Detection of the Synthetic Cannabinoids AB-CHMINACA, ADB-CHMINACA, MDMB-CHMICA, and 5F-MDMB-PINACA in Biological Matrices: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Hair
3.2. Oral Fluid
3.3. Blood, Serum, and Plasma
3.4. Urine
4. Discussion
Author Contributions
Funding
 .
.Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5F-MDMB-PINACA | Methyl 2-(1-(5-fluoropentyl)-1H-indazole-3-carboxamido)-3,3-dimethylbutanoate |
| AB-CHMINACA | N-[(2S)-1-amino-3-methyl-1-oxobutan-2-yl]-1-(cyclohexylmethyl)indazole-3-carboxamide |
| ADB-CHMNACA | N-(1-Amino-3,3-dimethyl-1-oxo-2-butanyl)-1-(cyclohexylmethyl)-1H-indazole-3-carboxamide |
| CB1 | Cannabinoid receptor type 1 |
| CB2 | Cannabinoid receptor type 2 |
| DUID | Driving under the influence of drugs |
| EMCDDA | European Monitoring Centre for Drugs and Drug Addiction |
| GC-IMS | Gas chromatography-ion mobility spectrometry |
| GC-MS/MS | Gas chromatography-tandem mass spectrometry |
| HLM | Human liver microsomes |
| HRMS | High-resolution mass spectrometry |
| IA | Immunoassays |
| LC-HRMS | Liquid chromatography/high-resolution mass spectrometry |
| LC-MS/MS | Liquid chromatography-tandem mass spectrometry |
| LC-QTOF-MS | Liquid chromatography-quadrupole time-of-flight mass spectrometry |
| LLE | Liquid-liquid extraction |
| LLOQ | Lower limit of quantification |
| LOD | Limit of detection |
| LOI | Limit of identification |
| MDMB-CHMICA | Methyl (2S)-2-{[1-(cyclohexylmethyl)-1H-indol-3-yl]formamido}-3,3-dimethylbutanoate |
| MEPS | Semi-automated microextraction by packed sorbent |
| MS | Mass spectrometry |
| MS/MS | Tandem mass spectrometry |
| MTBE | Methyl tertiary-butyl ether |
| NPS | New psychoactive substances |
| OF | Oral fluid |
| PRISMA | Preferred reporting items for systematic reviews and meta-analysis |
| QTOF | Quadruple time of flight |
| SC/SCs | Synthetic cannabinoid/synthetic cannabinoids |
| SLE | Supported liquid extraction |
| SLE | Supported liquid extraction |
| SPDE | Solid-phase dispersive extraction |
| SPE | Solid-phase extraction |
| THC | Tetrahydrocannabinol |
| UHPLC-QTOF-MS | Ultra-high pressure liquid chromatography quadrupole time-of-flight mass spectrometry |
References
- European Monitoring Centre for Drugs and Drug Addiction EMCDDA. Operating Guidelines for the Risk Assessment of New Psychoactive Substances; EMCDDA: Luxembourg, 2006; Volume 2006.
- European Monitoring Centre for Drugs and Drug Addiction. New Psychoactive Substances in Europe: An Update from the EU Early Warning System; Publications Office of the European Union: Luxembourg, 2015.
- Mechoulam, R. (Ed.) Cannabinoids as Therapeutic Agents, 1st ed.; Chapman and Hall/CRC: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Auwärter, V. JMS Letter. J. Mass Spectrom. 2009, 44, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, B.; Ravi, J.; Ganju, R.K. Cannabinoids as therapeutic agents in cancer: Current status and future implications. Oncotarget 2014, 5, 5852–5872. [Google Scholar] [CrossRef] [PubMed]
- EMCDDA. European Drug Report 2015: Perspectives on Drugs: Synthetic Cannabinoids in Europe; EMCDDA: Luxembourg, 2015; Volume 9.
- Alves, V.L.; Gonçalves, J.; Aguiar, J.; Teixeira, H.M.; Câmara, J.S. The synthetic cannabinoids phenomenon: From structure to toxicological properties. A review. Crit. Rev. Toxicol. 2020, 50, 359–382. [Google Scholar] [CrossRef] [PubMed]
- EMCDDA. Perspectives on Drugs—Synthetic Cannabinoids in Europe; EMCDDA: Lisbon, Portugal, 2013.
- Malaca, S.; Busardò, F.P.; Nittari, G.; Sirignano, A.; Ricci, G. Fourth generation of synthetic cannabinoid receptor agonists: A review on the latest insights. Curr. Pharm. Des. 2022, 28. [Google Scholar] [CrossRef] [PubMed]
- Castaneto, M.S.; Gorelick, D.A.; Desrosiers, N.A.; Hartman, R.L.; Pirard, S.; Huestis, M.A. Synthetic cannabinoids: Epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 2014, 144, 12–41. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. Report on the Risk Assessment of Mephedrone in the Framework of the Council Decision on New Psychoactive Substances; EMCDDA Risk Assesments; EMCDDA: Luxembourg, 2011.
