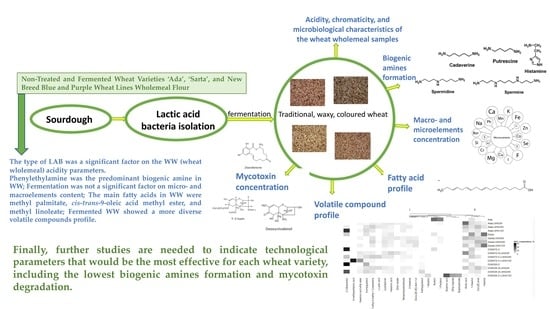
Comparison Study of Nontreated and Fermented Wheat Varieties ‘Ada’, ‘Sarta’, and New Breed Blue and Purple Wheat Lines Wholemeal Flour
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
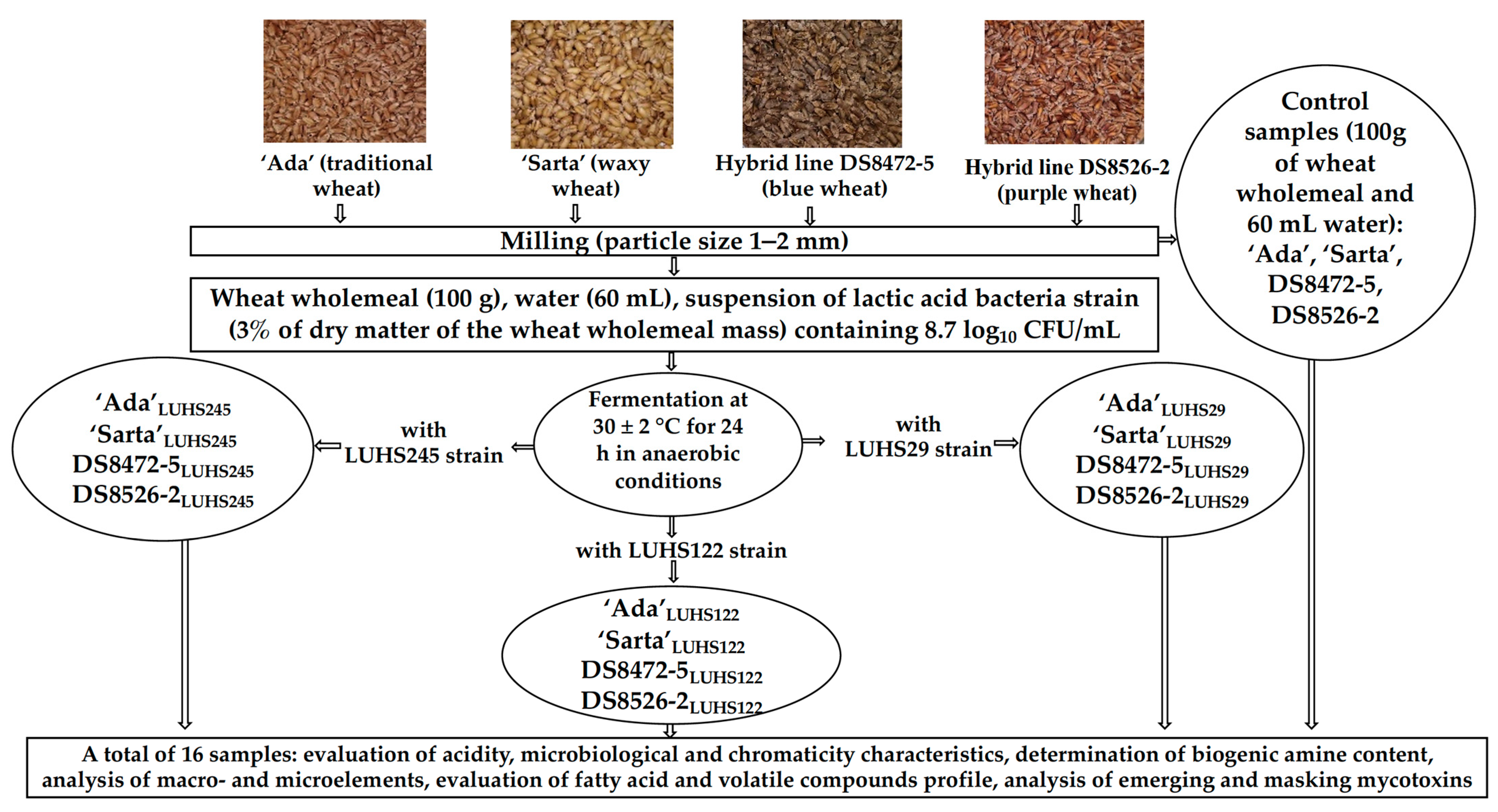
2.1. Wheat and Lactic Acid Bacteria Strains Used in the Experiments and Wheat Wholemeal Preparation and Fermentation
2.2. Evaluation of Acidity, Microbiological, and Chromaticity Characteristics of Wheat Wholemeal Samples
2.3. Determination of the Biogenic Amine Content in Wheat Wholemeal Samples Nonfermented and Fermented with Different Lactic Acid Bacteria Strains
2.4. Analysis of Macro- and Microelements of Wheat Wholemeal Samples Nonfermented and Fermented with Different Lactic Acid Bacteria Strains Using Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
2.5. Fatty Acid Composition Analysis
2.6. Evaluation of Volatile Compound Profile
2.7. High-Performance Liquid Chromatography Coupled to Triple Quadrupole Mass Spectrometry (HPLC-MS/MS) for Mycotoxin Analysis
2.7.1. Materials and Chemicals
2.7.2. Sample Preparation
2.7.3. Chromatographic Method
2.8. Statistical Analysis
3. Results and Discussion
3.1. Acidity, Chromaticity, and Microbiological Characteristics of the Wheat Wholemeal Samples
3.2. Biogenic Amine Content in Wheat Wholemeal Samples
3.3. Macro- and Microelements of Wheat Wholemeal
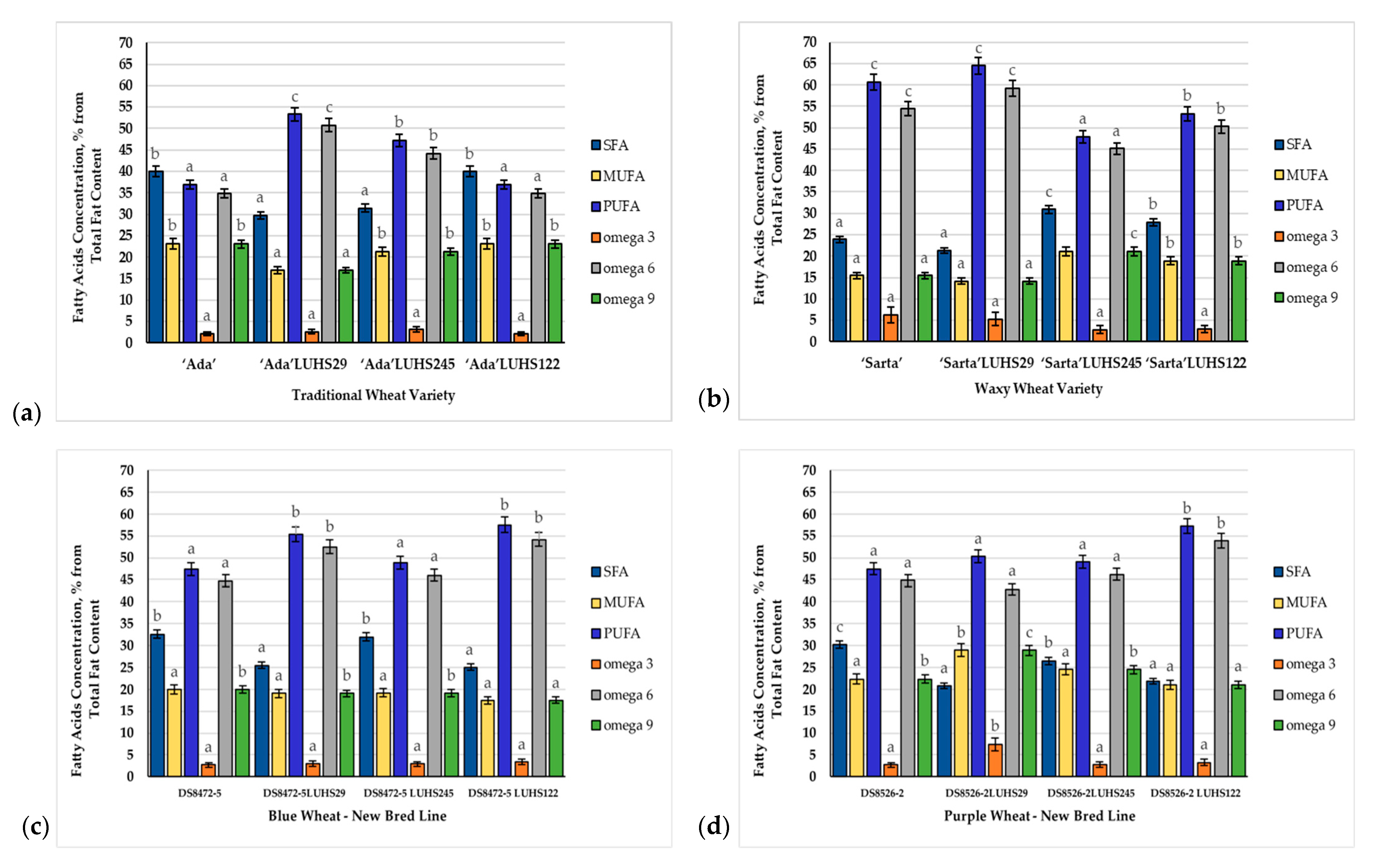
3.4. Fatty Acid Composition of the Wheat Wholemeal Samples
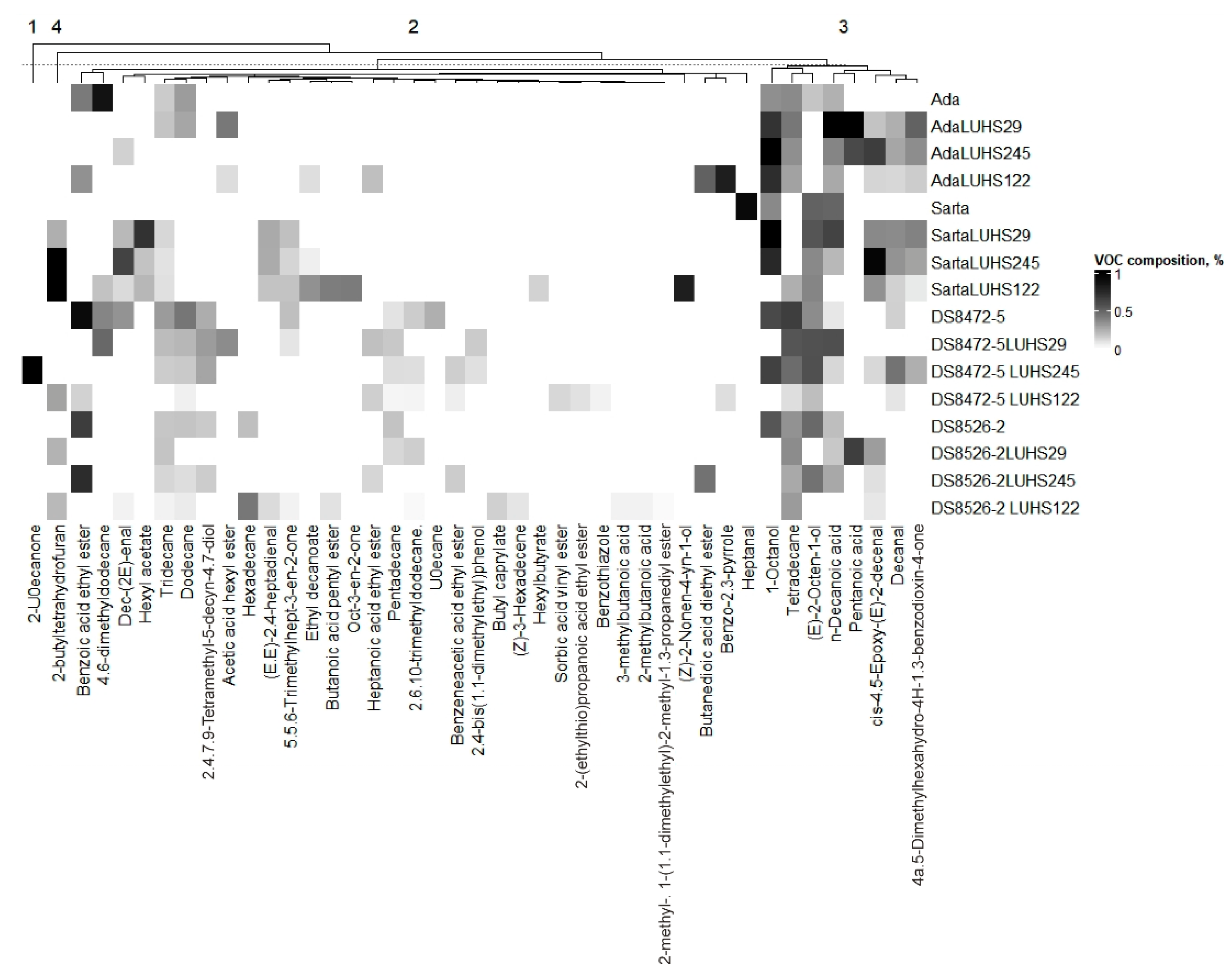
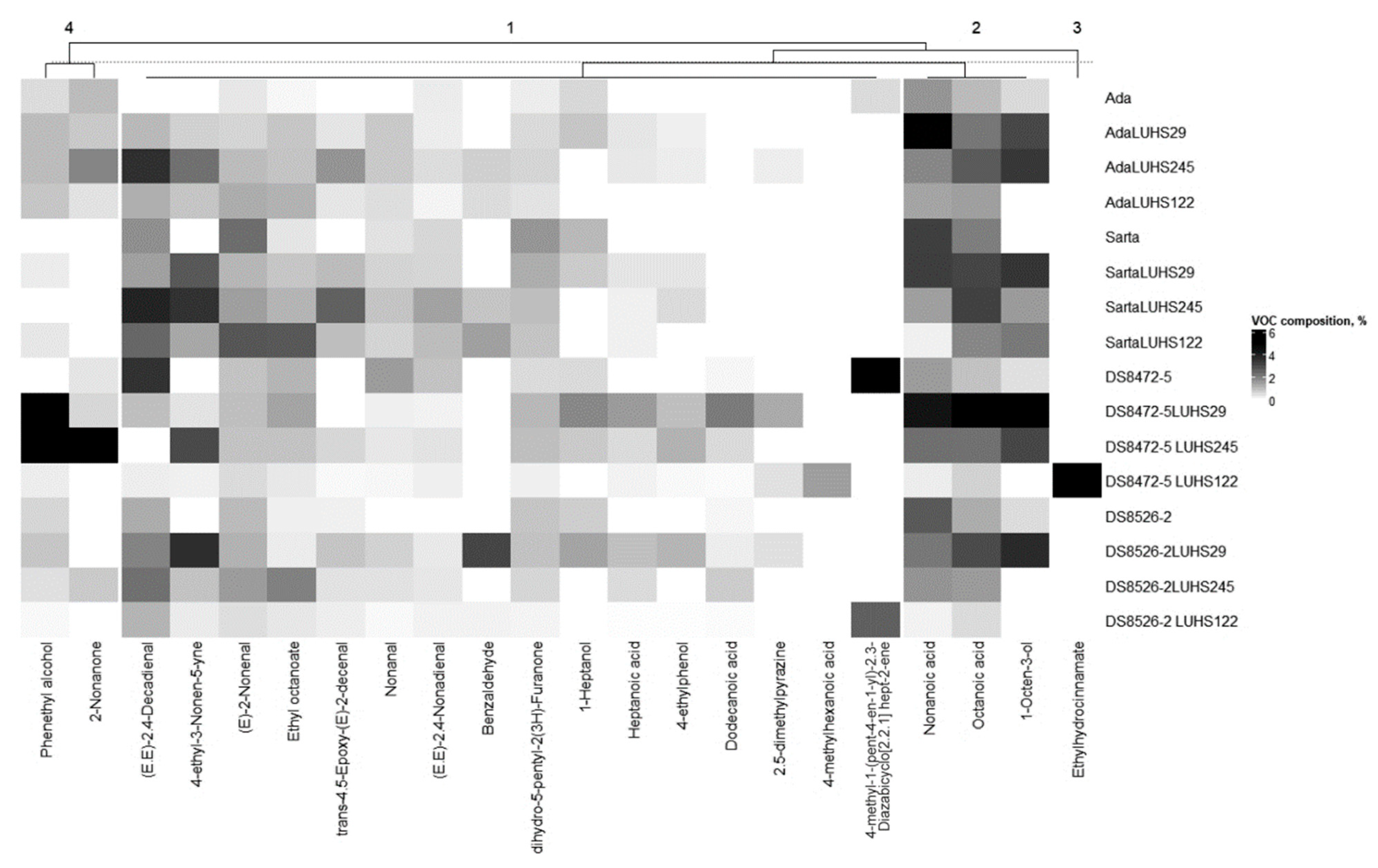
3.5. Volatile Compound Profile of the Different Wheat Wholemeal Varieties
3.6. Changes in the Mycotoxin Concentration in Wheat Wholemeal during Fermentation
3.7. The Limitations and Perspectives of the Current Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reynolds, M.P. Climate Change and Crop Production; CABI: Wallingford, UK, 2010; ISBN 978-1-84593-633-4. [Google Scholar]
- Shewry, P.R.; Hey, S.J. The Contribution of Wheat to Human Diet and Health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Bośko, P.; Živčák, M.; Brestic, M.; Smetanska, I. Bioactive Phytochemicals and Antioxidant Properties of the Grains and Sprouts of Colored Wheat Genotypes. Molecules 2018, 23, 2282. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, A.; Barreca, D.; Bellocco, E.; Smeriglio, A.; Trombetta, D.; Lagana, G. Chapter Eight—Colored Phytonutrients: Role and Applications in the Functional Foods of Anthocyanins|Elsevier Enhanced Reader. In Phytonutrients in Food, 1st ed.; Woodhead Publishing: Sawston, UK, 2020; pp. 177–195. [Google Scholar]
- Garg, M.; Chawla, M.; Chunduri, V.; Kumar, R.; Sharma, S.; Sharma, N.K.; Kaur, N.; Kumar, A.; Mundey, J.K.; Saini, M.K.; et al. Transfer of Grain Colors to Elite Wheat Cultivars and Their Characterization. J. Cereal Sci. 2016, 71, 138–144. [Google Scholar] [CrossRef]
- Wang, C.-J.; Wang, J.-M.; Lin, W.-L.; Chu, C.-Y.; Chou, F.-P.; Tseng, T.-H. Protective Effect of Hibiscus Anthocyanins against Tert-Butyl Hydroperoxide-Induced Hepatic Toxicity in Rats. Food Chem. Toxicol. 2000, 38, 411–416. [Google Scholar] [CrossRef]
- Burdulis, D.; Sarkinas, A.; Jasutiené, I.; Stackevicené, E.; Nikolajevas, L.; Janulis, V. Comparative Study of Anthocyanin Composition, Antimicrobial and Antioxidant Activity in Bilberry (Vaccinium Myrtillus L.) and Blueberry (Vaccinium Corymbosum L.) Fruits. Acta Pol. Pharm. 2009, 66, 399–408. [Google Scholar]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as Antimicrobial Agents of Natural Plant Origin. Nat. Prod. Commun. 2011, 6, 1934578X1100600136. [Google Scholar] [CrossRef]
- Adams, J.; Hofman, K.; Moubarac, J.-C.; Thow, A.M. Public Health Response to Ultra-Processed Food and Drinks. BMJ 2020, 369, m2391. [Google Scholar] [CrossRef]
- Hu, Y.; Ding, M.; Sampson, L.; Willett, W.C.; Manson, J.E.; Wang, M.; Rosner, B.; Hu, F.B.; Sun, Q. Intake of Whole Grain Foods and Risk of Type 2 Diabetes: Results from Three Prospective Cohort Studies. BMJ 2020, 370, m2206. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary Fibre and Whole Grains in Diabetes Management: Systematic Review and Meta-Analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef]
- Seal, C.J.; Thielecke, F. Health Benefits and Recommendations for Daily Whole Grain Intake. Cereal Foods World 2018, 63, 103–106. [Google Scholar] [CrossRef]
- Bartkiene, E.; Zokaityte, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Klupsaite, D.; Cernauskas, D.; Ruzauskas, M.; Bartkevics, V.; Pugajeva, I.; et al. Combination of Extrusion and Fermentation with Lactobacillus Plantarum and L. Uvarum Strains for Improving the Safety Characteristics of Wheat Bran. Toxins 2021, 13, 163. [Google Scholar] [CrossRef]
