Elevated CO2 Altered Rice VOCs Aggravate Population Occurrence of Brown Planthoppers by Improving Host Selection Ability
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. CO2 Level and Environmental Condition
2.2. Plant Materials and Insect Stocks
2.3. Host Selection Assays of N. lugen Adults for the Healthy and BPH-Damaged Rice Plants
2.4. RNA Extraction, cDNA Synthesis and qRT-PCR Analysis
2.5. Collection and Identification Assays of the VOCs from the Healthy and BPH-Damaged Rice Plants Grown under Ambient and Elevated CO2
2.6. Statistical Analysis
3. Results
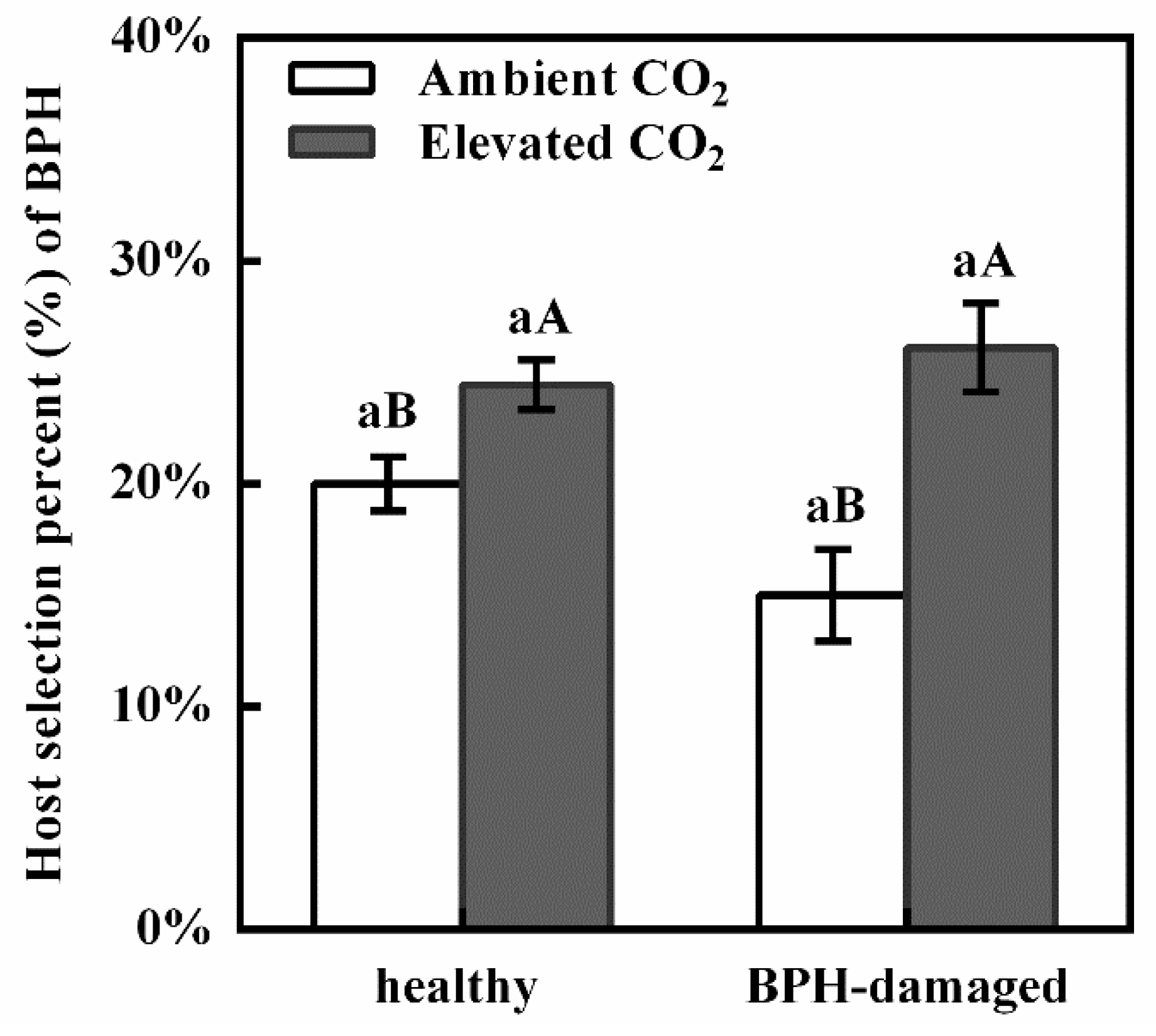
3.1. Effect of Elevated CO2 on the Host Selection of BPH for the Healthy and BPH-Damaged Rice Plants
