Novel Glycosylation by Amylosucrase to Produce Glycoside Anomers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Enzymes and Chemicals
2.2. Biotransformation
2.3. HPLC Analysis
2.4. Purification and Identification
3. Results and Discussion
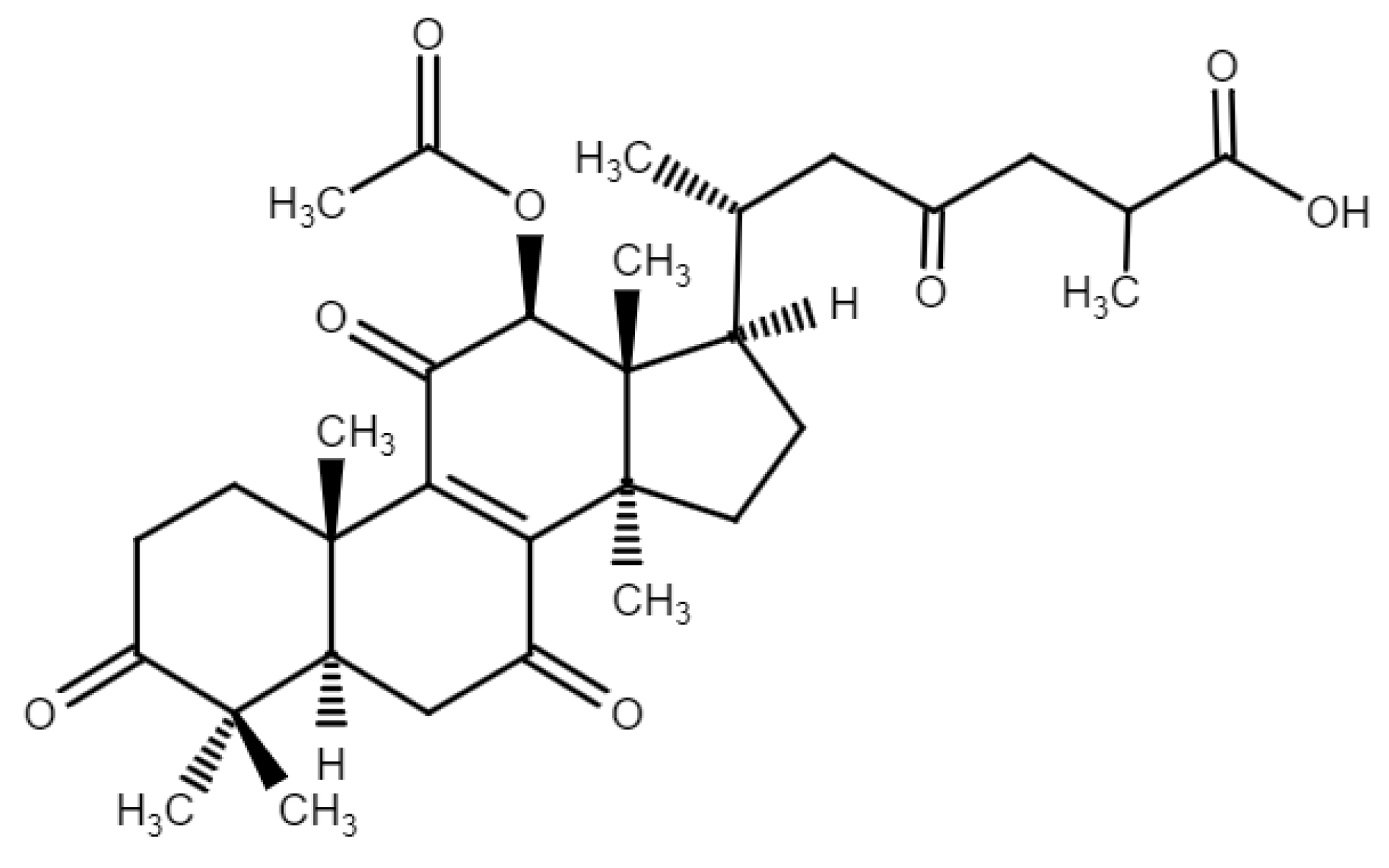
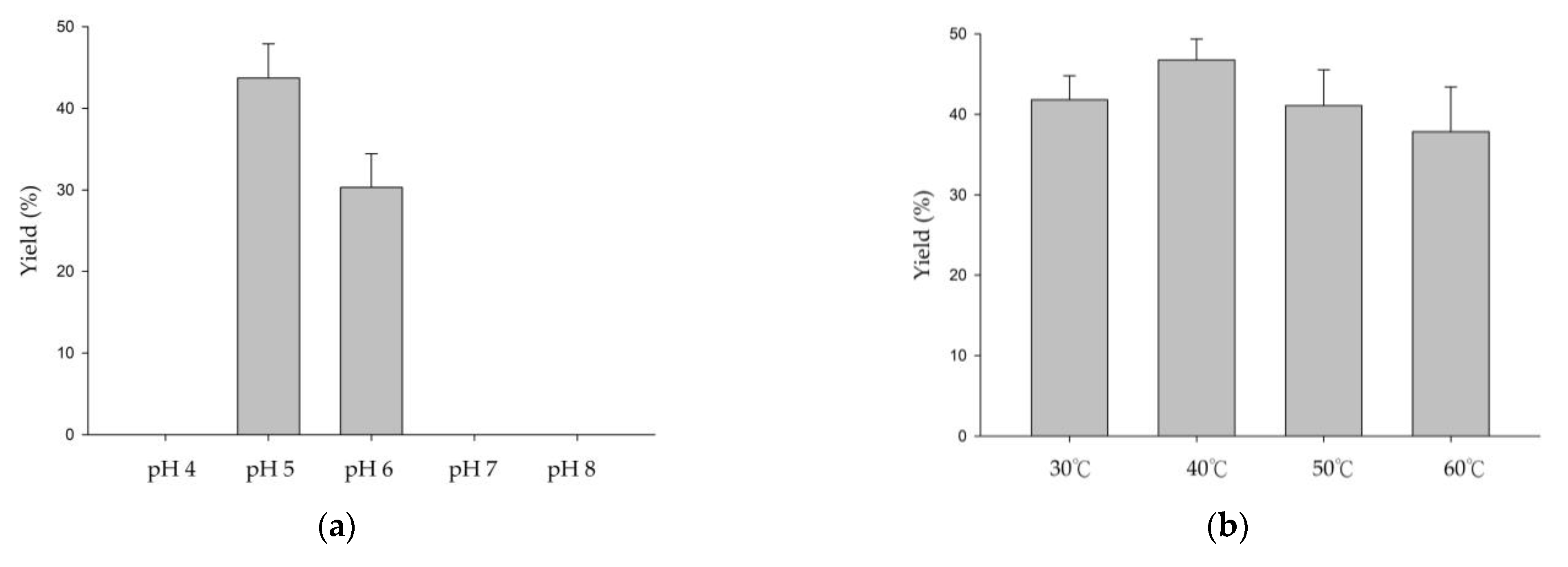
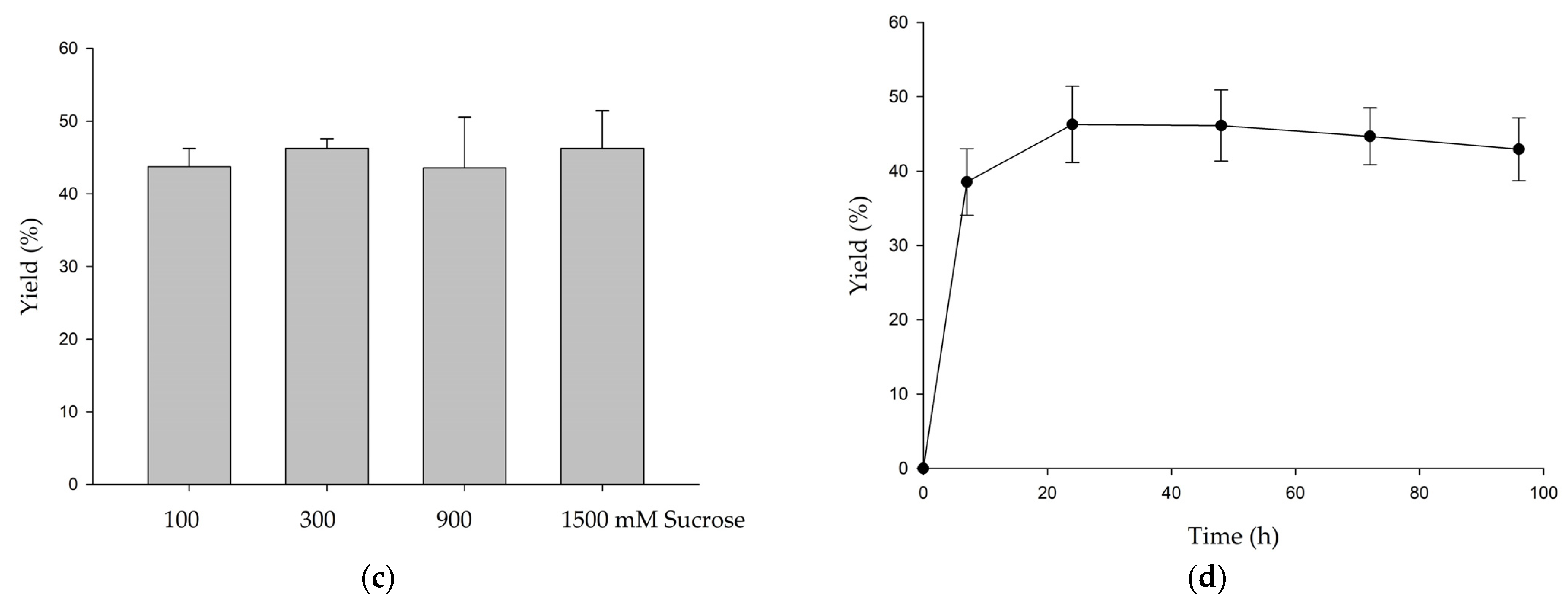
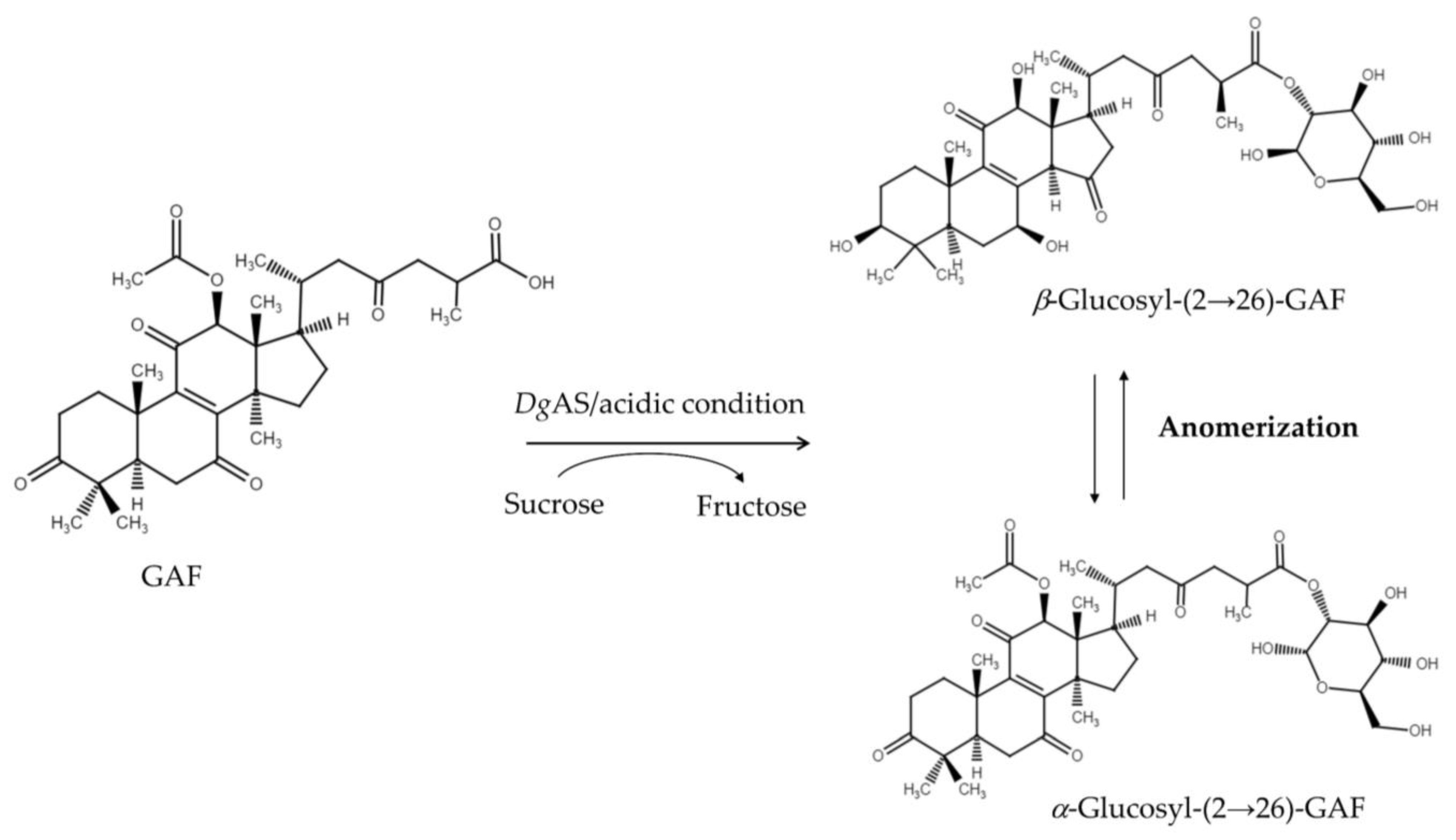
3.1. Biotransformation of GAF by DgAS
3.2. Purification and Identification of the Biotransformation Product
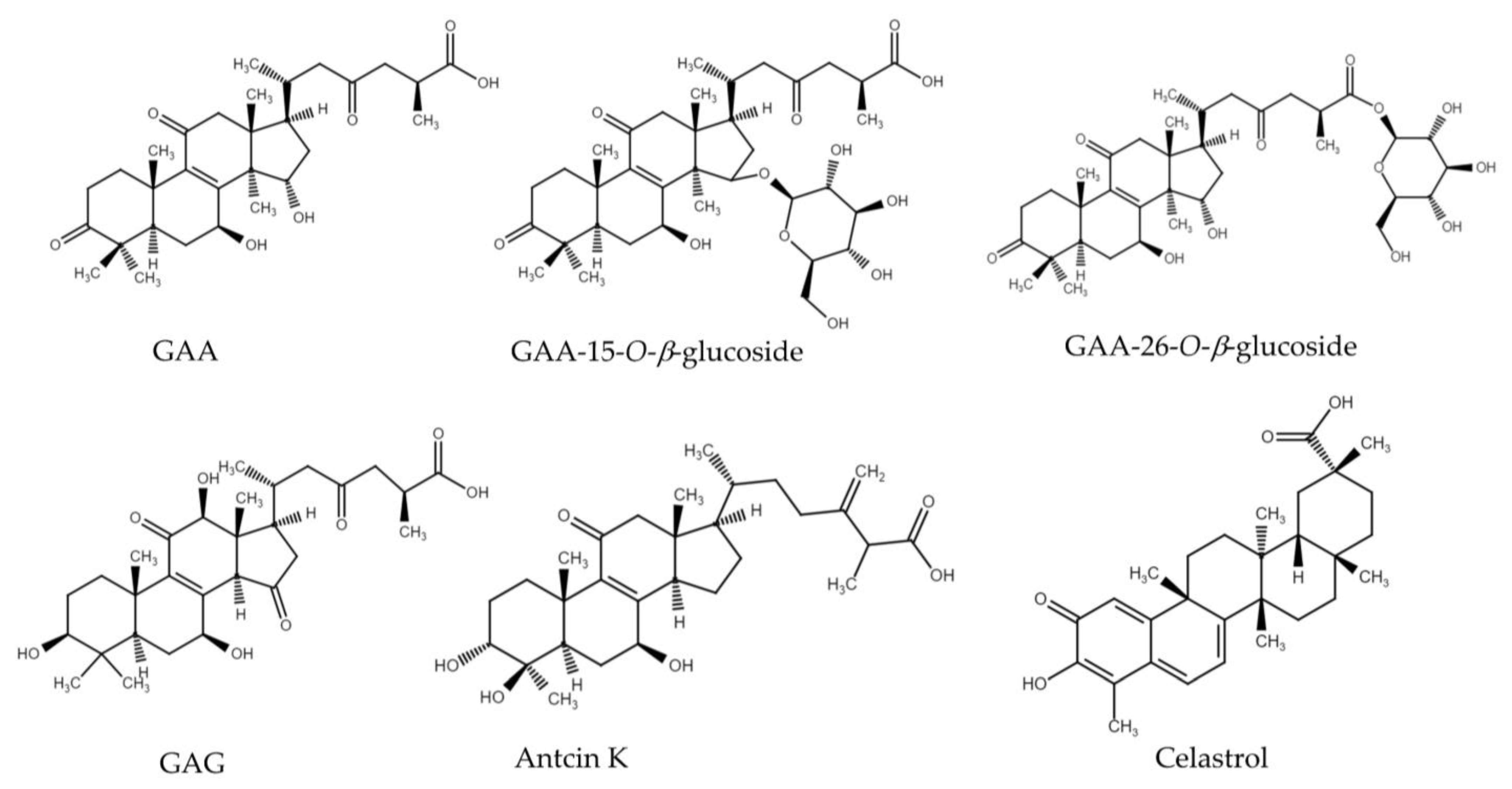
3.3. Evaluation of the Glycosylation Activity of DgAS toward Other Triterpenoids
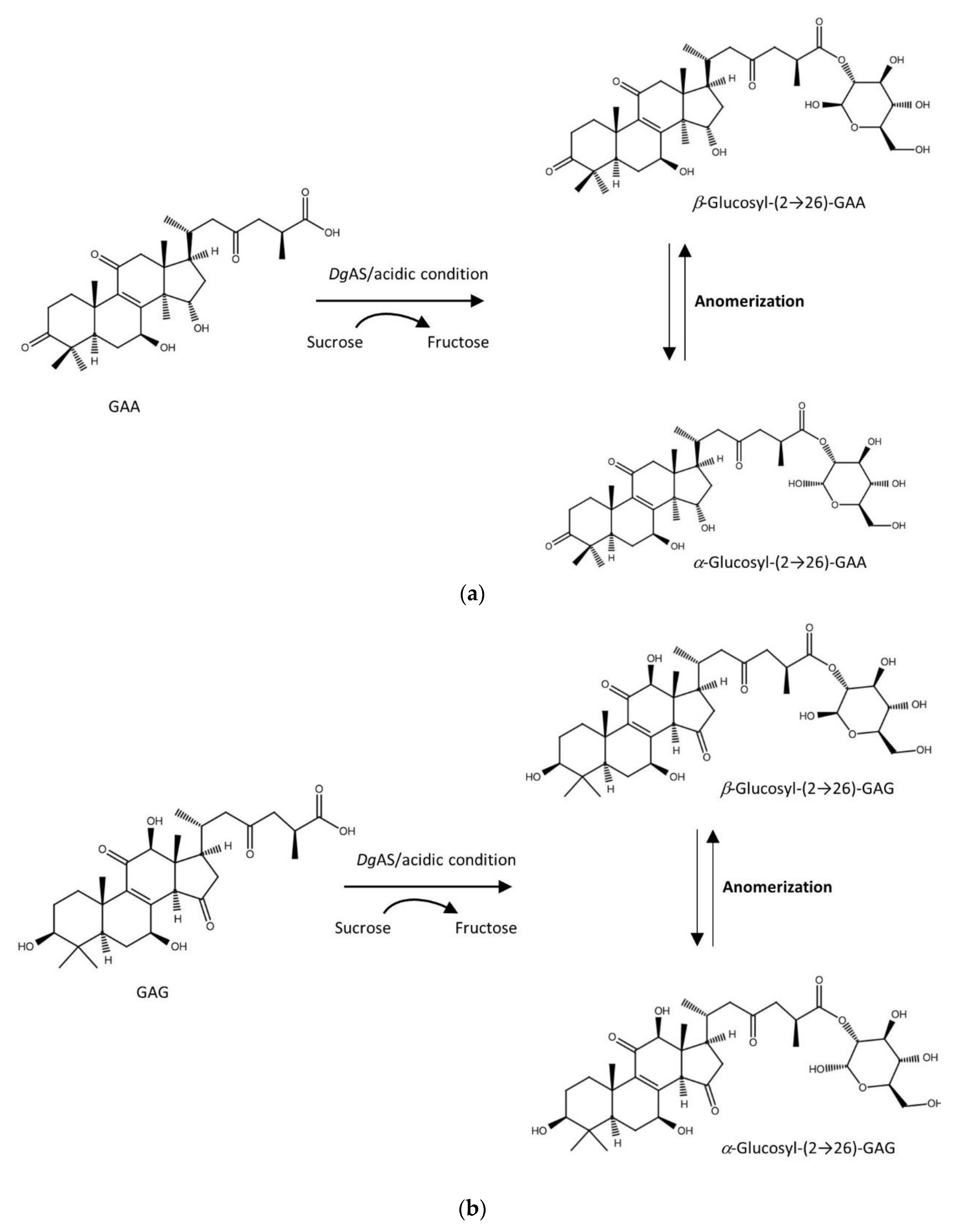
3.4. Purification and Identification of Biotransformation Products from GAA and GAG
3.5. Proposed Reaction Mechanism of DgAS toward Ganoderic Acids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benini, S. Carbohydrate-active enzymes: Structure, activity, and reaction products. Int. J. Mol. Sci. 2020, 21, 2727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mestrom, L.; Przypis, M.; Kowalczykiewicz, D.; Pollender, A.; Kumpf, A.; Marsden, S.R.; Bento, I.; Jarzębski, A.B.; Szymańska, K.; Chruściel, A.; et