Consistent Site-Specific Foraging Behaviours of Yellow-eyed Penguins/Hoiho Breeding on Stewart Island, New Zealand
Abstract
:Simple Summary
Abstract
1. Introduction
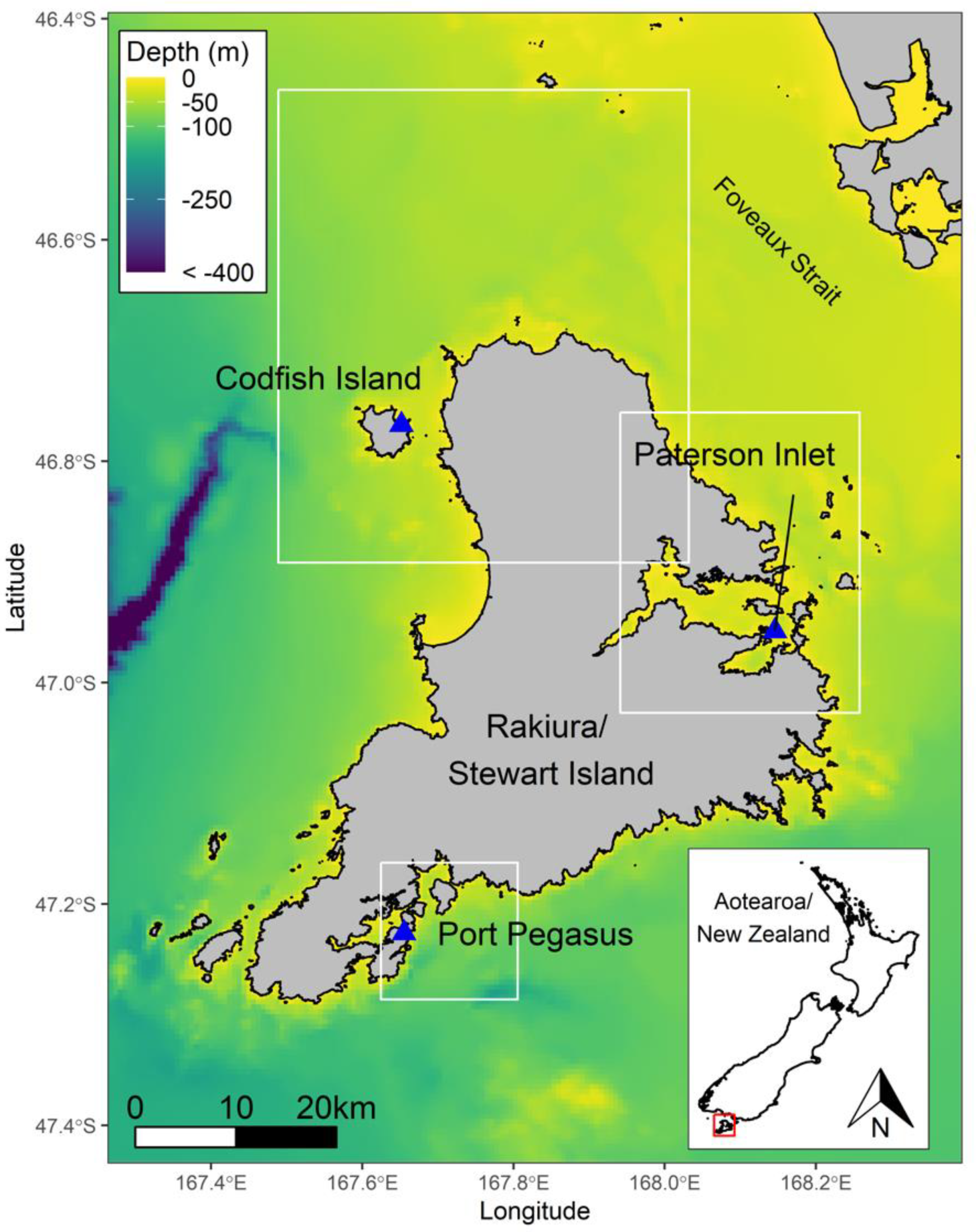
2. Materials and Methods
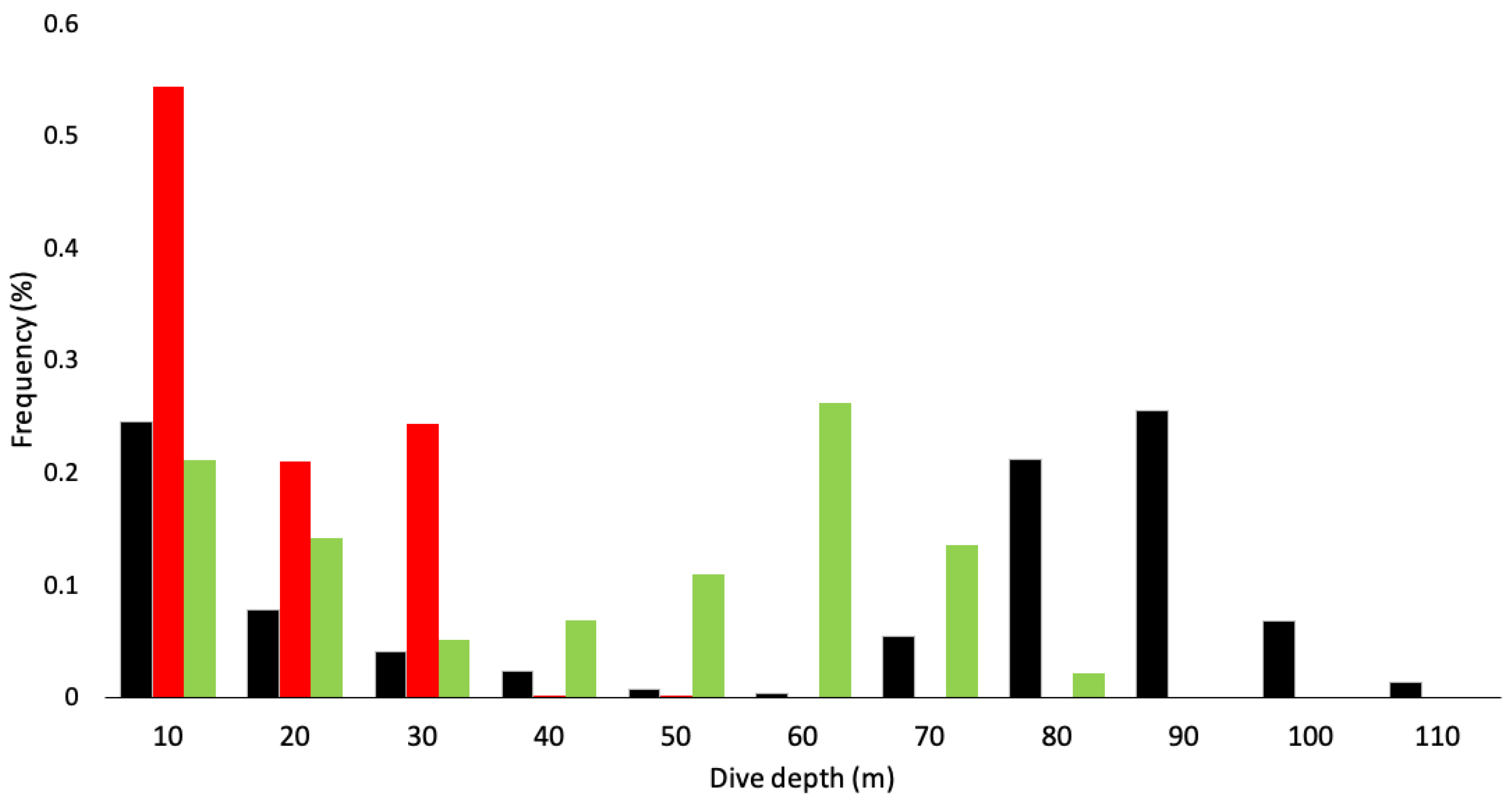
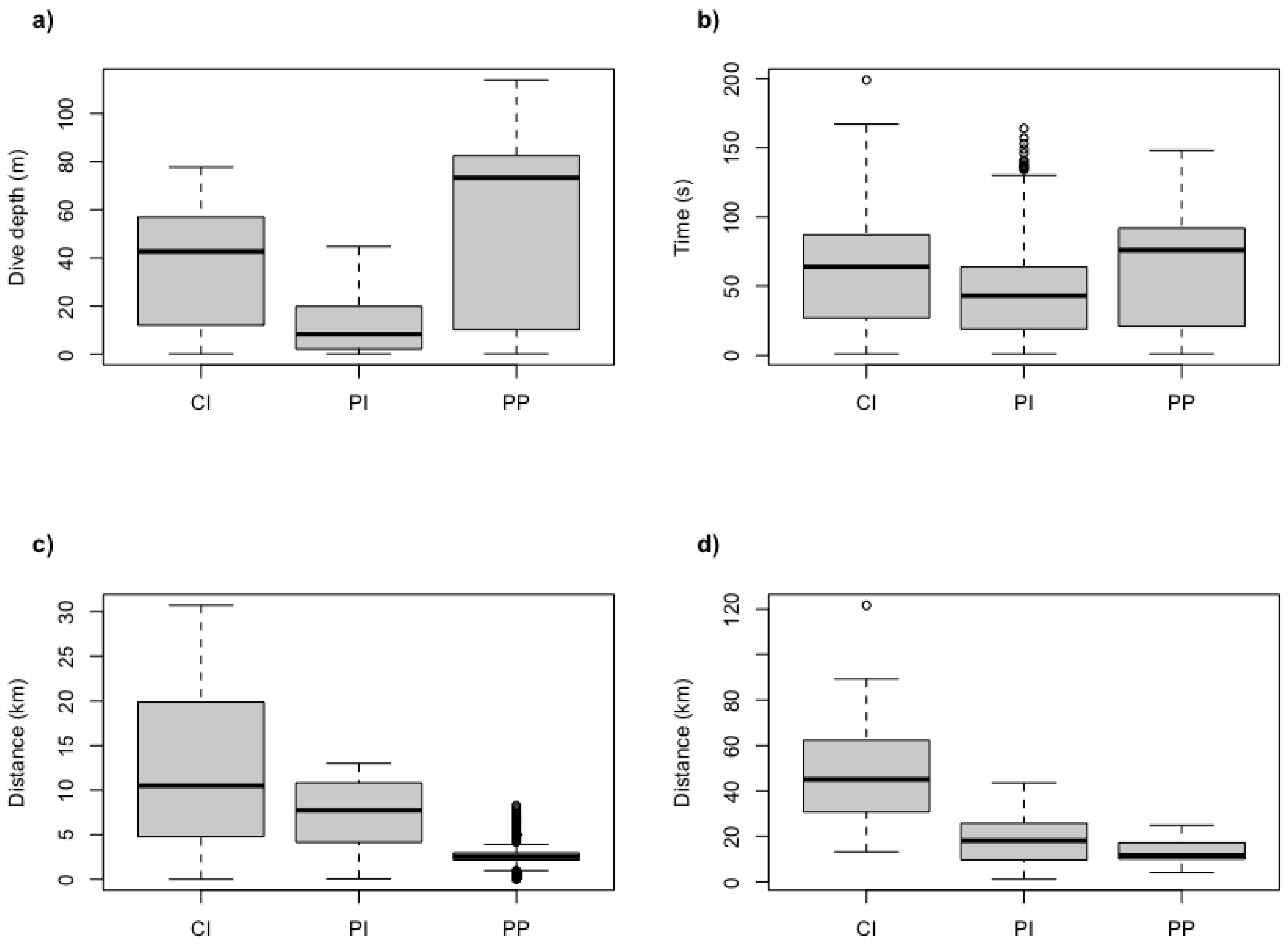
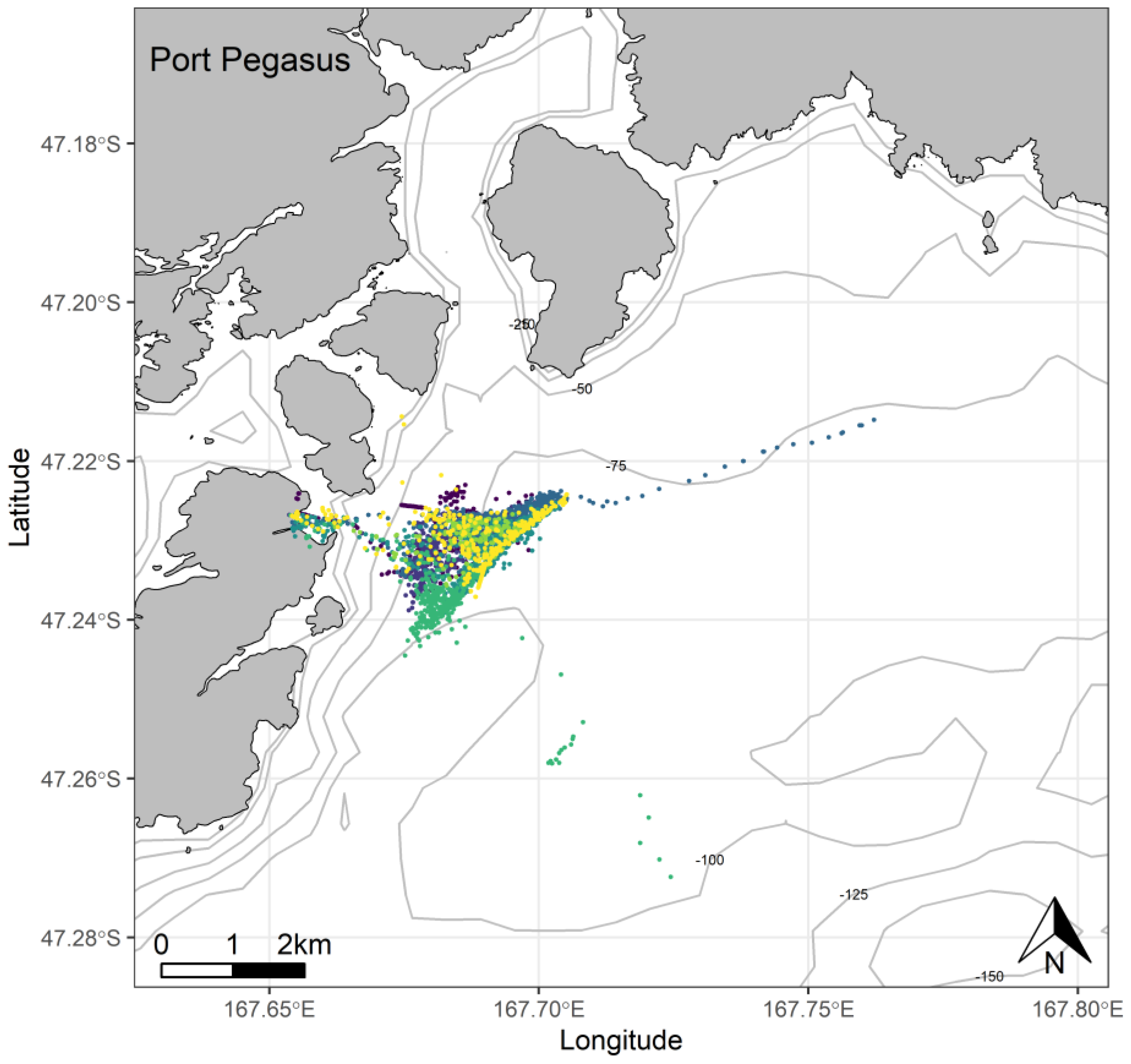
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barry, J.; Newton, M.; Dodd, J.A.; Hooker, O.E.; Boylan, P.; Lucas, M.C.; Adams, C.E. Foraging specialisms influence space use and movement patterns of the European eel Anguilla anguilla. Hydrobiologia 2016, 766, 333–348. [Google Scholar] [CrossRef] [Green Version]
- Schofield, G.; Hobson, V.J.; Lilley, M.K.; Katselidis, K.A.; Bishop, C.M.; Brown, P.; Hays, G.C. Inter-annual variability in the home range of breeding turtles: Implications for current and future conservation management. Biol. Conserv. 2010, 143, 722–730. [Google Scholar] [CrossRef]
- Biro, D.; Meade, J.; Guilford, T. Familiar route loyalty implies visual pilotage in the homing pigeon. Proc. Natl. Acad. Sci. USA 2004, 101, 17440–17443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattern, T. Marine Ecology of Offshore and Inshore Foraging Penguins: The Snares Penguin Eudyptes robustus and Yellow-Eyed Penguin Megadyptes antipodes. Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2007. [Google Scholar]
- Merkle, J.A.; Fortin, D.; Morales, J.M. A memory-based foraging tactic reveals an adaptive mechanism for restricted space use. Ecol. Lett. 2014, 17, 924–931. [Google Scholar] [CrossRef]
