Simple Summary
The presence of the oak pinhole borer, the insect Platypus cylindrus, in Portuguese cork oak stands has drastically increased in the past few decades. This beetle excavates long galleries in the trunk while inoculating fungi (called ambrosia fungi) transported in special organs (mycangia) that will serve as food source for its offspring. The combined action of extensive boring into the heartwood and the inoculation of fungi leads to an increase in tree mortality. A new ambrosial fungus, Ceratocystiopsis quercina, was isolated from the insects’ mycangia and from wilting trees, namely from the staining patches it causes on wood.
Abstract
Platypus cylindrus is the most common ambrosia beetle in stands of Quercus suber in Portugal. This insect farms specialized fungi in sapwood galleries, using its mycangia to carry and store these organisms. Some ectosymbiotic fungi carried by P. cylindrus are phytopathogenic and cause extensive tree mortality and severe economic losses. To understand the role of P. cylindrus fungal symbionts in stands of Q. suber we examined beetle galleries present in declining and/or dying cork oak trees during field surveys. Logs with active galleries were obtained in situ and from captured emerging beetles. Insects were aseptically dissected, and their mycangia and intestine were retrieved. Morphological and molecular profiles of fungal isolates obtained from cultured insect parts were carried out to accurately characterize and identify isolated fungi. Molecular characterizations were performed with DNA sequence data from four loci, i.e., LSU, SSU, 5.8S-ITS2-28S, and TUB. Morphological results consistently showed a collection of Ophiostoma-like fungal axenic isolates, while phylogenies inferred that this collection constitutes an undescribed taxon reported herein for the first time in association with P. cylindrus in Portuguese cork oak stands. The novel species was erected as Ceratocystiopsis quercina sp. nov. and constitutes a new phytopathogenic fungal species associated with symptoms of vegetative cork oak decline.
1. Introduction
Platypus cylindrus Fab. (Coleoptera: Curculionidae) is a ubiquitous ambrosia beetle in stands of Quercus suber L. in the Mediterranean basin. This insect was initially considered a secondary pest in Portuguese stands as its impact was generally limited to dead or weakened trees [1,2,3]. However, since the 1980s, severe infestations were observed in apparently healthy cork oaks, causing widespread tree death within three months to one year and a half after infestation [4,5,6]. Cork oak forests are a very specific, delicately balanced ecosystem which mainly persists in the Mediterranean basin. It is therefore of major concern that over the last three decades an alarming decline of trees has increased across its distribution area, namely in the representative Portuguese cork oak stands. Platypus cylindrus emerged as a determinant factor in the decline of stands since adults attack trees of all ages, especially those recently decorked or weakened, but retaining wood humidity. The attacks of P. cylindrus are localized in the trunk and branches of larger diameter (Figure 1a,b). The males begin the colonization of trees through gallery excavations, while the females carry fungi, called ambrosia fungi, in specialized structures in their thorax, known as mycangia [7,8,9,10] (Figure 1c). These symbiotic fungi will grow on the walls of the galleries providing not only a significant food source to both insect larvae and adults, but also giving the fungi a continuous mean of dispersal [11] (Figure 1d,e). Platypus cylindrus lives in close association with ambrosia fungi, which in turn are a determinant factor in the decline of cork oak stands, causing severe economic losses in the Mediterranean region [12,13]. The most important group of ambrosia fungi are members of the heterogeneous Ophiostomatales group, a so-called ophiostomatoid fungi. These include genera with similar morphological and ecological characteristics, i.e., Ceratocystiopsis, Graphilbum, Leptographium, Ophiostoma, Raffaelea, and Sporothrix [14,15]. The taxonomy of ophiostomatoid fungi was intricate since the first descriptions were reported [16,17,18]. At present they are grouped in two fungal families, i.e., Ceratocystidaceae and Ophiostomataceae. The genus Ceratocystiopsis includes nearly 20 taxa, most of which obtained from plants infested by phloem-and-wood-breeding beetles.

Figure 1.
(a) Cork oak affected by Platypus cylindrus and its ambrosia fungi; (b) cross section of the trunk with wood staining along the galleries; (c) emerged beetle with the ambrosial mycelium coming out of the mycangia; (d) transversal cut showing the gallery lined by a silky white mycelium, (e) older gallery (bars = 1 mm).
Several ophiostomatoid fungi have already been reported to be associated with P. cylindrus and its galleries, in Quercus spp. in France, Algeria, Greece, Portugal, and Tunisia, namely the Raffaelea species [19,20,21,22,23,24,25,26,27]. Studies of oak decline in Europe have also shown that the fungi complex Ophiostoma/Ceratocystis was pathogenic to Quercus trees [28,29,30]. In this study, the newly collected Ophiostoma-like fungi isolates were accurately identified based on morphological characters and DNA sequences for four loci (LSU, SSU, 5.8S-ITS2-28S and TUB) [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45] with the overall objective of contributing to the knowledge of the etiology of cork oak decline in Portuguese stands.
2. Materials and Methods
2.1. Sampling and Fungal Isolation
In total, 12 cork oaks infested by P. cylindrus and exhibiting vegetative decline symptoms were selected in 2 main cork-producing regions of Portugal, i.e., Ribatejo province and Alentejo province. One log from each tree (30 cm diam. and 0.5 m length) was collected and the sampling was repeated during 2005, 2006, and 2007. Beetles were captured from active galleries with fine mesh nets attached to the logs and observed under a binocular microscope to confirm their identity. A total of 100 insects per year were aseptically dissected with sterilized ophthalmic scalpels (Feather ®, Sacramento, CA, USA) under a stereo binocular microscope × 40 LEICA MZ6 (Wetzlar, Germany) to obtain their mycangia, intestine, and parts of the exoskeleton (elytra).
Collection of beetles was followed by sampling of fungi from gallery walls by cutting logs in sections exposing the gallery system where P. cylindrus larvae feed. Wood fragments containing galleries were excised from the logs. Fungal isolation from the beetle parts and wood fragments was made after surface sterilization of these pieces for 1 min in 1% sodium hypochlorite. Surface sterilized samples were subsequently plated directly on 1.5% malt extract agar (10 g Difco MEA, Franklin, NJ, USA; 15 g agar in 1 L dH2O) amended with 500 mg/L cycloheximide (Sigma-Aldrich, St. Louis, MO, USA) and 500 mg/L streptomycin (Sigma-Aldrich, St. Louis, MO, USA). Plates were incubated at room temperature in dark conditions for 1 to 2 weeks, when visible fungal growth was observed and axenic cultures of each putative strain were obtained.