- Zimmermann, U.S.; Winkelmann, P.R.; Pilhatsch, M.; Nees, J.A.; Spanagel, R.; Schulz, K. Withdrawal Phenomena and Dependence Syndrome After the Consumption of “Spice Gold”-konsum. Dtsch. Arztebl. 2009, 106, 464–467. [Google Scholar] [CrossRef]
- Weaver, M.F.; Hopper, J.A.; Gunderson, E.W. Designer drugs 2015: Assessment and management. Addict. Sci. Clin. Pr. 2015, 10, 8. [Google Scholar] [CrossRef]
- Cooper, Z.D. Adverse Effects of Synthetic Cannabinoids: Management of Acute Toxicity and Withdrawal. Curr. Psychiatry Rep. 2016, 18, 52. [Google Scholar] [CrossRef]
- Oliveira, P.; Morais, A.S.F.; Madeira, N. Synthetic Cannabis Analogues and Suicidal Behavior: Case Report. J. Addict. Med. 2017, 11, 408–410. [Google Scholar] [CrossRef]
- Thomas, S.; Bliss, S.; Malik, M. Suicidal ideation and self-harm following K2 use. J. Okla. State Med. Assoc. 2012, 105, 430–433. [Google Scholar]
- Ti, L.; Tobias, S.; Maghsoudi, N.; Milloy, M.; McDonald, K.; Shapiro, A.; Beriault, D.; Stefan, C.; Lysyshyn, M.; Werb, D. Detection of synthetic cannabinoid adulteration in the unregulated drug supply in three Canadian settings. Drug Alcohol Rev. 2021, 40, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Zuba, D.; Byrska, B.; Maciow, M. Comparison of “herbal highs” composition. Anal. Bioanal. Chem. 2011, 400, 119–126. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, M.A.; Ahmed, S.; Gul, S.W.; Gul, W. Review of synthetic cannabinoids on the illicit drug market. In Critical Issues in Alcohol and Drugs of Abuse Testing; Elsevier: Amsterdam, The Netherlands, 2019; pp. 273–319. ISBN 9780128156070. [Google Scholar]
- Franz, F.; Jechle, H.; Angerer, V.; Pegoro, M.; Auwärter, V.; Neukamm, M.A. Synthetic cannabinoids in hai—Pragmatic approach for method updates, compound prevalences and concentration ranges in authentic hair samples. Anal. Chim. Acta 2018, 1006, 61–73. [Google Scholar] [CrossRef]
- Salomone, A.; Luciano, C.; Di Corcia, D.; Gerace, E.; Vincenti, M. Hair analysis as a tool to evaluate the prevalence of synthetic cannabinoids in different populations of drug consumers. Drug Test. Anal. 2014, 6, 126–134. [Google Scholar] [CrossRef]
- Zhou, L.; Shen, M.; Shen, B.; Chen, H.; Wang, X.; Deng, H.; Xiang, P.; Shi, Y. Application of a UPLC-MS/MS method for quantitative analysis of 29 synthetic cannabinoids and their metabolites, such as ADB-BUTINACA and MDMB-4en-PINACA in human hair in real cases. Forensic Sci. Int. 2021, 331, 111139. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Shin, I.; Kim, J.; Yang, W. Simultaneous quantification of 37 synthetic cannabinoid metabolites in human urine by liquid chromatography-tandem mass spectrometry. Forensic Toxicol. 2015, 33, 221–234. [Google Scholar] [CrossRef]
- Castaneto, M.S.; Wohlfarth, A.; Desrosiers, N.A.; Hartman, R.L.; Gorelick, D.A.; Huestis, M.A. Synthetic cannabinoids pharmacokinetics and detection methods in biological matrices. Drug Metab. Rev. 2015, 47, 124–174. [Google Scholar] [CrossRef]
- Gundersen, P.O.M.; Spigset, O.; Josefsson, M. Screening, quantification, and confirmation of synthetic cannabinoid metabolites in urine by UHPLC-QTOF-MS. Drug Test. Anal. 2019, 11, 51–67. [Google Scholar] [CrossRef]
- Gottardo, R.; Sorio, D.; Musile, G.; Trapani, E.; Seri, C.; Serpelloni, G.; Tagliaro, F. Screening for synthetic cannabinoids in hair by using LC-QTOF MS: A new and powerful approach to study the penetration of these new psychoactive substances in the population. Med. Sci. Law 2014, 54, 22–27. [Google Scholar] [CrossRef]
- EMCDDA. Europol EMCDDA–Europol 2011 Annual Report on the Implementation of Council Decision 2005/387/JHA; EMCDDA: Lisbon, Portigal, 2012.
- Giorgetti, A.; Busardò, F.P.; Tittarelli, R.; Auwärter, V.; Giorgetti, R. Post-Mortem Toxicology: A Systematic Review of Death Cases Involving Synthetic Cannabinoid Receptor Agonists. Front. Psychiatry 2020, 11, 464. [Google Scholar] [CrossRef]
- Maeda, H.; Kikura-Hanajiri, R.; Kawamura, M.; Nagashima, E.; Yoshida, K.-I. AB-CHMINACA-induced sudden death from non-cardiogenic pulmonary edema. Clin. Toxicol. 2018, 56, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Gaunitz, F.; Andresen-Streichert, H. Analytical findings in a non-fatal intoxication with the synthetic cannabinoid 5F-ADB (5F-MDMB-PINACA): A case report. Int. J. Legal Med. 2022, 136, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, P.; Gieroń, J. Acute intoxication of four individuals following use of the synthetic cannabinoid MAB-CHMINACA. Clin. Toxicol. 2016, 54, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, P. Fatal intoxication with synthetic cannabinoid MDMB-CHMICA. Forensic Sci. Int. 2016, 261, e5–e10. [Google Scholar] [CrossRef]
- World Health Organization. AB-CHMINACA Critical Review Report; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Bäckberg, M.; Tworek, L.; Beck, O.; Helander, A. Analytically Confirmed Intoxications Involving MDMB-CHMICA from the STRIDA Project. J. Med Toxicol. 2017, 13, 52–60. [Google Scholar] [CrossRef][Green Version]
- Dybowski, M.P.; Typek, R.; Dawidowicz, A.L.; Holowinski, P. On practical problems in precise estimation of 5F-ADB in urine samples. Forensic Toxicol. 2020, 39, 213–221. [Google Scholar] [CrossRef]
- Carlier, J.; Diao, X.; Sempio, C.; Huestis, M.A. Identification of New Synthetic Cannabinoid ADB-CHMINACA (MAB-CHMINACA) Metabolites in Human Hepatocytes. AAPS J. 2017, 19, 568–577. [Google Scholar] [CrossRef]
- Report on the Risk Assessment of N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-1H-Indazole-3-Carboxamide (AB-CHMINACA) in the Framework of the Council Decision on New Psychoactive Substances. Available online: https://www.emcdda.europa.eu/publications/risk-assessments/ab-chminaca_en (accessed on 27 April 2022).
- Report on the Risk Assessment of methyl 2-[[1-(cyclohexylmethyl)-1H-indole-3-carbonyl]amino]-3,3-dimethylbutanoate (MDMB-CHMICA) in the Framework of the Council Decision on New Psychoactive Substances. Available online: https://www.emcdda.europa.eu/publications/risk-assessments/mdmb-chmica_en (accessed on 27 April 2022).
