Pelagic Bacteria and Viruses in a High Arctic Region: Environmental Control in the Autumn Period
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Processing
2.3. Statistical Analyses
3. Results
3.1. Environmental Parameters
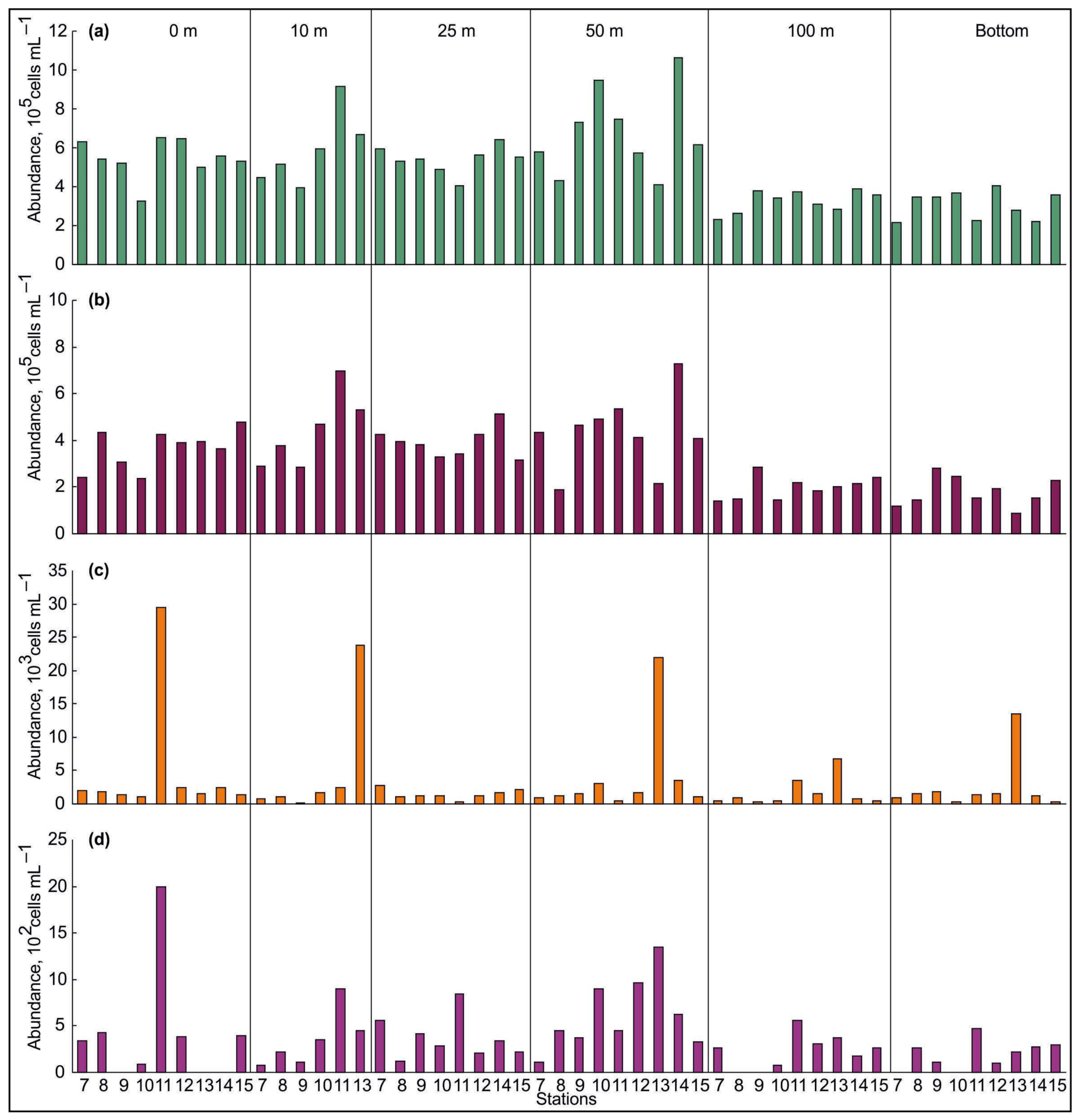
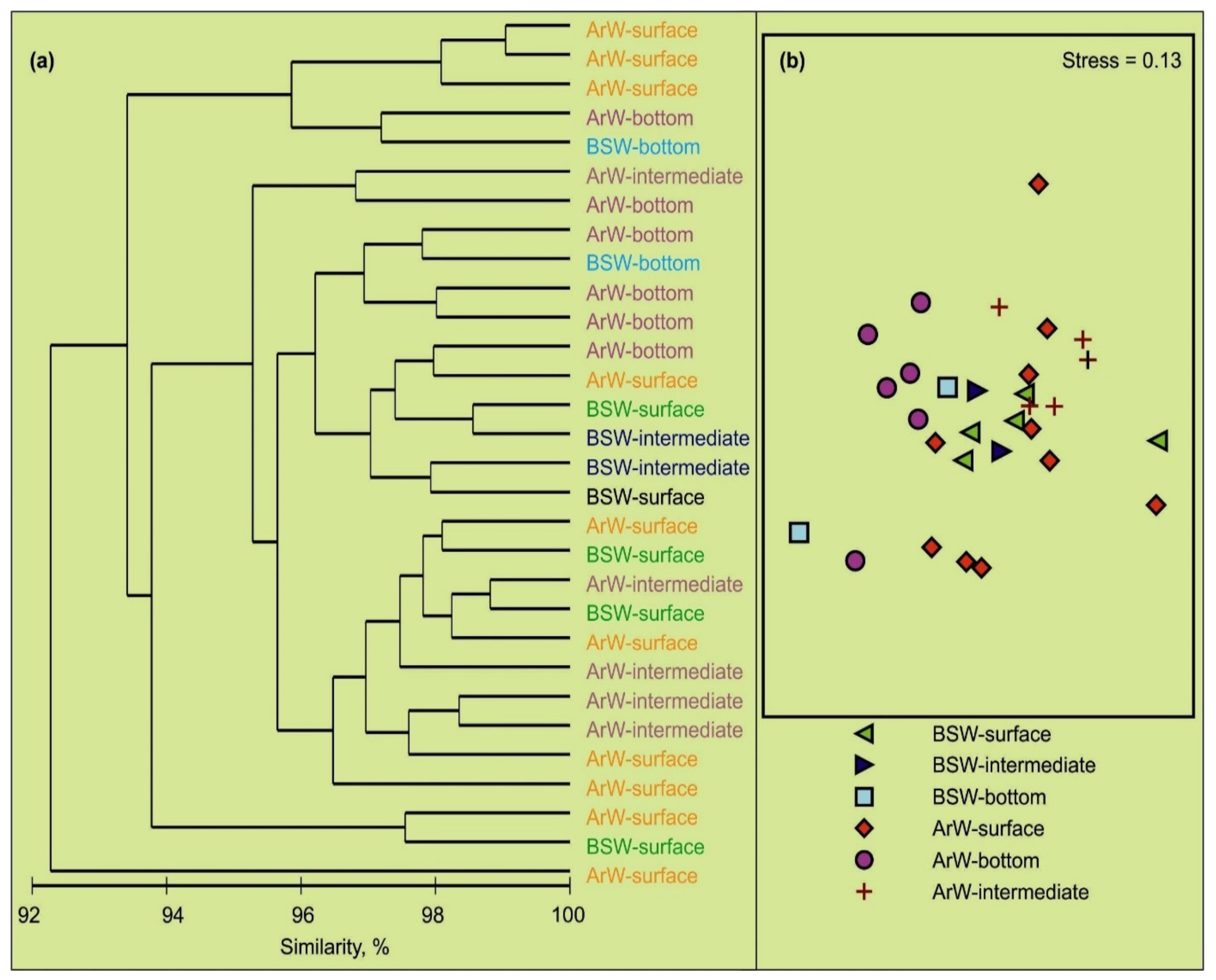
3.2. Spatial Variations of Microbial Plankton
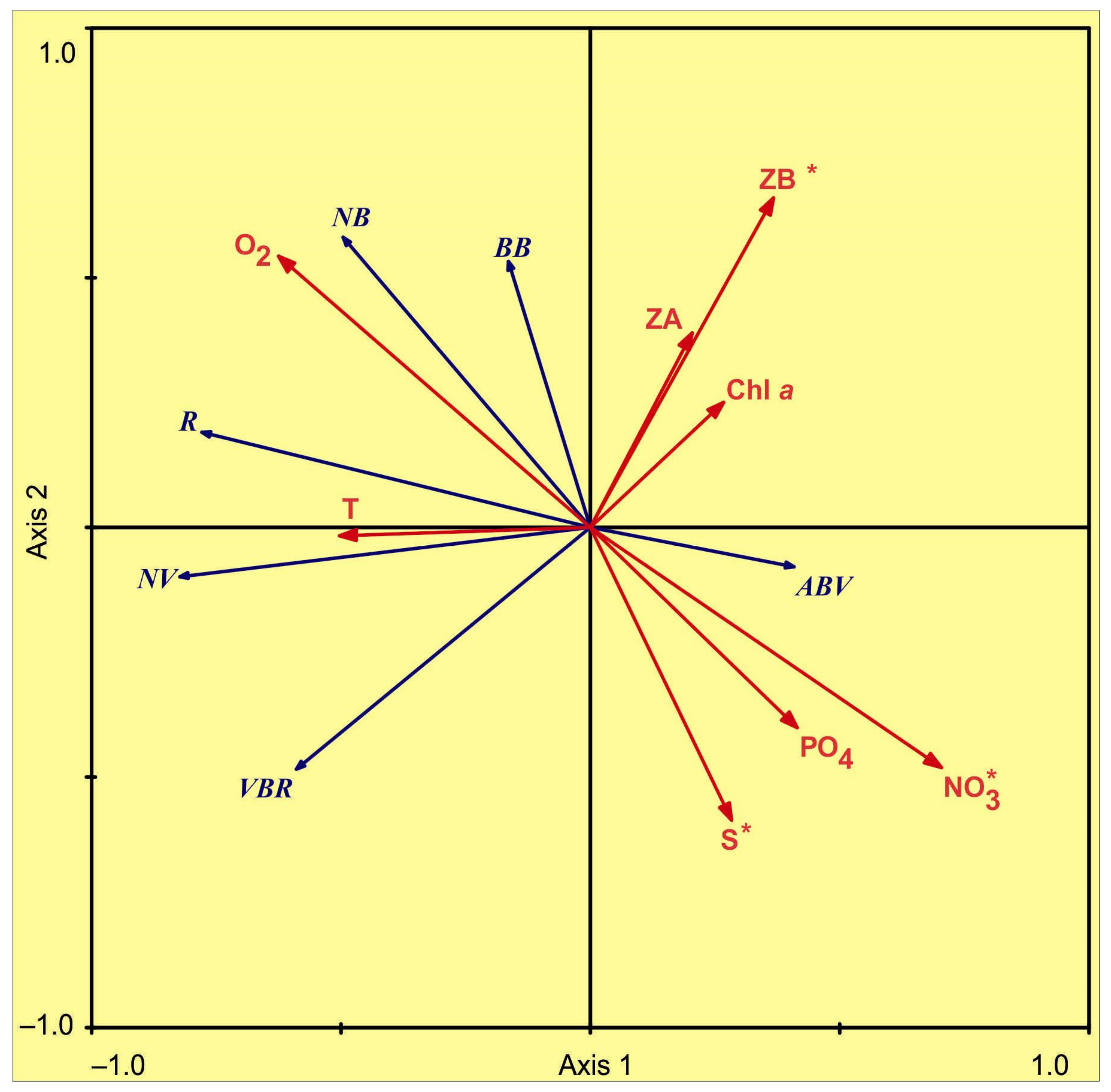
3.3. Environmental Control of Microbial Plankton
4. Discussion
4.1. Environmental Parameters
4.2. Spatial Variation of Microbial Plankton
4.3. Environmental Control of Microbial Plankton
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakshaug, E.; Johnsen, G.; Kovacs, K. (Eds.) Ecosystem Barents Sea; Tapir Academic Press: Trondheim, Norway, 2009. [Google Scholar]
- Jakobsen, T.; Ozhigin, V.K. (Eds.) The Barents Sea: Ecosystem, Resources, Management: Half a Century of Russian-Norwegian Cooperation; Tapir Academic Press: Trondheim, Norway, 2011. [Google Scholar]
- ICES. Working Group on the Integrated Assessments of the Barents Sea (WGIBAR). ICES Sci. Rep. 2021, 3, 77, 1–236. [Google Scholar]
- Ozhigin, V.; Ivshin, V.; Trofimov, A.; Karsakov, A.L.; Antsiferov, M. The Barents Sea Water: Structure, Circulation, Variability; PINRO Press: Murmansk, Russia, 2016. [Google Scholar]
- Polyakov, I.V.; Alkire, M.B.; Bluhm, B.A.; Brown, K.A.; Carmack, E.C.; Chierici, M.; Danielson, S.L.; Ellingsen, I.; Ershova, E.A.; Gårdfeldt, K.; et al. Borealization of the Arctic Ocean in response to anomalous advection from sub-arctic seas. Front. Mar. Sci. 2020, 7, 491. [Google Scholar] [CrossRef]
- Ardyna, M.; Arrigo, K. Phytoplankton dynamics in a changing Arctic Ocean. Nat. Clim. Change 2020, 10, 892–903. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Epiplankton in the Barents Sea: Summer variations of mesozooplankton biomass, community structure and diversity. Cont. Shelf Res. 2013, 52, 1–11. [Google Scholar] [CrossRef]
- Kirchman, D.L.; Morán, X.A.G.; Ducklow, H. Microbial growth in the polar oceans—Role of temperature and potential impact of climate change. Nat. Rev. Microbiol. 2009, 7, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Boras, J.A.; Sala, M.M.; Arrieta, J.M.; Sá, E.L.; Felipe, J.; Agustí, S.; Duarte, C.M.; Vaqué, D. Effect of ice melting on bacterial carbon fluxes channeled by viruses and protists in the Arctic Ocean. Polar Biol. 2010, 33, 1695–1707. [Google Scholar] [CrossRef]
- Ducklow, H.W. Minireview: The bacterial content of the oceanic euphotic zone. FEMS Microbiol. Ecol. 1999, 30, 1–10. [Google Scholar] [CrossRef]