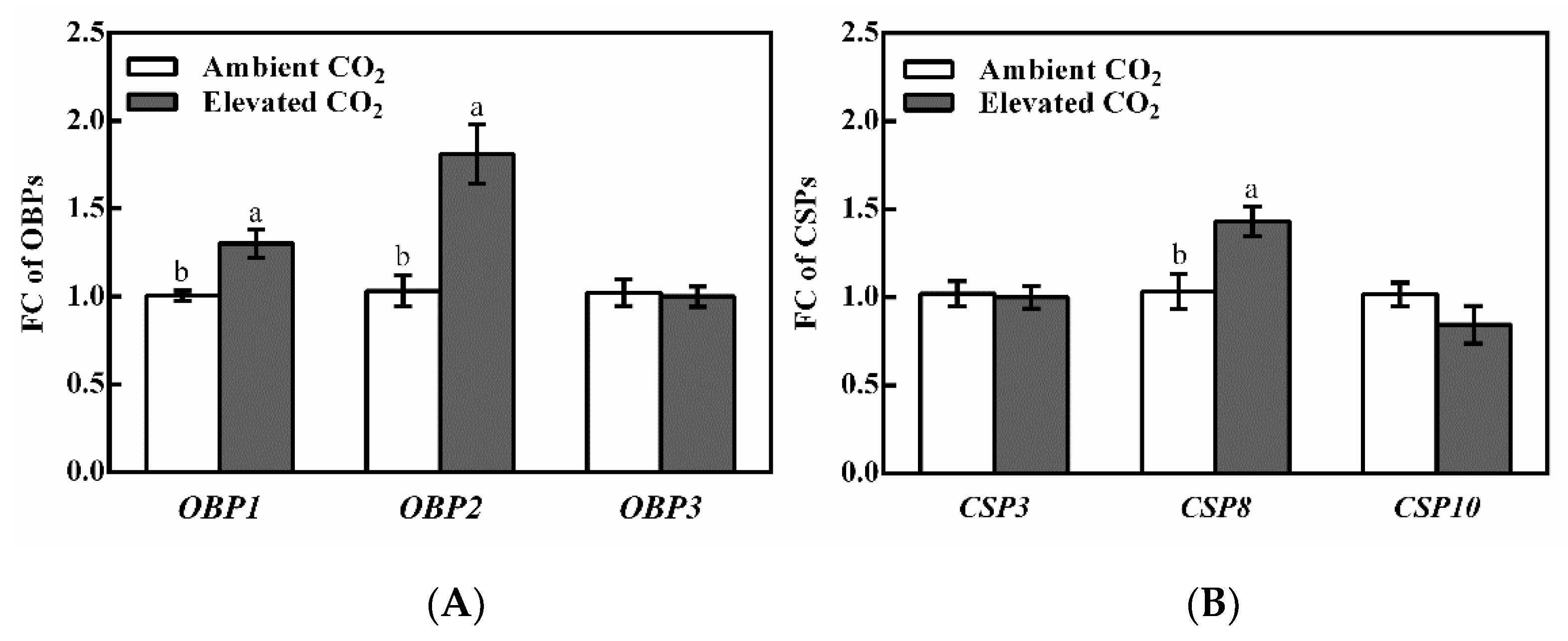
3.2. Relative Expression Levels of OBP and CSP Genes in BPH Adults Fed on Rice Plants Grown under Ambient and Elevated CO2
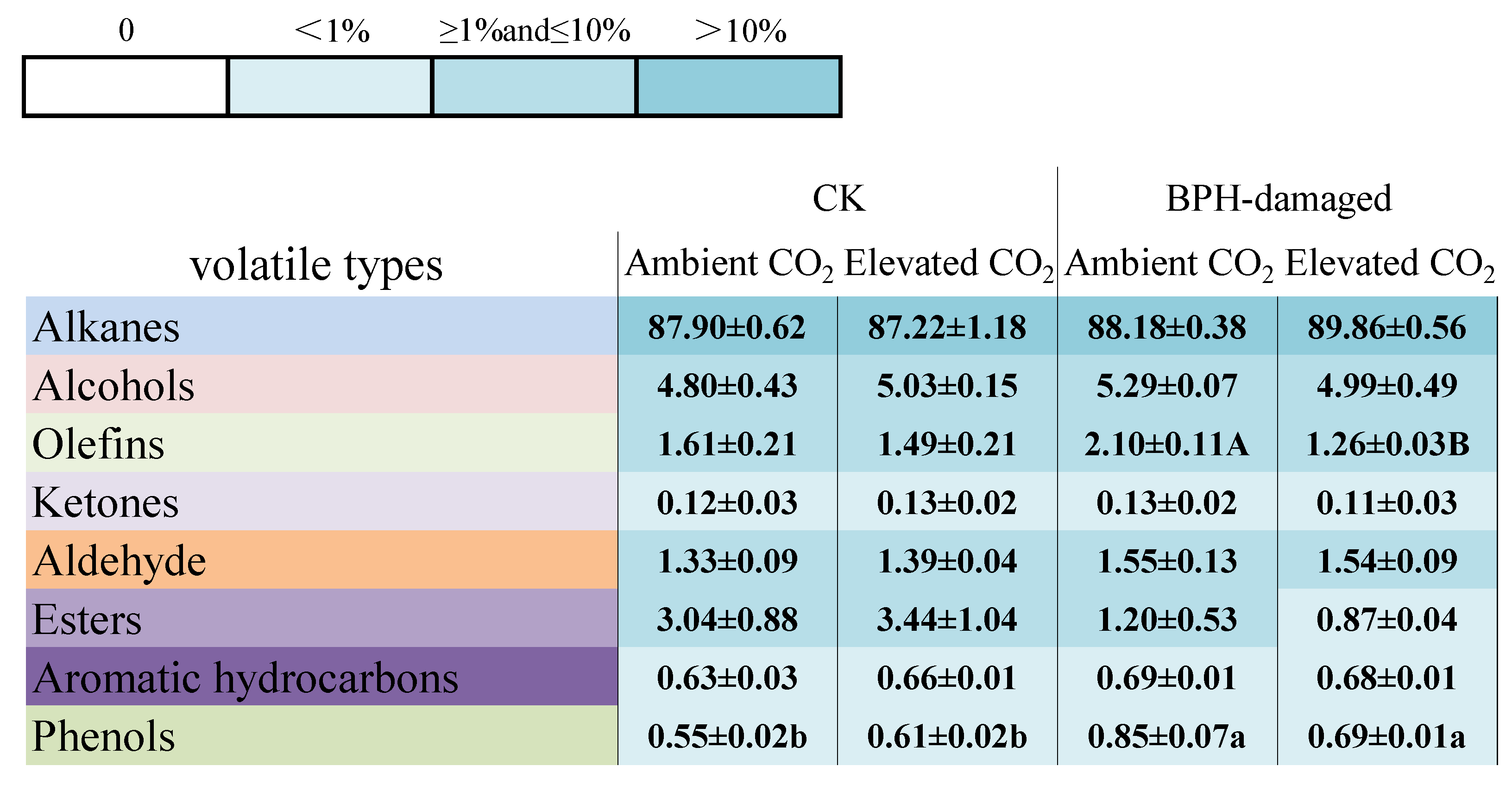
3.3. Effect of Elevated CO2 on the Components and Contents of VOCs from the Healthy and BPH-Damaged Rice Plants
3.4. Correlation Analysis among the Host-Selection Rate, the Transcript Expression Levels of OBPs and CSPs in BPH Adults and the Relative Percentages of Rice Plant VOCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NOAA: Ed Dlugokencky and Pieter Tans, NOAA/GML. Available online: https://gml.noaa.gov/ccgg/trends/ (accessed on 17 May 2022).