- Report on the Risk Assessment of methyl 2-{[1-(5-fluoropentyl)-1H-indazole-3-carbonyl]amino}-3,3-dimethylbutanoate (5F-MDMB-PINACA) in the Framework of the Council Decision on New Psychoactive Substances. Available online: https://www.emcdda.europa.eu/publications/risk-assessments/5f-mdmb-pinaca_en (accessed on 27 April 2022).
- Report on the Risk Assessment of N-(1-Amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-1H-indazole-3-carboxamide (ADB-CHMINACA) in the Framework of the Council Decision on New Psychoactive Substances. Available online: https://www.emcdda.europa.eu/publications/risk-assessments/adb-chminaca_en (accessed on 27 April 2022).
- Diao, X.; Huestis, M.A. New Synthetic Cannabinoids Metabolism and Strategies to Best Identify Optimal Marker Metabolites. Front. Chem. 2019, 7, 109. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Standard Practices for Method Validation in Forensic Toxicology|American Academy of Forensic Sciences. Available online: https://www.aafs.org/asb-standard/standard-practices-method-validation-forensic-toxicology (accessed on 10 May 2022).
- Cho, B.; Cho, H.S.; Kim, J.; Sim, J.; Seol, I.; Baeck, S.K.; In, S.; Shin, D.H.; Kim, E. Simultaneous determination of synthetic cannabinoids and their metabolites in human hair using LC-MS/MS and application to human hair. Forensic Sci. Int. 2020, 306, 110058. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.; Cho, H.S.; Lee, J.; In, S.; Kim, E. Determination of AB-CHMINACA and its metabolites in human hair and their deposition in hair of abusers. J. Pharm. Biomed. Anal. 2017, 140, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Franz, F.; Angerer, V.; Hermanns-Clausen, M.; Auwärter, V.; Moosmann, B. Metabolites of synthetic cannabinoids in hair—proof of consumption or false friends for interpretation? Anal. Bioanal. Chem. 2016, 408, 3445–3452. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Martin, J.; Galettis, P. A Validated Method for the Detection of Synthetic Cannabinoids in Oral Fluid. J. Anal. Toxicol. 2019, 43, 10–17. [Google Scholar] [CrossRef]
- Cooman, T.; Santos, H.; Cox, J.; Filho, J.F.A.; Borges, K.; Romão, W.; Arroyo-Mora, L.E. Development, validation and evaluation of a quantitative method for the analysis of twenty-four new psychoactive substances in oral fluid by LC–MS/MS. Forensic Chem. 2020, 19, 100231. [Google Scholar] [CrossRef]
- Sorribes-Soriano, A.; Verdeguer, J.; Pastor, A.; Armenta, S.; Esteve-Turrillas, F.A. Determination of Third-Generation Synthetic Cannabinoids in Oral Fluids. J. Anal. Toxicol. 2021, 45, 331–336. [Google Scholar] [CrossRef]
- Denia, A.; Esteve-Turrillas, F.A.; Armenta, S. Analysis of drugs including illicit and new psychoactive substances in oral fluids by gas chromatography-drift tube ion mobility spectrometry. Talanta 2022, 238, 122966. [Google Scholar] [CrossRef]
- Peterson, B.L.; Couper, F.J. Concentrations of AB-CHMINACA and AB-PINACA and Driving Behavior in Suspected Impaired Driving Cases. J. Anal. Toxicol. 2015, 39, 642–647. [Google Scholar] [CrossRef]
- Tynon, M.; Homan, J.; Kacinko, S.; Ervin, A.; McMullin, M.; Logan, B.K. Rapid and sensitive screening and confirmation of thirty-four aminocarbonyl/carboxamide (NACA) and arylindole synthetic cannabinoid drugs in human whole blood. Drug Test. Anal. 2017, 9, 924–934. [Google Scholar] [CrossRef]
- Hess, C.; Murach, J.; Krueger, L.; Scharrenbroch, L.; Unger, M.; Madea, B.; Sydow, K. Simultaneous detection of 93 synthetic cannabinoids by liquid chromatography-tandem mass spectrometry and retrospective application to real forensic samples. Drug Test. Anal. 2017, 9, 721–733. [Google Scholar] [CrossRef]
- Seywright, A.; Torrance, H.J.; Wylie, F.M.; McKeown, D.A.; Lowe, D.J.; Stevenson, R. Analysis and clinical findings of cases positive for the novel synthetic cannabinoid receptor agonist MDMB-CHMICA. Clin. Toxicol. 2016, 54, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Grapp, M.; Kaufmann, C.; Streit, F.; Binder, L. Systematic forensic toxicological analysis by liquid-chromatography-quadrupole-time-of-flight mass spectrometry in serum and comparison to gas chromatography-mass spectrometry. Forensic Sci. Int. 2018, 287, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Kokaji, Y.; Muranaka, Y.; Ito, R. Simultaneous determination of synthetic cannabinoids in illegal herbal products and blood by LC/TOF-MS, and linear regression analysis of retention time using log Pow. Forensic Chem. 2020, 17, 100202. [Google Scholar] [CrossRef]