- Fuhrman, J.A. Microbial community structure and its functional implications. Nature 2009, 459, 193–199. [Google Scholar] [CrossRef]
- Wommack, K.E.; Colwell, R.R. Virioplankton: Viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 2000, 64, 69–114. [Google Scholar] [CrossRef]
- Suttle, C.A. Marine viruses—Major players in the global ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef]
- Wilhelm, S.; Suttle, A.C. Viruses and Nutrient Cycles in the Sea. BioScience 1999, 49, 781–788. [Google Scholar] [CrossRef]
- Steward, G.F.; Fandino, L.B.; Hollibaugh, J.T.; Whitledge, T.E.; Azam, F. Microbial biomass and viral infections of heterotrophic prokaryotes in the sub-surface layer of the central Arctic Ocean. Deep-Sea Res. I 2007, 54, 1744–1757. [Google Scholar] [CrossRef]
- Steward, G.F.; Smith, D.C.; Azam, F. Abundance and production of bacteria and viruses in the Bering and Chukchi Seas. Mar. Ecol. Prog. Ser. 1996, 131, 287–300. [Google Scholar] [CrossRef]
- Sherr, B.F.; Sherr, E.B. Community respiration/production and bacterial activity in the upper water column of the central Arctic Ocean. Deep-Sea Res. I 2003, 50, 529–542. [Google Scholar] [CrossRef]
- Sherr, E.B.; Sherr, B.F.; Wheeler, P.A.; Thompson, K. Temporal and spatial variation in stocks of autotrophic and heterotrophic microbes in the upper water column of the central Arctic Ocean. Deep-Sea Res. I 2003, 50, 557–571. [Google Scholar] [CrossRef]
- Rokkan Iversen, K.; Seuthe, L. Seasonal microbial processes in a high-latitude fjord (Kongsfjorden, Svalbard): I. Heterotrophic bacteria, picoplankton and nanoflagellates. Polar Biol. 2011, 34, 731–749. [Google Scholar] [CrossRef]
- Yau, S.; Seth-Pasricha, M. Viruses of Polar Aquatic Environments. Viruses 2019, 11, 189. [Google Scholar] [CrossRef]
- Howard-Jones, M.H.; Ballard, V.D.; Allen, A.E.; Frischer, M.E.; Verity, P.G. Distribution of bacterial biomass and activity in the marginal ise zone of the central Barents Sea during summer. J. Mar. Syst. 2002, 38, 77–91. [Google Scholar] [CrossRef]
- Sturluson, M.; Nielsen, T.G.; Wassmann, P. Bacterial abundance, biomass and production during spring blooms in the northern Barents Sea. Deep-Sea Res. II 2008, 55, 2186–2198. [Google Scholar] [CrossRef]
- Tammert, H.; Olli, K.; Sturluson, M.; Hodal, H. Bacterial biomass and activity in the marginal ice zone of the northern Barents Sea. Deep-Sea Res. II 2008, 55, 2199–2209. [Google Scholar] [CrossRef]
