The ABC Model of Happiness—Neurobiological Aspects of Motivation and Positive Mood, and Their Dynamic Changes through Practice, the Course of Life
Abstract
:Simple Summary
Abstract
1. Introduction
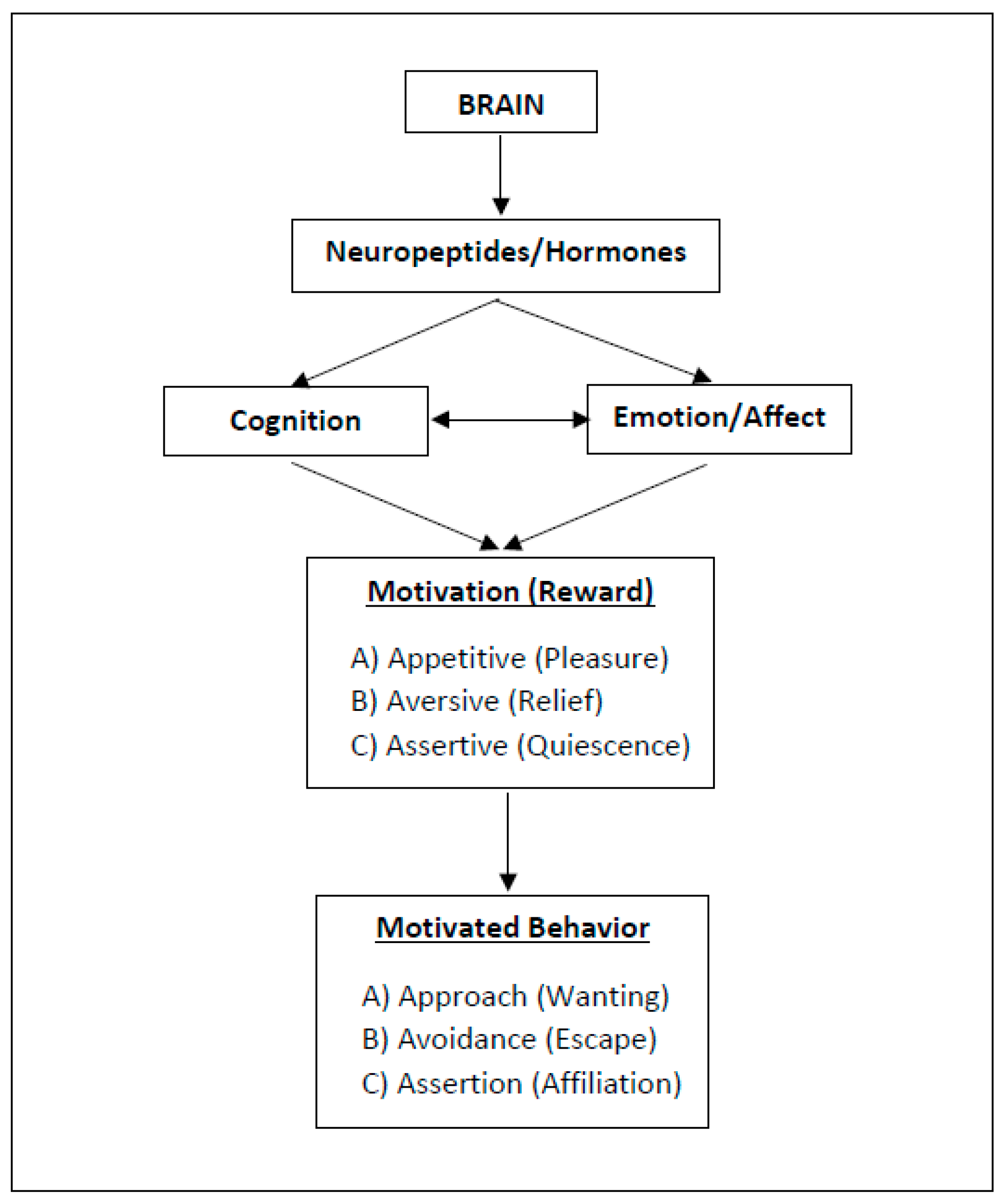
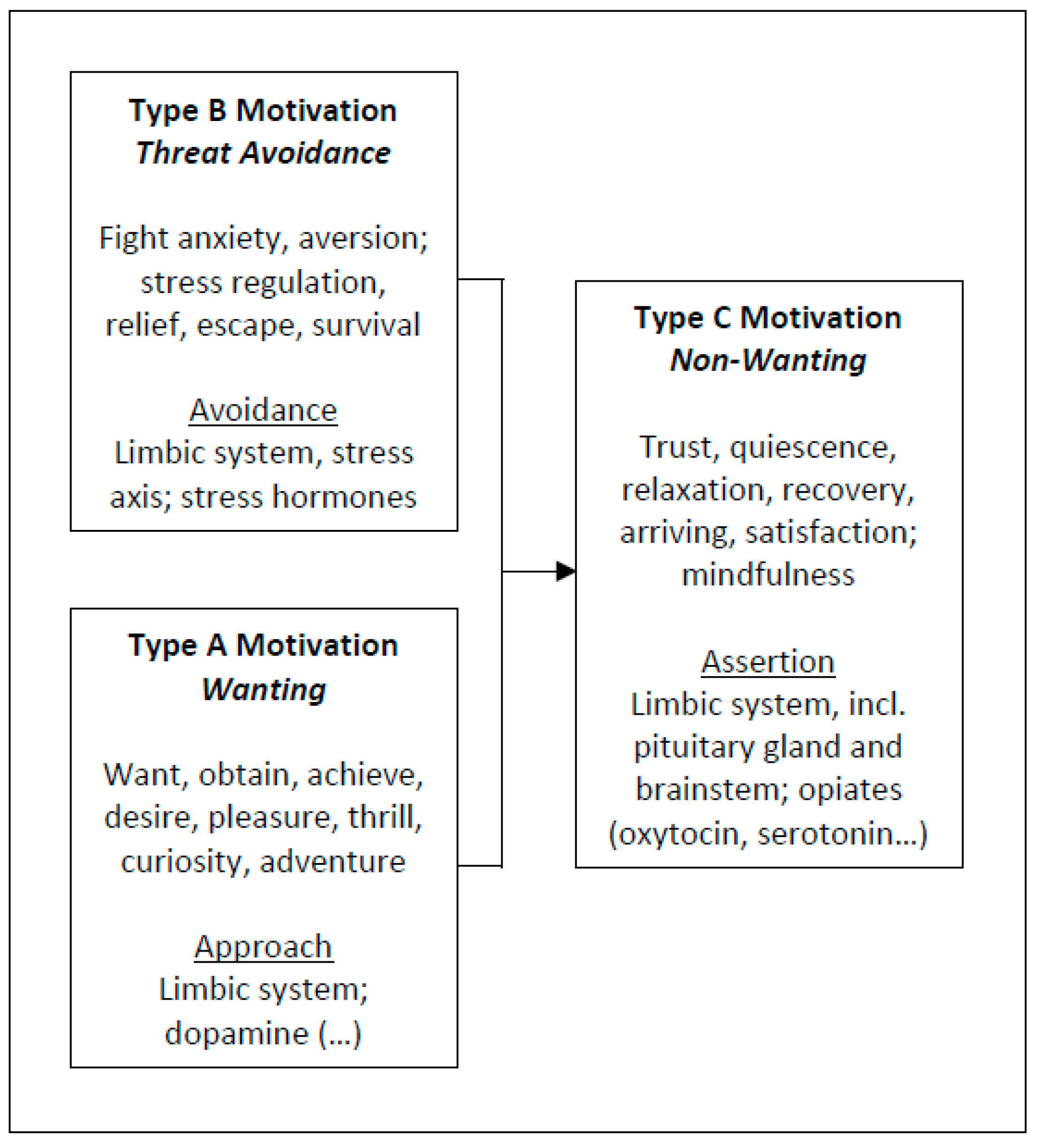
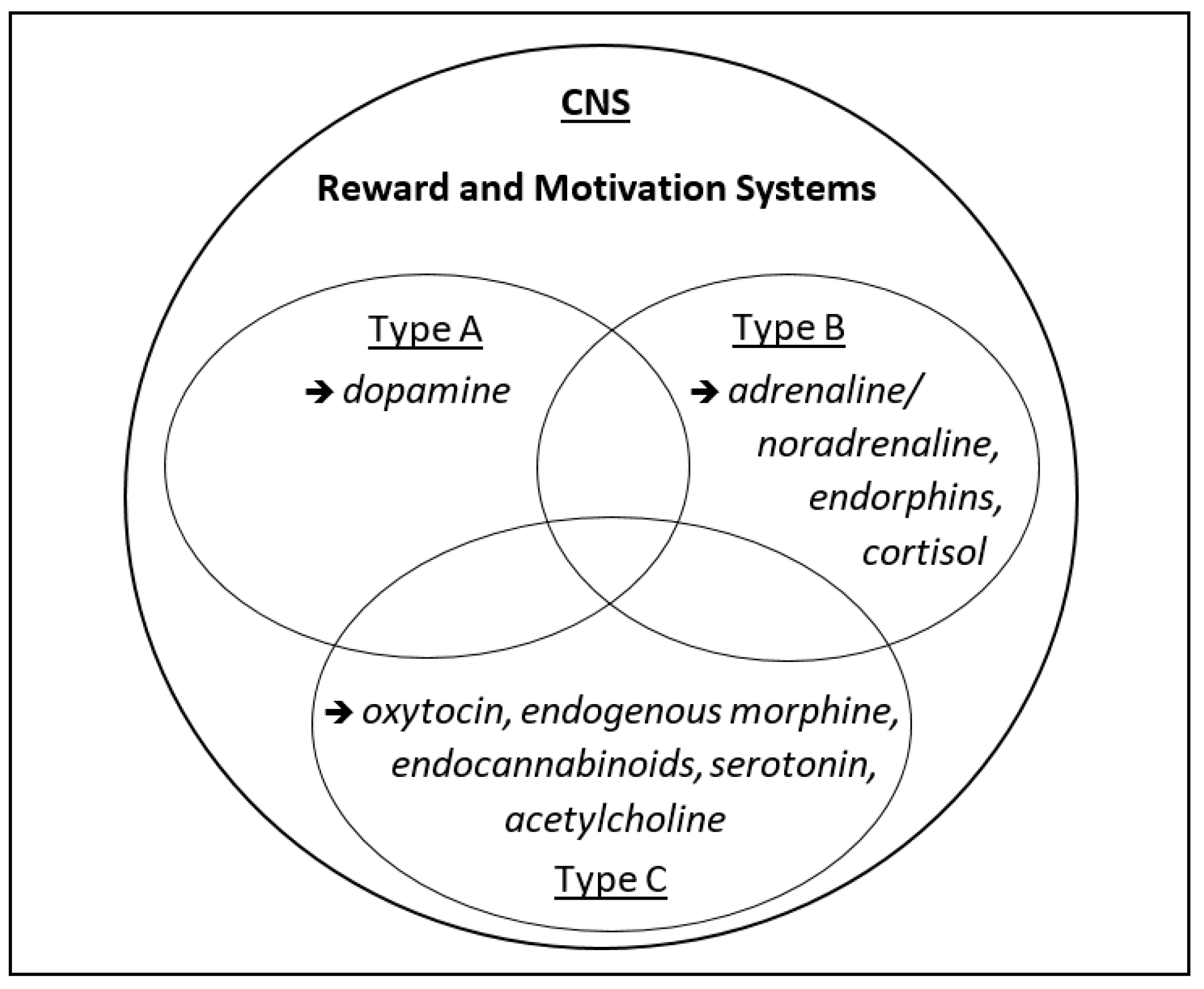
2. Three Types of Motivation and Rewarding (“Happy”) States
2.1. Approach Motivation
2.2. Avoidance Motivation
2.3. Assertion Motivation
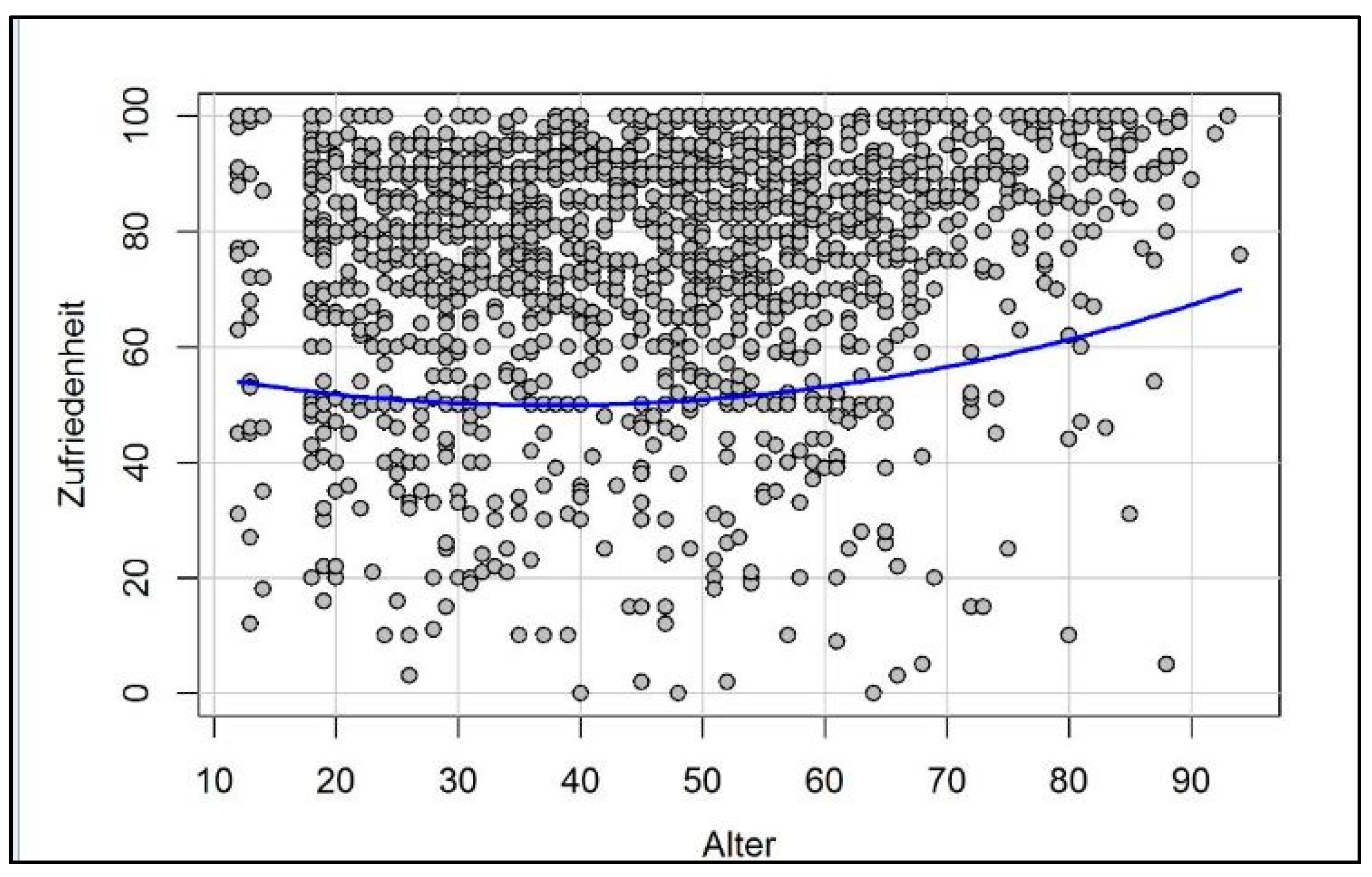
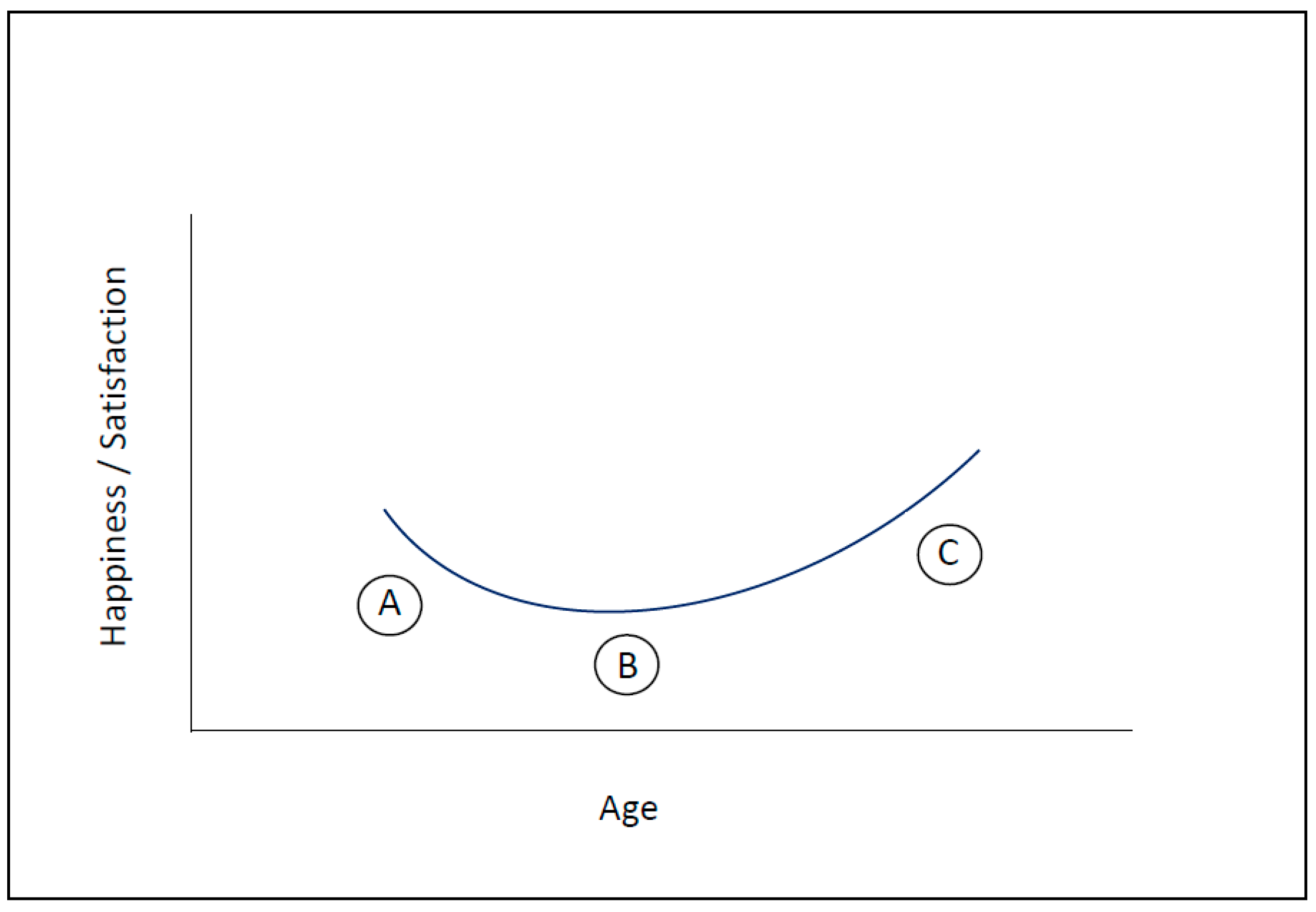
3. How Motivation and Happiness Change over the Course of Life
4. Can Happiness Be Learned? Long-Term Effects of Stress Reduction, Contemplative Practice
5. Discussion
6. Strengths, Weaknesses, Future Research
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, J.L. The Pursuit and Maintenance of Happiness. N. Engl. J. Med. 2021, 385, 1327–1328. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A. Happiness and Health. Annu. Rev. Public Health 2019, 40, 339–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helliwell, J.F.; Aknin, L.B. Expanding the social science of happiness. Nat. Hum. Behav. 2018, 2, 248–252. [Google Scholar] [CrossRef]
- Webb, L.E.; Veenhoven, R.; Harfeld, J.L.; Jensen, M.B. What is animal happiness? Ann. N. Y. Acad. Sci. 2019, 1438, 62–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esch, T.; Stefano, G.B. The neurobiology of pleasure, reward processes, addiction and their health implications. Neuroendocrinol. Lett. 2004, 25, 235–251. [Google Scholar] [PubMed]
- Esch, T.; Stefano, G.B. Endogenous reward mechanisms and their importance in stress reduction, exercise and the brain. Arch. Med. Sci. 2010, 6, 447–455. [Google Scholar] [CrossRef]
- Esch, T. The Neurobiology of Happiness, 3rd ed.; Thieme: Stuttgart, Germany, 2017. [Google Scholar]
- Michaelsen, M.M.; Esch, T. Motivation and reward mechanisms in health behavior change processes. Brain Res. 2021, 1757, 147309. [Google Scholar] [CrossRef]
- Karwetzky, C.; Werdecker, L.; Esch, T. What Matters Most in Life? A German Cohort Study on the Sources of Meaning and Their Neurobiological Foundations in Four Age Groups. Front. Psychol. 2021, 12, 777751. [Google Scholar] [CrossRef]
- Karwetzky, C.; Michaelsen, M.M.; Werdecker, L.; Esch, T. The U-curve of happiness revisited: Correlations and differences in life satisfaction over the span of life—an empirical evaluation based on data from 1,597 individuals aged 12 to 94 in Germany. Front. Psychol. 2022, 13, 837638. [Google Scholar] [CrossRef]
- Michaelsen, M.M.; Esch, T. Functional mechanisms of health behavior change techniques: A conceptual review. Front. Psychol. 2022, 13, 725644. [Google Scholar] [CrossRef]
- Esch, T.; Stefano, G.B. The neurobiology of stress management. Neuroendocrinol. Lett. 2010, 31, 19–39. [Google Scholar] [PubMed]
- Buss, D.M. The evolution of happiness. Am. Psychol. 2000, 55, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Loonen, A.J.M.; Ivanova, S.A. Circuits regulating pleasure and happiness: Evolution and role in mental disorders. Acta Neuropsychiatr. 2018, 30, 29–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loonen, A.J.; Ivanova, S.A. Circuits Regulating Pleasure and Happiness: The Evolution of the Amygdalar-Hippocampal-Habenular Connectivity in Vertebrates. Front. Neurosci. 2016, 10, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esch, T. Self-regulation as part of medicine. Dtsch Arztebl Int. 2016, 111, 2214–2220. [Google Scholar]
- Esch, T. Self-healing in health-care: Using the example of mind-body medicine. Bundesgesundheitsblatt Gesundh. Gesundh. 2020, 63, 577–585. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Li, Q.; Zou, Q.; Liu, Q. A road map for happiness: The psychological factors related cell types in various parts of human body from single cell RNA-seq data analysis. Comput. Biol. Med. 2022, 143, 105286. [Google Scholar] [CrossRef]
- Feicht, T.; Wittmann, M.; Jose, G.; Mock, A.; von Hirschhausen, E.; Esch, T. Evaluation of a seven-week web-based happiness training to improve psychological well-being, reduce stress, and enhance mindfulness and flourishing: A randomized controlled occupational health study. Evid. Based Complement. Alternat. Med. 2013, 2013, 676953. [Google Scholar] [CrossRef]
