Integrative Taxonomy of Armeria arenaria (Plumbaginaceae), with a Special Focus on the Putative Subspecies Endemic to the Apennines
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
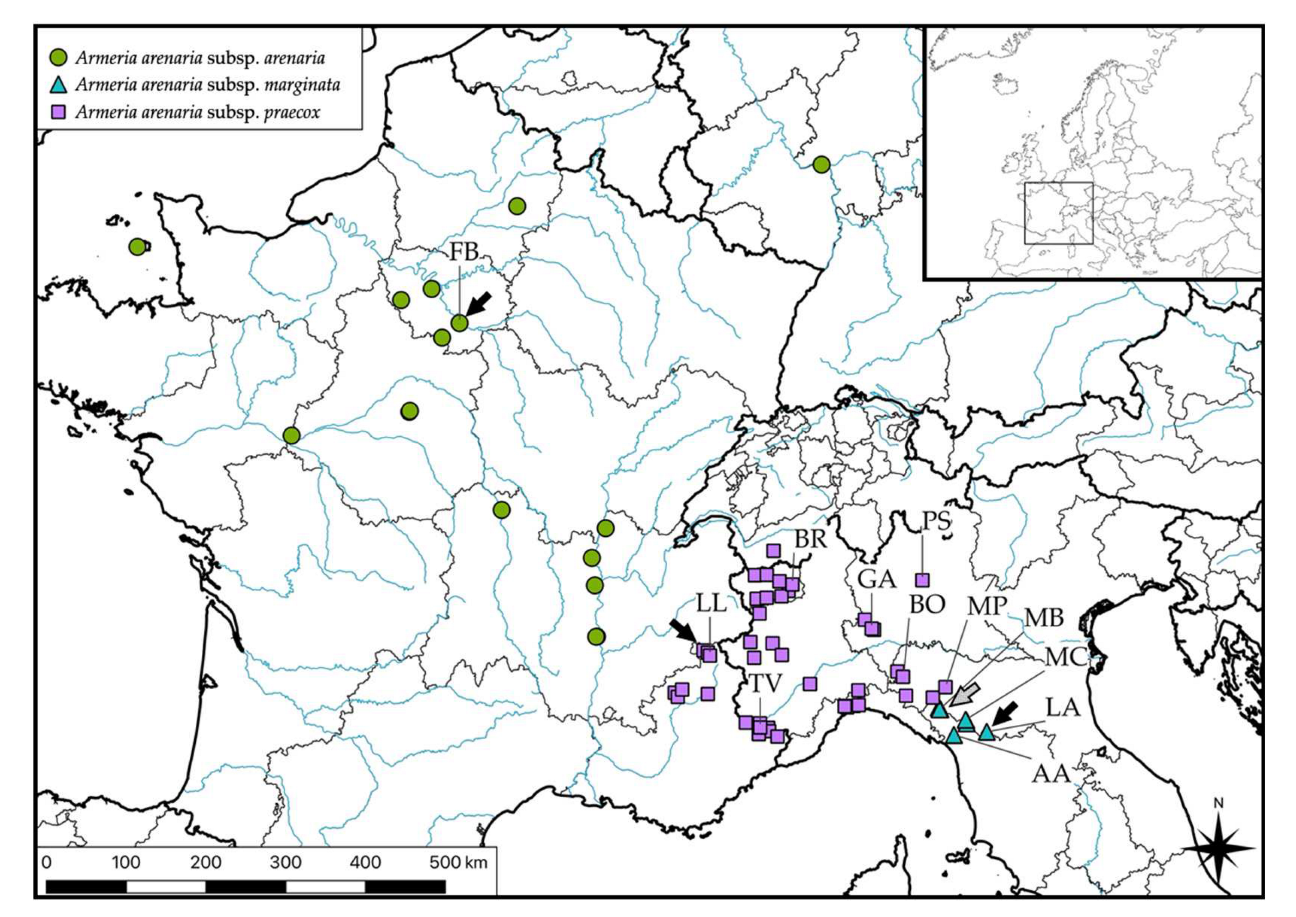
2.1. Sampling
2.2. Morphometric Analysis
2.3. Seed Morpho–Colorimetric Analysis
2.4. Karyological Analysis
2.5. DNA Extraction and Molecular Systematics
2.6. Comparative Niche Analysis
2.7. Nomenclature and Distribution
3. Results
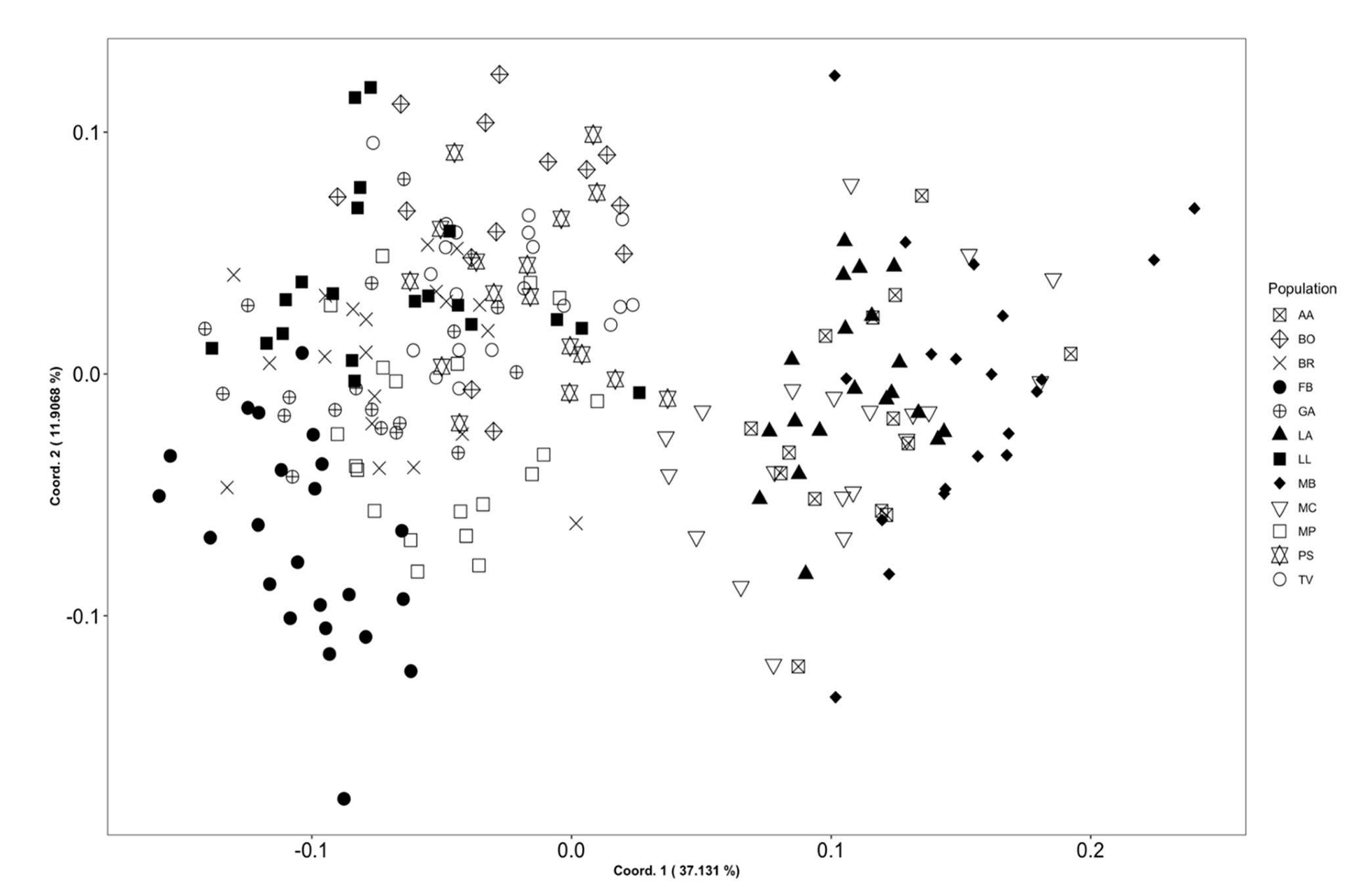
