The Status of Posidonia oceanica at Tremiti Islands Marine Protected Area (Adriatic Sea)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Meadow Density and Coverage
3.2. Phenological and Lepidochronological Analysis
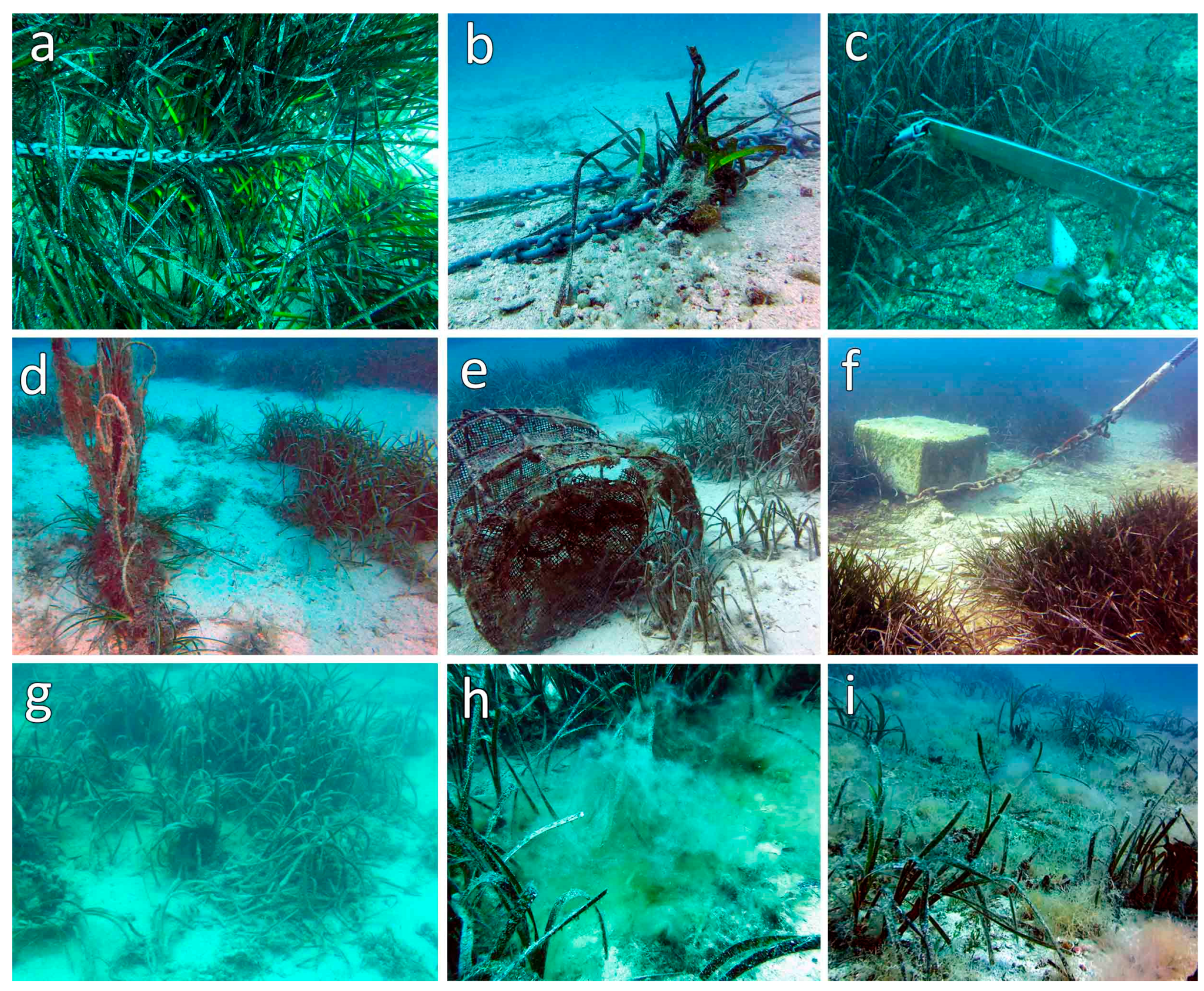
3.3. Expansion/Regression of P. oceanica and Anthropogenic Stressors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawton, J.H. What do species do in ecosystems? Oikos 1994, 71, 367–374. [Google Scholar] [CrossRef]
- Ingrosso, G.; Abbiati, M.; Badalamenti, F.; Bavestrello, G.; Belmonte, G.; Cannas, R.; Benedetti-Cecchi, L.; Bertolino, M.; Bevilacqua, S.; Bianchi, C.N.; et al. Mediterranean Bioconstructions Along the Italian Coast. Adv. Mar. Biol. 2018, 79, 61–136. [Google Scholar]
- Buia, M.C.; Gambi, M.C.; Zupo, V. Structure and functioning of Mediterranean seagrass ecosystems: An overview. Biol. Mar. Mediterr. 2000, 7, 167–190. [Google Scholar]
- Buia, M.C.; Gambi, M.C.; Dappiano, M. The seagrass systems. Biol. Mar. Mediterr. 2004, 11, 133–184. [Google Scholar]
- Mazzella, L.; Scipione, M.B.; Buia, M.C. Spatio-temporal distribution of algal and animal communities in a Posidonia oceanica (L.) Delile meadow. Mar. Ecol. 1989, 10, 107–129. [Google Scholar] [CrossRef]
- Gambi, M.C.; Lorenti, M.; Russo, G.F.; Scipione, M.B. Depth and seasonal distribution of some groups of the vagile fauna of the Posidonia oceanica leaf stratum: Structural and trophic analyses. Mar. Ecol. 1992, 13, 17–39. [Google Scholar] [CrossRef]
- Francour, P. Fish assemblages of Posidonia oceanica beds at Port-Cros (France, NW Mediterranean): Assessment of composition and long-term fluctuations by visual census. Mar. Ecol. 1997, 18, 157–173. [Google Scholar] [CrossRef]
- Pergent-Martini, C.; Pergent, G.; Monnier, B.; Boudouresque, C.F.; Mori, C.; Valette-Sansevin, A. Contribution of Posidonia oceanica meadows in the context of climate change mitigation in the Mediterranean Sea. Mar. Environ. 2021, 165, 10523. [Google Scholar] [CrossRef]
- Bay, D. Etude in situ de la production primaire d’un herbier de Posidonies, (Posidonia oceanica (L.) Delile) de la baie de Calvi-Corse. Progr. Rép. Stn. Océanogr. Stareso Univ. Liège Belg. 1978, 18, 1–251. [Google Scholar]
- Boudouresque, C.F.; Meinesz, A. Decouverte de l’herbier de Posidonie. Car. Parc. Natn. Port-Cros 1982, 4, 79. [Google Scholar]
- Dauby, P.; Bale, A.J.; Bloomer, N.; Canon, C.; Ling, R.D.; Norro, A.; Robertson, J.E.; Simon, A.; Théate, J.M.; Watson, A.J.; et al. Particle fluxes over a Mediterranean seagrass bed: A one-year sediment trap experiment. Mar. Ecol. Prog. Ser. 1995, 126, 233–246. [Google Scholar] [CrossRef]
- Chessa, L.A.; Fustier, V.; Fernandez, C.; Mura, F.; Pais, A.; Pergent, G.; Serra, S.; Vitale, L. Contribution to the knowledge of ‘banquettes’ of Posidonia oceanica (L.) Delile in Sardinia Island. Biol. Mar. Mediterr. 2000, 7, 35–38. [Google Scholar]
- Mateo, M.A.; Sanchez-Lizaso, J.L.; Romero, J. Posidonia oceanica “banquettes”: A preliminary assessment for an ecosystem carbon and nutrient budget. Mar. Ecol. Prog. Ser. 2003, 151, 43–45. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Berand, G.; Bonhomme, P.; Charbonnell, E.; Diviacco, G.; Meinesz, A.; Pergent, G.; Pergent-Martini, C.; Ruitton, S.; Tunesi, L. Préservation Et Conservation Des Herbiers A Posidonia Oceanica; Ramoge Pub, 2006; pp. 1–202. ISBN 2-905540-30-3. [Google Scholar]
