Deficiency in FTSJ1 Affects Neuronal Plasticity in the Hippocampal Formation of Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Open Field
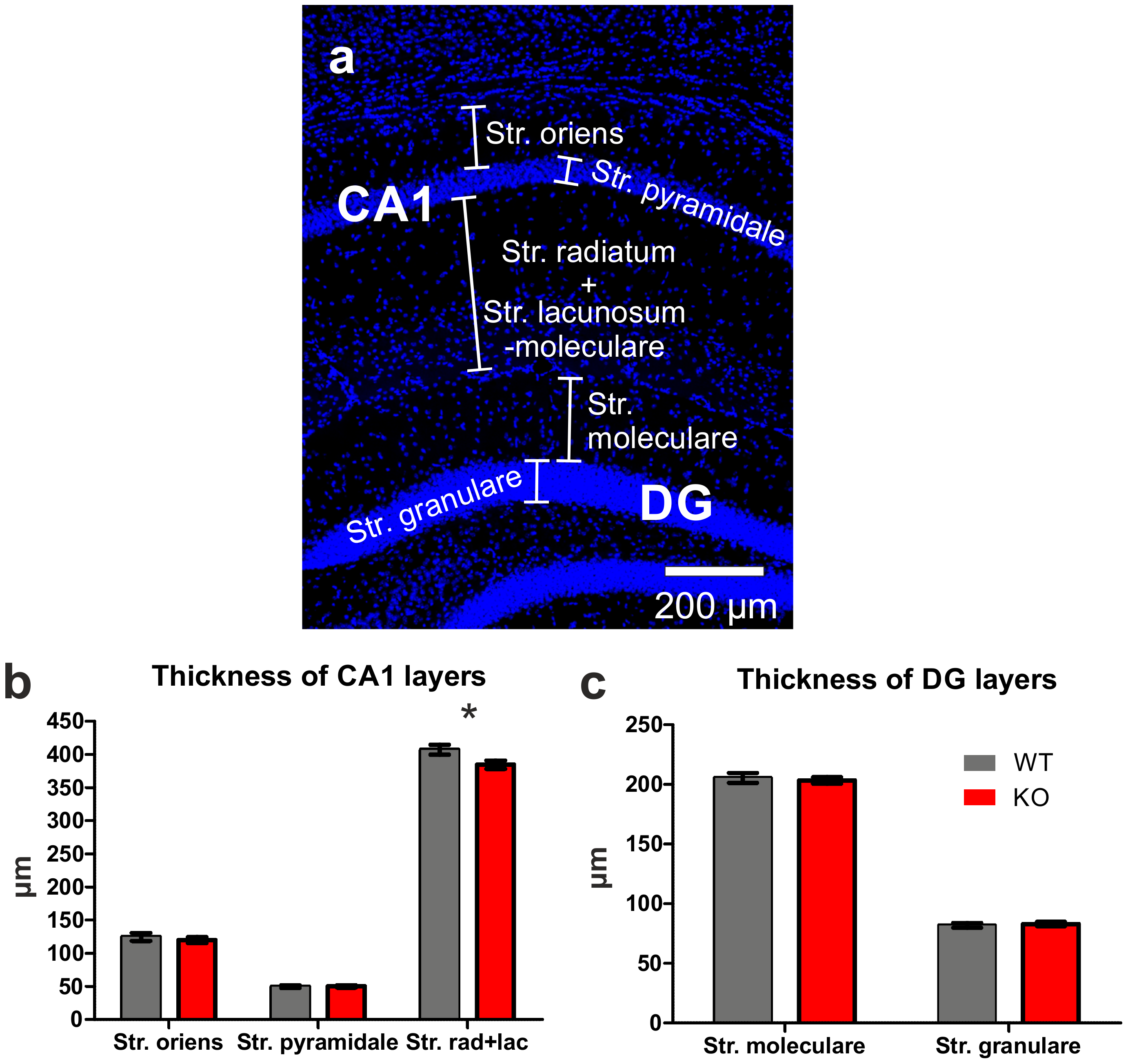
2.3. Layer Thicknesses in Area CA1 and the Dentate Gyrus (DG)
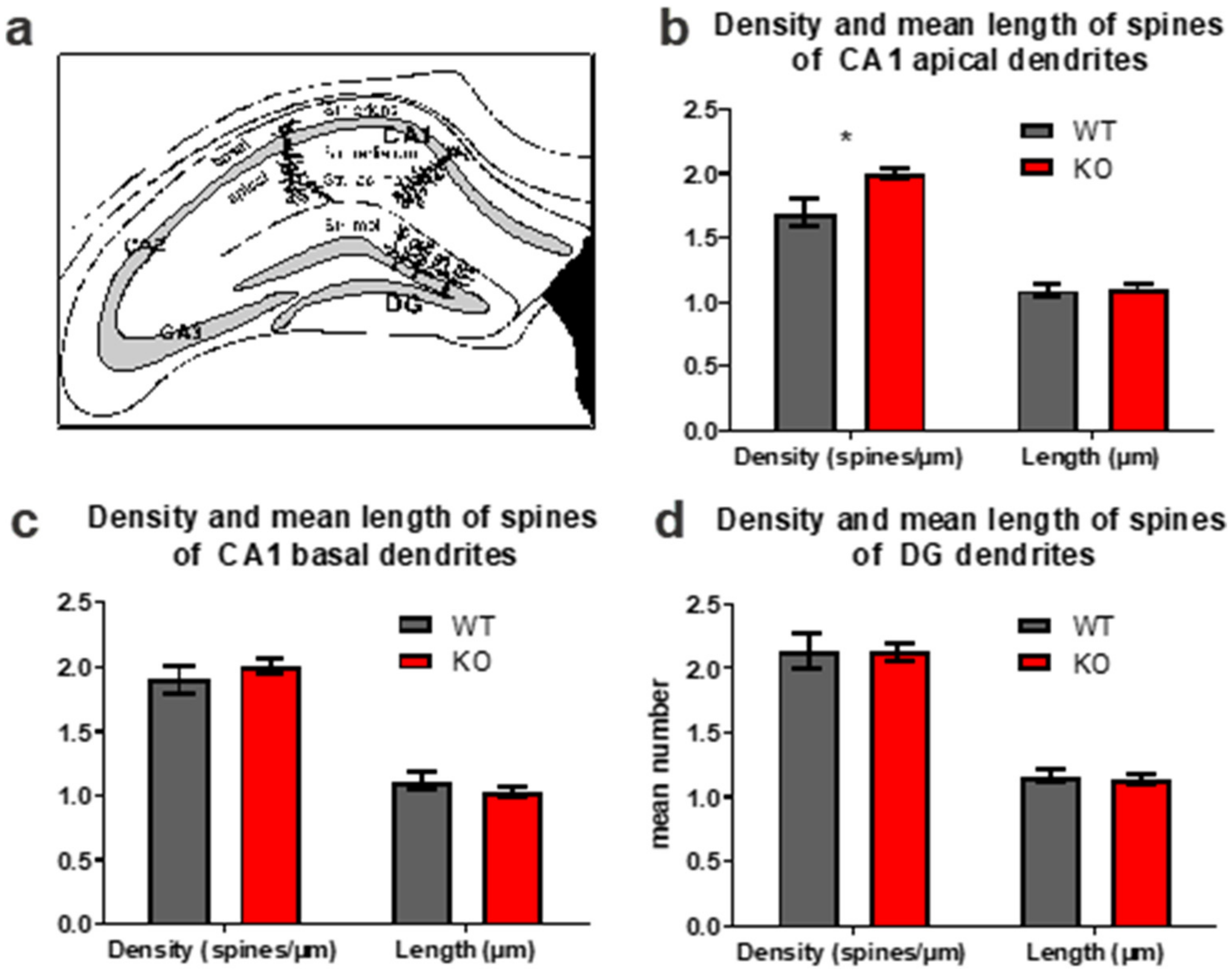
2.4. Golgi Impregnation and Analysis of Dendritic Spines
2.5. Electrophysiology
2.6. Mass Spectrometry (MS)
2.7. Statistical Analysis
3. Results
3.1. Open Field
3.2. Thickness of Layers in the Hippocampal Area CA1 and in the Dentate Gyrus (DG)
3.3. Dendritic Spines in the Hippocampal Area CA1 and in the DG
3.4. LTP within Hippocampal Area CA1
3.5. Mass Spectrometry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freude, K.; Hoffmann, K.; Jensen, L.-R.; Delatycki, M.B.; des Portes, V.; Moser, B.; Hamel, B.; van Bokhoven, H.; Moraine, C.; Fryns, J.-P.; et al. Mutations in the FTSJ1 Gene Coding for a Novel S-Adenosylmethionine–Binding Protein Cause Nonsyndromic X-Linked Mental Retardation. Am. J. Hum. Genet. 2004, 75, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Nagayoshi, Y.; Chujo, T.; Hirata, S.; Nakatsuka, H.; Chen, C.-W.; Takakura, M.; Miyauchi, K.; Ikeuchi, Y.; Carlyle, B.C.; Kitchen, R.R.; et al. Loss of Ftsj1 perturbs codon-specific translation efficiency in the brain and is associated with X-linked intellectual disability. Sci. Adv. 2021, 7, eabf3072. [Google Scholar] [CrossRef] [PubMed]
- Takano, K.; Nakagawa, E.; Inoue, K.; Kamada, F.; Kure, S.; Goto, Y.-I. Japanese Mental Retardation Consortium A loss-of-function mutation in theFTSJ1 gene causes nonsyndromic X-linked mental retardation in a japanese family. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2008, 147B, 479–484. [Google Scholar] [CrossRef]
- Guy, M.P.; Shaw, M.; Weiner, C.L.; Hobson, L.; Stark, Z.; Rose, K.; Kalscheuer, V.M.; Gecz, J.; Phizicky, E.M. Defects in tRNA Anticodon Loop 2′-O-Methylation Are Implicated in Nonsyndromic X-Linked Intellectual Disability due to Mutations in FTSJ1. Hum. Mutat. 2015, 36, 1176–1187. [Google Scholar] [CrossRef] [Green Version]
- Froyen, G.; Bauters, M.; Boyle, J.; Van Esch, H.; Govaerts, K.; van Bokhoven, H.; Ropers, H.H.; Moraine, C.; Chelly, J.; Fryns, J.P.; et al. Loss of SLC38A5 and FTSJ1 at Xp11.23 in three brothers with non-syndromic mental retardation due to a microdeletion in an unstable genomic region. Qual. Life Res. 2007, 121, 539–547. [Google Scholar] [CrossRef]