- IPCC. Climate Chang. 2013: Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Chang.; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; 1535p. [Google Scholar]
- Heena, R.; Rahul, S. Impact of elevated CO2 and temperature on plant carbon metabolism. Food Sci. Rep. 2020, 1, 25–28. [Google Scholar]
- Ainsworth, E.A.; Rogers, A.; Leakey, A.D.B.; Heady, L.E.; Gibon, Y.; Stitt, M.; Schurr, U. Does elevated atmospheric [CO2] alter diurnal C uptake and the balance of C and N metabolites in growing and fully expanded soybean leaves? J. Exp. Bot. 2007, 58, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.; Martínez-Carrasco, R.; Pérez, P.; Morcuende, R. New insights into the impacts of elevated CO2, nitrogen, and temperature levels on the regulation of C and N metabolism in durum wheat using network analysis. New Biotechnol. 2018, 40, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.T.; Sun, Y.; Chen, H.Y.H.; Ruan, H.H. Effects of elevated CO2 on the C:N stoichiometry of plants, soils, and microorganisms in terrestrial ecosystems. Catena 2021, 201, 105219. [Google Scholar] [CrossRef]
- Sun, Y.C.; Jing, B.B.; Ge, F. Response of amino acid changes in Aphis gossypii (Glover) to elevated CO2 levels. J. Appl. Entomol. 2009, 133, 189–197. [Google Scholar] [CrossRef]
- Lindroth, R.L. Impacts of elevated atmospheric CO2 and O3 on forests: Phytochemistry, trophic interactions, and ecosystem dynamics. J. Chem. Ecol. 2010, 36, 2–21. [Google Scholar] [CrossRef]
- Dáder, B.; Fereres, A.; Moreno, A.; TręBicki, P. Elevated CO2 impacts bell pepper growth with consequences to Myzus persicae life history, feeding behaviour and virus transmission ability. Sci. Rep. 2016, 6, 19120. [Google Scholar] [CrossRef] [PubMed]
- Vassiliadis, S.; Plummer, K.M.; Powell, K.S.; Trębicki, P.; Luck, J.E.; Rochfort, S.J. The effect of elevated CO2 and virus infection on the primary metabolism of wheat. Funct. Plant Biol. 2016, 43, 892–902. [Google Scholar] [CrossRef]
- Teawkul, P.; Hwang, S.Y. Carbon dioxide- and temperature-mediated changes in plant defensive compounds alter food utilization of herbivores. J. Appl. Entomol. 2019, 143, 289–298. [Google Scholar] [CrossRef]
- Peñuelas, J.; Estiarte, M. Can elevated CO2 affect secondary metabolism and ecosystem function? Trends Ecol. Evol. 1998, 13, 20–24. [Google Scholar] [CrossRef]
- Jamieson, M.A.; Burkle, L.A.; Manson, J.S.; Runyon, J.B.; Trowbridge, A.M.; Zientek, J. Global change effects on plant-insect interactions: The role of phytochemistry. Curr. Opin. Insect Sci. 2017, 23, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Stiling, P.; Cattell, M.; Moon, D.C.; Rossi, A.; Drake, B. Elevated atmospheric CO2 lowers herbivore abundance but increases leaf abscission rates. Glob. Chang. Biol. 2010, 8, 658–667. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Kozlov, M.V. Consequences of simultaneous elevation of carbon dioxide and temperature for plant–herbivore interactions: A metaanalysis. Glob. Chang. Biol. 2010, 12, 27–41. [Google Scholar] [CrossRef]
- Robinson, E.A.; Ryan, G.D.; Newman, J.A. A meta-analytical review of the effects of elevated CO2 on plant-arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol. 2012, 194, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.I.; Kost, C.; Boland, W. Herbivore-induced, indirect plant defences. Biochim. Biophys. Acta 2005, 1734, 91–111. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Sharma, H.C.; Paulraj, M.G.; War, M.Y. Herbivore induced plant volatiles: Their role in plant defense for pest management. Plant Signal. Behav. 2011, 6, 1973–1978. [Google Scholar] [CrossRef]
- Valkama, E.; Koricheva, J.; Oksanen, E. Effects of elevated O3, alone and in combination with elevated CO2, on tree leaf chemistry and insect herbivore performance: A meta-analysis. Glob. Chang. Biol. 2007, 13, 184–201. [Google Scholar] [CrossRef]
- Bidart-Bouzat, M.G.; Imeh-Nathaniel, A. Global change effects on plant chemical defenses against insect herbivores. J. Integr. Plant Biol. 2008, 50, 1339–1354. [Google Scholar] [CrossRef]