2.2. Microscopic Observation and Descriptions
Colonized agar plugs (5 mm diam.) were excised from actively growing 1 week old pure cultures of different isolates. These discs were transferred to the center of fresh plates containing 1.5% MEA. Growth rates were determined at temperatures ranging from 5 to 35 °C, at 5-degree intervals, 3 and 10 days after inoculation, in the dark. The colony diameter of six replicates was calculated by averaging the 12 measurements. Mycelial colours were described using the terminology from Saccardo (1891) [46]. Tolerance to cycloheximide was assessed by measuring fungal growth on MEA amended with 100, 500, and 1000 ppm cycloheximide after autoclaving. For fungal morphological characterization, 3 to 5-day-old slide cultures mounted in lactophenol were examined with light microscopy with differential interference contrast microscopy (Olympus BX-41 with Olympus DP11, Tokyo, Japan) [47]. Fifty measurements were obtained for each taxonomically informative structure. For scanning electron microscopy (SEM), small wood blocks (5 × 2 × 5 mm) bearing fungal structures were fixed according to Lee et al., (2003) [48] and Massoumi-Alamouti et al. (2009) [44]. After fixation, the samples were critical point dried, sputter coated twice with gold palladium (98:2), and examined using a JEOL 35 scanning electron microscope (JEOL, Peabody, MA, USA). Isolates used in this study are maintained at the Centraalbureau voor Schimmelcultures (CBS), Utrecht, The Netherlands, as well as in the culture collection of INIAV Institute (Micoteca da Estação Agronómica Nacional (MEAN)) (PC acronyms, Table 1). In addition, mycelial plugs were placed in the voucher specimen collection at Iowa State University (secondary collection; C acronyms, Table 1). Voucher information and GenBank accession numbers of all isolates included in this study and sequences used in the phylogenetic analyses are listed in Table 1.

Table 1.
Details of isolates obtained in this study (bold) and of strains representing species of Ophiostoma and Ceratocystiopsis retrieved from GenBank and used in phylogenetic analyses.
Based on unique culture morphology, representative isolates were selected for DNA sequence-based characterization. Fungal DNA extraction was performed using mycelia from pure cultures with the Puregene® DNA Purification Kit (Gentra Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions. Five genomic regions were amplified by polymerase chain reaction (PCR) and sequenced for phylogenetic analyses. The nuclear large subunit ribosomal DNA (LSU, 28S rDNA) was amplified using primers NL1 and NL4 [49] as well as LROR and LR5 [49,50]. The nuclear small subunit ribosomal DNA (SSU, 18S rDNA) was amplified with primers NS1, NS3, NS4, and NS6 [51,52]. The internal transcribed spacer 1 and 2 (ITS1-5.8S-ITS2) and the internal transcribed spacer 2 and large subunit (5.8S-ITS2-28S) were amplified with primers ITS5/NL4 [52], ITS1F [53], and ITSp3 [54]. Amplification of β-Tubulin (TUB) used primers T10 [55] and Bt2b [56]. All reactions run in a 25 μL volume, containing 12.5 μL of Supreme NZYTaq II DNA polymerase Master Mix (NZYTech,, Lisbon, Portugal), 2 μL of DNA template, 1 μL of each forward and reverse primer (10 μM) and 8.5 μL of molecular-grade water (Sigma-Aldrich, St. Louis, MI, USA). Amplification reactions were performed in the thermocycler Biometra TAdvanced (Analytik, Jena, Germany). Amplification of the various loci was performed under the following conditions: a denaturation step at 95 °C for 5 min followed by 35 cycles at 94 °C for 1 min, 1 min at 50–55 °C (depending on the primer annealing temperature), and 1 min at 72 °C, with a final extension step of 7 min at 72 °C. Amplified products were visualized under UV light on a 1.5% agarose gel to confirm successful amplification. PCR products were purified using ExoSAP-IT™ PCR Product Cleanup Reagent (ThermoFisher Scientific, Pittsburg, PA, USA) following the manufacturer’s protocols. These were submitted to the Sequencing facility at STABVIDA (Caparica, Portugal) for Sanger sequencing. Consensus sequences were assembled using SequencherTM (Gene Codes Corp., Ann Arbor, MI, USA). Consensus sequences were trimmed and a preliminary molecular identification was made by comparing the sequences of our isolates with those of the National Center for Biotechnology Information (NCBI) using the Basic Local Alignment Search Tool (BLAST: https://www.ncbi.nlm.nih.gov, accessed on 5 May 2021) and those in datasets referred by other authors [17,44,49,50,57]. Phylogenetic analyses were conducted separately for the five rDNA regions (nSSU, nLSU, ITS1-5.8S-ITS2, 5.8S-ITS2-28S, and TUB) with new sequences generated in this study and with other selected published sequences based on their genetic distance to Ophiostomatales [58,59,60]. Novel sequences from this study were deposited in GenBank (accession numbers are included in Table 1). A concatenated dataset analysis of the three adjacent regions was not done due to the high degree of divergence of sequences from our isolates and other Ophiostomatales available in GenBank (variance and length of the ITS1 region). We opt for a discrete phylogenetic analysis of the 5.8S-ITS2-28S region and partial nucleotide sequences from the large and small subunit rDNA, LSU, and SSU, and from β-tubulin, TUB. Only sequence fragments that could be aligned with certainty were used to generate alignments and included in the maximum likelihood phylogenetic analyses with MEGA, version X [61] with 1000 bootstrap replicates. The best evolutionary substitution model for LSU and ITS regions was Tamura 3-parameter (T92+G) and for SSU and TUB regions was Kimura 2-parameter (K2+G). Only bootstrap values above 50 were considered well supported in the final consensus tree.
