Post-Mortem Interval of Human Skeletal Remains Estimated with Handheld NIR Spectrometry
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
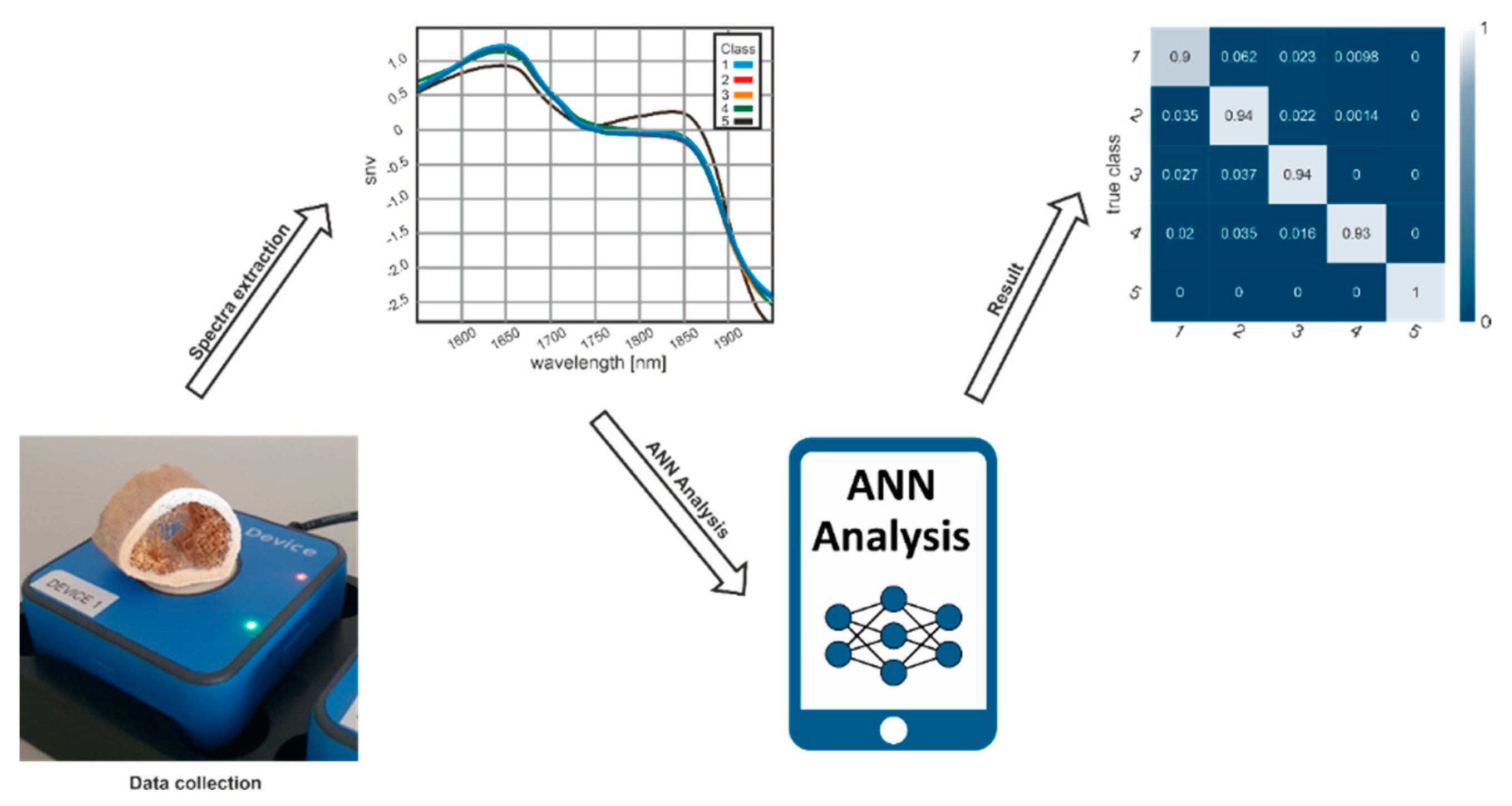
2.1. Sample Collection and Ethical Considerations
2.2. Measurement System
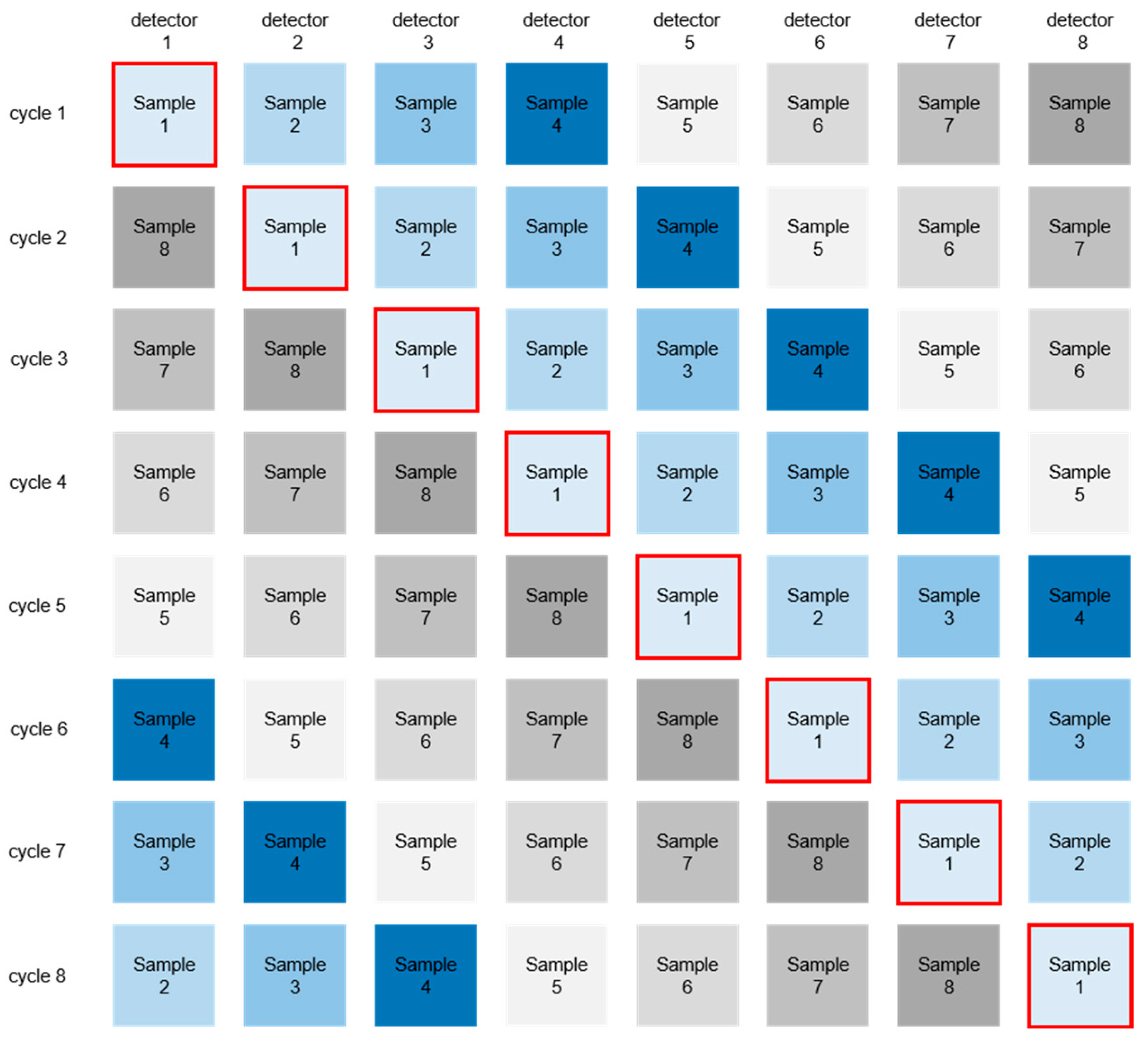
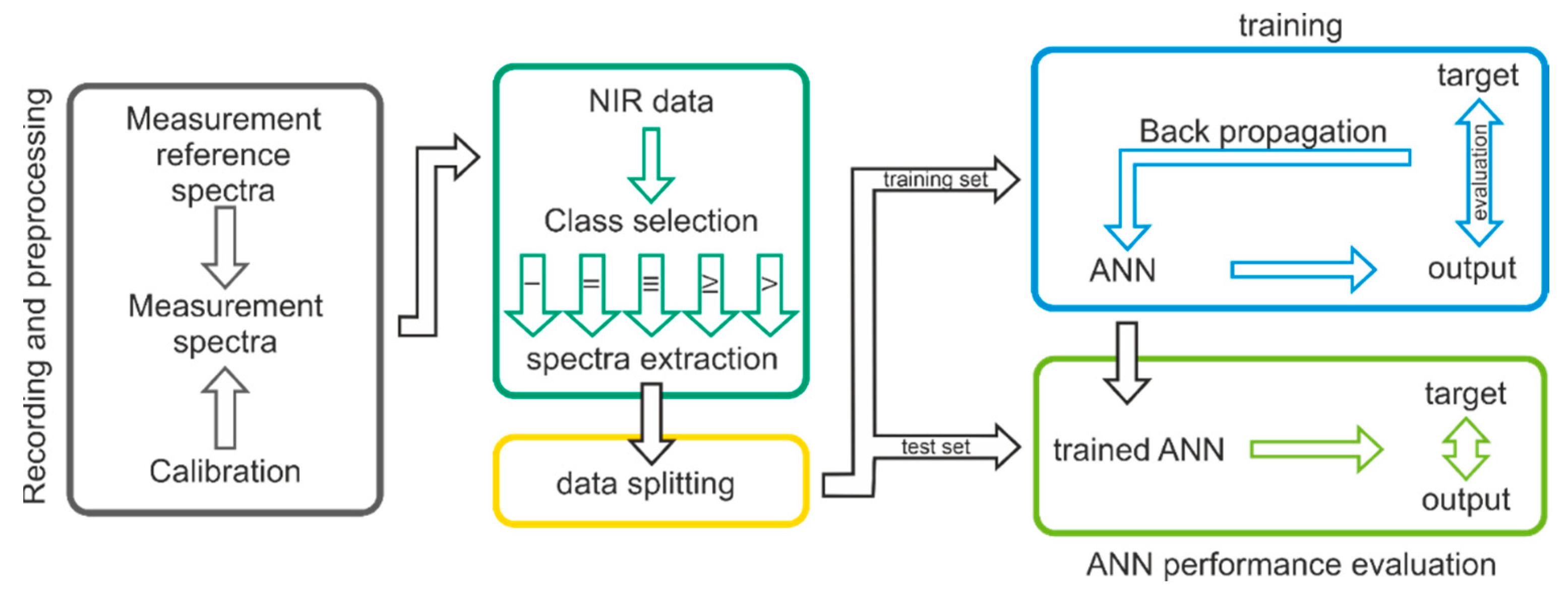
2.3. Data Preparation
2.4. Model Creation
3. Results
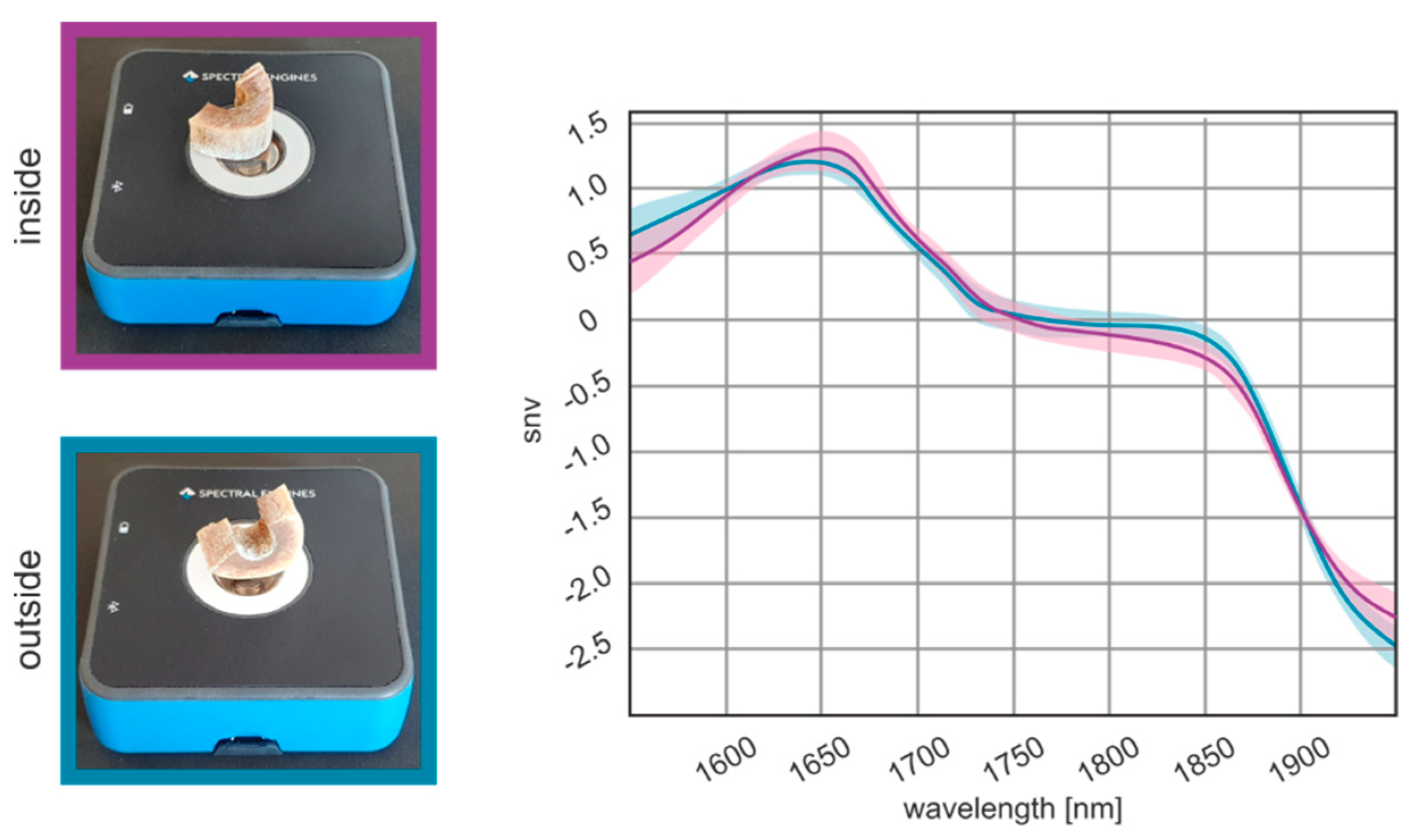
3.1. Optimization of the Measurement Location
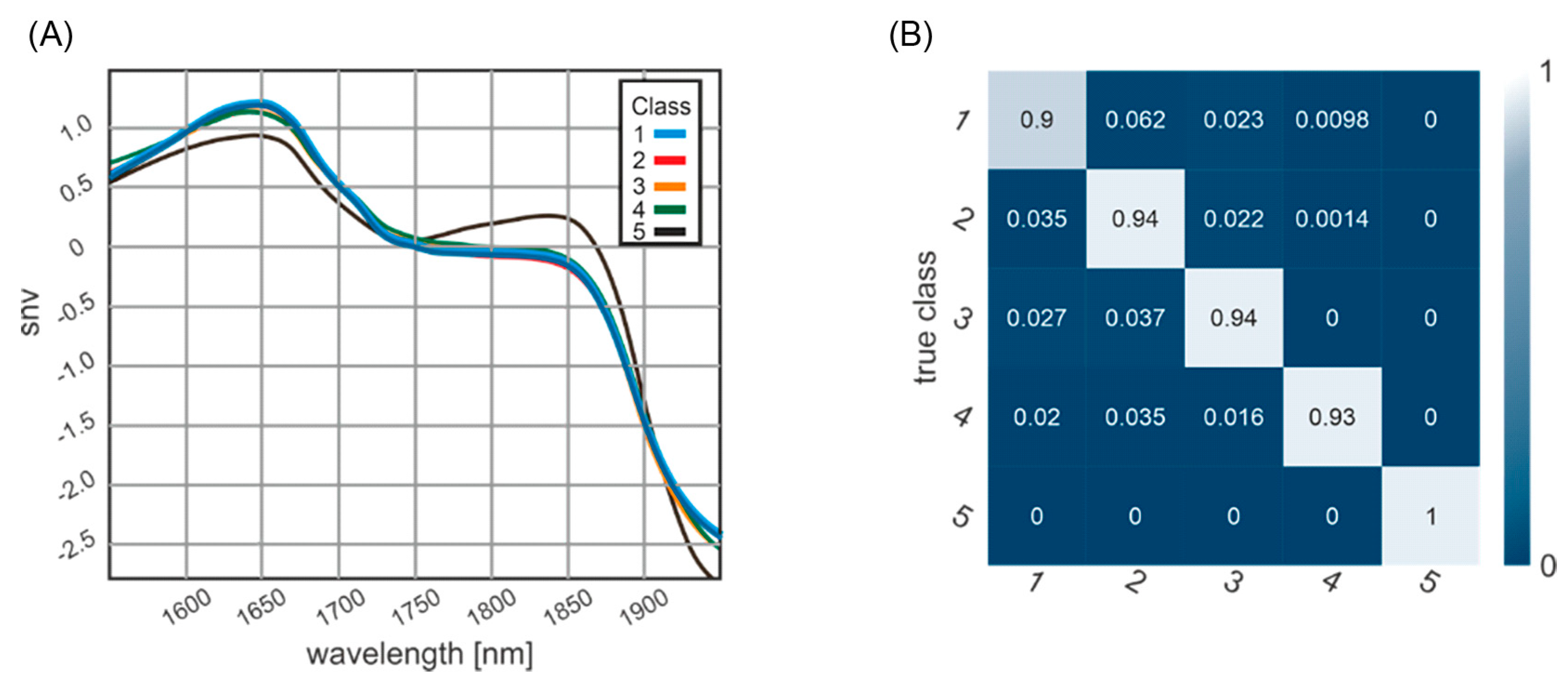
3.2. Classification of Post-Mortem Interval
3.3. Confusion Matrix of Classification Results
3.4. Practicability of Handheld NIR Spectrometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knight, B.; Lauder, I. Methods of dating skeletal remains. Hum. Biol. 1969, 41, 322–341. [Google Scholar] [CrossRef]
- Knight, B.; Lauder, I. Practical methods of dating skeletal remains: A preliminary study. Med. Sci. Law 1967, 7, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Introna, F., Jr.; Di Vella, G.; Campobasso, C.P. Determination of postmortem interval from old skeletal remains by image analysis of luminol test results. J. Forensic Sci. 1999, 44, 535–538. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Herrero, L.; Uribe, B.; Armas, L.H.; Alonso, M.L.; Sarmiento, A.; Irurita, J.; Alonso, R.M.; Maguregui, M.I.; Etxeberria, F.; Bartolome, L. Estimation of the post-mortem interval of human skeletal remains using Raman spectroscopy and chemometrics. Forensic Sci. Int. 2021, 329, 111087. [Google Scholar] [CrossRef]
- Sterzik, V.; Holz, F.; Ohlwärther, T.E.N.; Thali, M.; Birngruber, C.G. Estimating the postmortem interval of human skeletal remains by analyzing their fluorescence at 365 and 490 nm. Int. J. Leg. Med. 2018, 132, 933–938. [Google Scholar] [CrossRef]
- Ohlwärther, T.E.N.; Holz, F.; Heidorn, F.; Verhoff, M.A.; Birngruber, C.G. Rechtsmedizinische Begutachtung von Knochenfunden am Gießener Institut für Rechtsmedizin. Rechtsmedizin 2022, 32, 11–19. [Google Scholar] [CrossRef]
