Benthic Macrofauna Community Bioirrigation Potential (BIPc): Regional Map and Utility Validation for the South-Western Baltic Sea
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Biological Dataset and Environmental Predictors
2.3. Calculating Community Bioirrigation Potential (BIPc)
2.4. Assessing Temporal Variability in BIPc
2.5. Modelling Spatial Distribution of BIPc and Validating Index Performance
3. Results
3.1. Key Species
3.2. Temporal Variability in BIPc
3.3. Spatial Distribution of BIPc and Comparison with Patterns in BPc
3.4. Validating BIPc Index Sensitivity to Changes in Solute Fluxes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aller, R.C. Experimental studies of changes produced by deposit feeders on pore water, sediment, and overlying water chemistry. Am. J. Sci. 1978, 278, 1185–1234. [Google Scholar] [CrossRef]
- Koretsky, C.M.; Meile, C.; Cappellen, P.V. Quantifying bioirrigation using ecological parameters: A stochastic approach. Geochem. Trans. 2002, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Baranov, V. Influence of Bioturbation on Sediment Respiration in Advection and Diffusion Dominated Systems. Ph.D. Dissertation, Humboldt-Universität zu Berlin, Berlin, Germany, 8 February 2018. [Google Scholar] [CrossRef]
- Wei, C.L.; Rowe, G.T.; Escobar-Briones, E.; Boetius, A.; Soltwedel, T. Global patterns and predictions of seafloor biomass using Random Forests. PLoS ONE 2010, 5, e15323. [Google Scholar] [CrossRef] [PubMed]
- Meysman, F.J.R.; Galaktionov, E.S.; Gribsholt, B.; Middelburg, J.J. Bioirrigation in permeable sediments: Advective pore-water transport induced by burrow ventilation. Limnol. Oceanogr. 2006, 51, 142–156. [Google Scholar] [CrossRef] [Green Version]
- Baranov, V.; Lewandowski, J.; Romeijn, P.; Singer, G.; Krause, S. Effects of bioirrigation of non-biting midges (Diptera: Chironomidae) on lake sediment respiration. Sci. Rep. 2016, 6, 27329. [Google Scholar] [CrossRef] [Green Version]
- Granelli, W. Influence of Chironomus plumosus larvae on the oxygen-uptake of sediment. Arch. Hydrobiol. 1979, 87, 385–403. [Google Scholar]
- Soster, F.M.; Matisoff, G.; Schloesser, D.W.; Edwards, W.J. Potential impact of Chironomus plumosus larvae on hypolimnetic oxygen in the central basin of Lake Erie. J. Great Lakes Res. 2015, 41, 348–357. [Google Scholar] [CrossRef]
- McCall, P.L.; Tevesz, M.J. (Eds.) Sedimentary Geology 100. In Animal-Sediment Relations: The Biogenic Alteration of Sediments; Springer Science and Business Media: Berlin, Germany, 1985; pp. vii–xi. [Google Scholar]
- Rhoads, D.C.; Boyer, L.F. The Effects of Marine Benthos on Physical Properties of Sediments. In Animal-Sediment Relations; McCall, P.L., Tevesz, M.J., Eds.; Topics in Geobiology; Springer: Boston, MA, USA, 1982; Volume 100. [Google Scholar] [CrossRef]
- Solan, M.; Ward, E.R.; White, E.L.; Hibberd, E.E.; Cassidy, C.; Schuster, J.M.; Hale, R.; Godbold, J.A. Worldwide measurements of bioturbation intensity, ventilation rate, and the mixing depth of marine sediments. Sci. Data 2019, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Soetaert, K.; Herman, P.M.; Middelburg, J.J.; Heip, C.; deStigter, H.S.; van Weering, T.C.E.; Epping, E.; Helder, W. Modelling 210Pb–derived mixing activity in ocean margin sediments: Diffusive versus nonlocal mixing. J. Mar. Res. 1996, 54, 1207–1227. [Google Scholar] [CrossRef]
- Morys, C.; Forster, F.; Graf, G. Variabilty of bioturbation in various sediment types and on different spatial scales in the southwestern Baltic Sea. Mar. Ecol. Prog. Ser. 2016, 557, 31–49. [Google Scholar] [CrossRef]
- Toussaint, E.; De Borger, E.; Braeckman, U.; De Backer, A.; Soetaert, K.; Vanaverbeke, J. Faunal and environmental drivers of carbon and nitrogen cycling along a permeability gradient in shallow North Sea sediments. Sci. Total Environ. 2021, 767, 144994. [Google Scholar] [CrossRef]
- Butenschön, M.; Clark, J.; Aldridge, J.N.; Allen, J.I.; Artioli, Y.; Blackford, J.; Bruggeman, J.; Cazenave, P.; Ciavatta, S.; Kay, S.; et al. ERSEM 15.06: A generic model for marine biogeochemistry and the ecosystem dynamics of the lower trophic levels. Geosci. Model Dev. 2016, 9, 1293–1339. [Google Scholar] [CrossRef] [Green Version]