al. Leloir glycosyltransferases in applied biocatalysis: A multidisciplinary approach. Int. J. Mol. Sci. 2019, 20, 5263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-active EnZymes database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 2008, 37, D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, S. Nucleotide sugars in chemistry and biology. Molecules 2020, 25, 5755. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, P.D.; Almeida, A.; Bak, S. Evolution of structural diversity of triterpenoids. Front. Plant Sci. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-Y.; Zeng, J.-Z.; Wong, A.S.T. Chemical structures and pharmacological profiles of ginseng saponins. Molecules 2019, 24, 2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhang, H.; Zuo, J.; Gong, X.; Yi, F.; Zhu, W.; Li, L. Advances in research on the active constituents and physiological effects of Ganoderma lucidum. Biomed. Dermatol. 2019, 3, 6. [Google Scholar] [CrossRef]
- Liang, C.; Tian, D.; Liu, Y.; Li, H.; Zhu, J.; Li, M.; Xin, M.; Xia, J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur. J. Med. Chem. 2019, 174, 130–141. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, H.; Sun, X.; Zhao, H.; Wu, L.; Zhu, D.; Yang, G.; Shao, Y.; Zhang, X.; Mao, X.; et al. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules 2014, 19, 17478–17535. [Google Scholar] [CrossRef]
- Chang, T.S.; Chiang, C.M.; Kao, Y.H.; Wu, J.Y.; Wu, Y.W.; Wang, T.Y. A new triterpenoid glucoside from a novel acidic glycosylation of ganoderic acid A via recombinant glycosyltransferase of Bacillus subtilis. Molecules 2019, 24, 3457. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.S.; Wu, J.Y.; Wang, T.Y.; Wu, K.Y.; Chiang, C.M. Uridine diphosphate-dependent glycosyltransferases from Bacillus subtilis ATCC 6633 catalyze the 15-O-glycosylation of ganoderic acid A. Int. J. Mol. Sci. 2018, 19, 3469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, T.-S.; Wang, T.Y.; Hsueh, T.Y.; Lee, Y.W.; Chuang, H.M.; Cai, W.X.; Wu, J.Y.; Chiang, C.M.; Wu, Y.W. A genome-centric approach reveals a novel glycosyltransferase from the GA A07 strain of Bacillus thuringiensis responsible for catalyzing 15-O-glycosylation of ganoderic