- Mumm, R.; Dicke, M. Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can. J. Zool. 2010, 88, 628–667. [Google Scholar] [CrossRef]
- Rosenstiel, T.N.; Potosnak, M.J.; Griffin, K.L.; Fall, R.; Monson, R.K. Increased CO2 uncouples growth from isoprene emission in an agriforest ecosystem. Nature 2003, 421, 256–259. [Google Scholar] [CrossRef]
- Monson, R.; Trahan, N.; Rosenstiel, T.N.; Veres, P.; Moore, D.J.; Wilkinson, M.; Norby, R.J.; Volder, A.; Tjoelker, M.G.; Briske, D.D. Isoprene emission from terrestrial ecosystems in response to global change. Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 2007, 365, 1677–1695. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Fischbach, R.; Schnitzler, J.P.; Ciccioli, P.; Brancaleoni, E.; Calfapietra, C.; Seufert, G. Monoterpene emission and monoterpene synthase activities in the Mediterranean evergreen oak Quercus ilex L. grown at elevated CO2 concentrations. Glob. Chang. Biol. 2001, 7, 709–717. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Reisdorff, C.; Pfanz, H. Quantitative effects of enhanced CO2 on jasmonic acid induced plant volatiles of lima bean (Phaseolus lunatus L.). J. Appl. Bot. Food Qual. 2011, 84, 65–71. [Google Scholar] [CrossRef]
- Guerenstein, P.G.; Hildebrand, J.G. Roles and effects of environmental carbon dioxide in insect life. Annu. Rev. Entomol. 2008, 53, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Zavala, J.A.; Nabity, P.D.; Delucia, E.H. An emerging understanding of mechanisms governing insect herbivory under elevated CO2. Annu. Rev. Entomol. 2013, 58, 79–97. [Google Scholar] [CrossRef]
- Jackson, R.B.; Cook, C.W.; Pippen, J.S.; Palmer, S.M. Increased belowground biomass and soil CO2 fluxes after a decade of carbon dioxide enrichment in a warm-temperate forest. Ecology 2009, 90, 3352–3366. [Google Scholar] [CrossRef] [PubMed]
- Hansson, B.S. Olfaction in Lepidoptera. Experientia 1995, 51, 1003–1027. [Google Scholar] [CrossRef]
- Dicke, M.; Loon, J. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl. 2000, 97, 237–249. [Google Scholar] [CrossRef]
- Liu, Z.; Hua, B.Z.; Lu, L. Ultrastructure of the sensilla on larval antennae and mouthparts in the peach fruit moth, Carposina sasakii Matsumura (Lepidoptera: Carposinidae). Micron 2011, 42, 478–483. [Google Scholar] [CrossRef]
- Wang, Q.K.; Zhang, M.; Li, K.; Dong, Z. Olfactory sensilla on antennae and maxillary palps of Fannia hirticeps (Stein, 1892) (Diptera: Fanniidae). Microsc. Res. Tech. 2012, 75, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ulyshen, M.D.; Poland, T.M. Abundance of volatile organic compounds in white ash phloem and emerald ash borer larval frass does not attract Tetrastichus planipennisi in a Y-tube olfactometer. Insect Sci. 2016, 23, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Faisal, Y.; Claudia, S.; Nicolas, D.; Coppin, C.W.; Gunjan, P.; Oakeshott, J.G.; Martine, M. An antennal carboxylesterase from Drosophila melanogaster, esterase 6, is a candidate odorant-degrading enzyme toward food odorants. Front. Physiol. 2015, 6, 315. [Google Scholar] [CrossRef]
- Zhao, J.J.; Zhang, Y.; Fan, D.S.; Feng, J.N. Identification and expression profiling of odorant-binding proteins and chemosensory proteins of Daktulosphaira vitifoliae (Hemiptera: Phylloxeridae). J. Econ. Entomol. 2017, 110, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; Peng, H.; Lan, Z.; Fang, S.Q.; Li, F. Large-scale identification of odorant-binding proteins and chemosensory proteins from expressed sequence tags in insects. BMC Genom. 2009, 10, 632. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhang, J.; Liu, N.Y.; Zhang, Y.N.; Yang, K.; Dong, S.L. Distinct expression profiles and different functions of odorant binding proteins in Nilaparvata lugens Stål. PLoS ONE 2011, 6, e28921. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhang, Y.N.; Li, Z.Q.; Yang, K.; Zhu, J.Y.; Liu, S.J.; Dong, S.L. An antennae-enriched carboxylesterase from Spodoptera exigua displays degradation activity in both plant volatiles and female sex pheromones. Insect Mol. Biol. 2014, 23, 475–486. [Google Scholar] [CrossRef]