- Soderling, S.H.; Langeberg, L.K.; Soderling, J.A.; Davee, S.M.; Simerly, R.; Raber, J.; Scott, J.D. Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice. Proc. Natl. Acad. Sci. USA 2003, 100, 1723–1728. [Google Scholar] [CrossRef] [Green Version]
- Khelfaoui, M.; Denis, C.; Van Galen, E.; De Bock, F.; Schmitt, A.; Houbron, C.; Morice, E.; Giros, B.; Ramakers, G.; Fagni, L.; et al. Loss of X-Linked Mental Retardation Gene Oligophrenin1 in Mice Impairs Spatial Memory and Leads to Ventricular Enlargement and Dendritic Spine Immaturity. J. Neurosci. 2007, 27, 9439–9450. [Google Scholar] [CrossRef]
- Baker, K.B.; Wray, S.P.; Ritter, R.; Mason, S.; Lanthorn, T.H.; Savelieva, K. Male and female Fmr1 knockout mice on C57 albino background exhibit spatial learning and memory impairments. Genes Brain Behav. 2010, 9, 562–574. [Google Scholar] [CrossRef]
- Dahlhaus, R. Of Men and Mice: Modeling the Fragile X Syndrome. Front. Mol. Neurosci. 2018, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.R.; Garrett, L.; Hölter-Koch, S.; Rathkolb, B.; Rácz, I.; Adler, T.; Prehn, C.; Hans, W.; Rozman, J.; Becker, L.; et al. A mouse model for intellectual disability caused by mutations in the X-linked 2′-O-methyltransferase Ftsj1 gene. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 2083–2093. [Google Scholar] [CrossRef]
- Hammelrath, L.; Škokić, S.; Khmelinskii, A.; Hess, A.; van der Knaap, N.; Staring, M.; Lelieveldt, B.P.; Wiedermann, D.; Hoehn, M. Morphological maturation of the mouse brain: An in vivo MRI and histology investigation. NeuroImage 2016, 125, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Bohlen und Halbach, O.; Krause, S.; Medina, D.; Sciarretta, C.; Minichiello, L.; Unsicker, K. Regional- and age-dependent reduction in trkb receptor expression in the hippocampus is associated with altered spine morphologies. Biol. Psychiatry 2006, 59, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.V.P.; Lømo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 331–356. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.V.P.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Lømo, T. Discovering long-term potentiation (LTP)—Recollections and reflections on what came after. Acta Physiol. 2018, 222, e12921. [Google Scholar] [CrossRef]
- Malenka, R.C.; Nicoll, R.A. Long-term potentiation—A decade of progress? Science 1999, 285, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Baltaci, S.B.; Mogulkoc, R.; Baltaci, A.K. Molecular Mechanisms of Early and Late LTP. Neurochem. Res. 2019, 44, 281–296. [Google Scholar] [CrossRef]