- Mattern, T.; Ellenberg, U.; Houston, D.M.; Davis, L.S. Consistent foraging routes and benthic foraging behaviour in yellow-eyed penguins. Mar. Ecol. Prog. Ser. 2007, 343, 295–306. [Google Scholar] [CrossRef]
- Osland, M.J.; Stevens, P.W.; Lamont, M.M.; Brusca, R.C.; Hart, K.M.; Waddle, J.H.; Langtimm, C.A.; Williams, C.M.; Keim, B.D.; Terando, A.J.; et al. Tropicalization of temperate ecosystems in North America: The northward range expansion of tropical organisms in response to warming winter temperatures. Glob. Chang. Biol. 2021, 27, 3009–3034. [Google Scholar] [CrossRef]
- Bertram, D.F.; Mackas, D.L.; Welch, D.W.; Boyd, W.S.; Ryder, J.L.; Galbraith, M.; Hedd, A.; Morgan, K.; O’Hara, P.D. Variation in zooplankton prey distribution determines marine foraging distributions of breeding Cassin’s Auklet. Deep Sea Research Part I. Oceanogr. Res. Pap. 2017, 129, 32–40. [Google Scholar] [CrossRef]
- Weimerskirch, H.; Ancel, A.; Caloin, M.; Zahariev, A.; Spagiari, J.; Kersten, M.; Chastel, O. Foraging efficiency and adjustment of energy expenditure in a pelagic seabird provisioning its chick. J. Anim. Ecol. 2003, 72, 500–508. [Google Scholar] [CrossRef] [Green Version]
- Osborne, O.E.; Hara, P.D.; Whelan, S.; Zandbergen, P.; Hatch, S.A.; Elliott, K.H. Breeding seabirds increase foraging range in response to an extreme marine heatwave. Mar. Ecol. Prog. Ser. 2020, 646, 161–173. [Google Scholar] [CrossRef]
- Fayet, A.L.; Clucas, G.V.; Anker-Nilssen, T.; Syposz, M.; Hansen, E.S. Local prey shortages drive foraging costs and breeding success in a declining seabird, the Atlantic puffin. J. Anim. Ecol. 2021, 90, 1152–1164. [Google Scholar] [CrossRef]