- Venger, M.P.; Shirikolobova, T.I.; Makarevich, P.R.; Vodopyanova, V.V. Viruses in the pelagic zone of the Barents Sea. Dokl. Biol. Sci. 2012, 446, 345–349. [Google Scholar] [CrossRef]
- Venger, M.P.; Kopylov, A.I.; Zabotkina, E.A.; Makarevich, P.R. The influence of viruses on bacterioplankton of the offshore and coastal parts of the Barents Sea. Rus. J. Mar. Biol. 2016, 42, 26–35. [Google Scholar] [CrossRef]
- Shirikolobova, T.I.; Zhichkin, A.P.; Venger, M.P.; Vodopyanova, V.V.; Moiseev, D.V. Bacteria and viruses of the ice-free aquatic area of the Barents sea at the beginning of polar night. Dokl. Biol. Sci. 2016, 469, 182–186. [Google Scholar]
- Makarevich, P.R. (Ed.) Cruise Report of the Complex Expedition to the R/V Dalnie Zelentsy in the Barents Sea (October 01–October 15, 2020); MMBI: Murmansk, Russia, 2020. [Google Scholar]
- Dvoretsky, V.G.; Dvoretsky, A.G. Copepod communities off Franz Josef Land (northern Barents Sea) in late summer of 2006 and 2007. Polar Biol. 2011, 34, 1231–1238. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Arctic marine mesozooplankton at the beginning of the polar night: A case study for southern and south-western Svalbard waters. Polar Biol. 2020, 43, 71–79. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Mesozooplankton in the Kola Transect (Barents Sea): Autumn and winter structure. J. Sea Res. 2018, 142, 18–22. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Coastal mesozooplankton assemblages during spring bloom in the eastern Barents Sea. Biology 2022, 11, 204. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Sea Water Analysis; Pergamon Press: New York, NY, USA, 1992. [Google Scholar]
- Aminot, A.; Rey, F. Standard Procedure for the Determination of Chlorophyll A by Spectroscopic Methods; International Council for the Exploration of the Sea: Copenhagen, Denmark, 2000. [Google Scholar]
- Chislenko, L.L. Nomogrammes to Determine Weights of Aquatic Organisms Based on the Size and Form of Their Bodies (Marine Mesobenthos and Plankton); Nauka Press: Leningrad, USSR, 1968. (In Russian) [Google Scholar]
- Hanssen, H. Mesozooplankton of the Laptev Sea and the adjacent eastern Nansen Basin—Distribution and community structure in late summer. Ber. Polarforsch. 1997, 229, 1–131. [Google Scholar]
- Richter, C. Regional and seasonal variability in the vertical distribution of mesozooplankton in the Greenland Sea. Ber. Polarforsch. 1994, 154, 1–90. [Google Scholar]
