Trematodes of Genera Gyrabascus and Parabascus from Bats in European Russia: Morphology and Molecular Phylogeny
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasite Collection and Examination
2.2. DNA Extraction, Amplification, Sequencing, and Phylogenetic Analysis
3. Results
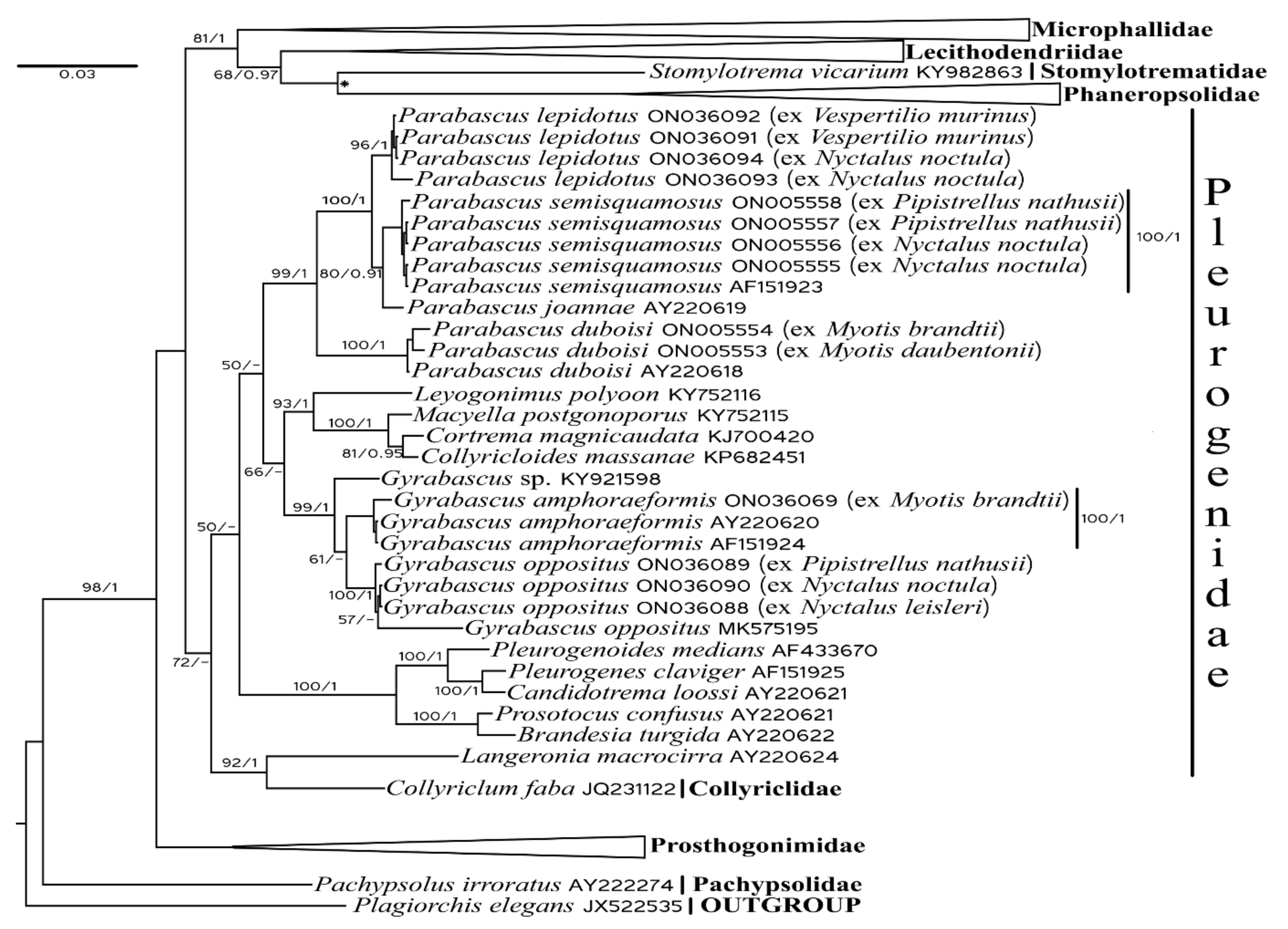
3.1. Molecular Phylogenetic Analysis
3.2. Systematics and Morphological Characteristics
- Superfamily Microphalloidea Ward, 1901
- Family Pleurogenidae Looss, 1899
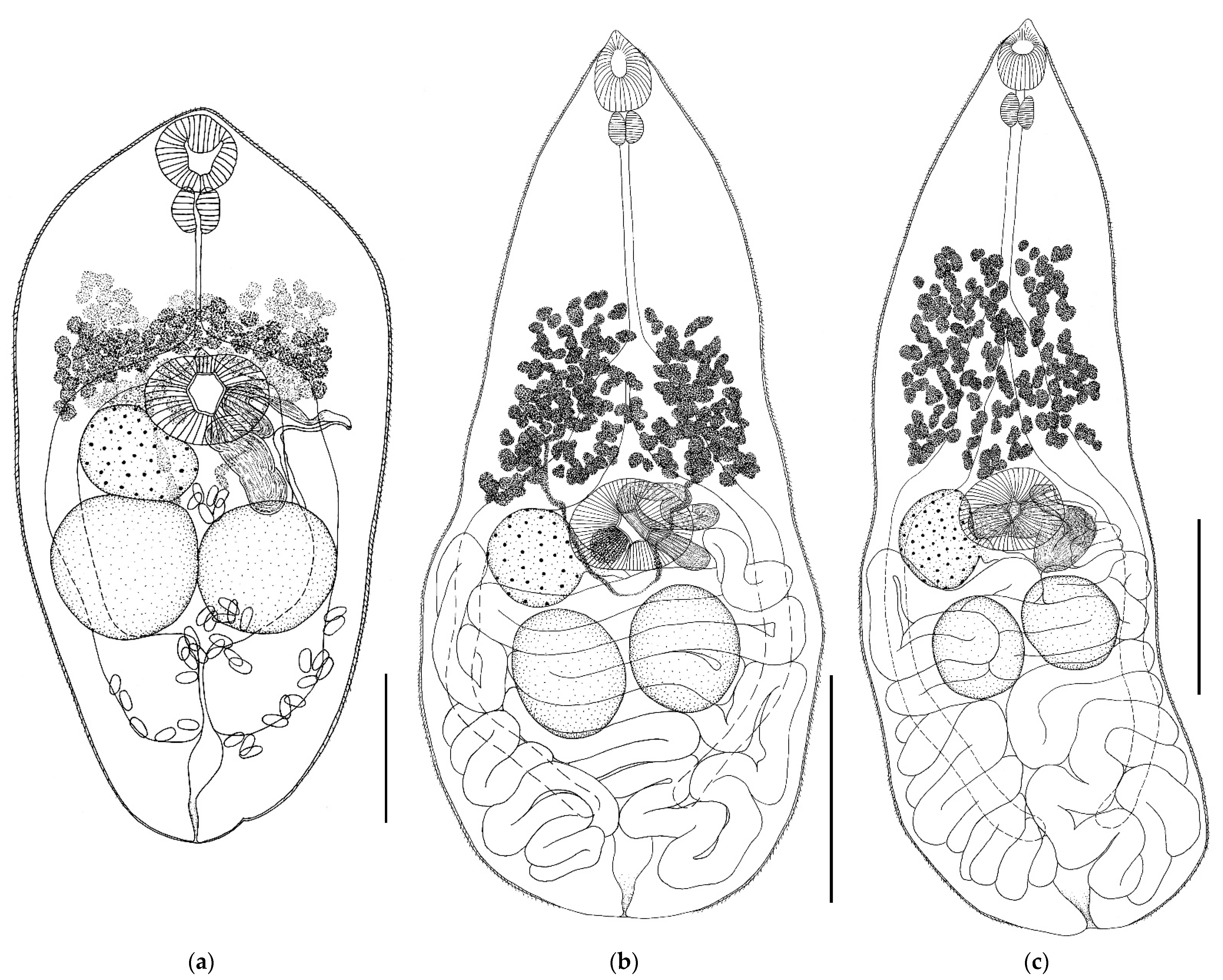
- Genus Gyrabascus Macy 1935
- Gyrabascus amphoraeformis (Mödlinger, 1930) (Figure 2)
- Host: Myotis brandtii.
- Geographical locality: Smolny village (Republic of Mordovia, Russia).
- Availability: GenBank No ON036069.
- Accession numbers in collection of IEVB RAS: No 2101.
- Gyrabascus oppositus (Zdzitowiecki, 1969) (Figure 2)
- Host: Nyctalus noctula, Nyctalus leisleri, Pipistrellus nathusii.
- Geographical locality: Smolny village (Republic of Mordovia, Russia).
- Availability: GenBank No ON036088—ON036090.