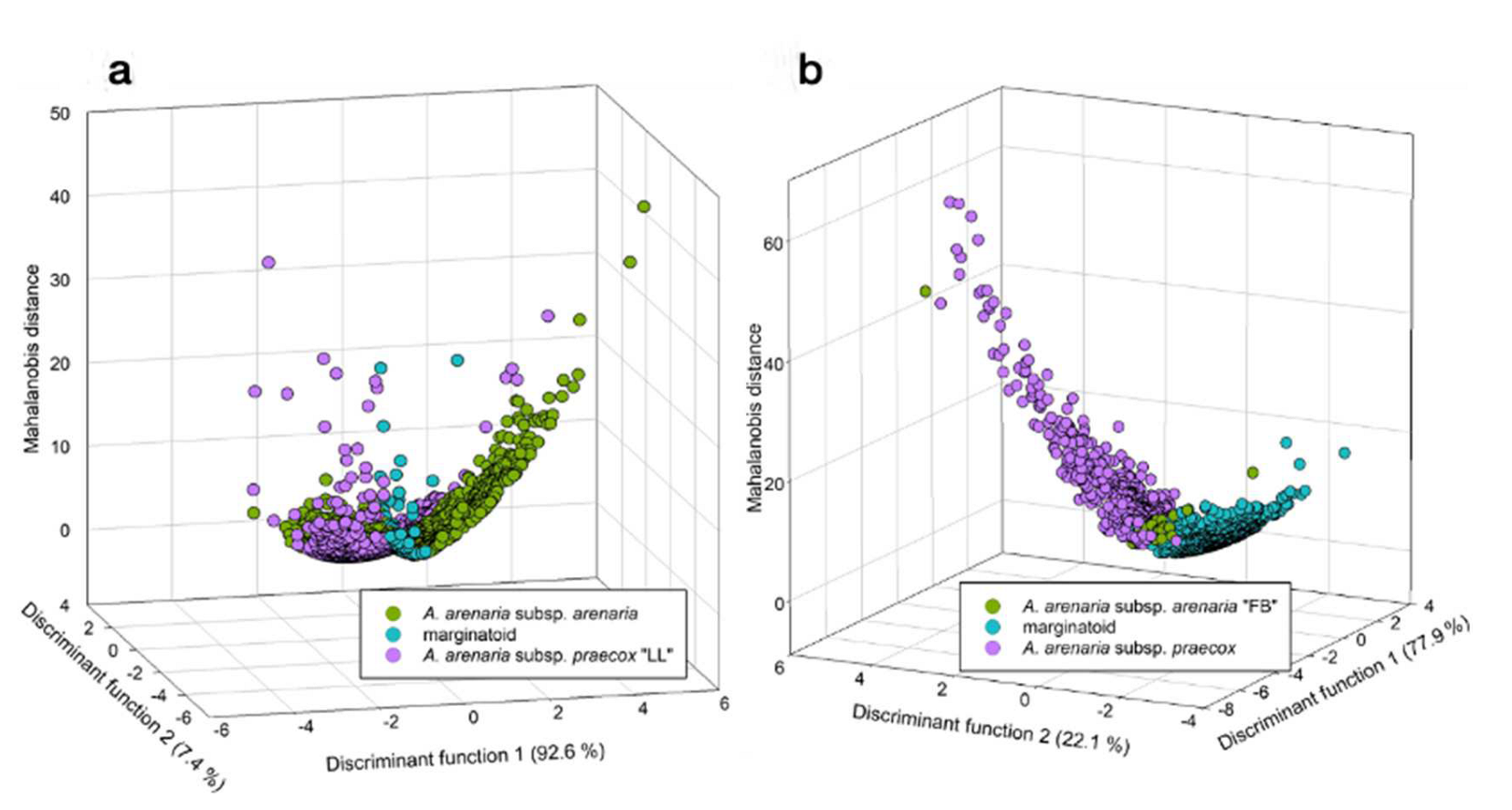
3.1. Morphometry
3.2. Seed Morpho–Colorimetry
3.3. Karyotype Structure and Asymmetry
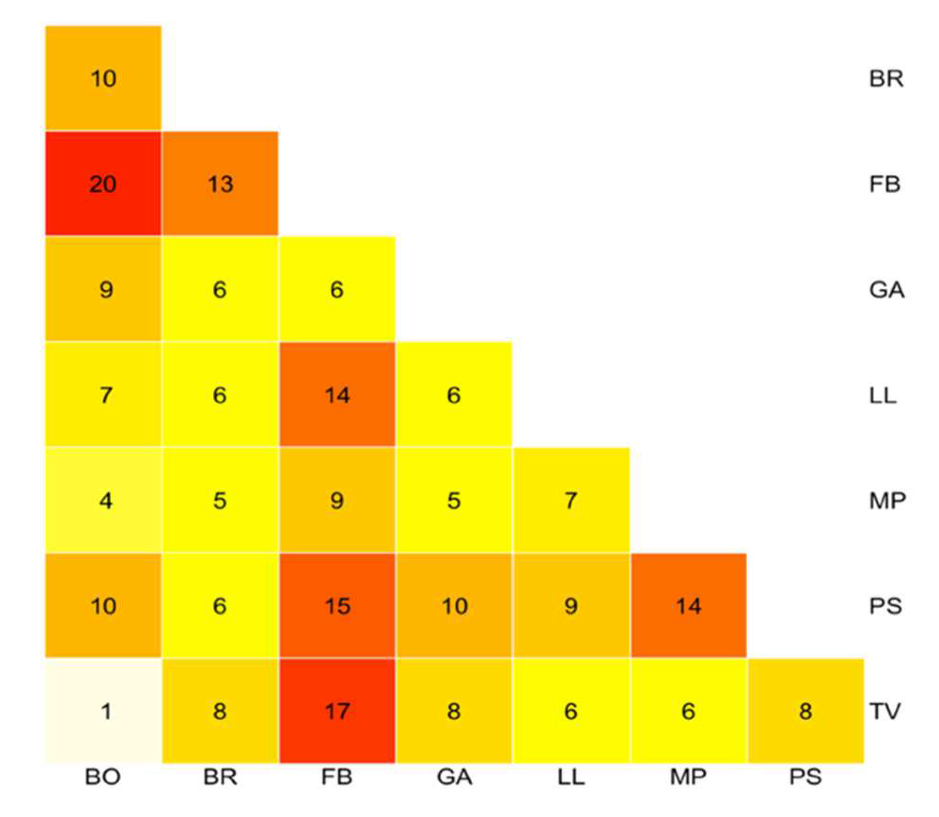
3.4. Molecular Systematics
3.5. Comparative Niche Analysis
4. Discussion
5. Taxonomic and Nomenclatural Scheme
5.1. Identification Key
- 1.
- Leaves with flat apex, middle vein translucid or nearly so, veins (section!) usually ≤ 5, bracts of the inner spikelet 8.78 ± 0.95 mm long, capitula 18.3 ± 1.5 mm …….…….……...…….…….…...….……………...….Armeria arenaria subsp. marginata
- 1.
- Leaves with cucullate apex, middle vein not translucid, veins (section!) usually > 5, bracts of the inner spikelet 6.67 ± 0.91 mm long, capitula 16.6 ± 2 mm wide……..……..……..……..…………………………...…………………………………... 2
- 2.
- Capitulum intermediate scales 3.87 ± 0.81 mm long; capitula 13.91 ± 1.5 mm wide; calyx 0.7 ± 0.15 mm wide, seeds 2.38 ± 0.07 mm long ……….……….……….………………………….…......Armeria arenaria subsp. arenaria
- 2.