- Krotulski, A.J.; Mohr, A.L.; Logan, B.K. Emerging Synthetic Cannabinoids: Development and Validation of a Novel Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry Assay for Real-Time Detection. J. Anal. Toxicol. 2020, 44, 207–217. [Google Scholar] [CrossRef]
- Ong, R.S.; Kappatos, D.C.; Russell, S.G.; Poulsen, H.A.; Banister, S.D.; Gerona, R.R.; Glass, M.; Johnson, C.S.; McCarthy, M. Simultaneous analysis of 29 synthetic cannabinoids and metabolites, amphetamines, and cannabinoids in human whole blood by liquid chromatography–tandem mass spectrometry—A New Zealand perspective of use in 2018. Drug Test. Anal. 2020, 12, 195–214. [Google Scholar] [CrossRef]
- Franz, F.; Angerer, V.; Jechle, H.; Pegoro, M.; Ertl, H.; Weinfurtner, G.; Janele, D.; Schlögl, C.; Friedl, M.; Gerl, S.; et al. Immunoassay screening in urine for synthetic cannabinoids – an evaluation of the diagnostic efficiency. Clin. Chem. Lab. Med. 2017, 55, 1375–1384. [Google Scholar] [CrossRef]
- Kakehashi, H.; Shima, N.; Ishikawa, A.; Nitta, A.; Asai, R.; Wada, M.; Nakano, S.; Matsuta, S.; Sasaki, K.; Kamata, H.; et al. Effects of lipophilicity and functional groups of synthetic cannabinoids on their blood concentrations and urinary excretion. Forensic Sci. Int. 2020, 307, 110106. [Google Scholar] [CrossRef]
- Institóris, L.; Hidvégi, E.; Dobos, A.; Sija, É.; Kereszty, M.; Tajti, L.B.; Somogyi, G.P.; Varga, T. The role of illicit, licit, and designer drugs in the traffic in Hungary. Forensic Sci. Int. 2017, 275, 234–241. [Google Scholar] [CrossRef]
- Franz, F.; Angerer, V.; Moosmann, B.; Auwärter, V. Phase I metabolism of the highly potent synthetic cannabinoid MDMB-CHMICA and detection in human urine samples. Drug Test. Anal. 2017, 9, 744–753. [Google Scholar] [CrossRef]
- Yeter, O.; Ozturk, Y.E. Metabolic profiling of synthetic cannabinoid 5F-ADB by human liver microsome incubations and urine samples using high-resolution mass spectrometry. Drug Test. Anal. 2019, 11, 847–858. [Google Scholar] [CrossRef]
- Tyndall, J.A.; Gerona, R.; De Portu, G.; Trecki, J.; Elie, M.-C.; Lucas, J.; Slish, J.; Rand, K.; Bazydlo, L.; Holder, M.; et al. An outbreak of acute delirium from exposure to the synthetic cannabinoid AB-CHMINACA. Clin. Toxicol. 2015, 53, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Cannaert, A.; Franz, F.; Auwärter, V.; Stove, C.P. Activity-Based Detection of Consumption of Synthetic Cannabinoids in Authentic Urine Samples Using a Stable Cannabinoid Reporter System. Anal. Chem. 2017, 89, 9527–9536. [Google Scholar] [CrossRef] [PubMed]
- Thorspecken, J.; Skopp, G.; Pötsch, L. In Vitro Contamination of Hair by Marijuana Smoke. Clin. Chem. 2004, 50, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Shah, I.; Al-Dabbagh, B.; Salem, A.E.; Hamid, S.A.; Muhammad, N.; Naughton, D.P. A review of bioanalytical techniques for evaluation of cannabis (Marijuana, weed, Hashish) in human hair. BMC Chem. 2019, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, K.F.; Oliveira, K.D.; Huestis, M.A.; Costa, J.L. Screening of 104 New Psychoactive Substances (NPS) and Other Drugs of Abuse in Oral Fluid by LC–MS-MS. J. Anal. Toxicol. 2020, 44, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Evaluation of Saliva/Oral Fluid as an Alternate Drug Testing Specimen|Office of Justice Programs. Available online: https://www.ojp.gov/ncjrs/virtual-library/abstracts/evaluation-salivaoral-fluid-alternate-drug-testing-specimen (accessed on 10 May 2022).
- Gundersen, P.O.M.; Broecker, S.; Slørdal, L.; Spigset, O.; Josefsson, M. Retrospective screening of synthetic cannabinoids, synthetic opioids and designer benzodiazepines in data files from forensic post mortem samples analysed by UHPLC-QTOF-MS from 2014 to 2018. Forensic Sci. Int. 2020, 311, 110274. [Google Scholar] [CrossRef]
- Kusano, M.; Zaitsu, K.; Taki, K.; Hisatsune, K.; Nakajima, J.; Moriyasu, T.; Asano, T.; Hayashi, Y.; Tsuchihashi, H.; Ishii, A. Fatal intoxication by 5F-ADB and diphenidine: Detection, quantification, and investigation of their main metabolic pathways in humans by LC/MS/MS and LC/Q-TOFMS. Drug Test. Anal. 2018, 10, 284–293. [Google Scholar] [CrossRef]
- Schedules of controlled substances: Temporary placement of three synthetic cannabinoids into schedule I. Final order. Fed. Regist. 2015, 80, 5042–5047.