- Accession numbers in collection of IEVB RAS: No 2102–2105.
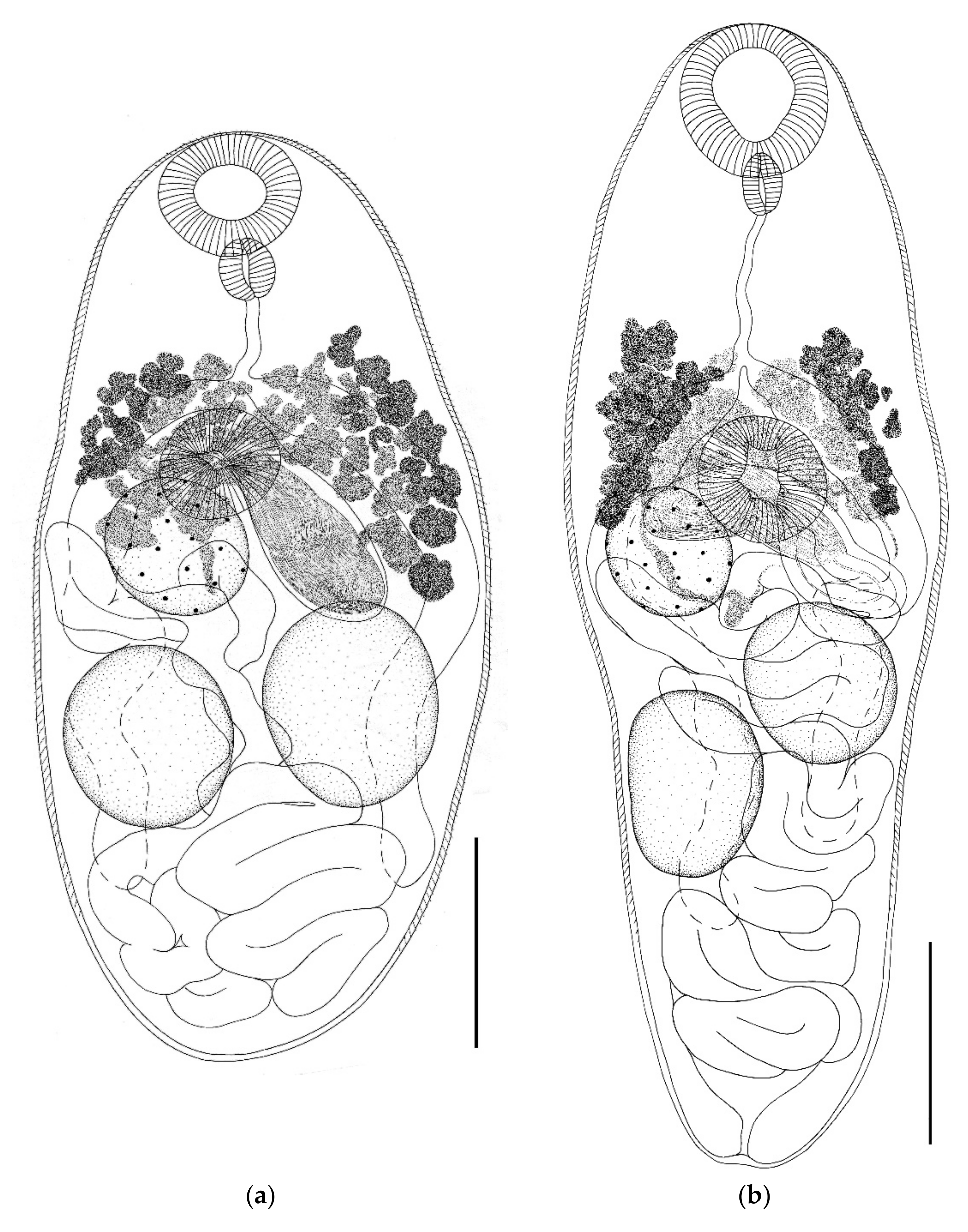
- Genus Parabascus Looss, 1907
- Host: Myotis brandtii, Myotis daubentonii.
- Geographical locality: Smolny and Pushta villages (Republic of Mordovia, Russia).
- Availability: GenBank No ON005553, ON005554.
- Accession numbers in collection of IEVB RAS: No 2106–2108.
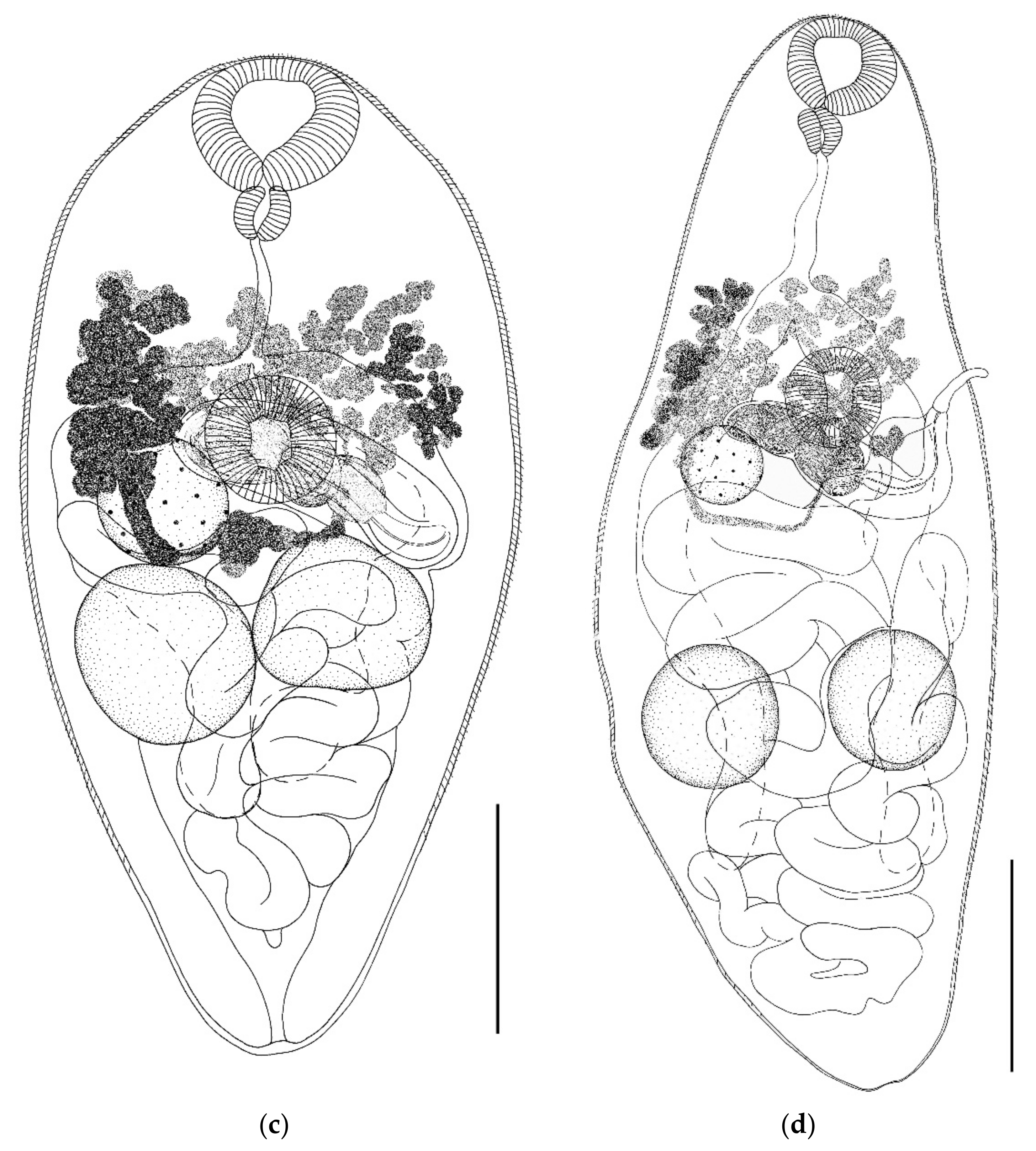
- Parabascus semisquamosus (Braun, 1900) (Figure 4)
- Host: Nyctalus noctula, Pipistrellus nathusii.
- Geographical locality: Smolny and Pushta villages (Republic of Mordovia, Russia).
- Availability: GenBank No ON005555—ON005558.
- Accession numbers in collection of IEVB RAS: No 2109–2112.
- Parabascus lepidotus Looss, 1907 (Figure 5)
- Host: Nyctalus noctula, Vespertilio murinus.
- Geographical locality: Smolny and Pushta villages (Republic of Mordovia, Russia).
- Availability: GenBank No ON036091—ON036094.
- Accession numbers in collection of IEVB RAS: No 2113–2119.