- Capitulum intermediate scales 5.98 ± 0.89 mm long; capitula 16.57 ± 2 mm wide; calyx 1 ± 0.16 mm wide, seeds 2.89 ± 0.25 mm long ……….……….……….……….……….…...…...…........Armeria arenaria subsp. praecox
5.2. Nomenclature and Distribution
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christenhusz, M.; Byng, J. The number of known plant species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Mayo, S.J. Plant taxonomic species and their role in the workflow of integrative species delimitation. Kew Bull. 2022, 77, 1–26. [Google Scholar] [CrossRef]
- Klayman, J. Varieties of confirmation bias. In Psychology of Learning and Motivation; Elsevier: Amsterdam, The Netherlands, 1995; Volume 32, pp. 385–418. ISBN 978-0-12-543332-7. [Google Scholar]
- Ariely, D.; Carmon, Z. Gestalt characteristics of experiences: The defining features of summarized events. J. Behav. Decis. Mak. 2000, 13, 191–201. [Google Scholar] [CrossRef]
- Bernis, F. El genero Armeria Willd. en Portugal. Bol. Soc. Brot. 1949, 23, 225–263. [Google Scholar]
- Bernis, F. Revisión del género Armeria Willd. con especial referencia a los grupos ibéricos. Anales Inst. Bot. Cavanilles 1954, 5–287. [Google Scholar]
- Bernis, F. Revisión del género Armeria Willd. con especial referencia a los grupos ibéricos. Parte Secunda (Conclusión). Anales Inst. Bot. Cavanilles 1957, 14, 259–432. [Google Scholar]
- Garnett, S.T.; Christidis, L. Taxonomy anarchy hampers conservation. Nature 2017, 546, 25–27. [Google Scholar] [CrossRef]
- Dayrat, B. Towards integrative taxonomy. Biol. J. Linn. Soc. 2005, 85, 407–415. [Google Scholar] [CrossRef]
- Popper, K. The Logic of Scientific Discovery; Routledge: Abingdon-on-Thames, UK, 2005; ISBN 0-203-99462-0. [Google Scholar]
- Turrill, W.B. The investigation of plant species. Proc. Linn. Soc. London 1935, 147, 1–144. [Google Scholar] [CrossRef]
- Turrill, W.B. The expansion of taxonomy with special reference to spermatophyta. Biol. Rev. Biol. Proc. Camb. Phil. Soc. 1938, 13, 342–373. [Google Scholar] [CrossRef]
- Knapp, S. Taxonomy as a team sport. In The New Taxonomy; Systematics Association Special Volumes; CRC Press: Broken Sound Park, NY, USA, 2008; pp. 33–53. ISBN 978-1-4200-0856-2. [Google Scholar]
- Pupulin, F. Why we have no serious alternatives but cooperative taxonomy. Lankesteriana 2016, 16, 279–291. [Google Scholar] [CrossRef]
- WFO, World Flora Online. Available online: http://www.worldfloraonline.org (accessed on 4 December 2021).
- Arrigoni, P.V. Contributo alla conoscenza delle Armerie Sardo-Corse. Webbia 1970, 25, 137–182. [Google Scholar] [CrossRef]
- Arrigoni, P.V. Contribution to the study of the genus Armeria (Plumbaginaceae) in the Italian peninsula. Fl. Medit. 2015, 25, 7–32. [Google Scholar] [CrossRef]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Nieto Feliner, G.; Aguilar, F.A.; Rosselló, J.A. A new species of Armeria (Plumbaginaceae) from southern spain with molecular and morphometric evidence on its origin. Bot. J. Linn. Soc. 2001, 135, 71–84. [Google Scholar] [CrossRef][Green Version]
- Nieto Feliner, G. Natural and experimental hybridization in Armeria (Plumbaginaceae): Armeria salmantica. Int. J. Plant Sci. 1997, 158, 585–592. [Google Scholar] [CrossRef]
- Nieto Feliner, G.; Álvarez, I.; Fuertes-Aguilar, J.; Heuertz, M.; Marques, I.; Moharrek, F.; Piñeiro, R.; Riina, R.; Rosselló, J.A.; Soltis, P.S.; et al. Is homoploid hybrid speciation that rare? An empiricist’s view. Heredity 2017, 118, 513–516. [Google Scholar] [CrossRef]
- Nieto Feliner, G.; Rosato, M.; Alegre, G.; San Segundo, P.; Rosselló, J.A.; Garnatje, T.; Garcia, S. Dissimilar molecular and morphological patterns in an introgressed peripheral population of a sand dune species (Armeria pungens, Plumbaginaceae). Plant Biol. 2019, 21, 1072–1082. [Google Scholar] [CrossRef]
- Tauleigne-Gomes, C.; Lefèbvre, C. Natural hybridisation between two coastal endemic species of Armeria (Plumbaginaceae) from Portugal. 1. Populational in situ investigations. Plant Syst. Evol. 2005, 250, 215–230. [Google Scholar] [CrossRef]
- Tauleigne-Gomes, C.; Lefèbvre, C. Natural hybridisation between two coastal endemic species of Armeria (Plumbaginaceae) from Portugal. 2. Ecological investigations on a hybrid zone. Plant Syst. Evol. 2008, 273, 225–236. [Google Scholar] [CrossRef]
- Nieto Feliner, G. Armeria Willd. In Flora Ibérica: Plantas Vasculares de la Península Ibérica e Islas Baleares; Castroviejo, S., Laínz, M., López, G., Montserrat, P., Muñoz Garmendia, F., Paiva, J., Villar, L., Eds.; Consejo Superior de Investigaciones Científicas: Madrid, Spain, 1990; Volume 2, pp. 642–721. [Google Scholar]
- Donadille, P. Contribution à l’étude du genre Armeria Willd. (Plumbaginaceae). III. Clé générale des taxons français. Bull. Soc. Bot. France 1969, 116, 511–521. [Google Scholar] [CrossRef]
- De Giorgi, P.; Giacò, A.; Astuti, G.; Minuto, L.; Varaldo, L.; De Luca, D.; De Rosa, A.; Bacchetta, G.; Sarigu, M.; Peruzzi, L. An integrated taxonomic approach points towards a single-species hypothesis for Santolina (Asteraceae) in Corsica and Sardinia. Biology 2022, 11, 356. [Google Scholar] [CrossRef]
- Liu, L.; Astuti, G.; Coppi, A.; Peruzzi, L. Different chromosome numbers but slight morphological differentiation and genetic admixture among populations of the Pulmonaria hirta complex (Boraginaceae). Taxon 2022, in press. [Google Scholar] [CrossRef]
- POWO—Plants of the World Online, Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 15 November 2021).