- Guala, I.; Simeone, S.; Buia, M.C.; Flagella, S.; Baroli, M.; De Falco, G. Posidonia oceanica ‘banquette’ removal: Environmental impact and management implications. Biol. Mar. Mediterr. 2006, 13, 149–153. [Google Scholar]
- Fonseca, M.S.; Koehl, M.A.R.; Kopp, B.S. Biomechanical factors contributing to self-organization in seagrass landscapes. J. Exp. Mar. Biol. Ecol. 2007, 340, 227–246. [Google Scholar] [CrossRef]
- Blanc, J.J.; Jeudy De Grissac, A. Réflexion Géologique sur la Régression des Herbiers à Posidonies (Départements du Var et des Bouches-du-Rhône). In Second International Workshop on Posidonia Beds; Boudouresque, C.F., Meinesz, A., Fresi, E., Gravez, V., Eds.; GIS Posidonie: Marseille, France, 1989; Volume 2, pp. 273–285. [Google Scholar]
- Leriche, A.; Pasqualini, V.; Boudouresque, C.F.; Bernard, G.; Bonhomme, P.; Clabaut, P.; Denis, J. Spatial, temporal and structural variations of a Posidonia oceanica seagrass meadow facing human activities. Aquat. Bot. 2006, 84, 287–293. [Google Scholar] [CrossRef]
- Montefalcone, M.; Parravicini, V.; Vacchi, M.; Albertelli, G.; Morri, C.; Nike Bianchi, C. Human influence on seagrass habitat fragmentation in NW Mediterranean Sea. Estuar. Coast. Shelf Sci. 2010, 86, 292–298. [Google Scholar] [CrossRef]
- Telesca, L.; Belluscio, A.; Criscoli, A.; Ardizzone, G.; Apostolaki, E.T.; Fraschetti, S.; Gristina, M.; Knittweis, L.; Martin, C.S.; Pergent, G.; et al. Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Sci. Rep. 2015, 5, 12505. [Google Scholar] [CrossRef] [PubMed]
- De los Santos, C.B.; Krause-Jensen, D.; Alcoverro, T.; Marbà, N.; Duarte, C.M.; van Katwijk, M.M.; Pérez, M.; Romero, J.; Sánchez-Lizaso, J.L.; Roca, G.; et al. Recent trend reversal for declining European seagrass meadows. Nat. Commun. 2019, 10, 3356. [Google Scholar] [CrossRef]
- Ardizzone, G.D.; Pelusi, P. Yield and damage evaluation of bottom trawling on Posidonia meadows. In Proceedings of the First International Workshop on Posidonia Oceanica Beds, Porquerolles, France, 12–15 October 1983. [Google Scholar]
- Ceccherelli, G.; Campo, D. Valutazione sperimentale dell’effetto dell’ancoraggio su Posidonia oceanica L. (Delile). Biol. Mar. Mediterr. 2002, 9, 672–673. [Google Scholar]
- Ceccherelli, G.; Campo, D.; Milazzo, M. Short-term response of the slow growing seagrass Posidonia oceanica to simulated anchor impact. Mar. Environ. 2007, 63, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Montefalcone, M.; Chiantore, M.; Lanzone, A.; Morri, C.; Bianchi, C.N.; Albertelli, G. BACI design reveals the decline of the seagrass Posidonia oceanica induced by anchoring. Mar. Pollut. 2008, 56, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Panayotidis, P.; Makris, P.; Catsiki, V.A. Cycle de bioaccumulation du Cu, Cd, et Cr dans les écailles de Posidonia oceanica. Rapp. Comm. Int. Mer Medit. 1990, 32, 13. [Google Scholar]
