Overview and Update on Extracellular Vesicles: Considerations on Exosomes and Their Application in Modern Medicine
Simple Summary
Abstract
1. Introduction
2. Natural Nanoparticles Versus Synthetic Nanoparticles: The EVs Benefits
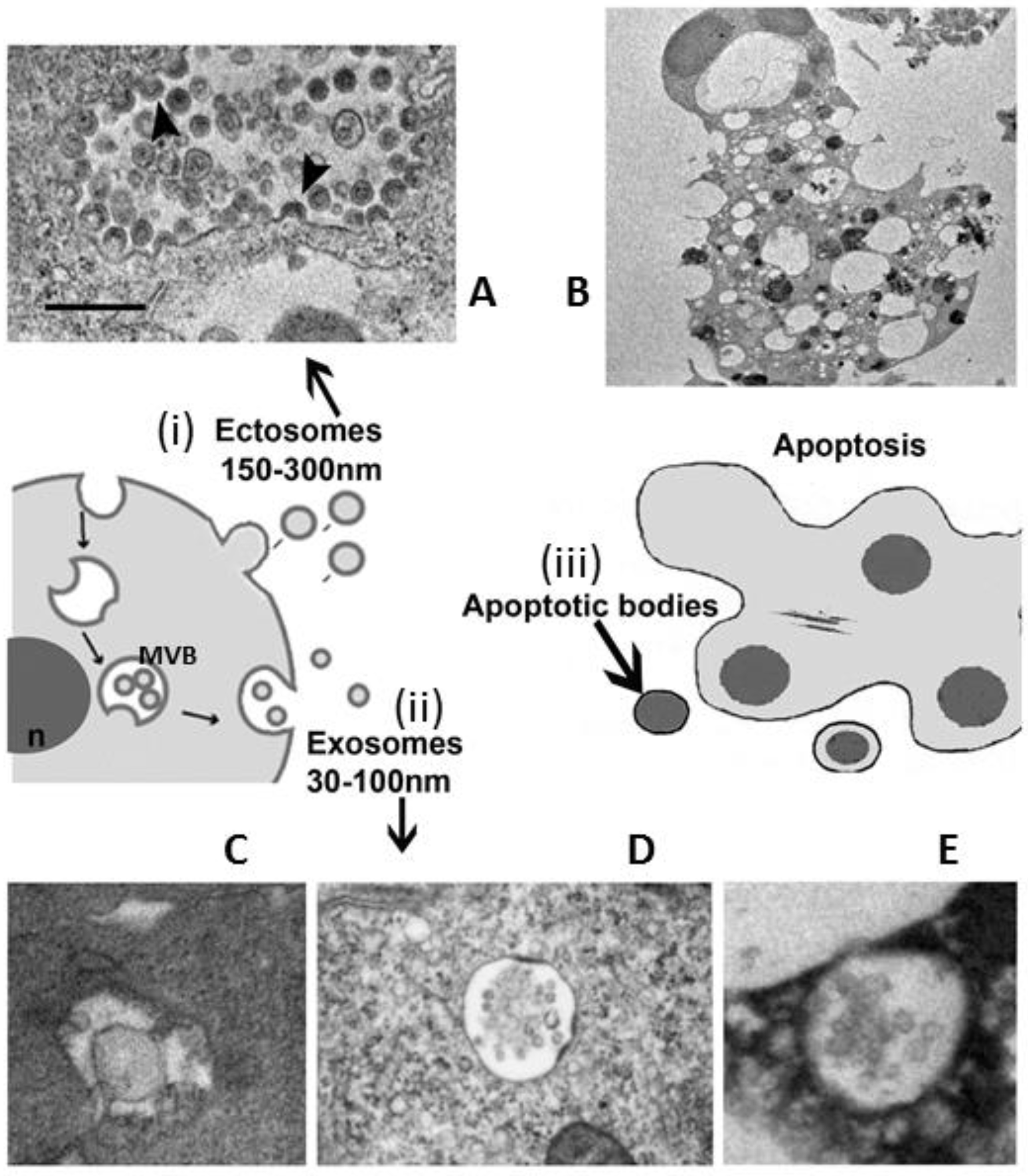
3. EVs Population under Microscope
- apoptotic bodies (ApoBDs): vesicles of relatively large size (1–5 μm), variable in structure and composition; they are released by the blebbing process of cells undergoing apoptosis (Figure 1B)
- microvesicles (MVs): diameter size of 150 nm–1 μm, they are shed directly by the outward budding and fission of the plasma membrane (Figure 1A)
- exosomes (EXOs): EVs with a diameter size ranging from 30 to 150 nm, with a density of 1.13 to 1.19 g/mL in sucrose; they originate from the late endosomal trafficking machinery. They are intracellularly produced into organelles called multivesicular bodies (MVBs) and ultimately, they are released into the extracellular milieu as a result of MVBs fusion with the plasma membrane (Figure 1C–E) [9,10,11,28,29].
4. The Use of Exosomes for Clinic Aims
4.1. EXOs Characteristics
4.2. EXOs Source
- Macrophages are mononuclear phagocytes that have critical roles in innate immunity. Macrophage-derived EXOs are known to express functional immune proteins; they can interact with brain vessel endothelial cells and cross the blood–brain barrier, an ability mediated in part by surface components; they can deliver some factors such as anti-inflammatory cytokines (i.e., IL-4). Moreover, they exhibit strong anti-tumor and anti-inflammatory effects [73,74].
- Mesenchymal stem cells are a popular choice for cell therapy. Indeed, they are easily obtained from different human tissues such as bone marrow, dental pulp, and adipose tissue. Mesenchymal stem cells are capable of self-renewal and are involved in modulating the immune response. EXOs isolated from these cells are extremely beneficial in promoting wound healing and in repairing tissue such as skin and cardiac tissue. Cao et al. [75] found that mesenchymal stem cell-derived exosomal miR-125b-5p could promote the repair of renal tubules in acute kidney injury. These vesicles also seem to inhibit cancer progression and have an inflammation melioration capacity. Additionally, these cells are known to secrete relatively high numbers of EXOs [76,77,78,79].
- Cancer cell lines such as melanoma cells are commonly used to produce EXOs. As reported before, tumor cell-derived EXOs can either block tumor growth or be involved in cancer progression and are capable of converting a normal cell into a transformed one. Thus, more importantly, tumor cells may be a double-edged sword when used for delivering therapeutics agents because their EXOs could show potential risk in aggravating a patient’s malignity instead of improving it or conferring drug resistance [80,81].
- To overcome the risk of horizontal gene transfer when EXOs are recovered from tumor cells or immortalized cells, some researchers have investigated the potential of human Red Blood Cells (RBC) as a source of vesicles. RBCs are abundant in the body, easy to obtain, and available in blood banks. A strategy to generate large-scale amounts of RBC-EXOs for the delivery of RNA and drugs was demonstrated by Usman et al. [82].
- Plasma exosomes are also derived from Platelets (PLT). These originate from bone marrow megakaryocytes and have no nucleus and a short half-life. PLT-derived EXOs can be obtained from animals, healthy volunteers, and from platelets in disease states. The functions of PLT-EXOs depend mainly on their source as they are rich in a variety of cargos. Platelets in disease states often contain pathogenic factors that can be used as biomarkers for disease diagnosis. EXOs obtained from healthy volunteers or mice can inhibit platelet activation and endothelial inflammation, while human PLT-EXOs have been shown to increase cell proliferation and migration of mesenchymal stromal cells (MSCs) from human bone marrow. PLT-EXOs could present advantageous therapeutic properties, including homologous administration in the clinical setting, thus overcoming the restrictive requirement of other biological products. Although procedures such as high-speed centrifugation of plasma induce the aggregation of PLT-derived EVs more than erythrocytes EVs and washing for preparing ‘washed’ platelets shows that most EXOs will be removed, nowadays isolation protocols with the use of specific commercial kits can avoid this effect [19].
4.3. EXOs Isolation and Storage
- Ultrafiltration: is a method based on the vesicle size, involving the use of fluid pressure to drive the migration through a polymeric membrane with defined pore size; vesicles are separated selectively from the samples with the simultaneous retention of larger molecules. It is simple and fast, but EXOs can be degraded and lost [92].
- Immunoaffinity: is a capture isolation technique based on the recognition by antibodies or ligands of EXO marker components (antigen) that are exposed on the vesicle surface. The immunoaffinity method has the advantages of rapid isolation, simplicity, and high specificity, and the sample volume can be very small in comparison to ultracentrifugation, but it is very expensive due to the cost of antibodies [93].
- Size-Exclusion Chromatography (SEC) techniques can isolate EXOs based upon molecular size and density, mainly by means of a column filled with a porous stationary phase with a specific pore size distribution. When the sample enters the gel, small particles with small hydrodynamic radii diffuse into the pores while large molecules with large hydrodynamic radii will not. Hence, the passage of proteins and other smaller contaminating molecules is delayed while larger molecules or larger vesicles (>75 nm) exit the column and will be eluted earlier in the void volume The porous stationary phase contained in the column can be cross-linked dextrans, polyacrylamide, agarose beads (commercially named as Sepharose), and allyldextran in which small particles can penetrate. The primary advantages of this technique are the screening of high-purity EXOs with less protein contamination compared to ultracentrifugation, and the preservation of vesicle integrity, structure, and biological activity as it relies on gravity rather than sheer force for isolation. However, this technique is limited by: (1) the need for dedicated equipment; (2) the accessibility of the chromatography column to contamination; therefore, aseptic working conditions should be ensured especially if the isolated EVs are intended for therapeutic use; (3) an initial large volume is required; (4) low yield; (5) difficulty in scaling up; (6) inability to separate EXOs from vesicles of the same size. Research efforts have been performed to overcome those challenges and enhance SEC efficacy and speed. For instance, the EXO pellet is re-suspended after enrichment by ultracentrifugation in combination with ultrafiltration methods and then further purified using SEC. This combined strategy resulted in improved purity and preserved EXO function. Moreover, commercially available columns and kits based on size-exclusion chromatography were designed to simplify EV isolation; iZON Science produced the qEV Exosome Isolation Kit that, as well as the PURE-EVs kit (Hansa Biomed), allows rapid, high-precision isolation within less than half an hour so the SEC methodology is nowadays relatively easy and fast. However, this combination is not suitable for scale-up production [94].