- Geinisman, Y. Structural synaptic modifications associated with hippocampal LTP and behavioral learning. Cereb. Cortex 2000, 10, 952–962. [Google Scholar] [CrossRef] [Green Version]
- Muller, D.; Toni, N.; Buchs, P.A. Spine changes associated with long-term potentiation. Hippocampus 2000, 10, 596–604. [Google Scholar] [CrossRef]
- Toni, N.; Buchs, P.-A.; Nikonenko, I.; Bron, C.; Muller, D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 1999, 402, 421–425. [Google Scholar] [CrossRef]
- Moretti, P.; Levenson, J.M.; Battaglia, F.; Atkinson, R.; Teague, R.; Antalffy, B.; Armstrong, D.; Arancio, O.; Sweatt, J.D.; Zoghbi, H. Learning and Memory and Synaptic Plasticity Are Impaired in a Mouse Model of Rett Syndrome. J. Neurosci. 2006, 26, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Lauterborn, J.C.; Rex, C.S.; Kramár, E.; Chen, L.Y.; Pandyarajan, V.; Lynch, G.; Gall, C.M. Brain-Derived Neurotrophic Factor Rescues Synaptic Plasticity in a Mouse Model of Fragile X Syndrome. J. Neurosci. 2007, 27, 10685–10694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bracke, A.; Domanska, G.; Bracke, K.; Harzsch, S.; Brandt, J.V.D.; Bröker, B.; Halbach, O.V.B.U. Obesity Impairs Mobility and Adult Hippocampal Neurogenesis. J. Exp. Neurosci. 2019, 13, 1179069519883580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busch, R.; Baldus, M.; Vogt, M.A.; Berger, S.M.; Bartsch, D.; Gass, P.; Halbach, O.V.B.U. Effects of p75NTR deficiency on cholinergic innervation of the amygdala and anxiety-like behavior. J. Neurochem. 2017, 141, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Sielaff, M.; Kuharev, J.; Bohn, T.; Hahlbrock, J.; Bopp, T.; Tenzer, S.; Distler, U. Evaluation of FASP, SP3, and iST Protocols for Proteomic Sample Preparation in the Low Microgram Range. J. Proteome Res. 2017, 16, 4060–4072. [Google Scholar] [CrossRef]
- Hughes, C.S.; Foehr, S.; Garfield, D.A.; Furlong, E.E.; Steinmetz, L.M.; Krijgsveld, J. Ultrasensitive proteome analysis using paramagnetic bead technology. Mol. Syst. Biol. 2014, 10, 757. [Google Scholar] [CrossRef]
- Blankenburg, S.; Hentschker, C.; Nagel, A.; Hildebrandt, P.; Michalik, S.; Dittmar, D.; Surmann, K.; Völker, U. Improving Proteome Coverage for Small Sample Amounts: An Advanced Method for Proteomics Approaches with Low Bacterial Cell Numbers. Proteomics 2019, 19, e1900192. [Google Scholar] [CrossRef] [Green Version]