- Harris, R.P.; Wiebe, P.H.; Lenz, J.; Skjoldal, H.R.; Huntley, M. (Eds.) ICES Zooplankton Methodology Manual; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Porter, K.G.; Feig, Y.S. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 1980, 25, 943–948. [Google Scholar] [CrossRef]
- Norland, S. The Relationships between Biomass and Volume of Bacteria. In Handbook of Methods in Aquatic Microbial Ecology; Kemp, P., Sherr, B., Sherr, E., Cole, J., Eds.; Lewis Publishing: Boca Raton, FL, USA, 1993; pp. 303–308. [Google Scholar]
- Noble, R.T.; Fuhrman, J.A. Virus decay and its causes in coastal waters. Appl. Environ. Microbiol. 1997, 63, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.G.; Jackson, G.A. Viral dynamics: A model of the effects of size, shape, motion and abundance of single-celled planktonic organisms and other particles. Mar. Ecol. Prog. Ser. 1992, 89, 103–116. [Google Scholar] [CrossRef]
- Lee, S.; Fuhrman, J.A. Relationships between biovolume and biomass of naturally derived marine bacterioplankton. Appl. Environ. Microbiol. 1987, 53, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Kruskal, W.; Wallis, W. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v5: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2001. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Changes in Marine Communities: An Approach to Statistical Analysis and Interpretation; Plymouth Marine Laboratory, NERC: Plymouth, UK, 1994. [Google Scholar]
- Anderson, M.J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manualand CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Rao, C.R. The use and interpretation of principal component analysis in applied research. Sankhy Na Ser. A 1964, 26, 329–358. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier Science: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Legendre, P.; Anderson, M.J. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Matishov, G.; Zuyev, A.; Golubev, V.; Adrov, N.; Timofeev, S.; Karamusko, O.; Pavlova, L.; Fadyakin, O.; Buzan, A.; Braunstein, A.; et al. Climatic Atlas of the Arctic Seas 2004: Part I. Database of the Barents, Kara, Laptev, and White Seas—Oceanography and Marine Biology; NOAA Atlas NESDIS 58; U.S. Government Printing Office: Washington, DC, USA, 2004.
- Olsen, A.; Johannessen, T.; Rey, F. On the nature of the factors that control spring bloom development at the entrance to the Barents Sea and their interannual variability. Sarsia 2003, 88, 379–393. [Google Scholar] [CrossRef]