- Sogawa. Windborn displacements of the rice planthoppers related to the seasonal weather patterns in Kyushu district. Bull. Kushu Natl. Agric. Exp. Stn. 1995, 28, 219–278. [Google Scholar] [CrossRef][Green Version]
- Sun, B.; Zhang, L.X.; Yang, L.Z.; Zhang, F.S.; Norse, D.; Zhu, Z. Agricultural non-point source pollution in China: Causes and mitigation measures. Ambio 2012, 41, 370–379. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Q.; Lu, W.; Liu, F. Sublethal effects of four synthetic insecticides on the generalist predator Cyrtorhinus lividipennis. J. Pest Sci. 2015, 88, 383–392. [Google Scholar] [CrossRef]
- Khan, Z.R.; Ampong-Nyarko, K.; Chiliswa, P.; Hassanali, A.; Kimani, S.; Lwande, W.; Overholt, W.A.; Picketta, J.A.; Smart, L.E.; Woodcock, C.M. Intercropping increases parasitism of pests. Nature 1997, 388, 631–632. [Google Scholar] [CrossRef]
- Pickett, J.A.; Khan, Z.R. Plant volatile-mediated signalling and its application in agriculture: Successes and challenges. New Phytol. 2016, 212, 856–870. [Google Scholar] [CrossRef]
- Mastrandrea, M.D.; Mach, K.J.; Plattner, G.K.; Edenhofer, O.; Stocker, T.F.; Field, C.B.; Ebi, K.L.; Matschoss, P.R. The IPCC AR5 guidance note on consistent treatment of uncertainties: A common approach across the working groups. Clim. Chang. 2011, 108, 675. [Google Scholar] [CrossRef]
- Singh, S.K.; Badgujar, G.; Reddy, V.R.; Fleisher, D.H.; Bunce, J.A. Carbon dioxide diffusion across stomata and mesophyll and photo-biochemical processes as affected by growth CO2 and phosphorus nutrition in cotton. J. Plant Physiol. 2013, 170, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Liu, X.W.; Huang, Z.J.; Wang, L.; Zhang, Y.F.; Gao, Y.L.; Gui, F.R.; Chen, F.J. Elevated CO2 enhances the host resistance against the western flower thrips, Frankliniella occidentalis, through increased callose deposition. J. Pest Sci. 2019, 94, 55–68. [Google Scholar] [CrossRef]
- Qian, L.; Huang, Z.J.; Liu, X.W.; Li, C.X.; Gao, Y.L.; Gui, F.R.; Chang, X.L.; Chen, F.J. Effect of elevated CO2 on interactions between the host plant Phaseolus vulgaris and the invasive western flower thrips, Frankliniella occidentalis. J. Pest Sci. 2021, 94, 43–54. [Google Scholar] [CrossRef]
- Kumar, A.; Silim, S.N.; Okamoto, M.; Siddiqi, M.Y.; Glass, A.D.M. Differential expression of three members of the AMT1 gene family encoding putative high-affinity NH4+ transporters in roots of Oryza sativa subspecies indica. Plant Cell Environ. 2003, 26, 907–914. [Google Scholar] [CrossRef]
- Waris, M.I.; Younas, A.; Ameen, A.; Rasool, F.; Wang, M.Q. Expression profiles and biochemical analysis of chemosensory protein 3 from Nilaparvata lugens (Hemiptera: Delphacidae). J. Chem. Ecol. 2020, 46, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Waris, M.I.; Younas, A.; Ul Qamar, M.T.; Hao, L.; Ameen, A.; Ali, S.; Abdelnabby, H.E.; Zeng, F.F.; Wang, M.Q. Silencing of chemosensory protein gene NlugCSP8 by RNAi induces declining behavioral responses of Nilaparvata lugens. Front. Physiol. 2018, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Waris, M.I.; Younas, A.; Adeel, M.M.; Duan, S.G.; Quershi, S.R.; Kaleem Ullah, R.M.; Wang, M.Q. The role of chemosensory protein 10 in the detection of behaviorally active compounds in brown planthopper, Nilaparvata lugens. Insect Sci. 2020, 27, 531–544. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Yang, Y.X.; Sun, H.H.; Liu, Z.W. Metabolic imidacloprid resistance in the brown planthopper, Nilaparvata lugens, relies on multiple P450 enzymes. Insect Biochem. Mol. Biol. 2016, 79, 50–56. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Jallow, M.; Zalucki, M. Within and between population variation in host-plant preference and specificity in Australian Helicoverpa Armigera (Hubner) (Lepidoptera: Noctuidae). Aust. J. Zool. 1996, 44, 503–519. [Google Scholar] [CrossRef]
- Cao, Y.; Zhi, J.R.; Zhang, R.Z.; Li, C.; Liu, Y.; Lv, Z.Y.; Gao, Y. Different population performances of Frankliniella occidentalis and Thrips hawaiiensis on flowers of two horticultural plants. J. Pest Sci. 2018, 91, 79–91. [Google Scholar] [CrossRef]