- Zak, P.J.; Curry, B.; Owen, T.; Barraza, J.A. Oxytocin release increases with age and is associated with life satisfaction and prosocial behaviors. Front. Behav. Neurosci. 2022, 16, 846234. [Google Scholar] [CrossRef]
- Park, S.; Kahnt, T.; Dogan, A.; Strang, S.; Fehr, E.; Tobler, P.N. A neural link between generosity and happiness. Nat. Commun. 2017, 8, 15964. [Google Scholar] [CrossRef] [Green Version]
- Rutledge, R.B.; Skandali, N.; Dayan, P.; Dolan, R.J. Dopaminergic modulation of decision making and subjective well-being. J. Neurosci. 2015, 35, 9811–9822. [Google Scholar] [CrossRef] [PubMed]
- Burgdorf, J.; Panksepp, J. The neurobiology of positive emotions. Neurosci. Biobehav. Rev. 2006, 30, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.M.; Tanabe, A.; Iwahori, Y. A systematic review of the effect of L-tryptophan supplementation on mood and emotional functioning. J. Diet. Suppl. 2021, 18, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.A.; Fong, G.T. Temporal self-regulation theory: A model for individual health behavior. Health Psychol. Rev. 2007, 1, 6–52. [Google Scholar] [CrossRef]
- Kahneman, D.; Tversky, A. Prospect Theory: An Analysis of Decision under Risk. Econometrica 1979, 47, 263–291. [Google Scholar] [CrossRef] [Green Version]
- Marteau, T.M.; Hollands, G.J.; Fletcher, P.C. Changing Human Behavior to Prevent Disease: The Importance of Targeting Automatic Processes. Science 2012, 337, 1492–1495. [Google Scholar] [CrossRef] [Green Version]
- Sheeran, P.; Gollwitzer, P.M.; Bargh, J.A. Nonconscious processes and health. Health Psychol. 2013, 32, 460–473. [Google Scholar] [CrossRef]
- Strack, F.; Deutsch, R. Reflective and impulsive determinants of social behavior. Pers. Soc. Psychol. Rev. 2004, 8, 220–247. [Google Scholar] [CrossRef] [Green Version]
- Cohn, M.A.; Fredrickson, B.L. In search of durable positive psychology interventions: Predictors and consequences of long-term positive behavior change. J. Posit. Psychol. 2010, 5, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Fredrickson, B.L.; Arizmendi, C.; van Cappellen, P. Same-day, cross-day, and upward spiral relations between positive affect and positive health behaviours. Psychol. Health 2020, 36, 444–460. [Google Scholar] [CrossRef]
- Rhodes, R.E.; Kates, A. Can the Affective Response to Exercise Predict Future Motives and Physical Activity Behavior? A Systematic Review of Published Evidence. Ann. Behav. Med. 2015, 49, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Woolley, K.; Fishbach, A. Immediate Rewards Predict Adherence to Long-Term Goals. Pers. Soc. Psychol. Bull. 2017, 43, 151–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puglisi-Allegra, S.; Ventura, R. Prefrontal/accumbal catecholamine system processes emotionally driven attribution of motivational salience. Rev. Neurosci. 2012, 23, 509–526. [Google Scholar] [CrossRef]
- Eder, A.B.; Hommel, B. Anticipatory control of approach and avoidance: An ideomotor approach. Emot. Rev. 2013, 5, 276–280. [Google Scholar] [CrossRef]
- Elliot, A.J.; Eder, A.B.; Harmon-Jones, E. Approach-avoidance motivation and emotion: Convergence and divergence. Emot. Rev. 2013, 5, 308–311. [Google Scholar] [CrossRef]
- Scholer, A.A.; Higgins, E.T. Dodging monsters and dancing with dreams: Success and failure at different levels of approach and avoidance. Emot. Rev. 2013, 5, 254–258. [Google Scholar] [CrossRef] [Green Version]
- Ekman, P.; Cordaro, D. What is meant by calling emotions basic. Emot. Rev. 2011, 3, 364–370. [Google Scholar] [CrossRef]
- Ekman, P. Basic Emotions. In Handbook of Cognition and Emotion; Dalgleish, T., Power, M.J., Eds.; Wiley-Interscience: Hoboken, NJ, USA, 2005; pp. 45–60. [Google Scholar]
- Bourgeois, A.; Neveu, R.; Vuilleumier, P.; Chelazzi, L. How does awareness modulate goal-directed and stimulus-driven shifts of attention triggered by value learning? PLoS ONE 2016, 11, e0160469. [Google Scholar]
- McCall, C.; Singer, T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat. Neurosci. 2012, 15, 681–688. [Google Scholar] [CrossRef]
- Bozarth, M. Pleasure systems in the brain. In Pleasure: The Politics and the Reality; Warburton, D.M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1994; pp. 5–14. [Google Scholar]