- Makarevich, P.R.; Vodopianova, V.V.; Bulavina, A.S.; Vashchenko, P.S.; Ishkulova, T.G. Features of the distribution of chlorophyll-a concentration along the western coast of the Novaya Zemlya Archipelago in spring. Water 2021, 13, 3648. [Google Scholar] [CrossRef]
- Makarevich, P.; Druzhkova, E.; Larionov, V. Primary producers of the Barents Sea. In Diversity of Ecosystems; Mahamane, A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 367–392. [Google Scholar]
- Wassmann, P.; Ratkova, T.; Andreassen, I.; Vernet, M.; Pedersen, G.; Rey, F. Spring bloom development in the Marginal Ice Zone and the Central Barents Sea. Mar. Ecol. 1999, 20, 321–346. [Google Scholar] [CrossRef]
- Dalpadado, P.; Arrigo, K.R.; van Dijken, G.L.; Skjoldal, H.R.; Bagøien, E.; Dolgov, A.V.; Prokopchuk, I.P.; Sperfeld, E. Climate effects on temporal and spatial dynamics of phytoplankton and zooplankton in the Barents Sea. Progr. Oceanogr. 2020, 182, 102320. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Checklist of fauna found in zooplankton samples from the Barents Sea. Polar Biol. 2010, 33, 991–1005. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Regional differences of mesozooplankton communities in the Kara Sea. Cont. Shelf Res. 2015, 105, 26–41. [Google Scholar] [CrossRef]
- Raymont, J.E.G. Plankton and Productivity in the Oceans, 2nd ed.; Pergamon Press: Southampton, UK, 1983; Volume 2. [Google Scholar]
- Payet, J.P.; Suttle, C.A. Physical and biological correlates of virus dynamics in the southern Beaufort Sea and Amundsen Gulf. J. Mar. Syst. 2008, 74, 933–945. [Google Scholar] [CrossRef]
- Sazhin, A.F.; Romanova, N.D.; Mosharov, S.A. Bacterial and primary production in the pelagic zone of the Kara Sea. Oceanology 2010, 50, 759–765. [Google Scholar] [CrossRef]
- Mosharova, I.V.; Mosharov, S.A.; Ilinskiy, V.V. Distribution of bacterioplankton with active metabolism in waters of the St. Anna Trough, Kara Sea, in autumn 2011. Oceanology 2017, 57, 114–121. [Google Scholar] [CrossRef]
- Kopylov, A.I.; Sazhin, A.F.; Zabotkina, E.A.; Romanova, N.D. Virioplankton in the Kara Sea: The Impact of Viruses on Mortality of Heterotrophic Bacteria. Oceanology 2015, 55, 620–631. [Google Scholar] [CrossRef]
- Kopylov, A.I.; Zabotkina, E.A.; Romanenko, A.V.; Kosolapov, D.B.; Sazhin, A.F. Viruses in the water column and the sediment of the eastern part of the Laptev Sea. Estuar. Coast. Shelf Sci. 2020, 242, 106836. [Google Scholar] [CrossRef]