- Mercolini, L.; Protti, M. Biosampling strategies for emerging drugs of abuse: Towards the future of toxicological and forensic analysis. J. Pharm. Biomed. Anal. 2016, 130, 202–219. [Google Scholar] [CrossRef]
- Kintz, P.; Ameline, A.; Gheddar, L.; Escudero, P.; Ferrari, L.; Raul, J.-S. Cocaine External Contamination Can Be Documented by a Hair Test. J. Anal. Toxicol. 2020, 44, e4–e5. [Google Scholar] [CrossRef]
- Cobo-Golpe, M.; De-Castro-Ríos, A.; Cruz, A.; López-Rivadulla, M.; Lendoiro, E. Determination and Distribution of Cannabinoids in Nail and Hair Samples. J. Anal. Toxicol. 2021, 45, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Busardò, F.P.; Gottardi, M.; Pacifici, R.; Varì, M.R.; Tini, A.; Volpe, A.R.; Giorgetti, R.; Pichini, S. Nails Analysis for Drugs Used in the Context of Chemsex: A Pilot Study. J. Anal. Toxicol. 2020, 44, 619. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, A.G. Detection Times of Drugs of Abuse in Blood, Urine, and Oral Fluid. Ther. Drug Monit. 2004, 26, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Institóris, L.; Kovács, K.; Sija, É.; Berkecz, R.; Körmöczi, T.; Németh, I.; Elek, I.; Bakos, Á.; Urbán, I.; Pap, C.; et al. Clinical symptoms and blood concentration of new psychoactive substances (NPS) in intoxicated and hospitalized patients in the Budapest region of Hungary (2018-19). Clin. Toxicol. 2022, 60, 18–24. [Google Scholar] [CrossRef]
- Krotulski, A.J.; Bishop-Freeman, S.C.; Mohr, A.L.; Logan, B.K. Evaluation of Synthetic Cannabinoid Metabolites in Human Blood in the Absence of Parent Compounds: A Stability Assessment. J. Anal. Toxicol. 2021, 45, 60–68. [Google Scholar] [CrossRef]
- Franz, F.; Haschimi, B.; King, L.A.; Auwärter, V. Extraordinary long detection window of a synthetic cannabinoid metabolite in human urine—Potential impact on therapeutic decisions. Drug Test. Anal. 2020, 12, 391–396. [Google Scholar] [CrossRef]
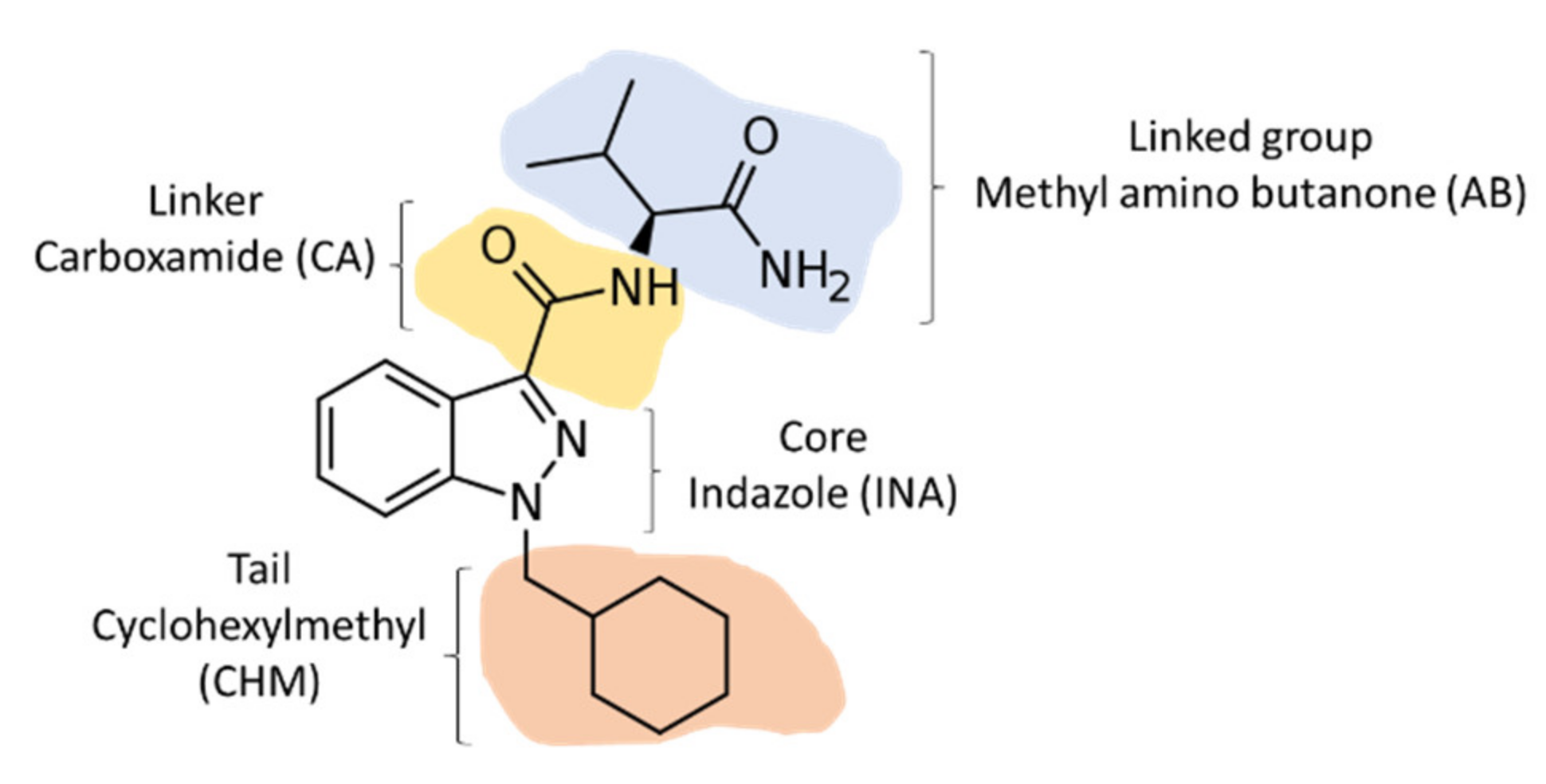
| AB-CHMINACA | MDMB-CHMICA | 5F-MDMB-PINACA * | ADB-CHMINACA | |
|---|---|---|---|---|
| IUPAC name | N-(1-Amino-3-methyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-1H-indazole-3-carboxamide | Methyl 2-({[1-(cyclohexylmethyl)-1H-indol-3-yl] carbonyl}amino)-3,3-dimethylbutanoate | Methyl 2-{[1-(5-fluoropentyl)-1Hindazole-3-carbonyl]amino}-3,3-dimethylbutanoate | N-(1-Amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-1H-indazole-3-carboxamide |
| Street names ** | Aromatic Pot Pourri, Jamaican Gold Supreme, Bonzai Citrus, Blaze, Bubblegum, Manga Xtreme, Matt Hardener, Aura mystic Bulc | Godfather, CUSHCottonCandy, KUSHSecondGereration, KUSHherbal incense, Ninja, Sirius, SKIHIGH, CRITICAL haze | ANNIHILATION, BLACK MAMBA ULTRA, Blueberry Blitz, CHERRY BOMB, Dutchy, EXODUS FORMULA 6-A, Sky High, Spike 99 ULTRA, and Vanilla Ice | ADB-CHMINACA, MAB-CHMINACA |
| Molecular formula | C20H28N4O2 | C23H32N2O3 | C20H28FN3O3 | C21H30N4O2 |
| Molecular weight (g/mol) | 356.5 | 384.5 | 377.5 | 370.5 |
| Structure |  Linked group: methyl amino butanone (AB) Tail: cyclohexylmethyl (CHM) Core: indazole (INA) Linker: carboxamide (CA) | 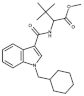 Linked group: methyldimethylbutanoate (MDMB) Tail: cyclohexylmethyl (CHM) Core: indole (I) Linker: carboxamide (CA) | 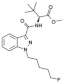 Linked group: dimethyl methyl butanoate (MDMB) Tail: pentyl (P), with a fluoro moiety at the position 5 Core: indazole (INA) Linker: carboxamide (CA) |  Linked group: dimethylaminobutanone (ADB) Tail: cyclohexylmethyl tail (CHM) Core: indazole (INA) Linker: carboxamide (CA) |
| Pharmacology and toxicology | Full and partial agonist of the CB1 and CB2 receptors, respectively; 11–58 times more potent than THC [33] | Potent and full agonist of the CB1 receptor and agonist at the CB2 receptor; 400 times more potent than THC, two times more potent than AB-CHMINACA [34] | Potent full agonist at the CB1 and CB2 receptor; 289 times more potent than THC, 17 times more potent than MDMB-CHMICA [35] | Potent and full agonist of the CB1 receptor and agonist at the CB2 receptor; 270 times more potent than THC [36] |
| Detection and evaluation by the EMCDDA | First detected: February 2014 (Riga, Latvia) First notified to the EMCDDA: April 2014 Risk-assessed by the EMCDDA in 2017 | First detected: August 2014 (Hungary) First notified to the EMCDDA: September 2014 Risk-assessed by the EMCDDA in 2016 | First detected: September 2014 (Hungary) First notified to the EMCDDA: January 2015 Risk-assessed by the EMCDDA in 2017 | First detected: Synthesis of ADB-CHMINACA was first described in a 2009 patent by Pfizer First notified to the EMCDDA: September 2014 Risk-assessed by the EMCDDA in 2017 |
| Psychological and behavioral effects | Duration of the effect: 1–2 h after smoking Effects: cannabis- and THC-like (relaxation, confusion, anxiety…); psychotic episodes and aggressive behaviors have also been reported | Duration of the effect: 120 min after smoking Effects: more pronounced in comparison to cannabis; most common paranoia, euphoria, visual hallucinations, and anxiety | Duration of the effect: 1–2 h after smoking. Effects lasting more than 10 h have been described Effects: cannabis- and THC-like (relaxation, confusion, anxiety); psychotic episodes and aggressive behaviors have also been reported. | Effects: cannabis- and THC-like (relaxation, confusion, anxiety…); psychotic episodes and aggressive behaviors have also been reported |