- Ropert-Coudert, Y.; Kato, A.; Wilson, R.P.; Cannell, B. Foraging strategies and prey encounter rate of free-ranging Little Penguins. Mar. Biol. 2006, 149, 139–148. [Google Scholar] [CrossRef]
- Chiaradia, A.F.; Kerry, K.R. Daily nest attendance and breeding performance in the little penguin Eudyptula minor at Phillip Island, Australia. Mar. Ornithol. 1999, 27, 13–20. [Google Scholar]
- McCutcheon, C.; Dann, P.; Salton, M.; Renwick, L.; Hoskins, A.J.; Gormley, A.M.; Arnould, J.P. The foraging range of Little Penguins (Eudyptula minor) during winter. Emu 2011, 111, 321–329. [Google Scholar] [CrossRef]
- Oliver, M.J.; Kohut, J.T.; Bernard, K.; Fraser, W.; Winsor, P.; Statscewich, H.; Fredj, E.; Cimino, M.; Patterson-Fraser, D.; Carvalho, F. Central place foragers select ocean surface convergent features despite differing foraging strategies. Sci. Rep. 2019, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.G.; Chilvers, B.L.; French, R.K.; Battley, P.F. Diving plasticity in the ancestral range of the yellow-eyed penguin Megadyptes antipodes, an endangered marine predator. Mar. Ecol. Prog. Ser. 2020, 648, 191–205. [Google Scholar] [CrossRef]
- Crawford, R.; Ellenberg, U.; Frere, E.; Hagen, C.; Baird, K.; Brewin, P.; Crofts, S.; Glass, J.; Mattern, T.; Pompert, J.; et al. Tangled and drowned: A global review of penguin bycatch in fisheries. Endanger. Species Res. 2017, 34, 373–396. [Google Scholar] [CrossRef] [Green Version]
- BirdLife International. Species Factsheet: Megadyptes Antipodes. 2022. Available online: http://www.birdlife.org (accessed on 16 February 2022).
- NIWA. From New Zealand Bathymetry Data Set. NIWA. Available online: https://niwa.co.nz/our-science/oceans/bathymetry (accessed on 14 September 2021).