- Berridge, K.C.; Kringelbach, M.L. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology 2008, 199, 457–480. [Google Scholar] [CrossRef] [Green Version]
- Schultz, W. Neuronal reward and decision signals: From theories to data. Physiol. Rev. 2015, 95, 853–951. [Google Scholar] [CrossRef] [PubMed]
- Cacioppo, J.T.; Gardner, W.L.; Berntson, G.G. The affect system has parallel and integrative processing components: Form follows function. J. Pers. Soc. Psychol. 1999, 76, 839–855. [Google Scholar] [CrossRef]
- Lang, P.J.; Bradley, M.M. Appetitive and defensive motivation: Goal-directed or goal-determined? Emot. Rev. 2013, 5, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. What are emotional states, and why do we have them? Emot. Rev. 2013, 5, 241–247. [Google Scholar] [CrossRef]
- Schneirla, T.C. An evolutionary and developmental theory of biphasic processes underlying approach and withdrawal. In Nebraska Symposium on Motivation; Jones, M.R., Ed.; University Nebraska Press: Lincoln, NE, USA, 1959; pp. 1–42. [Google Scholar]
- Kringelbach, M.L. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat. Rev. Neurosci. 2005, 6, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2001, 2, 119–128. [Google Scholar] [CrossRef]
- Nestler, E.J.; Malenka, R.C.; Hyman, S.E. Molecular Basis of Neuropharmacology; McGraw-Hill Medical: New York, NY, USA, 2001. [Google Scholar]
- Berridge, K.C. The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology 2007, 191, 391–431. [Google Scholar] [CrossRef]
- Smith, K.S.; Berridge, K.C.; Aldridge, J.W. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc. Natl. Acad. Sci. USA 2011, 108, E255–E264. [Google Scholar] [CrossRef] [Green Version]
- Nestler, E.J.; Malenka, R.C. The addicted brain. Sci. Am. 2004, 290, 78–85. [Google Scholar] [CrossRef]
- Seymour, B.; Singer, T.; Dolan, R. The neurobiology of punishment. Nat. Rev. Neurosci. 2007, 8, 300–311. [Google Scholar] [CrossRef] [Green Version]
- Berridge, K.C. Evolving concepts of emotion and motivation. Front. Psychol. 2018, 9, 1647. [Google Scholar] [CrossRef] [Green Version]
- Hirschberg, J.; Manning, C.D. Advances in natural language processing. Science 2015, 349, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J. The emotion probe: Studies of motivation and attention. Am. Psychol. 1995, 50, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.; Wiese, D.; Vaidya, J.; Tellegen, A. The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. J. Pers. Soc. Psychol. 1999, 76, 820–838. [Google Scholar] [CrossRef]
- LeDoux, J. Fear and the brain: Where have we been, and where are we going? Biol Psychiatry 1998, 44, 1229–1238. [Google Scholar] [CrossRef]
- Krisam, M.; Meder, B.; von Philipsborn, P. Nudging in Primary Prevention: Overview and Perspectives for Germany. Gesundheitswesen 2017, 79, 117–123. [Google Scholar]
- Levenson, R.W. Basic emotion questions. Emot. Rev. 2011, 3, 379–386. [Google Scholar] [CrossRef]
- Deutsch, R.; Smith, K.J.M.; Kordts-Freudinger, R.; Reichardt, R. How absent negativity relates to affect and motivation: An integrative relief model. Front. Psychol. 2015, 6, 152. [Google Scholar] [CrossRef] [Green Version]
- Esch, T.; Stefano, G.B.; Fricchione, G.L. The therapeutic use of the relaxation response in stress-related diseases. Med. Sci. Monit. 2003, 9, RA23–RA34. [Google Scholar]
- Stefano, G.B.; Fricchione, G.L.; Esch, T. Relaxation: Molecular and physiological significance. Med. Sci. Monit. 2006, 12, HY21–HY31. [Google Scholar]
- Kim, J.; Lee, S.; Park, K.; Hong, I.; Song, B.; Son, G.; Park, H.; Kim, W.R.; Park, E.; Choe, H.K.; et al. Amygdala depotentiation and fear extinction. Proc. Natl. Acad. Sci. USA 2007, 104, 20955–20960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangha, S. Plasticity of fear and safety neurons of the amygdala in response to fear extinction. Front. Behav. Neurosci. 2015, 9, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esch, T. Meditation in Complementary and Integrative Medicine: Taxonomy of Effects and Methods. Complement. Med. Res. 2021, 28, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Esch, T. The Neurobiology of Meditation and Mindfulness. In Meditation—Neuroscientific Approaches and Philosophical Implications/Springer Series: Studies in Neuroscience, Consciousness and Spirituality; Schmidt, S., Walach, H., Eds.; Springer International Publishing: New York, NY, USA, 2014; Volume 2, pp. 153–173. [Google Scholar]