- Lawrence, G.H.M. Armerias, native and cultivated. Gentes Herbarum 1940, 11, 391–418. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R.; RStudio, Inc.: Boston, MA, USA, 2020. [Google Scholar]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research; CRAN, 2020. [Google Scholar]
- Johannes Maier, M. R: Einführung Durch Angewandte Statistik, Wiley series Scientific Tools; 2nd ed.; Pearson Studium: Munchen, Germany, 2015. [Google Scholar]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Podani, J. Multivariate Exploratory Analysis of Ordinal Data in Ecology: Pitfalls, Problems and Solutions. Journal of Vegetation Science 2005, 16, 497–510. [Google Scholar] [CrossRef]
- Cailliez, F. The analytical solution of the additive constant problem. Psychometrika 1983, 48, 305–308. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. ape 5.0: An Environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Koul, A. PredPsych: Predictive Approaches in Psychology; CRAN, 2019. Available online: https://cran.r-project.org/web/packages/PredPsych/PredPsych.pdf (accessed on 10 July 2022).
- Cohen, J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Fleiss, J.L.; Levin, B.; Paik, M.C. The measurement of interrater agreement. In Statistical Methods for Rates and Proportions; Wiley Series in Probability and Statistics; Wiley: Hoboken, NJ, USA, 2003; ISBN 978-0-471-45861-6. [Google Scholar]
- Mangiafico, S. Rcompanion: Functions to Support Extension Education Program Evaluation; CRAN, 2020. Available online: https://cran.r-project.org/web/packages/rcompanion/rcompanion.pdf (accessed on 10 July 2022).
- Landini, G. Quantitative analysis of the epithelial lining architecture in radicular cysts and odontogenic keratocysts. Head Face Med. 2006, 2, 4. [Google Scholar] [CrossRef]
- Terral, J.-F.; Tabard, E.; Bouby, L.; Ivorra, S.; Pastor, T.; Figueiral, I.; Picq, S.; Chevance, J.-B.; Jung, C.; Fabre, L.; et al. Evolution and history of grapevine (Vitis vinifera) under domestication: New morphometric perspectives to understand seed domestication syndrome and reveal origins of ancient european cultivars. Ann. Bot. 2010, 105, 443–455. [Google Scholar] [CrossRef]
- Sarigu, M.; Grillo, O.; Bianco, M.L.; Ucchesu, M.; d’Hallewin, G.; Loi, M.C.; Venora, G.; Bacchetta, G. Phenotypic identification of plum varieties (Prunus domestica L.) by endocarps morpho-colorimetric and textural descriptors. Comput. Electron. Agric. 2017, 136, 25–30. [Google Scholar] [CrossRef]
- Sarigu, M.; Porceddu, M.; Schmitt, E.; Camarda, I.; Bacchetta, G. Taxonomic discrimination of the Paeonia mascula group in the tyrrhenian islands by seed image analysis. Syst. Biodiv. 2019, 17, 801–810. [Google Scholar] [CrossRef]
- Altınordu, F.; Peruzzi, L.; Yu, Y.; He, X. A Tool for the analysis of chromosomes: KaryoType. Taxon 2016, 65, 586–592. [Google Scholar] [CrossRef]
- Aceto, S.; Caputo, P.; Cozzolino, S.; Gaudio, L.; Moretti, A. Phylogeny and evolution of Orchis and allied genera based on ITS DNA variation: Morphological gaps and molecular continuity. Mol. Phylog. Evol. 1999, 13, 67–76. [Google Scholar] [CrossRef]
- Shaw, J.; Lickey, E.B.; Beck, J.T.; Farmer, S.B.; Liu, W.; Miller, J.; Siripun, K.C.; Winder, C.T.; Schilling, E.E.; Small, R.L. The tortoise and the hare II: Relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. Amer. J. Bot. 2005, 92, 142–166. [Google Scholar] [CrossRef]
- Shaw, J.; Lickey, E.B.; Schilling, E.E.; Small, R.L. Comparison of whole Chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: The tortoise and the hare III. Amer. J. Bot. 2007, 94, 275–288. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. Spec. Publ. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Sympos. Ser. 1999, 41, 95–98. [Google Scholar]
- Goloboff, P. NONA ver. 2. Published by the author: Tucumán, Argentina, 1999. [Google Scholar]
- Nixon, K. WinClada Ver. 1.0000. Published by the author: Ithaca, Greece, 2002. [Google Scholar]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. JModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, G. Estimating the dimension of a model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in bayesian phylogenetics using tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree 1.4. 2 Software; Institute of Evolutionary Biology, University Edinburgh: Edinburgh, UK, 2014. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef]
- Peruzzi, L.; Roma-Marzio, F.; Pinzani, L.; Bedini, G. Wikiplantbase #Italia v1.0. Available online: http://bot.biologia.unipi.it/wpb/italia/index.html (accessed on 4 December 2021).