- Meyer, D.J.; Bijlani, S.; de Sautu, M.; Spontarelli, K.; Young, V.C.; Gatto, C.; Artigas, P. FXYD protein isoforms differentially modulate human Na/K pump function. J. Gen. Physiol. 2020, 152, e202012660. [Google Scholar] [CrossRef]
- Zhao, W.-Q.; Waisman, D.; Grimaldi, M. Specific localization of the annexin II heterotetramer in brain lipid raft fractions and its changes in spatial learning. J. Neurochem. 2004, 90, 609–620. [Google Scholar] [CrossRef]
- Ji, J.; Maren, S. Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learn. Mem. 2008, 15, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Nicoll, R.A. A Brief History of Long-Term Potentiation. Neuron 2017, 93, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holscher, C. Synaptic plasticity and learning and memory: Ltp and beyond. J. Neurosci. Res. 1999, 58, 62–75. [Google Scholar] [CrossRef]
- Nkomozepi, P.; Mazengenya, P.; Ihunwo, A.O. Age-related changes in Ki-67 and DCX expression in the BALB/c mouse (Mus Musculus) brain. Int. J. Dev. Neurosci. 2019, 72, 36–47. [Google Scholar] [CrossRef]
- Choudhury, K.; McQuillin, A.; Puri, V.; Pimm, J.; Datta, S.; Thirumalai, S.; Krasucki, R.; Lawrence, J.; Bass, N.J.; Quested, D.; et al. A Genetic Association Study of Chromosome 11q22-24 in Two Different Samples Implicates the FXYD6 Gene, Encoding Phosphohippolin, in Susceptibility to Schizophrenia. Am. J. Hum. Genet. 2007, 80, 664–672. [Google Scholar] [CrossRef] [Green Version]
- Chaumette, B.; Ferrafiat, V.; Ambalavanan, A.; Goldenberg, A.; Dionne-Laporte, A.; Spiegelman, D.; Dion, P.A.; Gerardin, P.; Laurent, C.; Cohen, D.; et al. Missense variants in ATP1A3 and FXYD gene family are associated with childhood-onset schizophrenia. Mol. Psychiatry 2020, 25, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Biesemann, C.; Gronborg, M.; Luquet, E.; Wichert, S.P.; Bernard, V.; Bungers, S.R.; Cooper, B.; Varoqueaux, F.; Li, L.; Byrne, J.A.; et al. Proteomic screening of glutamatergic mouse brain synaptosomes isolated by fluorescence activated sorting. EMBO J. 2014, 33, 157–170. [Google Scholar] [CrossRef]
- Glushchenko, T.S.; Izvarina, N.L. Na+, K+-ATPase activity in neurons and glial cells of the olfactory cortex of the rat brain during the development of long-term potentiation. Neurosci. Behav. Physiol. 1997, 27, 49–52. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Nakanishi, H.; Kimura, K.; Matsubara, K.; Ozaki-Kuroda, K.; Katata, T.; Honda, T.; Kiyohara, Y.; Heo, K.; Higashi, M.; et al. Nectin: An adhesion molecule involved in formation of synapses. J. Cell Biol. 2002, 156, 555–565. [Google Scholar] [CrossRef]