- Esch, T.; Kream, R.M.; Stefano, G.B. Chromosomal processes in mind-body medicine: Chronic stress, cell aging, and telomere length. Med. Sci. Monit. Bas. Res. 2018, 24, 134–140. [Google Scholar] [CrossRef]
- Stefano, G.B.; Benson, H.; Fricchione, G.L.; Esch, T. The Stress Response: Always Good and When It Is Bad; Medical Science International: New York, NY, USA, 2005. [Google Scholar]
- Stefano, G.B.; Esch, T. Integrative medical therapy: Examination of meditation’s therapeutic and global medicinal outcomes via nitric oxide. Int. J. Mol. Med. 2005, 16, 621–630. [Google Scholar]
- Esch, T.; Guarna, M.; Bianchi, E.; Zhu, W.; Stefano, G.B. Commonalities in the central nervous system’s involvement with complementary medical therapies: Limbic morphinergic processes. Med. Sci. Monit. 2004, 10, MS6–MS17. [Google Scholar]
- Esch, T. The neuronal basis of meditation and mindfulness. Sucht 2014, 60, 21–28. [Google Scholar] [CrossRef]
- Stefano, G.B.; Esch, T.; Cadet, P.; Zhu, W.; Mantione, K.; Benson, H. Endocannabinoids as autoregulatory signaling molecules: Coupling to nitric oxide and a possible association with the relaxation response. Med. Sci. Monit. 2003, 9, RA63–RA75. [Google Scholar]
- Huffmeijer, R.; van Ijzendoorn, M.H.; Bakermans-Kranenburg, M.J. Ageing and oxytocin: A call for extending human oxytocin research to ageing populations—a mini review. Gerontology 2013, 59, 32–39. [Google Scholar] [CrossRef]
- Stefano, G.B.; Fricchione, G.; Goumon, Y.; Esch, T. Pain, immunity, opiate and opioid compounds and health. Med. Sci. Monit. 2005, 11, MS47–MS53. [Google Scholar]
- Zhu, W.; Ma, Y.; Bell, A.; Esch, T.; Guarna, M.; Bilfinger, T.V.; Bianchi, E.; Stefano, G.B. Presence of morphine in rat amygdala: Evidence for the mu3 opiate receptor subtype via nitric oxide release in limbic structures. Med. Sci. Monit. 2004, 10, BR433–BR439. [Google Scholar] [PubMed]
- Mantione, K.J.; Goumon, Y.; Esch, T.; Stefano, G.B. Morphine 6beta glucuronide: Fortuitous morphine metabolite or preferred peripheral regulatory opiate? Med. Sci. Monit. 2005, 11, MS43–MS46. [Google Scholar] [PubMed]
- Zhu, W.; Mantione, K.J.; Shen, L.; Cadet, P.; Esch, T.; Goumon, Y.; Bianchi, E.; Sonetti, D.; Stefano, G.B. Tyrosine and tyramine increase endogenous ganglionic morphine and dopamine levels in vitro and in vivo: cyp2d6 and tyrosine hydroxylase modulation demonstrates a dopamine coupling. Med. Sci. Monit. 2005, 11, BR397–BR404. [Google Scholar] [PubMed]
- Mantione, K.J.; Cadet, P.; Zhu, W.; Kream, R.M.; Sheehan, M.; Fricchione, G.L.; Goumon, Y.; Esch, T.; Stefano, G.B. Endogenous morphine signaling via nitric oxide regulates the expression of CYP2D6 and COMT: Autocrine/paracrine feedback inhibition. Addict. Biol. 2008, 13, 118–123. [Google Scholar] [CrossRef]
- Mantione, K.J.; Zhu, W.; Kream, R.M.; Esch, T.; Stefano, G. Regulation of the transcription of the Catechol-O-Methyltransferase gene by morphine and epinephrine. Act. Nerv. Super. Rediviva 2010, 52, 51–56. [Google Scholar]
- Esch, T.; Kream, R.M.; Stefano, G.B. Emerging regulatory roles of opioid peptides, endogenous morphine, and opioid receptor subtypes in immunomodulatory processes: Metabolic, behavioral, and evolutionary perspectives. Immunol. Lett. 2020, 227, 28–33. [Google Scholar] [CrossRef]
- Stefano, G.B.; Bianchi, E.; Guarna, M.; Fricchione, G.L.; Zhu, W.; Cadet, P.; Mantione, K.J.; Casares, F.M.; Kream, R.M.; Esch, T. Nicotine, alcohol and cocaine coupling to reward processes via endogenous morphine signaling: The dopamine-morphine hypothesis. Med. Sci. Monit. 2007, 13, RA91–RA102. [Google Scholar]
- Stefano, G.B.; Kream, R.M.; Mantione, K.J.; Sheehan, M.; Cadet, P.; Zhu, W.; Bilfinger, T.V.; Esch, T. Endogenous morphine/nitric oxide-coupled regulation of cellular physiology and gene expression: Implications for cancer biology. Semin. Cancer Biol. 2008, 18, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Von Hirschhausen, E.; Esch, T. The Better Half; Rowohlt: Reinbek, Germany, 2018. [Google Scholar]
