Should Endometrial Cancer Treatment Be Centralized?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Pathology
3. Transvaginal Ultrasound
4. Surgery
5. Fertility-Sparing Surgery
6. Multidisciplinary Evaluation
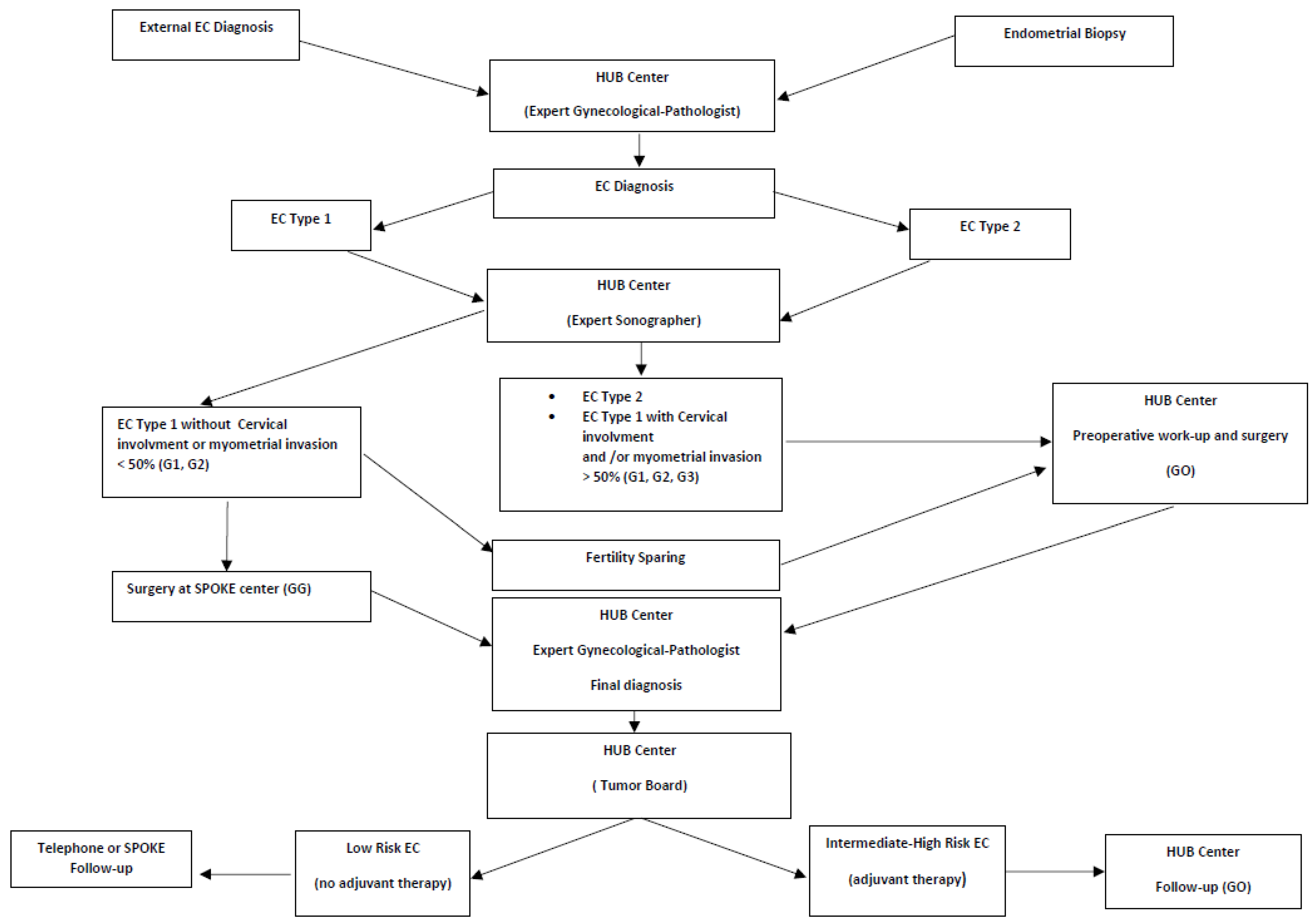
7. Hub-and-Spoke Model
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matsuura, M.; Suzuki, T.; Morishita, M.; Tanaka, R.; Ito, E. Chemotherapy (CT) with radiotherapy versus CT alone for FIGO stage3c endometrial cancer. Eur. J. Gynaecol. Oncol. 2009, 30, 40–44. [Google Scholar] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandato, V.D.; Mastrofilippo, V.; Palicelli, A.; Silvotti, M.; Serra, S.; Giaccherini, L.; Aguzzoli, L. Solitary vulvar metastasis from early-stage endometrial cancer: Case report and literature review. Medicine 2021, 100, e25863. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Murali, R.; Soslow, R.A.; Weigelt, B. Classification of endometrial carcinoma: More than two types. Lancet Oncol. 2014, 15, e268-78. [Google Scholar] [CrossRef]
- Creasman, W. Revised FIGO staging for carcinoma of the endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 109. [Google Scholar] [CrossRef]
- Sorosky, J.I. Endometrial cancer. Obstet. Gynecol. 2008, 111, 436–447. [Google Scholar] [CrossRef] [Green Version]
- Mandato, V.D.; Farnetti, E.; Torricelli, F.; Abrate, M.; Casali, B.; Ciarlini, G.; Pirillo, D.; Gelli, M.C.; Nicoli, D.; Grassi, M.; et al. HNF1B polymorphism influences the prognosis of endometrial cancer patients: A cohort study. BMC Cancer 2015, 15, 229. [Google Scholar] [CrossRef] [Green Version]
- Torricelli, F.; Mandato, V.D.; Farnetti, E.; Abrate, M.; Casali, B.; Ciarlini, G.; Pirillo, D.; Gelli, M.C.; Costagliola, L.; Nicoli, D.; et al. Polymorphisms in cyclooxygenase-2 gene in endometrial cancer patients. Tumour Biol. 2015, 36, 7423–7430. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73, Erratum in Nature 2013, 500, 242. [Google Scholar] [PubMed] [Green Version]
- Torricelli, F.; Nicoli, D.; Bellazzi, R.; Ciarrocchi, A.; Farnetti, E.; Mastrofilippo, V.; Zamponi, R.; La Sala, G.B.; Casali, B.; Mandato, V.D. Computational development of a molecular-based approach to improve risk stratification of endometrial cancer patients. Oncotarget 2018, 9, 25517–25528. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Scottish Cancer Intelligence Unit 2000: Trends in Cancer Survival in Scotland 1971–1995 Edinburgh: Information and Statistics Division of National Health Service in Scotland. 2000. Available online: https://www.isdscotland.org/Health-Topics/Cancer/Publications/2010-08-31/trends_1971-95.pdf (accessed on 3 December 2020).