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.; Randin, C.; Zimmermann, N.E. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef]
- Schoener, T.W. Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology 1970, 51, 408–418. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Euro+Med PlantBase. Available online: https://www.emplantbase.org/home.html (accessed on 20 May 2021).
- Stafleu, F.A.; Cowan, R.S. Taxonomic Literature: A Selective Guide to Botanical Publications and Collections with Dates, Commentaries and Types, 2nd ed.; Bohn, Scheltema & Holkema: Utrecht, The Netherlands, 1976. [Google Scholar]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. Available online: http://sweetgum.nybg.org/science/ih/ (accessed on 4 December 2021).
- Nieto Feliner, G. El género Armeria Willd. (Plumbaginaceae) en la Península Ibérica: Aclaraciones y novedades para una síntesis. Anal. Jard. Bot. Madr. 1987, 44, 319–348. [Google Scholar]
- Baumel, A.; Auda, P.; Torre, F.; Médail, F. Morphological polymorphism and RDNA internal transcribed spacer (ITS) sequence variation in Armeria(Plumbaginaceae) from South-Eastern France. Bot. J. Linn. Soc. 2009, 159, 255–267. [Google Scholar] [CrossRef][Green Version]
- Tison, J.-M.; Jauzein, P.; Michaud, H. Flore de la France Méditerranéenne Continentale; Naturalia Publications: Turriers, France, 2014; ISBN 978-2-909717-90-6. [Google Scholar]
- Tison, J.-M.; de Foucault, B. Flora Gallica: Flore de France; Biotope Editions: Mèze, France, 2014; Volume 1, ISBN 2-36662-012-8. [Google Scholar]
- HUH Index of Botanists. Available online: https://kiki.huh.harvard.edu/databases/botanist_search.php?mode=details&id=47839 (accessed on 4 April 2022).
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile; Koeltz Botanical Books: Glashütten, Germany, 2019; p. 159. [Google Scholar] [CrossRef]
- Giacò, A.; Astuti, G.; Peruzzi, L. Typification and nomenclature of the names in the Santolina chamaecyparissus species complex (Asteraceae). Taxon 2021, 70, 189–201. [Google Scholar] [CrossRef]
- Domina, G.; Astuti, G.; Bacchetta, G.; Barone, G.; Rešetnik, I.; Terlević, A.; Thiébaut, M.; Peruzzi, L. Typification of 14 names in the Dianthus virgineus group (Caryophyllaceae). Phytokeys 2021, 187, 1–14. [Google Scholar] [CrossRef]


| Code | Current Taxonomic Hypothesis | Population | Voucher | n |
|---|---|---|---|---|
| MB * | A. arenaria subsp. apennina | Italy, Emilia–Romagna, between M. Marmagna & M. Braiola (WGS84: 44.401250 N, 9.994250 E) | M. Tiburtini et S. Quitarrá; 27 June 2020; PI53990–PI54009 | 20 |
| MC | A. arenaria subsp. apennina | Italy, Emilia–Romagna, Monte Cusna (WGS84: 44.288194 N, 0.390055 E) | M. Tiburtini et S. Quitarrá; 26 June 2020; PI54653–PI54672 | 20 |
| MP | A. arenaria subsp. apennina | Italy, Emilia–Romagna, Monte Prinzera (WGS84: 44.640833 N, 10.083772 E) | M. Tiburtini et G. Astuti; 12 June 2020; PI53010–PI54029 | 20 |
| BO | A. arenaria subsp. arenaria | Italy, Emilia–Romagna, Monte Tre Abati, loc. Bobbio (WGS84: 44.752425 N, 9.436694 E) | S. Orsenigo; 3 June 2020; PI54050–PI54063 | 14 |
| BR | A. arenaria subsp. arenaria | Italy, Val d’Aosta, Brusson (WGS84: 45.738166 N, 7.754111 E) | S. Orsenigo; 16 June 2020; PI53970–PI53989 | 20 |
| FB * | A. arenaria subsp. arenaria | France, Île–de–France, Fontainebleau (WGS84: 48.437194 N, 2.690166 E) | M. Tiburtini et F. Losacco; 18 June 2020; PI56593–PI56615 | 23 |
| GA | A. arenaria subsp. arenaria | Italy, Lombardia, Gambolò (WGS84: 45.268611 N, 8.961027 E) | S. Orsenigo; 26 May 2020; PI54032–PI54049 | 18 |
| PS | A. arenaria subsp. arenaria | Italy, Lombardia, Piana di Salmezza (WGS84: 45.782583 N, 9.732277 E) | S. Orsenigo; 12 June 2020; PI53950–PI53969 | 20 |
| TV | A. arenaria subsp. arenaria | Italy, Piemonte, Terme di Valdieri (WGS84: 44.204083 N, 7.265000 E) | S. Orsenigo; 26 June 2020; PI55569–PI56573 | 20 |
| AA | A. arenaria subsp. marginata | Italy, Toscana, Alpi Apuane (WGS84: 44.124166 N, 10.212000 E) | M. Tiburtini et S. Quitarrá; 28 June 2020; PI54673–PI54686 | 14 |