- Pazzaglia, J.; Santillán-Sarmiento, A.; Helber, S.B.; Ruocco, M.; Terlizzi, A.; Marín-Guirao, L.; Procaccini, G. Does Warming Enhance the Effects of Eutrophication in the Seagrass Posidonia oceanica? Front. Mar. Sci. 2020, 7, 1067. [Google Scholar] [CrossRef]
- Piazzi, L.; Ceccherelli, G.; Cinelli, F. Threat to macroalgal diversity: Effects of the introduced green alga Caulerpa racemosa in the Mediterranean. Mar. Ecol. Prog. Ser. 2001, 210, 149–159. [Google Scholar] [CrossRef]
- Duarte, C.M. The future of seagrass meadow. Environ. Conserv. 2003, 29, 192–196. [Google Scholar] [CrossRef]
- Marín-Guirao, L.; Entrambasaguas, L.; Ruiz, J.M.; Procaccini, G. Heat-stress induced flowering can be a potential adaptive response to ocean warming for the iconic seagrass Posidonia oceanica. Mol. Ecol. 2019, 28, 2486–2501. [Google Scholar] [CrossRef]
- Johnson, E.A.; Williams, S.L. Sexual reproduction in seagrasses: Reports for five Caribbean species with details for Halodule wrightii Ascherson and Syringodium filiforme, Kutz. Carib. J. Sci. 1982, 18, 61–70. [Google Scholar]
- Gallegos, M.; Merino, M.; Marbà, N.; Duarte, C.M. Flowering of Thalassia testudinum Banks ex König in the Mexican Caribbean: Age-dependence and interannual variability. Aquat. Bot. 1992, 43, 249–255. [Google Scholar] [CrossRef]
- Marbà, N.; Walker, D.I. Growth, flowering, and population dynamics of temperate Western Australian seagrasses. Mar. Ecol. Progr. Ser. 1999, 184, 105–118. [Google Scholar] [CrossRef][Green Version]
- Ruiz, J.M.; Marín-Guirao, L.; García-Muñoz, R.; Ramos-Segura, A.; Bernardeau-Esteller, J.; Pérez, M.; Procaccini, G. Experimental evidence of warming-induced flowering in the Mediterranean seagrass Posidonia oceanica. Mar. Pollut. 2018, 134, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Procaccini, G.; Ruggiero, M.V.; Orsini, L. Genetic structure and distribution of microsatellite diversity in Posidonia oceanica over the whole Mediterranean basin. Bull. Mar. Sci. 2002, 71, 1291–1297. [Google Scholar]
- Guillén, J.E.; Sánchez Lizaso, J.L.; Jiménez, S.; Martínez, J.; Codina, A.; Montero, M.; Triviño, A.; Soler, G.; Zubcoff, J.J. Evolution of Posidonia oceanica seagrass meadows and its implications for management. J. Sea Res. 2013, 83, 65–71. [Google Scholar] [CrossRef]
- Abadie, A.; Pace, M.; Gobert, S.; Borg, J.A. Seascape ecology in Posidonia oceanica seagrass meadows: Linking structure and ecological processes for management. Ecol. Indic. 2018, 87, 1–13. [Google Scholar] [CrossRef]
- Gobert, S.; Sartoretto, S.; Rico-Raimondino, V.; Andral, B.; Chery, A.; Lejeune, P.; Boissery, P. Assessment of the ecological status of Mediterranean French coastal waters as required by the Water Framework Directive using the Posidonia oceanica Rapid Easy Index: PREI. Mar. Pollut. Bull. 2009, 58, 1727–1733. [Google Scholar] [CrossRef]
- Boero, F.; Foglini, F.; Fraschetti, S.; Goriup, P.; Macpherson, E.; Planes, S.; Soukissian, T.; The CoCoNet Consortium. CoCoNet: Towards coast to coast networks of marine protected areas (from the shore to the high and deep sea), coupled with sea-based wind energy potential. Sci. Res. 2016, 6, 95. [Google Scholar]
- Ruiz, J.M.; Romero, J. Effects of disturbances caused by coastal constructions on spatial structure, growth dynamics and photosynthesis of the seagrass Posidonia oceanica. Mar. Pollut. Bull. 2003, 46, 1523–1533. [Google Scholar] [CrossRef]