- Belanger, C.F.; Hennekens, C.H.; Rosner, B.; Speizer, F.E. The nurses’ health study. Am. J. Nurs. 1978, 78, 1039–1040. [Google Scholar] [CrossRef]
- Colditz, G.A.; Hankinson, S.E. The Nurses’ Health Study: Lifestyle and health among women. Nat. Rev. Cancer 2005, 5, 388–396. [Google Scholar] [CrossRef]
- Landes, S.D.; Ardelt, M.; Vaillant, G.E.; Waldinger, R.J. Childhood adversity, midlife generativity, and later life well-being. J. Gerontol. B Psychol. Sci. Soc. Sci. 2014, 69, 942–952. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Floud, S.; Pirie, K.; Green, J.; Peto, R.; Beral, V. Million Women Study Collaborators. Does happiness itself directly affect mortality? The prospective UK Million Women Study. Lancet 2016, 387, 874–881. [Google Scholar] [CrossRef] [Green Version]
- Shmotkin, D.; Shrira, A.; Eyal, N.; Blumstein, T.; Shorek, A. The prediction of subjective wellness among the old-old: Implications for the "fourth-age" conception. J. Gerontol. B Psychol. Sci. Soc. Sci. 2014, 69, 719–729. [Google Scholar] [CrossRef]
- Blanchflower, D.G. Is happiness U-shaped everywhere? Age and subjective well-being in 145 countries. J. Popul. Econ. 2021, 34, 575–624. [Google Scholar] [CrossRef]
- Baltes, P.B.; Smith, J. New frontiers in the future of aging: From successful aging of the young old to the dilemmas of the fourth age. Gerontology 2003, 49, 123–135. [Google Scholar] [CrossRef]
- Jopp, D.S.; Rott, C.; Boerne, K.; Boch, K.; Kruse, A. Zweite Heidelberger Hundertjährigen-Studie: Herausforderungen und Stärken des Lebens mit 100 Jahren; Robert Bosch Stiftung: Stuttgart, Germany, 2013. [Google Scholar]
- Wurm, S. Lebensalter, drittes und viertes. In Dorsch: Lexikon der Psychologie; Wirtz, M.A., Ed.; Hogrefe: Göttingen, Germany, 2021. [Google Scholar]
- Wahl, H.W. Die Neue Psychologie des Alterns: Überraschende Erkenntnisse über Unsere Längste Lebensphase; Kösel: München, Germany, 2017. [Google Scholar]
- Kahneman, D.; Riis, J. Living, and Thinking about It: Two Perspectives on Life. In The Science of Well-Being; Huppert, F.A., Baylis, N., Keverne, B., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 285–304. [Google Scholar]
- Staudinger, U. There are many reasons against it, and yet many people are doing well: The paradox of subjective well-being. Psychol. Rundsch. 2000, 51, 185–197. [Google Scholar] [CrossRef]
- Herschbach, P. The ‘satisfaction paradox’ in the Quality of Life research—what does our well-being depend on? Psychother. Psychosom. Med. Psychol. 2002, 52, 141–150. [Google Scholar] [CrossRef]
- Schilling, O. Development of life satisfaction in old age: Another view on the ‘paradox’. Soc. Indicat. Res. 2006, 75, 241–271. [Google Scholar] [CrossRef] [Green Version]
- Ferring, D.; Boll, T. Subjective Well-being in Older Adults: Current State and Gaps of Research. In Ageing, Health and Pensions in Europe; Bovenberg, L., Van Soest, A., Zaidi, A., Eds.; Palgrave MacMillan: Basingstoke, UK, 2010; pp. 173–205. [Google Scholar]
- Gerstorf, D.; Hoppmann, C.A.; Löckenhoff, C.E.; Infurna, F.J.; Schupp, J.; Wagner, G.G.; Ram, N. Terminal decline in well-being: The role of social orientation. Psychol. Aging 2016, 31, 149–165. [Google Scholar] [CrossRef] [Green Version]
- Brandmaier, A.M.; Ram, N.; Wagner, G.G.; Gerstorf, D. Terminal decline in well-being: The role of multi-indicator constellations of physical health and psychosocial correlates. Dev. Psychol. 2017, 53, 996–1012. [Google Scholar] [CrossRef] [Green Version]
- Kotre, J.N. Outliving the Self; Johns Hopkins University Press: Baltimore, MD, USA, 1984. [Google Scholar]
- Erikson, E.H. The Life Cycle Completed; W.W. Norton: New York, NY, USA, 1982. [Google Scholar]
- Frijters, P.; Beatton, T. The mystery of the U-shaped relationship between happiness and age. J. Econom. Behav. Org. 2012, 82, 525–542. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S. Stress, adaptation, and disease: Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef]
- Esch, T. Health in stress: Change in the stress concept and its significance for prevention, health and life style. Gesundheitswesen 2002, 64, 73–81. [Google Scholar] [CrossRef]