| LA * | A. arenaria subsp. marginata | Italy, Emilia–Romagna, Libro Aperto (WGS84: 44.157402 N, 10.712021 E) | M. Tiburtini et S. Quitarrá; 25 June 2020; PI56573–PI56592 | 20 |
| LL * | A. arenaria subsp. praecox | France, Hautes Alpes, Le Lauzet (WGS84: 44.980166 N, 6.499611 E) | M. Tiburtini et F. Losacco; 16 June 2020; PI55549–PI55568 | 20 |
| Character Name | Description of the Character | Type | Tool |
|---|---|---|---|
| SCAB | Calyx awns scabrous (yes/no) | BI | Stereo |
| AWN | Awn presence on the calyx’s limb (yes/no) | BI | Stereo |
| CALYX_HAIRINESS | Calyx hairiness (holotrichous/pleurotrichous) | BI | Stereo |
| CALYX_VEINS | Number of calyx veins with hairs (10/5) | BI | Stereo |
| CAP_SHAPE | Shape of capitulum (hemispherical/subspherical) | BI | Stereo |
| DIMORP | Leaf dimorphism (yes/no) | BI | Stereo |
| INNER_SPI_BRACT_HAIR | Presence of hairs on inner spikelet bract (yes/no) | BI | Stereo |
| MAR_SUM_LEAF | Margin of the summer leaf (hyaline/dentate) | BI | Stereo |
| MAR_WIN_LEAF | Margin of the winter leaf (hyaline/dentate) | BI | Stereo |
| OUTER_SPI_BRACT_HAIR | Presence of hairs on the outer spikelet bract (yes/no) | BI | Stereo |
| INNER_SPI_BRACT_APEX | Shape of the inner spikelet bract apex (crenulate/undulate) | BI | Stereo |
| OUT_SPI_BRACT_APEX | Shape of the outer spikelet bract apex (crenulate/undulate) | BI | Stereo |
| SUM_LEAF_APEX | Shape of the summer leaf apex (acute/cucullate) | BI | Stereo |
| VEINS_HAIRS | Presence of hairs along the leaf veins (yes/no) | BI | Stereo |
| WIN_LEAF_APEX | Shape of the winter leaf apex (acute/cucullate) | BI | Stereo |
| COL_INV | Involucre colour (green/variegated/reddish/dry) | CN | Stereo |
| COL_PET | Petal colour (white/pink/fuchsia) | CN | Stereo |
| GEN_SHAPE_OUT_INV_BRACT | Shape of the outer involucral bract (deltate < triangular < strictly triangular) | CO | Stereo |
| SHAPE_APEX_INN_INV_BRACT | Shape of the inner involucral bract apex (round < acute < mucronate < apiculate) | CO | Stereo |
| SHAPE_APEX_INT_INV_BRACT | Shape of the intermediate involucral bract apex (round < acute < mucronate < apiculate) | CO | Stereo |
| SHAPE_APEX_OUT_INV_BRACT | Shape of the outer involucral bract apex (acute < mucronate < apiculate < acuminated < subulate < long subulate) | CO | Stereo |
| ANG_SUM_TIP | Summer leaf tip angle (°) | QC | Fiji |
| ANG_WIN_TIP | Winter leaf tip angle (°) | QC | Fiji |
| AWN_LENG | Awn length (mm) | QC | Fiji |
| DIAM_CAP | Capitulum diameter (mm) | QC | Calliper |
| HEIGTH | Plant height (mm) | QC | Ruler |
| LENG_CAL _PED | Calyx pedicel length (mm) | QC | Fiji |
| LENG_CAL_TUBE | Calyx tube length (mm) | QC | Fiji |
| LENG_INNER_INV_BRACT | Inner involucral bract length (mm) | QC | Calliper |
| LENG_INNER_SPI_BRACLE | Inner spikelet bracteole length (mm) | QC | Calliper |
| LENG_INNER_SPI_BRACT | Inner spikelet bract length (mm) | QC | Calliper |
| LENG_INTER_INV_BRACT | Intermediate involucral bract length (mm) | QC | Calliper |
| LENG_OUT_INV_BRACT | Outer involucral bract length (mm) | QC | Calliper |
| LENG_OUTER_SPI_BRACLE | Outer spikelet bracteole length (mm) | QC | Calliper |
| LENG_OUTER_SPI_BRACT | Outer spikelet bract length (mm) | QC | Calliper |
| LENG_SUM_LEAF | Summer leaf length (mm) | QC | Ruler |
| LENG_WIN_LEAF | Winter leaf length (mm) | QC | Ruler |
| LIMB_LENG | Limb length (mm) | QC | Fiji |
| SCA_DIAM | Scape diameter at 1 cm from the base (mm) | QC | Calliper |
| SCA_LENG | Scape length (mm) | QC | Ruler |
| SHEATH_LENG | Sheath length (mm) | QC | Calliper |
| WIDTH_CAL_TUBE | Calyx tube width below the limb (mm) | QC | Fiji |
| WIDTH_IAL_SUM | Width of the hyaline margin in summer leaf (mm) | QC | Fiji |
| WIDTH_IAL_WIN | Width of the hyaline margin in winter leaf (mm) | QC | Fiji |
| WIDTH_INNER_INV_BRACT | Inner involucral bract width at the middle (mm) | QC | Calliper |
| WIDTH_INNER_SPI_ BRACT | Inner spikelet bract width at the middle (mm) | QC | Calliper |