- Cao, Y.; Li, C.; Yang, H.; Li, J.; Li, S.; Wang, Y.W. Laboratory and field investigation on the orientation of Frankliniella occidentalis (Thysanoptera: Thripidae) to more suitable host plants driven by volatiles and component analysis of volatiles. Pest. Manag. Sci. 2019, 75, 598–606. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, M.F.; Jiang, S.L.; Zhang, Y.F.; Parajulee, M.N.; Chen, F.J. Host-selection behavior and physiological mechanisms of the cotton aphid, Aphis gossypii, in response to rising atmospheric carbon dioxide levels. J. Insect Physiol. 2018, 109, 149–156. [Google Scholar] [CrossRef]
- Qian, L.; Huang, Z.J.; Liu, H.; Liu, X.W.; Jin, Y.X.; Pokharel, S.S.; Chen, F.J. Elevated CO2-mediated plant VOCs change aggravates invasive thrips occurrence by altering their host-selection behaviour. J. Appl. Entomol. 2021, 145, 777–788. [Google Scholar] [CrossRef]
- Hu, X.Y.; Su, S.L.; Liu, Q.S.; Jiao, Y.Y.; Peng, Y.F.; Li, Y.H.; Turlings, T.C.J. Caterpillar-induced rice volatiles provide enemy-free space for the offspring of the brown planthopper. eLife 2020, 9, e55421. [Google Scholar] [CrossRef]
- Veyrat, N.; Robert, C.; Turlings, T.; Erb, M.; Deyn, G.D. Herbivore intoxication as a potential primary function of an inducible volatile plant signal. J. Ecol. 2016, 104, 591–600. [Google Scholar] [CrossRef]
- Aartsma, Y.; Bianchi, F.; Werf, W.; Poelman, E.H.; Dicke, M. Herbivore-induced plant volatiles and tritrophic interactions across spatial scales. New Phytol. 2017, 216, 1054–1063. [Google Scholar] [CrossRef]
- Chapman, R.F. Contact chemoreception in feeding by phytophagous insects. Annu. Rev. Entomol. 2003, 48, 455–484. [Google Scholar] [CrossRef]
- Honson, N.S.; Gong, Y.; Plettner, E. Chapter nine-structure and function of insect odorant and pheromone-binding proteins (OBPs and PBPs) and chemosensory-specific proteins (CSPs). Recent Adv. Phytochem. 2005, 39, 227–268. [Google Scholar]
- Sachse, S.; Krieger, P. Olfaction in insects. e-Neuroforum 2011, 2, 49–60. [Google Scholar] [CrossRef]
- You, L.; Wang, G.L.; Wei, H.Y. Advances in neuron transferring pathways in insects’ olfactory signals. Biol. Disaster Sci. 2012, 35, 7–11. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Xu, S.F.; Wubie, A.J.; Li, W.; Guo, Z.B.; Zhou, T. Research advance of olfactory proteins and olfactory mechanism in insects. Genom. Appl. Biol. 2013, 32, 667–676. [Google Scholar]
- Li, Z.Q.; Zhang, S.; Luo, J.Y.; Cui, J.J.; Ma, Y.; Dong, S.L. Two minus-C odorant binding proteins from Helicoverpa armigera, display higher ligand binding affinity at acidic pH than neutral pH. J. Insect Physiol. 2013, 59, 263–272. [Google Scholar] [CrossRef]
- Pelosi, P.; Zhou, J.J.; Ban, L.P.; Calvello, M. Soluble proteins in insect chemical communication. Cell. Mol. Life Sci. CMLS 2006, 63, 1658–1676. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Ghaninia, M.; Tabari, M.A. Olfactory cues explain differential attraction of the striped rice stem borer to different varieties of rice plant. J. Appl. Entomol. 2016, 140, 376–385. [Google Scholar] [CrossRef]
- Vuorinen, T.; Reddy, G.; Nerg, A.M.; Holopainen, J.K. Monoterpene and herbivore-induced emissions from cabbage plants grown at elevated atmospheric CO2 concentration. Atmos. Environ. 2004, 38, 675–682. [Google Scholar] [CrossRef]
- Zhang, J.J.; Li, Y.; Guo, J.P.; Du, B.; He, G.C.; Zhang, Y.J.; Chen, R.Z.; Li, J.R. Lipid profiles reveal different responses to brown planthopper infestation for pest susceptible and resistant rice plants. Metabolomics 2018, 14, 120. [Google Scholar] [CrossRef]
- Li, C.Z.; Sun, H.; Gao, Q.; Bian, F.Y.; Noman, A.; Xiao, W.H.; Zhou, G.X.; Lou, Y.G. Host plants alter their volatiles to help a solitary egg parasitoid distinguish habitats with parasitized hosts from those without. Plant Cell Environ. 2020, 43, 1740–1750. [Google Scholar] [CrossRef] [PubMed]


| Primer | Sequence (5′-3′) | Description |
|---|---|---|
| NlugOBP1-F | TTTGGCACAGAAACGATTTGGAG | Odorant-binding protein gene (OBPs) |
| NlugOBP1-R | CATTGGGCACTTGTCTTTGGAG | |