acid A. Int. J. Mol. Sci. 2019, 20, 5192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.Y.; Ding, H.Y.; Wang, T.Y.; Zhang, Y.R.; Chang, T.S. Glycosylation of ganoderic acid G by Bacillus glycosyltransferases. Int. J. Mol. Sci. 2021, 22, 9744. [Google Scholar] [CrossRef] [PubMed]
- Moulis, C.; Guieysse, D.; Morel, S.; Severac, E.; Remaud-Simeon, M. Natural and engineered transglycosylases: Green tools for the enzyme-based synthesis of glycoproducts. Curr. Opin. Chem. Biol. 2021, 61, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tian, Y.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Recent progress on biological production of alpha-arbutin. Appl. Microbiol. Biotechnol. 2018, 102, 8145–8152. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Yoo, S.H.; Choi, S.J.; Kim, Y.R.; Park, C.S. Versatile biotechnological applications of amylosucrase, a novel glucosyltransferase. Food Sci. Biotechnol. 2020, 29, 1–16. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, W.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Amylosucrase as a transglucosylation tool: From molecular features to bioengineering applications. Biotechnol. Adv. 2018, 36, 1540–1552. [Google Scholar] [CrossRef]
- Chang, T.S.; Wang, T.Y.; Yang, S.Y.; Kao, Y.H.; Wu, J.Y.; Chiang, C.M. Potential industrial production of a well-soluble, alkaline-stable, and anti-inflammatory isoflavone glucoside from 8-hydroxydaidzein glucosylated by recombinant amylosucrase of Deinococcus geothermalis. Molecules 2019, 24, 2236. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.-M.; Wang, T.-Y.; Ke, A.-N.; Chang, T.-S.; Wu, J.-Y. Biotransformation of ergostane triterpenoid antcin K from Antrodia cinnamomea by soil-isolated Psychrobacillus sp. AK 1817. Catalysts 2017, 7, 299. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.M.; Wang, T.Y.; Wu, J.Y.; Zhang, Y.R.; Lin, S.Y.; Chang, T.S. Production of new isoflavone diglucosides from glycosylation of 8-hydroxydaidzein by Deinococcus geothermalis amylosucrase. Fermentation 2021, 7, 232. [Google Scholar] [CrossRef]
- Rha, C.S.; Kim, H.G.; Baek, N.I.; Kim, D.O.; Park, C.S. Using amylosucrase for the controlled synthesis of novel isoquercitrin glycosides with different glycosidic linkages. J. Agric. Food Chem. 2020, 68, 13798–13805. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Matsuda, S.; Kodota, S. Ganoderic acid D, E, F, and H and lucidenic aicd D, E, and F, new triterpenoids from Ganoderma lucidum. Chem. Pharm. Bull. 1985, 33, 2624–2627. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Taniguchi, M.; Baba, K. Antitumor and antimetastatic effects on liver triterpenoid fractions of Ganoderma lucidum: Mechanism of action and isolation of an active substance. Anticancer Res. 2002, 22, 3309–3318. [Google Scholar] [PubMed]
- Chang, T.S.; Wang, T.Y.; Chiang, C.M.; Lin, Y.J.; Chen, H.L.; Wu, Y.W.; Ting, H.J.; Wu, J.Y. Biotransformation of celastrol to a novel, well-soluble, low-toxic and anti-oxidative celastrol-29-O-β-glucoside by Bacillus glycosyltransferases. J. Biosci. Bioeng. 2021, 131, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Kohda, H.; Tokumoto, W.; Sakamoto, K.; Fujii, M.; Hirai, Y.; Yamasaki, K.; Komoda, Y.; Nakamura, H.; Ishihara, S.; Uchida, M. The biologically active constituents of Ganoderma lucidum (FR.) Karst. histamine release-inhibitory triterpenes. Chem. Pharm. Bull. 1985, 33, 1367–1374. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, T.; Matsuda, S.; Murai, Y.; Ogita, Z. Ganoderic acid G and I and ganolucidic acid A and B, new triterpenoids from Ganoderma lucidum. Chem. Pharm. Bull. 1985, 33, 2628–2631. [Google Scholar] [CrossRef] [Green Version]
- Bassanini, I.; Kapešová, J.; Petrásková, L.; Pelantová, H.; Markošová, K.; Rebroš, M.; Valentová, K.; Kotik, M.; Káňová, K.; Bojarová, P.; et al. Glycosidase-catalyzed synthesis of glycosyl esters and phenolic glycosides of aromatic acids. Adv. Synth. Catal. 2019, 361, 2627–2637. [Google Scholar] [CrossRef] [Green Version]

| Triterpenoid | Sucrose (mM) | pH 4 | pH 5 | pH 6 | pH 7 | pH 8 |
|---|---|---|---|---|---|---|
| GAA | 100 | N.D. 2 | 53.3 ± 4.7 | 31.1 ± 2.6 | 3.1 ± 0.0 | N.D. |
| 1500 | N.D. | 50.7 ± 2.7 | 27.7 ± 0.9 | 3.2 ± 0.1 | N.D. | |
| GAG | 100 | N.D. | 52.4 ± 3.6 | 17.2 ± 1.0 | 2.1 ± 0.0 | N.D. |
| 1500 | N.D. | 47.6 ± 1.5 | 18.0 ± 0.8 | 0.8 ± 0.0 | N.D. | |
| GAA-15-O-β-glucoside | 100 | N.D. | N.D. | N.D. | N.D. | N.D. |
| 1500 | N.D. | N.D. | N.D. | N.D. | N.D. | |
| GAA-26-O-β-glucoside | 100 | N.D. | N.D. | N.D. | N.D. | N.D. |
| 1500 | N.D. | N.D. | N.D. | N.D. | N.D. | |