- Wrede, A.; Beermann, J.; Dannheim, J.; Gutow, L.; Brey, T. Organism functional traits and ecosystem supporting services—A novel approach to predict bioirrigation. Ecol. Indic. 2018, 91, 737–743. [Google Scholar] [CrossRef]
- Renz, J.R.; Powilleit, M.; Gogina, M.; Zettler, M.L.; Morys, C.; Forster, S. Community bioirrigation potential (BIPc), an index to quantify the potential for solute exchange at the sediment-water interface. Mar. Environ. Res. 2018, 141, 214–224. [Google Scholar] [CrossRef]
- Solan, M.; Cardinale, B.J.; Downing, A.L.; Engelhardt, K.A.M.; Ruesink, J.L.; Srivastava, D.S. Extinction and ecosystem function in the marine benthos. Science 2004, 306, 1177–1180. [Google Scholar] [CrossRef] [Green Version]
- Gogina, M.; Morys, C.; Forster, S.; Gräwe, U.; Friedland, R.; Zettler, M.L. Towards benthic ecosystemfunctioning maps: Quantifying bioturbation potential in the German part of the Baltic Sea. Ecol. Ind. 2017, 73, 574–588. [Google Scholar] [CrossRef]
- Gogina, M.; Zettler, M.L.; Vanaverbeke, J.; Dannheim, J.; Van Hoey, G.; Desroy, N.; Wrede, A.; Reiss, H.; Degraer, S.; Van Lancker, V.; et al. Interregional comparison of benthic ecosystem functioning: Community bioturbation potential in four regions along the NE Atlantic shelf. Ecol. Ind. 2020, 110, 105945. [Google Scholar] [CrossRef]
- Raymond, C.; Samuelsson, G.S.; Agrenius, S.; Schaanning, M.T.; Gunnarsson, J.S. Impaired benthic macrofauna function 4 years after sediment capping with activated carbon in the Grenland fjords, Norway. Environ. Sci. Pollut. Res. Int. 2021, 28, 16181–16197. [Google Scholar] [CrossRef]
- De Borger, E.; Tiano, J.; Braeckman, U.; Ysebaert, T.; Soetaert, K. Biological and biogeochemical methods for estimating bio-irrigation: A case study in the Oosterschelde estuary. Biogeosciences 2020, 17, 1701–1715. [Google Scholar] [CrossRef] [Green Version]
- Mermillod-Blondin, F.; Rosenberg, R.; François-Carcaillet, F.; Norling, K.; Mauclaire, L. Influence of bioturbation by three benthic infaunal species on microbial communities and biogeochemical processes in marine sediment. Aquat. Microb. Ecol. 2004, 36, 271–284. [Google Scholar] [CrossRef]
- Braeckman, U.; Provoost, P.; Gribsholt, B.; Van Gansbeke, D.; Middelburg, J.J.; Soetaert, K.; Vincx, M.; Vanaverbeke, J. Role of macrofauna functional traits and density in biogeochemical fluxes and bioturbation. Mar. Ecol. Prog. Ser. 2010, 399, 173–186. [Google Scholar] [CrossRef]
- Dargahi, B.; Kolluru, V.; Cvetkovic, V. Multi-layered stratification in the Baltic Sea: Insight from a modelling study with reference to environmental conditions. J. Mar. Sci. Eng. 2017, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Darr, A.; Gogina, M.; Zettler, M.L. Detecting hot-spots of bivalve biomass in the south-western Baltic Sea. J. Mar. Syst. 2014, 134, 69–80. [Google Scholar] [CrossRef]
- Zettler, M.L.; Friedland, R.; Gogina, M.; Darr, A. Variation in benthic long–term data of transitional waters: Is interpretation more than speculation? PLoS ONE 2017, 12, e0175746. [Google Scholar] [CrossRef]
- Vaquer-Sunyer, R.; Duarte, C.M. Thresholds of hypoxia for marine biodiversity. Proc. Natl. Acad. Sci. USA 2008, 105, 15452–15457. [Google Scholar] [CrossRef] [Green Version]
- Klingbeil, K.; Mohammadi-Aragh, M.; Gräwe, U.; Burchard, H. Quantification of spurious dissipation and mixing—Discrete variance decay in a finite–volume framework. Ocean Model. 2014, 81, 49–64. [Google Scholar] [CrossRef]
- Gräwe, U.; Naumann, M.; Mohrholz, V.; Burchard, H. Anatomizing one of the largest saltwater inflows into the Baltic Sea in December 2014. J. Geophys. Res. Oceans 2015, 120, 7676–7697. [Google Scholar] [CrossRef] [Green Version]
- Tauber, F. Meeresbodenrelief in der Deutschen Ostsee/Seabed Relief in the German Baltic Sea. Bundesamt für Seeschifffahrt und Hydrographie. Hamburg, Germany. 2012. Available online: https://www.geoseaportal.de/ (accessed on 10 September 2016).
- Tauber, F. Meeresbodensedimente in der Deutschen Ostsee/Seabed Sediments in the German Baltic Sea. Bundesamt für Seeschifffahrt und Hydrographie. Hamburg, Germany. 2012. Available online: https://www.geoseaportal.de/ (accessed on 10 September 2016).