3. Results
3.1. Fungal Isolation and Identification
Fungal isolations were performed from 300 adults P. cylindrus emerged from the cork oak logs and from pieces of their galleries during sampling seasons of three years. The most frequent fungi were species of the ophiostomatoid Raffaelea genus, including a novel species erected as Raffaelea quercina Inácio, Sousa, and Nóbrega (2021), a new pathogenic fungus associated with P. cylindrus [62]. Other Ophiostoma-like colonies were the second most frequent species, being mainly present in the mycangia in the insect and also prevalent on the intestine and on the ambrosial mat lining of the galleries. A total of seven axenic Ophiostoma-like colonies were selected for further molecular analyses and morphological characterization.
3.2. Phylogenetic Analyses
Seven unidentified Ophiostoma-like isolates were phylogenetically placed using four loci (LSU, SSU, 5.8-ITS2-28S, and TUB) corresponding to four nuclear genes, i.e., 28S rDNA, 18S rDNA, ITS2, and β-Tubulin. We aimed to identify isolates at the species level.
Datasets for phylogenetic analyses included available sequences for reference species in the genus Ceratocystiopsis, as well as other representative taxa in the Ophiostoma genus (Table 1). The first aligned dataset (Figure 2a), contained LSU 28S rDNA sequences of Ceratocystiopsis species and related species in closely related genera (451 characters, 82 parsimony informative). The second alignment (Figure 2b), contained SSU 18S rDNA sequences (1024 characters, 51 parsimony informative). For the phylogenetic analysis of the 5.8S-ITS2-28S region (Figure 2c), the alignment had 311 characters, including gaps, of which 116 were parsimony informative. The fourth alignment (Figure 2d), contained the β-tubulin (TUB) sequences (242 characters, 133 parsimony informative).
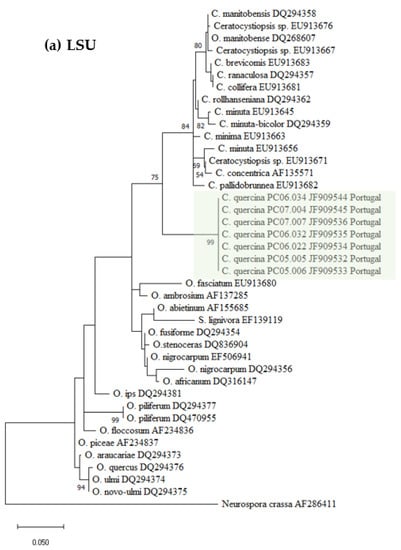
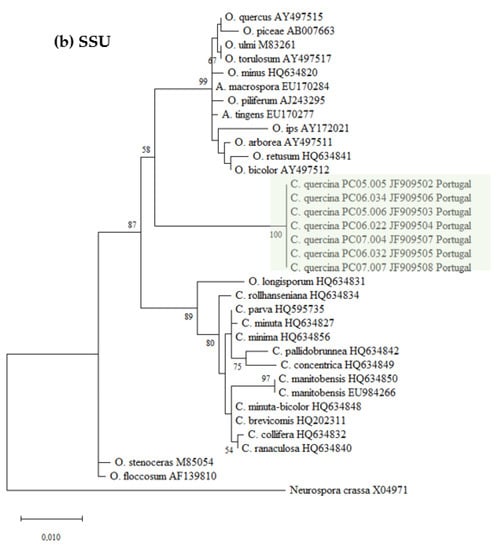
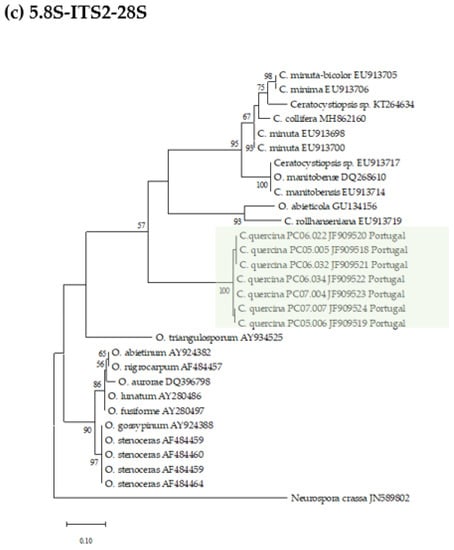
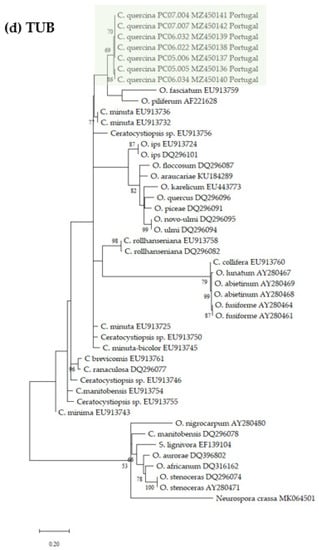
Figure 2.
Phylogenetic trees resulting from maximum likelihood analyses of the (a) LSU, (b) SSU, (c) ITS2, and (d) TUB regions for species of Ophiostoma and Ceratocystiopsis. Bootstrap support values above 50 are indicated on the nodes. New sequences and new species proposed in this study are indicated in color. In all the phylogenies three clades were distinctly present, i.e., one represented by known isolates of Ophiostoma spp., a second one containing isolates of known Ceratocystiopsis spp., and a third clade with the new seven isolates from this study.
The seven isolates from this study, included in all the separate analyses of the rDNA and β-Tubulin gene regions, resulted in trees with similar topologies (Figure 2). The novel fungal isolates derived from this study claded apart from all pre-existing and known clades (57–89% bootstrap support. See Figure 2).
The phylogenic analyses presented in this study support the existence of a new Ophiostoma-like species clearly discriminated from pre-existing species and clades. The different phylogenetic trees consistently group the Portuguese isolates in a single and distinct clade, closely related to Ceratocystiopsis species, therefore supporting the novel status of these isolates as a distinct new species of Ceratocystiopsis, herein described and referred to as Ceratocystiopsis quercina sp. nov.
3.3. Morphology and Taxonomy
Ceratocystiopsis quercina M.L. Inácio, E. Sousa, and F. Nóbrega, sp. nov (Figure 2).
- (1)
- MycoBank: MB 841995;
- (2)
- Holotype: LISE 96335;
- (3)
- Etymology: Named after the host genus from which it was isolated, Quercus;
- (4)
- Host trees/distribution: On galleries of Quercus suber in Portugal = on mycangia of Platypus cylindrus.