- Balestri, E.; Benedetti-Cecchi, L.; Lardicci, C. Variability in patterns of growth and morphology of Posidonia oceanica meadows exposed to urban and industrial waste: Contrasts with two reference locations. J. Exp. Mar. Biol. Ecol. 2004, 308, 1–21. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Bernard, G.; Pergent, G.; Shili, A.; Verlaque, M. Regression of Mediterranean seagrasses caused by natural processes and anthropogenic disturbances and stress: A critical review. Bot. Mar. 2009, 52, 395–418. [Google Scholar] [CrossRef]
- Martínez-Crego, B.; Vergés, A.; Alcoverro, T.; Romero, J. Selection of multiple seagrass indicators for environmental biomonitoring. Mar. Ecol. Prog. Ser. 2008, 361, 93–109. [Google Scholar] [CrossRef]
- Montefalcone, M.; Albertelli, G.; Morri, C.; Parravicini, V.; Bianchi, C.N. Legal protection is not enough: Posidonia oceanica meadows in marine protected areas are not healthier than those in unprotected areas of the northwest Mediterranean Sea. Mar. Pollut. Bull. 2009, 58, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Murillo, F.; Jimenez-Gutierrez, S.; Martínez-Vidal, J.; Guillén, J.E.; Sánchez-Lizaso, J.L. Spatiotemporal Trends Observed in 20 Years of Posidonia oceanica Monitoring along the Alicante Coast, Spain. Water 2022, 14, 274. [Google Scholar] [CrossRef]
- Giraud, G. Contribution à la Description et à la Phénologie Quantitative des Herbiers de Posidonia oceanica (L.) Delile. Ph.D. Thesis, University Aix-Marseille II, Marseille, France, 1977. [Google Scholar]
- Pergent, G.; Pergent-Martini, C.; Bouderesque, C.F. Utilisation de l’herbier a Posidonia oceanica comme indicateur biologique de la qualité du milieu littoral en méditerranée: État des connaissances. Mésogée 1995, 54, 3–27. [Google Scholar]
- Pergent, G.; Pergent-Martini, C.; Casalta, B.; Lopez, Y.; Royo, C.; Mimault, B.; Salivas-Decaux, M.; Short, F. Comparison of three seagrass monitoring systems: SeagrassNet, “Posidonia” programme and RSP. In Proceedings of the Third Mediterranean Symposium on Marine Vegetation, Marseilles, France, 2–29 March 2007; pp. 141–150. [Google Scholar]
- Romero, J.; Martínez-Crego, B.; Alcoverro, T.; Pérez, M. A multivariate index based on the seagrass Posidonia oceanica (POMI) to assess ecological status of coastal waters under the water framework directive (WFD). Mar. Pollut. Bull. 2007, 55, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Torquemada, Y.; Díaz-Valdés, M.; Colilla, F.; Luna, B.; Sánchez-Lizaso, J.L.; Ramos-Esplá, A.A. Descriptors from Posidonia oceanica (L.) Delile meadows in coastal waters of Valencia, Spain, in the context of the EU Water Framework Directive. J. Mar. Sci. 2008, 65, 1492–1497. [Google Scholar] [CrossRef]
- Lopez y Royo, C.; Casazza, G.; Pergent-Martini, C.; Pergent, G. A biotic index using the seagrass Posidonia oceanica (BiPo) to evaluate ecological status of coastal waters. Ecol. Ind. 2010, 10, 380–389. [Google Scholar] [CrossRef]
- CAR/ASP Publ. UNEP-MAP-RAC/SPA. Action Plan for the Conservation of Marine Vegetation in the Mediterranean Sea; CAR/ASP, Ed.; SPA/RAC: Tunis, Tunisia, 1999; p. 47. [Google Scholar]