- Kopylov, A.I.; Kosolapov, D.B.; Zabotkina, E.A.; Romanenko, A.V.; Sazhin, A.F. Viruses and viral infection of heterotrophic prokaryotes in shelf waters of the western part of the East Siberian Sea. J. Mar. Syst. 2021, 218, 103544. [Google Scholar] [CrossRef]
- Hodges, L.R.; Bano, N.; Hollibaugh, J.T.; Yager, P. Illustrating the importance of particulate organic matter to pelagic microbial abundance and community structure—An Arctic case study. Aquat. Microb. Ecol. 2005, 40, 217–227. [Google Scholar] [CrossRef][Green Version]
- Kirchman, D.L.; Elifantz, H.; Dittel, A.I.; Malmstrom, R.R.; Cottrell, M.T. Standing stocks and activity of Archaea and Bacteria in the western Arctic Ocean. Limnol. Oceanogr. 2007, 52, 495–507. [Google Scholar] [CrossRef]
- Turner, J.T. The importance of small planktonic copepods and their roles in pelagic marine food webs. Zool. Stud. 2004, 43, 255–266. [Google Scholar]
- Biswas, S.; Tiwari, P.K.; Bona, F.; Pal, S.; Venturino, E. Modeling the avoidance behavior of zooplankton on phytoplankton infected by free viruses. J. Biol. Phys. 2020, 46, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Tiwari, P.K.; Kang, Y.; Pal, S. Effects of zooplankton selectivity on phytoplankton in an ecosystem affected by free-viruses and environmental toxins. Math. Biosci. Eng. 2020, 17, 1272–1317. [Google Scholar] [CrossRef]
- Rivkin, R.B.; Anderson, M.R.; Lajzerowicz, C. Microbial processes in cold oceans: Relationship between temperature and bacterial growth rate. Aquat. Microb. Ecol. 1996, 10, 243–254. [Google Scholar] [CrossRef][Green Version]
- He, J.; Zhang, F.; Lin, L.; Ma, Y.; Chen, J. Bacterioplankton and picophytoplankton abundance, biomass, and distribution in the Western Canada Basin during summer 2008. Deep-Sea Res. II 2012, 81–84, 36–45. [Google Scholar] [CrossRef]
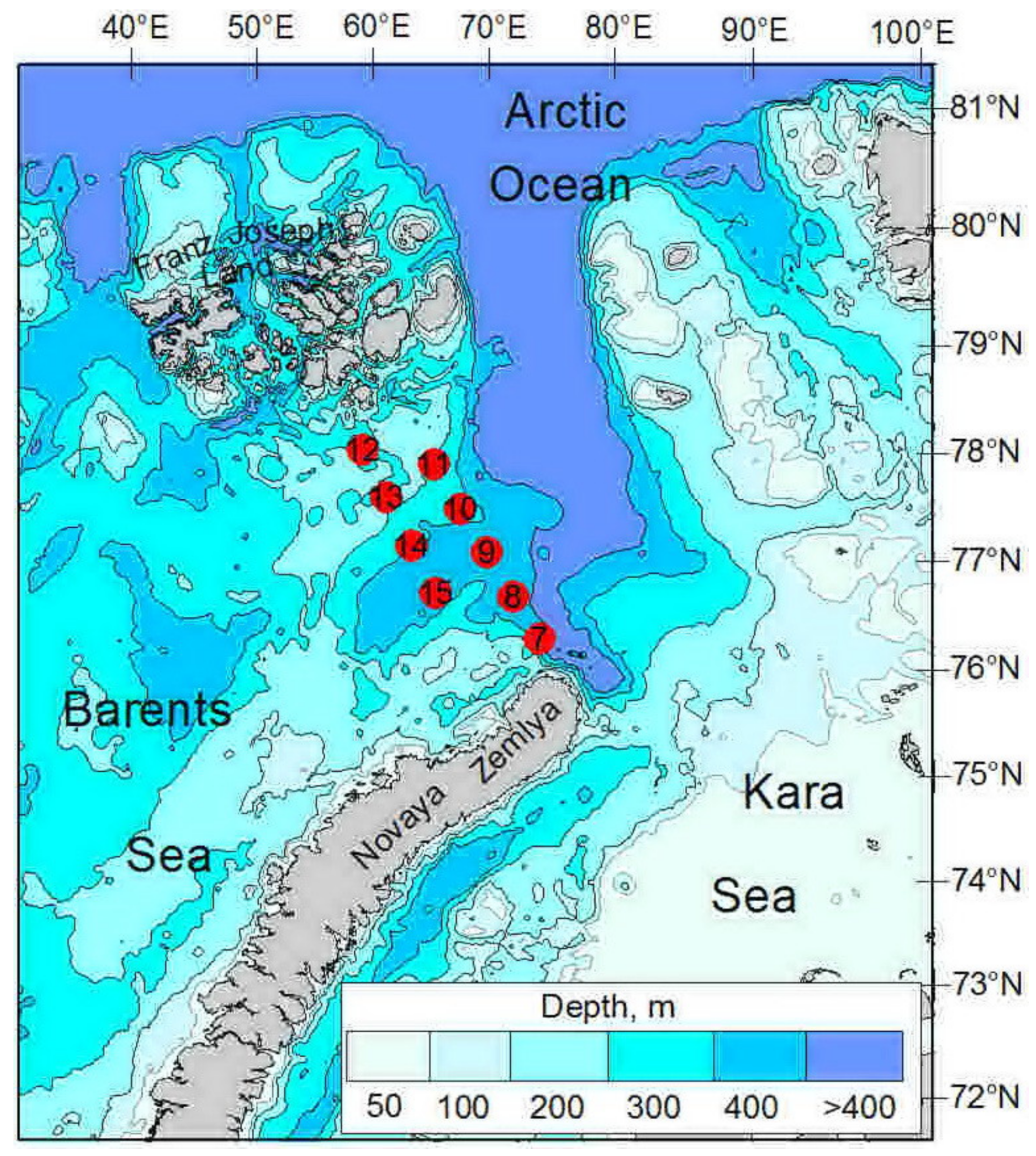
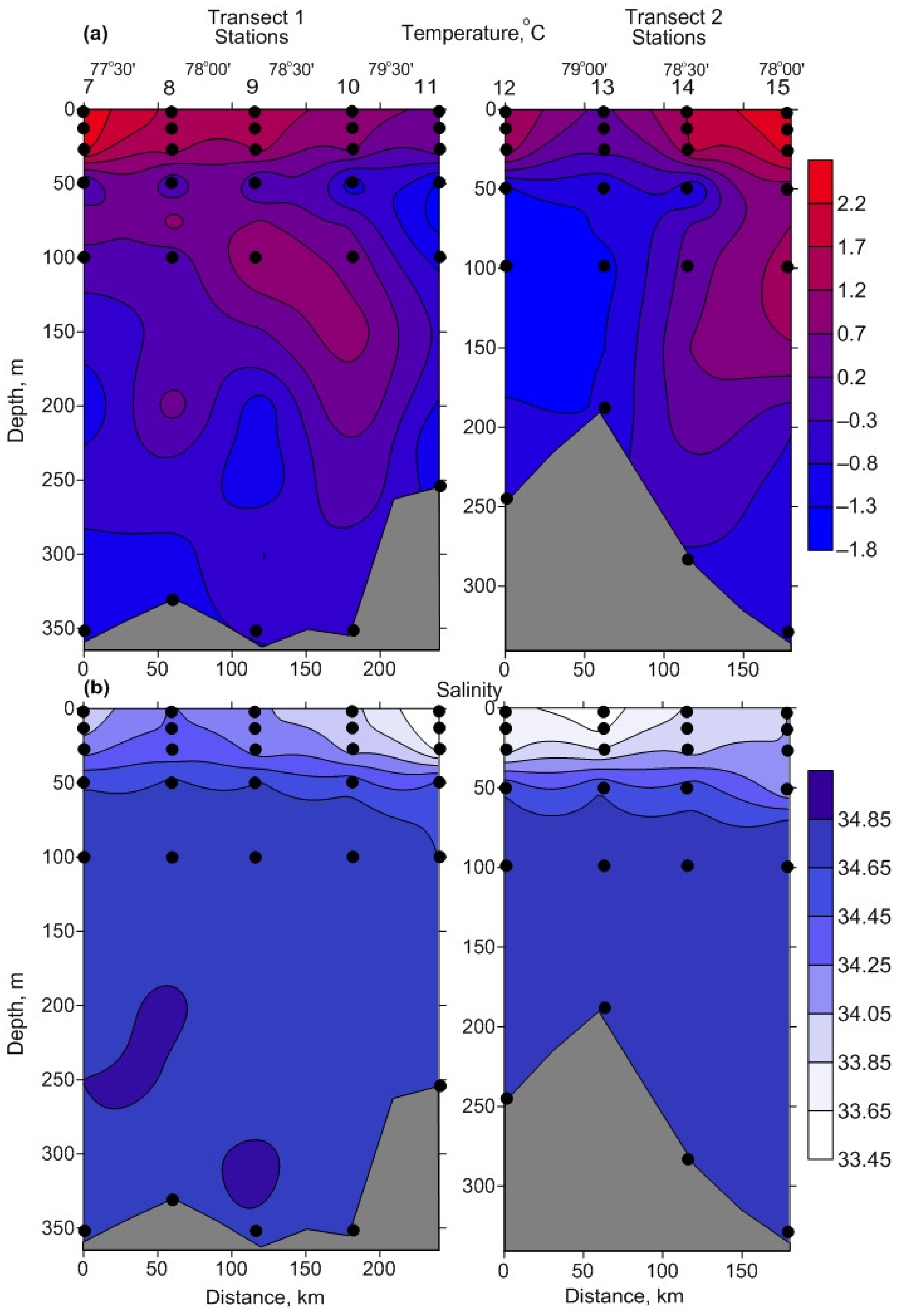
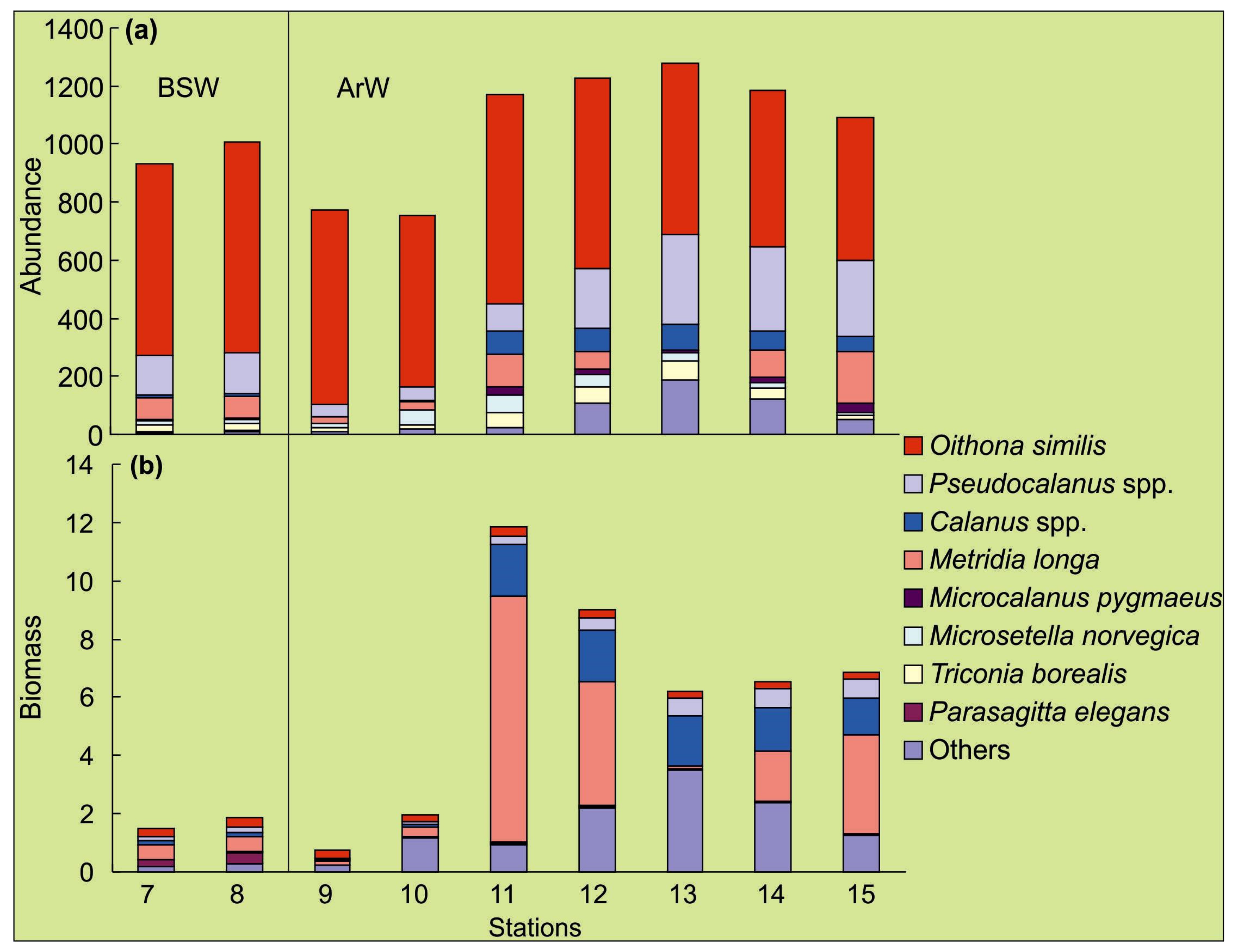
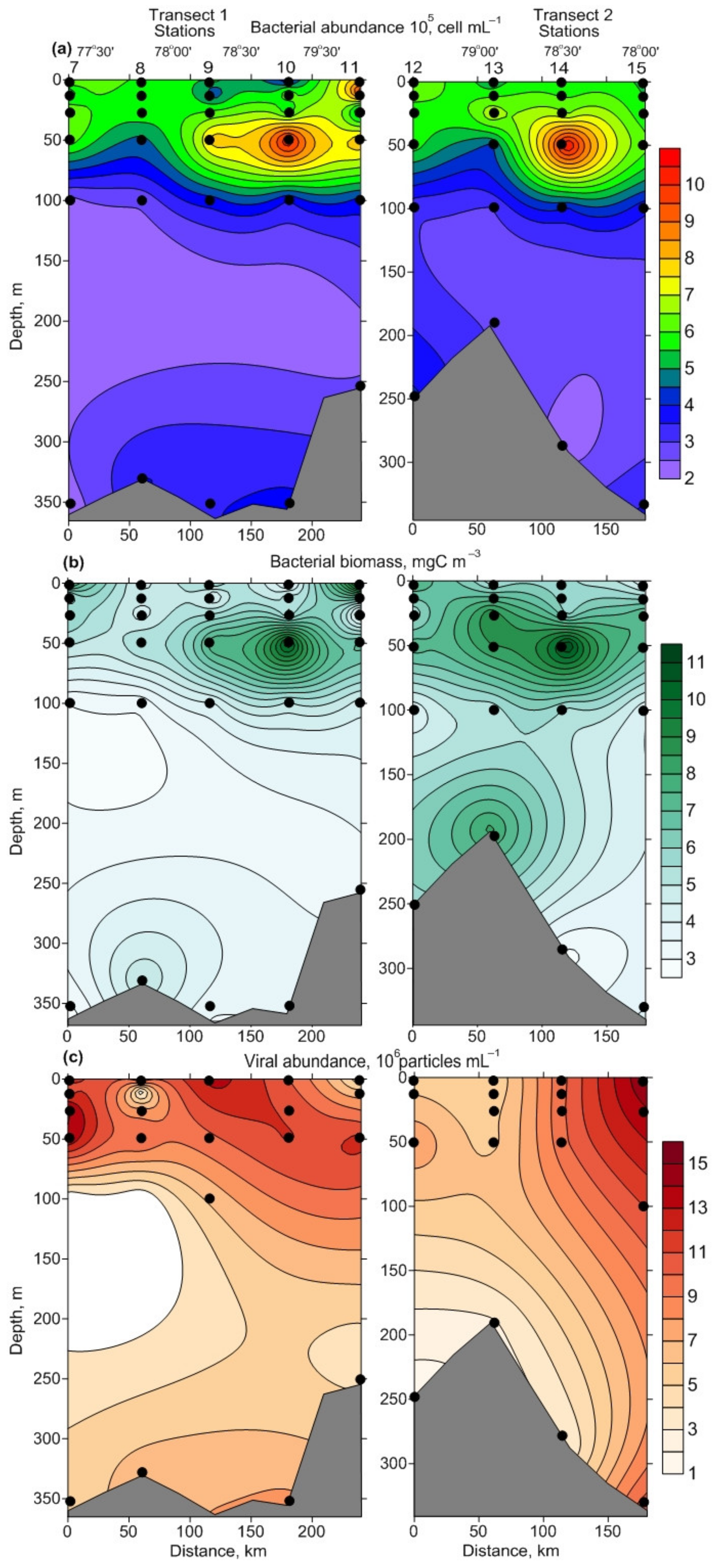
| ID | Date | N | E | Depth, m | Temp | Sal | Water Mass |
|---|---|---|---|---|---|---|---|
| 7 | 08.10.2020 | 77°23′ | 67°44′ | 360 | −1.28–2.51 | 33.95–34.89 | BSW |
| 8 | 09.10.2020 | 77°50′ | 66°46′ | 330 | −0.98–1.55 | 34.21–34.94 | BSW |
| 9 | 09.10.2020 | 78°19′ | 65°41′ | 365 | −1.50–1.43 | 34.08–34.88 | ArW |
| 10 | 09.10.2020 | 78°47′ | 64°32′ | 355 | −1.27–0.98 | 33.92–34.84 | ArW |
| 11 | 09.10.2020 | 79°15′ | 63°16′ | 255 | −1.54–0.61 | 33.49–34.83 | ArW |
| 12 | 10.10.2020 | 79°25′ | 59°24′ | 247 | −1.79–1.07 | 33.72–34.83 | ArW |
| 13 | 10.10.2020 | 78°56′ | 60°41′ | 192 | −1.66–0.09 | 33.57–34.81 | ArW |