- Pleger, B.; Ruff, C.C.; Blankenburg, F.; Klöppel, S.; Driver, J.; Dolan, R. Influence of Dopaminergically Mediated Reward on Somatosensory Decision-Making. PLoS Biol. 2009, 7, e1000164. [Google Scholar] [CrossRef] [Green Version]
- De Neve, J.E.; Christakis, N.A.; Fowler, J.H.; Frey, B.S. Genes, Economics, and Happiness. J. Neurosci. Psychol. Econ. 2012, 5, 193–211. [Google Scholar] [CrossRef] [Green Version]
- Bartels, M. Genetics of wellbeing and its components satisfaction with life, happiness, and quality of life: A review and meta-analysis of heritability studies. Behav. Genet. 2015, 45, 137–156. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.Y.; Hölzel, B.K.; Posner, M.I. The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 2015, 16, 213–225. [Google Scholar] [CrossRef]
- Moss, A.S.; Reibel, D.K.; Wintering, N.; Vedaei, F.; Porter, H.; Khosravi, M.; Heholt, J.; Alizadeh, M.; Mohamed, F.B.; Newberg, A.B. Cerebral Blood Flow and Brain Functional Connectivity Changes in Older Adults Participating in a Mindfulness-Based Stress Reduction Program. Behav. Sci. 2022, 12, 48. [Google Scholar] [CrossRef]
- Sezer, I.; Pizzagalli, D.A.; Sacchet, M.D. Resting-state fMRI functional connectivity and mindfulness in clinical and non-clinical contexts: A review and synthesis. Neurosci. Biobehav. Rev. 2022, 135, 104583. [Google Scholar] [CrossRef]
- Singleton, O.; Newlon, M.; Fossas, A.; Sharma, B.; Cook-Greuter, S.R.; Lazar, S.W. Brain Structure and Functional Connectivity Correlate with Psychosocial Development in Contemplative Practitioners and Controls. Brain Sci. 2021, 11, 728. [Google Scholar] [CrossRef]
- Sevinc, G.; Rusche, J.; Wong, B.; Datta, T.; Kaufman, R.; Gutz, S.E.; Schneider, M.; Todorova, N.; Gaser, C.; Thomalla, G.; et al. Mindfulness Training Improves Cognition and Strengthens Intrinsic Connectivity Between the Hippocampus and Posteromedial Cortex in Healthy Older Adults. Front. Aging Neurosci. 2021, 13, 702796. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.; Engert, V. It matters what you practice: Differential training effects on subjective experience, behavior, brain and body in the ReSource Project. Curr. Opin. Psychol. 2019, 28, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Engen, H.G.; Bernhardt, B.C.; Skottnik, L.; Ricard, M.; Singer, T. Structural changes in socio-affective networks: Multi-modal MRI findings in long-term meditation practitioners. Neuropsychologia 2018, 116, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Fehr, E.; Fischbacher, U. The nature of human altruism. Nature 2003, 425, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Morishima, Y.; Schunk, D.; Bruhin, A.; Ruff, C.C.; Fehr, E. Linking brain structure and activation in temporoparietal junction to explain the neurobiology of human altruism. Neuron 2012, 75, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Isaacowitz, D.M.; Vaillant, G.E.; Seligman, M.E. Strengths and satisfaction across the adult lifespan. Int. J. Aging Hum. Dev. 2003, 57, 181–201. [Google Scholar] [CrossRef]
- Waldinger, R.J.; Schulz, M.S. What’s love got to do with it? Social functioning, perceived health, and daily happiness in married octogenarians. Psychol. Aging 2010, 25, 422–431. [Google Scholar] [CrossRef] [Green Version]
- Danner, D.D.; Snowdon, D.A.; Friesen, W.V. Positive emotions in early life and longevity: Findings from the nun study. J. Pers. Soc. Psychol. 2001, 80, 804–813. [Google Scholar] [CrossRef]
- Benzo, R.P.; Kirsch, J.L.; Nelson, C. Compassion, Mindfulness, and the Happiness of Healthcare Workers. Explore 2017, 13, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Michaelsen, M.M.; Graser, J.; Onescheit, M.; Tuma, M.; Pieper, D.; Werdecker, L.; Esch, T. Iga.Report 45: Effectiveness of Mindfulness Techniques in the Work Context; Initiative Gesundheit und Arbeit (iga): Berlin, Germany, 2021. [Google Scholar]
- Janssen, M.; Heerkens, Y.; Kuijer, W.; van der Heijden, B.; Engels, J. Effects of Mindfulness-Based Stress Reduction on employees’ mental health: A systematic review. PLoS ONE 2018, 13, e0191332. [Google Scholar] [CrossRef]