- Microfluidics platforms represent emerging isolation methods developed to separate EVs from large cellular debris and protein aggregates. Microfluidics techniques enable the differentiation, capture, enrichment, and isolation of particles of very similar shapes and sizes. Different isolation principles have been designed: size based, immune-affinity based, and dynamic categories that make use of emerging nanomaterials. Size-based exosome separation devices allow the separation of highly pure EXOs driving the plasma inside a channel where nanofilters, nanoporous membranes, or nanoarrays can trap vesicles when fluids flow through them. In another device, an acoustofluidics device, using ultrasound standing waves, in a contact-free continuous flow manner, EXOs are directly isolated from undiluted small blood samples based on their size, density, and compressibility. The result is the formation of clusters of EVs. These clusters are then washed and released upon deactivation of an ultrasound. This device maintains the structures, characteristics, and functions of the EXOs with a purity of about 98%. In addition, it enables the separation time, reagent consumption, and sample volume for isolating EXOs to be significantly reduced with short processing times with decreasing human intervention. In an immunoaffinity-based microfluidic device, the vesicle separation relies on specific biomarkers on the EXOs membrane. A commercial immune-microfluidics chip (ExoChip) allows the isolation of EXOs from mixed cultures because it is functionalized with a commonly expressed antigen, CD63. The specific interactions between CD63 and antibodies immobilized on the chip allow the capture of the vesicles. Unfortunately, to separate them efficiently, the immunoaffinity-based separation microfluidic devices need highly represented antigens (targeted proteins) on the vesicle surface. Other innovative and attractive separation approaches that have the ability to isolate EXOs based on their physical and biochemical properties are being simultaneously developed: some microfluidic isolation methods typically require small starting volumes from serum and cell culture (10 s–100 s of μL), while others can be performed on larger volume samples; they can reduce reagent consumption, are fast, and efficient. However, scalability, validation, sample pretreatments, and standardization are still considered bottlenecks for these devices, which are mainly applied in the field of diagnosis [95,96].
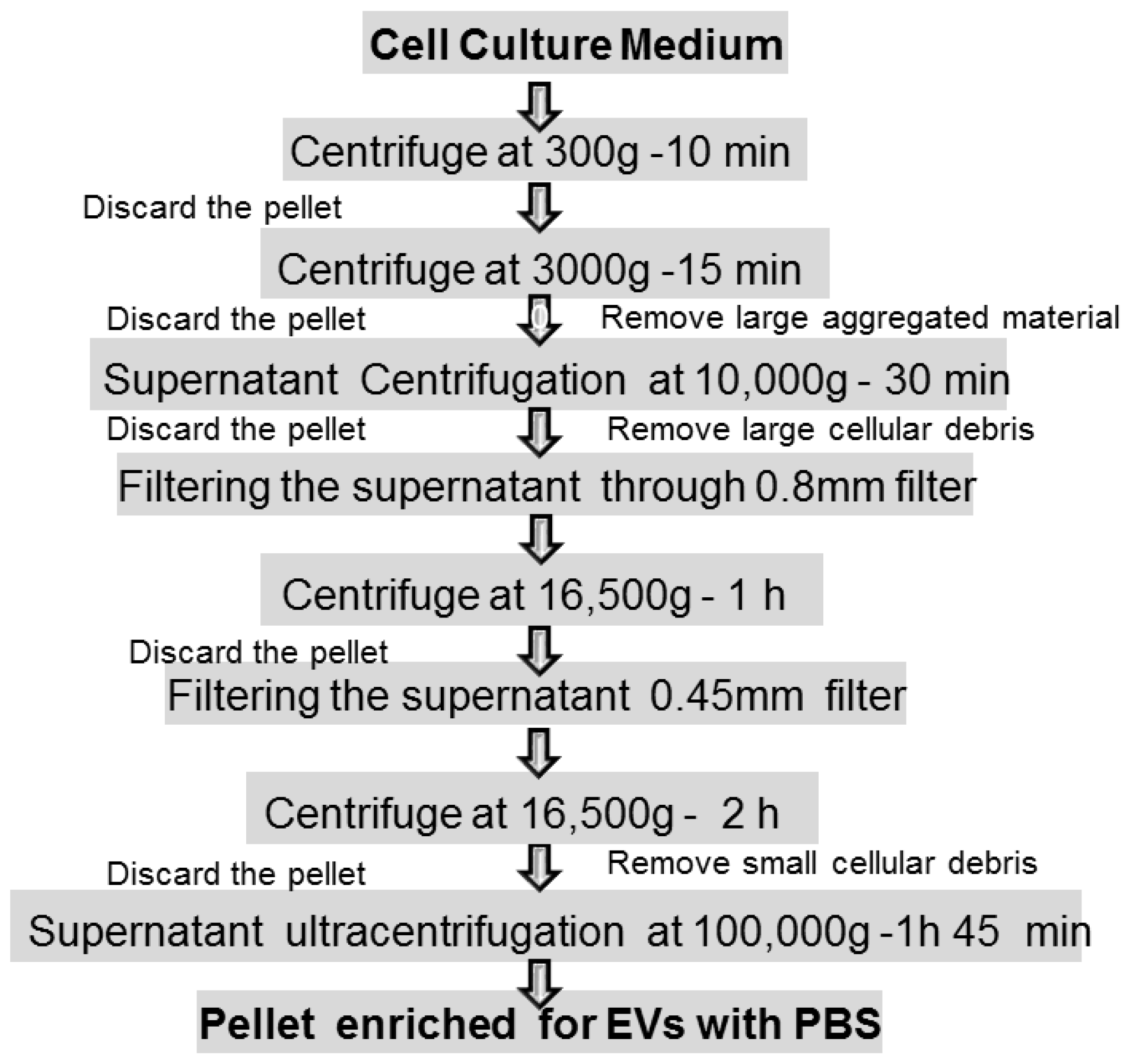
- Ultracentrifugation (100,000× g or greater) is currently the most widely used purification method that mainly depends on vesicle density, size, and shape. It consists of two steps after pelleting down cells: a pre-cleaning and filtering of samples centrifuged at low and intermediate speed centrifugation (500–10,000× g) to remove dead cells and cell debris, followed by the flotation in a density gradient centrifugation to precipitate and enrich EXOs. High-speed centrifugation (40,000–100,000× g) is often combined with a density gradient using commonly iodixanol or sucrose as a medium to remove contaminants such as proteins, protein/RNA aggregates, and lipoproteins. The EXOs can be collected in the density range of 1.1 to 1.2 g/mL Depending on the rotor utilized, this procedure is suitable for large sample processing; it requires little sample pretreatment and has the characteristics of low contamination risk. Moreover, the affordability is high since only one ultracentrifuge is needed for long time use. Apart from the access to expensive equipment, it is of low cost. At the same time, however, the density gradient centrifugation method is time consuming and requires extra care to prevent gradient damage. In addition, damage to EXOs by high-speed centrifugation might occur if used for long times (more than 3–4 h) [97] (Figure 4).
- Co-precipitation is an appealing precipitation-based isolation method thanks to the simple protocol and high yield. Polyethylene glycol (PEG) is generally used as a co-precipitator by decreasing the solubility of EXOs. The method lacks specificity and results in low purity of vesicles [98].
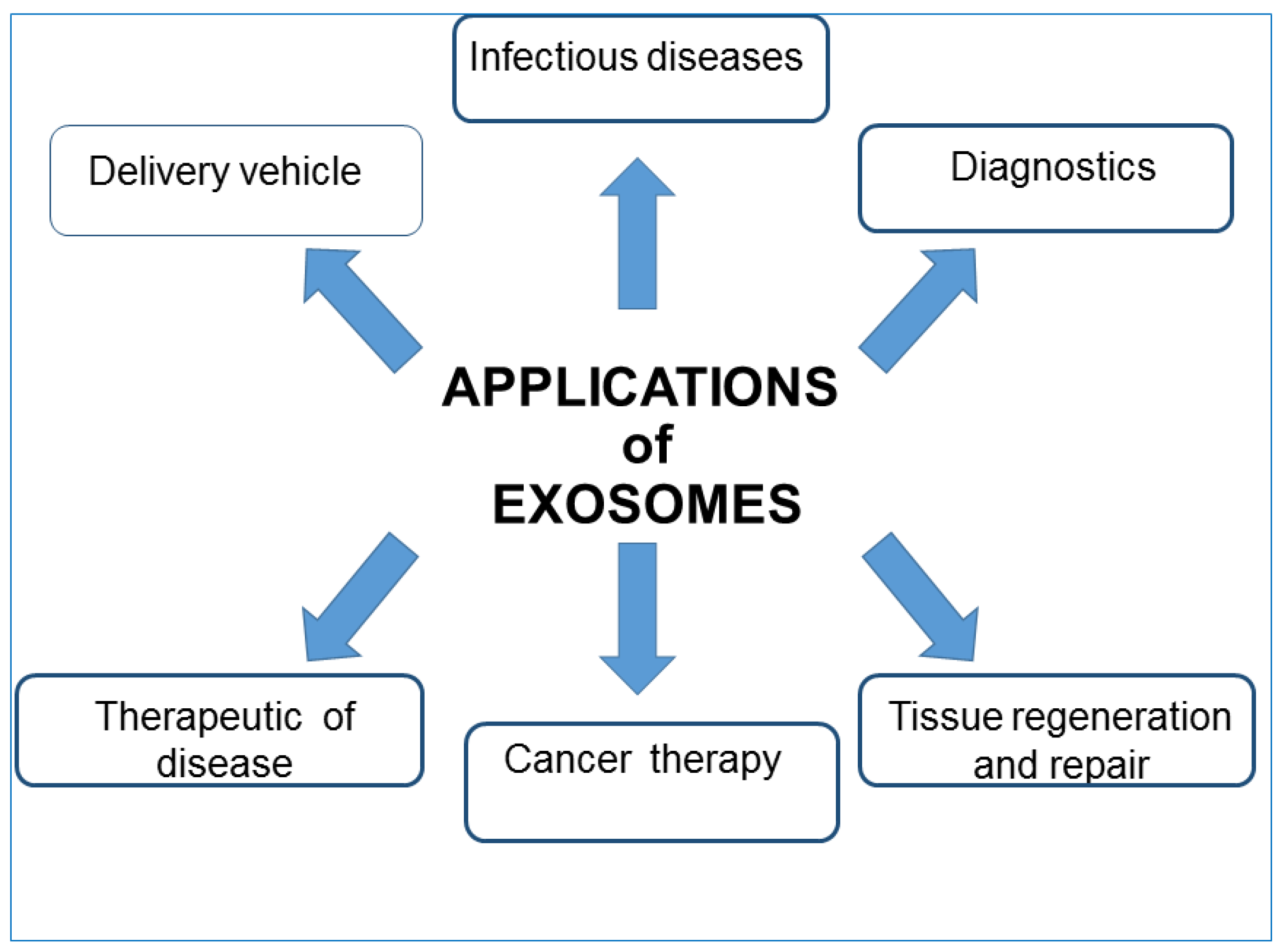
4.4. EXOs Clinical Applications
- Liquid biopsies: because EXOs differ in their composition based on the current state of the secreting cells, being able to isolate them from different body fluids can be considered a potent screening tool. Compared with traditional solid biopsy, liquid biopsy has a number of advantages: firstly, minimal trauma. Thus, EXOs isolated from liquid biopsies can be used as both diagnostic and prognostic non-invasive biomarkers. EXOs released from normal and cancer cell lines have different nucleic acid contents and membrane structures in accordance with their surface proteins, cholesterol contents, and cholesterol/phospholipid ratios. This enables the early detection of many pathological conditions, and their regression or progression in response to therapy. EXOs originating from tumor cells possess active molecules and specific genomic and proteomic features characteristic of a particular tumor type; therefore, their analysis could predict the potential presence of the tumor. For example, human serum exosomal long noncoding RNAs-UCA1 and exosomal miRNAs can be used as diagnostic biomarkers for cancer risk [101,102,103]. Epidermal growth factor receptor (EGFR), placental alkaline phosphatase (PLAP), and leucine-rich alpha-2 glycoproteins (LRG1) are potential biomarkers for non-small cell lung cancer, as they are all overexpressed in patients. Moreover, Grimolizzi et al. found that in both early and advanced-stage non-small cell lung cancer patients, miR-126 was mainly present in EXOs, while in healthy controls, circulating miR-126 was equally distributed between EXOs and exosome-free serum fractions. The detection of prostate cancer can also be achieved, evidencing the presence of exosomal miRNA-141 and miRNA-375 [104,105,106]. EXOs can find application as biomarkers also in cardiovascular diseases, and exosomal miRNAs may be beneficial for diagnosing heart diseases. Another important disease that could benefit from the study and application of EXOs is diabetes mellitus. Recent literature demonstrates that the content of exosomal miRNA is remarkably different in the sera of type I diabetes patients in comparison with that of healthy control. In addition, a pre-clinical study has indicated that exosomes also participate in type 2 diabetes pathogenesis. Certain EXOs biomarkers (P-S396-tau, P-T181-tau, and Ab1–42) seem to predict the development of Alzheimer’s disease up to 10 years in advance; EXOs secreted by various parts of the kidney, contain several biomolecules that might be markers of abnormality present in the kidney [107,108].