- Verhoff, M.A.; Heyne, M.; Kreutz, K.; Ramsthaler, F. Liegezeiteingrenzung anhand postmortaler Verletzungen. Rechtsmedizin 2009, 19, 223–227. [Google Scholar] [CrossRef]
- Nagy, G.; Lorand, T.; Patonai, Z.; Montsko, G.; Bajnoczky, I.; Marcsik, A.; Mark, L. Analysis of pathological and non-pathological human skeletal remains by FT-IR spectroscopy. Forensic Sci. Int. 2008, 175, 55–60. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, G.; Lednev, I.K. Potential application of Raman spectroscopy for determining burial duration of skeletal remains. Anal. Bioanal. Chem. 2011, 401, 2511–2518. [Google Scholar] [CrossRef]
- Prieto-Castelló, M.J.; Hernández del Rincón, J.P.; Pérez-Sirvent, C.; Álvarez-Jiménez, P.; Pérez-Cárceles, M.D.; Osuna, E.; Luna, A. Application of biochemical and X-ray diffraction analyses to establish the postmortem interval. Forensic Sci. Int. 2007, 172, 112–118. [Google Scholar] [CrossRef]
- Boaks, A.; Siwek, D.; Mortazavi, F. The temporal degradation of bone collagen: A histochemical approach. Forensic Sci. Int. 2014, 240, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Krogman, W.M.; Iscan, M.Y. The Human Skeleton in Forensic Medicine; CC Thomas Springfield: Springfield, IL, USA, 1986. [Google Scholar]
- Blau, S.; Ubelaker, D.H. Handbook of Forensic Anthropology and Archaeology; Left Coast Press: Walnut Creek, CA, USA, 2009. [Google Scholar]
- Berg, S. The determination of bone age. In Methods of Forensic Science; Interscience: Hoboken, NJ, USA, 1963; Volume 2, pp. 231–252. [Google Scholar]
- Behrensmeyer, A.K. Taphonomic and ecologic information from bone weathering. Paleobiology 1978, 4, 150–162. [Google Scholar] [CrossRef] [Green Version]
- Hare, P. Organic geochemistry of bone and its relation to the survival of bone in the natural environment. Foss. Mak. Vertebr. Taphon. Paleoecol. 1988, 69, 208–222. [Google Scholar]
- Gordon, C.C.; Buikstra, J.E. Soil pH, bone preservation, and sampling bias at mortuary sites. Am. Antiq. 1981, 46, 566–571. [Google Scholar] [CrossRef]