- Shaffer, M.R.; Rovellini, A. A Review of Habitat Use, Home Range and Connectivity for Selected New Zealand Species. 2020. Available online: https://dxcprod.doc.govt.nz/globalassets/documents/conservation/marine-and-coastal/marine-protected-areas/mpa-publications/habitat-use-and-movement-patterns-2020.pdf (accessed on 16 March 2021).
- Ryan, P.G.; Petersen, S.L.; Peters, G.; Grémillet, D. GPS tracking a marine predator: The effects of precision, resolution and sampling rate on foraging tracks of African Penguins. Mar. Biol. 2004, 145, 215–223. [Google Scholar] [CrossRef]
- Rey, A.R.; Bost, C.A.; Schiavini, A.; Pütz, K. Foraging movements of Magellanic Penguins Spheniscus magellanicus in the Beagle Channel, Argentina, related to tide and tidal currents. J. Ornithol. 2010, 151, 933–943. [Google Scholar] [CrossRef]
- Chilvers, B.L.; Dobbins, M.L.; Edmonds, H.K. Diving behaviour of yellow-eyed penguins, Port Pegasus/Pikihatītī, Stewart Island/Rakiura, New Zealand. N. Z. J. Zool. 2014, 41, 161–170. [Google Scholar] [CrossRef]
- van Heezik, Y.; Davis, L.S. Effects of food variability on growth rates, fledging sizes and reproductive success in the Yellow-eyed Penguin Megadyptes antipodes. Ibis 1990, 132, 354–365. [Google Scholar] [CrossRef]
- Mori, Y. Optimal diving behaviour for foraging in relation to body size. J. Evol. Biol. 2002, 15, 269–276. [Google Scholar] [CrossRef]
- Schreer, J.F.; Kovacs, K.M.; O’Hara Hines, R.J. Comparative diving patterns of pinnipeds and seabirds. Ecol. Monogr. 2001, 71, 137–162. [Google Scholar] [CrossRef]
- Mori, Y.; Boyd, I.L. The behavioural basis for nonlinear functional responses and optimal foraging in Antarctic fur seals. Ecology 2004, 85, 398–410. [Google Scholar] [CrossRef]
- Saino, N.; Romano, M.; Ambrosini, R.; Rubolini, D.; Boncoraglio, G.; Caprioli, M.; Romano, A. Longevity and lifetime reproductive success of barn swallow offspring are predicted by their hatching date and phenotypic quality. J. Anim. Ecol. 2012, 81, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Mattern, T.; Ellenberg, U. Hoiho Population and Tracking. POP2018-02 Final Report Prepared by Eudyptes Consulting Ltd for the Conservation Services Programme, Department of Conservation. 2021; 50p. Available online: https://www.doc.govt.nz/globalassets/documents/conservation/marine-and-coastal/marine-conservation-services/reports/final-reports/pop2018-02-hoiho-tracking-final-report.pdf (accessed on 14 September 2021).
- Mattern, T.; Ellenberg, U. Utilisation of the Marine Habitat by Hoiho/Yellow-Eyed Penguins from Rakiura/Stewart Island. Final Report for POP2020-05, Prepared by Eudyptes Consulting for the Conservation Services Programme, Department of Conservation. 2022; 45p. Available online: https://www.doc.govt.nz/globalassets/documents/conservation/marine-and-coastal/marine-conservation-services/reports/final-reports/pop2020-05-hoiho-tracking-rakiura-final-report.pdf (accessed on 14 September 2021).
- Marchant, S.; Higgins, P.J. Megadyptes antipodes yellow-eyed penguin. In Handbook of Australian, New Zealand and Antarctic Birds; Oxford University Press: Melbourne, VIC, Australia, 1990; Volume 1, pp. 236–246. Available online: https://www.abebooks.com/book-search/title/the-handbook-of-australian-new-zealand-and-antarctic-birds/ (accessed on 14 September 2021).
- Seddon, P.J.; Ellenberg, U.; van Heezik, Y. Yellow-eyed penguin (Megadyptes antipodes). In Penguins: Natural History and Conservation; Garcia Borboroglu, P., Boersma, P.D., Eds.; University of Washington Press: Seattle, WA, USA; London, UK, 2013; pp. 91–110. [Google Scholar]
- Stein, A.M.; Young, M.J.; Darby, J.T.; Seddon, P.J.; van Heezik, Y. Evidence for high inter-generational individual quality in yellow-eyed penguins. PeerJ 2017, 5, e2935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darby, J.T.; Seddon, P.J.; Davis, L.S. Breeding biology of yellow-eyed penguins (Megadyptes antipodes). In Penguin Biology, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 1990; pp. 45–62. [Google Scholar]
- Browne, T.; Lalas, C.; Mattern, T.; Van Heezik, Y. Chick starvation in yellow- eyed penguins: Evidence for poor diet quality and selective provisioning of chicks from conventional diet analysis and stable isotopes. Austral Ecol. 2011, 36, 99–108. [Google Scholar] [CrossRef]
- Darby, J.T.; Dawson, S.M. Bycatch of yellow-eyed penguins (Megadyptes antipodes) in gillnets in New Zealand waters 1979–1997. Biol. Conserv. 2000, 93, 327–332. [Google Scholar] [CrossRef]
- Ratz, H.; Murphy, B. Effects of habitat and introduced mammalian predators on the breeding success of Yellow-eyed Penguins Megadyptes antipodes, South Island, New Zealand. Pac. Conserv. Biol. 1999, 5, 16–27. [Google Scholar] [CrossRef]
- Alley, M.R.; Suepaul, R.B.; McKinlay, B.; Young, M.J.; Wang, J.; Morgan, K.J.; Hunter, S.A.; Gartrell, B.D. Diphtheritic stomatitis in Yellow-eyed penguins (Megadyptes antipodes) in New Zealand. J. Wildl. Dis. 2017, 53, 102–110. [Google Scholar] [CrossRef]
- Richdale, E. A Population Study of Penguins; Oxford University Press: Oxford, UK, 1957. [Google Scholar]
- Cranfield, H.J.; Manighetti, B.; Michael, K.P.; Hill, A. Effects of oyster dredging on the distribution of bryozoan biogenic reefs and associated sediments in Foveaux Strait, southern New Zealand. Cont. Shelf Res. 2003, 23, 1337–1357. [Google Scholar] [CrossRef]
- Carbines, G.; Jiang, W.; Beentjes, M.P. The impact of oyster dredging on the growth of blue cod, Parapercis colias, in Foveaux Strait, New Zealand. Aquat. Conserv. Mar. Freshw. Ecosyst. 2004, 14, 491–504. [Google Scholar] [CrossRef]
- Mattern, T.; Wilson, K.J. New Zealand Penguins–Current Knowledge and Research Priorities; A report compiled for Birds New Zealand; Penguin Archive: Dunedin, New Zealand, 2018. [Google Scholar] [CrossRef]
- King, S.D.; Harper, G.A.; Wright, J.B.; McInnes, J.C.; van der Lubbe, J.E.; Dobbins, M.L.; Murray, S.J. Site-specific reproductive failure and decline of a population of the Endangered yellow-eyed penguin: A case for foraging habitat quality. Mar. Ecol. Prog. Ser. 2012, 467, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Alley, M.R.; Morgan, K.J.; Gill, J.M.; Hocken, A.G. Diseases and causes of mortality in yellow-eyed penguins, Megadyptes antipodes. Kokako 2004, 11, 18–23. [Google Scholar]
- Hill, A.G.; Howe, L.; Gartrell, B.D.; Alley, M.R. Prevalence of Leucocytozoon spp, in the endangered yellow-eyed penguin Megadyptes antipodes. Parasitology 2010, 137, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Stats NZ. Asset Value of Commercial Fish Resources Caught in New Zealand. Figure.NZ. Available online: https://figure.nz/chart/LM7iOMLt6SEQscZR (accessed on 1 March 2022).