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Tomaszewski, J.E.; Bear, H.D.; Connally, J.A.; Epstein, J.I.; Feldman, M.; Foucar, K.; Layfield, L.; LiVolsi, V.; Sirota, R.L.; Stoler, M.H.; et al. Consensus conference on second opinions in diagnostic anatomic pathology. Who, What, and When. Am. J. Clin. Pathol. 2000, 114, 329–335. [Google Scholar] [CrossRef]
- Spoor, E.; Cross, P. Audit of Endometrial Cancer Pathology for a Regional Gynecological Oncology Multidisciplinary Meeting. Int. J. Gynecol. Pathol. 2019, 38, 514–519. [Google Scholar] [CrossRef]
- Rivasi, F.; Palicelli, A. Peritoneal keratin granulomas: Cytohistological correlation in a case of endometrial adenocarcinoma with squamous differentiation. Cytopathology 2012, 23, 342–344. [Google Scholar] [CrossRef]
- Grevenkamp, F.; Kommoss, F.; Kommoss, F.; Lax, S.; Fend, F.; Wallwiener, D.; Schönfisch, B.; Krämer, B.; Brucker, S.Y.; Taran, F.A.; et al. Second Opinion Expert Pathology in Endometrial Cancer: Potential Clinical Implications. Int. J. Gynecol. Cancer 2017, 27, 289–296. [Google Scholar] [CrossRef]
- Kommoss, S.; Pfisterer, J.; Reuss, A.; Diebold, J.; Hauptmann, S.; Schmidt, C.; du Bois, A.; Schmidt, D.; Kommoss, F. Specialized pathology review in patients with ovarian cancer: Results from a prospective study. Int. J. Gynecol. Cancer 2013, 23, 1376–1382. [Google Scholar] [CrossRef]
- Mandato, V.D.; Torricelli, F.; Mastrofilippo, V.; Palicelli, A.; Ciarlini, G.; Pirillo, D.; Annunziata, G.; Aguzzoli, L. Accuracy of preoperative endometrial biopsy and intraoperative frozen section in predicting the final pathological diagnosis of endometrial cancer. Surg. Oncol. 2020, 35, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Jónsdóttir, B.; Marcickiewicz, J.; Borgfeldt, C.; Bjurberg, M.; Dahm-Kähler, P.; Flöter-Rådestad, A.; Hellman, K.; Holmberg, E.; Kjølhede, P.; Rosenberg, P.; et al. Preoperative and intraoperative assessment of myometrial invasion in endometrial cancer-A Swedish Gynecologic Cancer Group (SweGCG) study. Acta Obstet. Gynecol. Scand. 2021, 100, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Gastón, B.; Muruzábal, J.C.; Lapeña, S.; Modroño, A.; Guarch, R.; García de Eulate, I.; Alcázar, J.L. Transvaginal Ultrasound Versus Magnetic Resonance Imaging for Assessing Myometrial Infiltration in Endometrioid Low Grade Endometrial Cancer: A Prospective Study. J. Ultrasound Med. 2022, 41, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Cubo-Abert, M.; Díaz-Feijoo, B.; Bradbury, M.; Rodríguez-Mías, N.L.; Vera, M.; Pérez-Hoyos, S.; Gómez-Cabeza, J.J.; Gil-Moreno, A. Diagnostic performance of transvaginal ultrasound and magnetic resonance imaging for preoperative evaluation of low-grade endometrioid endometrial carcinoma: Prospective comparative study. Ultrasound Obstet. Gynecol. 2021, 58, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Cerovac, A.; Ljuca, D.; Arnautalic, L.; Habek, D.; Bogdanovic, G.; Mustedanagic-Mujanovic, J.; Grgic, G. Efficacy of transvaginal ultrasound versus magnetic resonance imaging for preoperative assessment of myometrial invasion in patients with endometrioid endometrial cancer: A prospective comparative study. Radiol. Oncol. 2022, 56, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Amin, T.; Thanatsis, N.; Naftalin, J.; Jurkovic, D. A prospective comparison of the diagnostic accuracies of ultrasound and magnetic resonance imaging in preoperative staging of endometrial cancer. J. Gynecol. Oncol. 2022, 33, e22. [Google Scholar] [CrossRef]
- Lalwani, N.; Dubinsky, T.; Javitt, M.C.; Gaffney, D.K.; Glanc, P.; Elshaikh, M.A.; Kim, Y.B.; Lee, L.J.; Pannu, H.K.; Royal, H.D.; et al. ACR Appropriateness Criteria® pretreatment evaluation and follow-up of endometrial cancer. Ultrasound Q. 2014, 30, 21–28. [Google Scholar] [CrossRef]