- Friedland, R.; Neumann, T.; Schernewski, G. Climate change and the Baltic Sea Action Plan: Model simulations on the future of the western Baltic Sea. J. Mar. Syst. 2012, 105–108, 175–186. [Google Scholar] [CrossRef]
- Schernewski, G.; Friedland, R.; Carstens, M.; Hirt, U.; Leujak, W.; Nausch, G.; Neumann, T.; Petenati, T.; Sagert, S.; Wasmund, N.; et al. Implementation of European marine policy: New water quality targets for German Baltic water. Mar. Policy 2015, 51, 305–321. [Google Scholar] [CrossRef]
- Leipe, T.; Tauber, F.; Vallius, H.; Virtasalo, J.; Uscinowicz, S.; Kowalski, N.; Hille, S.; Lindgren, S.; Myllyvirta, T. Particulate organic carbon (POC) in surface sediments of the Baltic Sea. Geo-Mar. Lett. 2011, 31, 175–188. [Google Scholar] [CrossRef]
- Neumann, T.; Koponen, S.; Attila, J.; Brockmann, C.; Kallio, K.; Kervinen, M.; Mazeran, C.; Müller, D.; Philipson, P.; Thulin, S.; et al. Optical model for the Baltic Sea with an explicit CDOM state variable: A case study with Model ERGOM (version 1.2). Geosci. Model Dev. 2021, 14, 5049–5062. [Google Scholar] [CrossRef]
- Forster, S.; Khalili, A.; Kitlar, J. Variation of nonlocal irrigation in a subtidal benthic community. J. Mar. Res. 2003, 61, 335–357. [Google Scholar] [CrossRef] [Green Version]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 1 November 2021).
- Lipka, M. Current Biogeochemical Processes and Element Fluxes in Surface Sediments of Temperate Marginal Seas (Baltic Sea and Black Sea). Ph.D. Dissertation, Ernst-Moritz-Arndt-Universität, Greifswald, Germany, 2018. Available online: https://nbn-resolving.org/urn:nbn:de:gbv:9-opus-23761 (accessed on 21 May 2020).
- Legendre, P.; Anderson, M.J. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Powilleit, M.; Forster, S. Continuous and High Transport of Particles and Solutes by Benthos in Coastal Eutrophic Sediments of the Pomeranian Bay. Front. Mar. Sci. 2018, 5, 472. [Google Scholar] [CrossRef]