Material examined: Portugal, Chamusca (Santarém), on mycangia and in galleries of the insect Platypus cylindrus on declining Quercus suber, Maria L. Inácio, May 2006 (LISE 96335 holotype; ex-type culture PC05.032 = MEAN 1336 = C2508 = CBS 148604); Portugal, Chamusca (Santarém) in galleries of the insect Platypus cylindrus on declining Quercus suber, Maria L. Inácio, May 2005 (living culture, PC05.005 = MEAN 1335 = C2510 = CBS 148603); Portugal, Chamusca (Santarém) in the mycangia of Platypus cylindrus emerged from Quercus suber, Maria L. Inácio, May 2005 (living culture, PC05.006 = MEAN 1297 = C2511); Portugal, Chamusca (Santarém) in the mycangia of Platypus cylindrus emerged from Quercus suber, Maria L. Inácio, May 2006 (living culture, PC06.022 = MEAN 1298 = C2519); Portugal, Montemor (Alentejo) in the mycangia of Platypus cylindrus emerged from Quercus suber, Maria L. Inácio, May 2006 (living culture, PC06.034 = MEAN 1337 = C2507); Portugal, Montemor (Alentejo) in galleries of the insect Platypus cylindrus on declining Quercus suber, Maria L. Inácio, May 2007 (living culture, PC07.004 = MEAN 1338 = C2517); Portugal, Montemor (Alentejo) in the intestine of the insect Platypus cylindrus emerged from Quercus suber, Maria L. Inácio, May 2007 (living culture, PC07.007 = MEAN 1299 = C2509 = CBS 148605).
Description: Colonies effuse, yeast-like, ivory-white to cream-colored, smooth, later mucilaginous, with light concentric zonation, few with a light olive-green mottling appearing in the center or a sporodochium-like both in culture and in wood (Figure 3a–d), corresponding to a Hyalorhinocladiella anamorph which initially formed protoperithecia in culture that did not developed necks even when isolates were paired (Figure 3e,f); perithecia develop abundantly in 30 days, dark brown, superficial on wood and in culture on the superficial mycelium; globe-shaped, (55-)60-70(-80) μm diameter; neck short (15–25 μm long), conical, with an obtuse apex. Ascospores extrude through the ostiole in a narrow cirrhus; hyaline, one-celled, fusiform with a hyaline sheath, (9.2-)10.0-11.3(-12.2) × (0.8-)1.0-1.2(-1.4) μm (Figure 3H–K). Colonies grow slowly on MEA, 37–38 mm after 10 d at 25 °C. Hyphae hyaline and septate that bound together forming compact hyphal ropes with cluster of conidia. Conidiophores micronematous and mononematous or synnematous, erect, septate, slender with a tapered apex. Conidial development occurring through both annellidic percurrent or sympodial proliferation but not leaving conspicuous scars (Figure 3G,L–O). Conidia with various shape being the triangular the most prevalent, with (4.2-)5.2-5.8(-8.4) × (1.7-)1.8-2.2(-3.3) μm (Figure 3P–Q). Our SEM micrographs do not help in the clarification on the mode of conidial development since we found hyaline conidiophores and primary annellidic conidiogenous cells as well as proliferation sympodial.
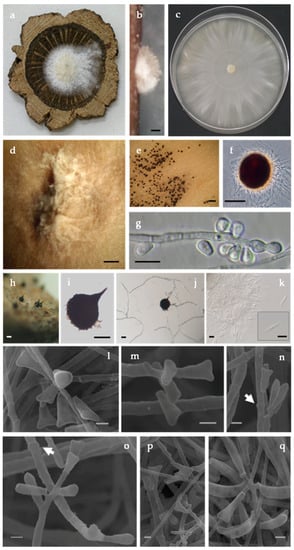
Figure 3.
Culture, conidiophores, and conidia of Ceratocystiopsis quercina (isolate PC06.032 = C2508). (a) Culture growing on a wood disc of Quercus suber. (b) Sporodochia-like of conidiophores on wood (bar = 250 μm). (c) Colony morphology after 2 weeks on malt extract agar in 90 mm diameter plate. (d) Mass of conidiophores, conidia, and yeast-like growth on malt extract agar (d,e bar = 500 μm). (e,f) Protoperithecia formed on malt extract agar, (f) Differential interference contrast (bar = 50 μm). (g) Newly formed conidia through percurrent proliferation without conspicuous scars (annellations) at the point of conidial dehiscence (bar = 10 μm). (h–j) Perithecia formation in 30 days, (h) Superficial on autoclaved wood (bar = 50μm) and (i) on culture, (j) Ascospores extruding from the ostiole (i,j bar = 25 μm). (k) Ascospores fusiform with sheath (bar = 5 μm). (l–q) Scanning electron micrographs of conidia of various shape and conidiogenous with percurrent and sympodial proliferation and some conidiogenous cells showing annellations (arrows in (n) and (o)) (bar = 1 μm). Note: Morphological comparisons and growth data indicates that this Ceratocystiopsis species, with Hyalorhinocladiella anamorph, retrieved from P. cylindrus and their galleries in Q. suber is different from any Ophiostomatales previously described. This fungus could also be distinguished from previously described species based on DNA comparisons, as documented in this study. The new Portuguese Ceratocystiopsis species presents a stable and distinct position in the inferred phylogenetic relationships.
4. Discussion
This study identified, characterized, and erected a new species of ambrosia fungus with a Hyalorhinocladiella anamorph associated with Platypus cylindrus in declining cork oak trees in Portugal. Morphological features and phylogenetic analyses supported the assignation of the axenic isolates retrieved during this study to a novel species. The species was named Ceratocystiopsis quercina based on clade relationships with other Ceratocystiopsis species.
Ceratocystiopsis quercina was found closely associated with the mycangia of P. cylindrus, being also isolated from their intestinal tract. Genome fragment sequences of the cycloheximide-tolerant Hyalorhinocladiella isolates did not match known available genome sequences. These divergences corroborated the differences found in the morphology, i.e., the absence of sprout cells or sporodochia typical of Raffaelea species, common symbionts of P. cylindrus. Based on these findings, the species is new to science, and is the first known Ceratocystiopsis associated with this ambrosia beetle.