- Eriksson, L.S.; Lindqvist, P.G.; Flöter Rådestad, A.; Dueholm, M.; Fischerova, D.; Franchi, D.; Jokubkiene, L.; Leone, F.P.; Savelli, L.; Sladkevicius, P.; et al. Transvaginal ultrasound assessment of myometrial and cervical stromal invasion in women with endometrial cancer: Interobserver reproducibility among ultrasound experts and gynecologists. Ultrasound Obstet. Gynecol. 2015, 45, 476–482. [Google Scholar] [CrossRef] [Green Version]
- Antonsen, S.L.; Jensen, L.N.; Loft, A.; Berthelsen, A.K.; Costa, J.; Tabor, A.; Qvist, I.; Hansen, M.R.; Fisker, R.; Andersen, E.S.; et al. MRI, PET/CT and ultrasound in the preoperative staging of endometrial cancer—A multicenter prospective comparative study. Gynecol. Oncol. 2013, 128, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Celik, C.; Ozdemir, S.; Kiresi, D.; Emlik, D.; Tazegül, A.; Esen, H. Evaluation of cervical involvement in endometrial cancer by transvaginal sonography, magnetic resonance imaging and frozen section. J. Obstet. Gynaecol. 2010, 30, 302–307. [Google Scholar] [CrossRef]
- Savelli, L.; Ceccarini, M.; Ludovisi, M.; Fruscella, E.; De Iaco, P.A.; Salizzoni, E.; Mabrouk, M.; Manfredi, R.; Testa, A.C.; Ferrandina, G. Preoperative local staging of endometrial cancer: Transvaginal sonography vs. magnetic resonance imaging. Ultrasound Obstet. Gynecol. 2008, 31, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.N.; Fleischer, A.C.; Reed, G.W. Depth of myometrial invasion in endometrial cancer: Preoperative assessment by transvaginal ultrasonography. Gynecol. Oncol. 1990, 39, 321–327. [Google Scholar] [CrossRef]
- Karlsson, B.; Norström, A.; Granberg, S.; Wikland, M. The use of endovaginal ultrasound to diagnose invasion of endometrial carcinoma. Ultrasound Obstet. Gynecol. 1992, 2, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, J.L.; Galván, R.; Albela, S.; Martinez, S.; Pahisa, J.; Jurado, M.; López-García, G. Assessing myometrial infiltration by endometrial cancer: Uterine virtual navigation with three-dimensional US. Radiology 2009, 250, 776–783. [Google Scholar] [CrossRef] [Green Version]
- Mascilini, F.; Testa, A.C.; Van Holsbeke, C.; Ameye, L.; Timmerman, D.; Epstein, E. Evaluating myometrial and cervical invasion in women with endometrial cancer: Comparing subjective assessment with objective measurement techniques. Ultrasound Obstet. Gynecol. 2013, 42, 353–358. [Google Scholar] [CrossRef]
- Alcazar, J.L.; Carazo, P.; Pegenaute, L.; Gurrea, E.; Campos, I.; Neri, M.; Pascual, M.A.; Guerriero, S. Preoperative Assessment of Cervical Involvement in Endometrial Cancer by Transvaginal Ultrasound and Magnetic Resonance Imaging: A Systematic Review and Meta-Analysis. Ultraschall Med. 2021, 23. [Google Scholar] [CrossRef]
- Green, R.W.; Valentin, L.; Alcazar, J.L.; Chiappa, V.; Erdodi, B.; Franchi, D.; Frühauf, F.; Fruscio, R.; Guerriero, S.; Graupera, B.; et al. Endometrial cancer off-line staging using two-dimensional transvaginal ultrasound and three-dimensional volume contrast imaging: Intermethod agreement, interrater reliability and diagnostic accuracy. Gynecol. Oncol. 2018, 150, 438–445. [Google Scholar] [CrossRef]
- Partridge, E.; Jessup, J.; Donaldson, E.; Taylor, P.; Randal, M.; Braley, P. 1996 Patient care evaluation study (PCE) of cancer of the corpus uteri, National Cancer Database (NCDB), American College of Surgery. Gynecol. Oncol. 1999, 72, 445. [Google Scholar]
- Roland, P.Y.; Kelly, F.J.; Kulwicki, C.Y.; Blitzer, P.; Curcio, M.; Orr, J.W., Jr. The benefits of a gynecologic oncologist: A pattern of care study for endometrial cancer treatment. Gynecol. Oncol. 2004, 93, 125–130. [Google Scholar] [CrossRef]
- Macdonald, O.K.; Sause, W.T.; Lee, R.J.; Dodson, M.K.; Zempolich, K.; Gaffney, D.K. Does oncologic specialization influence outcomes following surgery in early stage adenocarcinoma of the endometrium? Gynecol. Oncol. 2005, 99, 730–735. [Google Scholar] [CrossRef]