| 14 | 10.10.2020 | 78°26′ | 61°55′ | 290 | −1.33–1.41 | 33.88–34.84 | ArW |
| 15 | 10.10.2020 | 77°57′ | 63°27′ | 340 | −1.10–2.05 | 34.02–34.85 | ArW |
| Parameter | A Surface (0–25 m) | B Intermediate (50–100 m) | C Bottom (192–365 m) | |||
|---|---|---|---|---|---|---|
| Range | Mean ± SE | Range | Mean ± SE | Range | Mean ± SE | |
| Arctic Water | ||||||
| NB, 105 cell·mL−1 | 3.33–9.25 | 5.67 ± 0.35 C | 2.96–10.68 | 6.12 ± 0.77 C | 2.27–4.09 | 3.20 ± 0.27 AB |
| BB, mgC·m−3 | 3.4–12.5 | 6.2 ± 0.6 C | 4.1–12 | 7.7 ± 0.8 C | 2.9–8.2 | 4.5 ± 0.7 AB |
| VBR | 1–28 | 13 ± 2 | 7–14 | 11 ± 1 | 4–14 | 9 ± 1 |
| ABV, µm3 | 0.02–0.074 | 0.037 ± 0.003 BC | 0.033–0.085 | 0.049 ± 0.005 A | 0.031–0.136 | 0.055 ± 0.014 A |
| R | 0.8–7.1 | 4.0 ± 0.5 | 3.0–9.6 | 5.7 ± 0.7 | 0.3–1.4 | 0.9 ± 0.2 AB |
| NV, 106 particles·mL−1 | 0.9–15 | 7.1 ± 1.0 C | 5.7–10.1 | 7.6 ± 0.5 C | 1.4–4.3 | 2.9 ± 0.5 AB |
| Temperature, °C | −0.2–2.0 | 1.0 ± 0.2 BC | −1.7–0.4 | −1.2 ± 0.2 A | −1.3–−0.8 | −1.0 ± 0.1 A |
| Salinity | 33.49–34.08 | 33.84 ± 0.06 BC | 34.06–34.74 | 34.63 ± 0.06 | 34.73–34.84 | 34.81 ± 0.02 |
| PO4, µM | 0.0–0.2 | 0.1 ± 0.0 BC | 0.0–0.7 | 0.4 ± 0.1 AC | 0.3–0.9 | 0.6 ± 0.1 AB |
| O2, mL·L−1 | 7.8–8.0 | 8.0 ± 0.0 C | 7.0–8.2 | 7.6 ± 0.1 C | 6.8–7.0 | 6.9 ± 0.0 AB |
| NO3, µM | 0.1–1.3 | 0.6 ± 0.1 BC | 0.4–16.1 | 8.4 ± 1.4 AC | 15–18.1 | 17.2 ± 0.4 AB |
| Chl a, mg·m−3 | 0.19–0.36 | 0.28 ± 0.02 | – | – | – | – |
| ZA, ind.·m−3 (0–100 m) | 752–1278, 1067 ± 35 | |||||
| ZB, mgC·m−3 (0–100 m) | 0.8–11.8, 6.3 ± 0.6 | |||||
| Barents Sea Water | ||||||
| NB, 105 cell·mL−1 | 4.46–6.36 | 5.47 ± 0.27 C | 4.33–5.80 | 5.06 ± 0.73 C | 2.20–3.53 | 2.87 ± 0.67 AB |
| BB, mgC·m−3 | 4.6–9.6 | 6.1 ± 0.8 C | 5.6–6.1 | 5.8 ± 0.2 C | 2.7–5.5 | 4.1 ± 1.4 AB |
| VBR | 0–15 | 11 ± 2 | 14–15 | 15 ± 1 C | 10–11 | 10 ± 0 |
| ABV, µm3 | 0.026–0.056 | 0.037 ± 0.005 C | 0.03–0.051 | 0.041 ± 0.01 C | 0.042–0.059 | 0.051 ± 0.008 AB |
| R | 2.2–6.1 | 4.3 ± 0.7 C | 2.8–4.7 | 3.7 ± 0.9 C | 0.5–1.4 | 0.9 ± 0.4 AB |
| NV, 106 particles·mL−1 | 4.4–9.1 | 7.0 ± 0.7 C | 6.0–8.9 | 7.5 ± 1.4 C | 2.4–3.6 | 3.0 ± 0.6 AB |
| Temperature, °C | 1.5–2.5 | 2.0 ± 0.2 BC | −0.4–−0.2 | –0.3 ± 0.1 AC | −1–−0.9 | –0.9 ± 0.0 AB |
| Salinity | 33.95–34.27 | 34.14 ± 0.06 BC | 34.62–34.7 | 34.66 ± 0.04 A | 34.83–34.83 | 34.83 ± 0.01 A |
| P-PO4, µM | 0.1–0.1 | 0.1 ± 0.0 BC | 0.3–0.5 | 0.4 ± 0.1 AC | 0.6–0.7 | 0.7 ± 0.0 AB |
| O2, mL·L−1 | 7.6–7.8 | 7.7 ± 0.0 C | 7.0–7.7 | 7.4 ± 0.3 C | 6.8–6.9 | 6.8 ± 0.0 AB |
| N-NO3, µM | 0.7–1.4 | 0.9 ± 0.1 BC | 2.5–3.9 | 3.2 ± 0.7 AC | 18.1–18.7 | 18.4 ± 0.3 BC |