- Volkenborn, N.; Woodin, S.A.; Wethey, D.S.; Polerecky, L. Bioirrigation. In Encyclopedia of Ocean Sciences, 3rd ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 2, pp. 663–670. [Google Scholar] [CrossRef]
- Miatta, M.; Snelgrove, P.V.R. Benthic nutrient fluxes in deep-sea sediments within the Laurentian Channel MPA (eastern Canada): The relative roles of macrofauna, environment and sea pen octocorals. Deep Sea Res. Part I 2021, 178, 103655. [Google Scholar] [CrossRef]
- Bohlen, L.; Dale, A.W.; Wallmann, K. Simple transfer functions for calculating benthic fixed nitrogen losses and C:N:P regeneration ratios in global biogeochemical models. Glob. Biogeochem. Cycles 2012, 26, GB3029. [Google Scholar] [CrossRef] [Green Version]
- Wallmann, K. Phosphorus imbalance in the global ocean? Glob. Biogeochem. Cycles 2010, 24, GB4030. [Google Scholar] [CrossRef] [Green Version]
- Dale, A.W.; Paul, K.M.; Clemens, D.; Scholz, F.; Schroller-Lomnitz, U.; Wallmann, K.; SGeilert, S.; Hensen, C.; Plass, A.; Liebetrau, V.; et al. Recycling and burial of biogenic silica in an open margin oxygen minimum zone. Glob. Biogeochem. Cycles 2021, 35, e2020GB006583. [Google Scholar] [CrossRef]
- Baumfalk, Y.A. Heterogeneous grain size distribution in tidal flat sediment caused by bioturbation activity of Arenicola marina (Polychaeta). Neth. J. Sea Res. 1979, 13, 428–440. [Google Scholar] [CrossRef]
- Fish, J.D.; Fish, S. (Eds.) A Students Guide to the Seashore; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Chaffin, J.D.; Kane, D.D. Burrowing mayfly (Ephemeroptera: Ephemeridae: Hexagenia spp.) bioturbation and bioirrigation: A source of internal phosphorus loading in Lake Erie. J. Great Lakes Res. 2010, 36, 57–63. [Google Scholar] [CrossRef]
- Miatta, M.; Snelgrove, P.V.R. Sedimentary Organic Matter Shapes Macrofaunal Communities but Not Benthic Nutrient Fluxes in Contrasting Habitats Along the Northwest Atlantic Continental Margin. Front. Mar. Sci. 2021, 8, 756054. [Google Scholar] [CrossRef]