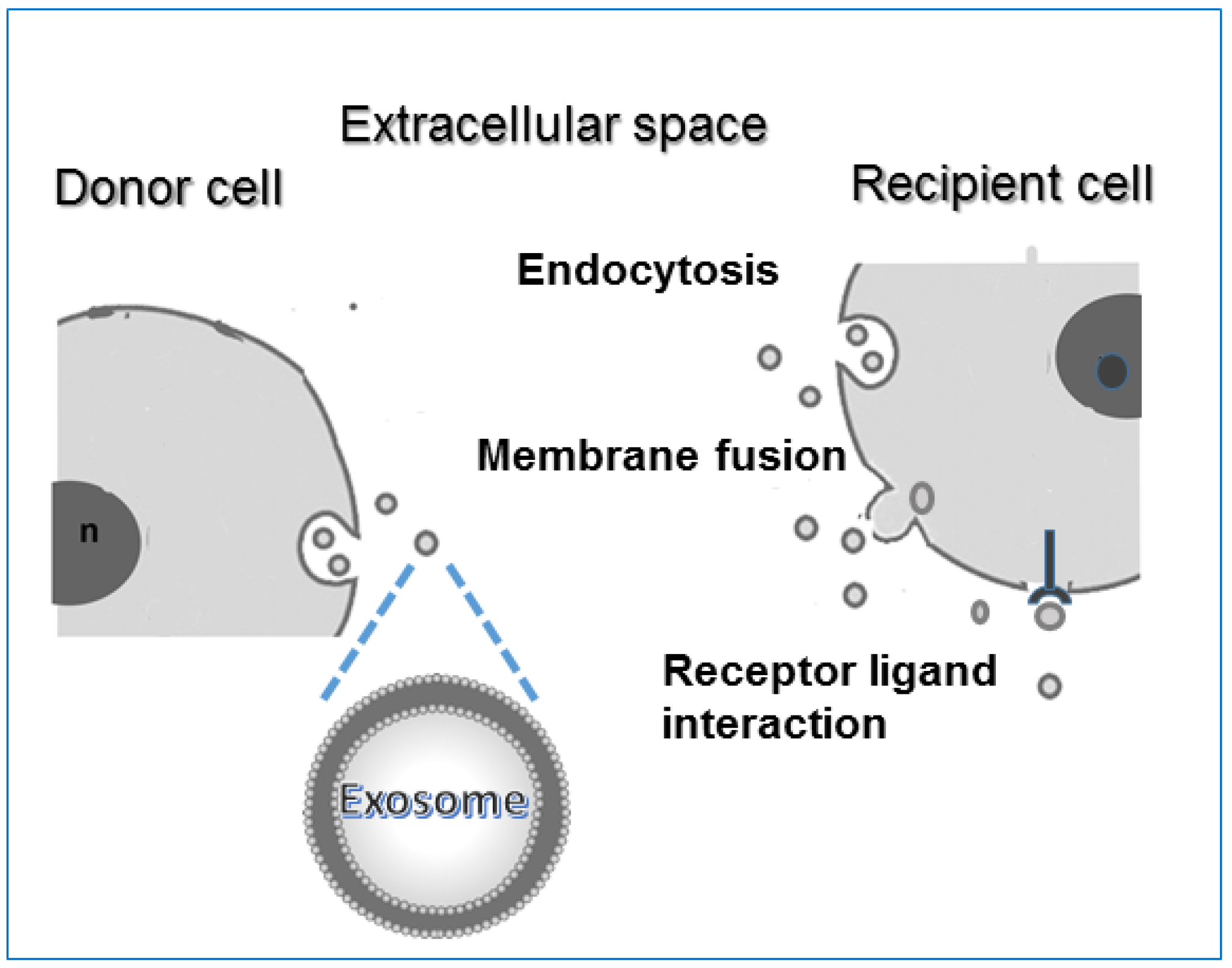
- Therapeutic intervention: Several studies have highlighted the therapeutic importance of EXOs. Being able to redirect vesicles to tissues of interest, EXO administration could be used to degrade pathological signals or focus their intrinsic therapeutic activity. EVs regulate various normal physiological and pathological activities; thus they can be used as natural therapeutic agents for treating a variety of common diseases. There are sufficient pre-clinical studies to support the application of dendritic cell-derived EXOs to treat different types of cancer such as metastatic melanoma and non-small cell lung cancer. For example, EXOs derived from mature dendritic cells prevent the production of cancer cells as they contain DHA (C22:16 docosahexaenoic acid, fatty acid), which enhances the antigen-presenting ability of cells and thus inhibits tumor cell proliferation. However, as EXOs participate in the progression of tumors and promote various stages of tumorigenesis, some research aims to regulate the process of EXOs secretion and reduce their release from tumor cells to normal levels or inhibit their uptake by the target cells [109]. The results of a preclinical trial indicated that by using dimethylamiloride (DMA), the secretion of EXOs can be repressed in murine tumor models by blocking intracellular Ca2+ and Na+/Ca2+ and Na+/H+ channels. Indeed, the increase in intracellular Ca2+ and reduction in intercellular and intracellular pH values lead to an increase in EXOs secretion, and the consequent uptake by recipient cells. Moreover, in order to remove the metastatic effect of cancer, a biotechnology company named Aethlon Medical has developed an adjuvant therapy called HER2O-some, which decreases HER2-positive EXOs secreted by cancer cells in circulation and thus interrupts the progression of HER2-positive breast cancer. Although the technique based on EXOs removal has achieved great progress, further research is still needed to assess its clinical safety. EXOs derived from bone marrow mesenchymal stem cells could produce protective effects in brain injury models, multiple sclerosis, and other neurological disorders thanks to their ability to enter biological barriers such as the BBB. In epilepsy, the administration of native EXOs can result in a reduction in inflammation, memory preservation, and a decrease in neuronal loss. The regenerative properties of EXOs have been shown after stroke injury in both rat and mouse models. By means of proteomics analysis, EXOs derived from mesenchymal stem cells were found to contain various proteins involved in the process of brain repair function. EXOs may accelerate and stimulate regeneration in several tissues, for instance, kidneys, and also seem to modulate transplant rejection [78,110,111]. Furthermore, EXOs have shown a protective effect on joint damage in a collagenase-induced OA model and in several cardiovascular diseases [112,113]. EXOs can act as a decoy for virus and bacterial toxins, thus suggesting a potential role as therapeutic agents [114,115]. These days, well-designed EXOs against COVID-19 may be feasible to prevent initial infection or further internal dissemination of the virus, thus reducing the virus burden and disease severity. Interestingly, EVs can be used in the treatment of COVID-19-associated brain damage due to their unique ability to penetrate the BBB and their potential to be engineered and targeted to a specific part of the CNS [116,117,118,119,120]. Recently, clinical trials that point to the use of EXOs as therapeutic agents against COVID-19 infection are currently ongoing. Moreover, EXOs are also being explored for their vaccine potential. In order to overcome the shortcomings of existing vaccines and contain escalating cases of COVID-19, several biotechnology companies are focusing on vaccine development using EXOs as a platform against SARS-CoV-2 [121]. In the following section, it will be in short reported how the potential use of natural EXOs is largely improved when they are modified and used as carriers of therapeutic agents.