To date, only Raffaelea spp. and Ambrosiella spp. have been documented as alimentary mycangial symbionts of ambrosia beetles. The sexual state of some of these ambrosia beetle symbionts was only found recently [63,64]. Previously, it was believed that a sexual state would not be an advantage for dispersal by an ambrosia beetle with mycangia [50]. It is probable that species of Raffaelea have derived from an ancestor with an ophiostomatoid sexual state and conidiogenesis similar to extant species of Hyalorhinocladiella or Pesotum [60]. The accurate isolation technique from the mycangia and intestinal content of the beetles employed in this study, and the use of cycloheximide in the isolation medium allowed a good recovery of the ophiostomatoid symbionts. Ceratocystiopsis quercina found in association with P. cylindrus was also isolated from the galleries of the insect in declining oaks. It was also recovered from declining cork oaks with visible aborted attacks of P. cylindrus. Thus, even if insects do not succeed in colonizing the tree, they are able to inoculate the pathogen into a susceptible host. Without P. cylindrus as a vector for dissemination, it would not be possible for these fungi to reach new hosts, as they are enclosed within the tree. In addition, fungi in the Ophiostomatales require pre-existing wounds in order to infect their hosts. The beetles enable infection by carrying the fungi into pre-existing wounds on trees or produce these wounds themselves while excavating galleries [65]. Thus, without P. cylindrus, it would not be possible for the fungal species to continue its life cycle, just as without the fungi, the beetles would have an extremely difficult time colonizing new trees. It is, indeed, an obligatory symbiosis. Inside the host, the fungus can rapidly spread from several points.
In the wood of their host trees, ambrosia fungi usually penetrate only a few mm into the xylem and their growth is usually restricted to areas surrounding the galleries [66]. However, C. quercina penetrates several cm into the sapwood of its cork oak hosts and causes a brown discoloration in the xylem. It is likely that, as it occurs with fungi such as R. lauricola, aborted attacks by the insect in the sapwood of healthy trees vector thousands of spores of the fungus, which oozees from the mycangia of the beetles, infecting severed vessels, and ultimately cause the systemic colonization of the host [67,68]. This most likely new strategy allows the insect to spread wilt disease in cork oak stands, facilitating the beetles’ establishment in the host plant. In this manner, P. cylindrus does not need to wait another decorking cycle to establish new populations in Q. suber stands, and trees could be potentially colonized from the decorking until they rebuilt a thick cork layer. In addition, the beetle’s success has most likely been further enhanced with climate change, with continuous mild winters causing less offspring mortality, and summer droughts causing stressed trees, thus making host trees more susceptible to attacks [69].
It has generally been accepted that one or a few fungal species are associated with a particular ambrosia beetle species, however, more recent studies note that fungal symbionts of ambrosia beetles are more diverse, more generalist and more competitive than previously assumed, and that ambrosia fungi may compete among each other for entrance to, and growth within, the mycangia of the vectoring beetles [50,63]. If so, it would be expected that more species could be isolated from P. cylindrus, especially if collected in other parts of its distribution range. Additionaly, new taxa of Ophiostomatales are being revealed and more comprehensive and robust phylogenies are being provided [70].
In terms of role in oak decline, the combined action of P. cylindrus massive attacks and extensive gallery excavation with a successful parallel inoculation of ambrosia fungi into the plant hosts, leads to an increase of tree mortality enhanced through new associations with wilt-causing fungi. Understanding the ecology and population dynamics of P. cylindrus-associated fungi is important for the surveillance and management of the beetle-fungal complex and it impacts on forest stands, and could improve prediction and modeling of disease dissemination. Biological control of these fungal phytopathogens may be possible through manipulation of the mycangial mycoflora. However, with the consistent isolation of Ophiostomatales. from all beetles sampled, the incidence of cork oak wilt appears to be driven only by the population level of P. cylindrus, and disease management should focus on this parameter rather than mycoflora manipulation.
5. Conclusions
Cork oak forests are very specific, delicately-balanced ecosystems that only persist in the Mediterranean basin. It is therefore of major concern that over the last three decades an alarming decline of trees has increased across its distribution area, namely in the representative Portuguese cork oak stands. Due to cork oak decline being a multifactorial process, several causes have been pointed out as contributors to tree mortality and loss of vigour, namely biotic factors. The insect Platypus cylindrus emerged as a determinant factor in the decline of stands and its population outbreaks in the last decades have caused heavy economic damages since cork loses its quality and ultimately trees death overcomes.
The symptoms and signs exhibited by cork oaks attacked by P. cylindrus, including the presence of numerous entry holes and profuse sawdust emerging from these holes, do not reveal the real dimension of the attack intensity within the trunk. Coupled with this extensive boring activity, the inoculation of ambrosia fungi (Ophiostomatales) is part of the insect’s strategy to establish its offspring in the host trees. Ambrosia beetles and their associated fungi constitute a small part of a much larger food web, the complexities of which we have barely started to understand. There are many questions about the extraordinary complexity of the interactions between these wood-inhabiting beetles, the assembly of fungi which they transmit, and the tree which supports the whole community. We believe that the research described herein with the discovery of a new ambrosia fungi, Ceratocystiopsis quercina, improves the knowledge on the mycobiota associated with the oak pinhole borer in Portuguese cork oak stands, and can help to avoid costly mistakes in the management of these emblematic stands, preserving the economic and cultural heritage of the unique cork oak stands and landscapes present in the Mediterranean.
Author Contributions
Conceptualization, M.L.I., E.S. and F.N.; methodology, M.L.I., E.S. and F.N.; software, F.N. and J.M.; validation, M.L.I., J.M., A.L., E.S. and F.N.; formal analysis, M.L.I., J.M., A.L., E.S. and F.N.; resources, E.S. and F.N.; data curation, M.L.I. and F.N.; writing—original draft preparation, M.L.I. and F.N.; writing—review and editing, M.L.I., F.N., J.M., A.L. and E.S.; visualization, M.L.I., F.N., J.M., E.S. and A.L.; supervision, E.S. and A.L.; project administration, M.L.I.; funding acquisition, M.L.I., E.S. and F.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by the Portuguese Foundation for Science and Technology (FCT) through BD/26033/2005 and the R&D Unit, UIDB/04551/2020 (GREEN-IT—Bioresources for Sustainability) and by Instituto Nacional de Investigação Agrária, I.P.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The generated sequences are in GenBank® nucleic acid sequence database, the National Center for Biotechnology Information (NCBI) at https://www.ncbi.nlm.nih.gov/, accessed on 6 April 2022.