- Piontek, J.; Endres, S.; Le Moigne, F.A.C.; Schartau, M.; Engel, A. Relevance of Nutrient-Limited Phytoplankton Production and Its Bacterial Remineralization for Carbon and Oxygen Fluxes in the Baltic Sea. Front. Mar. Sci. 2019, 6, 581. [Google Scholar] [CrossRef] [Green Version]
- Norkko, J.; Shumway, S.E. (Eds.) Chapter 10. Bivalves as bioturbators and bioirrigators. In Shellfish Aquaculture and the Environment, 1st ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 297–317. [Google Scholar]
- Wrede, A.; Andresen, H.; Asmus, R.; Wiltshire, K.H.; Brey, T. Macrofaunal irrigation traits enhance predictability of nutrient fluxes across the sediment-water interface. Mar. Ecol. Prog. Ser. 2019, 632, 27–42. [Google Scholar] [CrossRef]
- Garcia, C.; Solan, M.; Bolam, S.G.; Sivyer, D.; Parker, R.; Godbold, J.A. Exploration of multiple post-extinction compensatory scenarios improves the likelihood of determining the most realistic ecosystem future. Environ. Res. Commun. 2021, 3, 045001. [Google Scholar] [CrossRef]
- Roskosch, A.; Hette, N.; Hupfer, M.; Lewandowski, J. Alteration of Chironomus plumosus ventilation activity and bioirrigation-mediated benthic fluxes by changes in temperature, oxygen concentration, and seasonal variations. Freshw. Sci. 2012, 31, 269–281. [Google Scholar] [CrossRef]
- Zabel, M.; Hensen, C. Quantification and Regionalization of Benthic Reflux. In Marine Geochemistry; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
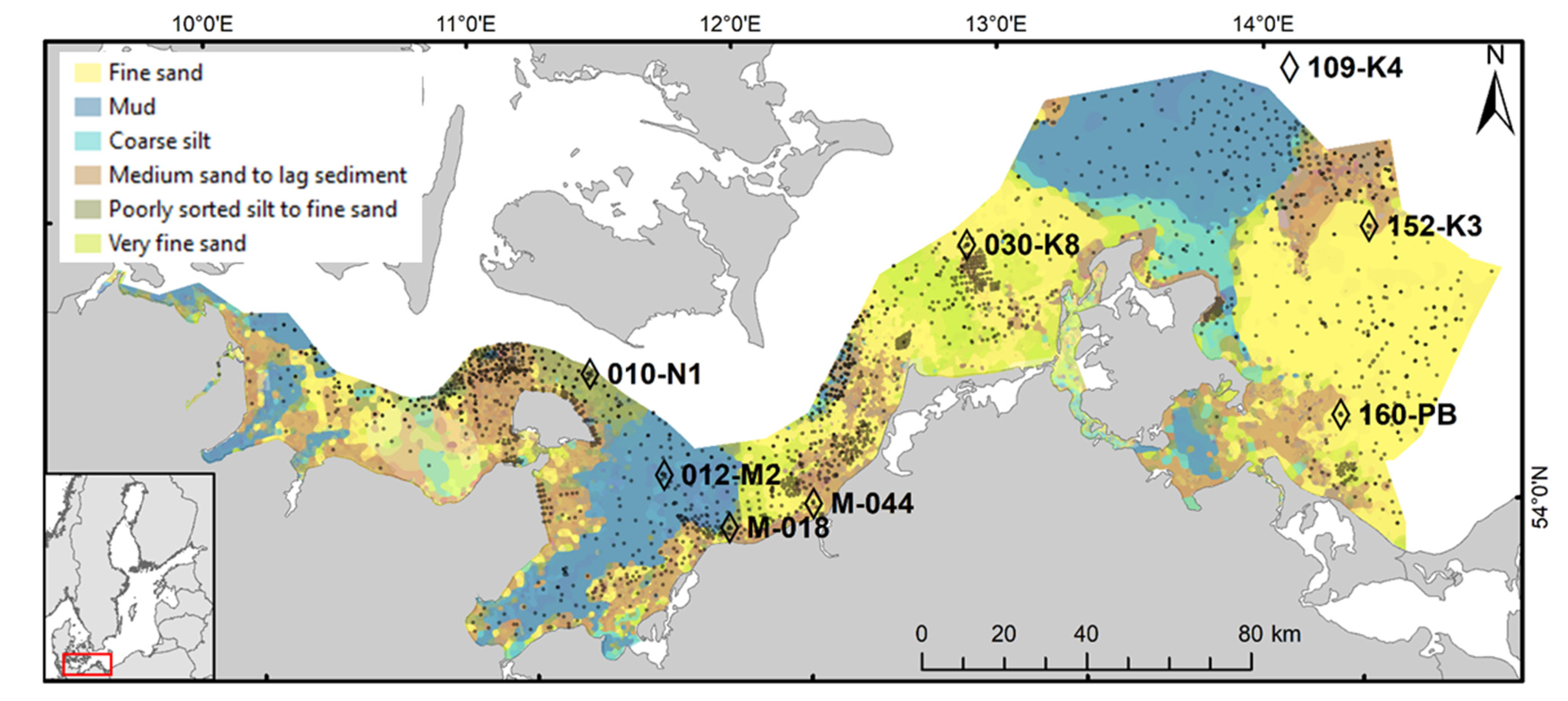
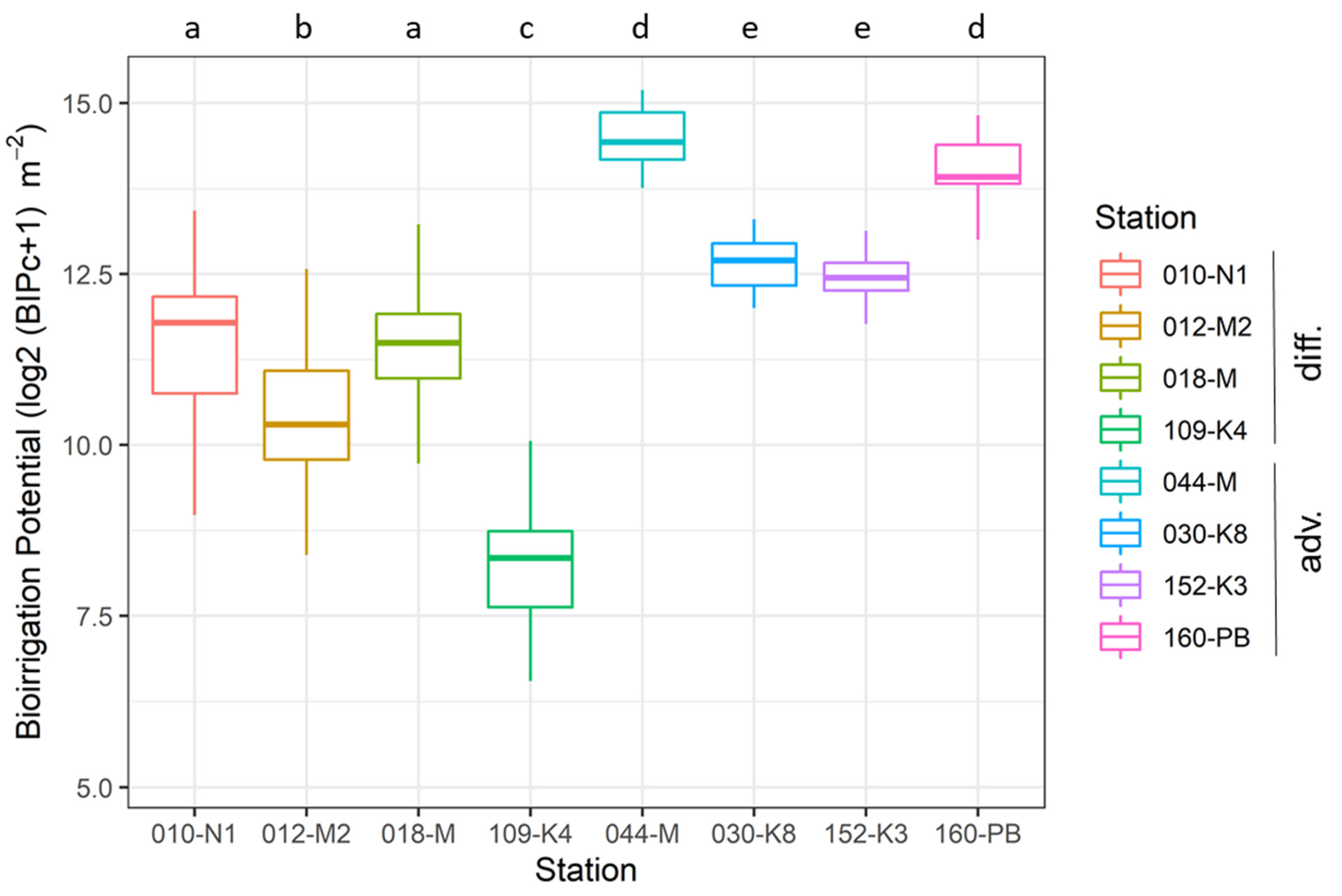
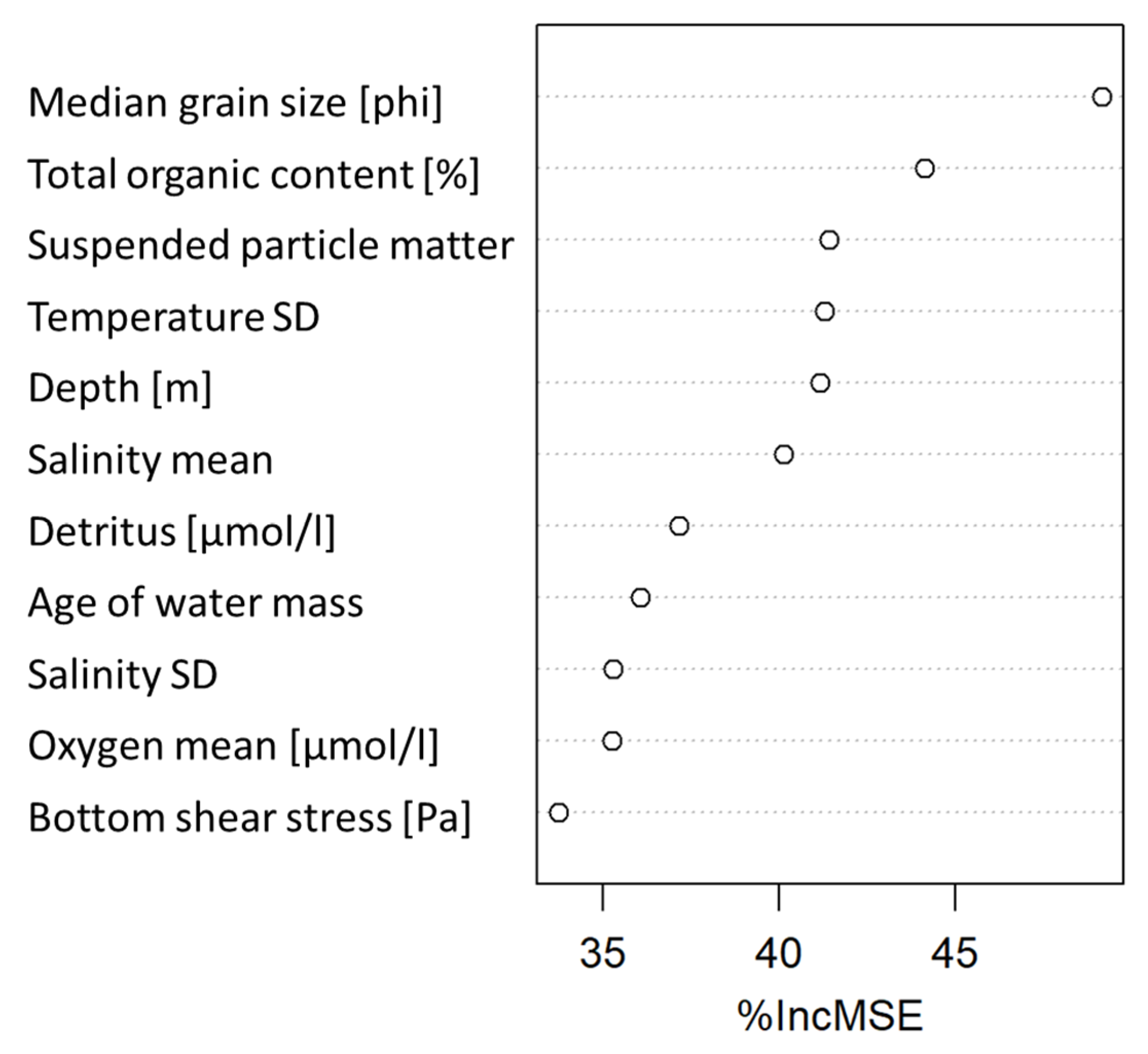
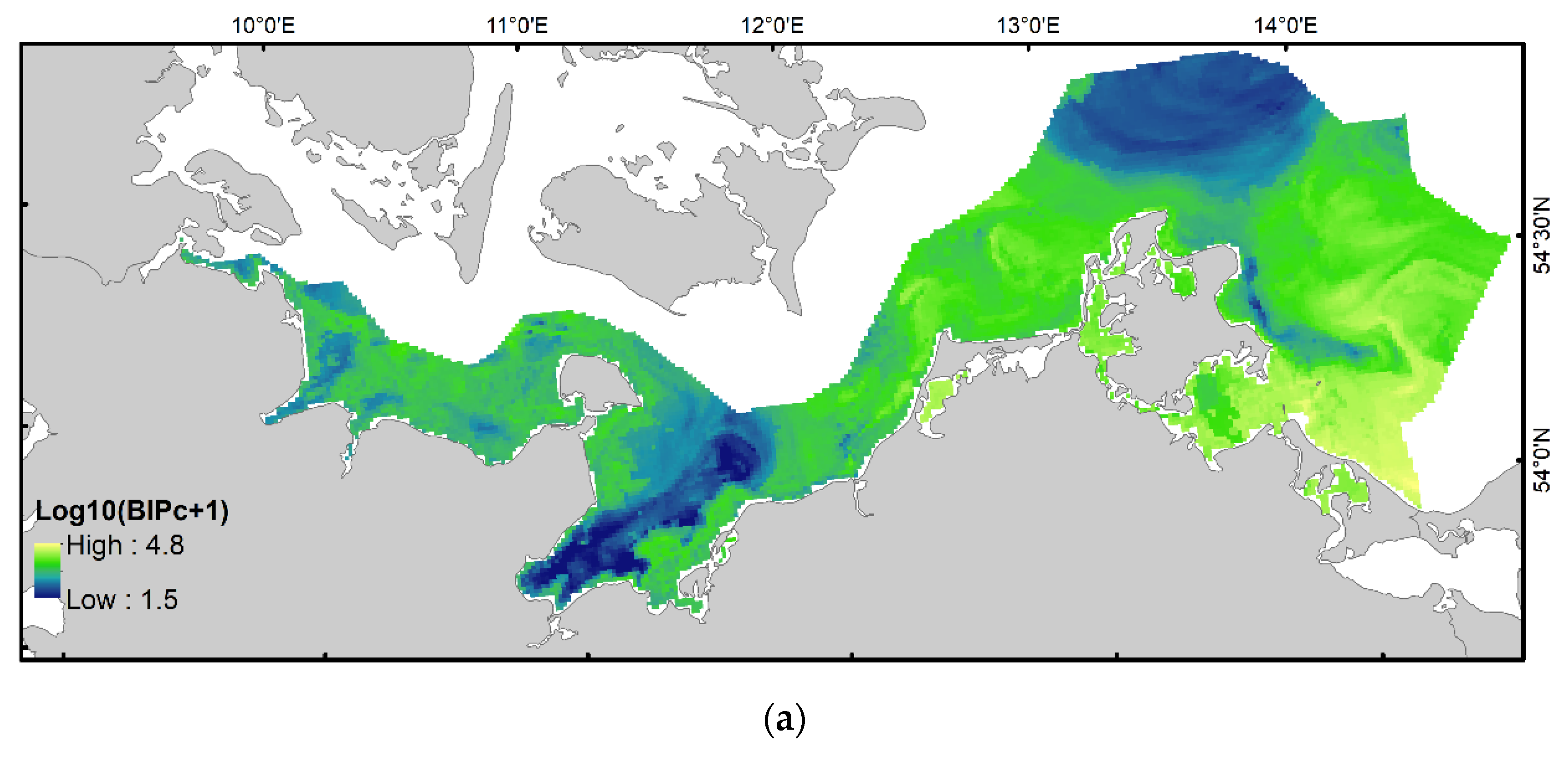

| Monitoring Station | 010-N1 | 012-M2 | M-018 | M-044 * | 030-K8 | 109-K4 | 152-K3 | 160-PB |
|---|---|---|---|---|---|---|---|---|
| Diffusive (D) vs. advective (A) | D | D | D | A | A | D | A | A |
| Number of sampling events | 22 | 24 | 35 | 9 | 21 | 21 | 20 | 17 |
| Median grain size (μm) | 146 | 21 | 108 | 197 | 224 | 19 | 218 | 191 |