| Some analytical identification techniques used based on the EMCDDA | GC-MS; FTIR-ATR; HPLC-TOF; NMR; LC-MS; UV-VIS; LRMS; HRMS; DART-MS [37] | NMR and HPLC-DAD for quantification in products. LC-MS/MS for detection in biological samples [38] | HPLC-DAD for quantification in products. LC-MS/MS for detection in biological samples [39] | In products: GC-MS, LC-QqQ-MS/MS, GC-TOF-MS, GC-EI-MS, NMR, LC-MS/MS, IR, UV In biological samples: LC-QqQ-MS/MS, LC-MS/MS, LC-QTRAP-MS/MS, GC-MS, LC-QTOF-MS [40] |
| Matrix | Study/Country | Qual./ Quant. | Analyzed SCs | Sample Preparation | Detection Method | Type and Details of Samples | Study Limitations as Reported by the Authors |
|---|---|---|---|---|---|---|---|
| HAIR | Cho et al., 2020 [45]/(South Korea) | Quant. | 18 SCs and 41 of their metabolites, including: AB-CHMINACA and AB-CHMINACA M1A, M2 and M4 | Washed with methanol and cut finely (1 mm) and dried at room temperature. Incubation with 2 mL methanol at 38 °C, evaporation under nitrogen gas, and filtration | LC-MS/MS | Hair samples from 43 individuals who were suspected of using SCs. (January 2016–December 2018) | Not reported |
| Sim et al., 2017 [46] (South Korea) | Quant. | AB-CHMINACA and its six metabolites: M2, M4, M3A, M5A, M6, and M7 | Washed with methanol and distilled water, through-flow dried, and cut into 1–2 mm pieces. Incubation with 2 mL of methanol at 38 °C, evaporation under nitrogen gas at 45 °C and filtration | LC-MS/MS | 122 hair samples from suspects who were suspected of using SCs and had been arrested by the police. (November 2015–November 2016) | Not reported | |
| Franz et al., 2016 [47] (Germany) | Qual. and semi-quant. for parent compounds Qual. for metabolites | AB-CHMINACA and its metabolite AB-CHMINACA M2 | Washed by shaking in deionized water, acetone, and petroleum ether. Dried, cut into 1 mm pieces, and extracted by ultrasonication. Dried under nitrogen at 40 °C | LC-MS/MS | Hair sample collected from a 16-year-old female withdrawal patient for abstinence control | Findings in the hair segments do not correlate with use of the drug in the period at which the corresponding hair segments had grown The distribution of the detected compounds is suggestive of incorporation via sebum and sweat | |
| Franz et al., 2018 [20] (Germany) | Qual. and semi-quant. | AB-CHMINACA, ADB-CHMINACA, MDMB-CHMICA, and 5F-MDMB-PINACA | Washed by shaking in deionized water, acetone, and petroleum ether. Dried, cut into 1 mm pieces, and extracted by ultrasonication. Dried under nitrogen at 40 °C | LC-MS/MS | 294 hair samples (drug abstinence testing) (January 2012–December 2016) | High matrix effects The exact LODs were not determined individually (estimated to be around one order of magnitude lower for most analytes compared to the LLOQs). | |
| ORAL FLUID | Williams et al., 2019 [48] (Australia) | Quant. | 19 SCs, including AB-CHMINACA | Protein precipitation | LC-MS/MS | 12 authentic samples submitted for routine testing in which no cannabinoids were detected | Lack of confirmed positive samples, lack of an external quality assurance program |
| Cooman et al., 2020 [49] (USA and Brazil) | Quant. | 24 SCs and cathionine derivatives, including AB-CHMINACA | SPE | LC-MS/MS | Blind study that included 10 OF samples from volunteers, prepared with varying concentrations of analytes | LLOQ bias of 33.6% for AB-CHMINACA AB-CHMINACA values > 20% greater than the highest calibrator due to matrix and ion suppression/enhancement effects or to samples being prepared at higher concentrations than expected. | |
| Sorribes-Soriano et al., 2021 [50] (Spain) | Quant. | 5 SCs, including 5F-MDMB-PINACA | SPE by MEPS | GC-MS | Pool of 15 saliva samples from different volunteers spiked with a synthetic cannabinoid at 125 and 250 μg/L | Not reported | |
| Denia et al., 2022 [51] (Spain) | Quant. | 5F-MDMB-PINACA | Extraction by chloroform mixture and phase separation by centrifugation | GC-IMS | Pool of OF samples from five non-consumer volunteers with known concentrations of the added SCs | Not reported | |
| BLOOD | Peterson and Couper, 2015 [52] (USA) | Quant. | 40 SCs, including AB-CHMINACA | LLE | LC-MS/MS | 6815 blood samples from suspected impaired driving cases | Tests were no uniformity in the performed tests among all cases, as the number of compounds screened increased over the year |
| Tynon et al., 2017 [53] (USA) | Qual. | 34 SCs, including AB-CHMINACA and ADB-CHMINACA | LLE using MTBE | LC-MS/MS | 1497 blood samples from forensic investigations, including postmortem examinations and driving impairment cases (March 2015–December 2015) | AB-CHMINACA and ABD-CHMINACA did not meet the requirements for quantitative confirmation | |