- Piepenbrink, H. Two examples of biogenous dead bone decomposition and their consequences for taphonomic interpretation. J. Archaeol. Sci. 1986, 13, 417–430. [Google Scholar] [CrossRef]
- Dollerup, E. Chemical analyses and microradiographic investigations on bone biopsies from cases of osteoporosis and osteomalacia as compared with normal. Part I. Calcium, phophorus and nitrogen content of normal and osteoporotic human bone. In Bone and Booth; The Macmillan Company: New York, NY, USA, 1964; Volume 399, pp. 399–404. [Google Scholar]
- Schultz, M. Microscopic investigation of excavated skeletal remains: A contribution to paleopathology and forensic medicine. In Forensic Taphonomy: The Postmortem Fate of Human Remains; CRC Press: Boca Raton, FL, USA, 1997; pp. 201–222. [Google Scholar]
- Yoshino, M.; Kimijima, T.; Miyasaka, S.; Sato, H.; Seta, S. Microscopical study on estimation of time since death in skeletal remains. Forensic Sci. Int. 1991, 49, 143–158. [Google Scholar] [CrossRef]
- Chibnall, A.; Rees, M.; Williams, E. The total nitrogen content of egg albumin and other proteins. Biochem. J. 1943, 37, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camps, F.E.; Cameron, J.M.; Lanham, D.J. Practical Forensic Medicine; Hutchinson: Paris, France, 1971. [Google Scholar]
- Cattaneo, C.; Gelsthorpe, K.; Phillips, P.; Sokol, R.J. Reliable identification of human albumin in ancient bone using ELISA and monoclonal antibodies. Am. J. Phys. Anthropol. 1992, 87, 365–372. [Google Scholar] [CrossRef]
- Procopio, N.; Williams, A.; Chamberlain, A.T.; Buckley, M. Forensic proteomics for the evaluation of the post-mortem decay in bones. J. Proteom. 2018, 177, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Bonete, G.; Pérez-Cárceles, M.D.; Maurandi-López, A.; Pérez-Martínez, C.; Luna, A. Association between protein profile and postmortem interval in human bone remains. J. Proteom. 2019, 192, 54–63. [Google Scholar] [CrossRef]