- Güreşen, A.; Pergent, G.; Güreşen, S.O.; Aktan, Y. Evaluating the coastal ecosystem status of two Western and Eastern Mediterranean islands using the seagrass Posidonia oceanica. Ecol. Ind. 2020, 108, 105734. [Google Scholar] [CrossRef]
- Costantino, G.; Mastrototaro, F.; Tursi, A.; Torchia, G.; Pititto, F.; Salerno, G.; Lembo, G.; Sion, L.; D’Onghia, G.; Carlucci, R.; et al. Distribution and Bio-Ecological Features of Posidonia oceanica meadows along the coasts of the southern Adriatic and northern Ionian seas. Chem. Ecol. 2010, 26, 91–104. [Google Scholar] [CrossRef]
- Chimienti, G.; Stithou, M.; Dalle Mura, I.; Mastrototaro, F.; D’Onghia, G.; Tursi, A.; Izzi, C.; Fraschetti, S. An explorative assessment of the importance of Mediterranean Coralligenous habitat to local economy: The case of recreational diving. J. Environ. Account. Manag. 2017, 5, 315–325. [Google Scholar] [CrossRef]
- Chimienti, G.; De Padova, D.; Mossa, M.; Mastrototaro, F. A mesophotic black coral forest in the Adriatic Sea. Sci. Rep. 2020, 10, 8504. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Mastrototaro, F.; Montesanto, F.; Chimienti, G. Monitoring the seagrass Posidonia oceanica to understand the effects of local disturbances in a marine protected area. In Proceedings of the 2021 IEEE International Workshop on Metrology for the Sea (MetroSea), Virtual Conference, 4–6 October 2021. [Google Scholar]
- Chimienti, G.; Mastrototaro, F.; D’Onghia, G. Mesophotic and Deep-Sea Vulnerable Coral Habitats of the Mediterranean Sea: Overview and Conservation Perspectives. In Advances in the Studies of the Benthic Zone; Soto, L.A., Ed.; IntechOpen: London, UK, 2019; pp. 1–20. [Google Scholar]
- Matarrese, A.; Panza, M.; Mastrototaro, F.; Costantino, G. Preliminary benthic charting of Tremiti Islands (Adriatic Sea). Biol. Mar. Med. 2000, 7, 590–593. [Google Scholar]
- Pergent, G.; Boudouresque, C.F.; Crouzet, A. Variation cycliques dans les écailles des rhizome orthotrope de Posidonia oceanica. Tra Sci. Parc Nation. Port-Cros Fr. 1983, 9, 107–148. [Google Scholar]
- Boudouresque, C.F.; Jeudy De Grissac, A.; Meinesz, A. Relations entre la sédimentation et l’allongement des rhizomes orthotropes de Posidonia oceanica dans la baie d’Elbu (Corse). In Proceedings of the International Workshop on Posidonia oceanica Beds, Porquerolles, France, 12–15 October 1983; Volume 1, pp. 185–191. [Google Scholar]
- Pergent, G.; Boudouresque, C.F.; Crouzet, A.; Meinesz, A. Cyclic changes along Posidonia oceanica rhizomes (lepidochronology): Present state and perspectives. Mar. Ecol. 1989, 10, 221–230. [Google Scholar] [CrossRef]
- Pergent, G. Recherches Lepidochronologiques Chez Posidonia oceanica (Potamogetonaceae). Fluctuations des Paramètres Anatomiques et Morphologiques des Écailles des Rhizomes. Ph.D. Thesis, University Aix-Marseille II, Marseille, France, 1987. [Google Scholar]
- Bertrandy, M.C.; Boudouresque, C.F.; Foret, P.; Lefevre, J.R.; Meinesz, A. Réseau De Surveillance Posidonies. Rapport. 1985. Conseille Reg. Paca, Gis Posidonie. Cipalm, Capvar, Celcop, Gis Posidonie Ed., Marseille, Fr. 1986, pp. 1–61. Available online: https://gisposidonie.osupytheas.fr (accessed on 6 March 2022).