- Zokaityte, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Klupsaite, D.; Bartkevics, V.; Pugajeva, I.; Bērziņa, Z.; Gruzauskas, R.; Sidlauskiene, S.; et al. The Influence of Combined Extrusion and Fermentation Processes on the Chemical and Biosafety Parameters of Wheat Bran. LWT 2021, 146, 111498. [Google Scholar] [CrossRef]
- Lancetti, R.; Sciarini, L.; Pérez, G.T.; Salvucci, E. Technological Performance and Selection of Lactic Acid Bacteria Isolated from Argentinian Grains as Starters for Wheat Sourdough. Curr. Microbiol. 2021, 78, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Ebrahimi, M.; Mortazavi, S.A.; Abedfar, A. Application of the Selected Antifungal LAB Isolate as a Protective Starter Culture in Pan Whole-Wheat Sourdough Bread. Food Control 2019, 95, 298–307. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel Insights on the Functional/Nutritional Features of the Sourdough Fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef]
- Gänzle, M.G. Enzymatic and Bacterial Conversions during Sourdough Fermentation. Food Microbiol. 2014, 37, 2–10. [Google Scholar] [CrossRef]
- Pétel, C.; Onno, B.; Prost, C. Sourdough Volatile Compounds and Their Contribution to Bread: A Review. Trends Food Sci. Technol. 2017, 59, 105–123. [Google Scholar] [CrossRef]
- Clarke, C.I.; Arendt, E.K. A Review of the Application of Sourdough Technology to Wheat Breads. Adv. Food Nutr. Res. 2005, 49, 137–161. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic Acid Bacteria Isolation from Spontaneous Sourdough and Their Characterization Including Antimicrobial and Antifungal Properties Evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef]
- Weegels, P.L. The Future of Bread in View of Its Contribution to Nutrient Intake as a Starchy Staple Food. Plant. Foods Hum. Nutr. 2019, 74, 1–9. [Google Scholar] [CrossRef]
- Zhu, F. Staling of Chinese Steamed Bread: Quantification and Control. Trends Food Sci. Technol. 2016, 55, 118–127. [Google Scholar] [CrossRef]
- Ayua, E.O.; Kazem, A.E.; Hamaker, B.R. Whole Grain Cereal Fibers and Their Support of the Gut Commensal Clostridia for Health. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100245. [Google Scholar] [CrossRef]
- Gong, L.; Cao, W.; Chi, H.; Wang, J.; Zhang, H.; Liu, J.; Sun, B. Whole Cereal Grains and Potential Health Effects: Involvement of the Gut Microbiota. Food Res. Int 2018, 103, 84–102. [Google Scholar] [CrossRef]
- Capurso, A.; Capurso, C. The Mediterranean Way: Why Elderly People Should Eat Wholewheat Sourdough Bread-a Little Known Component of the Mediterranean Diet and Healthy Food for Elderly Adults. Aging Clin. Exp. Res. 2020, 32, 1–5. [Google Scholar] [CrossRef]
- Shalaby, A.R. Significance of Biogenic Amines to Food Safety and Human Health. Food Res. Int. 1996, 29, 675–690. [Google Scholar] [CrossRef]
- Taylor, S.L.; World Health Organization. Histamine Poisoning Associated with Fish, Cheese, and Other Foods; World Health Organization: Geneva, Switzerland, 1985. [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on Risk Based Control of Biogenic Amine Formation in Fermented Foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef]
- Rodriguez, M.B.R.; da Silva Carneiro, C.; da Silva Feijó, M.; Júnior, C.A.C.; Mano, S.B. Bioactive Amines: Aspects of Quality and Safety in Food. Food Nutr. Sci. 2014, 5, 138–146. [Google Scholar] [CrossRef]
- Black, B.A.; Zannini, E.; Curtis, J.M.; Gänzle, M.G. Antifungal Hydroxy Fatty Acids Produced during Sourdough Fermentation: Microbial and Enzymatic Pathways, and Antifungal Activity in Bread. Appl. Environ. Microbiol. 2013, 79, 1866–1873. [Google Scholar] [CrossRef]
- Ekwomadu, T.I.; Akinola, S.A.; Mwanza, M. Fusarium Mycotoxins, Their Metabolites (Free, Emerging, and Masked), Food Safety Concerns, and Health Impacts. Int. J. Environ. Res. Public Health 2021, 18, 11741. [Google Scholar] [CrossRef]
- Ekwomadu, T.I.; Dada, T.A.; Nleya, N.; Gopane, R.; Sulyok, M.; Mwanza, M. Variation of Fusarium Free, Masked, and Emerging Mycotoxin Metabolites in Maize from Agriculture Regions of South Africa. Toxins 2020, 12, 149. [Google Scholar] [CrossRef]