| (91) | (7) | (41) | (67) | (6) | (7) | (17) | (11) | |
| Fraction finer 63 μm (%) | 34.2 | 84.5 | 26.5 | 0.0 | 1.8 | 84.3 | 2.5 | 1.8 |
| (27.7) | (22.7) | (16.2) | (-) | (1.9) | (24.7) | (3.3) | (2.6) | |
| Fraction coarser 2000 μm (%) | 0.28 | 0.84 | 0.04 | 0.00 | 0.01 | 0.61 | 0.84 | 0.03 |
| (0.92) | (3.36) | (0.15) | (-) | (0.02) | (2.29) | (1.23) | (0.1) | |
| Sorting (phi) | 1.64 | 1.76 | 1.31 | 0.56 | 0.49 | 1.67 | 0.60 | 0.50 |
| (0.74) | (0.47) | (0.42) | (-) | (0.12) | (0.43) | (0.14) | (0.14) | |
| Skewness (phi) | −0.40 | −0.37 | −0.49 | 0.04 | −0.16 | −0.46 | −0.07 | −0.04 |
| (0.61) | (0.44) | (0.48) | (-) | (0.11) | (0.32) | (0.23) | (0.12) | |
| Total organic content (%) | 2.97 | 9.27 | 1.58 | 0.01 | 0.29 | 12.05 | 0.44 | 0.25 |
| (0.85) | (1.13) | (0.36) | (-) | (0.1) | (1.55) | (0.18) | (0.1) | |
| Oxygen (near bottom) (ml/l) | 5.07 | 4.84 | 5.26 | 7.79 | 6.34 | 3.84 | 5.45 | 7.15 |
| (1.47) | (1.73) | (1.75) | (0.84) | (0.53) | (1.03) | (1.28) | (1.69) | |
| Salinity (near bottom) | 21.1 | 20.0 | 18.9 | 14.91 | 12.6 | 18.0 | 10.7 | 8.1 |
| (2.5) | (2.6) | (2.1) | (1.7) | (3.2) | (2.6) | (2.2) | (1.4) | |
| Water depth (m) | 28.1 | 24.3 | 20.3 | 10.8 | 22.6 | 47.4 | 30.6 | 14.3 |
| Latitude (WGS 84), N | 54°33.08′ | 54°18.86′ | 54°10.99′ | 54°12.94′ | 54°43.41′ | 55°0.01′ | 54°37.96′ | 54°14.41′ |
| Longitude (WGS 84), E | 11°19.17′ | 11°33′ | 11°46.01′ | 12°5.14′ | 12°47.02′ | 14°4.96′ | 14°16.96′ | 14°4.11′ |
| Key Taxa and Contribution to Overall Total BIPc | Key Taxa and Contribution to Total per Sediment Type | ||||
|---|---|---|---|---|---|
| overall | Mud | Medium sand | |||
| Mya arenaria | 22.40% | Arctica islandica | 22.90% | Marenzelleria viridis | 19.80% |
| Marenzelleria viridis | 18.80% | Scoloplos armiger | 11.70% | Mya arenaria | 18.10% |
| Arctica islandica | 9.90% | Terebellides stroemii | 10.50% | Arctica islandica | 13.40% |
| Scoloplos armiger | 7.70% | Macoma balthica | 10.10% | Scoloplos armiger | 10.00% |