- Drug delivery and nanotherapy: any shuttle used for drug delivery must possess several necessary characteristics: (i) can encapsulate an adequate amount of drug to obtain therapeutic effect; (ii) must possess a prolonged inherent stability of size, structure, and bioactivity of the therapeutic agent during circulation before reaching the target organ; (iii) can evade macrophages’ phagocytosis, must have non-toxic properties, be biocompatible with the immune response, and be non-immunogenic. For the past few years, several new nanoscale systems to deliver therapeutic drugs or genes have been designed to improve bioavailability, reduce the toxicity of traditional drugs, and target specific sites. The first clinical success in nanotechnology occurred in 1995 with the approval of Doxil (a formulation of liposomal doxorubicin). Since then, new therapies and biocompatible nanocarriers have been designed (silver nanoparticles, polymer nanoparticles, nanotubes) and used, but until now, an ideal drug delivery system, with long-term safety and biocompatibility, remains to be planned. EXOs are good candidates for delivering vehicles of chemotherapeutics agents to specific cells and tissues and trigger phenotypic changes. EXOs have lower immunogenicity than virus-based delivery systems and liposomes. As aforementioned, the lipid bilayer gives EVs an amphiphilic nature that allows them to store and dissolve both hydrophobic and hydrophilic compounds. Compared with free drugs, exosomes loaded with chemotherapeutic drugs showed a higher efficacy. Examples of such systems are doxorubicin-loaded EXOs, EXO-curcumin, and paclitaxel-loaded EXOs that were shown to exert stronger anti-proliferative activities or cytotoxicity in cancer cells than drugs alone. Curcumin is a polyphenol compound made from turmeric, a flowering plant of the ginger family. Curcumin loaded onto exosomes forms a complex that improves its solubility, stability, and bioavailability enabling to exert its antioxidant, antineoplastic, anti-inflammatory, and chemopreventive properties. Sun et al. treated mice with this complex and found that mice were able to resist lipopolysaccharide-induced septic shock [122]. Paclitaxel is a highly hydrophilic molecule used as an antitumor drug, but its clinical application is limited because of dose-dependent toxic side effects. The toxicity resulted in reduced exosomes loaded with this drug. Yang et al. using the zebrafish model demonstrated that exosomes loaded with the doxorubicin drug were able to cross the BBB and inhibit the growth of tumors. Doxorubicin is an amphiphilic drug that inhibits angiogenesis and controls tumor growth [123]. In addition, molecules such as catalase with antioxidant properties, anthocyanins with anti-cancer activity against ovarian cancer, and other molecules can exert increased therapeutic effects when loaded on exosomes. EXOs can also deliver DNA and RNA as genetic therapeutic agents. These molecules have sizes that obstacles passive diffusion and they are susceptible to enzyme degradation. Thus, they can be delivered and protected by the double membranes of the exosomal carrier. A summary of EXOs clinical applications is reported in Figure 5.
- An active approach: donor cells are co-incubated with small molecular weight drugs or other chemical compounds. Cargos may passively diffuse across the cell membrane and concentrate in the cytoplasm; after appropriate stimulation such as heat or hypoxia, cells release EXOs loaded with the desired cargo. A simple over-expression in the parental cells of desired cargo is most of the time sufficient. The gene transfection approach is used for loading exogenous nucleic acids into donor cells; the cells are transfected with DNA plasmid vectors, noncoding RNAs, etc., that are easily packaged by the natural biomolecular synthesis processes within EXOs. Then, EXOs can be rapidly isolated and purified [124]. This approach is simple but can result in poor loading and thus is not suitable for wide applications.
- A passive approach: EXOs previously isolated from different sources are incubated with various molecules, preferentially hydrophobic, that can easily penetrate inside and localize in their lumen. To improve EXOs permeabilization, different chemical or physical methods can be used. For example, saponin permeabilization, sonication, and mechanical extrusion over a polycarbonate membrane. A method often used for loading siRNA is electroporation; following the application of high-voltage electricity to the suspension of EXOs and therapeutic agents, temporary pores are created in the membrane through which molecules can pass inside the vesicles [125]. Another approach to modifying vesicles and improving their specific targeting ability is surface membrane modification. Using procedures such as chemical modifications to the EXO membrane, click chemistry, etc., target molecules, peptides, and ligand aptamers are allowed to directly anchor on the exosomal membrane [126,127].
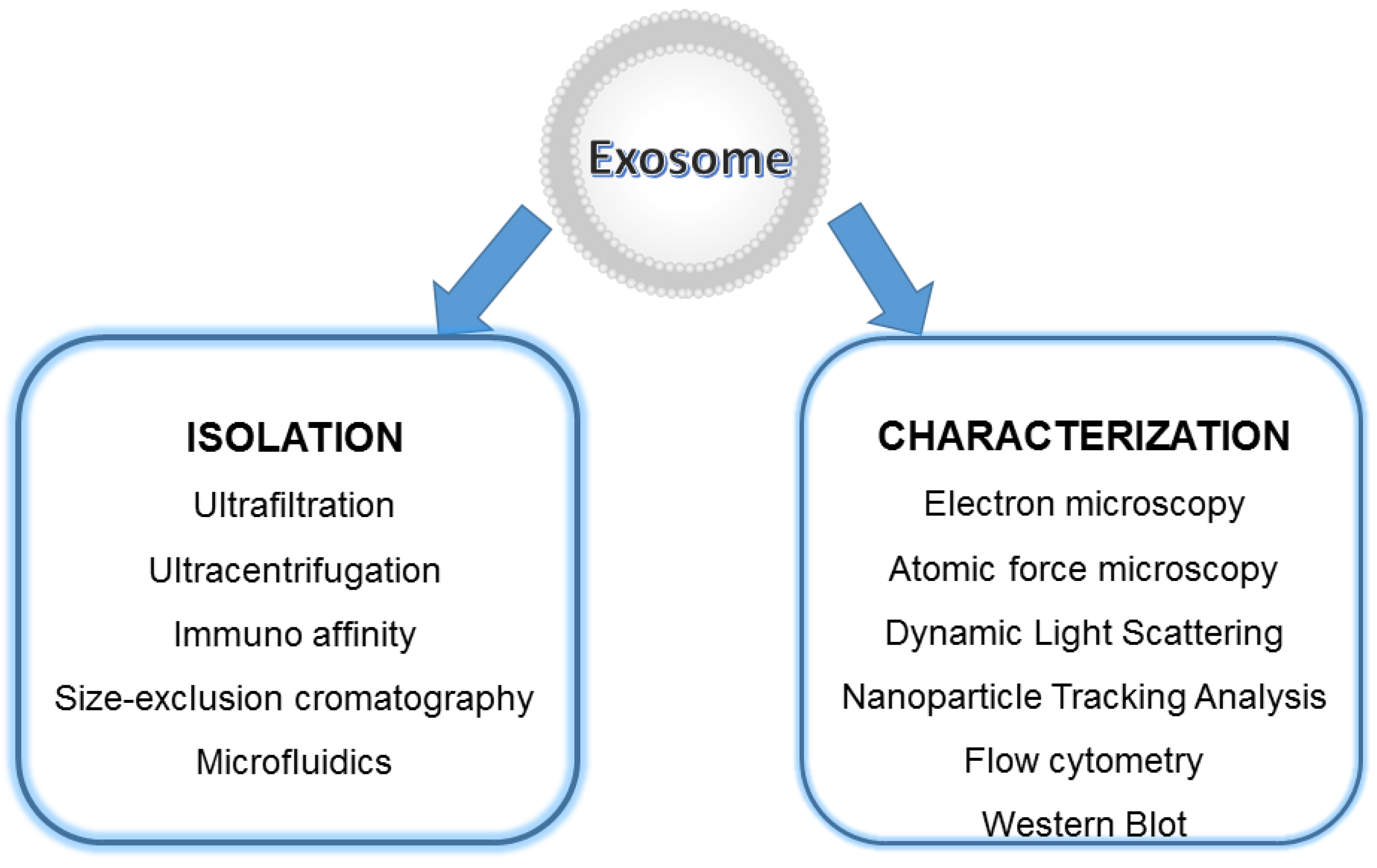
5. Exosomes Characterization and Detection Techniques
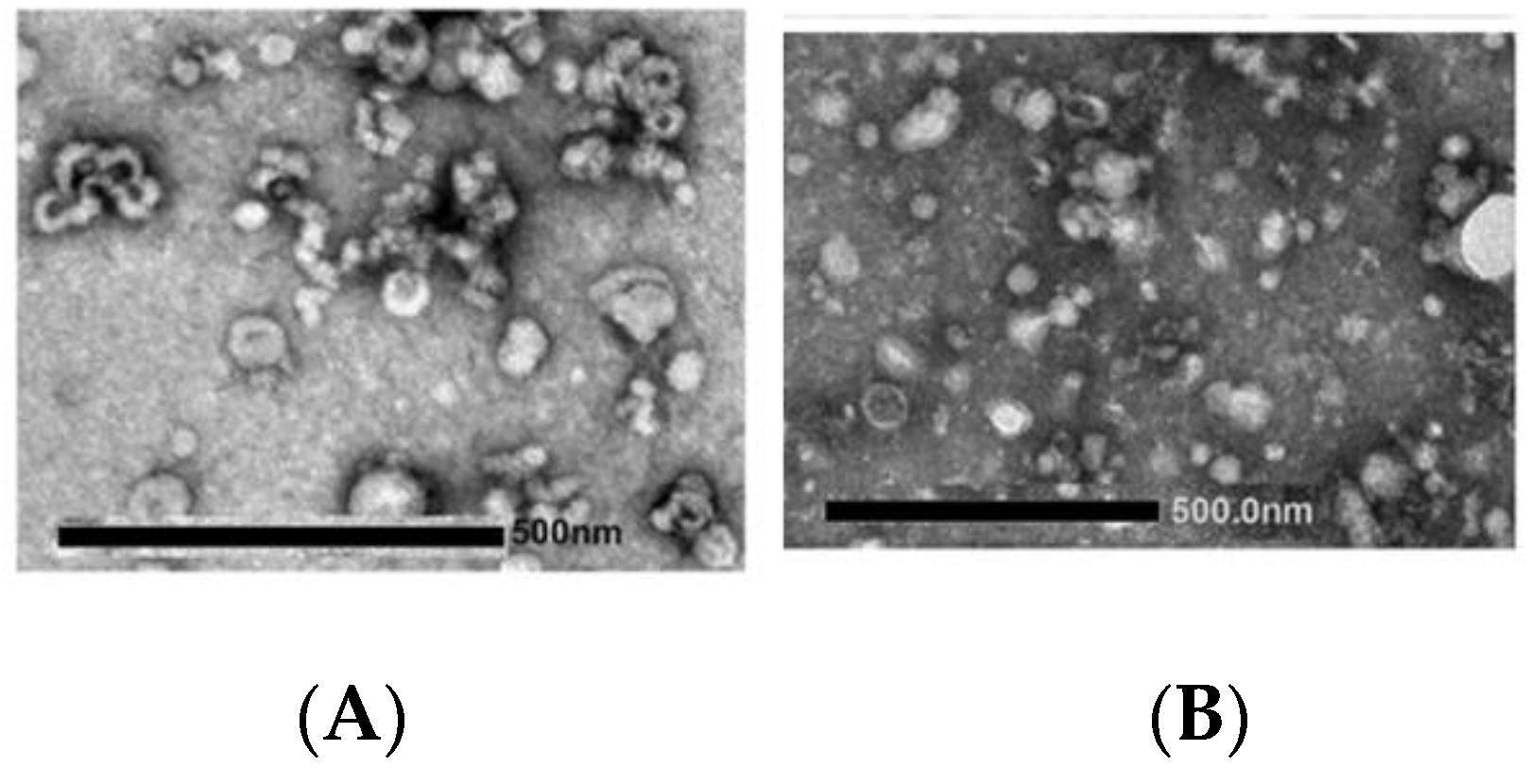
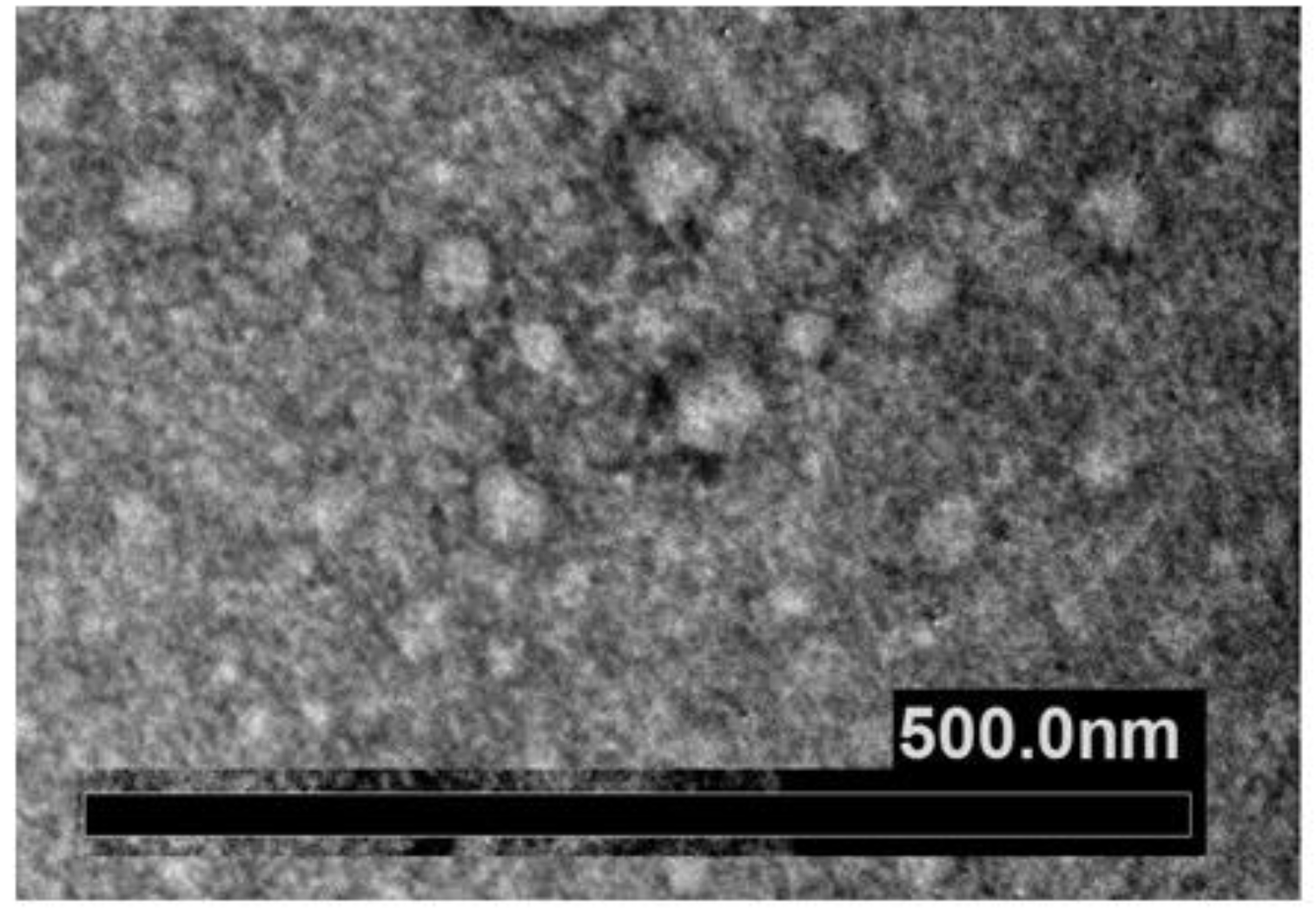
- Imaging approaches are critical steps in EXOs’ characterization; these usually include whole-mount SEM and TEM. The most accessible and simplest method of TEM imaging is conventional negative stain, which evidences a morphology called “cup-shaped”, because of a divot in the center of the exosomal vesicles (Figure 7). As reported by Raposo and Stoorvogel [9], the appearance of cup-shaped EXOs is likely an artifact due to the drying process associated with the sample preparation. Indeed, “whole-mount” samples are deposited on electron microscopy grids and allowed to desiccate on the surface. The nonuniformity of the capillary forces leads to a collapse resulting in a shape distortion. Accordingly, isolated frozen vesicles examined by cryo-EM and kept in their native state appear without ultrastructural changes; they show a spherical geometry rather than a cup-shaped feature. Moreover, 3D images generated by cryo-electron tomography and observations with other standard techniques verify the spherical shape.