- Geng, X.; Mandai, K.; Maruo, T.; Wang, S.; Fujiwara, T.; Mizoguchi, A.; Takai, Y.; Mori, M. Regulatory role of the cell adhesion molecule nectin-1 in GABAergic inhibitory synaptic transmission in the CA3 region of mouse hippocampus. Genes Cells 2016, 21, 88–98. [Google Scholar] [CrossRef] [Green Version]
- VanGuilder, H.D.; Bixler, G.V.; Sonntag, W.; Freeman, W.M. Hippocampal expression of myelin-associated inhibitors is induced with age-related cognitive decline and correlates with deficits of spatial learning and memory. J. Neurochem. 2012, 121, 77–98. [Google Scholar] [CrossRef]
- Long, K.L.P.; Chao, L.L.; Kazama, Y.; An, A.; Hu, K.Y.; Peretz, L.; Muller, D.C.Y.; Roan, V.D.; Misra, R.; Toth, C.E.; et al. Regional gray matter oligodendrocyte- and myelin-related measures are associated with differential susceptibility to stress-induced behavior in rats and humans. Transl. Psychiatry 2021, 11, 631. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, L.I.; Perrone-Bizzozero, N.I.; Neve, R.L.; Rodriguez, W. Chapter 26 GAP-43 as a marker for structural plasticity in the mature CNS. Prog. Brain Res. 1990, 86, 309–320. [Google Scholar] [CrossRef]
- Strata, P.; Buffo, A.; Rossi, F. Mechanisms of axonal plasticity. Arch. Ital. Biol. 1999, 137, 181–192. [Google Scholar] [PubMed]
- Korte, M.; Carroll, P.; Wolf, E.; Brem, G.; Thoenen, H.; Bonhoeffer, T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. USA 1995, 92, 8856–8860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Smith, G.M.; Chen, J. Impaired dendritic development and synaptic formation of postnatal-born dentate gyrus granular neurons in the absence of brain-derived neurotrophic factor signaling. Exp. Neurol. 2009, 215, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-G.; Son, H. Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep. 2009, 42, 239–244. [Google Scholar] [CrossRef] [Green Version]



| Categories | Diseases or Functions Annotation | p-Value | Molecules p < 0.05; FC 1.3 | Molecules p < 0.05 |
|---|---|---|---|---|
| Neurological Disease | Hypomyelination of axons | 7.84 × 10−5 | MAG, MBP | MAG, MBP |
| Neurological Disease, Organismal Injury and Abnormalities | Progressive encephalopathy | 3.70 × 10−3 | ANXA2, GAP43, MAG, MBP | ALDH1L1, ANXA2, ATP5F1A, ATP8A1, ENO2, FTH1, GAK, GAP43, GNAS, MAG, MBP, NAE1, TUBA1A |