- Hoekstra, A.; Singh, D.K.; Garb, M.; Arekapudi, S.; Rademaker, A.; Lurain, J.R. Participation of the general gynecologist in surgical staging of endometrial cancer: Analysis of cost and perioperative outcomes. Gynecol. Oncol. 2006, 103, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Mandato, V.D.; Torricelli, F.; Palomba, S.; Uccella, S.; Pirillo, D.; Ciarlini, G.; De Iaco, P.; Lucia, E.; Giorda, G.; Ditto, A.; et al. Uterine Papillary Serous Carcinoma Arising in a Polyp: A Multicenter Retrospective Analysis on 75 Patients. Am. J. Clin. Oncol. 2019, 42, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Mandato, V.D.; Torricelli, F.; Mastrofilippo, V.; Pirillo, D.; Annunziata, G.; Ciarlini, G.; D’Ippolito, G.; Bartolomeo, E.D.; Aguzzoli, L. Impact of a Province-wide Endometrial Cancer Guideline on Daily Practice. Anticancer Res. 2021, 41, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Mandato, V.D.; Formisano, D.; Pirillo, D.; Ciarlini, G.; Cerami, L.B.; Ventura, A.; Spreafico, L.; Palmieri, T.; La Sala, G.B.; Abrate, M. Province wide clinical governance network for clinical audit for quality improvement in endometrial cancer management. Int. J. Gynecol. Cancer 2012, 22, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Doll, K.M.; Meng, K.; Gehrig, P.A.; Brewster, W.R.; Meyer, A.M. Referral patterns between high- and low-volume centers and associations with uterine cancer treatment and survival: A population-based study of Medicare, Medicaid, and privately insured women. Am. J. Obstet. Gynecol. 2016, 215, 447.e1–447.e13. [Google Scholar] [CrossRef] [Green Version]
- McCrum, A.; Howe, K.; Weeks, J.; Kirkpatrick, A.; Murdoch, J. A prospective regional audit of surgical management of endometrial cancer in the South and West of England. J. Obstet. Gynaecol. 2001, 21, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.C.; De Caestecker, L.; Gillis, C.R.; Hole, D.; Davis, J.A.; Penney, G.; Siddiqui, N.A. Staging quality is related to the survival of women with endometrial cancer: A Scottish population based study. Deficient surgical staging and omission of adjuvant radiotherapy is associated with poorer survival of women diagnosed with endometrial cancer in Scotland during 1996 and 1997. Br. J. Cancer 2002, 86, 1837–1842. [Google Scholar]
- Parkin, D.E.; Warraich, Q.; Fleming, D.J.; Chew, G.K.; Cruickshank, M.E. An audit of the quality of endometrial cancer care in a specialised unit. Scott. Med. 2006, 51, 22–24. [Google Scholar]
- Wright, J.D.; Lewin, S.N.; Deutsch, I.; Burke, W.M.; Sun, X.; Herzog, T.J. Effect of surgical volume on morbidity and mortality of abdominal hysterectomy for endometrial cancer. Obstet. Gynecol. 2011, 117, 1051–1059. [Google Scholar] [CrossRef]
- Wright, J.D.; Hershman, D.L.; Burke, W.M.; Lu, Y.S.; Neugut, A.I.; Lewin, S.N.; Herzog, T.J. Influence of surgical volume on outcome for laparoscopic hysterectomy for endometrial cancer. Ann. Surg. Oncol. 2012, 19, 948–958. [Google Scholar] [CrossRef]
- Wright, J.D.; Ruiz, M.P.; Chen, L.; Gabor, L.R.; Tergas, A.I.; St Clair, C.M.; Hou, J.Y.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L. Changes in Surgical Volume and Outcomes Over Time for Women Undergoing Hysterectomy for Endometrial Cancer. Obstet. Gynecol. 2018, 132, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, S.C.; Borah, B.J.; Bakkum-Gamez, J.N.; Weaver, A.L.; McGree, M.E.; Haas, L.R.; Keeney, G.L.; Mariani, A.; Podratz, K.C. Prospective assessment of survival, morbidity, and cost associated with lymphadenectomy in low-risk endometrial cancer. Gynecol. Oncol. 2012, 127, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Yost, K.J.; Cheville, A.L.; Al-Hilli, M.M.; Mariani, A.; Barrette, B.A.; McGree, M.E.; Weaver, A.L.; Dowdy, S.C. Lymphedema after surgery for endometrial cancer: Prevalence, risk factors, and quality of life. Obstet. Gynecol. 