| Antcin K | 100 | N.D. | N.D. | N.D. | N.D. | N.D. |
| 1500 | N.D. | N.D. | N.D. | N.D. | N.D. | |
| Celastrol | 100 | N.D. | N.D. | N.D. | N.D. | N.D. |
| 1500 | N.D. | N.D. | N.D. | N.D. | N.D. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-Y.; Ding, H.-Y.; Luo, S.-Y.; Wang, T.-Y.; Tsai, Y.-L.; Chang, T.-S. Novel Glycosylation by Amylosucrase to Produce Glycoside Anomers. Biology 2022, 11, 822. https://doi.org/10.3390/biology11060822
Wu J-Y, Ding H-Y, Luo S-Y, Wang T-Y, Tsai Y-L, Chang T-S. Novel Glycosylation by Amylosucrase to Produce Glycoside Anomers. Biology. 2022; 11(6):822. https://doi.org/10.3390/biology11060822
Chicago/Turabian StyleWu, Jiumn-Yih, Hsiou-Yu Ding, Shun-Yuan Luo, Tzi-Yuan Wang, Yu-Li Tsai, and Te-Sheng Chang. 2022. "Novel Glycosylation by Amylosucrase to Produce Glycoside Anomers" Biology 11, no. 6: 822. https://doi.org/10.3390/biology11060822
APA StyleWu, J.-Y., Ding, H.-Y., Luo, S.-Y., Wang, T.-Y., Tsai, Y.-L., & Chang, T.-S. (2022). Novel Glycosylation by Amylosucrase to Produce Glycoside Anomers. Biology, 11(6), 822. https://doi.org/10.3390/biology11060822