- Non-imaging methods, such as dynamic light scattering (DLS), nanoparticle tracking analysis (NTA), Raman spectroscopy, tunable resistive pulse sensing (TRPS), and other biophysical approaches are being developed or adapted for EXOs data acquisition [134]. All these methods provide particles’ size distribution and concentration measurements; however, they indirectly estimate parameters via the use of some basic assumptions to interpret data. For example, NTA uses laser light to irradiate a nanoparticle suspension and estimates the size of EVs as their hydrodynamic diameters, which correlate the resistance to the Brownian mobility of EVs in the solution. A larger hydrodynamic diameter of a vesicle implies its lower mobility in liquid. Surface proteins and other molecules anchored or adsorbed to the membrane surface, the so-called the “coronal layer” around vesicles, and some debris can substantially influence the mobility and increases the hydrodynamic size of vesicles. Thus, their Brownian motion becomes difficult to track. DLS can quickly measure the average hydrodynamic diameter from a small volume of samples. An inherent weakness of DLS is its low resolution. To obtain satisfactory peak resolution, a particle size difference of at least three times is generally required. All these techniques are individually unable to determine the phenotype of the vesicles and their biochemical composition, and most commonly they need to be complemented with microscopy techniques.
6. Considerations and Challenges in the Research
- How are EXOs sorted to disparate targets?
- How can recipient cells discriminate among EVs? What are the mechanisms that mediate addressability?
- Which is the detailed mechanism of EXO uptake by recipient cells?
- The decision of EVs cargos’ destination in the recipient cells is not completely known. EXOs can release their cargo within the cytosol or directly within the nucleus or acidic organelles. Moreover, EXOs can also bind to extracellular matrix proteins regulating cell differentiation or promoting organ function repair. Fundamental questions remain about the fate of the biological cargo of EXOs.
- Do molecular cargos function singly or in combination with other EVs?
- Do EVs from different sources and distinct subtypes present different organ bio-distribution patterns and biological functions?
- Could the process of modification and loading with compounds of choice compromise EXOs biological functionality and induce immunogenicity?
- In addition, more data need to be collected on the characteristics of circulating EXOs and their spatiotemporal properties.
- Inconsistent isolation methodologies and insufficient massive and stable production of engineered EXOs with constant characteristics for clinical use; methods currently available are time consuming and expensive;
- Lack of standardization of engineered EXO preparations, especially in a purified form, to ensure quality control;
- It is still not clear which cell source is mostly suitable for generation of engineered vesicles;
- Efficiency of cargo or inefficient loading in engineering EXOs: for example, it is currently lower than that for liposomes;
- Lack of selection methods of EVs subtypes as there is a high heterogeneity of subpopulations. The diverse subpopulations of EVs represent a major challenge for EVs-based theranostics applications. Apart from their heterogeneity in size, density, and shape that can be overcome by improving combinations of different separation methods, cell heterogeneity is an important question. The biogenesis of EXOs within each cell type heavily depends on the microenvironment as well as the physiological states, and EXOs are like a “fingerprint” of the releasing cell and its metabolic status. EVs cargo can be affected by changes in gene expression resulting from environmental cues such as oxygen levels or inflammation. Based on surface marker expression, Gebara et al. [147] found that amniotic fluid-derived EVs showed a heterogeneous origin; vesicles expressed markers of fetal, placental cells, and also mesenchymal and stem cells markers. Investigations on tetraspanin marker expression profiles in individual EVs have evidenced seven subpopulations. EXOs composition is not limited to proteins; lipids are other major components of EXOs, so the analysis of lipid expression in individual EVs may provide insight into their nature. Lipid biomolecules may affect target cell function via direct activation of cell surface receptors or as secondary messengers following endocytosis. Subpopulations may also be separated based on RNA profiles. Subpopulations of EXOs with different components could also originate from the different sorting machinery involved during their biogenesis. Indeed, EXOs formed from ESCRT-based pathways are associated with different envelope-distinct biomolecules compared to the EXOs that are formed independently from the ESCRT. Even when vesicles are isolated from a single cell source (e.g., from cells cultured in vitro), spatial and temporal changes in confluency, cell cycle stage, and stress may contribute to the observed heterogeneity. Circulating EXOs are released from a large variety of different cell types, and in a given disease, EVs from the relevant cell type or tissue may be masked or confounded by the contribution of cargo from multiple other cells. For such reason, which subpopulations of vesicles from blood provide information for disease diagnostics and which subpopulation is actually dictating the fate of the target cells remain unclear. To help overcome the heterogeneity challenges in EXOs-based applications, further investigations with a wide variety of additional EV markers are required. Various types of omics technologies to analyze at the single EXOs level need to be improved. Super-resolution microscopy is a promising approach that allows rapid and direct visualization of individual EVs and their surface protein markers in aqueous solution where the native structure is retained. Unfortunately, intra-EV visualization is extremely technically challenging due to the small and heterogeneous nature of EVs. A different new direction would be in vivo tracking and real-time imaging of subpopulations of EVs; studying the in vivo at a single vesicle level without disrupting their physiological environments is with a very high degree of complexity. The highly relevant question of whether EXOs subpopulations are truly functionally distinct remains to be answered. The complexity of EXOs requires a lot of effort to acquire the desired accuracy in isolation and separation procedures;
- Pharmaceutical parameters such as EV storage and stability are still not standardized. EVs obtained from different sources may require different storage conditions. Freeze/thaw cycles should be minimized, as they may damage the EV membranes. Adding cryoprotectants seems to be positive. To increase commercial availability, novel preservation methods are encouraged. Lyophilization seems to be a promising method for the preservation of vesicles, but its application requires further investigations, as studies reporting on this technique are still preliminary;
- Route of administration is an important factor affecting the safety of EXOs.