2014, 124, 307–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matanes, E.; Eisenberg, N.; Amajoud, Z.; Gupta, V.; Yasmeen, A.; Ismail, S.; Racovitan, F.; Raban, O.; Lau, S.; Salvador, S.; et al. Sentinel Lymph Node Sampling as an Alternative to Lymphadenectomy in Patients With Endometrial Cancer and Obesity. J. Obstet. Gynaecol. Can. 2021, 43, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Tait, D.L.; Lehman, A.; Brown, J.; Crane, E.K.; Kemp, E.V.; Taylor, V.D.; Naumann, R.W. Comparison of Perioperative Outcomes between Minimally Invasive Sentinel Node Biopsy and Full Lymphadenectomy for Endometrial Cancer. J. Minim. Invasive Gynecol. 2021, 28, 1514–1518. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, R.; Khalil, A.; Ghunaim, S.; El Housheimi, A.; Khalife, D.; Sassine, D.; Khoury, K.; Mailhac, A.; Nassour, F.; Saliba, M.; et al. The accuracy and clinical impact of intraoperative frozen section in determining the extent of surgical intervention in patients with early stage endometrial cancer. J. Obstet. Gynaecol. 2022, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nagar, H.; Wietek, N.; Goodall, R.J.; Hughes, W.; Schmidt-Hansen, M.; Morrison, J. Sentinel node biopsy for diagnosis of lymph node involvement in endometrial cancer. Cochrane Database Syst. Rev. 2021, 6, CD013021. [Google Scholar] [CrossRef]
- Lecointre, L.; Lodi, M.; Faller, É.; Boisramé, T.; Agnus, V.; Baldauf, J.J.; Gallix, B.; Akladios, C. Diagnostic Accuracy and Clinical Impact of Sentinel Lymph Node Sampling in Endometrial Cancer at High Risk of Recurrence: A Meta-Analysis. J. Clin. Med. 2020, 9, 3874. [Google Scholar] [CrossRef]
- Khoury-Collado, F.; Glaser, G.E.; Zivanovic, O.; Sonoda, Y.; Levine, D.A.; Chi, D.S.; Gemignani, M.L.; Barakat, R.R.; Abu-Rustum, N.R. Improving sentinel lymph node detection rates in endometrial cancer: How many cases are needed? Gynecol. Oncol. 2009, 115, 453–455. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, K.J.; Min, K.J.; Lee, S.; Jung, U.S.; Hong, J.H.; Song, J.Y.; Lee, J.K.; Lee, N.W. Learning curve for sentinel lymph node mapping in gynecologic malignancies. J. Surg. Oncol. 2020, 121, 599–604. [Google Scholar] [CrossRef]
- Tucker, K.; Staley, S.A.; Gehrig, P.A.; Soper, J.T.; Boggess, J.F.; Ivanova, A.; Rossi, E. Defining the learning curve for successful staging with sentinel lymph node biopsy for endometrial cancer among surgeons at an academic institution. Int. J. Gynecol. Cancer 2020, 30, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Casarin, J.; Multinu, F.; Abu-Rustum, N.; Cibula, D.; Cliby, W.A.; Ghezzi, F.; Leitao, M.; Konishi, I.; Nam, J.H.; Querleu, D.; et al. Factors influencing the adoption of the sentinel lymph node technique for endometrial cancer staging: An international survey of gynecologic oncologists. Int. J. Gynecol. Cancer 2019, 29, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Piedmonte, M.R.; Spirtos, N.M.; Eisenkop, S.M.; Schlaerth, J.B.; Mannel, R.S.; Spiegel, G.; Barakat, R.; Pearl, M.L.; Sharma, S.K. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J. Clin. Oncol. 2009, 10, 5331–5336. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zeng, D.; Ou, H.; Tang, Y.; Li, J.; Zhong, H. Laparoscopic treatment of endometrial cancer: Systematic review. J. Minim. Invasive Gynecol. 2013, 20, 413–423. [Google Scholar] [CrossRef]
- Wright, J.D.; Barrena Medel, N.I.; Sehouli, J.; Fujiwara, K.; Herzog, T.J. Contemporary management of endometrial cancer. Lancet 2012, 379, 1352–1360. [Google Scholar] [CrossRef]
- Graves, N.; Janda, M.; Merollini, K.; Gebski, V.; Obermair, A. LACE trial committee. The cost-effectiveness of total laparoscopic hysterectomy compared to total abdominal hysterectomy for the treatment of early stage endometrial cancer. BMJ Open 2013, 18, e001884. [Google Scholar] [CrossRef] [Green Version]