Acknowledgments
The authors would like to thank Marina Cardoso for the technical assistance and Joana Henriques for the contribution in some parts of the practical work. We are also very grateful to Thomas Harrington of Iowa State University (Ames, IA, USA) and Michael Wingfield and Wilhelm de Beer of FABI University of Pretoria (Pretoria, South Africa), for their patience, support, and teachings about Ophiostomatales.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Seabra, A.F. Contribuição para a história da Entomologia em portugal. Publicações Dir. Geral Serv. Florestais Aquícolas 1939, 6, 1–20. [Google Scholar]
- Baeta-Neves, C.M. Lista dos insectos da biocenose do sobreiro (Quercus suber L.). Bol. Soc. Port. Ciên. Nat. 1950, 111, 33–46. [Google Scholar]
- Español, F. Los Platipodidos de Cataluña (Col. Phytophagoidea). Bol. Ser. Plagas 1964, 7, 115–117. [Google Scholar]
- Ferreira, M.C.; Ferreira, G.W. Platypus cylindrus F. (Coleóptera: Platipodidae) Plaga de Quercus suber. Bol. San. Veg. Plagas 1989, 4, 301–305. [Google Scholar]
- Sousa, E.M. Contribuition à l’étude de la biologie de populations de Platypus cylindrus (Coleoptera: Platypodidae) dans des peuplements de chênes liège au Portugal. In Thèse Pour L’Obtention du Diplôme de Doctorat; Université de Lyon: Lyon, France, 1996; p. 153. [Google Scholar]
- Sousa, E.M.; Débouzie, D. Caractéristiques bioécologiques de Platypus cylindrus au Portugal. IOBC/Wprs Bull. 2002, 25, 75–83. [Google Scholar]
- Batra, L.R. Ecology of ambrosia fungi and their dissemination by beetles. Trans. Kans. Acad. Sci. 1963, 66, 213–236. [Google Scholar] [CrossRef]
- Beaver, R.A. Insect-fungus relationships in the bark and ambrosia beetles. In Insect-Fungus Interactions; Wilding, N., Collins, N.M., Hammond, P.M., Webber, J.F., Eds.; Academic Press: London, UK, 1989; pp. 121–143. [Google Scholar]
- Francke-Grosmann, H. Ectosymbiosis in wood-inhabiting insects. In Symbiosis; Henry, S.M., Ed.; Academic Press: New York, NY, USA, 1967; Volume 11, pp. 142–206. [Google Scholar]
- Fraedrich, S.W.; Harrington, T.C.; Rabaglia, R.J.; Ulyshen, M.D.; Mayfield, A.E.; Hanula, J.L.; Eickwort, J.M.; Miller, D.R. A fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the southeastern United States. Plant. Dis. 2008, 92, 215–224. [Google Scholar] [CrossRef]
- Inácio, M.L.; Henriques, J.; Sousa, E. Mycobiota associated with Platypus cylindrus Fab. (Coleoptera: Platypodidae) on cork oak in Portugal. IOBC/Wprs Bull. 2010, 57, 87–95. [Google Scholar]
- Sousa, E.M.; Débouzie, D.D.; Pereira, H. The role of the insect Platypus cylindrus F. (Coleoptera, Platypodidae) in the decline of cork oak stands in Portugal. IOBC/Wprs Bull. 1995, 18, 24–37. [Google Scholar]
- Sousa, E.M.; Débouzie, D.D. Biological characteristics of Platypus cylindrus F. in Portugal. IOBC/Wprs Bull. 2002, 25, 75–83. [Google Scholar]
- Hyde, K.D.; Norphanphoun, C.; Maharachchikumbura, S.S.N.; Bhat, D.J.; Jones, E.B.G.; Bundhun, D.; Chen, Y.J.; Bao, D.F.; Boonmee, S.; Calabon, M.S.; et al. Refined families of Sordariomycetes. Mycosphere 2020, 11, 305–1059. [Google Scholar] [CrossRef]
- Strzałka, B.; Jankowiak, R.; Bilański, P.; Patel, N.; Hausner, G.; Linnakoski, R.; Solheim, H. Two new species of Ophiostomatales (Sordariomycetes) associated with the bark beetle Dryocoetes alni from Poland. MycoKeys 2020, 68, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Kok, L.T. Lipids of ambrosia fungi in the life of the mutualistic beetles. In Insect-Fungus Symbioses; Batra, L.R., Ed.; Halsted Press: Sussex, UK, 1979; pp. 33–52. [Google Scholar]
- Plattner, A.; Kim, J.; Reid, J.; Hausner, G.; Lim, Y.W.; Yamaoka, Y.; Breuil, C. Resolving taxonomic and phylogenetic incongruence within species Ceratocystiopsis minuta. Mycologia 2009, 101, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, M.J.; Barnes, I.; de Beer, Z.W.; Roux, J.; Wingfield, B.D.; Taerum, S.J. Novel associations between ophiostomatoid fungi, insects and tree hosts: Current status—future prospects. Biol. Invasions 2017, 19, 3215–3228. [Google Scholar] [CrossRef]
- Baker, J.M. Ambrosia beetle and their fungi, with particular reference to Platypus cylindrus Fab. Symp. Soc. Gen. Microbiol. 1963, 13, 323–354. [Google Scholar]
- Cassier, P.; Lévieux, J.; Morelet, M.; Rougon, D. The Mycangia of Platypus cylindrus Fab. and P. oxyurus Dufour (Coleoptera: Platypodidae). Structure and associated fungi. J. Insect Physiol. 1996, 42, 171–179. [Google Scholar] [CrossRef]
- Sousa, E.M.; Tomaz, I.L.; Moniz, F.A.; Basto, S. La Répartition Spatiale des Champignons Associés à Platypus cylindrus Fab. (Coleoptera: Platypodidae). Phytopath. Medit. 1997, 36, 153. Available online: https://www.jstor.org/stable/42685302 (accessed on 12 December 2021).