- Szelecz, I.; Lösch, S.; Seppey, C.V.W.; Lara, E.; Singer, D.; Sorge, F.; Tschui, J.; Perotti, M.A.; Mitchell, E.A.D. Comparative analysis of bones, mites, soil chemistry, nematodes and soil micro-eukaryotes from a suspected homicide to estimate the post-mortem interval. Sci. Rep. 2018, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Procopio, N.; Ghignone, S.; Williams, A.; Chamberlain, A.; Mello, A.; Buckley, M. Metabarcoding to investigate changes in soil microbial communities within forensic burial contexts. Forensic Sci. Int. Genet. 2019, 39, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Castellano, M.A.; Villanueva, E.C.; von Frenckel, R. Estimating the date of bone remains: A multivariate study. J. Forensic Sci. 1984, 29, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martínez, C.; Pérez-Cárceles, M.D.; Legaz, I.; Prieto-Bonete, G.; Luna, A. Quantification of nitrogenous bases, DNA and Collagen type I for the estimation of the postmortem interval in bone remains. Forensic Sci. Int. 2017, 281, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.E.; Suchey, J.M.; Payen, L.A.; Slota, P.J., Jr. The use of radiocarbon (14C) to identify human skeletal materials of forensic science interest. J. Forensic Sci. 1989, 34, 1196–1205. [Google Scholar] [CrossRef]
- Kanz, F.; Reiter, C.; Risser, D.U. Citrate Content of Bone for Time Since Death Estimation: Results from Burials with Different Physical Characteristics and Known PMI. J. Forensic Sci. 2014, 59, 613–620. [Google Scholar] [CrossRef]
- Schwarcz, H.P.; Agur, K.; Jantz, L.M. A New Method for Determination of Postmortem Interval: Citrate Content of Bone*. J. Forensic Sci. 2010, 55, 1516–1522. [Google Scholar] [CrossRef]
- Sterzik, V.; Jung, T.; Jellinghaus, K.; Bohnert, M. Estimating the postmortem interval of human skeletal remains by analyzing their optical behavior. Int. J. Leg. Med. 2016, 130, 1557–1566. [Google Scholar] [CrossRef]
- Swift, B. Dating human skeletal remains: Investigating the viability of measuring the equilibrium between 210Po and 210Pb as a means of estimating the post-mortem interval. Forensic Sci. Int. 1998, 98, 119–126. [Google Scholar] [CrossRef]