- Vadopalas, L.; Ruzauskas, M.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Bartkevics, V.; Pugajeva, I.; Reinolds, I.; Badaras, S.; et al. Combination of Antimicrobial Starters for Feed Fermentation: Influence on Piglet Feces Microbiota and Health and Growth Performance, Including Mycotoxin Biotransformation in Vivo. Front. Vet. Sci. 2020, 7, 528990. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Bartkevics, V.; Pugajeva, I.; Krungleviciute, V.; Mayrhofer, S.; Domig, K. The Contribution of P. Acidilactici, L. Plantarum, and L. Curvatus Starters and L-(+)-Lactic Acid to the Acrylamide Content and Quality Parameters of Mixed Rye—Wheat Bread. LWT 2017, 80, 43–50. [Google Scholar] [CrossRef]
- Ben-Gigirey, B.; Vieites Baaptista de Sousa, J.M.; Villa, T.G.; Barros-Velazquez, J. Histamine and Cadaverine Production by Bacteria Isolated from Fresh and Frozen Albacore (Thunnus Alalunga). J. Food Prot. 1999, 62, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Thomson Brooks/Cole Publishing Co.: Belmont, CA, USA, 1996; p. 600. ISBN 0-534-23100-4. [Google Scholar]
- Kidoń, M.; Grabowska, J. Bioactive Compounds, Antioxidant Activity, and Sensory Qualities of Red-Fleshed Apples Dried by Different Methods. LWT 2021, 136, 110302. [Google Scholar] [CrossRef]
- Gao, L.; Girard, B.; Mazza, G.; Reynolds, A.G. Changes in Anthocyanins and Color Characteristics of Pinot Noir Wines during Different Vinification Processes. J. Agric. Food Chem. 1997, 45, 2003–2008. [Google Scholar] [CrossRef]
- Lachman, J.; Martinek, P.; Kotíková, Z.; Orsák, M.; Šulc, M. Genetics and Chemistry of Pigments in Wheat Grain—A Review. J. Cereal Sci. 2017, 74, 145–154. [Google Scholar] [CrossRef]
- Antognoni, F.; Mandrioli, R.; Potente, G.; Taneyo Saa, D.L.; Gianotti, A. Changes in Carotenoids, Phenolic Acids and Antioxidant Capacity in Bread Wheat Doughs Fermented with Different Lactic Acid Bacteria Strains. Food Chem. 2019, 292, 211–216. [Google Scholar] [CrossRef]
- McDonald, C.E. Lipoxygenase and Lutein Bleaching Activity of Durum Wheat Semolina. Cereal Chem. 1979, 2, 84–89. [Google Scholar]
- Capuani, A.; Behr, J.; Vogel, R.F. Influence of Lactic Acid Bacteria on the Oxidation–Reduction Potential of Buckwheat (Fagopyrum Esculentum Moench) Sourdoughs. Eur. Food Res. Technol. 2012, 235, 1063–1069. [Google Scholar] [CrossRef]
- Stanzer, D.; Ivanuša, I.; Kazazić, S.; Hanousek Čiča, K.; Mrvčić, J. Diversity of Lactic Acid Bacteria on Organic Flours and Application of Isolates in Sourdough Fermentation. Hrvat. Časopis Za Prehrambenu Tehnol. Biotehnol. I Nutr. 2017, 12, 44–51. [Google Scholar]
- De Vuyst, L.; Van Kerrebroeck, S.; Harth, H.; Huys, G.; Daniel, H.-M.; Weckx, S. Microbial Ecology of Sourdough Fermentations: Diverse or Uniform? Food Microbiol. 2014, 37, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Paramithiotis, S.; Chouliaras, Y.; Tsakalidou, E.; Kalantzopoulos, G. Application of Selected Starter Cultures for the Production of Wheat Sourdough Bread Using a Traditional Three-Stage Procedure. Process. Biochem. 2005, 40, 2813–2819. [Google Scholar] [CrossRef]
- Chavan, R.S.; Chavan, S.R. Sourdough Technology—A Traditional Way for Wholesome Foods: A Review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 169–182. [Google Scholar] [CrossRef]
- Hammes, W.P.; Gänzle, M.G. Sourdough Breads and Related Products. In Microbiology of Fermented Foods; Wood, B.J.B., Ed.; Springer: Boston, MA, USA, 1998; pp. 199–216. ISBN 978-1-4613-0309-1. [Google Scholar]
- Spicher, G. Baked Goods; Biotechnology, Food and Feed Productions with Microorganisms; Verlag Chemie: Weinheim, Germany, 1983; Volume 5, pp. 1–80. [Google Scholar]
- Silla Santos, M.H. Biogenic Amines: Their Importance in Foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M.; Tofalo, R.; Suzzi, G. Biogenic Amines in Raw and Processed Seafood. Front. Microbiol. 2012, 3, 188. [Google Scholar] [CrossRef]
- Özogul, F.; Hamed, I. The Importance of Lactic Acid Bacteria for the Prevention of Bacterial Growth and Their Biogenic Amines Formation: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1660–1670. [Google Scholar] [CrossRef]
- Marijan, A.; Džaja, P.; Bogdanović, T.; Škoko, I.; Cvetnić, Ž.; Dobranić, V.; Zdolec, N.; Šatrović, E.; Severin, K. Influence of Ripening Time on the Amount of Certain Biogenic Amines in Rind and Core of Cow Milk Livno Cheese. Mljekarstvo Časopis Za Unaprjeđenje Proizv. I Prerade Mlijeka 2014, 64, 159–169. [Google Scholar] [CrossRef][Green Version]
- Moret, S.; Smela, D.; Populin, T.; Conte, L.S. A Survey on Free Biogenic Amine Content of Fresh and Preserved Vegetables. Food Chem. 2005, 89, 355–361. [Google Scholar] [CrossRef]
- Mantis, F.; Tsachev, I.; Sabatakou, O.; Burriel, A.; Vacalopoulos, A.; Ramantanis, S. Safety and shelflife of widely distributed vacuum packed, heat treated sausages. Bulg. J. Vet. Med. 2006, 4, 245–254. [Google Scholar]