7. Conclusions and Outlooks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drexler, E.K.; Peterson, C.; Pergamit, G. Unbounding the Future: The Nanothechnology Revolution; William Morrow and Company, Inc.: New York, NY, USA, 1991. [Google Scholar]
- Weissing, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (part 1): Products on the market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef]
- Rizzo, L.Y.; Theek, B.; Storm, G.; Kiessling, F.; Lammers, T. Recent progress in nanomedicine: Therapeutic, diagnostic and theranostic applications. Curr. Opin. Biotechnol. 2013, 24, 1156–1166. [Google Scholar] [CrossRef]
- Kumar, V.; Bayda, S.; Hadla, M.; Caligiuri, I.; Russo Spena, C.; Palazzolo, S.; Kempter, S.; Corona, G.; Toffoli, G.; Rizzolio, F. Enhanced Chemotherapeutic behavior of Open-caged DNA@Doxorubicin nanostructures for cancer cells. J. Cell Physiol. 2016, 231, 106–110. [Google Scholar] [CrossRef]
- Chidambaran, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Sci. 2011, 14, 67–77. [Google Scholar]
- Choi, Y.H.; Hang, H. Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Investig. 2018, 48, 43–60. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Shen, P.; Wang, J.; Shen, Y.; Shen, Y.; Webster, T.J.; Deng, J. Applications of inorganic nanomaterials in pathothermal therapy based on combinational cancer treatment. Int. J. Nanomed. 2020, 15, 1903–1914. [Google Scholar] [CrossRef]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome- mediated transfer of mRNAs and microRNAs is a novel mechanisms of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Gangoda, L.; Boukouris, S.; Liem, M.; Kalra, H.; Mathivanan, S. Extracellular vesicles including exosomes are mediators of signal transduction: Are they protective or pathogenic? Proteomics 2015, 15, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Théry, C. Communication by extracellular vesicles: Where we are and where we need to go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.D.; Homesley, H.D.; Doellgast, G.J. Binding of specific peroxidase-labe{}led antibody to placental-type phosphatase on tumor-derived membrane fragments. Cancer Res. 1980, 40, 4064–4069. [Google Scholar] [PubMed]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Lasser, C.; Alikhani, V.S.; Ekstrom, K.; Eldh, M.; Paredes, P.T.; Bossios, A.; Sjöstrand, M.; Gabrielsson, S.; Lötvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011, 9, 9. [Google Scholar] [CrossRef]
- Yuana, Y.; Koning, R.I.; Kuil, M.E.; Rensen, P.C.; Koster, A.; Bertina, R.M.; Osanto, S. Cryo-electron microscopy of extracellular vesicles in fresh plasma. J. Extracell. Vesicles 2013, 2, 21494. [Google Scholar] [CrossRef]
- Arraud, N.; Linares, R.; Tan, S.; Gounou, C.; Pasquet, J.M.; Mornet, S.; Brisson, A.R. Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 2014, 12, 614–627. [Google Scholar] [CrossRef]
- Greening, D.W.; Gopal, S.K.; Mathias, R.A.; Liu, L.; Sheng, J.; Zhu, H.J.; Simpson, R.J. Emerging roles of exosomes during epithelial-mesenchymal transition and cancer progression. Semin. Cell Dev. Biol. 2015, 40, 60–71. [Google Scholar] [CrossRef]
- Hoog, J.L.; Lotvall, J. Diversity of extracellular vesicles in human ejaculates revealed by cryo-electron microscopy. J. Extracell. Vesicles 2015, 4, 28680. [Google Scholar] [CrossRef]
- Van Dommelen, S.M.; Vader, P.; Lakhal, S.; Kooijmans, S.A.; van Solinge, W.W.; Wood, M.J.; Schiffelers, R.M. Microvesicles and exosomes: Opportunities for cell-derived membrane vesicles in drug delivery. J. Control. Release 2012, 161, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Rajadas, J.; Seifalian, A.M. Exosomes as nano-theranostic delivery platforms for gene therapy. Adv. Drug Deliv. Rev. 2013, 65, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, Y.; Shi, J. Exosome biochemistry and advances nanotechnology for next–generation theranostic platform. Adv. Mater. 2019, 31, 1802896. [Google Scholar] [CrossRef]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef]
- Shi, M.; Sheng, l.; Stewart, T.; Zabetian, C.P.; Zhang, J. New windows into the brain: Central nervous system-derived extracellular vesicles in blood. J. Prog. Neurobiol. 2019, 175, 96–106. [Google Scholar] [CrossRef]
- Pan, B.T.; Teng, K.; Wu, C.; Adam, M.; Johnstone, R.M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 1985, 101, 942–948. [Google Scholar] [CrossRef]
- Crescitelli, R.; Lasser, C.; Szabo, T.G.; Kittel, A.; Eldh, M.; Dianzani, I.; Buzás, E.I.; Lötvall, J. Distinct RNA profiles in subpopulations of extracellular vesicles: Apoptotic bodies, microvesicles and exosomes. J. Extracell. Vesicles 2013, 2, 20677. [Google Scholar] [CrossRef]
- Brydson, R.; Brown, A.; Hodges, C.; Abellan, P.; Hondow, N. Microscopy of nanoparticulate dispersions. J. Microsc. 2015, 260, 238–247. [Google Scholar] [CrossRef]
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J.; Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J. The methods of choice for Extracellular Vesicles (EVs) Characterization. Int. J. Mol. Sci. 2017, 18, 1153. [Google Scholar] [CrossRef]
- Meldolesi, J. Exosomes and ectosomes in intercellular communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide ranging implications in tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Poon, I.K.H.; Lucas, C.D.; Rossi, A.G.; Ravichandran, K.S. Apoptotic cell clearance: Basic biology and therapeutic potential. Nat. Rev. Immunol. 2014, 14, 166–180. [Google Scholar] [CrossRef]
- Xu, X.B.; Lai, Y.Y.; Zi, C.H. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef]
- Lee, Y.; El Andaloussi, S.; Wood, M.J. Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012, 21, R125–R134. [Google Scholar] [CrossRef]
- Cocucci, E.; Meldolesi, J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.L.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug. Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.T.; Johnstone, R.M. Fate of the tranferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Harding, C.; Sthahl, P. Tranferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing. Biochem. Biophys. Res. Commun. 1983, 113, 650–658. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Théry, C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From garbage bins to promising therapeutic targets. Int. J. Mol. Sci. 2017, 18, 538. [Google Scholar] [CrossRef]
- Van Niel, G.; Porto-Carreiro, I.; Simoes, S.; Raposo, G. Exosomes: A common pathway for a specialized function. J. Biochem. 2006, 140, 13–21. [Google Scholar] [CrossRef]
- Lotwall, J.; Hill, A.F.; Hochberg, F.; Buzàs, E.J.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morat, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Thery, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicles subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Kalluri, R.; le Bleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, 6478. [Google Scholar] [CrossRef]
- Jeppesen, D.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of exosome composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [PubMed]
- Mulkahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; d’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta 2014, 1841, 108–120. [Google Scholar] [CrossRef]
- Zhou, C.F.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.; Chin, A.R.; et al. Cancer secreted miR-105 destroys vascular endothelium barriers to promote metastasis. Cancer Cell 2014, 25, 505–515. [Google Scholar] [CrossRef]
- Lafourcade, C.; Ramirez, J.P.; Luarte, A.; Fernandez, A.; Wyneken, U. MiRNA in astrocyte-derived-exosomes as possible mediators of neuronal plasticity. J. Exp. Neurosci. 2016, 10, JEN-S39916. [Google Scholar] [CrossRef]
- Herrera, M.; Llorens, C.; Rodriguez, M.; Herrera, A.; Ramos, R.; Gil, B.; Candia, A.; Larriba, M.J.; Garre, P.; Earl, J.; et al. Differential distribution and enrichment of non-coding RNAs in exosomes from normal and cancer-associated fibroblasts in colorectal cancer. Mol. Cancer 2018, 17, 114. [Google Scholar] [CrossRef]
- Chen, Z.; Larregina, A.T.; Morelli, A.E. Impact of extracellular vesicles on innate immunity. Curr. Opin. Organ Transplant. 2019, 24, 670–678. [Google Scholar] [CrossRef]
- Zhou, C.F.; Ma, J.; Huang, L.; Yi, H.Y.; Zhang, Y.M.; Wu, X.G.; Yan, R.M.; Liang, L.; Zhong, M.; Yu, Y.H.; et al. Cervical squamous cell carcinoma-secreted exosomal miR-221-3p promotes lymphangiogenesis and lymphatic metastasis by targeting VASH1. Oncogene 2019, 38, 1256–1268. [Google Scholar] [CrossRef]
- Rezaie, J.; Rahbarghazi, R.; Pezeshki, M.; Mazhar, M.; Yekani, F.; Khaksar, M.; Shokrollahi, E.H.; Hashemzadeh, S.; Sokullu, S.; Tokac, M. Cardioprotective role of extracellular vesicles: A highlight on exosome beneficial effects in cardio-vascular diseases. J. Cell Physiol. 2019, 234, 21732–21745. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, C.; Wang, S.; Wang, Z.; Jiang, J.; Wang, W.; Li, X.; Chen, J.; Liu, K.; Li, C.; et al. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res. 2016, 76, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Taverna, S.; Pucci, M.; Giallombardo, M.; Di Bella, M.A.; Santarpia, M.; Reclusa, P.; Bazo, I.B.; Rolfo, C.; Alessandro, R. Amphiregulin contained in NSCLC exosomes induces osteoclast differentiation through the activation of EGFR pathway. Sci. Rep. 2017, 7, 3170. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, C.; Harikumar, K.B. The origin and functions of exosomes in cancer. Front. Oncol. 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Li, L.M.; Liu, Z.X.; Cheng, Q.Y. Exosome plays an important role in the development of hepatocellular carcinoma. Pathol. Res. Pract. 2019, 215, 152468. [Google Scholar] [CrossRef] [PubMed]