- Cappella, A.; Gibelli, D.; Muccino, E.; Scarpulla, V.; Cerutti, E.; Caruso, V.; Sguazza, E.; Mazzarelli, D.; Cattaneo, C. The comparative performance of PMI estimation in skeletal remains by three methods (C-14, luminol test and OHI): Analysis of 20 cases. Int. J. Leg. Med. 2018, 132, 1215–1224. [Google Scholar] [CrossRef]
- Maclaughlin-Black, S.M.; Herd, R.J.; Willson, K.; Myers, M.; West, I.E. Strontium-90 as an indicator of time since death: A pilot investigation. Forensic Sci. Int. 1992, 57, 51–56. [Google Scholar] [CrossRef]
- Ramsthaler, F.; Kreutz, K.; Zipp, K.; Verhoff, M.A. Dating skeletal remains with luminol-chemiluminescence. Validity, intra- and interobserver error. Forensic Sci. Int. 2009, 187, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Sarabia, J.; Pérez-Martínez, C.; Hernández del Rincón, J.P.; Luna, A. Study of chemiluminescence measured by luminometry and its application in the estimation of postmortem interval of bone remains. Leg. Med. 2018, 33, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Ramsthaler, F.; Ebach, S.C.; Birngruber, C.G.; Verhoff, M.A. Postmortem interval of skeletal remains through the detection of intraosseal hemin traces. A comparison of UV-fluorescence, luminol, Hexagon-OBTI®, and Combur® tests. Forensic Sci. Int. 2011, 209, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Piga, G.; Malgosa, A.; Thompson, T.; Enzo, S. A new calibration of the XRD technique for the study of archaeological burned human remains. J. Archaeol. Sci. 2008, 35, 2171–2178. [Google Scholar] [CrossRef]
- Longato, S.; Woss, C.; Hatzer-Grubwieser, P.; Bauer, C.; Parson, W.; Unterberger, S.H.; Kuhn, V.; Pemberger, N.; Pallua, A.K.; Recheis, W.; et al. Post-mortem interval estimation of human skeletal remains by micro-computed tomography, mid-infrared microscopic imaging and energy dispersive X-ray mapping. Anal. Methods 2015, 7, 2917–2927. [Google Scholar] [CrossRef] [Green Version]
- Surovell, T.A.; Stiner, M.C. Standardizing infra-red measures of bone mineral crystallinity: An experimental approach. J. Archaeol. Sci. 2001, 28, 633–642. [Google Scholar] [CrossRef] [Green Version]