- Morelet, M. Une espèce nouvelle de Raffaelea, isolée de Platypus cylindrus, coléoptère xylomycétophage des chênes. Ann. Soc. Sci. Nat. Archéol. Toulon 1998, 50, 185–193. [Google Scholar]
- Henriques, J.; Inácio, M.L.; Sousa, E. Ambrosia fungi in the insect-fungi symbiosis in relation to cork oak decline. Rev. Iberoam. Micol. 2006, 23, 185–188. [Google Scholar] [CrossRef]
- Belhoucine, L.; Bouhraoua, R.T.; Meijer, M.; Houbraken, J.; Harrak, M.J.; Samson, R.A.; Equihua-Martinez, A.; Pujade-Villar, J. Mycobiota associated with Platypus cylindrus (Coleoptera: Curculionidae, Platypodidae) in cork oak stands of North West Algeria, Africa. Afr. J. Microbiol. Res. 2011, 5, 4411–4423. [Google Scholar] [CrossRef]
- Inácio, M.L.; Henriques, J.; Lima, A.; Sousa, E.B. Ophiostomatoid fungi associated with cork oak mortality in Portugal. IOBC/WPRS Bull. 2012, 76, 89–92. [Google Scholar]
- Soulioti, N.; Tsopelas, P.; Woodward, S.; Holdenrieder, O. Platypus cylindrus, a vector of Ceratocystis platani in Platanus orientalis stands in Greece. For. Pathol. 2015, 45, 367–372. [Google Scholar] [CrossRef]
- Bellahirech, A.; Inácio, M.L.; Woodward, S.; Ben Jamâa, M.L.; Nóbrega, F. Ophiostoma tsotsi and Ophiostoma quercus associated with Platypus cylindrus F. (Coleoptera: Curculionidae) in cork oak stands in Tunisia. For. Pathol. 2018, 49, 856–872. [Google Scholar] [CrossRef]
- Badler, H. Pathogenicity of Ceratocystis spp. in oaks under stress. In Proceedings of an International Congress Recent Advances in Studies on Oak Decline, Selva di Fasano, Brindisi, Italy, 13–18 September 1992; Luisi, N., Lerario, P., Vannini, A., Eds.; pp. 31–37. [Google Scholar]
- Degreef, J. Isolation of Ophiostoma querci (Georgev.) Nannfeldt from declining oaks in Belgium: Selection techniques and pathogenicity test. In Proceedings of an International Congress Recent Advances in Studies on Oak Decline, Selva di Fasano, Brindisi, Italy, 13–18 September 1992; Luisi, N., Lerario, P., Vannini, A., Eds.; pp. 471–473. [Google Scholar]
- Delatour, C.; Menard, A.; Vautrot, A.; Simonin, G. Pathogenicity assessment of Ophiostomatales: Ophiostoma querci on oak compared to O. novo-ulmi on elm. In Proceedings of an International Congress Recent Advances in Studies on Oak Decline, Selva di Fasano, Brindisi, Italy, 13–18 September 1992; Luisi, N., Lerario, P., Vannini, A., Eds.; 1992; pp. 59–65. [Google Scholar]
- Harrington, T.C.; McNew, D.; Steimel, J.; Hofstra, D.; Farrell, R. Phylogeny and taxonomy of the Ophiostoma piceae complex and the Dutch elm disease fungi. Mycologia 2001, 93, 111–136. [Google Scholar] [CrossRef]
- de Beer, Z.W.; Wingfield, B.D.; Wingfield, M. The Ophiostoma piceae complex in the Southern Hemisphere: A phylogenetic study. Mycol. Res. 2003, 107, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, R.D.; de Beer, Z.W.; Jacobs, K.; Wingfield, B.D.; Wingfield, M.J. Multigene phylogenies define Ceratocystiopsis and Grosmannia distinct from Ophiostoma. Stud. Mycol. 2006, 55, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kim, S.H.; Lee, S.; Breuil, C. Distinguishing Ophiostoma ips and Ophiostoma montium, two bark beetle-associated sapstain fungi. FEMS Microbiol. Lett. 2003, 222, 187–192. [Google Scholar] [CrossRef]
- Gorton, C.; Kim, S.H.; Henricot, B.; Webber, J.; Breuil, C. Phylogenetic analysis of the bluestain fungus Ophiostoma minus based on partial ITS rDNA and β-tubulin gene sequences. Mycol. Res. 2004, 108, 759–765. [Google Scholar] [CrossRef]
- Lim, Y.W.; Alamouti, S.M.; Kim, J.J.; Lee, S.; Breuil, C. Multigene phylogenies of Ophiostoma clavigerum and closely related species from bark beetle-attacked Pinus in North America. FEMS Microbiol. Lett. 2004, 237, 89–96. [Google Scholar] [CrossRef][Green Version]
- Zhou, X.; de Beer, Z.W.; Wingfield, M.J. DNA sequence comparisons of Ophiostoma spp., including Ophiostoma aurorae sp. nov., associated with pine bark beetles in South Africa. Stud. Mycol. 2006, 55, 269–277. [Google Scholar] [CrossRef]
- Wingfield, B.D.; Viljoen, C.D.; Wingfield, M.J. Phylogenetic relationships of ophiostomatoid fungi associated with Protea infructescences in South Africa. Mycol. Res. 1999, 103, 1616–1620. [Google Scholar] [CrossRef]
- Nel, W.J.; Wingfield, M.J.; de Beer, Z.W.; Duong, T.A. Ophiostomatalean fungi associated with wood boring beetles in South Africa including two new species. Antonie Leeuwenhoek 2021, 114, 667–686. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Duong, T.; Taerum, S.; Wingfield, M.; Zhou, X.; Yin, M.; de Beer, Z. Ophiostomatoid fungi associated with the spruce bark beetle Ips typographus, including 11 new species from China. Persoonia 2019, 42, 50–74. [Google Scholar] [CrossRef] [PubMed]
- Jankowiak, R.; Solheim, H.; Bilański, P.; Marincowitz, S.; Wingfield, M.J. Seven new species of Graphilbum from conifers in Norway, Poland, and Russia. Mycologia 2020, 112, 1240–1262. [Google Scholar] [CrossRef] [PubMed]