- Maintz, L.; Novak, N. Histamine and Histamine Intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef]
- Hungerford, J.M. Scombroid Poisoning: A Review. Toxicon 2010, 56, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Linares, D.M.; del Rio, B.; Redruello, B.; Ladero, V.; Martin, M.C.; Fernandez, M.; Ruas-Madiedo, P.; Alvarez, M.A. Comparative Analysis of the in Vitro Cytotoxicity of the Dietary Biogenic Amines Tyramine and Histamine. Food Chem. 2016, 197, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Glória, M.B.A. Bioactive Amines. In Handbook of Food Science, Technology and Engineering; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Landete, J.M.; de las Rivas, B.; Marcobal, A.; Muñoz, R. Molecular Methods for the Detection of Biogenic Amine-Producing Bacteria on Foods. Int. J. Food Microbiol. 2007, 117, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Li, H.; Tong, Y.; Jing, R.; Zhang, F.; Zou, C. Identification of Quantitative Trait Locus of Zinc and Phosphorus Density in Wheat (Triticum Aestivum L.) Grain. Plant Soil 2008, 306, 95–104. [Google Scholar] [CrossRef]
- Sieprawska, A.; Filek, M.; Walas, S.; Tobiasz, A.; Mrowiec, H.; Miszalski, Z. Does Micro- and Macroelement Content Differentiate Grains of Sensitive and Tolerant Wheat Varieties? Acta Physiol. Plant. 2014, 36, 3095–3100. [Google Scholar] [CrossRef]
- Verni, M.; Rizzello, C.G.; Coda, R. Fermentation Biotechnology Applied to Cereal Industry By-Products: Nutritional and Functional Insights. Front. Nutr. 2019, 6, 42. [Google Scholar] [CrossRef]
- Foucaud, C.; Francois, A.; Richard, J. Development of a Chemically Defined Medium for the Growth of Leuconostoc Mesenteroides. Appl. Environ. Microbiol. 1997, 63, 301–304. [Google Scholar] [CrossRef]
- Von Wright, A.; Axelsson, L. Lactic Acid Bacteria: An Introduction. In Lactic Acid Bacteria: Microbiological and Functional Aspects; CRC Press: Boca Raton, FL, USA, 2011; Volume 4, pp. 1–16. [Google Scholar]
- Guo, Z.; Zhang, Z.; Xu, P.; Guo, Y. Analysis of Nutrient Composition of Purple Wheat. Cereal Res. Commun. 2012, 41, 293–303. [Google Scholar] [CrossRef]
- He, Y.Z.; Ning, J.F. Analysis of Nutrition Composition in the Special Purple Grain Wheat “Qinhei 1” Containing Rich Fe and Zn. J. Northwest A F Univ. Nat. Sci. Ed. 2003, 31, 87–90. [Google Scholar]
- Tian, S.; Chen, Z.; Wei, Y. Measurement of Colour-Grained Wheat Nutrient Compounds and the Application of Combination Technology in Dough. J. Cereal Sci. 2018, 83, 63–67. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, J.; Hou, J.; Li, Y.; Huang, X.; Wang, C.; Lu, H.; Zhu, Y.; Guo, T. Evaluation of Yield, Processing Quality, and Nutritional Quality in Different-Colored Wheat Grains under Nitrogen and Phosphorus Fertilizer Application. Crop. Sci. 2018, 58, 402–415. [Google Scholar] [CrossRef]
- Lafiandra, D.; Masci, S.; Sissons, M.; Dornez, E.; Delcour, J.; Courtin, C.; Caboni, M.F. Kernel Components of Technological Value. In Durum Wheat Chemistry and Technology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 85–124. [Google Scholar]
- Narducci, V.; Finotti, E.; Galli, V.; Carcea, M. Lipids and Fatty Acids in Italian Durum Wheat (Triticum Durum Desf.) Cultivars. Foods 2019, 8, 223. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L. Dietary N-6 and n-3 Polyunsaturated Fatty Acids: From Biochemistry to Clinical Implications in Cardiovascular Prevention. Biochem. Pharm. 2009, 77, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 Fatty Acids and Cardiovascular Disease: Effects on Risk Factors, Molecular Pathways, and Clinical Events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Armanino, C.; De Acutis, R.; Rosa Festa, M. Wheat Lipids to Discriminate Species, Varieties, Geographical Origins and Crop Years. Anal. Chim. Acta 2002, 454, 315–326. [Google Scholar] [CrossRef]
- Beleggia, R.; Platani, C.; Nigro, F.; De Vita, P.; Cattivelli, L.; Papa, R. Effect of Genotype, Environment and Genotype-by-Environment Interaction on Metabolite Profiling in Durum Wheat (Triticum Durum Desf.) Grain. J. Cereal Sci. 2013, 57, 183–192. [Google Scholar] [CrossRef]
- Nejadsadeghi, L.; Maali-Amiri, R.; Zeinali, H.; Ramezanpour, S.; Sadeghzade, B. Membrane Fatty Acid Compositions and Cold-Induced Responses in Tetraploid and Hexaploid Wheats. Mol. Biol Rep. 2015, 42, 363–372. [Google Scholar] [CrossRef]