- Thompson, T.; Islam, M.; Piduru, K.; Marcel, A. An investigation into the internal and external variables acting on crystallinity index using Fourier Transform Infrared Spectroscopy on unaltered and burned bone. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 299, 168–174. [Google Scholar] [CrossRef]
- Gourion-Arsiquaud, S.; Faibish, D.; Myers, E.; Spevak, L.; Compston, J.; Hodsman, A.; Shane, E.; Recker, R.R.; Boskey, E.R.; Boskey, A.L. Use of FTIR spectroscopic imaging to identify parameters associated with fragility fracture. J. Bone Miner. Res. 2009, 24, 1565–1571. [Google Scholar] [CrossRef] [Green Version]
- Thompson, T.; Gauthier, M.; Islam, M. The application of a new method of Fourier Transform Infrared Spectroscopy to the analysis of burned bone. J. Archaeol. Sci. 2009, 36, 910–914. [Google Scholar] [CrossRef] [Green Version]
- Pucéat, E.; Reynard, B.; Lécuyer, C. Can crystallinity be used to determine the degree of chemical alteration of biogenic apatites? Chem. Geol. 2004, 205, 83–97. [Google Scholar] [CrossRef]
- Patonai, Z.; Maasz, G.; Avar, P.; Schmidt, J.; Lorand, T.; Bajnoczky, I.; Mark, L. Novel dating method to distinguish between forensic and archeological human skeletal remains by bone mineralization indexes. Int. J. Legal Med. 2013, 127, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Verhoff, M.A.; Kreutz, K.; Jopp, E.; Kettner, M. Forensische Anthropologie im 21. Jahrhundert. Rechtsmedizin 2013, 23, 79–84. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Lin, H.; Zha, S.; Fang, R.; Wei, X.; Fan, S.; Wang, Z. Estimation of the late postmortem interval using FTIR spectroscopy and chemometrics in human skeletal remains. Forensic Sci. Int. 2017, 281, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Creagh, D.; Cameron, A. Estimating the Post-Mortem Interval of skeletonized remains: The use of Infrared spectroscopy and Raman spectro-microscopy. Radiat. Phys. Chem. 2017, 137, 225–229. [Google Scholar] [CrossRef]