- Michael, K.P. A Strategic Research Plan (2010–15) to Underpin Management Goals of the 2009 Fisheries Plan for Foveaux Strait Oysters (Ostrea chilensis, OYU 5). New Zealand Fisheries Assessment Report. 2010, p. 21. Available online: https://docs.niwa.co.nz/library/public/FAR2010-21.pdf (accessed on 1 March 2022).
- Yellow-Eyed Penguin Trust. Value to the Economy-Yellow-Eyed Penguin Trust. Yellow-Eyed Penguin Trust. Retrieved 17 May 2022. Available online: https://www.yellow-eyedpenguin.org.nz/penguins/value-to-the-economy/ (accessed on 31 October 2017).
- Froude, V.A.; Smith, R. Area-Based Restrictions in the New Zealand Marine Environment. Department of Conservation MCU Report; Pacific Eco-Logic Ltd.: Bay Of Islands, New Zealand, 2004; Volume 18, p. 3. Available online: https://www.doc.govt.nz/globalassets/documents/conservation/marine-and-coastal/fishing/area-based-restrictions-hi-res.pdf (accessed on 1 March 2022).
- Mello, H.L.; Smith, A.M.; Wood, A.C.; Tidey, E.J. Enhanced biodiversity and abundance of benthic invertebrate macrofauna in a New Zealand marine reserve. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1854–1867. [Google Scholar] [CrossRef]
- Richard, Y.; Abraham, E.; Filippi, D. Assessment of the Risk to Seabird Populations from New Zealand Commercial Fisheries. Ministry of Fisheries. 2011. Available online: http://files.dragonfly.co.nz/publications/pdf/Richardetal_2011a_IPA2009-20.pdf (accessed on 1 March 2022).
- Ellenberg, U.; Mattern, T. Yellow-Eyed Penguin—Review of Population Information; In Final Report of Contract; Department of Conservation: Wellington, New Zealand, 2012; p. 4350. Available online: https://www.researchgate.net/publication/258106472_Yellow-eyed_penguin_Review_of_population_information_Marine_Conservation_Services_Programme_Department_of_Conservation_Wellington (accessed on 1 March 2022).
- Setiawan, A.N.; Massaro, M.; Darby, J.T.; Davis, L.S. Mate and territory retention in yellow-eyed penguins. Condor 2005, 107, 703–709. [Google Scholar] [CrossRef]
- Fisheries (Southland and Sub-Antarctic Areas Commercial Fishing) Regulations 1986). Available online: https://legislation.govt.nz/regulation/public/1986/0220/22.0/DLM111064.html#DLM112270 (accessed on 10 February 2021).
- Gilpin, M.E. Minimal viable populations: Processes of species extinction. In Conservation Biology: The Science of Scarcity and Diversity; WonderBook: Gaithersburg, MD, USA, 1986. [Google Scholar]
- Barbraud, C.; Rolland, V.; Jenouvrier, S.; Nevoux, M.; Delord, K.; Weimerskirch, H. Effects of climate change and fisheries bycatch on Southern Ocean seabirds: A review. Mar. Ecol. Prog. Ser. 2012, 454, 285–307. [Google Scholar] [CrossRef] [Green Version]
- Setiawan, A.N.; Darby, J.T.; Lambert, D.M. The use of morphometric measurements to sex yellow-eyed penguins. Waterbirds 2004, 27, 96–101. [Google Scholar] [CrossRef]
- Wilson, R.P.; Wilson, M.P.T. Tape: A package-attachment technique for penguins. Wildl. Soc. Bull. 1989, 17, 77–79. Available online: https://www.jstor.org/stable/3782045 (accessed on 10 February 2021).
- Wilson, R.P.; Pütz, K.; Peters, G.; Culik, B.; Scolaro, J.A.; Charrassin, J.B.; Ropert-Coudert, Y. Long-term attachment of transmitting and recording devices to penguins and other seabirds. Wildl. Soc. Bull. 1997, 25, 101–106. Available online: https://www.jstor.org/stable/3783290 (accessed on 10 February 2021).
- Pistorius, P.A.; Green, D.B.; Seddon, P.J.; Thiebault, A. In situ observation of a record-sized squid prey consumed by a gentoo penguin. Polar Biol. 2020, 43, 279–283. [Google Scholar] [CrossRef]
- Lescroël, A.; Schmidt, A.; Elrod, M.; Ainley, D.G.; Ballard, G. Foraging dive frequency predicts body mass gain in the Adélie penguin. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar] [CrossRef]
- Gelman, A.; Su, Y.S. Arm: Data Analysis Using Regression and Multilevel/Hierarchical Models. R Package Version 1.8–6. 2015. Available online: http://CRAN.R-project.org/package=arm (accessed on 6 November 2021).
- Gelman, A. Scaling regression inputs by dividing by two standard deviations. Stat. Med. 2008, 27, 2865–2873. [Google Scholar] [CrossRef]
- Burn, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Pittman, S.J.; Costa, B.M.; Battista, T.A. Using lidar bathymetry and boosted regression trees to predict the diversity and abundance of fish and corals. J. Coast. Res. 2009, 53, 27–38. [Google Scholar] [CrossRef]
- Evans, R.; Lea, M.A.; Hindell, M.A. Predicting the distribution of foraging seabirds during a period of heightened environmental variability. Ecol. Appl. 2021, 31, e02343. [Google Scholar] [CrossRef]
- Lescroel, A.; Ridoux, V.; Bost, C.A. Spatial and temporal variation in the diet of the gentoo penguin (Pygoscelis papua) at Kerguelen Islands. Polar Biol. 2004, 27, 206–216. [Google Scholar] [CrossRef]
- Moore, P.J.; Wakelin, M.; Douglas, M.E.; McKinlay, B.; Nelson, D.; Murphy, B. Yellow-Eyed Penguin Foraging Study, South-Eastern New Zealand. Science and Research Series. 1995. Available online: https://www.doc.govt.nz/Documents/science-and-technical/sr83.pdf (accessed on 6 November 2021).