- CoNISMa. Risivisitazione di Alcune Praterie di Posidonia oceanica (L.) Délile lungo le Coste Delle Regioni: Liguria, Toscana, Lazio, Basilicata e Puglia e Progetto Pilota per L’armonizzazione dei Relativi Dati Cartografici Esistenti; CoNISMa: Rome, Italy, 2003; p. 72. [Google Scholar]
- CoNISMa. Monitoraggio delle Praterie di Posidonia Oceanica Presso l’AMP Isole Tremiti; CoNISMa: Rome, Italy, 2020; p. 152. [Google Scholar]
- Chimienti, G.; De Padova, D.; Adamo, M.; Mossa, M.; Bottalico, A.; Lisco, A.; Ungaro, N.; Mastrototaro, F. Effects of global warming on Mediterranean coral forests. Sci. Rep. 2021, 11, 20703. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, E.; Cebrián, E.; Alcoverro, T. Mortality of shoots of Posidonia oceanica following meadow invasion by red alga Lophocladia lallemandii. Bot. Mar. 2007, 50, 8–13. [Google Scholar] [CrossRef]
- Cushman-Roisin, B.; Gacic, M.; Poulain, P.M.; Artegiani, A. Physical Oceanography of the Adriatic Sea: Past, Present and Future; Springer: Dordrecht, The Netherlands, 2001; pp. 1–304. [Google Scholar]
- Millot, C.; Taupier-Letage, I. Circulation in the Mediterranean Sea. In The Mediterranean Sea. Handbook of Environmental Chemistry; Sailot, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 5, pp. 29–66. [Google Scholar]
- Chimienti, G.; Rizzo, L.; Kaleb, S.; Falace, A.; Fraschetti, S.; Giosa, F.D.; Tursi, A.; Barbone, E.; Ungaro, N.; Mastrototaro, F. Rhodolith Beds Heterogeneity along the Apulian Continental Shelf (Mediterranean Sea). J. Mar. Sci. Eng. 2020, 8, 813. [Google Scholar] [CrossRef]
- Garrabou, J.; Gómez-Gras, D.; Ledoux, J.-B.; Linares, C.; Bensoussan, N.; López-Sendino, P.; Bazairi, H.; Espinosa, F.; Ramdani, M.; Grimes, S.; et al. Collaborative Database to Track Mass Mortality Events in the Mediterranean Sea. Front. Mar. Sci. 2019, 6, 707. [Google Scholar] [CrossRef]
- González-Correa, J.; Bayle Sempere, J.; Sánchez-Jerez, P.; Valle, C. Posidonia oceanica meadows are not declining globally. Analysis of population dynamics in marine protected areas of the Mediterranean Sea. Mar. Ecol. Prog. Ser. 2007, 336, 111–119. [Google Scholar]
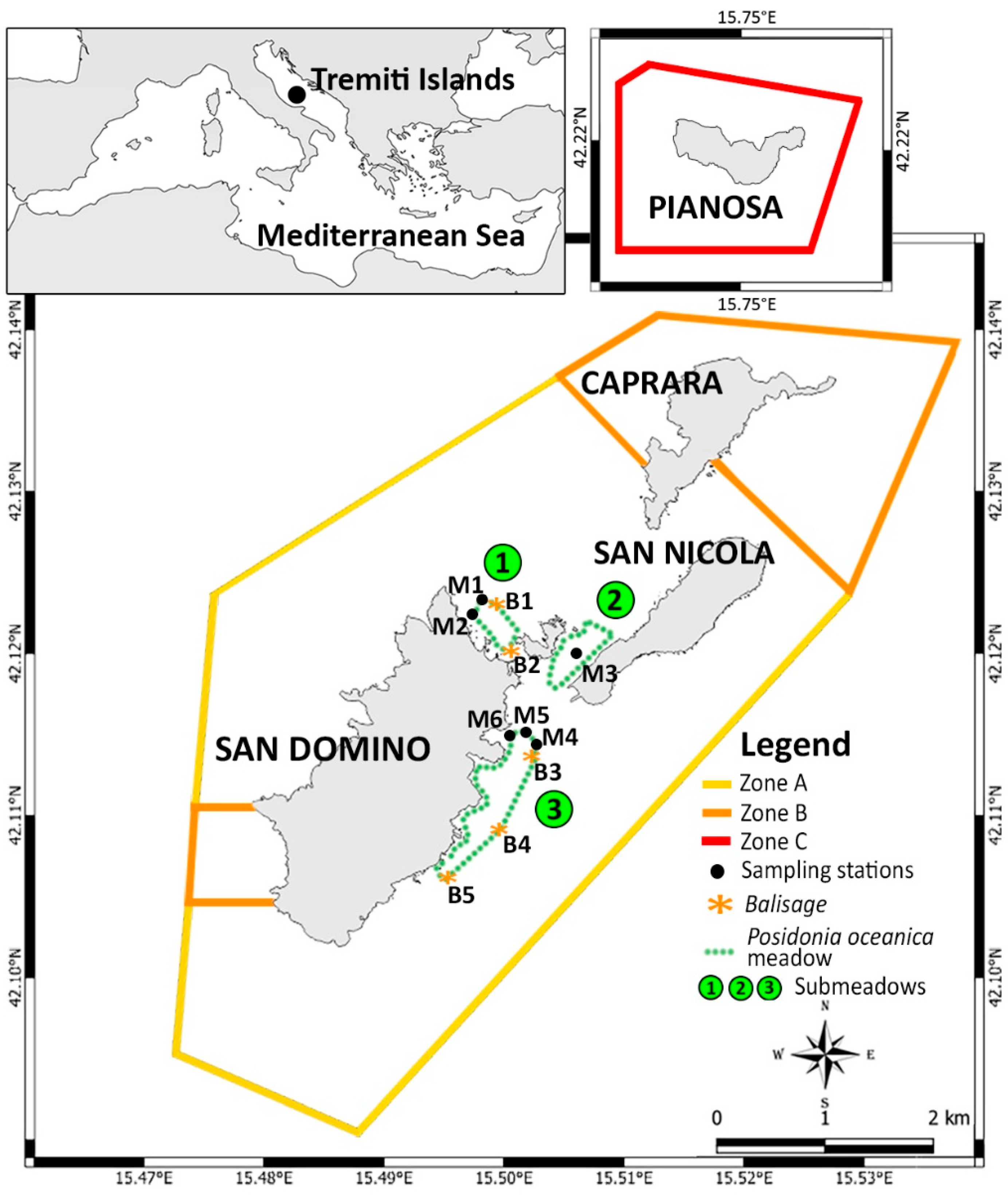



| ID | Type | Submeadow | Coordinates | Depth (m) |
|---|---|---|---|---|
| M1 | Lower limit | 1 | 42°07′25.73″ N–15°29′39.67″ E | 19 |
| M2 | Upper limit | 1 | 42°07′24.85″ N–15°29′38.92″ E | 15 |
| M3 | Middle zone | 2 | 42°07′18.12″ N–15°30′9.36″ E | 8 |
| M4 | Lower limit | 3 | 42°07′0.26″ N–15°29′52.05″ E | 21 |
| M5 | Middle zone | 3 | 42°07′0.43″ N–15°29′50.62″ E | 15 |
| M6 | Upper limit | 3 | 42°07′1.17″ N–15°29′46.74″ E | 8 |