| Cellular Development, Cellular Growth and Proliferation, Nervous System Development and Function, Tissue Development | Outgrowth of neurites | 4.85 × 10−3 | GAP43, MAG, RAB22A, VCAM1 | BASP1, GAP43, GNAS, HRAS, MAG, PLXNA4, PRKACA, RAB22A, SLC25A5, SRC, SYN1, TUBA1A, VCAM1 |
| Cell Morphology, Cellular Assembly and Organization | Elongation of cellular protrusions | 8.21 × 10−3 | GAP43, MAG | ALCAM, GAP43, GNAS, MAG |
| Cell Death and Survival, Neurological Disease, Organismal Injury and Abnormalities | Cell death of hippocampal neurons | 1.03 × 10−3 | ATP2C1, VPS33A | ATP2C1, CAT, GSK3A, KSR1, NAE1, PLXNA4, SLC25A12, SRC, VPS33A |
| Neurological Disease, Skeletal and Muscular Disorders | Neuromuscular disease | 2.58 × 10−2 | ANXA2, GAP43, MBP, NDUFB9 | ALCAM, ANXA2, AP1S1, ATP5F1A, ATP5F1B, BASP1, ENO2, FKBP4, FTH1, GAP43, GNAS, MAP2K4, MBP, NDUFB9, NDUFS6, Tmsb4x, TUBA1A, UQCRC2 |
| Nervous System Development and Function, Neurological Disease | Abnormal morphology of central nervous system | 2.59 × 10−2 | CTSB, GAP43, MAG, MBP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Bohlen und Halbach, V.; Venz, S.; Nwakor, S.; Hentschker, C.; Hammer, E.; Junker, H.; Kuss, A.W.; von Bohlen und Halbach, O.; Jensen, L.R. Deficiency in FTSJ1 Affects Neuronal Plasticity in the Hippocampal Formation of Mice. Biology 2022, 11, 1011. https://doi.org/10.3390/biology11071011
von Bohlen und Halbach V, Venz S, Nwakor S, Hentschker C, Hammer E, Junker H, Kuss AW, von Bohlen und Halbach O, Jensen LR. Deficiency in FTSJ1 Affects Neuronal Plasticity in the Hippocampal Formation of Mice. Biology. 2022; 11(7):1011. https://doi.org/10.3390/biology11071011
Chicago/Turabian Stylevon Bohlen und Halbach, Viola, Simone Venz, Simon Nwakor, Christian Hentschker, Elke Hammer, Heike Junker, Andreas W. Kuss, Oliver von Bohlen und Halbach, and Lars R. Jensen. 2022. "Deficiency in FTSJ1 Affects Neuronal Plasticity in the Hippocampal Formation of Mice" Biology 11, no. 7: 1011. https://doi.org/10.3390/biology11071011
APA Stylevon Bohlen und Halbach, V., Venz, S., Nwakor, S., Hentschker, C., Hammer, E., Junker, H., Kuss, A. W., von Bohlen und Halbach, O., & Jensen, L. R. (2022). Deficiency in FTSJ1 Affects Neuronal Plasticity in the Hippocampal Formation of Mice. Biology, 11(7), 1011. https://doi.org/10.3390/biology11071011