- Moss, E.L.; Morgan, G.; Martin, A.; Sarhanis, P.; Ind, T. Economic evaluation of different routes of surgery for the management of endometrial cancer: A retrospective cohort study. BMJ Open 2021, 13, e045888. [Google Scholar] [CrossRef]
- Greggi, S.; Franchi, M.; Aletti, G.; Biglia, N.; Ditto, A.; Fagotti, A.; Giorda, G.; Mangili, G.; Odicino, F.; Salerno, M.G.; et al. Management of endometrial cancer in Italy: A national survey endorsed by the Italian Society of Gynecologic Oncology. Int. J. Surg. 2014, 12, 1038–1044. [Google Scholar] [CrossRef] [Green Version]
- Monterossi, G.; Ghezzi, F.; Vizza, E.; Zannoni, G.F.; Uccella, S.; Corrado, G.; Restaino, S.; Quagliozzi, L.; Casarin, J.; Dinoi, G.; et al. Minimally Invasive Approach in Type II Endometrial Cancer: Is It Wise and Safe? J. Minim. Invasive Gynecol. 2017, 24, 438–445. [Google Scholar] [CrossRef]
- Chan, J.K.; Gardner, A.B.; Taylor, K.; Blansit, K.; Thompson, C.A.; Brooks, R.; Yu, X.; Kapp, D.S. The centralization of robotic surgery in high-volume centers for endometrial cancer patients--a study of 6560 cases in the U.S. Gynecol. Oncol. 2015, 138, 128–132. [Google Scholar] [CrossRef]
- Yu, H.Y.; Hevelone, N.D.; Lipsitz, S.R.; Kowalczyk, K.J.; Nguyen, P.L.; Hu, J.C. Hospital volume, utilization, costs and outcomes of robot-assisted laparoscopic radical prostatectomy. J. Urol. 2012, 187, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Ananth, C.V.; Tergas, A.I.; Herzog, T.J.; Burke, W.M.; Lewin, S.N.; Lu, Y.S.; Neugut, A.I.; Hershman, D.L. An economic analysis of robotically assisted hysterectomy. Obstet. Gynecol. 2014, 123, 1038–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parham, G.; Phillips, J.L.; Hicks, M.L.; Andrews, N.; Jones, W.B.; Shingleton, H.M.; Menck, H.R. The National Cancer Data Base report on malignant epithelial ovarian carcinoma in African-American women. Cancer 1997, 80, 816–826. [Google Scholar] [CrossRef]
- Kokoska, E.R.; Bird, T.M.; Robbins, J.M.; Smith, S.D.; Corsi, J.M.; Campbell, B.T. Racial disparities in the management of pediatric appendicitis. J. Surg. Res. 2007, 137, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.; Bell, T.; Martin, J.; Bhuva, K.; Grim, R.; Ahuja, V. Use, cost, complications, and mortality of robotic versus nonrobotic general surgery procedures based on a nationwide database. Am. Surg. 2013, 79, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Stephan, J.M.; Goodheart, M.J.; McDonald, M.; Hansen, J.; Reyes, H.D.; Button, A.; Bender, D. Robotic surgery in supermorbidly obese patients with endometrial cancer. Am. J. Obstet. Gynecol. 2015, 213, 49. [Google Scholar] [CrossRef]
- Subramaniam, A.; Kim, K.H.; Bryant, S.A.; Zhang, B.; Sikes, C.; Kimball, K.J.; Kilgore, L.C.; Huh, W.K.; Straughn, J.M., Jr.; Alvarez, R.D. A cohort study evaluating robotic versus laparotomy surgical outcomes of obese women with endometrial carcinoma. Gynecol. Oncol. 2011, 122, 604–607. [Google Scholar] [CrossRef]
- Bixel, K.; Barrington, D.A.; Vetter, M.H.; Suarez, A.A.; Felix, A.S. Determinants of Surgical Approach and Survival Among Women with Endometrial Carcinoma. J. Minim. Invasive Gynecol. 2021, 1, 219–230. [Google Scholar] [CrossRef]
- Santandrea, G.; Piana, S.; Valli, R.; Zanelli, M.; Gasparini, E.; De Leo, A.; Mandato, V.D.; Palicelli, A. Immunohistochemical Biomarkers as a Surrogate of Molecular Analysis in Ovarian Carcinomas: A Review of the Literature. Diagnostics 2021, 11, 199. [Google Scholar] [CrossRef]
- Duggan, M.A.; Anderson, W.F.; Altekruse, S.; Penberthy, L.; Sherman, M.E. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship. Am. J. Surg. Pathol. 2016, 40, e94–e102. [Google Scholar] [CrossRef]
- Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20200515-2 (accessed on 16 October 2021).