- Upchurch, R.G. Fatty Acid Unsaturation, Mobilization, and Regulation in the Response of Plants to Stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Rosicka-Kaczmarek, J.; Mi, K.; Makowski, B. Composition and Functional Properties of Lipid Components from Selected Cereal Grains. Res. Signpost 2015. Available online: https://www.semanticscholar.org/paper/Composition-and-functional-properties-of-lipid-from-Rosicka-Kaczmarek-Mi%C5%9Bkiewicz/207d83d4582d72cf8a545348b50a641f6bfedc09 (accessed on 28 May 2022).
- Kaseleht, K.; Paalme, T.; Mihhalevski, A.; Sarand, I. Analysis of Volatile Compounds Produced by Different Species of Lactobacilli in Rye Sourdough Using Multiple Headspace Extraction. Int. J. Food Sci. Technol. 2011, 46, 1940–1946. [Google Scholar] [CrossRef]
- Damiani, P.; Gobbetti, M.; Cossignani, L.; Corsetti, A.; Simonetti, M.S.; Rossi, J. The Sourdough Microflora. Characterization of Hetero- and Homofermentative Lactic Acid Bacteria, Yeasts and Their Interactions on the Basis of the Volatile Compounds Produced. LWT—Food Sci. Technol. 1996, 29, 63–70. [Google Scholar] [CrossRef]
- Hansen, B.; Hansen, Å. Volatile Compounds in Wheat Sourdoughs Produced by Lactic Acid Bacteria and Sourdough Yeasts. Z. Für Lebensm. -Unters. Und-Forsch. 1994, 198, 202–209. [Google Scholar] [CrossRef]
- Corona, O.; Alfonzo, A.; Ventimiglia, G.; Nasca, A.; Francesca, N.; Martorana, A.; Moschetti, G.; Settanni, L. Industrial Application of Selected Lactic Acid Bacteria Isolated from Local Semolinas for Typical Sourdough Bread Production. Food Microbiol. 2016, 59, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Schieberle, P. Important Aroma Compounds in Freshly Ground Wholemeal and White Wheat FlourIdentification and Quantitative Changes during Sourdough Fermentation. J. Agric. Food Chem. 2002, 50, 6835–6840. [Google Scholar] [CrossRef]
- Vermeulen, N.; Czerny, M.; Gänzle, M.G.; Schieberle, P.; Vogel, R.F. Reduction of (E)-2-Nonenal and (E,E)-2,4-Decadienal during Sourdough Fermentation. J. Cereal Sci. 2007, 45, 78–87. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Z.; Guo, X.; Wang, F.; Huang, J.; Sun, B.; Wang, X. Sourdough Improves the Quality of Whole-Wheat Flour Products: Mechanisms and Challenges—A Review. Food Chem. 2021, 360, 130038. [Google Scholar] [CrossRef]
- Ripari, V.; Gänzle, M.G.; Berardi, E. Evolution of Sourdough Microbiota in Spontaneous Sourdoughs Started with Different Plant Materials. Int. J. Food Microbiol. 2016, 232, 35–42. [Google Scholar] [CrossRef]
- Prückler, M.; Lorenz, C.; Endo, A.; Kraler, M.; Dürrschmid, K.; Hendriks, K.; Soares da Silva, F.; Auterith, E.; Kneifel, W.; Michlmayr, H. Comparison of Homo- and Heterofermentative Lactic Acid Bacteria for Implementation of Fermented Wheat Bran in Bread. Food Microbiol. 2015, 49, 211–219. [Google Scholar] [CrossRef]
- Spaggiari, M.; Ricci, A.; Calani, L.; Bresciani, L.; Neviani, E.; Dall’Asta, C.; Lazzi, C.; Galaverna, G. Solid State Lactic Acid Fermentation: A Strategy to Improve Wheat Bran Functionality. LWT 2020, 118, 108668. [Google Scholar] [CrossRef]
- Messens, W.; De Vuyst, L. Inhibitory Substances Produced by Lactobacilli Isolated from Sourdoughs—a Review. Int. J. Food Microbiol. 2002, 72, 31–43. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Valerio, F.; Evidente, A.; Lazzaroni, S.; Corsetti, A.; Gobbetti, M. Purification and Characterization of Novel Antifungal Compounds from the Sourdough Lactobacillus Plantarum Strain 21B. Appl. Environ. Microbiol. 2000, 66, 4084–4090. [Google Scholar] [CrossRef] [PubMed]
- Ryan, L.A.M.; Zannini, E.; Dal Bello, F.; Pawlowska, A.; Koehler, P.; Arendt, E.K. Lactobacillus Amylovorus DSM 19280 as a Novel Food-Grade Antifungal Agent for Bakery Products. Int. J. Food Microbiol. 2011, 146, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Dalié, D.K.D.; Deschamps, A.M.; Richard-Forget, F. Lactic Acid Bacteria—Potential for Control of Mould Growth and Mycotoxins: A Review. Food Control. 2010, 21, 370–380. [Google Scholar] [CrossRef]
- Guimaraes, J.T.; Silva, E.K.; Ranadheera, C.S.; Moraes, J.; Raices, R.S.; Silva, M.C.; Ferreira, M.S.; Freitas, M.Q.; Meireles, M.A.A.; Cruz, A.G. Effect of High-Intensity Ultrasound on the Nutritional Profile and Volatile Compounds of a Prebiotic Soursop Whey Beverage. Ultrason. Sonochemistry 2019, 55, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Haskard, C.A.; El-Nezami, H.S.; Kankaanpää, P.E.; Salminen, S.; Ahokas, J.T. Surface Binding of Aflatoxin B1 by Lactic Acid Bacteria. Appl. Environ. Microbiol. 2001, 67, 3086–3091. [Google Scholar] [CrossRef]

| Wheat Samples | pH | TTA, °N | Color Coordinates, NBS | LAB Count, log10 CFU/g | ||
|---|---|---|---|---|---|---|