4. Discussion
- 1(6) Cirrus sac absent.
- 2(4) Genital pore marginal.
- 3(2) Body oval with a maximum width at the intestinal bifurcation level. Ventral sucker pre-equatorial ...................... Gyrabascus amphoraeformis (Mödlinger, 1930).
- 4(2) Genital pore submedial.
- 5(4) Body pear-shaped with a maximum width at the testes level. Ventral sucker equatorial ………….………………….... Gyrabascus oppositus (Zdzitowiecki 1969).
- 6(1) Cirrus sac present.
- 7(12) Ventral sucker pre-equatorial.
- 8(10) Ventral sucker larger than oral sucker.
- 9(8) Body elongated, narrow. Intestinal branches extend far beyond the testes level. The body length to width ratio is 3.2–5.2: 1. The oral sucker to ventral sucker ratio is 0.6–0.7:1. The oral sucker to pharynx ratio is 1.6–2.3:1 ......................................................................................... Parabascus semisquamosus (Braun, 1900).
- 10(8) Ventral sucker equal to or less than oral sucker.
- 11(10) Body oval, shortened. Intestinal branches end directly behind the testes. The body length to width ratio is 1.5–3.0:1. The oral sucker to ventral sucker ratio is 1.0–1.4:1. The oral sucker to pharynx ratio is 2.0–3.0:1 ……………….…..……..………………………………………………….. Parabascus duboisi (Hurkova, 1961).
- 12(7) Ventral sucker equatorial.
- 13(12) Body pear-shaped with a maximum width at the testes level. The body length to width ratio is 1.1–2.5:1. The oral sucker to ventral sucker ratio is 0.7–0.9:1. The oral sucker to pharynx ratio is 1.6–2.3:1 ....... Parabascus lepidotus (Looss, 1907).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khotenovsky, I.A. On the evolution of trematodes from bats. Parazitologiia 1972, 6, 79–82. [Google Scholar]
- Thewissen, J.G.M.; Babcock, S.K. The origin of flight in bats. BioScience 1992, 42, 340–345. [Google Scholar] [CrossRef]
- Mammals of Russia. Available online: http://rusmam.ru/mammal/index?sort=sort (accessed on 20 January 2022).
- Il’in, V.Y.; Vekhnik, V.P.; Smirnov, D.G.; Kurmaeva, N.M.; Zolina, N.F.; Matrosova, O.M. Dynamics of abundance of bats (Chiroptera: Vespertilionidae) during hibernation in caves of the Samarian Luka over a 20-year period. Rus. J. Ecol. 1999, 30, 428–431. [Google Scholar]
- Artaev, O.N.; Smirnov, D.G. Bats (Chiroptera; Mammalia) of Mordovia: Specific structure and features of distribution. Nat. Conserv. Res. 2016, 1, 38–51. [Google Scholar] [CrossRef]
- Smirnov, D.G.; Vekhnik, V.P.; Kurmaeva, N.M.; Shepelev, A.A.; Il’in, V.Y. Species structure and dynamics of bat communities (Chiroptera: Vespertilionidae) hibernating in artificial caves of Samara Luka. Biol. Bull. 2007, 34, 507–516. [Google Scholar] [CrossRef]
- Smirnov, D.G.; Vekhnik, V.P. Ecology of nutrition and differentiation of the trophic niches of bats (Chiroptera: Vespertilionidae) in floodplain ecosystems of the Samara Bend. Biol. Bull. 2014, 41, 60–70. [Google Scholar] [CrossRef]
- Orlova, M.V.; Smirnov, D.G.; Vekhnik, V.P.; Lukyanenko, A.M.; Zabashta, A.V. Ectoparasites and pathogens of Kuhl’s pipistrelle Pipistrellus kuhlii (Kuhl, 1817) (Chiroptera: Vespertilionidae): Our own and published data review. Russ. J. Biol. Invasions 2020, 11, 348–362. [Google Scholar] [CrossRef]
- Orlova, M.V.; Klimov, P.B.; Moskvitina, N.S.; Orlov, O.L.; Zhigalin, A.V.; Smirnov, D.G.; Dzhamirzoyev, H.S.; Vekhnik, V.P.; Pavlov, A.V.; Emelyanova, A.A.; et al. New records of bat flies (Diptera: Nycteribiidae), with an updated checklist of the nycteribiids of Russia. Zootaxa 2021, 4927, 410–430. [Google Scholar] [CrossRef]
- Orlova, M.V.; Klimov, P.B.; Orlov, O.L.; Smirnov, D.G.; Zhigalin, A.V.; Budaeva, I.V.; Emelyanova, A.A.; Anisimov, N.V. A checklist of bat-associated macronyssid mites (Acari: Gamasina: Macronyssidae) of Russia, with new host and geographical records. Zootaxa 2021, 4974, 537–564. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, D.G.; Vekhnik, V.P.; Dzhamirzoyev, G.S.; Titov, S.V. On the taxonomic status of species from the group Myotis nattereri» (Chiroptera, Vespertilionidae) in the Eastern Caucasus. Nat. Conserv. Res. 2020, 5, 30–42. [Google Scholar] [CrossRef]
- Bazhenov, Y.A. Ecology of bat species in the arid region of the Daurian steppe at the peak of drought. Nat. Conserv. Res. 2021, 6, 42–49. [Google Scholar] [CrossRef]
- Belkin, V.V.; Fyodorov, F.V.; Ilyukha, V.A.; Yakimova, A.E. Characteristics of the bat (Chiroptera) population in Protected Areas in the northern and middle taiga subzones of European Russia. Nat. Conserv. Res. 2021, 6 (Suppl. 1), 17–31. [Google Scholar] [CrossRef]
- Smirnov, D.G.; Bezrukov, V.A.; Kurmaeva, N.M. Use of habitat and foraging time by females of Eptesicus nilssonii (Chiroptera, Vespertilionidae). Russ. J. Theriol. 2021, 20, 1–10. [Google Scholar] [CrossRef]
- Kirillova, N.Y.; Kirillov, A.A.; Vekhnik, V.P. Nematodes (Nematoda) from bats (Chiroptera) of the Samarskaya Luka peninsula (Russia). Parazitologiia 2008, 42, 526–532. [Google Scholar] [PubMed]
- Kirillov, A.A.; Kirillov, N.Y.; Vekhnik, V.P. Trematodes (Trematoda) of bats (Chiroptera) from the Middle Volga region. Parazitologiia 2012, 46, 384–413. [Google Scholar] [PubMed]
- Kirillov, A.A.; Kirillova, N.Y.; Chikhlyaev, I.V. Trematodes of Terrestrial Vertebrates of the Middle Volga Region; Cassandra: Togliatti, Russia, 2012; pp. 3–329. [Google Scholar]
- Kirillov, A.A.; Ruchin, A.B.; Artaev, O.N. Helminths of bats (Chiroptera) from Mordovia. Bull. Vol. Univ. Tatishch. 2015, 4, 319–328. [Google Scholar]
- Kirillov, A.A.; Ruchin, A.B.; Artaev, O.N. To the study of helminths of bats (Chiroptera) from Mordovia. Proceed. Sam. Sci. Cent Rus. Acad. Sci. 2015, 17, 859–866. [Google Scholar]
- Ruchin, A.B.; Kirillov, A.A.; Chikhlyaev, I.V.; Kirillova, N.Y. Parasitic worms of land vertebrates of the Mordovia Nature Reserve. In Flora and Fauna of Reserves; Committee of RAS for the Conservation of Biological Diversity: Moscow, Russia, 2016; Volume 124, pp. 3–72. [Google Scholar]
- Kirillova, N.Y.; Kirillov, A.A. Overview of helminths in small mammals in the Zhiguli State Reserve. Nat. Conserv. Res. 2017, 2, 24–37. [Google Scholar] [CrossRef]