| B1 | Balisage | 1 | 42°07′26.04″ N–15°29′40.37″ E | 19 |
| B2 | Balisage | 1 | 42°07′18.63” N–15°29′48.59″ E | 10 |
| B3 | Balisage | 3 | 42°07′00.13″ N–15°29′53.22″ E | 21 |
| B4 | Balisage | 3 | 42°06′38.92″ N–15°29′35.71″ E | 22 |
| B5 | Balisage | 3 | 42°06′28.75″ N–15°29′26.07″ E | 25 |
| Parameters | Analysis and Purpose | 2003 | 2015 | 2020 |
|---|---|---|---|---|
| Absolute density (rhizomes m−2) | Number of living rhizomes present in a 40 × 40 cm sampler frame to quantify the number of rhizomes per surface unit | • | • | • |
| Relative density (rhizomes m−2) | Absolute rhizome density in relation to the percentage coverage as an indicator of the actual density of the whole meadow | • | • | • |
| Leaf area index (m2m−2) | Mean leaf surface of each bundle in relation to the relative density to assess the potential photosynthetic surface of the meadow | • | • | • |
| Rhizome age (years) | Number of lepidochronological cycles per rhizome, identified by two thickness scale minimums, to assess the age of the rhizomes, with particular attention to the occurrence of young rhizomes as an indication of an active meadow renovation | • | • | • |
| Habitat mapping | Side-scan sonar survey to assess the distribution and the extension of the meadow | • | • | |
| Balisage systems | Measurement of distances between pickets and meadow limits over time to assess any progression or regression of the meadow | • | • |
| ID | Year | Absolute Rhizome Density (Rhizomes m−2) | Relative Rhizome Density (Rhizomes m−2) | Leaf Area Index (m2m−2) | Rhizome Age (Years) |
|---|---|---|---|---|---|
| M1 | 2003 vs. 2015 | 6.25 × 10−5 *** | 4.52 × 10−5 *** | 6.38 × 10−7 *** | 0.171 |
| 2015 vs. 2020 | 0.053 | 0.149 | 0.156 | 0.013 * | |
| 2003 vs. 2020 | 0.038 * | 6.1 × 10−3 * | 1.0 × 10−3 * | 2.6 × 10−3 * | |
| M2 | 2003 vs. 2015 | 2.31 × 10−7 *** | 8.40 × 10−8 *** | 1.32 × 10−5 *** | 0.172 |
| 2015 vs. 2020 | 0.773 | 4.0 × 10−3 * | 0.071 | 0.863 | |
| 2003 vs. 2020 | 1.79 × 10−7 *** | 3.87 × 10−10 *** | 8.76 × 10−7 *** | 0.102 | |
| M3 | 2003 vs. 2015 | 2.64 × 10−5 *** | 3.13 × 10−6 *** | 6.0 × 10−3 * | 0.296 |
| 2015 vs. 2020 | 0.087 | 5.67 × 10−6 *** | 3.24 × 10−8 *** | 0.013 * | |
| 2003 vs. 2020 | 5.11 × 10−4 *** | 0.060 | 0.278 | 0.029 * | |
| M4 | 2003 vs. 2015 | 8.09 × 10−6 *** | 5.01 × 10−8 *** | 1.06 × 10−6 *** | 0.424 |
| 2015 vs. 2020 | 0.148 | 3.60 × 10−4 *** | 1.41 × 10−5 *** | 4.58 × 10−4 *** | |
| 2003 vs. 2020 | 2.10 × 10−5 *** | 2.32 × 10−8 *** | 1.68 × 10−7 *** | 4.2 × 10−3 * | |