| Adamowicz and Gieroń, 2016 [31] (Poland) | Quant. | ADB-CHMINACA | Protein precipitation | LC-MS/MS | Blood samples from four adolescents who had smoked a substance labeled “AM-2201” | Not reported | |
| Adamowicz, 2016 [32] (Poland) | Quant. | MDMB-CHMICA | Protein precipitation | LC-MS/MS | Antemortem and postmortem blood sample of a 25-year-old male with fatal intoxication due to SC abuse | Not reported | |
| Hess et al., 2017 [54] (Germany) | Qual. and quant. | 93 SCs, including AB-CHMINACA, MDMB-CHMICA, 5F-MDMB-PINACA, and ADB-CHMINACA | LLE | LC-MS/MS | 189 blood samples from suspected drugged individuals while diving (January 2013–November 2015) | When applied to real case samples, quantification ranges of many of the compounds were lower than LLOQ. | |
| Seywright et al., 2016 [55] (U.K.) | Quant. | MDMB-CHMICA | LLE | LC-MS/MS | 26 cases suspected of having consumed SC at the Emergency Department of Glasgow RoyalInfirmary | Small number of cases No metabolite screening because no reference standards were available. This may have increased the detection window | |
| Bäckberg et al., 2017 [34] (Sweden) | Quant. | MDMB-CHMICA | Protein precipitation | LC-HRMS | Eight intoxication cases involving MDMB-CHMICA from the pool of samples from the STRIDA project (2014–2015) | Small sample size Possible interferences by other psychoactive substances Difficulty in the identification of MDMB-CHMICA due to the unknown stability of the compound and inter-individual variability of drug metabolism | |
| Grapp et al., 2018 [56] (Germany) | Quant. | 950 compounds (185 drugs and metabolites), including AB-CHMINACA and MDMB-CHMICA | LLE | LC-QTOF-MS | Analysis 247 drug-positive serum and 12 post mortem femoral blood samples submitted by the police of Lower Saxony with the request for drug analysis | For the correct identification of compounds, data verification by a toxicologist was needed. | |
| Saito et al., 2020 [57] (Japan) | Qual. | 47 SCs, including AB-CHMINACA | SPDE | LC/TOF-MS | Blood samples (no additional specifications) | Not reported | |
| Krotulski et al., 2020 [58] (USA) | Qual. | 247 SCs, including AB-CHMINACA, 5F-MDMB-PINACA, MDMB-CHMICA, ADB-CHMINACA in blood and AB-CHMINACA M2, 5F-MDMB-PINACA M20, ADB-CHMINACA M2 in urine | LLE | LC-QTOF-MS | 200 authentic blood samples suspected of containing synthetic cannabinoids; 104 were compared against the results provided by the toxicology laboratory (June 2018) | Not reported | |
| Ong et al., 2020 [59] (New Zealand) | Qual. and semi-quant. | 29 SCs and metabolites including 5F-MDMB-PINACA, 5F-MDMB-PINACA M20, AB-CHMNACA, AB-CHMINACA M1A, MDMB-CHMICA and MDMB-CHMICA M30 | SLE | LC-MS/MS | 564 authentic human blood samples: Postmortem examinations (n = 180); criminal cases (n = 8); impaired drivers (n = 343); emergency department admissions (n = 19); psychiatric care patients (n = 14) | The validation evaluated an inadequate distribution of concentration points; therefore, exact quantitative values were not reported | |
| URINE | Franz et al., 2017 [60] (Germany) | Qual. and quant. | Qual.: 130 metabolites from 45 SCs Quant.: 31 metabolites from 14 SCs Including metabolites from AB-CHMINACA, ADB-CHMINACA, and MDMB-CHMICA | SPE | Immunoassay confirmed by LC-MS/MS | Study A: 549 urine samples from a regular drug screening (October–November 2014) Study B: 100 negative and 100 positive urine samples included in the study from a regular drug screening (January–June 2015) | LC-MS/MS was not fully validated for the assessed analytes (reference standards not commercially available): a similar fragmentation pattern of a parent compound was assumed. A limited number of positive samples was analyzed because samples positive for metabolites of more than one SC were excluded |
| Dybowski et al., 2021 [35] (Poland) | Qual. and quant. | 5F-MDMB-PINACA and its degradation products | QuEChERS extraction (combination of LLE + d-SPE) | GC-MS/MS | Urine samples from volunteers spiked with 5F-MDMB-PINACA | Very low recovery (<30%) of the drug from alkaline urine. | |
| Kakehashi et al., 2020 [61] (Japan) | Quant. | AB-CHMINACA, 5F-MDMB-PINACA | LLE | LC-MS/MS | 27 urine samples from drivers involved in car crashes allegedly under the influence of SCs (2011–2014) | Quantification was impossible for some urine specimens due to insufficient sample volume | |
| Institóris et al., 2017 [62] (Hungary) | Qual. and quant. | 100 SCs, including AB-CHMINACA ADB-CHMINACA MDMB-CHMICA | Enzymatic hydrolysis and SLE | UHPLC-MS/MS | 271 urine samples from drivers suspected to have used DUID (2014–2015) | Incomplete clinical data collection Unanalyzed substances may have affected the half-life of the analyzed ones | |
| Franz et al., 2017 [63] (Germany) | Qual. | MDMB-CHMICA and the M25 and M30 metabolites | LLE | LC-MS/MS | 5717 authentic urine samples in controls of abstinence control (October 2014–November 2015) | Exact structure of some metabolites is unknown (impossible by NMRS) Phase II metabolites could not be covered because of the glucuronide cleavage step in sample preparation. Polymorphisms in CYP450 isoenzymes were not studied but could influence individual metabolic profiles. | |