- Yang, B.; Xie, L.; Zhang, H.; Zhu, Q.; Du, Y.; Luo, X.; Chen, X. Insulin resistance and overweight prolonged fertility-sparing treatment duration in endometrial atypical hyperplasia patients. J. Gynecol. Oncol. 2018, 29, e35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Guo, T.; Cui, R.; Feng, Y.; Bai, H.; Zhang, Z. Weight control is vital for patients with early-stage endometrial cancer or complex atypical hyperplasia who have received progestin therapy to spare fertility: A systematic review and meta-analysis. Cancer Manag. Res. 2019, 11, 4005–4021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalogera, E.; Dowdy, S.C.; Bakkum-Gamez, J.N. Preserving fertility in young patients with endometrial cancer: Current perspectives. Int. J. Womens Health 2014, 6, 691–701. [Google Scholar] [PubMed] [Green Version]
- Gunderson, C.C.; Fader, A.N.; Carson, K.A.; Bristow, R.E. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: A systematic review. Gynecol. Oncol. 2012, 125, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Mandato, V.D.; Palomba, S.; Nucera, G.S.; La Sala, G.B. Hysteroscopic Resection in Fertility-Sparing Surgery for Atypical Hyperplasia and Endometrial Cancer: How Important Are Intrauterine Adhesions? J. Minim. Invasive Gynecol. 2016, 23, 453–454. [Google Scholar] [CrossRef]
- Nucera, G.; Mandato, V.D.; Gelli, M.C.; Palomba, S.; La Sala, G.B. Gonadotropin releasing hormone agonist and levonorgestrel-intrauterine device followed by in vitro fertilization program as management strategy for an infertile endometrial cancer patient: A case report. Gynecol. Endocrinol. 2013, 29, 219–221. [Google Scholar] [CrossRef]
- Rodolakis, A.; Biliatis, I.; Morice, P.; Reed, N.; Mangler, M.; Kesic, V.; Denschlag, D. European Society of Gynecological Oncology Task Force for fertility preservation: Clinical recommendations for fertility-sparing management in young endometrial cancer patients. Int. J. Gynecol. Cancer 2015, 25, 1258–1265. [Google Scholar] [CrossRef]
- Park, J.Y.; Seong, S.J.; Kim, T.J.; Kim, J.W.; Kim, S.M.; Bae, D.S.; Nam, J.H. Pregnancy outcomes after fertility sparing management in young women with early endometrial cancer. Obstet. Gynecol. 2013, 121, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Chiva, L.; Sastre, F.L.; Galan, V.C.; Galainena, L.G.; Martín, A.G.; Cortijo, L.G.; González, N.C. Conservative management of patients with early endometrial carcinoma: A systematic review. Clin. Transl. Oncol. 2008, 10, 155–162. [Google Scholar]
- Shao, R. Progesterone receptor isoforms A and B: New insights into the mechanism of progesterone resistance for the treatment of endometrial carcinoma. Ecancermedicalscience 2013, 7, 381. [Google Scholar]
- Floyd, J.L.; Campbell, S.; Rauh-Hain, J.A.; Woodard, T. Fertility preservation in women with early-stage gynecologic cancer: Optimizing oncologic and reproductive outcomes. Int. J. Gynecol. Cancer 2021, 31, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.esgo.org/media/2015/12/Endometrial_broz_A6_b.pdf (accessed on 16 October 2021).
- Ibanez, E.; Chiva, L.; Rodriguez-Escudero, F.J. Resultados de la Encuesta Nacional sobre Carcinoma de Endometrio Diagnosticado en 1993. Adv. Ginecol. Oncol. 1993, 9, 505. [Google Scholar]
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Jürgenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer-Combined Analysis of the PORTEC Cohorts. Clin. Cancer Res. 2016, 22, 4215–4224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAlpine, J.N.; Chiu, D.S.; Nout, R.A.; Church, D.N.; Schmidt, P.; Lam, S.; Leung, S.; Bellone, S.; Wong, A.; Brucker, S.Y.; et al. Evaluation of treatment effects in patients with endometrial cancer and POLE mutations: An individual patient data meta-analysis. Cancer 2021, 15, 2409–2422. [Google Scholar] [CrossRef]
- Church, D.N.; Stelloo, E.; Nout, R.A.; Valtcheva, N.; Depreeuw, J.; ter Haar, N.; Noske, A.; Amant, F.; Tomlinson, I.P.; Wild, P.J.; et al. Prognostic signi-ficance of POLE proofreading mutations in endometrial cancer. J. Natl. Cancer Inst. 2014, 107, 402. [Google Scholar] [PubMed]
- Ardighieri, L.; Palicelli, A.; Ferrari, F.; Bugatti, M.; Drera, E.; Sartori, E.; Odicino, F. Endometrial Carcinomas with Intestinal-Type Metaplasia/Differentiation: Does Mismatch Repair System Defects Matter? Case Report and Systematic Review of the Literature. J. Clin. Med. 2020, 9, 2552. [Google Scholar] [CrossRef]
- Seagle, B.L.; Strohl, A.E.; Dandapani, M.; Nieves-Neira, W.; Shahabi, S. Survival Disparities by Hospital Volume among American Women with Gynecologic Cancers. JCO Clin. Cancer Inform. 2017, 1, 1–15. [Google Scholar] [CrossRef]
- Zola, P.; Ciccone, G.; Piovano, E.; Fuso, L.; Peirano, E.; Di Cuonzo, D.; Perrone, A.M.; Mandato, V.D.; Zavallone, L.; Chiudinelli, F.; et al. Intensive versus minimalist follow-up in patients treated for endometrial cancer: A multicentric randomized controlled trial (The TOTEM study—NCT00916708). J. Clin. Oncol. 2021, 39, 5506. [Google Scholar] [CrossRef]
- Dolly, D.; Mihai, A.; Rimel, B.J.; Fogg, L.; Rotmensch, J.; Guirguis, A.; Yordan, E.; Dewdney, S. A Delay from Diagnosis to Treatment Is Associated with a Decreased Overall Survival for Patients with Endometrial Cancer. Front. Oncol. 2016, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Shalowitz, D.I.; Epstein, A.J.; Buckingham, L.; Ko, E.M.; Giuntoli, R.L., 2nd. Survival implications of time to surgical treatment of endometrial cancers. Am. J. Obstet. Gynecol. 2017, 216, 268.e1–268.e18. [Google Scholar] [CrossRef] [Green Version]