- Kirillov, A.A.; Kirillova, N.Y.; Krasnobayev, Y.P.; Vekhnik, V.P. Parasitic Worms of Small Mammals in Zhiguli State Nature Reserve; Committee of RAS for the Conservation of Biological Diversity, A.N. Severtzov Institute of Ecology and Evolution of RAS: Moscow, Russia, 2017; pp. 3–81. [Google Scholar]
- Kirillov, N.Y.; Kirillov, A.A.; Vekhnik, V.P.; Ruchin, A.B.; Grishutkin, G.F. Helminths of bats (Chiroptera) in “Smolny” National Park: Preliminary Data. Proceed. Mord. St. Nat. Res. 2018, 21, 223–230. [Google Scholar]
- Skvortsov, V.G. Trematodes of the family Lecithodendriidae from bats in Moldavia. Paras. Anim. Plant. Mold. 1970, 5, 17–36. [Google Scholar]
- Sharpilo, V.P.; Tkach, V.V. About type species of genus Plagiorchis Luhe, 1899 (Trematoda, Plagiorchiidae). Vest. Zool. 1992, 5, 8–15. [Google Scholar]
- Tkach, V.V.; Pawlowski, J.; Sharpilo, V.P. Molecular and morphological differentiation between species of the Plagiorchis vespertilionis group (Digenea, Plagiorchiidae) occurring in European bats, with a re-description of P. vespertilionis (Muller, 1780). Syst. Parasitol. 2000, 47, 9–22. [Google Scholar] [CrossRef]
- Odening, K. Exkretionssystem und systematische stellung exkretionssystem und systematische stellung einiger eledermaustrematoden aus Berlin und umgebung nebst bemerkungen zum Lecithodendrioiden komplex. Z. Parasitenkd. 1964, 24, 453–483. [Google Scholar] [CrossRef] [PubMed]
- Zdzietowiecki, K. Helminths of bats in Poland. III. Trematodes of the subfamily Lecithodendriidae, except for Lecithodendriinae. Acta Parasitol. Pol. 1969, 16, 227–237. [Google Scholar]
- Khotenovsky, I.A. Trematodes of the genus Parabascus (Trematoda, Pleurogenidae) from bats in Holarctic. Parasitol. Coll. Pap. 1985, 33, 125–133. [Google Scholar]
- Sokolov, S.G.; Shchenkov, S.V.; Kalmykov, A.P.; Smirnova, A.D. Morphology and phylogenetic position of two microphalloid trematode species, parasites of the vesper bat Pipistrellus kuhlii in the Lower Volga region of Russia. Zool. Zhurnal 2020, 99, 261–274. [Google Scholar] [CrossRef]
- Lotz, J.M.; Font, W.F. Family Gyrabascidae Macy, 1935. In Keys to Trematoda; Bray, R.A., Gibson, D.J., Jones, A., Eds.; CAB International and Natural History Museum: London, UK, 2008; Volume 3, pp. 523–525. [Google Scholar]
- Lotz, J.M.; Font, W.F. Family Phaneropsolidae Mehra, 1935. In Keys to Trematoda; Bray, R.A., Gibson, D.J., Jones, A., Eds.; CAB International and Natural History Museum: London, UK, 2008; Volume 3, pp. 545–562. [Google Scholar]
- Sharpilo, V.P.; Iskova, N.I. Trematodes. Plagiorchiata. Fauna of Ukraine; Naukova Dumka: Kiev, Ukraine, 1989; Volume 34, pp. 3–280. [Google Scholar]
- Braun, M. Trematoden der Chiroptera. Ann. Naturhist. Mus. Wien. 1900, 15, 215–236. [Google Scholar]
- Looss, A. Ueber einige neue Trematoden der aegyptischen fauna. Notizen zur Helminthologie Egyptens. Zbl. Bakteriol. Parasitenkd. 1907, 43, 478–490. [Google Scholar]
- Hurkova, J.A. A contribution to the knowledge of bat trematodes in Czechoslovakia. Acta Univ. Carol.-Biol. 1959, 1, 29–36. [Google Scholar]
- Hurkova, J.A. A contribution to the knowledge of bat trematodes of the g. Parabascus Looss and g. Limatulum Travassos (fam. Lecithodendriidae) with a description of a new species. Vest. Ceskosl. Zool. Spol. 1961, 25, 277–288. [Google Scholar]
- Modlinger, G. Trematoden ungarischer Chiropteren. Stud. Zool. Bud. 1930, 1, 191–203. [Google Scholar]
- Mituch, J. Beitrag zur Ercenntnis der Helminthenfauna von Miniopterus schreibersi (Kuhl, 1819) in der Slovakei (CSSR). Helminthologia 1965, 6, 109–119. [Google Scholar]
- Tkach, V.V.; Pawlowski, J.; Mariaux, J. Phylogenetic analysis of the suborder Plagiorchiata (Platyhelminthes, Digenea) based on partial lsrDNA sequences. Int. J. Parasitol. 2000, 30, 83–93. [Google Scholar] [CrossRef]
- Tkach, V.V.; Littlewood, D.T.J.; Olson, P.D.; Kinsella, J.M.; Swiderski, Z. Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Syst. Parasitol. 2003, 56, 1–15. [Google Scholar] [CrossRef]
- Lord, J.S.; Parker, S.; Parker, F.; Brooks, D.R. Gastrointestinal helminths of pipistrelle bats (Pipistrellus pipistrellus/Pipistrellus pygmaeus) (Chiroptera: Vespertilionidae) of England. Parasitology 2012, 139, 366–374. [Google Scholar] [CrossRef]
- Sümer, N.; Yildirimhan, H.S. DNA sequencing of Digenea nuclear lsrDNA of the whiskered brown bat, Myotis aurescens (Vespertilionidae: Chiroptera), from Turkey. Turk. J. Zool. 2017, 41, 64–66. [Google Scholar] [CrossRef]
- Sümer, N.; Yildirimhan, H.S. Intestinal Helminth Parasites of Two Bat Species of the Genus Myotis Kaup, 1829 (Chiroptera: Vespertilionidae) from Turkey, with DNA Sequencing of Helminth Nuclear LSR rDNA. Acta Zool. Bulg. 2019, 71, 273–278. [Google Scholar]
- Ivashkin, V.M.; Kontrimavichus, V.L.; Nasarova, N.S. Methods of the Collection and Studies of Helminths of Land Mammals; Nauka: Moscow, Russia, 1971; pp. 3–123. [Google Scholar]
- Anikanova, V.S.; Bugmyrin, S.V.; Ieshko, E.P. Methods of the Collection and Studies of Helminths of Small Mammals; Karelian Scientific Center of RAS: Petrozavodsk, Russia, 2007; pp. 3–145. [Google Scholar]
- Khotenovsky, I.A. Family Pleurogenidae Looss, 1899. In Trematodes of Animals and Man. Essentials of Trematodology; Skryabin, K.I., Ed.; Nauka: Moscow, Russia, 1970; Volume 23, pp. 135–306. [Google Scholar]
- Khotenovsky, I.A. Family Allassogonoporidae Skarbilovich, 1947. In Trematodes of Animals and Man. Essentials of Trematodology; Skryabin, K.I., Ed.; Nauka: Moscow, Russia, 1974; Volume 25, pp. 245–258. [Google Scholar]
- Skvortsov, V.G. Critical overview of the helminth fauna in bats of the USSR and European countries. Proceed. Acad. Mold. SSR. Biol. Chem. Ser. 1971, 6, 53–59. [Google Scholar]
- Fauna Europaea. Available online: https://fauna-eu.org/ (accessed on 22 February 2022).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: http://www.r-project.org/index.html (accessed on 22 February 2022).
- Paradis, E.; Schliep, K. Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Bodenhofer, U.; Bonatesta, E.; Horejs-Kainrath, C.; Hochreiter, S. Msa: An R package for multiple sequence alignment. Bioinformatics 2015, 31, 3997–3999. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop, New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Resource Center “Computer Center of SpbU”. Available online: http://cc.spbu.ru (accessed on 23 February 2022).