| Hediste diversicolor | 7.40% | Lagis koreni | 5.20% | Pygospio elegans | 5.00% |
| Pygospio elegans | 4.70% | Fine sand | Coarse sand | ||
| Macoma balthica | 3.90% | Mya arenaria | 31.80% | Arctica islandica | 15.70% |
| Arenicola marina | 2.30% | Marenzelleria viridis | 19.80% | Marenzelleria viridis | 15.60% |
| Astarte borealis | 2.30% | Hediste diversicolor | 10.00% | Scoloplos armiger | 10.80% |
| Heteromastus filiformis | 1.60% | Arctica islandica | 6.70% | Mya arenaria | 7.40% |
| Sum | 81% | Scoloplos armiger | 5.70% | Pygospio elegans | 7.40% |
| Spearman Correlation Coefficients (n = 14) | Inventory Br mmol/m2 | Irrigation L/(m² d) Entire Core Depth | Irrigation L/(m² d) in 2 to 10 cm Sediment Depth Layer | BIPc Diff | BIPc Diff in 2 to 10 cm Sediment Depth Layer | BIPc Adv (Adv Scores in Fine Sands) | BPc | BPc in 2 to 10 cm Sediment Depth Layer | Abundance, ind/m2 | Wet Weight Biomass, g/m2 | Ash Free Dry Weight Biomass, g/m2 | Wet Weight Biomass in 2 to 10 cm Sediment Depth Layer, g/m2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corresponding p-Values | |||||||||||||
| Inventory Br mmol/m2 | 0.896 | 0.866 | 0.723 | 0.569 | 0.473 | 0.582 | 0.437 | 0.207 | 0.446 | 0.477 | 0.389 | ||
| Irrigation L/(m² d) entire core depth | 0.000 | 0.936 | 0.553 | 0.405 | 0.278 | 0.447 | 0.319 | 0.054 | 0.227 | 0.253 | 0.295 | ||
| Irrigation L/(m² d) in 2 to 10 cm sediment depth layer | 0.000 | 0.000 | 0.509 | 0.328 | 0.264 | 0.434 | 0.282 | 0.068 | 0.225 | 0.264 | 0.311 | ||
| BIPc diff | 0.003 | 0.040 | 0.063 | 0.789 | 0.516 | 0.895 | 0.789 | 0.169 | 0.763 | 0.780 | 0.578 | ||
| BIPc diff in 2 to 10 cm sediment depth layer | 0.034 | 0.151 | 0.252 | 0.001 | 0.248 | 0.538 | 0.903 | −0.315 | 0.934 | 0.903 | 0.859 | ||
| BIPc (adv scores in fine sands) | 0.088 | 0.337 | 0.361 | 0.059 | 0.392 | 0.415 | 0.257 | 0.453 | 0.231 | 0.253 | 0.125 | ||
| BPc | 0.029 | 0.109 | 0.121 | 0.000 | 0.047 | 0.140 | 0.644 | 0.343 | 0.552 | 0.600 | 0.284 | ||