| WIDTH_INNER_SPI_BRACLE | Inner spikelet bracteole width at the middle (mm) | QC | Calliper |
| WIDTH_INTER_INV_BRACT | Intermediate involucral bract width at the middle (mm) | QC | Calliper |
| WIDTH_OUT_INV_BRACT | Outer involucral bract width at the base (mm) | QC | Calliper |
| WIDTH_OUTER_SPI_ BRACT | Outer spikelet bract length at the middle (mm) | QC | Calliper |
| WIDTH_OUTER_SPI_BRATLE | Outer spikelet bracteole width at the middle (mm) | QC | Calliper |
| WIDTH_SUM_LEAF | Width of the summer leaf at the middle (mm) | QC | Calliper |
| WIDTH_WIN_LEAF | Width of the winter leaf at the middle (mm) | QC | Calliper |
| N_ WIN_VEINS | Number of veins (with sclerenchyma) of winter leaf | QD | Microscope |
| N_INV_BRACT | Number of involucral bracts | QD | — |
| N_SUM_VEINS | Number of veins (with sclerenchyma) of summer leaf | QD | Microscope |
| SCAP_NUM | Number of scapes | QD | — |
| A. arenaria subsp. | apennina | arenaria | marginata | praecox | Total |
|---|---|---|---|---|---|
| apennina | 46 | 5 | 8 | 0 | 59 |
| arenaria | 2 | 105 | 1 | 2 | 110 |
| marginata | 6 | 0 | 28 | 0 | 34 |
| praecox | 0 | 5 | 0 | 15 | 20 |
| Total | 54 | 115 | 37 | 17 | 223 |
| A. arenaria subsp. | apennina | marginata | arenaria | praecox | Total |
|---|---|---|---|---|---|
| apennina | 36.3 | 30 | 15 | 18.7 | 100 |
| marginata | 18.5 | 71.5 | 7.5 | 2.5 | 100 |
| arenaria | 15.5 | 8.2 | 48.7 | 27.7 | 100 |
| praecox | 20 | — | 15 | 65 | 100 |
| Group | Arenarioid | Marginatoid | Total |
|---|---|---|---|
| Arenarioid | 87.4 | 12.6 | 100 |
| Marginatoid | 16.3 | 83.3 | 100 |
| Groupings | Marginatoid | Arenarioid (LL Excluded) | LL | Total | |
|---|---|---|---|---|---|
| Grouping hypothesis I | Marginatoid | 81.3 | 12.3 | 6.5 | 100 |
| Arenarioid (LL excluded) | 13.1 | 52.3 | 34.6 | 100 | |
| LL | 1 | 21 | 78.0 | 100 | |
| Marginatoid | Arenarioid (FB Excluded) | FB | Total | ||
| Grouping hypothesis II | Marginatoid | 81.5 | 18.5 | — | 100 |
| Arenarioid (FB excluded) | 13.3 | 84.1 | 2.6 | 100 | |
| FB | — | 9 | 91.0 | 100 | |
| Armeria arenaria | subsp. | arenaria | praecox | marginata |
|---|---|---|---|---|
| Current taxonomic hypothesis | praecox | 0.036 ns/ns | ||
| marginata | 0.041 ns/ns | 0.044 ns/ns | ||
| apennina | 0.006 ns/ns | 0.000 ns/ns | 0.208 ns/ns | |
| Grouping hypothesis I | praecox | 0.036 ns/ns | ||
| marginata | 0.033 ns/ns | 0.012 ns/ns | ||
| Grouping hypothesis II | praecox | 0.005 ns/ns | ||
| marginata | 0.000 ns/ns | 0.107 ns/ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiburtini, M.; Astuti, G.; Bartolucci, F.; Casazza, G.; Varaldo, L.; De Luca, D.; Bottigliero, M.V.; Bacchetta, G.; Porceddu, M.; Domina, G.; et al. Integrative Taxonomy of Armeria arenaria (Plumbaginaceae), with a Special Focus on the Putative Subspecies Endemic to the Apennines. Biology 2022, 11, 1060. https://doi.org/10.3390/biology11071060
Tiburtini M, Astuti G, Bartolucci F, Casazza G, Varaldo L, De Luca D, Bottigliero MV, Bacchetta G, Porceddu M, Domina G, et al. Integrative Taxonomy of Armeria arenaria (Plumbaginaceae), with a Special Focus on the Putative Subspecies Endemic to the Apennines. Biology. 2022; 11(7):1060. https://doi.org/10.3390/biology11071060
Chicago/Turabian StyleTiburtini, Manuel, Giovanni Astuti, Fabrizio Bartolucci, Gabriele Casazza, Lucia Varaldo, Daniele De Luca, Maria Vittoria Bottigliero, Gianluigi Bacchetta, Marco Porceddu, Gianniantonio Domina, and et al. 2022. "Integrative Taxonomy of Armeria arenaria (Plumbaginaceae), with a Special Focus on the Putative Subspecies Endemic to the Apennines" Biology 11, no. 7: 1060. https://doi.org/10.3390/biology11071060
APA StyleTiburtini, M., Astuti, G., Bartolucci, F., Casazza, G., Varaldo, L., De Luca, D., Bottigliero, M. V., Bacchetta, G., Porceddu, M., Domina, G., Orsenigo, S., & Peruzzi, L. (2022). Integrative Taxonomy of Armeria arenaria (Plumbaginaceae), with a Special Focus on the Putative Subspecies Endemic to the Apennines. Biology, 11(7), 1060. https://doi.org/10.3390/biology11071060