| Chl a, mg·m−3 | 0.18–0.32 | 0.27 ± 0.03 | – | – | – | – |
| ZA, ind.·m−3 (0–100 m) | 930–1008, 970 ± 13 | |||||
| ZB, mgC·m−3 (0–100 m) | 1.5–1.9, 1.7 ± 0.1 | |||||
| Variable | LambdaA | P | F | Variance Inflation Factor (VIF) | Explained Variation, % |
|---|---|---|---|---|---|
| Nitrate | 0.27 | 0.001 | 10.23 | 9.455 | 27 |
| Zooplankton biomass | 0.12 | 0.005 | 5.44 | 4.959 | 12 |
| Salinity | 0.06 | 0.045 | 3.01 | 5.501 | 6 |
| Temperature | 0.05 | 0.069 | 2.77 | 5.368 | 5 |
| Dissolved oxygen | 0.05 | 0.068 | 2.65 | 9.845 | 5 |
| Phosphate | 0.05 | 0.11 | 2.39 | 8.011 | 5 |
| Zooplankton abundance | 0.01 | 0.514 | 0.7 | 2.702 | 1 |
| Chlorophyll a | 0.001 | 0.913 | 0.14 | 1.585 | <1 |
| Parameter | Temperature | Salinity | PO4 | O2 | NO3 | Chl a | ZA | ZB |
|---|---|---|---|---|---|---|---|---|
| NB, 105 cell·mL−1 | 0.26 | −0.50 | −0.44 | 0.64 | −0.63 | −0.03 | 0.08 | 0.19 |
| BB, mgC·m−3 | −0.02 | −0.28 | −0.23 | 0.34 | −0.34 | 0.15 | 0.25 | 0.28 |
| NV, 106 particles·mL−1 | 0.40 | −0.17 | −0.29 | 0.46 | −0.52 | −0.26 | −0.21 | −0.37 |
| ABV, µm3 | 0.29 | 0.13 | −0.04 | 0.10 | −0.18 | −0.26 | −0.29 | −0.54 |
| VBR | −0.35 | 0.27 | 0.24 | −0.35 | 0.38 | 0.31 | 0.32 | 0.15 |
| R | 0.42 | −0.35 | −0.43 | 0.60 | −0.65 | −0.13 | −0.07 | −0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvoretsky, V.G.; Venger, M.P.; Vashchenko, A.V.; Maksimovskaya, T.M.; Ishkulova, T.G.; Vodopianova, V.V. Pelagic Bacteria and Viruses in a High Arctic Region: Environmental Control in the Autumn Period. Biology 2022, 11, 845. https://doi.org/10.3390/biology11060845
Dvoretsky VG, Venger MP, Vashchenko AV, Maksimovskaya TM, Ishkulova TG, Vodopianova VV. Pelagic Bacteria and Viruses in a High Arctic Region: Environmental Control in the Autumn Period. Biology. 2022; 11(6):845. https://doi.org/10.3390/biology11060845
Chicago/Turabian StyleDvoretsky, Vladimir G., Marina P. Venger, Anastasya V. Vashchenko, Tatyana M. Maksimovskaya, Tatyana G. Ishkulova, and Veronika V. Vodopianova. 2022. "Pelagic Bacteria and Viruses in a High Arctic Region: Environmental Control in the Autumn Period" Biology 11, no. 6: 845. https://doi.org/10.3390/biology11060845
APA StyleDvoretsky, V. G., Venger, M. P., Vashchenko, A. V., Maksimovskaya, T. M., Ishkulova, T. G., & Vodopianova, V. V. (2022). Pelagic Bacteria and Viruses in a High Arctic Region: Environmental Control in the Autumn Period. Biology, 11(6), 845. https://doi.org/10.3390/biology11060845