| NlugOBP2-F | CATCAAGAGTGTACCAGAAGGAGAC | |
| NlugOBP2-R | AATCATCAGTTCATACCAGCAAGC | |
| NlugOBP3-F | AAGCCACTGACGAGGATGTAATG | |
| NlugOBP3-R | TTCACACCTTCCAAGTTGAGATTCTG | |
| NlugCSP3-F | TGATTGTGGTCGCGTTTGGA | Chemosensory protein gene (CSPs) |
| NlugCSP3-R | TAGGGCGTCCGGTATTGTTG | |
| NlugCSP8-F | TTTTGTGGCGGTTTTGTGCT | |
| NlugCSP8-R | CCACCCATCAGGCACTTGAA | |
| NlugCSP10-F | AGCTCTGAAAGCCGGACTAC | |
| NlugCSP10-R | ATGAACGCTTTGATGTGGGG | |
| Nlβ-Actin-F | ACTCCGGTGATGGTGTCTCT | Reference genes |
| Nlβ-Actin-R | GTCGGTCAAGTCACGACCA | |
| Nlug-Actin-F | TCAACCCAAAGGCCAACC | |
| Nlug-Actin-R | CACCGGAGTCAAGCACGATA |
| Indexes | CO2 Level (CO2) | BPH-Damaged Treatment | CO2 × BPH-Damaged Treatment | |
|---|---|---|---|---|
| Host-selection rate (%) | 22.022/<0.001 *** | 1.011/0.327 | 4.045/0.058 | |
| Odorant-binding proteins (OBPs) | OBP1 | 12.045/0.003 ** | / | / |
| OBP2 | 16.679/<0.001 *** | / | / | |
| OBP3 | 0.052/0.823 | / | / | |
| Chemosensory proteins (CSPs) | CSP3 | 0.042/0.840 | / | / |
| CSP8 | 9.462/0.007 ** | / | / | |
| CSP10 | 1.844/0.193 | / | / | |
| Volatile organic compounds (VOCs) | Alkane | 0.445/0.524 | 3.783/0.088 | 2.480/0.154 |
| Alcohols | 0.009/0.926 | 0.468/0.513 | 0.607/0.459 | |
| Alkenes | 9.195/0.016 * | 0.687/0.431 | 5.160/0.053 | |
| Ketones | 0.050/0.829 | 0.116/0.742 | 0.243/0.635 | |
| Aldehydes | 0.042/0.844 | 3.992/0.081 | 0.156/0.703 | |
| Esters | 0.002/0.964 | 9.069/0.017 * | 0.249/0.631 | |
| Aromatic hydrocarbons | 0.203/0.664 | 6.917/0.030 * | 1.404/0.27 | |
| Phenols | 1.914/0.204 | 27.186/<0.001 *** | 7.912/0.023 * | |
| Volatile Types | VOCs | CO2 | BPH-Damaged | CO2 × BPH-Damaged |
|---|---|---|---|---|
| Alkane | Undecane | 0.011/0.921 | 4.515/0.066 | 0.044/0.839 |
| Tridecane | 0.932/0.363 | 2.022/0.193 | 0.348/0.572 | |
| Pentadecane | 0.170/0.691 | 0.336/0.578 | 0.477/0.509 | |
| Hexadecane | 0.358/0.566 | 10.462/0.012 * | 0.357/0.567 | |
| Heptadecane | 1.670/0.232 | 0.021/0.888 | 10.157/0.013 * | |
| Octadecane | 0.203/0.664 | 0.164/0.696 | 0.510/0.495 | |
| Icosane | 0.491/0.504 | 2.573/0.147 | 2.515/0.151 | |
| Tetracosane | 0.023/0.884 | 0.203/0.664 | 1.505/0.255 | |
| Heptacosane | 0.000/0.995 | 0.011/0.920 | 0.983/0.350 | |
| 5-Ethyl-2,2,3-trimethylheptane | 0.302/0.598 | 0.852/0.383 | 0.137/0.721 | |
| 2,4,6-Trimethyldecane | 0.946/0.359 | 0.000/1.000 | 0.447/0.523 | |
| 4,7-Dimethylundecane | 0.037/0.852 | 2.039/0.191 | 0.156/0.703 | |
| 2,6-Dimethylundecane | 0.001/0.975 | 0.207/0.661 | 0.067/0.803 | |
| 3-Methyltetradecane | 0.907/0.369 | 0.992/0.348 | 0.920/0.366 | |
| 4-Methylpentadecane | 1.074/0.330 | 0.260/0.624 | 1.929/0.202 | |
| 2,6,8-Trimethyldecane | 0.003/0.956 | 0.111/0.748 | 0.597/0.462 | |
| 2,6-Dimethylheptadecane | 0.294/0.603 | 0.016/0.902 | 1.842/0.212 | |
| 4-Methylnonadecane | 0.013/0.912 | 0.040/0.847 | 0.114/0.744 | |
| Alcohols | Linalool | 21.416/0.002 ** | 6.098/0.039 * | 9.206/0.016 * |
| Trans-2-Undecen-1-ol | 0.333/0.580 | 0.858/0.381 | 0.008/0.929 | |
| Falcarinol | 1.482/0.258 | 0.686/0.432 | 0.035/0.855 | |
| 2-Hexyl-1-decanol | 0.000/0.992 | 0.136/0.721 | 0.061/0.811 | |
| Phytol | 0.411/0.539 | 0.612/0.457 | 1.691/0.230 | |
| Hexacosan-1-ol | 0.689/0.431 | 0.082/0.781 | 0.418/0.536 | |
| 1-Eicosanol | 0.531/0.487 | 0.383/0.553 | 0.894/0.372 | |
| Alkenes | Limonene | 12.893/0.007 ** | 1.758/0.222 | 7.430/0.026 * |
| Myrcene | 0.076/0.789 | 0.537/0.485 | 1.769/0.220 | |
| 1-Docosene | 0.171/0.690 | 0.288/0.606 | 0.456/0.518 | |
| Ketones | 2-Dodecanone | 0.050/0.829 | 0.116/0.742 | 0.243/0.635 |
| Aldehyde | Nonanal | 0.011/0.918 | 10.012/0.013 * | 0.105/0.754 |
| Decanal | 0.164/0.696 | 4.718/0.062 | 0.056/0.819 | |
| (Z)-9-Tetradecenal | 0.045/0.838 | 0.421/0.535 | 0.836/0.387 | |
| Esters | Methyl cis-9,10-epoxystearate | 0.003/0.955 | 9.180/0.016 * | 0.275/0.614 |
| Aromatic hydrocarbon | 1-Methyldecalin | 2.165/0.179 | 3.262/0.109 | 4.603/0.064 |
| 2-Methyltetralin | 0.298/0.600 | 3.136/0.115 | 0.031/0.865 | |
| Phenols | 2,6-Diphenylphenol | 1.914/0.204 | 27.186/<0.001 *** | 7.912/0.023 * |