- Woess, C.; Unterberger, S.H.; Roider, C.; Ritsch-Marte, M.; Pemberger, N.; Cemper-Kiesslich, J.; Hatzer-Grubwieser, P.; Parson, W.; Pallua, J.D. Assessing various Infrared (IR) microscopic imaging techniques for post-mortem interval evaluation of human skeletal remains. PLoS ONE 2017, 12, e0174552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamrogiewicz, M. Application of the near-infrared spectroscopy in the pharmaceutical technology. J. Pharm. Biomed. Anal. 2012, 66, 1–10. [Google Scholar] [CrossRef]
- Bec, K.B.; Grabska, J.; Huck, C.W. Near-Infrared Spectroscopy in Bio-Applications. Molecules 2020, 25, 2948. [Google Scholar] [CrossRef]
- Bec, K.B.; Grabska, J.; Huck, C.W. NIR spectroscopy of natural medicines supported by novel instrumentation and methods for data analysis and interpretation. J. Pharm. Biomed. Anal. 2021, 193, 113686. [Google Scholar] [CrossRef]
- Bec, K.B.; Grabska, J.; Huck, C.W. Principles and Applications of Miniaturized Near-Infrared (NIR) Spectrometers. Chemistry 2021, 27, 1514–1532. [Google Scholar] [CrossRef]
- Books, D.G.R.L. Strafgesetzbuch von Österreich; Books on Demand: Nordstedt, Germany, 2022. [Google Scholar]
- Bass, W.M. Human Osteology: A Laboratory and Field Manual of the Human Skeleton; Missouri Archaeological Society Springfield: Springfield, MO, USA, 1971. [Google Scholar]
- Macdonald, H.M.; Nishiyama, K.K.; Kang, J.; Hanley, D.A.; Boyd, S.K. Age-related patterns of trabecular and cortical bone loss differ between sexes and skeletal sites: A population-based HR-pQCT study. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2011, 26, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Cascant, M.M.; Rubio, S.; Gallello, G.; Pastor, A.; Garrigues, S.; Guardia, M.d.l. Burned bones forensic investigations employing near infrared spectroscopy. Vib. Spectrosc. 2017, 90, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Power, A.C.; Chapman, J.; Chandra, S.; Roberts, J.J.; Cozzolino, D. Illuminating the flesh of bone identification—An application of near infrared spectroscopy. Vib. Spectrosc. 2018, 98, 64–68. [Google Scholar] [CrossRef]