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Shchenkov, S.; Denisova, S.; Kremnev, G.; Dobrovolskij, A. Five new morphological types of virgulate and microcotylous xiphidiocercariae based on morphological and molecular phylogenetic analyses. J. Helminthol. 2020, 94, E94. [Google Scholar] [CrossRef]
- Fernandes, T.F.; dos Santos, J.N.; de Vasconcelos Melo, F.T.; Achatz, T.J.; McAllister, C.T.; Carrion Bonilla, C.; Tkach, V.V. Phylogenetic relationships of Ochoterenatrema Caballero, 1943 (Digenea: Lecithodendriidae) with descriptions of two new species. Parasitol. Int. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Dellagnola, F.; Campoy-Diaz, A.; Vega, I. First morphological and molecular identification of the cercaria of Stomylotrema vicarium from the endemic apple snail Pomacea americanista. Parasitology 2022, 149, 95–104. [Google Scholar] [CrossRef]
- Hurkova, J.A. Bat trematodes in Czechoslovakia. I. A systematical review of occurring species. Vest. Cescosl. Spolec. Zool. 1963, 27, 250–276. [Google Scholar]
- Dubois, G. Contribution à l’étude des Trématodes de Chiroptères. Rev. Suiss. Zool. 1956, 63, 683–695. [Google Scholar] [CrossRef]
- Dubois, G. Contribution à l’étude des Trématodes de Chiroptères. Revision du genre Alassogonoporus Olivier, 1938 et note additionelle sur le sous-genre Prosthodendrium Dollfus, 1931. Rev. Suiss. Zool. 1963, 70, 103–125. [Google Scholar] [CrossRef]
- Morozov, Y.F. To the helminth fauna of bats in the Belovezhskaya Pushcha. Bull. Acad. Sci. Bel. SSR 1961, 2, 92–98. [Google Scholar]
- Matskasi, I. A Bakony–Hegyseg kisemloseinek metely (trematodes) faunaja I. Veszprem Megyel Muz. Közl. 1973, 12, 253–256. [Google Scholar]
- Matskasi, I. The systematico-faunistical survey of the trematode fauna of hungarian bats. I. Ann. Hist.-Nat. Musei Nat. Hung. 1967, 59, 217–238. [Google Scholar]
- Styczynska-Jurewicz, E. The life cycle of Plagiorchis elegans (Rud., 1802) and revision of the genus Plagiorchis Luhe, 1889. Acta Parasitol. Pol. 1962, 10, 419–445. [Google Scholar]
- Blankespoor, H.D. Host-induced variation in Plagiorchis noblei Park, 1936 (Plagiorchiidae, Trematoda). Am. Midl. Nat. 1974, 92, 415–433. [Google Scholar] [CrossRef]
- Krasnolobova, T.A. Biological features of trematode taxonomy of genus Plagiorchis: Development of the trematode P. laricola in final hosts. Proceed. Gel. Lab. USSR 1971, 22, 92–118. [Google Scholar]
- Krasnolobova, T.A. Principles of trematode taxonomy of genus Plagiorchis Luhe, 1899. Proceed. Gel. Lab. USSR 1977, 27, 65–110. [Google Scholar]
- Krasnolobova, T.A. Trematodes of Fauna of the USSR. Genus Plagiorchis; Nauka: Moscow, Russia, 1987; pp. 3–165. [Google Scholar]
- Genov, T.; Somnaliev, P. Biology, morphology and taxonomy of Plagiorchis elegans (Rud., 1802) (Plagiorchiidae) in Bulgaria. In Fauna, Taxonomy and Ecology of Helminths in Birds; Bulgarian Academy of Sciences Publish: Sofia, Bulgaria, 1984; pp. 75–114. [Google Scholar]
- Filimonova, L.V. Life circle of Plagiorchis obtusus (Trematoda, Plagiorchiidae). Zool. Zhurn. 1973, 52, 766–769. [Google Scholar]
- Machado-Silva, J.R.; Galvao, C.; Presgrave, O.A.F.; Rey, L.; Gomes, D.C. Host-induced morphological changes of Schistosoma mansoni Sambon, 1907 male worms. Mem. Inst. Oswaldo Cruz 1994, 89, 411–416. [Google Scholar] [CrossRef]
- Perez-Ponce de Leon, G. Host-induced morphological variability in adult Posthodiplostomum minimum (Digenea: Neodiplostomidae). J. Parasitol. 1995, 81, 818–820. [Google Scholar] [CrossRef]
- Popovic, E.J.; Bjelic-Cabrilo, O.N.; Micic, R.M. Host-induced morphological variability of Pleurogenoides medians (Olson, 1876) Travassos, 1921 (Trematoda: Lecithodendriidae). Zbor. Mat. Srpsk. Prirod. Nauk. 2000, 98, 79–84. [Google Scholar]
- Hildebrand, J.; Adamczyk, M.; Laskowski, Z.; Zalesny, G. Host-dependent morphology of Isthmiophora melis (Schrank, 1788) Luhe, 1909 (Digenea, Echinostomatinae)–morphological variation vs. molecular stability. Parasites Vectors 2015, 8, 481. [Google Scholar] [CrossRef]
- Soltys, A. The helminth fauna of bats (Chiroptera) of Lublin palatinate. Acta Parasitol. Pol. 1959, 7, 599–613. [Google Scholar]
- León-Règagnon, V.; Brooks, D.R. Molecular phylogeny of Haematoloechus Looss, 1899 (Digenea: Plagiorchiidae), with emphasis on north American species. J. Parasitol. 2003, 89, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Littlewood, D.T.J. Molecular phylogenetics of cupped oysters based on partial 28S rRNA gene sequences. Mol. Phylogen. Evol. 1994, 3, 221–229. [Google Scholar] [CrossRef]
- Snyder, S.D.; Tkach, V.V. Phylogenetic and biogeographical relationships among some Holarctic frog lung flukes (Digenea: Haematoloechidae). J. Parasitol. 2001, 87, 1433–1440. [Google Scholar] [CrossRef]
- Kudlai, O.; Stunzenas, V.; Tkach, V. The taxonomic identity and phylogenetic relationships of Cercaria pugnax and C. helvetica XII (Digenea: Lecithodendriidae) based on morphological and molecular data. Fol. Parasitol. 2015, 62, 3. [Google Scholar] [CrossRef]
- Enabulele, E.E.; Lawton, S.P.; Walker, A.J.; Kirk, R.S. Molecular and morphological characterization of the cercariae of Lecithodendrium linstowi (Dollfus, 1931), a trematode of bats, and incrimination of the first intermediate snail host, Radix balthica. Parasitology 2018, 145, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Sasaki, M. Trematode diversity in freshwater snails from a stopover point for migratory waterfowls in Hokkaido, Japan: An assessment by molecular phylogenetic and population genetic analyses. Parasitol. Int. 2021, 83, 102329. [Google Scholar] [CrossRef] [PubMed]
- Dellagnola, F.A.; Montes, M.M.; Martorelli, S.R.; Vega, I.A. Morphological characterization and molecular phylogeny of zoonotic trematodes in the freshwater snail Asolene platae. Parasitology 2019, 146, 839–848. [Google Scholar] [CrossRef]
- Kanarek, G.; Zalesny, G.; Sitko, J.; Tkach, V.V. Phylogenetic relationships and systematic position of the families Cortrematidae and Phaneropsolidae (Platyhelminthes: Digenea). Fol. Parasitol. 2014, 61, 523. [Google Scholar] [CrossRef]
- Kanarek, G.; Zaleśny, G.; Sitko, J.; Tkach, V.V. The systematic position and structure of the genus Leyogonimus Ginetsinskaya, 1948 (Platyhelminthes: Digenea) with comments on the taxonomy of the superfamily Microphalloidea Ward, 1901. Acta Parasitol. 2017, 62, 617–624. [Google Scholar] [CrossRef]
- Al-Kandari, W.Y.; Al-Bustan, S.A.; Alnaqeeb, M. Ribosomal DNA sequence characterization of Maritrema cf. eroliae Yamaguti, 1939 (Digenea: Microphallidae) and its life cycle. J. Parasitol. 2011, 97, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Presswell, B.; Blasco-Costa, I.; Kostadinova, A. Two new species of Maritrema Nicoll, 1907 (Digenea: Microphallidae) from New Zealand: Morphological and molecular characterisation. Parasitol. Res. 2014, 113, 1641–1656. [Google Scholar] [CrossRef] [PubMed]
- Kudlai, O.; Cutmore, S.C.; Cribb, T.H. Morphological and molecular data for three species of the Microphallidae (Trematoda: Digenea) in Australia, including the first descriptions of the cercariae of Maritrema brevisacciferum Shimazu et Pearson, 1991 and Microphallus minutus Johnston, 1948. Fol. Parasitol. 2015, 62, 1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Olson, P.D.; Cribb, T.H.; Tkach, V.V.; Bray, R.A.; Littlewood, D.T.J. Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int. J. Parasitol. 2003, 33, 733–755. [Google Scholar] [CrossRef]
- Galaktionov, K.V.; Blasco-Costa, I.; Olson, P.D. Life cycles, molecular phylogeny and historical biogeography of the ‘pygmaeus’ microphallids (Digenea: Microphallidae): Widespread parasites of marine and coastal birds in the Holarctic. Parasitology 2012, 139, 1346–1360. [Google Scholar] [CrossRef]
- Galaktionov, K.V.; Blasco-Costa, I. Microphallus ochotensis sp. nov. (Digenea, Microphallidae) and relative merits of two-host microphallid life cycles. Parasitol. Res. 2018, 117, 1051–1068. [Google Scholar] [CrossRef]
- Athokpam, V.; (North-Eastern Hill University, Shillong, India); Tandon, V.; (North-Eastern Hill University, Shillong, India). Personal communication, 2022. Direct Submission.