| L* | a* | b* | ||||
| Traditional Wheat Variety | ||||||
| ‘Ada’ | 6.68 ± 0.03 d,D | 1.30 ± 0.02 a,C | 52.8 ± 1.21 b,C | 5.79 ± 0.11 c,D | 16.6 ± 0.13 c,D | 4.41 ± 0.11 a,B |
| ‘Ada’LUHS29 | 4.29 ± 0.02 c,C | 4.90 ± 0.08 b,B | 42.0 ± 1.10 a,C | 4.27 ± 0.10 a,C | 8.88 ± 0.11 a,C | 8.52 ± 0.15 b,C |
| ‘Ada’LUHS245 | 4.21 ± 0.01 b,C | 4.80 ± 0.09 b,A | 50.6 ± 1.50 b,C | 5.46 ± 0.16 b,B | 15.7 ± 0.21 b,D | 8.61 ± 0.14 b,A |
| ‘Ada’LUHS122 | 3.99 ± 0.02 a,B | 5.10 ± 0.15 c,A | 51.9 ± 0.97 b,D | 5.79 ± 0.13 c,D | 16.8 ± 0.19 c,C | 8.72 ± 0.18 b,A |
| Waxy Wheat Variety | ||||||
| ‘Sarta’ | 6.42 ± 0.03 c,C | 1.00 ± 0.03 a,A | 40.9 ± 1.19 a,A | 4.43 ± 0.09 c,B | 8.10 ± 0.20 a,A | 4.33 ± 0.09 a,A |
| ‘Sarta’LUHS29 | 4.19 ± 0.02 b,B | 4.50 ± 0.09 b,A | 44.9 ± 0.94 b,D | 3.85 ± 0.07 a,b,A | 9.59 ± 0.14 b,D | 8.11 ± 0.21 b,A |
| ‘Sarta’LUHS245 | 3.84 ± 0.01 a,A | 5.50 ± 0.16 c,C | 46.7 ± 1.17 c,B | 3.72 ± 0.11 a,A | 9.89 ± 0.11 c,B | 8.37 ± 0.18 b,A |
| ‘Sarta’LUHS122 | 3.81 ± 0.02 a,A | 5.70 ± 0.12 c,B,C | 48.9 ± 1.41 d,B | 3.94 ± 0.08 b,A | 14.3 ± 0.13 d,B | 8.64 ± 0.16 b,A |
| Blue Wheat—New BreedLine | ||||||
| DS8472-5 | 6.09 ± 0.02 d,A | 1.0 ± 0.02 a,A | 47.6 ± 0.32 b,B | 3.03 ± 0.07 c,A | 11.3 ± 0.09 b,C | 4.30 ± 0.06 a,A |
| DS8472-5LUHS29 | 4.07 ± 0.01 b,A | 5.40 ± 0.08 c,C | 40.9 ± 0.27 a,B | 4.08 ± 0.06 b,B | 6.12 ± 0.14 a,B | 8.31 ± 0.12 bA,B |
| DS8472-5LUHS245 | 4.15 ± 0.02 c,B | 5.10 ± 0.12 b,B | 50.3 ± 1.18 c,C | 6.85 ± 0.16 d,C | 13.8 ± 0.21 c,C | 8.53 ± 0.20 b,c,A |
| DS8472-5LUHS122 | 3.94 ± 0.03 a,B | 5.80 ± 0.04 d,C | 50.6 ± 0.34 c,C,D | 4.76 ± 0.07 c,C | 14.0 ± 0.32 c,B | 8.92 ± 0.21 c,A |
| Purple Wheat—New BreedLine | ||||||
| DS8526-2 | 6.18 ± 0.02 d,B | 1.20 ± 0.03 a,B | 46.1 ± 1.32 c,B | 5.12 ± 0.11 c,C | 9.52 ± 0.17 c,B | 4.53 ± 0.09 a,B |
| DS8526-2LUHS29 | 4.24 ± 0.03 a,B,C | 5.00 ± 0.11 b,B | 38.6 ± 0.77 a,A | 4.25 ± 0.12 b,B,C | 5.42 ± 0.08 a,A | 8.31 ± 0.11 b,A,B |
| DS8526-2LUHS245 | 4.86 ± 0.04 c,D | 5.90 ± 0.12 d,D | 40.3 ± 0.89 a,b,A | 3.58 ± 0.10 a,A | 5.29 ± 0.12 a,A | 8.45 ± 0.17 b,c,A |
| DS8526-2LUHS122 | 4.67 ± 0.02 b,C | 5.60 ± 0.14 c,B | 39.8 ± 1.14 a,A | 4.10 ± 0.09 b,B | 5.86 ± 0.14 b,A | 8.76 ± 0.09 c,A |
| Wheat Samples | Biogenic Amine, mg/kg | |||||||
|---|---|---|---|---|---|---|---|---|
| TRY | PHE | PUT | CAD | HIS | TYR | SPRMD | SPRM | |
| Traditional Wheat Variety | ||||||||
| ‘Ada’ | nd | 72.8 ± 5.4 b,C | 35.6 ± 2.9 b,A | nd | nd | nd | 23.4 ± 2.2 a,A | nd |
| ‘Ada’LUHS29 | nd | 56.9 ± 3.9 a,b,A | 20.3 ± 1.8 a,A | nd | nd | nd | 36.1 ± 3.3 b,B | nd |
| ‘Ada’LUHS245 | nd | 50.3 ± 3.4 a,B | 33.1 ± 2.5 b,B | 102.3 ± 6.9 a,B | nd | nd | 32.6 ± 3.8 b,A | nd |
| ‘Ada’LUHS122 | nd | 64.1 ± 4.7 b,B,C | 98.1 ± 6.3 c,B | 205.3 ± 9.8 a,B | 35.2 ± 2.6 A | nd | nd | nd |
| Waxy Wheat Variety | ||||||||
| ‘Sarta’ | nd | 68.8 ± 5.0 b,B | 32.6 ± 2.6 b,A | nd | nd | nd | nd | nd |
| ‘Sarta’LUHS29 | nd | 68.6 ± 5.2 b,B | 28.9 ± 2.1 a,b,B | nd | nd | nd | nd | nd |
| ‘Sarta’LUHS245 | nd | 46.9 ± 3.1 a,B | 26.7 ± 1.9 a,A | nd | nd | nd | 31.9 ± 3.4 A | nd |
| ‘Sarta’LUHS122 | nd | 55.6 ± 3.6 a,A | 26.6 ± 2.1 a,A | nd | 77.4 ± 5.4 B | nd | nd | nd |
| Blue Wheat—New BreedLine | ||||||||
| DS8472-5 | nd | 57.2 ± 3.9 a,b,A | nd | nd | nd | nd | nd | nd |
| DS8472-5LUHS29 | nd | 53.6 ± 3.5 a,A | nd | nd | nd | nd | 26.7 ± 3.2 a,A | nd |
| DS8472-5LUHS245 | nd | 62.2 ± 4.4 b,C | nd | nd | nd | nd | 36.5 ± 3.8 b,B | nd |
| DS8472-5LUHS122 | nd | 68.7 ± 5.1 b,C | nd | 88.0 ± 5.9 A | nd | nd | 31.9 ± 4.1 b,A | nd |
| Purple Wheat—New BreedLine | ||||||||
| DS8526-2 | nd | 58.0 ± 4.3 b,A | nd | nd | nd | nd | 21.4 ± 2.3 a,A | nd |
| DS8526-2LUHS29 | nd | 70.4 ± 5.3 c,B | nd | nd | nd | nd | 30.7 ± 3.5 c,A | nd |
| DS8526-2LUHS245 | nd | 26.4 ± 2.4 a,A | 26.8 ± 2.7 A | 84.0 ± 5.8 a,A | nd | nd | 27.2 ± 3.1 b,c,A | nd |
| DS8526-2LUHS122 | nd | 51.9 ± 3.2 b,A | nd | 99.9 ± 6.5 b,A | nd | 76.0 ± 5.4 | 26.3 ± 2.1 b,A | nd |
| Wheat Samples | Macroelements, d.m. | |||
|---|---|---|---|---|
| Na, g/100 g | Mg, g/kg | K, g/kg | Ca, g/kg | |
| Traditional Wheat Variety | ||||
| ‘Ada’ | <0.002 | 0.662 ± 0.066 a,B | 1.98 ± 0.194 a,B | 0.229 ± 0.023 a,C |
| ‘Ada’LUHS29 | 0.010 ± 0.001 a,B | 0.601 ± 0.060 a,B | 1.91 ± 0.187 a,A | 0.192 ± 0.019 a,B |
| ‘Ada’LUHS245 | 0.007 ± 0.002 a,B | 0.551 ± 0.055 a,A | 1.74 ± 0.170 a,A,B | 0.197 ± 0.018 a,B |
| ‘Ada’LUHS122 | 0.007 ± 0.002 a,A | 0.578 ± 0.058 a,D | 1.79 ± 0.175 a,B | 0.197 ± 0.020 a,C |
| Waxy Wheat Variety | ||||
| ‘Sarta’ | <0.002 | 0.555 ± 0.056 c,B | 1.98 ± 0.194 c,B | 0.305 ± 0.031 c,D |