- Roets, F.; De Beer, Z.W.; Crous, P.W.; Dreyer, L.L.; Wingfield, M.J. Ophiostoma gemellus and Sporothrix variecibatus from mites infesting Protea infructescences in South Africa. Mycologia 2008, 100, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Lücking, R.; Aime, M.C.; Robbertse, B.A.N.; Ariyawansa, H.A.; Aoki, T.; Cardinali, G.; Crous, P.W.; Druzhinina, I.; Geiser, D.M.; Hawksworth, D.L.; et al. Unambiguous identification of fungi: Where do we stand and how accurate and precise is fungal DNA barcoding? IMA Fungus 2020, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Massoumi Alamouti, S.; Tsui, C.M.; Breuil, C. Multigene phylogeny of filamentous ambrosia fungi associated with ambrosia and bark beetles. Mycol. Res. 2009, 113, 822–835. [Google Scholar] [CrossRef]
- Zanzot, J.W.; de Beer, Z.W.; Eckhardt, L.G.; Wingfield, M.J. A new Ophiostoma species from loblolly pine roots in the southeastern United States. Mycol. Progress 2010, 9, 447–457. [Google Scholar] [CrossRef]
- Saccardo, P.A. Chromotaxia seu nomenclator colorum polyglottus adclitis speciminibus coloratis ad botanicorum et zoologorum. Patavii 1891, 22. [Google Scholar]
- Riddell, R.W. Permanent stained mycological preparations obtained by slide culture. Mycologia 1950, 42, 265–270. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.J.; Fung, S.; Breuil, C. A PCR-RFLP marker distinguishing Ophiostoma clavigerum from morphologically similar Leptographium species associated with bark beetles. Can. J. Bot. 2003, 81, 1104–1112. [Google Scholar] [CrossRef]
- Matsuda, Y.; Kimura, K.; Ito, S. Genetic characterization of Raffaelea quercivora isolates collected from areas of oak wilt in Japan. Mycoscience 2010, 51, 310–316. [Google Scholar] [CrossRef]
- Harrington, T.C.; Aghayeva, D.N.; Fraedrich, S.W. New combinations in Raffaelea, Ambrosiella, and Hyalorhinocladiella, and four new species from the redbay ambrosia beetle. Xyleborus glabratus. Mycotaxon 2010, 111, 337–361. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: Genes for Phylogenetics; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 315–322. [Google Scholar]
- Rehner, S.A.; Samuels, G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- O’Donnell, K. Fusarium and its near relatives. In the Fungal Holomorph: Mitotic, Meiotic and Pleomorphic Speciation in Fungal Systematic; Reynolds, D.R., Taylor, J.W., Eds.; CAB International: Wallingford, UK, 1998; pp. 225–233. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Kusaba, M.; Tsuge, T. Phylogeny of Alternaria fungi known to produce host-specific toxins on the basis of variation in internal transcribed spacers of ribosomal DNA. Curr. Genet. 1995, 28, 491–498. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1330. [Google Scholar] [CrossRef]
- Gebhardt, H.; Begerow, D.; Oberwinkler, F. Identification of the ambrosia fungus of Xyleborus monographus and X. dryographus (Coleoptera: Curculionidae, Scolytinae). Mycol. Prog. 2004, 3, 95–102. [Google Scholar] [CrossRef]
- Gebhardt, H.; Oberwinkler, F. Conidial development in selected ambrosial species of the genus Raffaelea. Antonie Leeuwenhoek 2005, 88, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing 524 platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Inácio, M.L.; Marcelino, J.; Lima, A.; Sousa, E.; Nóbrega, F. Raffaelea quercina sp. nov. associated with cork oak (Quercus suber L.) decline in Portugal. Forests 2021, 12, 513. [Google Scholar] [CrossRef]
- Musvuugwa, T.; de Beer, Z.W.; Duong, T.A.; Dreyer, L.L.; Oberlander, K.C.; Roets, F. New species of Ophiostomatales from Scolytinae and Platypodinae beetles in the Cape Floristic Region, including the discovery of the sexual state of Raffaelea. Antonie Leeuwenhoek J. Microbiol. 2015, 108, 933–950. [Google Scholar] [CrossRef] [PubMed]
- Mayers, C.G.; Harrington, T.C.; Ranger, C. First report of a sexual state in an ambrosia fungus: Ambrosiella cleistominuta sp. nov. associated with the ambrosia beetle Anisandrus maiche. Botany 2017, 95, 503–512. [Google Scholar] [CrossRef]
- Nkuekam, G.K.; DeBeer, W.Z.; Wingfield, M.J.; Roux, J. A diverse assemblage of Ophiostoma species, including two new taxa on eucalypt trees in South Africa. Mycol. Progress 2011, 11, 515–533. [Google Scholar] [CrossRef]
- Kirisits, T. Fungal associates of European bark beetles with special emphasis on the ophiostomatoid fungi. In Bark and Wood Boring Insects in Living Trees in EUROPE, a Synthesis; Lieutier, F., Day, K.R., Battisti, A., Grégoire, J.-C., Evans, H.F., Eds.; Springer: Amsterdam, The Netherlands, 2007; pp. 181–235. [Google Scholar]
- Harrington, T.C.; Fraedrich, S.W.; Aghayeva, D.N. Raffaelea lauricola, a new ambrosia beetle symbiont and pathogen on the Lauraceae. Mycotaxon 2008, 104, 399–404. [Google Scholar]
- Kim, S.; Harrington, T.C.; Lee, J.C.; Seybold, S.J. Leptographium tereforme sp. nov. and other Ophiostomatales isolated from the root-feeding bark beetle Hylurgus ligniperda in California. Mycologia 2011, 103, 152–163. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.; Marçais, B.; Nageleisen, L.-M.; Piou, D.; Vannini, A. Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 597–612. [Google Scholar] [CrossRef]
- de Beer, W.; Procter, M.; Wingfield, M.J.; Marincowitz, S.; Duong, T.A. Generic boundaries in the Ophiostomatales reconsidered and revised. Stud. Mycol. 2022, 101, 57–120. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).