- Van Denderen, P.D.; Bolam, S.G.; Hiddink, J.G.; Jennings, S.; Kenny, A.; Rijnsdorp, A.D.; Van Kooten, T. Similar effects of bottom trawling and natural disturbance on composition and function of benthic communities across habitats. Mar. Ecol. Prog. Ser. 2015, 541, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Collie, J.; Hiddink, J.G.; van Kooten, T.; Rijnsdorp, A.D.; Kaiser, M.J.; Jennings, S.; Hilborn, R. Indirect effects of bottom fishing on the productivity of marine fish. Fish Fish. 2017, 18, 619–637. [Google Scholar] [CrossRef] [Green Version]
- van Heezik, Y. Seasonal, geographic and age-related variation in the diet of the yellow-eyed penguin (Megadyptes antipodes) on mainland New Zealand. N. Z. J. Zool. 1990, 17, 205–215. [Google Scholar] [CrossRef]
- van Heezik, Y. Diet of yellow-eyed, Fiordland crested and little blue penguins breeding sympatrically on Codfish Island, New Zealand. N. Z. J. Zool. 1990, 17, 543–548. [Google Scholar] [CrossRef]
- Horswill, C.; Trathan, P.N.; Ratcliffe, N. Linking extreme interannual changes in prey availability to foraging behaviour and breeding investment in a marine predator, the macaroni penguin. PLoS ONE 2017, 12, e0184114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattern, T.; Ellenberg, U.; Houston, D.M.; Lamare, M.; Davis, L.S.; van Heezik, Y.; Seddon, P.J. Straight line foraging in yellow-eyed penguins: New insights into cascading fisheries effects and orientation capabilities of marine predators. PLoS ONE 2013, 8, e84381. [Google Scholar] [CrossRef] [PubMed]
- Hickcox, R.P. Environmental, Climatic, and Biological Interactions Influencing the Marine Distribution of Yellow-Eyed Penguins (Megadyptes antipodes). Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2022. [Google Scholar]
- Pelletier, L.; Chiaradia, A.; Kato, A.; Ropert-Coudert, Y. Fine-scale spatial age segregation in the limited foraging area of an inshore seabird species, the little penguin. Oecologia 2014, 176, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.; Kim, J.H.; Kokubun, N.; Park, S.; Chung, H.; Lee, W.Y. (Group association and vocal behaviour during foraging trips in Gentoo penguins. Sci. Rep. 2017, 7, 7570. [Google Scholar] [CrossRef] [Green Version]
- McInnes, A.M.; McGeorge, C.; Ginsberg, S.; Pichegru, L.; Pistorius, P.A. Group foraging increases foraging efficiency in a piscivorous diver, the African penguin. R. Soc. Open Sci. 2017, 4, 170918. [Google Scholar] [CrossRef] [Green Version]
- McInnes, A.M.; Pistorius, P.A. Up for grabs: Prey herding by penguins facilitates shallow foraging by volant seabirds. R. Soc. Open Sci. 2019, 6, 190333. [Google Scholar] [CrossRef] [Green Version]
- Mattern, T.; McPherson, M.D.; Ellenberg, U.; van Heezik, Y.; Seddon, P.J. High definition video loggers provide new insights into behaviour, physiology, and the oceanic habitat of a marine predator, the yellow-eyed penguin. PeerJ 2018, 6, 6e54. [Google Scholar] [CrossRef]
- Mattern, T.; Ellenberg, U. Yellow-Eyed Penguin Diet and Indirect Effects Affecting Prey Composition. Collation of Biological Information; Department of Conservation: Wellington, New Zealand, 2018. [Google Scholar] [CrossRef]
- Muller, C.G.; Chilvers, B.L.; Chiardia, A.; French, R.K.; Kato, A.; Roupert-Coudert, Y.; Battley, P.F. Foraging areas and plasticity of yellow-eyed penguins Megadyptes antipodes in their subantarctic range. Mar. Ecol. Prog. Ser. 2021, 679, 149–162. [Google Scholar] [CrossRef]
- Hocken, A.G. Necropsy findings in yellow-eyed penguins (Megadyptes antipodes) from Otago, New Zealand. N. Z. J. Zool. 2005, 32, 1–8. [Google Scholar] [CrossRef]
- Vanstreels, R.E.T.; Hurtado, R.; Ewbank, A.; Bertozzi, C.P.; Catão-Dias, J.L. Lesions associated with drowning in bycaught penguins. Dis. Aquat. Org. 2016, 121, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willan, R.C. Soft-bottom assemblages of Paterson Inlet, Stewart Island. N. Zeal. J. Zool. 1981, 8, 229–248. [Google Scholar] [CrossRef] [Green Version]
- Mattern, T. (2022): Dive Analysis-Post-Guard 2018. Figshare. Dataset. Available online: https://figshare.com/articles/dataset/Dive_analysis_-_post-guard_2018/19775818/1 (accessed on 6 February 2022). [CrossRef]
- Harding, A.; Paredes, R.; Suryan, R.; Roby, D.; Irons, D.; Orben, R.; Renner, H.; Young, R.; Barger, C.; Dorresteijn, I.; et al. Does location really matter? An inter-colony comparison of seabirds breeding at varying distances from productive oceanographic features in the Bering Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2013, 94, 178–191. [Google Scholar] [CrossRef] [Green Version]
- Petersen, S.L.; Ryan, P.G.; Gremillet, D. Is food availability limiting African Penguins Spheniscus demersus at Boulders? A comparison of foraging effort at mainland and island colonies. Ibis 2006, 148, 14–26. [Google Scholar] [CrossRef]
- Gómez-Laich, A.; Wilson, R.P.; Sala, J.E.; Luzenti, A.; Quintana, F. Moving northward: Comparison of the foraging effort of Magellanic penguins from three colonies of northern Patagonia. Mar. Biol. 2015, 162, 1451–1461. [Google Scholar] [CrossRef]
- Cranfield, H.J.; Michael, K.P.; Doonan, I.J. Changes in the distribution of epifaunal reefs and oysters during 130 years of dredging for oysters in Foveaux Strait, southern New Zealand. Aquat. Conserv. Mar. Freshw. Ecosyst. 1999, 9, 461–483. [Google Scholar] [CrossRef]
- Jiang, W.; Carbines, G. Diet of blue cod, Parapercis colias, living on undisturbed biogenic reefs and on seabed modified by oyster dredging in Foveaux Strait, New Zealand. Aquat. Conserv. Mar. Freshw. Ecosyst. 2002, 12, 257–272. [Google Scholar] [CrossRef]
- Gregory, M.R. Marine debris: Notes from Chatham Island, and Mason and Doughboy Bays, Stewart Island. Tane 1999, 37, 201–210. [Google Scholar]
- Elley, T.; Mattern, T.; Ellenberg, U.; Young, M.J.; van Heezik, Y.; Seddon, P.J. Diet Disparity Evident Across a Restricted Range in Yellow-Eyed Penguins/Hoiho Breeding on Stewart Island, New Zealand; Department of Zoology: Dunedin, New Zealand, 2019. [Google Scholar]
- Mattern, T.; Meyer, S.; Ellenberg, U.; Houston, D.M.; Darby, J.T.; Young, M.; van Heezik, Y.; Seddon, P.J. Quantifying climate change impacts emphasises the importance of managing regional threats in the endangered Yellow-eyed penguin. PeerJ 2017, 5, e3272. [Google Scholar] [CrossRef]
- Massaro, M.; Blair, D. Comparison of population numbers of yellow-eyed penguins, Megadyptes antipodes, on Stewart Island and on adjacent cat-free islands. N. Z. J. Ecol. 2003, 27, 107–113. Available online: https://www.jstor.org/stable/24055335 (accessed on 6 February 2022).