| M5 | 2003 vs. 2015 | 1.15 × 10−8 *** | 2.79 × 10−10 *** | 2.60 × 10−6 *** | 1.3 × 10−3 * |
| 2015 vs. 2020 | 3.65 × 10−4 * | 4.41 × 10−7 *** | 5.89 × 10−10 *** | 0.622 | |
| 2003 vs. 2020 | 4.98 × 10−8 *** | 1.14 × 10−10 *** | 6.97 × 10−13 *** | 0.212 | |
| M6 | 2003 vs. 2015 | 2.87 × 10−5 *** | 1.23 × 10−5 *** | 3.481 × 10−5 *** | 0.352 |
| 2015 vs. 2020 | 0.096 | 0.030 * | 3.95 × 10−4 *** | 0.373 | |
| 2003 vs. 2020 | 2.11 × 10−4 *** | 1.26 × 10−4 *** | 2.0 × 10−3 * | 0.172 |
| Area (m2) | Area (ha) | Increase/Decrease (%) | |||
|---|---|---|---|---|---|
| Submeadow | 2015 | 2020 | 2015 | 2020 | |
| 1 | 11,010 | 11,666 | 1.101 | 1.167 | +5.6 |
| 2 | 42,291 | 45,083 | 4.229 | 4.508 | +6.2 |
| 3 | 103,046 | 77,405 | 10.305 | 7.740 | −33.1 |
| Total | 156,347 | 134,154 | 15.635 | 13.415 | −16.5 |
| Balisage | Picket ID | 2015 Distance (cm) | 2020 Distance (cm) | Variation (cm) |
|---|---|---|---|---|
| B1 | 11 | 27 | L | |
| 12 | 22 | 1 | +21 | |
| 13 | 32 | L | ||
| 14 | 16 | 23 | −7 | |
| 15 | 18 | L | ||
| B2 | 21 | 22 | 432 | −10 |
| 22 | 15 | 55 | −40 | |
| 23 | 9 | 23 | −14 | |
| 24 | 7 | 15 | −8 | |
| 25 | 14 | L | ||
| B3 | 6 | 10 | 1159 | −1149 |
| 7 | 12 | L | ||
| 8 | 15 | 1121 | −1106 | |
| 9 | 11 | L | ||
| 10 | 18 | L | ||
| B4 | 1 | 24 | 40 | −16 |
| 2 | 5 | 5 | 0 | |
| 3 | 5 | 40 | −35 | |
| 4 | 20 | 10 | +10 | |
| 5 | 17 | 8 | +9 | |
| B5 | 16 | 18 | 18 | 0 |
| 17 | 30 | 30 | 0 | |
| 18 | 32 | 45 | −13 | |
| 19 | 23 | 22 | +1 | |
| 20 | 20 | 11 | +9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tursi, A.; Mastrototaro, F.; Montesanto, F.; De Giosa, F.; Lisco, A.; Bottalico, A.; Chimienti, G. The Status of Posidonia oceanica at Tremiti Islands Marine Protected Area (Adriatic Sea). Biology 2022, 11, 923. https://doi.org/10.3390/biology11060923
Tursi A, Mastrototaro F, Montesanto F, De Giosa F, Lisco A, Bottalico A, Chimienti G. The Status of Posidonia oceanica at Tremiti Islands Marine Protected Area (Adriatic Sea). Biology. 2022; 11(6):923. https://doi.org/10.3390/biology11060923
Chicago/Turabian StyleTursi, Andrea, Francesco Mastrototaro, Federica Montesanto, Francesco De Giosa, Anna Lisco, Antonella Bottalico, and Giovanni Chimienti. 2022. "The Status of Posidonia oceanica at Tremiti Islands Marine Protected Area (Adriatic Sea)" Biology 11, no. 6: 923. https://doi.org/10.3390/biology11060923
APA StyleTursi, A., Mastrototaro, F., Montesanto, F., De Giosa, F., Lisco, A., Bottalico, A., & Chimienti, G. (2022). The Status of Posidonia oceanica at Tremiti Islands Marine Protected Area (Adriatic Sea). Biology, 11(6), 923. https://doi.org/10.3390/biology11060923