- Rahbarghazi, R.; Jabbari, N.; Sani, N.A. Tumor derived extracellular vesicles: Reliable tools for cancer diagnosis and clinical applications. Cell Commun. Signal 2019, 17, 73. [Google Scholar] [CrossRef]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef]
- Pitt, J.M.; André, F.; Amirogena, S.; Soria, J.C.; Eggermont, A.; Kroemer, G.; Zitvogel, L. Dendritic cell-derived exosomes for cancer therapy. J. Clin. Investig. 2016, 126, 1224–1232. [Google Scholar] [CrossRef]
- Liu, Q.; Rojas-Canales, D.M.; Divito, S.J.; Shufesky, W.J.; Stolz, D.B.; Erdos, G.; Sullivan, M.L.; Gibson, G.A.; Watkins, S.C.; Larregina, A.T.; et al. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J. Clin. Investig. 2016, 126, 2805–2820. [Google Scholar] [CrossRef]
- Khan, A.R.; Yang, X.; Fu, M.; Zhai, G.J. Recent progress of drug nano-formulations targeting to brain. J. Control. Release 2018, 291, 37–64. [Google Scholar] [CrossRef]
- Pullan, J.E.; Confeld, M.I.; Osborn, J.K.; Kim, J.; Sarkar, K.; Mallik, S. Exosomes as drug carriers for cancer therapy. Mol. Pharm. 2019, 16, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, Y.; Huang, L. Exosomes from M1-Polarized Macrophages Potentiate the Cancer Vaccine by Creating a Pro-inflammatory Microenvironment in the Lymph Node. Mol. Ther. 2017, 25, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.H.; Petree, J.R.; Lee, F.E.H.; Fan, X.; Salaita, K.; Guidot, D.M.; Sadikot, R.T. Macrophages exposed to HIV viral protein disrupt lung epithelial cell integrity and mitochondrial bioenergetics via exosomal microRNA shuttling. Cell Death Dis. 2019, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, B.; Tang, T.; Wen, Y.; Li, Z.; Feng, S.; Wu, M.; Liu, D.; Yin, D.; Ma, K.; et al. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics 2021, 11, 5248–5266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yin, Y.; Lai, R.C.; Tan, S.S.; Choo, A.B.H.; Lim, S.K. Mesenchymal stem cell secrete immunologically active exosomes. Stem Cells Dev. 2014, 23, 1233–1244. [Google Scholar] [CrossRef]
- Gégroire, V.; Langendijk, J.A. Advances in radiotherapy for head and neck cancer. J. Clin. Oncol. 2015, 33, 3277–3284. [Google Scholar] [CrossRef]
- Yao, K.; Ricardo, S.D. Mesenchymal stem cell as novel micro-ribonucleic acid delivery vehicles in kidney disease. Nephrology 2016, 21, 363–371. [Google Scholar] [CrossRef]
- Che, Y.; Shi, X.; Shi, Y.; Jiang, X.; Ai, Q.; Shi, Y.; Gong, F.; Jiang, W. Exosomes derived from miR-143-oerexpressing MSCs inhibit cell migration and invasion in human prostate cancer by downregulating TFF3. Mol. Ther. Nucleic Acids 2019, 18, 232–244. [Google Scholar] [CrossRef]
- Qu, L.; Ding, J.; Chen, C.; Wu, Z.J.; Liu, B.; Gao, Y.; Chen, W.; Liu, F.; Sun, W.; Li, X.F.; et al. Exosome-transmitted lncARSR promotes Sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016, 29, 653–668. [Google Scholar] [CrossRef]
- Qiao, L.; Hu, S.; Huang, K.; Su, T.; Li, Z.; Vandergriff, A.; Cores, J.; Dinh, P.U.; Allen, T.; Shen, D.; et al. Tumour cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics 2020, 10, 3474–3487. [Google Scholar] [CrossRef]
- Usman, W.M.; Pham, T.C.; Kwok, Y.Y. Efficient RNA drug delivery using red blood cells extracellular vesicles. Nat. Commun. 2018, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Gu, J.; Zhang, J.; Shi, H.; Quan, H.; Wang, D.; Xu, W.; Pan, J.; Santos, H.A. Engineered Extracellular vesicles for cancer therapy. Adv. Mater. 2021, 33, 2005709. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; John, S.M.; Schmitz, G. Milk: An exosomal microRNA transmitter promoting thymic regulatory T cell maturation preventing the development of atopy? J. Transl. Med. 2014, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine 2017, 13, 1627–1636. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Delivery of the therapeutic agents by nanoparticles made of grapefruit-derived lipid. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef]
- Di Gioia, S.; Hossain, N.; Conese, M. Biological properties and therapeutic effects of plant-derived nanovesicles. Open Med. 2020, 15, 1096–1122. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, M.; Merlin, D.A. Advances in plant-derived edible nanoparticle-based lipid nano-drug delivery systems as therapeutic nanomedicines. J. Mater. Chem. B 2018, 6, 1312–1321. [Google Scholar] [CrossRef]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus Limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL –mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef]
- Cheng, Y.; Zeng, Q.; Han, Q.; Xia, W. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell 2019, 10, 295–299. [Google Scholar] [CrossRef]
- Xu, R.; Simpson, R.J.; Greening, D.W.A. Protocol for isolation and proteomic characterization of distinct extracellular vesicle subtypes by sequential centrifugal ultrafiltration. Methods Mol. Biol. 2017, 1545, 91–116. [Google Scholar] [CrossRef] [PubMed]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Ji, H.; Mathivanan, S.; Scott, A.M.; Simpson, R.J. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 2012, 56, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Boing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.; Sturk, A.; Nieuwland, R. Single step isolation of extracellular vesicles by size- exclusion chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Liangsupree, T.; Multia, E.; Riekkola, M.L. Modern isolation and separation techniques for extracellular vesicles. J. Chromatogr. A 2021, 1636, 461773. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef]
- Rider, M.A.; Hurwitz, S.N.; Meckes, D.G. ExtraPEG: A polyethylene glycol-based method for enrichment of extracellular vesicles. Sci. Rep. 2016, 6, 23978. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.; Lu, W.; Xing, W. Isolation and visible detection of tumor-derived exosomes from plasma. Anal. Chem. 2018, 90, 14207–14215. [Google Scholar] [CrossRef]
- Wu, J.Y.; Li, Y.J.; Hu, X.B.; Huang, S.; Xian, D.X. Preservation of small extracellular vesicles for functional analysis and therapeutic applications: A comparative evaluation of storage conditions. Drug Deliv. 2021, 28, 162–170. [Google Scholar] [CrossRef]
- Ke, D.; Li, H.; Zhang, Y.; An, Y.; Fu, H.; Fang, X.; Zheng, X. The combination of circulating long noncoding RNAs AK001058, INHBA-AS1, MIR4435-2HG, and CEBPA-AS1 fragments in plasma serve as diagnostic markers for gastric cancer. Oncotarget 2017, 8, 21516–21525. [Google Scholar] [CrossRef]
- Kosaka, N.; Kogure, A.; Yamamoto, T.; Urabe, F.; Usuba, W.; Prieto-Vila, M.; Ochiya, T. Exploiting the message from cancer: The diagnostic value of extracellular vesicles for clinical applications. Exp. Mol. Med. 2019, 51, 31. [Google Scholar] [CrossRef] [PubMed]
- Shaimardanova, A.A.; Solovyeva, V.V.; Chulpanova, D.S.; James, V.; Kitaeva, K.V.; Rizvanov, A.A. Extracellular vesicles in the diagnosis and treatment of central nervous system diseases. Neural. Regen. Res. 2020, 15, 586–596. [Google Scholar] [PubMed]
- Sandfeld-Paulsen, N.; Aggerholm-Pedersen, R.; Bæk, K.R.; Jakobsen, P.; Meldgaard, P.; Folkersen, B.H.; Rasmussen, T.R.; Varming, K.; Jørgensen, M.M.; Sorensen, B.S. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol. Oncol. 2016, 10, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Grimolizzi, F.; Monaco, F.; Leoni, F.; Bracci, M.; Staffolani, S.; Bersaglieri, C.; Gaetani, S.; Valentino, M.; Amati, M.; Rubini, C.; et al. Exosomal miR-126 as a circulating biomarker in non-small-cell lung cancer regulating cancer progression. Sci. Rep. 2017, 7, 15277. [Google Scholar] [CrossRef] [PubMed]
- Porzycki, P.; Ciszkowicz, E.; Semik, M.; Tyrka, M. The combination of three miRNA (miR-141, miR-21, and miR-375) as potential diagnostic tool for prostate cancer recognition. Int. Urol. Nephrol. 2018, 50, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L.; et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimer’s Dement. 2015, 11, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Samoylenko, A.; Vainio, S.J. Exosomes as renal inductive signals in health and disease, and their application as diagnostic markers and therapeutic agents. Front. Cell Dev. Biol. 2015, 3, 65. [Google Scholar] [CrossRef]
- Dorronsoro, A.; Robbins, P.D. Regenerating the injured kidney with human umbilical cord mesenchymal stem cell-derived exosomes. Stem Cell Res. Ther. 2013, 4, 39. [Google Scholar] [CrossRef]
- Long, Q.; Upadhya, D.; Hattiangady, B.; Kim, D.K.; An, S.Y.; Shuai, B.; Prockop, D.J.; Shetty, A.K. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc. Natl. Acad. Sci. USA 2017, 114, E3536–E3545. [Google Scholar] [CrossRef]
- Otero-Ortega, L.; Laso-García, F.; Gómez-de Frutos, M.D.; Rodríguez-Frutos, B.; Pascual-Guerra, J.; Fuentes, B.; Díez-Tejedor, E.; Gutiérrez-Fernández, M. White Matter repair after Extracellular Vesicles administration in an experimental animal model of subcortical Stroke. Sci. Rep. 2017, 7, 44433. [Google Scholar] [CrossRef]