- Stewart, W.C.; Adams, M.P.; Stewart, J.A.; Nelson, L.A. Review of clinical medicine and religious practice. J. Relig. Health 2013, 52, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Woo, H. Stressors, social support, religious practice, and general well-being among Korean adult immigrants. J. Evid. Based Soc. Work 2013, 10, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Cobb, E.; Kor, A.; Miller, L. Support for adolescent spirituality: Contributions of religious practice and trait mindfulness. J. Relig. Health 2015, 54, 862–870. [Google Scholar] [CrossRef]
- Esch, T.; Stefano, G.B.; Fricchione, G.L.; Benson, H. Stress in cardiovascular diseases. Med. Sci. Monit. 2002, 8, RA93–RA101. [Google Scholar] [PubMed]
- Esch, T.; Stefano, G.B.; Fricchione, G.L.; Benson, H. The role of stress in neurodegenerative diseases and mental disorders. Neuroendocrinol. Lett. 2002, 23, 199–208. [Google Scholar]
- Esch, T.; Stefano, G.B.; Fricchione, G.L.; Benson, H. An overview of stress and its impact in immunological diseases. Mod. Asp. Immunobiol. 2002, 2, 187–192. [Google Scholar]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; INTERHEART Study Investigators; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Rosengren, A.; Hawken, S.; Ounpuu, S.; Sliwa, K.; Zubaid, M.; Almahmeed, W.A.; Blackett, K.N.; Sitthi-Amorn, C.; Sato, H.; INTERHEART Study Investigators; et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 953–962. [Google Scholar] [CrossRef]
- Queri, S.K. Is there a Divine Reward? Spirituality/Religiosity (S/R) Protects from Work Dissatisfaction and Reduces Stress Load. Gesundheitswesen 2021. [Google Scholar] [CrossRef]
- Mulligan, T.; Skidmore, F.M. Religiosity may alter the cold pressor stress response. Explore 2009, 5, 345–346. [Google Scholar] [CrossRef]
- Dedert, E.A.; Studts, J.L.; Weissbecker, I.; Salmon, P.G.; Banis, P.L.; Sephton, S.E. Religiosity may help preserve the cortisol rhythm in women with stress-related illness. Int. J. Psychiatry Med. 2004, 34, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Esch, T. Stress, adaptation, and self-organization: Balancing processes facilitate health and survival. Komplement. Kl. Nat. 2003, 10, 330–341. [Google Scholar]
- Esch, T.; Winkler, J.; Auwärter, V.; Gnann, H.; Huber, R.; Schmidt, S. Neurobiological Aspects of Mindfulness in Pain Autoregulation: Unexpected Results from a Randomized-Controlled Trial and Possible Implications for Meditation Research. Front. Hum. Neurosci. 2017, 10, 674. [Google Scholar] [CrossRef] [Green Version]
- Stefano, G.B.; Fricchione, G.L.; Slingsby, B.T.; Benson, H. The placebo effect and relaxation response: Neural processes and their coupling to constitutive nitric oxide. Brain Res. Rev. 2001, 35, 1–19. [Google Scholar] [CrossRef]
- Fricchione, G.; Stefano, G.B. Placebo neural systems: Nitric oxide, morphine and the dopamine brain reward and motivation circuitries. Med. Sci. Monit. 2005, 11, MS54–MS65. [Google Scholar] [PubMed]
- Enck, P.; Benedetti, F.; Schedlowski, M. New insights into the placebo and nocebo responses. Neuron 2008, 59, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Enck, P.; Bingel, U.; Schedlowski, M.; Rief, W. The placebo response in medicine: Minimize, maximize or personalize? Nat. Rev. Drug Discov. 2013, 12, 191–204. [Google Scholar] [CrossRef]
- Antonovsky, A.; Sagy, S. Aaron Antonovsky, the Scholar and the Man Behind Salutogenesis. In The Handbook of Salutogenesis; Mittelmark, M.B., Sagy, S., Eriksson, M., Bauer, G.F., Pelikan, J.M., Lindström, B., Espnes, G.A., Eds.; Springer: Cham, Switzerland, 2017; pp. 15–23. [Google Scholar]
- Werdecker, L.; Esch, T. Stress and Health. In Gesundheitswissenschaften; Haring, R., Ed.; Springer Nature: Heidelberg, Germany, 2019; pp. 347–360. [Google Scholar]
- Listopad, I.W.; Michaelsen, M.M.; Werdecker, L.; Esch, T. Bio-Psycho-Socio-Spirito-Cultural Factors of Burnout: A Systematic Narrative Review of the Literature. Front. Psychol. 2021, 12, 722862. [Google Scholar] [CrossRef]
- Listopad, I.W.; Esch, T.; Michaelsen, M.M. An Empirical Investigation of the Relationship Between Spirituality, Work Culture, and Burnout: The Need for an Extended Health and Disease Model. Front. Psychol. 2021, 12, 723884. [Google Scholar] [CrossRef]
- Esch, T.; Stefano, G.B. The Neurobiology of Love. Neuroendocrinol. Lett. 2005, 26, 175–192. [Google Scholar]