- Bilimoria, K.Y.; Ko, C.Y.; Tomlinson, J.S.; Stewart, A.K.; Talamonti, M.S.; Hynes, D.L.; Winchester, D.P.; Bentrem, D.J. Wait times for cancer surgery in the United States: Trends and predictors of delays. Ann. Surg. 2011, 253, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Knisely, A.; Huang, Y.; Melamed, A.; Tergas, A.I.; St Clair, C.M.; Hou, J.Y.; Khoury-Collado, F.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L.; et al. Effect of regionalization of endometrial cancer care on site of care and patient travel. Am. J. Obstet. Gynecol. 2020, 222, 58.e1–58.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, K.I.; Eska, J.S.; Harrison, R.F.; Suidan, R.; Abraham, A.; Chisholm, G.B.; Meyer, L.A.; Westin, S.N.; Fleming, N.D.; Frumovitz, M.; et al. Implementation of a sentinel lymph node mapping algorithm for endometrial cancer: Surgical outcomes and hospital charges. Int. J. Gynecol. Cancer 2020, 30, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Taylor, A.; Guttery, D.S.; Moss, E.L. Innovative Follow-up Strategies for Endometrial Cancer. Clin. Oncol. 2021, 33, e383–e392. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Country | Advantages | Disadvantages |
|---|---|---|---|
| Crawford SC et al. [48], 2001 | Scotland | Surgical staging, Lymphadenectomy, Adjuvant therapy | - |
| Roland PY et al. [40], 2003 | United States | Complete staging, Experience of gynecologists/oncologists, Minimization of the potential morbidity associated with adjuvant radiation | Geographical difficulties for access to the center |
| Macdonald OK et al [41], 2005 | United States | More appropriate adjuvant therapy | - |
| Parkin DE et al. [49], 2006 | Scotland | Complete staging, Correct selection of patients for adjuvant treatment (lymphadenectomy or radiotherapy), Multidisciplinary team | - |
| Hoekstra A. et al. [42], 2006 | United States | Operative time and cost, Experience of gynecologists/oncologists, Appropriate follow-up | - |
| Savelli L et al. [32], 2008 | Italy | Lower costs related to the presence of TVS performed by expert specialists | - |
| Mandato VD et al. [45], 2012 | Italy | Appropriate pre-surgical assessment, Multidisciplinary evaluation, Appropriate surgery treatment (laparoscopy, lymphadenectomy), Presence of expert pathologists | - |
| Wright JD et al. [50], 2011 | United States | Improved perioperative surgical/medical complications and ICU | - |
| Greggi et al. [69], 2014 | Italy | Optimal surgical treatment for high-risk cases | No benefit for low-risk cases |
| Chan JK et al. [71], 2015 | United States | Robotic surgery, Experience of gynecologists/oncologists, Cost-effectiveness. | Socio-economic barriers could delay the diagnosis and results |
| Eriksson et al. [29], 2015 | Europe | Improved preoperative ultrasound staging (ultrasound experts) | - |
| Doll KM et al. [46], 2016 | United States | Appropriate surgery treatment (lymphadenectomy), High survival for patients who, after centralization, undergo chemotherapy in small centers | Geographical difficulties for access to the center for racial/ethnic minorities who are more likely to live in close proximity to gynecologic oncologists |
| Seagle BLL et al. [100], 2017 | United States | Standardization of adjuvant therapy | - |
| Green RW et al. [38], 2018 | Europe | Superior diagnostic modalities (ultrasound experts) | - |
| Spoor E. and Cross P. [18], 2019 | UK | Greater diagnostic accuracy (expert pathologists) | - |
| Knisely A et al. [105], 2020 | United States | - | Increased travel distance may adversely affect care (limits or delayed access to care) |
| Mandato VD et al. [22], 2020 | Italy | Expert pathologists, Appropriateness of adjuvant therapy | |
| Mandato VD et al. [44], 2021 | Italy | Fewer peri- and post-operative complications, Expert pathologists, Laparoscopy, Appropriateness of adjuvant treatment | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandato, V.D.; Palicelli, A.; Torricelli, F.; Mastrofilippo, V.; Leone, C.; Dicarlo, V.; Tafuni, A.; Santandrea, G.; Annunziata, G.; Generali, M.; et al. Should Endometrial Cancer Treatment Be Centralized? Biology 2022, 11, 768. https://doi.org/10.3390/biology11050768
Mandato VD, Palicelli A, Torricelli F, Mastrofilippo V, Leone C, Dicarlo V, Tafuni A, Santandrea G, Annunziata G, Generali M, et al. Should Endometrial Cancer Treatment Be Centralized? Biology. 2022; 11(5):768. https://doi.org/10.3390/biology11050768
Chicago/Turabian StyleMandato, Vincenzo Dario, Andrea Palicelli, Federica Torricelli, Valentina Mastrofilippo, Chiara Leone, Vittoria Dicarlo, Alessandro Tafuni, Giacomo Santandrea, Gianluca Annunziata, Matteo Generali, and et al. 2022. "Should Endometrial Cancer Treatment Be Centralized?" Biology 11, no. 5: 768. https://doi.org/10.3390/biology11050768
APA StyleMandato, V. D., Palicelli, A., Torricelli, F., Mastrofilippo, V., Leone, C., Dicarlo, V., Tafuni, A., Santandrea, G., Annunziata, G., Generali, M., Pirillo, D., Ciarlini, G., & Aguzzoli, L. (2022). Should Endometrial Cancer Treatment Be Centralized? Biology, 11(5), 768. https://doi.org/10.3390/biology11050768