| ‘Sarta’LUHS29 | 0.011 ± 0.002 a,B | 0.607 ± 0.061 c,B | 1.82 ± 0.178 b,c,A | 0.293 ± 0.029 c,C |
| ‘Sarta’LUHS245 | 0.009 ± 0.001 a,B | 0.479 ± 0.048 b,A | 1.52 ± 0.152 b,A | 0.225 ± 0.025 b,B |
| ‘Sarta’LUHS122 | 0.011 ± 0.002 a,A | 0.373 ± 0.037 a,B | 1.15 ± 0.113 a,A | 0.160 ± 0.015 a,B |
| Blue Wheat—New BreedLine | ||||
| DS8472-5 | <0.002 | 0.401 ± 0.040 a,A | 1.45 ± 0.150 a,A | 0.160 ± 0.014 c,B |
| DS8472-5 LUHS29 | 0.008 ± 0.001 a,B | 0.445 ± 0.040 a,A | 1.96 ± 0.200 b,A | 0.148 ± 0.015 a,b,A |
| DS8472-5 LUHS245 | 0.009 ± 0.001 a,B | 0.431 ± 0.043 a,A | 1.71 ± 0.170 a,b,A,B | 0.126 ± 0.013 a,A |
| DS8472-5 LUHS122 | 0.007 ± 0.002 a,A | 0.456 ± 0.046 a,C | 1.76 ± 0.180 a,b,B | 0.147 ± 0.015 a,b,A,B |
| Purple Wheat—New BreedLine | ||||
| DS8526-2 | <0.002 | 0.518 ± 0.025 c,B | 1.93 ± 0.190 b,B | 0.146 ± 0.015 a,A |
| DS8526-2 LUHS29 | 0.005 ± 0.001 a,A | 0.376 ± 0.038 b,A | 1.96 ± 0.160 b,A | 0.124 ± 0.012 a,A |
| DS8526-2 LUHS245 | 0.004 ± 0.001 a,A | 0.500 ± 0.050 c,A | 2.00 ± 0.200 b,B | 0.129 ± 0.013 a,A |
| DS8526-2 LUHS122 | 0.006 ± 0.003 a,A | 0.292 ± 0.029 a,A | 1.11 ± 0.110 a,A | 0.134 ± 0.011 a,A |
| Wheat Samples | Essential Microelements, d.m. | |||||||
|---|---|---|---|---|---|---|---|---|
| Cr, mg/100 g | Mn, mg/kg | Fe, mg/kg | Co, mg/kg | Ni, mg/kg | Cu, mg/kg | Zn, mg/kg | Se, mg/kg | |
| Traditional Wheat Variety | ||||||||
| ‘Ada’ | <0.010 | 8.21 ± 0.820 c,A | 22.9 ± 2.60 a,B | <0.010 | <0.500 | 1.85 ± 0.190 a,D | 6.98 ± 0.70 b,A | <0.200 |
| ‘Ada’LUHS29 | <0.010 | 9.00 ± 0.900 c,A | 28.3 ± 2.80 a,C | <0.010 | <0.500 | 1.51 ± 0.150 a,C | 7.53 ± 0.75 b,B | <0.200 |
| ‘Ada’LUHS245 | <0.010 | 5.83 ± 0.580 a,A | 23.2 ± 2.28 a,B | <0.010 | <0.500 | 1.59 ± 0.160 a,C | 5.51 ± 0.55 a,A | <0.200 |
| ‘Ada’LUHS122 | 0.014 ± 0.001 | 7.81 ± 0.780 b,A | 38.7 ± 3.90 b,C | <0.010 | <0.500 | 1.61 ± 0.157 a,B | 7.05 ± 0.71 b,B | <0.200 |
| Waxy Wheat Variety | ||||||||
| ‘Sarta’ | 0.047 ± 0.001 a,B | 10.4 ± 1.00 a,b,A | 14.0 ± 1.40 b,A | <0.010 | <0.500 | 1.24 ± 0.120 b,C | 6.95 ± 0.70 c,A | <0.200 |
| ‘Sarta’LUHS29 | 0.052 ± 0.005 a | 13.4 ± 1.30 c,C | 15.4 ± 1.50 b,B | <0.010 | <0.500 | 1.32 ± 0.130 b,C | 7.14 ± 0.71 c,A | <0.200 |
| ‘Sarta’LUHS245 | <0.010 | 12.1 ± 1.20 b,c,B,C | 22.0 ± 2.20 c,B | <0.010 | <0.500 | 1.27 ± 0.128 b,C | 5.23 ± 0.52 b,A | <0.200 |
| ‘Sarta’LUHS122 | <0.010 | 8.25 ± 0.820 a,A | 5.48 ± 0.550 a,A | <0.010 | <0.500 | 0.830 ± 0.083 a,A | 3.61 ± 0.36 a,A | <0.200 |
| Blue Wheat—New BreedLine | ||||||||
| DS8472-5 | 0.015 ± 0.002 A | 10.7 ± 1.00 a,A | 14.9 ± 1.50 a,A | <0.010 | <0.500 | 0.763 ± 0.076 a,A | 7.25 ± 0.73 a,A | <0.200 |
| DS8472-5LUHS29 | <0.010 | 13.3 ± 1.30 b,C | 16.6 ± 1.70 a,B | <0.010 | <0.500 | 0.857 ± 0.086 a,B | 7.05 ± 0.71 a,A | <0.200 |
| DS8472-5LUHS245 | <0.010 | 10.9 ± 1.10 a,B | 13.7 ± 1.40 a,A | <0.010 | <0.500 | 0.695 ± 0.070 a,A | 6.64 ± 0.66 a,A,B | <0.200 |
| DS8472-5LUHS122 | <0.010 | 14.0 ± 1.40 b,B | 15.6 ± 1.60 a,B | <0.010 | <0.500 | 0.850 ± 0.085 a,A | 6.70 ± 0.67 a,B | <0.200 |
| Purple Wheat—New BreedLine | ||||||||
| DS8526-2 | <0.010 | 15.3 ± 1.50 c,B | 16.5 ± 1.70 b,A | <0.010 | <0.500 | 0.913 ± 0.091 b,B | 7.88 ± 0.79 b,A | <0.200 |
| DS8526-2LUHS29 | <0.010 | 11.1 ± 1.10 b,B | 10.3 ± 1.10 a,A | <0.010 | <0.500 | 0.692 ± 0.069 a,A | 6.03 ± 0.60 a,A | <0.200 |
| DS8526-2LUHS245 | <0.010 | 13.8 ± 1.40 c,C | 15.9 ± 1.60 b,A | <0.010 | <0.500 | 0.848 ± 0.085 a,b,A | 7.86 ± 0.79 b,B | <0.200 |
| DS8526-2LUHS122 | <0.010 | 8.71 ± 0.870 a,A | 17.6 ± 1.80 b,B | <0.010 | <0.500 | 0.728 ± 0.073 a,A | 6.11 ± 0.61 a,B | <0.200 |
| Wheat Samples | Non-Essential Macroelements, d.m. | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As, mg/100 g | V, mg/kg | Rb, mg/kg | Sr, mg/kg | Mo, mg/kg | Ag, mg/kg | Sb, mg/kg | Cs, mg/kg | TI, mg/kg | Cd, mg/kg | Ba, mg/kg | Pb, mg/kg | Al, mg/kg | Li, mg/kg | |
| Traditional Wheat Variety | ||||||||||||||
| ‘Ada’ | <0.050 | <2.00 | <1.00 | 1.00 ± 0.100 a,A | <0.500 | <2.00 | <0.500 | <2.00 | <2.00 | 0.038 ± 0.004 b,C | 2.71 ± 0.270 a,A | 0.037 ± 0.002 a | <5.00 | <0.050 |
| ‘Ada’LUHS29 | <0.050 | <2.00 | <1.00 | 0.949 ± 0.095 a,A | <0.500 | <2.00 | <0.500 | <2.00 | <2.00 | 0.029 ± 0.003 a,B | 2.36 ± 0.240 a,A | 0.032 ± 0.003 a | <5.00 | <0.050 |
| ‘Ada’LUHS245 | 0.009 ± 0.001 A | <2.00 | <1.00 | 0.854 ± 0.085 a,A | <0.500 | <2.00 | <0.500 | <2.00 | <2.00 | 0.037 ± 0.004 b,C | 2.27 ± 0.229 a,A | 0.038 ± 0.007 a | <5.00 | <0.050 |
| ‘Ada’LUHS122 | <0.050 | <2.00 | <1.00 | 0.909 ± 0.091 a,b,A | <0.500 | <2.00 | <0.500 | <2.00 | <2.00 | 0.033 ± 0.003 a,b,D | 2.30 ± 0.230 a,A | 0.034 ± 0.006 a | <5.00 | <0.050 |