- Esch, T.; Stefano, G.B. The neurobiological link between compassion and love. Med. Sci. Monit. 2011, 17, RA65–RA75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefano, G.B.; Stefano, J.M.; Esch, T. Anticipatory stress response: A significant commonality in stress, relaxation, pleasure and love responses. Med. Sci. Monit. 2008, 14, RA17–RA21. [Google Scholar] [PubMed]
- Heidl, C.M.; Landenberger, M.; Jahn, P. Life satisfaction in West Germany: A cross-sectional analysis using data from the Socio-Economic Panel. In SOEPpapers on Multidisciplinary Panel Data Research; Deutsches Institut für Wirtschaftsforschung (DIW): Berlin, Germany, 2012. [Google Scholar]
- Mroczek, D.K.; Spiro, A. Change in life satisfaction during adulthood: Findings from the veterans affairs normative aging study. J. Pers. Soc. Psychol. 2005, 88, 189–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanchflower, D.G.; Oswald, A.J. Is well-being U-shaped over the life cycle? Soc. Sci. Med. 2008, 66, 1733–1749. [Google Scholar] [CrossRef] [Green Version]
- Easterlin, R.A. Life cycle happiness and its sources. J. Econ. Psychol. 2006, 27, 463–482. [Google Scholar] [CrossRef] [Green Version]
- Seligman, M.E.; Steen, T.A.; Park, N.; Peterson, C. Positive psychology progress: Empirical validation of interventions. Am. Psychol. 2005, 60, 410–421. [Google Scholar] [CrossRef] [Green Version]
- Ryff, C.D. Psychological well-being revisited: Advances in the science and practice of eudaimonia. Psychother. Psychosom. 2014, 83, 10–28. [Google Scholar] [CrossRef] [Green Version]
- Sado, M.; Kosugi, T.; Ninomiya, A.; Nagaoka, M.; Park, S.; Fujisawa, D.; Shirahase, J.; Mimura, M. Mindfulness-Based Cognitive Therapy for Improving Subjective Well-Being Among Healthy Individuals: Protocol for a Randomized Controlled Trial. JMIR Res. Protoc. 2020, 9, e15892. [Google Scholar] [CrossRef]
- Kratz, F.; Brüderl, J. The Age Trajectory of Happiness. PsyArXiv Prepr. 2021, in press. [CrossRef]
- Jebb, A.T.; Morrison, M.; Tay, L.; Diener, E. Subjective Well-Being Around the World: Trends and Predictors Across the Life Span. Psychol. Sci. 2020, 31, 293–305. [Google Scholar] [CrossRef]
- Oh, J.; Chopik, W.J.; Konrath, S.; Grimm, K.J. Longitudinal changes in empathy across the life span in six samples of human development. Soc. Psychol. Pers. Sci. 2020, 11, 244–253. [Google Scholar] [CrossRef]
- Patel, S.; Pelletier-Bui, A.; Smith, S.; Roberts, M.B.; Kilgannon, H.; Trzeciak, S.; Roberts, B.W. Curricula for empathy and compassion training in medical education: A systematic review. PLoS ONE 2019, 14, e0221412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esch, T.; Sonntag, U.; Esch, S.M.; Thees, S. Stress management and mind-body medicine: A randomized controlled longitudinal evaluation of students’ health and effects of a behavioral group intervention at a middle-size German university (SM-MESH). Complementary Med. Res. 2013, 20, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Walach, H.; Schmidt, S.; Esch, T. Meditation intervention reviews. JAMA Intern. Med. 2014, 174, 1193–1194. [Google Scholar] [CrossRef] [PubMed]
- Schupp, J. The Socioeconomic Panel (SOEP). Bundesgesundheitsblatt Gesundh. Gesundh. 2012, 55, 767–774. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esch, T. The ABC Model of Happiness—Neurobiological Aspects of Motivation and Positive Mood, and Their Dynamic Changes through Practice, the Course of Life. Biology 2022, 11, 843. https://doi.org/10.3390/biology11060843
Esch T. The ABC Model of Happiness—Neurobiological Aspects of Motivation and Positive Mood, and Their Dynamic Changes through Practice, the Course of Life. Biology. 2022; 11(6):843. https://doi.org/10.3390/biology11060843
Chicago/Turabian StyleEsch, Tobias. 2022. "The ABC Model of Happiness—Neurobiological Aspects of Motivation and Positive Mood, and Their Dynamic Changes through Practice, the Course of Life" Biology 11, no. 6: 843. https://doi.org/10.3390/biology11060843
APA StyleEsch, T. (2022). The ABC Model of Happiness—Neurobiological Aspects of Motivation and Positive Mood, and Their Dynamic Changes through Practice, the Course of Life. Biology, 11(6), 843. https://doi.org/10.3390/biology11060843