- Department of Conservation. Te Mahere Rima Tau/Five Year Action Plan. 2020. Available online: https://www.doc.govt.nz/globalassets/documents/conservation/native-animals/birds/sea-and-shore/te-mahere-rima-tau-2020.pdf (accessed on 6 February 2022).
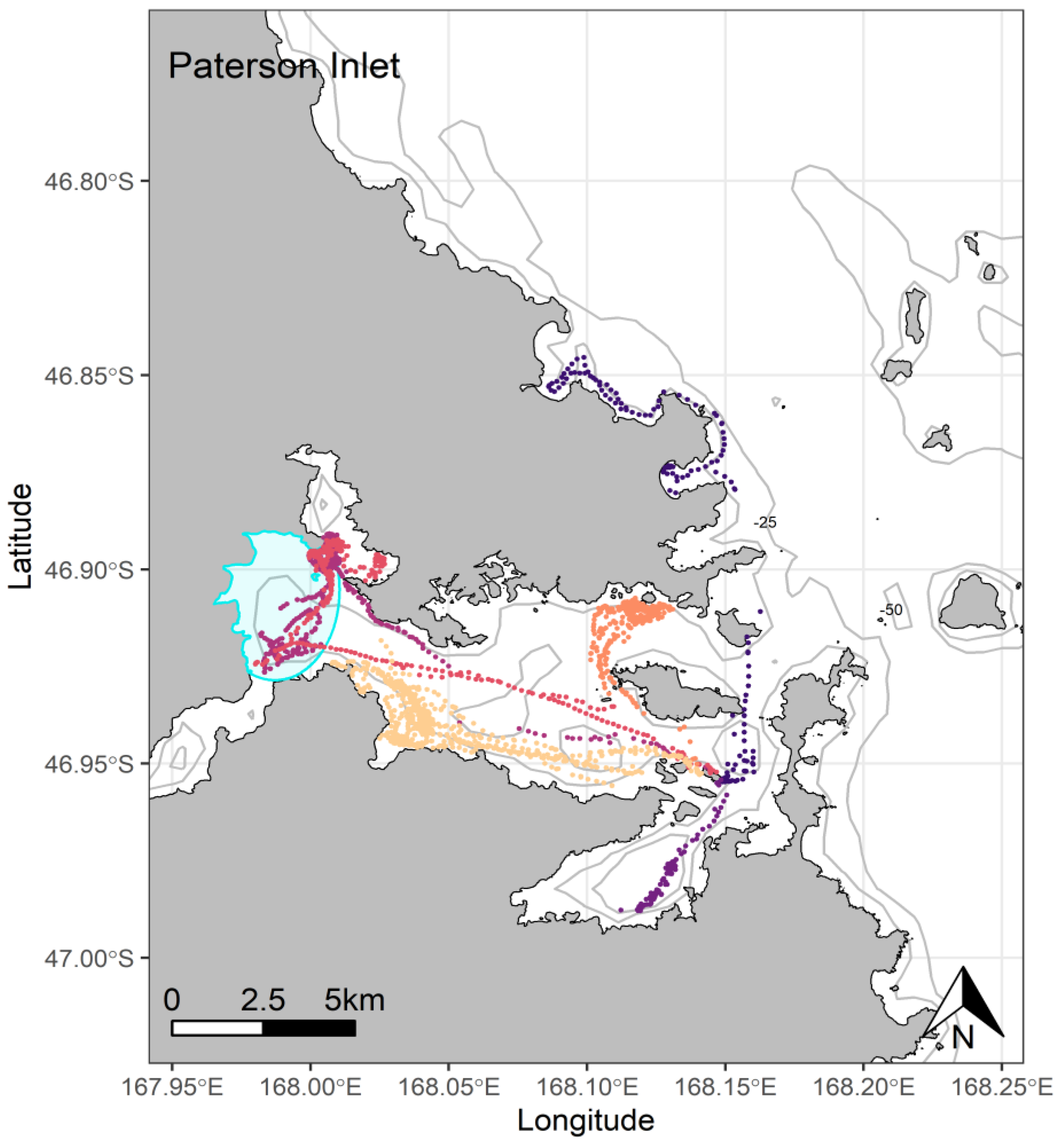
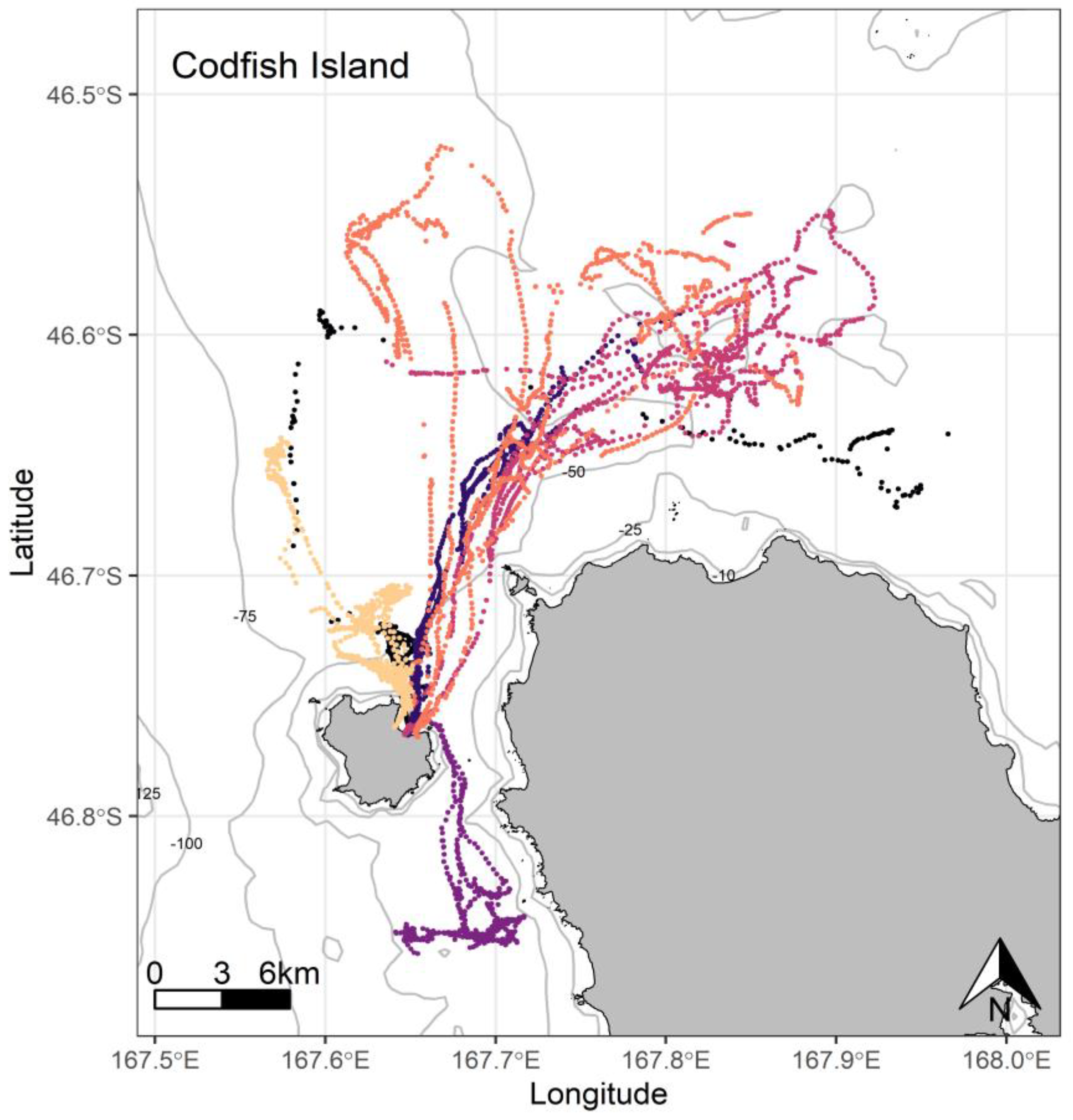
| Foraging Parameters | Port Pegasus | Paterson Inlet | Codfish Island |
|---|---|---|---|
| n | 7 | 6 | 6 |
| Total trips | 33 | 29 | 27 |
| Sex ratio (M:F) | (4:3) | (2:4) | (3:3) |
| Body mass (kg) | 5.6 ± 0.8 | 5.2 ± 0.5 | 5.4 ± 0.4 |
| Maximum depth (m) | 113.9 | 44.6 | 77.8 |
| Half day trips | 28 (81.8%) | 22 (75.9%) | 1 (3.7%) |
| Mean trip duration (h) | 7.8 ± 2.9 | 8.3 ± 3.1 | 13.9 ± 2.2 |
| Foraging radii (km) | 4.8 ± 2.1 | 9.9 ± 3.7 | 22.6 ± 8.1 |
| Trip length (km) | 13.1 ± 4.9 | 19.2 ± 11.6 | 49.5 ± 24.7 |
| % Benthic dives | 65.6 ± 0.1 | 62.8 ± 0.2 | 64.1 ± 0.2 |
| Dive frequency (dive/h) | 17.9 ± 3.5 | 34.0 ± 6.5 | 25.4 ± 3.8 |
| Dive duration (s) | 128.4 ± 7.2 | 72.9 ± 21.5 | 116.6 ± 17.5 |
| Bottom time (s) | 62.5 ± 4.9 | 43.9 ± 12.6 | 62.6 ± 15.6 |
| Mean depth (m) | 53.1 ± 4.3 | 11.6 ± 6.5 | 37.4 ± 7.5 |
| Diving Efficiency | 0.34 ± 0.1 | 0.46 ± 0.1 | 0.40 ± 0.1 |
| Foraging Effort | 0.73 ± 0.1 | 0.76 ± 0.1 | 0.78 ± 0.1 |
| Predictor Variable | Max Depth (m) | Bottom Time (s) | Foraging Radii (km) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C (SE) | t-value | p | C (SE) | t-value | p | C (SE) | t-value | p | |
| Intercept | 2.32 | 22.02 | <0.001 | 4.69 | 13.8 | <0.001 | 1.50 | 1.86 | 0.08 |
| Site: PI | 3.50 | −12.41 | <0.001 | 7.07 | −3.43 | <0.01 | 2.27 | 2.43 | 0.03 |
| Site: CI | 3.35 | −4.18 | <0.01 | 6.77 | 0.122 | 0.90 | 2.17 | 4.12 | <0.001 |
| Body Mass | 3.46 | −0.74 | 0.47 | 6.99 | −1.81 | 0.10 | 2.24 | 0.75 | 0.46 |
| Sex | 4.62 | −0.14 | 0.89 | 9.30 | 0.46 | 0.65 | 2.98 | −0.08 | 0.93 |
| Trip Length (km) | Diving Efficiency | Foraging Effort | |||||||
| C (SE) | t-value | p | C (SE) | t-value | p | C (SE) | t-value | p | |
| Intercept | 3.93 | 3.40 | <0.01 | 0.02 | 38.22 | <0.001 | 0.02 | 18.04 | <0.001 |