- Cantaluppi, V.; Gatti, S.; Medica, D.; Figliolini, F.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012, 82, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Cosenza, S.; Ruiz, M.; Toupet, K.; Jorgensen, C.; Noël, D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 2017, 7, 16214. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, J.V.; De Castro, R.O.; da Silva, E.Z.M.; Silveira, P.P.; Da Silva-Januário, M.E.; Arruda, E.; Jamur, M.C.; Oliver, C.; Aguiar, R.S.; Dasilva, L.L.P. Nef neutralizes the ability of exosomes from CD4+ T Cells to Act as decoys during HIV-1 infection. PLoS ONE 2014, 9, e113691. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.D.; Ching, K.L.; Liang, F.X.; Dhabaria, A.; Tam, K.; Ueberheide, B.M.; Unutmaz, D.; Torres, V.J.; Cadwell, K. Decoy exosomes provide protection against bacterial toxins. Nature 2020, 579, 260–264. [Google Scholar] [CrossRef]
- Cocozza, F.; Névo, N.; Piovesana, E.; Lahaye, X.; Buchrieser, J.; Schwartz, O.; Manel, N.; Tkach, M.; Théry, C.; Martin-Jaular, L. Extracellular vesicles containing ACE efficiently prevent infection by SARS-CoV-2 Spike protein-containing virus. J. Extracell. Vesicles 2020, 10, e12050. [Google Scholar] [CrossRef]
- Inal, J.M. Decoy ACE2-expressing extracellular vesicles that competitively bind SARS-CoV-2 as a possible COVID-19 therapy. Clin. Sci. 2020, 34, 1301–1304. [Google Scholar] [CrossRef]
- Kumar, S.; Zhi, K.; Mukherji, A.; Gerth, K. Repurposing antiviral protease inhibitors using extracellular vesicles for potential therapy of COVID-19. Viruses 2020, 12, 486. [Google Scholar] [CrossRef]
- Hassanpour, M.; Rezaie, J.; Nouri, M.; Panahi, Y. The role of extracellular vesicles in COVID-19 virus infection. Infect. Genet. Evol. 2020, 85, 104422. [Google Scholar] [CrossRef]
- Troyer, Z.; Alhusaini, N.; Tabler, C.O.; Sweet, T.; de Carvalho, K.I.L.; Schlatzer, D.M.; Carias, L.; King, C.L.; Matreyek, K.; Tilton, J.C. Extracellular vesicles carry SARS-CoV-2 spike protein and serve as decoys for neutralizing antibodies. J. Extracell. Vesicles 2021, 10, e12112. [Google Scholar] [CrossRef]
- Yoo, K.H.; Thapa, N.; Kim, B.J.; Lee, J.O.; Jang, Y.N.; Chwae, Y.J.; Kim, J. Possibility of exosome-based coronavirus disease 2019 vaccine. Mol. Med. Rep. 2022, 25, 26. [Google Scholar] [CrossRef]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, B.; Yuana, Y.; Schumacher, U. Extracellular vesicles-based drug delivery system for cancer treatment. Cogent Med. 2019, 6, 1635806. [Google Scholar] [CrossRef]
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact 2020, 20, 100261. [Google Scholar] [CrossRef]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Release 2015, 199, 145–155. [Google Scholar] [CrossRef]
- Wang, C.; Wang, A.; Wei, F.; Wong, D.T.W.; Tu, M. Electric field-induced disruption and realizing viable content from extracellular vesicles. Methods Mol. Biol. 2017, 1660, 367–376. [Google Scholar]
- Joshi, B.S.; de Beer, M.A.; Giepmans, B.N.G.; Zuhorn, I.S. Endocytosis of extracellular vesicles and release of their cargo from endosomes. ACS Nano 2020, 14, 4444–4455. [Google Scholar] [CrossRef]
- Hettich, B.F.; Bader, J.J.; Leroux, J.C. Encapsulation of hydrophilic compounds in small extracellular vesicles: Loading capacity and impact on vesicle functions. Adv. Healthc. Mater. 2021, 11, e2100047. [Google Scholar] [CrossRef]
- Zabeo, D.; Cvjetkovic, A.; Lasser, C.; Schorb, M.; Lotvall, J.; Hoog, J.L. Exosomes purified from a single cell type have diverse morphology. J. Extracell. Vesicles 2017, 6, 1329476. [Google Scholar] [CrossRef]
- Rikkert, L.G.; Nieuwland, R.; Terstappen, L.W.M.M.; Coumans, F.A.W. Quality of extracellular vesicle images by transmission electron microscopy is operator and protocol dependent. J. Extracell. Vesicles 2019, 8, 1555419. [Google Scholar] [CrossRef]
- Sharma, S.; LeClaire, M.; Gimzewski, J.K. Ascent of atomic force microscopy as a nanoanalytical tool for exosomes and other extracellular vesicles. Nanotechnology 2018, 29, 132001. [Google Scholar] [CrossRef] [PubMed]
- Skliar, M.; Chernyshev, V.S. Imaging of extracellular vesicles by atomic force microscopy. J. Vis. Exp. 2019, 151, e59254. [Google Scholar] [CrossRef] [PubMed]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.P.; Hole, P.; Carr, B.; Redman, C.W.G.; Harris, A.L.; Dobson, P.J.; et al. Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomed. Nanotechnol. Biol. Med. 2017, 7, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Orozco, A.F.; Lewis, D.E. Flow cytometric analysis of circulating microparticles in plasma. Cytometry A 2010, 77, 502–514. [Google Scholar] [CrossRef]
- Shen, W.; Guo, K.; Adkins, G.B.; Jiang, Q.; Liu, Y.; Sedano, S.; Duan, Y.; Yan, S.; Wang, E.; Bergersen, K.; et al. A single extracellular vesicle (EV) flow cytometry approach to reveal EV heterogeneity. Angew. Chem. 2018, 57, 15675–15680. [Google Scholar] [CrossRef]
- Choi, D.; Montermini, L.; Jeong, H.; Sharma, S.; Meehan, B.; Rak, J. Mapping subpopulations of cancer cell-derived extracellular vesicles and particles by nano-flow cytometry. ACS Nano 2019, 13, 10499–10511. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosomes isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Semina, S.E.; Scherbakov, A.M.; Vnukova, A.A.; Bagrov, D.V.; Evtushenko, E.G.; Safronova, V.M.; Golovina, D.A.; Lyubchenko, L.N.; Gudkova, M.V.; Krasil’nikov, M.A. Exosome-mediated transfer of cancer cell resistance to antiestrogen drugs. Molecules 2018, 3, 829. [Google Scholar] [CrossRef]
- Rappa, G.; Santos, M.F.; Green, T.M.; Karbanova, J.; Hassler, J.; Bai, Y.; Barsky, S.H.; Corbeil, D.; Lorico, A. Nuclear transport of cancer extracellular vesicle-derived biomaterials through nuclear envelope invagination-binding protein-associated late endosomes. Oncotarget 2017, 8, 14443–14461. [Google Scholar] [CrossRef]
- Koifman, N.; Biran, I.; Aharon, A.; Brenner, B.; Talmon, Y.A. Direct-imaging cryo-EM study of shedding extracellular vesicles from leukemic monocytes. J. Struct. Biol. 2017, 198, 177–185. [Google Scholar] [CrossRef]
- Cizmar, P.; Yuana, Y. Detection and characterization of extracellular vesicles by transmission and cryo-transmission electron microscopy. Methods Mol. Biol. 2017, 1660, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.M.; Roberts, L.D.; Vidavsky, N.; Chiou, A.E.; Fischbach, C.; Paszeka, M.J.; Estroffc, L.; Kourkoutis, L.F. Direct comparison of optical and electron microscopy methods for structural characterization of extracellular vesicles. J. Struct. Biol. 2020, 210, 107474. [Google Scholar] [CrossRef] [PubMed]
- Polishchuk, E.V.; Polishchuk, R.S.; Luini, A. Correlative light-electron microscopy as a tool to study in vivo dynamics and ultrastructure of intracellular structures. Methods Mol. Biol. 2013, 931, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, O.; Fontana, S.; Monteleone, F.; Taverna, S.; Di Bella, M.A.; Di Vizio, D.; Alessandro, R. Exosomes from metastatic cancer cells transfer amoeboid phenotype to non-metastatic cells and increase endothelial permeability: Their emerging role in tumor heterogeneity. Sci. Rep. 2017, 7, 4711. [Google Scholar] [CrossRef]
- Cully, M. Exosome-based candidates move into the clinic. Nat. Rev. Drug Discov. 2021, 20, 6–7. [Google Scholar] [CrossRef]
- Gebara, N.; Scheel, J.; Skovronova, R.; Grange, C.; Marozio, L.; Gupta, S.; Giorgione, V.; Caicci, F.; Benedetto, C.; Khalil, A.; et al. Single extracellular vesicle analysis in human amniotic fluid shows evidence of phenotype alterations in preeclampsia. J. Extracell. Vesicles 2022, 11, e12217. [Google Scholar] [CrossRef]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Garcia-Manrique, P.; Matos, M.; Gutierrez, G.; Pazos, C.; Blanco-Lopez, M.C. Therapeutic biomaterials based on extracellular vesicles: Classification of bio-engineering and mimetic preparation routes. J. Extracell. Vesicles 2018, 7, 1422676. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, B.; Ocansey, D.K.W.; Xu, W.; Qian, H. Extracellular vesicles: A bright star of nanomedicine. Biomaterials 2021, 269, 120467. [Google Scholar] [CrossRef]
- Chan, M.H.; Chang, Z.X.; Huang, C.F.; Lee, L.J.; Liu, R.S.; Hsiao, M. Integrated therapy platform of exosomal system: Hybrid inorganic/organic nanoparticles with exosomes for cancer treatment. Nanoscale Horiz. 2022, 7, 352–367. [Google Scholar] [CrossRef]
- Foulkers, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The regulation of nanomaterials and nanomedicine for clinical application: Current and future perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Bella, M.A. Overview and Update on Extracellular Vesicles: Considerations on Exosomes and Their Application in Modern Medicine. Biology 2022, 11, 804. https://doi.org/10.3390/biology11060804
Di Bella MA. Overview and Update on Extracellular Vesicles: Considerations on Exosomes and Their Application in Modern Medicine. Biology. 2022; 11(6):804. https://doi.org/10.3390/biology11060804
Chicago/Turabian StyleDi Bella, Maria Antonietta. 2022. "Overview and Update on Extracellular Vesicles: Considerations on Exosomes and Their Application in Modern Medicine" Biology 11, no. 6: 804. https://doi.org/10.3390/biology11060804
APA StyleDi Bella, M. A. (2022). Overview and Update on Extracellular Vesicles: Considerations on Exosomes and Their Application in Modern Medicine. Biology, 11(6), 804. https://doi.org/10.3390/biology11060804