- Kakui, K. A novel transmission pathway: First report of a larval trematode in a tanaidacean crustacean. Fauna Ryukyuana 2014, 17, 13–22. [Google Scholar]
- Pina, S.; Russell-Pinto, F.; Rodrigues, P. Morphological and molecular study of Microphallus primas (Digenea: Microphallidae) metacercaria, infecting the shore crab Carcinus maenas from northern Portugal. Fol. Parasitol. 2011, 58, 48–54. [Google Scholar] [CrossRef]
- O’Dwyer, K.; Faltýnková, A.; Georgieva, S.; Kostadinova, A. An integrative taxonomic investigation of the diversity of digenean parasites infecting the intertidal snail Austrolittorina unifasciata Gray, 1826 (Gastropoda: Littorinidae) in Australia. Parasitol. Res. 2015, 114, 2381–2397. [Google Scholar] [CrossRef]
- Kudlai, O.; Cribb, T.H.; Cutmore, S.C. A new species of microphallid (Trematoda: Digenea) infecting a novel host family, the Muraenidae, on the northern Great Barrier Reef, Australia. Syst. Parasitol. 2016, 93, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.A.; González-Acuña, D.; Tkach, V.V. First record of Gyrabascus (Digenea, Pleurogenidae) from Dromiciops bozinovici D’Elia et al., 2016 (Marsupialia: Microbiotheriidae) in Chile and its phylogenetic relationships. Comp. Parasitol. 2018, 85, 58–65. [Google Scholar] [CrossRef]
- Kanarek, G.; Zaleśny, G.; Czujkowska, A.; Sitko, J.; Harris, P.D. On the systematic position of Collyricloides massanae Vaucher, 1969 (Platyhelminthes: Digenea) with notes on distribution of this trematode species. Parasitol. Res. 2015, 114, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Boyce, K.; Hide, G.; Craig, P.S.; Reynolds, C.; Hussain, M.; Bodell, A.J.; Bradshaw, H.; Pickles, A.; Rogan, M.T. A molecular and ecological analysis of the trematode Plagiorchis elegans in the wood mouse Apodemus sylvaticus from a periaquatic ecosystem in the UK. J. Helminthol. 2014, 88, 310–320. [Google Scholar] [CrossRef][Green Version]
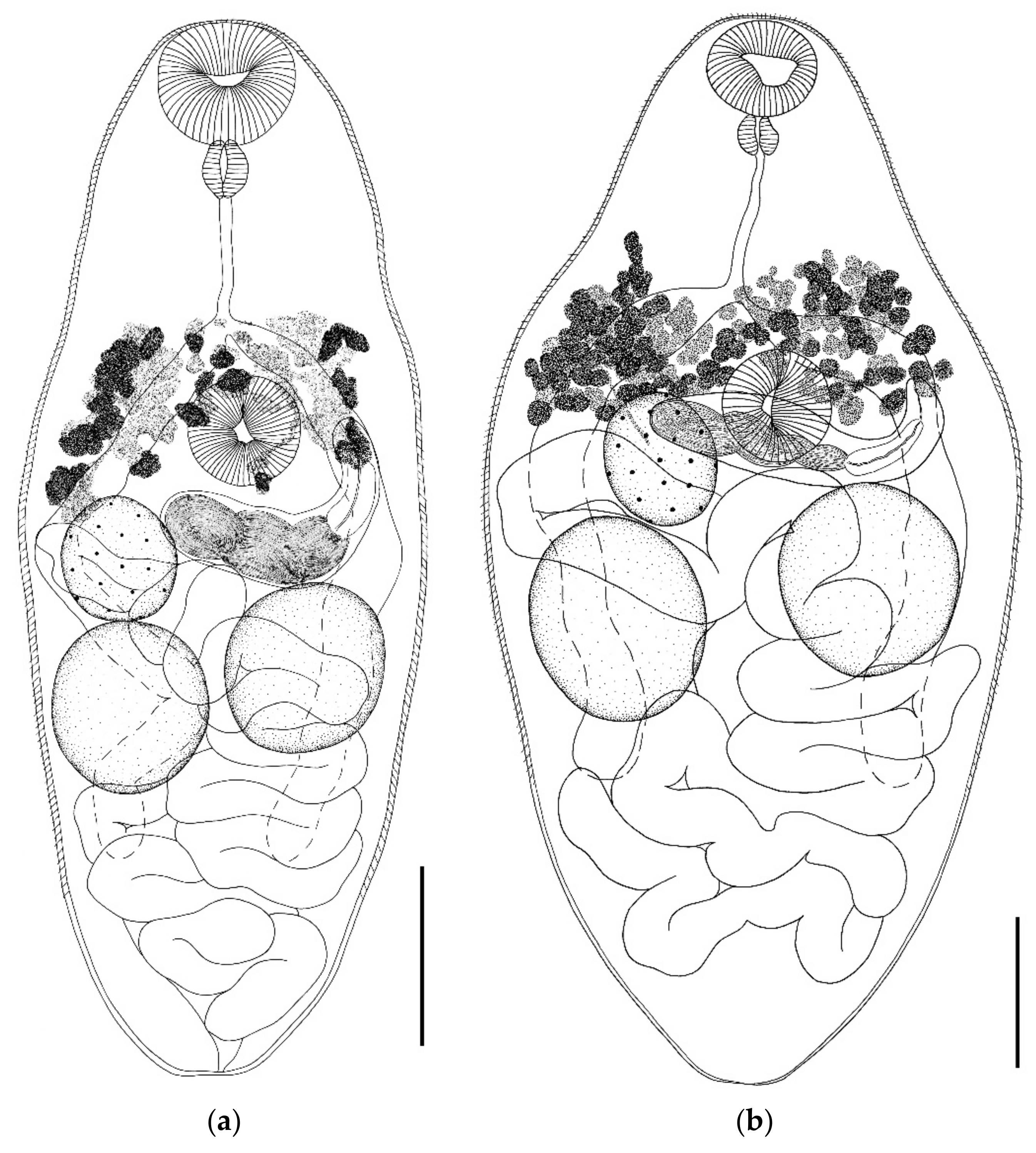
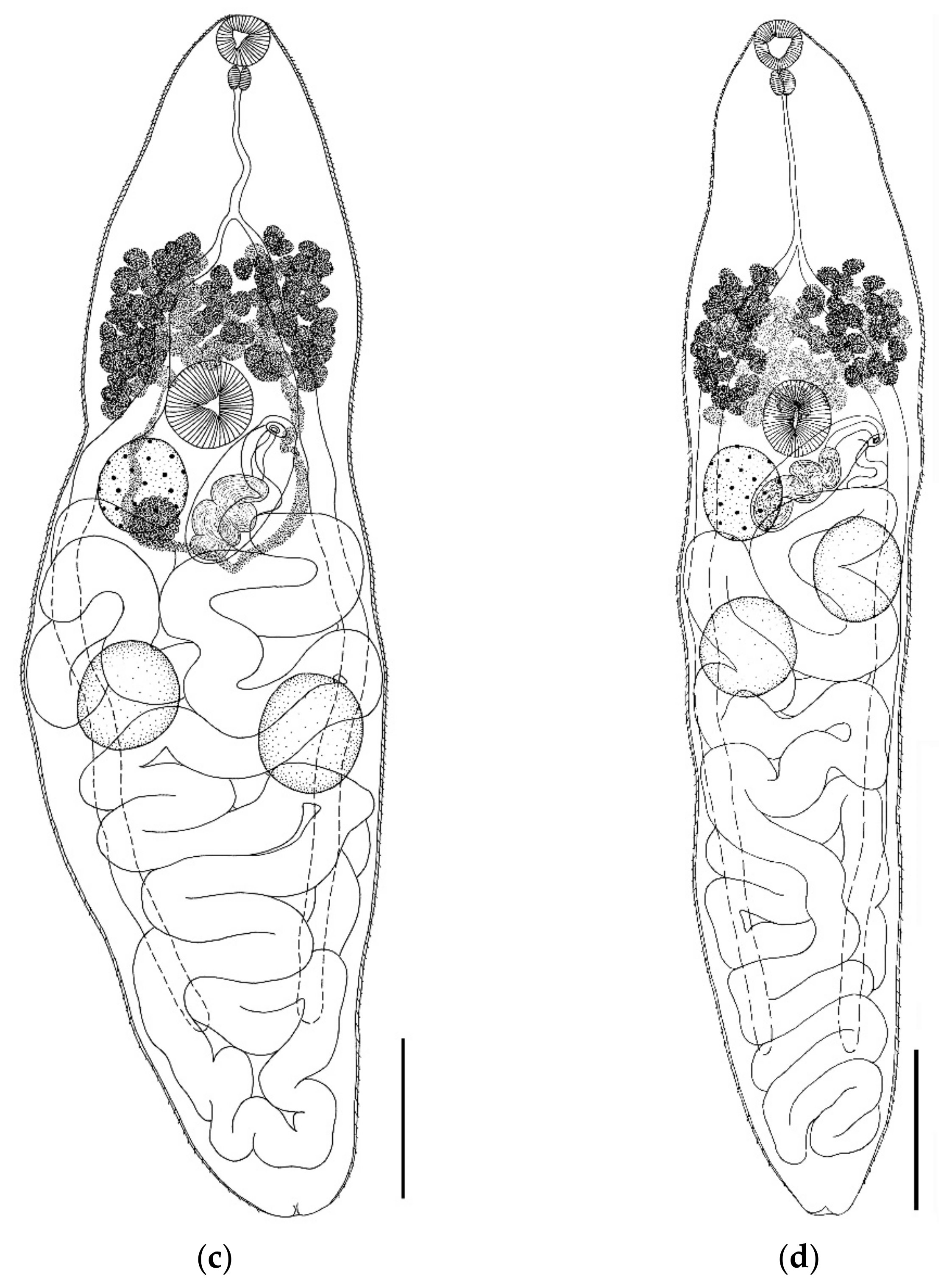


| Gyrabascus oppositus | Parabascus duboisi | Parabascus semisquamosus | Parabascus lepidotus | |||||
|---|---|---|---|---|---|---|---|---|
| Host | N. noctula | P. nathusii | M. daubentonii | M. brandtii | P. nathusii | N. noctula | V. murinus | N. noctula |
| Locality | Smolny | Smolny | Pushta | Smolny | Smolny | Pushta | Pushta | Pushta |
| Body length | 941–1230 (1073) | 652–948 (776) | 394–1020 (611) | 520–852 (679) | 1031–1523 (1343) | 1354–1892 (1608) | 441–770 (599) | 844–1160 (967) |
| Body width | 326–450 (382) | 348–460 (394) | 187–415 (245) | 217–422 (348) | 246–415 (362) | 277–460 (354) | 272–519 (386) | 385–619 (479) |
| OS length | 79–110 (94) | 69–91 (81) | 56–95 (68) | 55–79 (67) | 40–63 (53) | 47–63 (55) | 43–63 (52) | 49–79 (64) |
| OS width | 54–75 (67) | 49–69 (60) | 63–98 (74) | 59–87 (75) | 40–68 (54) | 49–67 (59) | 43–67 (54) | 51–83 (68) |
| VS length | 91–114 (104) | 102–126 (110) | 49–95 (64) | 49–79 (67) | 73–110 (90) | 75–106 (92) | 45–75 (60) | 63–98 (77) |
| VS width | 110–138 (126) | 79–106 (91) | 51–91 (64) | 47–79 (66) | 79–113 (93) | 75–110 (98) | 43–69 (58) | 55–106 (78) |
| Pharynx length | 39–51 (44) | 30–37 (33) | 24–39 (31) | 24–35 (29) | 24–39 (31) | 30–39 (34) | 24–32 (27) | 24–43 (32) |
| Pharynx width | 39–51 (45) | 30–37 (34) | 24–39 (31) | 22–35 (31) | 24–39 (31) | 26–41 (33) | 24–34 (29) | 24–45 (37) |
| Esophagus | 148–217 (178) | 126–177 (156) | 40–122 (71) | 51–118 (87) | 150–268 (189) | 187–335 (268) | 102–181 (135) | 217–293 (240) |
| Testes length | 100–158 (125) | 98–126 (114) | 63–138 (101) | 87–146 (114) | 118–165 (135) | 118–193 (151) | 71–130 (106) | 106–185 (143) |
| Testes width | 91–130 (109) | 86–106 (96) | 67–118 (86) | 87–138 (106) | 87–134 (115) | 91–165 (126) | 75–118 (103) | 106–169 (133) |
| Cirrus sac length | – | – | 158–275 (198) | 197–276 (234) | 138–226 (153) | 177–267 (215) | 154–205 (175) | 191–311 (248) |
| Cirrus sac width | – | – | 34–67 (46) | 37–55 (45) | 51–93 (60) | 59–110 (80) | 51–79 (64) | 67–85 (78) |
| Ovary length | 110–138 (120) | 86–118 (97) | 47–98 (66) | 51–102 (75) | 87–146 (113) | 96–157 (126) | 60–102 (91) | 102–165 (126) |
| Ovary width | 87–114 (103) | 78–110 (91) | 47–106 (68) | 55–98 (76) | 79–134 (102) | 83–139 (114) | 55–98 (82) | 104–165 (117) |
| Eggs length | 24–30 (27) | 23–26 (25) | 20–26 (23) | 20–26 (24) | 19–24 (21) | 19–24 (21) | 19–24 (20) | 20–24 (21) |