| Volatile Types | VOCs | Healthy (CK) Rice Plants | BPH-Damaged Rice Plants | ||
|---|---|---|---|---|---|
| Ambient CO2 | Elevated CO2 | Ambient CO2 | Elevated CO2 | ||
| Alkane | Undecane | 16.61 ± 1.12 | 16.43 ± 0.10b | 17.72 ± 0.18 | 17.78 ± 0.21a |
| Tridecane | 21.51 ± 0.69 | 21.70 ± 0.48 | 21.94 ± 0.64 | 22.76 ± 0.04 | |
| Pentadecane | 6.83 ± 0.09 | 6.68 ± 0.03 | 6.81 ± 0.19 | 6.85 ± 0.16 | |
| Hexadecane | 3.78 ± 0.10 | 3.78 ± 0.05b | 3.95 ± 0.04 | 4.03 ± 0.04a | |
| Heptadecane | 8.47 ± 0.03 | 9.26 ± 0.34 | 9.06 ± 0.08A | 8.72 ± 0.02B | |
| Octadecane | 2.47 ± 0.17 | 2.36 ± 0.01 | 2.37 ± 0.05 | 2.39 ± 0.01 | |
| Icosane | 10.58 ± 0.41 | 9.84 ± 0.46 | 9.55 ± 0.13 | 9.84 ± 0.14 | |
| Tetracosane | 4.84 ± 0.31 | 4.47 ± 0.23 | 4.40 ± 0.28 | 4.68 ± 0.23 | |
| Heptacosane | 3.61 ± 0.29 | 3.37 ± 0.31 | 3.40 ± 0.16 | 3.64 ± 0.18 | |
| 5-Ethyl-2,2,3-trimethylheptane | 0.19 ± 0.01 | 0.20 ± 0.02 | 0.21 ± 0.01 | 0.21 ± 0.02 | |
| 2,4,6-Trimethyldecane | 0.21 ± 0.01 | 0.21 ± 0.01 | 0.22 ± 0.00 | 0.21 ± 0.00 | |
| 4,7-Dimethylundecane | 0.94 ± 0.11 | 0.96 ± 0.02 | 1.07 ± 0.03 | 1.03 ± 0.09 | |
| 2,6-Dimethylundecane | 0.53 ± 0.03 | 0.52 ± 0.01 | 0.53 ± 0.02 | 0.54 ± 0.01 | |
| 3-Methyltetradecane | 0.63 ± 0.00 | 1.14 ± 0.53 | 0.62 ± 0.02 | 0.62 ± 0.02 | |
| 4-Methylpentadecane | 0.83 ± 0.05 | 0.75 ± 0.01 | 0.77 ± 0.04 | 0.78 ± 0.02 | |
| 2,6,8-Trimethyldecane | 2.26 ± 0.21 | 2.19 ± 0.03 | 2.14 ± 0.06 | 2.23 ± 0.04 | |
| 2,6-Dimethylheptadecane | 3.19 ± 0.23 | 2.94 ± 0.08 | 3.03 ± 0.08 | 3.14 ± 0.08 | |
| 4-Methylnonadecane | 0.42 ± 0.05 | 0.41 ± 0.06 | 0.39 ± 0.03 | 0.41 ± 0.03 | |
| Alcohols | Linalool | 0.19 ± 0.003b | 0.17 ± 0.01 | 0.23 ± 0.00Aa | 0.17 ± 0.01B |
| Trans-2-Undecen-1-ol | 0.29 ± 0.01 | 0.29 ± 0.01 | 0.30 ± 0.01 | 0.30 ± 0.01 | |
| Falcarinol | 0.59 ± 0.07 | 0.52 ± 0.02 | 0.65 ± 0.08 | 0.56 ± 0.06 | |
| 2-Hexyl-1-decanol | 1.28 ± 0.19 | 1.26 ± 0.06 | 1.22 ± 0.02 | 1.25 ± 0.02 | |
| Phytol | 0.37 ± 0.07b | 0.87 ± 0.22 | 0.91 ± 0.10a | 0.74 ± 0.45 | |
| Hexacosan-1-ol | 1.24 ± 0.06 | 1.16 ± 0.09 | 1.22 ± 0.04 | 1.21 ± 0.02 | |
| 1-Eicosanol | 0.83 ± 0.06 | 0.76 ± 0.05 | 0.76 ± 0.04 | 0.77 ± 0.01 | |
| Alkenes | Limonene | 0.78 ± 0.19 | 0.67 ± 0.15 | 1.30 ± 0.08A | 0.49 ± 0.02B |
| Myrcene | 0.16 ± 0.03 | 0.18 ± 0.03 | 0.17 ± 0.03 | 0.13 ± 0.02 | |
| 1-Docosene | 0.67 ± 0.05 | 0.63 ± 0.04 | 0.63 ± 0.02 | 0.64 ± 0.03 | |
| Ketones | 2-Dodecanone | 0.12 ± 0.03 | 0.13 ± 0.02 | 0.13 ± 0.02 | 0.11 ± 0.03 |
| Aldehyde | Nonanal | 0.44 ± 0.03 | 0.42 ± 0.00b | 0.56 ± 0.08 | 0.57 ± 0.03a |
| Decanal | 0.18 ± 0.02b | 0.20 ± 0.06 | 0.26 ± 0.02a | 0.27 ± 0.02 | |
| (Z)-9-Tetradecenal | 0.72 ± 0.07 | 0.77 ± 0.02 | 0.73 ± 0.03 | 0.70 ± 0.06 | |
| Esters | Methyl cis-9,10-epoxystearate | 3.05 ± 0.88 | 3.48 ± 1.04 | 1.21 ± 0.55 | 0.87 ± 0.04 |
| Aromatic hydrocarbon | 1-Methyldecalin | 0.29 ± 0.01b | 0.33 ± 0.01 | 0.33 ± 0.01a | 0.32 ± 0.01 |
| 2-Methyltetralin | 0.34 ± 0.02 | 0.33 ± 0.02 | 0.36 ± 0.01 | 0.36 ± 0.00 | |
| Phenols | 2,6-Diphenylphenol | 0.55 ± 0.02b | 0.61 ± 0.02b | 0.85 ± 0.07a | 0.69 ± 0.01a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, R.; Wang, X.; Liu, X.; Chen, F. Elevated CO2 Altered Rice VOCs Aggravate Population Occurrence of Brown Planthoppers by Improving Host Selection Ability. Biology 2022, 11, 882. https://doi.org/10.3390/biology11060882
Wang Y, Li R, Wang X, Liu X, Chen F. Elevated CO2 Altered Rice VOCs Aggravate Population Occurrence of Brown Planthoppers by Improving Host Selection Ability. Biology. 2022; 11(6):882. https://doi.org/10.3390/biology11060882
Chicago/Turabian StyleWang, Yanhui, Runzhao Li, Xiaohui Wang, Xiaowei Liu, and Fajun Chen. 2022. "Elevated CO2 Altered Rice VOCs Aggravate Population Occurrence of Brown Planthoppers by Improving Host Selection Ability" Biology 11, no. 6: 882. https://doi.org/10.3390/biology11060882
APA StyleWang, Y., Li, R., Wang, X., Liu, X., & Chen, F. (2022). Elevated CO2 Altered Rice VOCs Aggravate Population Occurrence of Brown Planthoppers by Improving Host Selection Ability. Biology, 11(6), 882. https://doi.org/10.3390/biology11060882