| Waxy Wheat Variety | ||||||||||||||
| ‘Sarta’ | 0.007 ± 0.003 a,A | <2.00 | <1.00 | 1.22 ± 0.11 c,A | <0.500 | <2.00 | <0.500 | <2.00 | <2.00 | 0.027 ± 0.003 a,B | 2.30 ± 0.225 a,A | <0.010 | <5.00 | <0.050 |
| ‘Sarta’LUHS29 | 0.011 ± 0.002 a,A | <2.00 | <1.00 | 1.20 ± 0.12 c,A,B | <0.500 | <2.00 | <0.500 | <2.00 | <2.00 | 0.026 ± 0.004 a,B | 2.38 ± 0.240 a,A | <0.010 | <5.00 | <0.050 |
| ‘Sarta’LUHS245 | <0.050 | <2.00 | <1.00 | 1.02 ± 0.10 b,A | <0.500 | <2.00 | <0.500 | <2.00 | <2.00 | 0.028 ± 0.003 a,B | 2.11 ± 0.210 a,A | <0.010 | <5.00 | <0.050 |
| ‘Sarta’LUHS122 | 0.013 ± 0.003 a,A | <2.00 | <1.00 | 0.719 ± 0.072 a,A | <0.500 | <2.00 | <0.500 | <2.00 | <2.00 | 0.022 ± 0.003 a,C | 2.13 ± 0.209 a,A | <0.010 | <5.00 | <0.050 |
| Blue Wheat—New BreedLine | ||||||||||||||
| DS8472-5 | <0.050 | <2.00 | <1.00 | 1.46 ± 0.15 a,B | <0.500 | <2.00 | <0.500 | <2.00 | <2.00 | 0.009 ± 0.001 a,A | <2.00 | <0.010 | <5.00 | <0.050 |
| DS8472-5LUHS29 | <0.050 | <2.00 | <1.00 | 1.61 ± 0.16 a,B | <0.500 | <2.00 | <0.500 | <2.00 | <2.00 | 0.010 ± 0.002 a,A | <2.00 | <0.010 | <5.00 | <0.050 |
| DS8472-5LUHS245 | <0.050 | <2.00 | <1.00 | 1.44 ± 0.14 a,B | <0.500 | <2.00 | <0.500 | <2.00 | <2.00 | 0.009 ± 0.001 a,A | <2.00 | <0.010 | <5.00 | <0.050 |
| DS8472-5LUHS122 | <0.050 | <2.00 | <1.00 | 1.67 ± 0.17 a,D | <0.500 | <2.00 | <0.500 | <2.00 | <2.00 | 0.012 ± 0.002 a,B | <2.00 | <0.010 | <5.00 | <0.050 |
| Purple Wheat—New BreedLine | ||||||||||||||
| DS8526-2 | <0.050 | <2.00 | <1.00 | 1.90 ± 0.19 b,B | <0.500 | <2.00 | <0.500 | <2.00 | <2.00 | 0.009 ± 0.001 a,A | <2.00 | <0.010 | <5.00 | <0.050 |
| DS8526-2LUHS29 | <0.050 | <2.00 | <1.00 | 1.45 ± 0.15 a,B | <0.500 | <2.00 | <0.500 | <2.00 | <2.00 | 0.008 ± 0.001 a,A | <2.00 | <0.010 | <5.00 | <0.050 |
| DS8526-2LUHS245 | <0.050 | <2.00 | <1.00 | 1.84 ± 0.18 b,C | <0.500 | <2.00 | <0.500 | <2.00 | <2.00 | 0.009 ± 0.001 a,A | <2.00 | <0.010 | <5.00 | <0.050 |
| DS8526-2LUHS122 | <0.050 | <2.00 | <1.00 | 1.32 ± 0.13 a,C | <0.500 | <2.00 | <0.500 | <2.00 | <2.00 | 0.008 ± 0.001 a,A | <2.00 | <0.010 | <5.00 | <0.050 |
| Wheat Samples | Mycotoxin Concentration, μg/kg | ||||||
|---|---|---|---|---|---|---|---|
| DON | HT2 | T2 | FB1 | FB2 | ZEA | OTA | |
| Traditional Wheat Variety | |||||||
| ‘Ada’ | <15 | <1.5 | <1.5 | <15 | <15 | <3 | <1.5 |
| ‘Ada’LUHS29 | |||||||
| ‘Ada’LUHS245 | |||||||
| ‘Ada’LUHS122 | |||||||
| Waxy Wheat Variety | |||||||
| ‘Sarta’ | <15 | <1.5 | <1.5 | <15 | <15 | <3 | <1.5 |
| ‘Sarta’LUHS29 | 21.5 ± 2.1 a,B | ||||||
| ‘Sarta’LUHS245 | 17.4 ± 1.8 a,B | ||||||
| ‘Sarta’LUHS122 | <15 | ||||||
| Blue Wheat—New BreedLine | |||||||
| DS8472-5 | 96.4 ± 4.2 a,A | <1.5 | <1.5 | <15 | <15 | <3 | <1.5 |
| DS8472-5LUHS29 | 163.0 ± 7.8 d,A | ||||||
| DS8472-5LUHS245 | 134.0 ± 6.5 c,A | ||||||
| DS8472-5LUHS122 | 116.0 ± 9.2 b,A | ||||||
| Purple Wheat—New BreedLine | |||||||
| DS8526-2 | 274.4 ± 9.8 d,B | <1.5 | <1.5 | <15 | <15 | <3 | <1.5 |
| DS8526-2LUHS29 | 392.3 ± 11.3 b,B | ||||||
| DS8526-2LUHS245 | 323.9 ± 14.5 c,B | ||||||
| DS8526-2LUHS122 | 226.0 ± 11.6 a,B | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartkiene, E.; Starkute, V.; Zokaityte, E.; Klupsaite, D.; Mockus, E.; Bartkevics, V.; Borisova, A.; Gruzauskas, R.; Liatukas, Ž.; Ruzgas, V. Comparison Study of Nontreated and Fermented Wheat Varieties ‘Ada’, ‘Sarta’, and New Breed Blue and Purple Wheat Lines Wholemeal Flour. Biology 2022, 11, 966. https://doi.org/10.3390/biology11070966
Bartkiene E, Starkute V, Zokaityte E, Klupsaite D, Mockus E, Bartkevics V, Borisova A, Gruzauskas R, Liatukas Ž, Ruzgas V. Comparison Study of Nontreated and Fermented Wheat Varieties ‘Ada’, ‘Sarta’, and New Breed Blue and Purple Wheat Lines Wholemeal Flour. Biology. 2022; 11(7):966. https://doi.org/10.3390/biology11070966
Chicago/Turabian StyleBartkiene, Elena, Vytaute Starkute, Egle Zokaityte, Dovile Klupsaite, Ernestas Mockus, Vadims Bartkevics, Anastasija Borisova, Romas Gruzauskas, Žilvinas Liatukas, and Vytautas Ruzgas. 2022. "Comparison Study of Nontreated and Fermented Wheat Varieties ‘Ada’, ‘Sarta’, and New Breed Blue and Purple Wheat Lines Wholemeal Flour" Biology 11, no. 7: 966. https://doi.org/10.3390/biology11070966
APA StyleBartkiene, E., Starkute, V., Zokaityte, E., Klupsaite, D., Mockus, E., Bartkevics, V., Borisova, A., Gruzauskas, R., Liatukas, Ž., & Ruzgas, V. (2022). Comparison Study of Nontreated and Fermented Wheat Varieties ‘Ada’, ‘Sarta’, and New Breed Blue and Purple Wheat Lines Wholemeal Flour. Biology, 11(7), 966. https://doi.org/10.3390/biology11070966