| BPc in 2 to 10 cm sediment depth layer | 0.118 | 0.266 | 0.329 | 0.001 | 0.000 | 0.375 | 0.013 | −0.312 | 0.846 | 0.829 | 0.771 | ||
| Abundance, ind/m2 | 0.478 | 0.854 | 0.816 | 0.563 | 0.273 | 0.104 | 0.230 | 0.277 | −0.271 | −0.229 | −0.495 | ||
| Wet weight biomass, g/m2 | 0.110 | 0.435 | 0.440 | 0.002 | 0.000 | 0.427 | 0.041 | 0.000 | 0.349 | 0.991 | 0.890 | ||
| Ash free dry weight biomass, g/m2 | 0.085 | 0.382 | 0.361 | 0.001 | 0.000 | 0.383 | 0.023 | 0.000 | 0.431 | 0.000 | 0.873 | ||
| Wet weight biomass in 2 to 10 cm sediment depth layer, g/m2 | 0.169 | 0.306 | 0.280 | 0.030 | 0.000 | 0.670 | 0.326 | 0.001 | 0.072 | 0.000 | 0.000 | ||
| Oxygen | Silica | Ammonium | Phosphate | Manganese | |
|---|---|---|---|---|---|
| BPc | −0.13 | −0.39 | 0.17 | 0.71 | 0.15 |
| BIPc | −0.20 | −0.33 | 0.18 | 0.73 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gogina, M.; Renz, J.R.; Forster, S.; Zettler, M.L. Benthic Macrofauna Community Bioirrigation Potential (BIPc): Regional Map and Utility Validation for the South-Western Baltic Sea. Biology 2022, 11, 1085. https://doi.org/10.3390/biology11071085
Gogina M, Renz JR, Forster S, Zettler ML. Benthic Macrofauna Community Bioirrigation Potential (BIPc): Regional Map and Utility Validation for the South-Western Baltic Sea. Biology. 2022; 11(7):1085. https://doi.org/10.3390/biology11071085
Chicago/Turabian StyleGogina, Mayya, Judith Rahel Renz, Stefan Forster, and Michael L. Zettler. 2022. "Benthic Macrofauna Community Bioirrigation Potential (BIPc): Regional Map and Utility Validation for the South-Western Baltic Sea" Biology 11, no. 7: 1085. https://doi.org/10.3390/biology11071085
APA StyleGogina, M., Renz, J. R., Forster, S., & Zettler, M. L. (2022). Benthic Macrofauna Community Bioirrigation Potential (BIPc): Regional Map and Utility Validation for the South-Western Baltic Sea. Biology, 11(7), 1085. https://doi.org/10.3390/biology11071085