| Site: PI | 6.28 | 1.26 | 0.23 | 0.02 | 1.37 | 0.19 | 0.02 | 4.10 | <0.001 |
| Site: CI | 5.98 | 6.47 | <0.001 | 0.02 | 1.87 | 0.08 | 0.02 | 2.16 | 0.045 |
| Body Mass | 5.94 | −0.17 | 0.86 | - | - | - | - | - | - |
| Sex | 7.89 | 0.09 | 0.93 | - | - | - | - | - | - |
| Site | Distance to Foraging Grounds (km) | Trip Length (km) | Trip Duration (h) | Sampling Period | Source |
|---|---|---|---|---|---|
| Otago Peninsula | c. 15 | 76.2 ± 71.3 | 70.6 ± 89.6 | 2019–2020 | [29,30,87] |
| Catlins | c. 20 | 82.0 ± 43.5 | 30.9 ± 16.0 | 2018–2020 | [29,30,87] |
| Port Pegasus | 4.8 ± 2.1 | 13.1 ± 4.9 | 7.8 ± 2.9 | 2020/21 | [this study] |
| Paterson Inlet | 9.9 ± 3.7 | 19.2 ± 11.6 | 8.3 ± 3.1 | 2020/21 | [this study] |
| Codfish Island | 22.6 ± 8.1 | 49.5 ± 24.7 | 13.9 ± 2.2 | 2020/21 | [this study] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elley, T.; Mattern, T.; Ellenberg, U.; Young, M.J.; Hickcox, R.P.; van Heezik, Y.; Seddon, P.J. Consistent Site-Specific Foraging Behaviours of Yellow-eyed Penguins/Hoiho Breeding on Stewart Island, New Zealand. Biology 2022, 11, 844. https://doi.org/10.3390/biology11060844
Elley T, Mattern T, Ellenberg U, Young MJ, Hickcox RP, van Heezik Y, Seddon PJ. Consistent Site-Specific Foraging Behaviours of Yellow-eyed Penguins/Hoiho Breeding on Stewart Island, New Zealand. Biology. 2022; 11(6):844. https://doi.org/10.3390/biology11060844
Chicago/Turabian StyleElley, Thor, Thomas Mattern, Ursula Ellenberg, Melanie J. Young, Rachel P. Hickcox, Yolanda van Heezik, and Philip J. Seddon. 2022. "Consistent Site-Specific Foraging Behaviours of Yellow-eyed Penguins/Hoiho Breeding on Stewart Island, New Zealand" Biology 11, no. 6: 844. https://doi.org/10.3390/biology11060844
APA StyleElley, T., Mattern, T., Ellenberg, U., Young, M. J., Hickcox, R. P., van Heezik, Y., & Seddon, P. J. (2022). Consistent Site-Specific Foraging Behaviours of Yellow-eyed Penguins/Hoiho Breeding on Stewart Island, New Zealand. Biology, 11(6), 844. https://doi.org/10.3390/biology11060844