| Eggs width | 12–16 (14) | 11–14 (12) | 12–16 (13) | 12–16 (14) | 8–12 (10) | 8–12 (10) | 10–14 (12) | 11–14 (12) |
| Body length/width ratio | 2.6–3.1 (2.8) | 1.8–2.2 (2.0) | 1.5–3.0 (2.5) | 1.6–2.8 (2.0) | 3.2–4.9 (3.7) | 3.3–5.2 (4.6) | 1.1–1.8 (1.6) | 1.5–2.5 (2.1) |
| OS/VS width ratio | 0.5–0.6 (0.5) | 0.5–0.6 (0.6) | 1.0–1.4 (1.2) | 1.0–1.2 (1.1) | 0.6–0.7 (0.6) | 0.6–0.7 (0.7) | 0.7–0.9 (0.8) | 0.7–0.9 (0.8) |
| OS/pharynx width ratio | 1.3–1.7 (1.5) | 1.5–1.9 (1.8) | 2.0–2.8 (2.5) | 2.1–3.0 (2.4) | 1.6–1.9 (1.7) | 1.6–2.3 (1.8) | 1.7–2.1 (1.9) | 1.6–2.3 (1.8) |
| Modlinger [38] | Dubois [64,65] | Hurkova [37,63] | Odening [27] | Zdzietowiecki [28] | Matskasi [67] | Khotenovsky [48] | Sharpilo, Iskova [33] | |
|---|---|---|---|---|---|---|---|---|
| Host | M. blythii | M. mystacinus, M. daubentonii | M. mystacinus, M. daubentonii, M. emarginatus, M. myotis, P. austriacus | M. myotis | M. myotis, M. dasycneme, M. daubentonii, M. mystacinus, B. barbastellus | M. daubentonii | M. daubentonii | M. bechsteinii, M. myotis, N. noctula, E. serotinus |
| Locality | Hungary | France, Switzerland | former Czechoslovakia | Germany | Poland | Hungary | Russia | Ukraine |
| Body size | 580–590 × 390 | 600–720 × 370–540 | 370–770 × 270–610 | 1050–1210 × 650–820 | 351–865 × 207–572 | 555 × 475 | 420–630 × 240–340 | 820–950 × 550–680 |
| Oral sucker | 50–60 | 60–90 | 40–70 | 80–90 | 39–94 × 44–84 | 43×50 | 40–70 | 66–88 × 77–93 |
| Ventral sucker | 140 1 | 100–200 × 120–160 | 80 × 140 | 120–170 × 140–180 | 63–129 × 80–173 | 95 × 125 | 60–80 × 80–100 | 140–150 × 130–150 |
| Pharynx | 50–60 | 40–50 | 30–40 | 40–50 × 500–600 | 23–55 × 26–58 | – | 30–50 | 33–44 × 38–49 |
| Esophagus | Very short | 60–100 | – | 140–230 | 44–171 | – | 50–150 | 110–160 |
| Testes | 260–270 × 180–190 | 100–160 × 160–200 | 110–200 × 80–140 | 260–340 × 150–210 | 58–182 × 46–202 | 125×87 | 60–120 × 70–110 | 170–270 × 140–170 |
| Ovary | 130 1 | 60–100 × 90–130 | 80–140 × 60–90 | 110–220×100–230 | 43–110 × 44–110 | 80 × 65 | 60–80 1 | 0.11–0.16 × 0.11–0.15 |
| Eggs | 26 × 11 | 21–26 × 10–12 | 20–26 × 8–13 | 19–23 × 9–12 | 21–27 × 10–14 | – | 20–28 × 11–14 | 20–22 × 10–13 |
| Sokolov et al. [30] | Mituch [39] | Zdzietowiecki [28] | |
|---|---|---|---|
| Host | Pipistrellus kuhlii | Miniopterus schreibersii | Eptesicus serotinus |
| Locality | Russia | former Czechoslovakia | Poland |
| Body size | 620–1010 × 330–440 | 1077 × 385 | 763–1070 × 370–547 |
| Oral sucker | 89–108 × 57–70 | 93 × 66 | 63–72 × 71–73 |
| Ventral sucker | 82–108 × 108–133 | 146 × 106 | 103–121 × 137–148 |
| Pharynx | 38–51 × 38–51 | – | 42–48 × 39–49 |
| Esophagus | 120–215 | 266 | 125–178 |
| Testes | 90–152 × 76–138 | 106 × 66 | 94–158 × 90–142 |
| Ovary | 82–114 × 95–152 | 100 × 60 | 69–105 × 77–87 |
| Eggs | 24–26 × 13–16 | 15 × 9 | 24–28 × 12–14 |
| Morozov [66] | Hurkova [63] | Odening [27] | Zdzietowiecki [28] | Skvortsov [24] | Matskasi [67] | Khotenovsky [29] | Kirillov et al. [17] | |
|---|---|---|---|---|---|---|---|---|
| Host | M. daubentonii, P. pipistrellus | M. dasycneme, M. dasycneme, M. mystacinus | M. daubentonii, M. myotis | M. daubentonii, M. dasycneme, M. emarginatus | M. daubentonii | M. daubentonii | M. daubentonii | M. dasycneme |
| Locality | Belarus | former Czechoslovakia | Germany | former Czechoslovakia, Poland | Moldova | Hungary | Russia | Russia |
| Body size | 700–820 × 350–460 | 550–910 × 220–370 | 323–595 × 198–330 | 478–984 × 191–432 | 292–986 × 132–520 | 950 × 580 | length 290–900 | 667–815 × 274–414 |
| Oral sucker | 70–80 × 60–90 | 50–80 1 | 49–62 × 42–72 | 54–86 × 65–94 | 42–74 × 45–93 | 62 × 75 | 53–100 1 | 74–82 × 78–85 |
| Ventral sucker | 70 × 60–70 | 50–70 1 | 40–60 × 46–58 | 54–78 × 56–87 | 39–98 × 42–101 | 63 × 100 | 46–90 1 | 67–76 × 74–79 |
| Pharynx | 30–40 1 | 20–40 1 | 23–26 × 21–33 | 24–43 × 26–43 | 21–39 × 26–42 | – | 22–24 1 | 24–39 × 28–43 |
| Esophagus | 80–110 | 60–100 | 40–99 | 52–125 | 34–133 | – | – | 52–98 |
| Testes | 100–170 × 80–140 | 80–130 × 80–140 | 67–107 × 56–90 | 69–161 × 64–142 | 58–172 × 79–207 | 150 × 125 | – | 72–93 × 76–122 |
| Cirrus sac | 190–260 × 80–140 | 120–220 × 50–100 | 86–151 × 28–51 | 38–81 | 86–255 × 42–79 | – | Small | 143–212 × 47–58 |
| Ovary | 90–110 × 80–140 | 60–80 × 60–100 | 37–81 × 33–74 | 54–116 × 48–119 | 53–138 × 58–159 | 90 × 130 | Spherical | 62–86 × 71–94 |
| Eggs | 22–25 × 11–13 | 22–26 × 12–16 | 19–23 × 9–12 | 21–26 × 12–15 | 21–26 × 18–21 | – | 22–28 × 12–16 | 18–24 × 8–12 |
