2005, 51, 150–156. [Google Scholar] [CrossRef]
- Omaña-Molina, M.; González Robles, A.; Salazar Villatoro, L.I.; Lorenzo Morales, J.; Cristóbal Ramos, A.R.; Hernández Ramírez, V.I. Reevaluating the role of Acanthamoeba proteases in tissue invasion: Observation of cytopathogenic mechanisms on MDCK cell. monolayers and hamster corneal cells. BioMed Res. Int. 2013, 2013, 461329. [Google Scholar] [CrossRef] [Green Version]
- Das, P.; De, T.; Chakraborti, T. Leishmania donovani secretory serine protease alters macrophage inflammatory response via COX-2 mediated PGE-2 production. Indian J. Biochem. Biophys. 2014, 51, 542–551. [Google Scholar]
- Cano, A.; Mattana, A.; Henriquez, F.L.; Alexander, J.; Roberts, C.W. Acanthamoeba proteases contribute to macrophage activation through PAR1, but not PAR2. Parasite Immunol. 2019, 41, e12612. [Google Scholar] [CrossRef] [Green Version]
- Koussis, K.; Withers-Martinez, C.; Yeoh, S.; Child, M.; Hackett, F.; Knuepfer, E.; Juliano, L.; Woehlbier, U.; Bujard, H.; Blackman, M.J. A multifunctional serine protease primes the malaria parasite for red blood cell invasion. EMBO J. 2009, 28, 725–735. [Google Scholar] [CrossRef] [Green Version]
- Collins, C.R.; Hackett, F.; Strath, M.; Penzo, M.; Withers-Martinez, C.; Baker, D.; Blackman, M.J. Malaria parasite cGMP-dependent protein kinase regulates blood stage merozoite secretory organelle discharge and egress. PLoS Pathog. 2013, 9, e1003344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, C.R.; Hackett, F.; Atid, J.; Tan, M.S.Y.; Blackman, M.J. The Plasmodium falciparum pseudoprotease SERA5 regulates the kinetics and efficiency of malaria parasite egress from host erythrocytes. PLoS Pathog. 2017, 13, e1006453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackman, M.J. Proteases in host cell invasion by the malaria parasite. Cell Microbiol. 2004, 6, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Buguliskis, J.S.; Lee, T.D.; Sibley, L.D. Functional analysis of rhomboid proteases during Toxoplasma invasion. mBio 2014, 5, e01795-14. [Google Scholar] [CrossRef] [Green Version]
- Buxbaum, L.U.; Denise, H.; Coombs, G.H.; Alexander, J.; Mottram, J.C.; Scott, P. Cysteine protease B of Leishmania mexicana inhibits host Th1 responses and protective immunity. J. Immunol. 2003, 171, 3711–3717. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, R.; Das, P.; De, T.; Chakraborti, T. Immunolocalization and characterization of two novel proteases in Leishmania donovani: Putative roles in host invasion and parasite development. Biochimie 2010, 92, 1274–1286. [Google Scholar] [CrossRef]
- Serrano-Luna, J.; Piña-Vázquez, C.; Reyes-López, M.; Ortiz-Estrada, G.; de la Garza, M. Proteases from Entamoeba spp. and pathogenic free-living amoebae as virulence factors. J. Trop. Med. 2013, 2013, 890603. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.A.; Nam, Y.H.; Min, A.; Kim, K.A.; Nozaki, T.; Saito-Nakano, Y.; Mirelman, D.; Shin, M.H. Entamoeba histolytica-secreted cysteine proteases induce IL-8 production in human mast cells via a PAR2-independent mechanism. Parasite 2014, 21, 1. [Google Scholar] [CrossRef] [Green Version]
- Visvesvara, G.S.; Moura, H.; Schuster, F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Betanzos, A.; Bañuelos, C.; Orozco, E. Host invasion by pathogenic amoebae: Epithelial disruption by parasite proteins. Genes 2019, 10, 618. [Google Scholar] [CrossRef] [Green Version]
- Bazzan, E.; Tinè, M.; Casara, A.; Biondini, D.; Semenzato, U.; Cocconcelli, E. Critical review of the evolution of extracellular vesicles’ knowledge: From 1946 to Today. Int. J. Mol. Sci. 2021, 22, 6417. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).