| Yeter and Ozturk, 2019 [64] (Turkey) | Quant. | 5F-MDMB-PINACA and the M20 metabolite | SPE +/− enzymatic hydrolysis | LC-HRMS | 30 samples chosen from screening of 8235 authentic urine samples from drug use suspects (January 2017–June 2018) | Not reported | |
| Gundersen et al., 2019 [25] (Norway) | Qual. and quant. | 35 SC metabolites, including AB-CHMINACA M1A and AB-CHMINACA M4. | SPE | UHPLC-QTOF-MS | 1000 urine samples from individuals in drug withdrawal programs (throughout 2014 and the first half of January 2015) | Due to matrix effects, low recoveries and linearities, and lack of isotopically labeled internal standards, the method should be considered semi-quantitative for AB-CHMINACA M1A and AB-CHMINACA 3-carboxyindazole | |
| Tyndall et al., 2015 [65] (USA) | Quant. | 50 SCs and metabolites + formula matches for 157 other SC parent compounds and 13 predicted AB-CHMINACA metabolites (including M2, M6, M11) Other drugs of abuse | Dilute and shoot method | LC-QTOF-MS | 21 urine samples from patients presenting to the emergency department with a documented suspicion of SCs use (May–June 2014) | Not reported | |
| Cannaert et al., 2017 [66] (Belgium and Germany) | Quant. | Four SCs, including AB-CHMINACA and its metabolites M1A, M1B, M2, M3A. ADB-CHMINACA and its metabolites M1, M2, M3 | LLE | LC-MS/MS (+CB Reporter Assays) | 74 authentic urine samples from suspected SC users | High concentrations of metabolites in urine are required for detection. False negative results for low concentrations of AB-CHMINACA Useful as a pre-screening tool but requires other analytical techniques for confirmation |
| Studies | LOD | LLOQ | Accuracy | Linearity | Matrix Effect | Precision | Process Efficiency | Recovery | Selectivity | Sensitivity | Specificity | Stability | Carryover |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cho et al., 2020 [45] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Sim et al., 2017 [46] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Franz et al., 2016 [47] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Franz et al., 2018 [20] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Williams et al., 2019 [48] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Cooman et al., 2020 [49] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Sorribes-Soriano et al., 2021 [50] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Denia et al., (2022) [51] | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Peterson and Couper, 2015 [52] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Tynon et al., 2017 [53] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Adamowicz and Gieroń, 2016 [31] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Adamowicz, 2016 [32] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Hess et al., 2017 [54] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Seywright et al., 2016 [55] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Grapp et al., 2018 [56] | ✓ | ✓ | ✓ | ✓ | |||||||||
| Saito et al., 2020 [57] | ✓ | ✓ | ✓ | ||||||||||
| Krotulski et al., 2020 [58] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Ong et al., 2020 [59] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Franz et al., 2017 [60] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Dybowski et al., 2021 [35] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Kakehashi et al., 2020 [61] | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Institóris et al., 2017 [62] | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Franz et al., 2017 [63] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Yeter and Ozturk 2019 [64] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Gundersen et al., 2019 [25] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Cannaert et al., 2017 [66] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Tapia, E.; Codina, J.; Villanueva-Blasco, V.J.; García-Algar, Ó.; Andreu-Fernández, V. Detection of the Synthetic Cannabinoids AB-CHMINACA, ADB-CHMINACA, MDMB-CHMICA, and 5F-MDMB-PINACA in Biological Matrices: A Systematic Review. Biology 2022, 11, 796. https://doi.org/10.3390/biology11050796
Navarro-Tapia E, Codina J, Villanueva-Blasco VJ, García-Algar Ó, Andreu-Fernández V. Detection of the Synthetic Cannabinoids AB-CHMINACA, ADB-CHMINACA, MDMB-CHMICA, and 5F-MDMB-PINACA in Biological Matrices: A Systematic Review. Biology. 2022; 11(5):796. https://doi.org/10.3390/biology11050796
Chicago/Turabian StyleNavarro-Tapia, Elisabet, Jana Codina, Víctor José Villanueva-Blasco, Óscar García-Algar, and Vicente Andreu-Fernández. 2022. "Detection of the Synthetic Cannabinoids AB-CHMINACA, ADB-CHMINACA, MDMB-CHMICA, and 5F-MDMB-PINACA in Biological Matrices: A Systematic Review" Biology 11, no. 5: 796. https://doi.org/10.3390/biology11050796
APA StyleNavarro-Tapia, E., Codina, J., Villanueva-Blasco, V. J., García-Algar, Ó., & Andreu-Fernández, V. (2022). Detection of the Synthetic Cannabinoids AB-CHMINACA, ADB-CHMINACA, MDMB-CHMICA, and 5F-MDMB-PINACA in Biological Matrices: A Systematic Review. Biology, 11(5), 796. https://doi.org/10.3390/biology11050796