| Looss [35] | Modlinger [38] | Hurkova [37,63] | Zdzietowiecki [28] | Skvortsov [24] | Khotenovsky [47] | Sharpilo, Iskova [33] | Kirillov et al. [17] | |
|---|---|---|---|---|---|---|---|---|
| Host | P. kuhlii | E. serotinus | N. noctula, P. pipistrellus | E. serotinus, M. nattereri | E. serotinus | E. serotinus | E. serotinus | E. nilssoni |
| Locality | Egypt | Hungary | former Czechoslovakia | Poland | Moldova | Uzbekistan | Ukraine | Russia |
| Body size | 1100 × 430 | 860 × 350 | 1030–1300 × 460–650 | 836–847 × 366–424 | 854–1396 × 530–686 | 790–1300 × 360–600 | 1000–1400 × 670–780 | 907–1277 × 461–675 |
| Oral sucker | 80 1 | 70 1 | 70–80 1 | 66–68 × 56–77 | 69–93 × 79–98 | 60–90 1 | 70–75 × 82–115 | 63–85 × 78–91 |
| Ventral sucker | 150 1 | 150 1 | 130–140 1 | 82–101 × 74–94 | 96–127 × 111–148 | 100–150 1 | 105–110 × 90–120 | 83–94 × 86–98 |
| Pharynx | 60 × 30 | 50 1 | 40–50 1 | 32–41 × 28–43 | 37–53 × 47–58 | 30–60 1 | 38–43 1 | 32–36 × 35–49 |
| Esophagus | Long | Long | 220–230 | 141–170 | 172–287 | 110–230 | 220–270 | 220–272 |
| Testes | Large | 200 1 | 100–120 × 150–170 | 117–198 × 101–157 | 106–178 × 172–226 | 120–220 × 130–210 | 140–240 1 | 114–137 × 128–143 |
| Cirrus sac | – | – | 220–230 × 90 | Width 110 | 212–411 × 85–118 | 200–300 × 80–110 | 270–300 × 80–120 | 216–305 × 78–83 |
| Ovary | – | 150 1 | 130–140 × 90–100 | 86–144 × 75–81 | 118–145 × 122–178 | 110–160 1 | 130–150 × 110–130 | 98–116 × 102–125 |
| Eggs | 22–23 × 13 | 29–30 × 11 | 24–25 × 13–15 | 18–23 × 11–15 | 18–23 × 10–13 | 20–25 × 11–14 | 21–23 × 11–13 | 20–24 × 11–14 |
| Braun [34] | Soltys [80] | Dubois [64] | Odening [27] | Zdzietowiecki [28] | Skvortsov [24] | Khotenovsky [29] | Sharpilo, Iskova [33] | Kirillov et al. [17] | |
|---|---|---|---|---|---|---|---|---|---|
| Host | N. noctula | N. noctula | N. noctula | P. pipistrellus | N. noctula | N. noctula | M. daubentonii | N. noctula | P. nathusii |
| Locality | Germany | Poland | France | Germany | Poland | Moldova | Russia | Ukraine | Russia |
| Body size | 1500 × 260 | 1000–1560 × 250–400 | 1470–1960 × 310–370 | 1210–1630 × 470–630 | 1347 × 254 | 266–1712 × 118–421 | 900–2600 | 1300–1800 × 330–470 | 1061–1617 × 215–446 |
| Oral sucker | 70–90 1 | 50–80 1 | 70–80 × 80 | 51–52 × 62–72 | 63–66 × 65–74 | 45–74 × 47–82 | 40–90 1 | 55–77 × 60–88 | 47–60 × 55–71 |
| Ventral sucker | 110 × 90 | – | 90–100 × 130–140 | 106–110 × 109–114 | 87–107 × 82–105 | 39–106 × 47–118 | 52–140 1 | 93–125 × 99–130 | 59–88 × 67–90 |
| Pharynx | 40 1 | 30 1 | 40–50 1 | 28–32 × 26–38 | 32–35 × 33–36 | 21–26 × 31–39 | 19–38 1 | 30–40 1 | 25–31 × 30–35 |
| Esophagus | Medium length | 160–240 | 260 | 106–131 | 282 | 85–250 | – | 220–360 | 192–268 |
| Testes | – | 150–200 | 160–200 × 140–170 | 144–228 × 104–172 | 103–135 × 73–91 | 63–140 × 87–174 | – | 80–190 × 100–170 | 90–118 × 74–87 |
| Cirrus sac | – | 120–200 × 70 | 310–350 × 90–100 | 224–259 × 93–104 | Width 74–75 | 106–258 × 47–98 | – | 220–300 × 60–100 | 190–200 × 58–79 |
| Ovary | Postacetobular | 150–180 | 160–170 × 130–140 | 127–135 × 90–143 | 96–166 × 78–94 | 61–106 × 54–159 | – | 65–160 × 66–140 | 67–83 × 75–98 |
| Eggs | 23–18 | 20 × 10 | 20–22 × 10–14 | 18–21 × 9–14 | 18–22 × 11–13 | 18–23 × 13–15 | 18–25 × 9–15 | 19–22 × 11 | 17–20 × 9–11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirillova, N.Y.; Shchenkov, S.V.; Kirillov, A.A.; Ruchin, A.B. Trematodes of Genera Gyrabascus and Parabascus from Bats in European Russia: Morphology and Molecular Phylogeny. Biology 2022, 11, 878. https://doi.org/10.3390/biology11060878
Kirillova NY, Shchenkov SV, Kirillov AA, Ruchin AB. Trematodes of Genera Gyrabascus and Parabascus from Bats in European Russia: Morphology and Molecular Phylogeny. Biology. 2022; 11(6):878. https://doi.org/10.3390/biology11060878
Chicago/Turabian StyleKirillova, Nadezhda Yu., Sergei V. Shchenkov, Alexander A. Kirillov, and Alexander B. Ruchin. 2022. "Trematodes of Genera Gyrabascus and Parabascus from Bats in European Russia: Morphology and Molecular Phylogeny" Biology 11, no. 6: 878. https://doi.org/10.3390/biology11060878
APA StyleKirillova, N. Y., Shchenkov, S. V., Kirillov, A. A., & Ruchin, A. B. (2022). Trematodes of Genera Gyrabascus and Parabascus from Bats in European Russia: Morphology and Molecular Phylogeny. Biology, 11(6), 878. https://doi.org/10.3390/biology11060878