| PMI | 0–2 Weeks | 2 Weeks–6 Months | 6 Months–1 Year | 1 Year–10 Years | >100 Years |
|---|---|---|---|---|---|
| flat | 25 | 34 | 5 | 0 | 0 |
| flat bathtub | 1 | 0 | 0 | 0 | 0 |
| plane crash | 4 | 0 | 0 | 0 | 0 |
| drowned | 2 | 8 | 1 | 1 | 0 |
| forest | 0 | 3 | 3 | 7 | 0 |
| forest hut | 0 | 0 | 0 | 1 | 0 |
| mountain | 0 | 1 | 1 | 1 | 0 |
| soil | 0 | 0 | 0 | 0 | 1 |
| Σ | 32 | 46 | 11 | 10 | 5 |
| median age | 61 | 59 | 52.9 | 49 | n.a |
| mean age | 58.44 | 61.98 | 57.5 | 44.28 | n.a |
| STD age | 16.95 | 13.81 | 20.74 | 10.70 | n.a |
| not identified age | 0 | 0 | 1 | 3 | n.a |
| female | 3 | 9 | 2 | 1 | 1 |
| male | 29 | 39 | 9 | 9 | 2 |
| not identified sex | 0 | 0 | 0 | 0 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, V.M.; Zelger, P.; Wöss, C.; Huck, C.W.; Arora, R.; Bechtel, E.; Stahl, A.; Brunner, A.; Zelger, B.; Schirmer, M.; et al. Post-Mortem Interval of Human Skeletal Remains Estimated with Handheld NIR Spectrometry. Biology 2022, 11, 1020. https://doi.org/10.3390/biology11071020
Schmidt VM, Zelger P, Wöss C, Huck CW, Arora R, Bechtel E, Stahl A, Brunner A, Zelger B, Schirmer M, et al. Post-Mortem Interval of Human Skeletal Remains Estimated with Handheld NIR Spectrometry. Biology. 2022; 11(7):1020. https://doi.org/10.3390/biology11071020
Chicago/Turabian StyleSchmidt, Verena Maria, Philipp Zelger, Claudia Wöss, Christian Wolfgang Huck, Rohit Arora, Etienne Bechtel, Andreas Stahl, Andrea Brunner, Bettina Zelger, Michael Schirmer, and et al. 2022. "Post-Mortem Interval of Human Skeletal Remains Estimated with Handheld NIR Spectrometry" Biology 11, no. 7: 1020. https://doi.org/10.3390/biology11071020
APA StyleSchmidt, V. M., Zelger, P., Wöss, C., Huck, C. W., Arora, R., Bechtel, E., Stahl, A., Brunner, A., Zelger, B., Schirmer, M., Rabl, W., & Pallua, J. D. (2022). Post-Mortem Interval of Human Skeletal Remains Estimated with Handheld NIR Spectrometry. Biology, 11(7), 1020. https://doi.org/10.3390/biology11071020









